1. Introduction
Inflammation is a condition that has been a burden
for decades. Research on the pathophysiology of inflammation has
been gradually deepening over the recent decades (1,2).
However, for certain chronic refractory inflammations, such as
Crohn's disease, difficult-to-treat rheumatoid arthritis, severe
asthma, atopic dermatitis or cutaneous lupus (3-6),
no suitable cure has been found. At present, there are various
treatments for persistent inflammation, including immunotherapy to
restore normal lymphocyte count by using granulocyte-macrophage
colony-stimulating factor and granulocyte-colony-stimulating factor
pair, anti-inflammatory and antioxidant therapy, anabolic and
anti-catabolic therapy and nutritional support therapy (7). For chronic inflammation of the
respiratory system, especially chronic obstructive pulmonary
disease (COPD), the current use of home oxygen therapy,
expectorants, long-acting bronchodilators, such as long-acting
muscarinic antagonists or long-acting β2-agonists,
antibiotics, influenza and pneumococcal vaccines, can only relieve
the clinical symptoms of patients and prevent the further
aggravation of the disease (8).
Similarly, the current treatment strategy for asthma, a common
chronic non-communicable respiratory disease, is limited to
controlling asthma symptoms and reducing the risk of recurrence
(9).
By contrast, cancer has become a major public health
issue in the 21st century, accounting for 25% of the global
mortality cases from non-communicable diseases, according to a 2022
Global Cancer Statistics report (10). With the continuous exploration of
cancer treatment, various ILs have been previously shown to serve a
critical role in the development of cancer, including IL-2, IL-6,
IL-10, IL-12 and IL-35 (11-15).
Amongst them, tumors frequently jointly produce IL-8, a chemotactic
factor, which serves different roles in promoting tumor processes,
such as angiogenesis and survival signal transduction in cancer
stem cells (16,17).
Evidence have been accumulating that gradually
deepened the knowledge into the various cytokine signaling
pathways, which has led to the development of a series of small
molecule antagonists and small molecule inhibitors targeting such
signaling pathways. For inflammation and cancers, IL-1R1
(MEDI8968), IL-5 (mepolizumab) or IL-5R (benralizumab), IL-8
(ABX-IL8), IL-18 (MEDI2338), IL-33 (MEDI3506) have been developed
for the treatment of COPD (18),
whereas AZD5069 is in the clinical development stage of cancer such
as triple-negative breast cancer (18,19).
In addition, AZD8039, danirixin (GSK1325756), ladarixin (DF2156A),
reparixin and SB656933(20), which
are more effective compared with traditional treatments for acute
respiratory distress syndrome, COVID-19 pneumonia, inflammatory
bowel disease (IBD), liver cancer and lung cancer have been
garnering interest.
At present, a novel and selective C-X-C motif
chemokine receptor (CXCR)1/2 antagonist that can exert therapeutic
effects against both inflammation and cancers, SCH527123, which can
inhibit the binding (or activation) of chemokines to CXCR1/2 in a
high-affinity manner, has been discovered (21,22).
It can inhibit signal transduction, neutrophil chemotaxis and
myeloperoxidase release (23,24).
It may have clinical value for the treatment of diseases such as
acquired immune deficiency syndrome, IBD and non-small cell lung
cancer directly (or indirectly) mediated by CXCR1/2-expressing
cells (25,26).
To the best of our knowledge, the present review is
the earliest of SCH527123 in the field of inflammation and cancer
treatment. It aims to discuss the history of the development of
SCH527123 and its mechanism of action, whilst summarizing the
application and achievements of SCH527123 in asthma, COPD,
pancreatic cancer and liver cancer and animal models (namely in
mice and rabbits) over recent years. In addition, limitations of
SCH5271213 development are discussed, where the clinical value of
SCH527123 in the treatment of diseases in the future was also
explored, to lay a foundation for future research.
2. C-X-C motif chemokine ligand (CXCL)8/IL-8
is involved in inflammation and tumorigenesis
IL-8 is a basic protein containing four cysteine
residues and two intramolecular disulfide bonds which stabilize the
structure of IL-8, that has strong resistance to deformation
processing, such as plasma peptidase, high temperature and
acid-base environment (27-31).
In addition, IL-8 is a neutrophil chemotactic polypeptide (32). In in vitro experiments, IL-8
has shown chemotactic activity to T lymphocytes, basophils and
neutrophils Although it can promote the migration of T cells and
neutrophils, it requires additional factors to do so (33).
A study performed in 1989 injected IL-8 into the
skin of rabbits and observed the accumulation of neutrophils and
formation of neutrophil-dependent edema in the rabbit skin
(34). Subsequently, 2 years
later, clinical trials demonstrated for the first time the presence
of bioactive substances such as melanoma growth stimulatory
activity, P-thromboglobulin and platelet factor 4 in the synovial
fluid of patients with rheumatoid arthritis and the expression of
IL-8 mRNA in synovial cells (35).
The level of IL-8 detected in patients with metastatic melanoma,
among 56 confirmed cases, showed that some had markedly elevated
serum IL-8 which correlated with tumour load but was independent of
the site of metastasis (36). This
clinical observation is supported by mechanistic studies summarized
by Waugh and Wilson (37), who
described how IL-8, through its receptors CXCR1 and CXCR2, promotes
melanoma cell migration and invasion. Additionally, IL-8
contributes to an immunosuppressive tumor microenvironment by
recruiting myeloid-derived suppressor cells (MDSCs) and inhibiting
lymphocytic infiltration, which together facilitate tumor
progression and metastasis (37).
Using cell culture, IL-8 has also been found to be involved in
airway mucosal tumor progression (38). These aforementioned experimental
data all suggest the important role of IL-8 in inflammation and
tumor development. The following sections of the present review
will discuss the role of IL-8 in inflammation and tumor
development.
Inflammation is the typical bodily response to
injury, resulting from various causes, such as physical injury,
ischemia-reperfusion injury, infection, toxin exposure, malignant
tumors and autoimmune reactions, all of which would otherwise lead
to tissue damage (39). The
capacity of IL-8 to attract and activate neutrophils has rendered
it a key inflammatory mediator from the initial stages of this
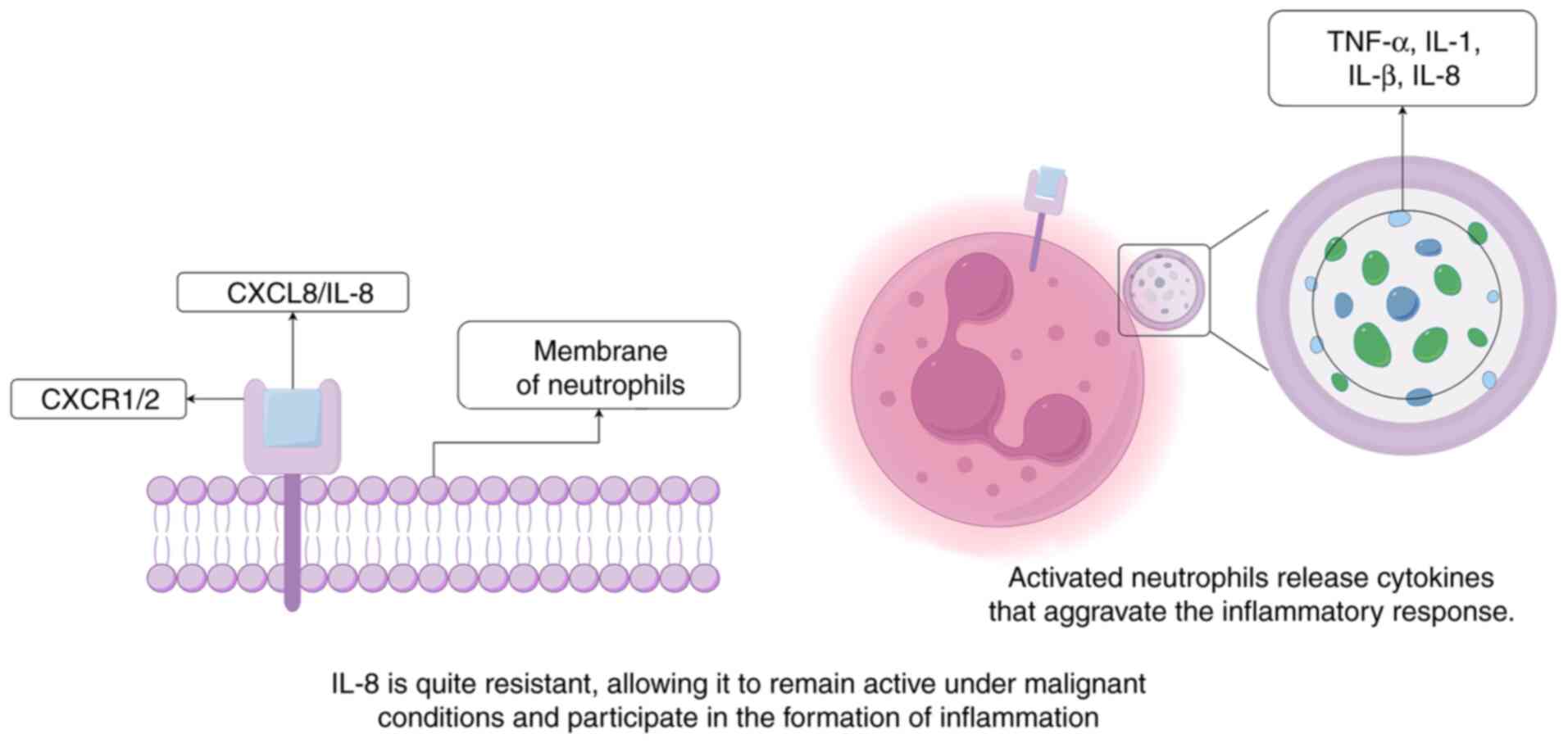
process (33,40). IL-8 can bind to the CXCR2 receptor
on the surface of neutrophils, leading to chemotaxis and the
activation of neutrophils, causing them to secrete various
cytokines, such as TNF-α, IL-1α, IL-1β and IL-8, exacerbating the
occurrence of inflammation (Fig.
1) (41). IL-8 is relatively
resistant to temperature, protein hydrolysis and acidic
environments. These resistances enable IL-8 to maintain its
activity under malignant conditions, making it a main molecule at
sites of acute inflammation (40).
Furthermore, IL-8 is frequently produced early in inflammation and
can remain active at the site of inflammation for extended periods,
up to several weeks. In acute inflammatory responses, such as
bacterial infections or tissue injury, IL-8 activity generally
lasts several weeks, whereas in chronic conditions, such as
rheumatoid arthritis, chronic obstructive pulmonary disease (COPD),
or certain cancers, it may remain elevated for several months. This
is in contrast to the majority of the other inflammatory cytokines,
such as TNF-α, IL-1β and interleukin-6 (IL-6), which will typically
disappear from the site of inflammation within a few hours in the
body (42). The prolonged activity
of IL-8 may be closely associated with its important role in the
occurrence of inflammation.
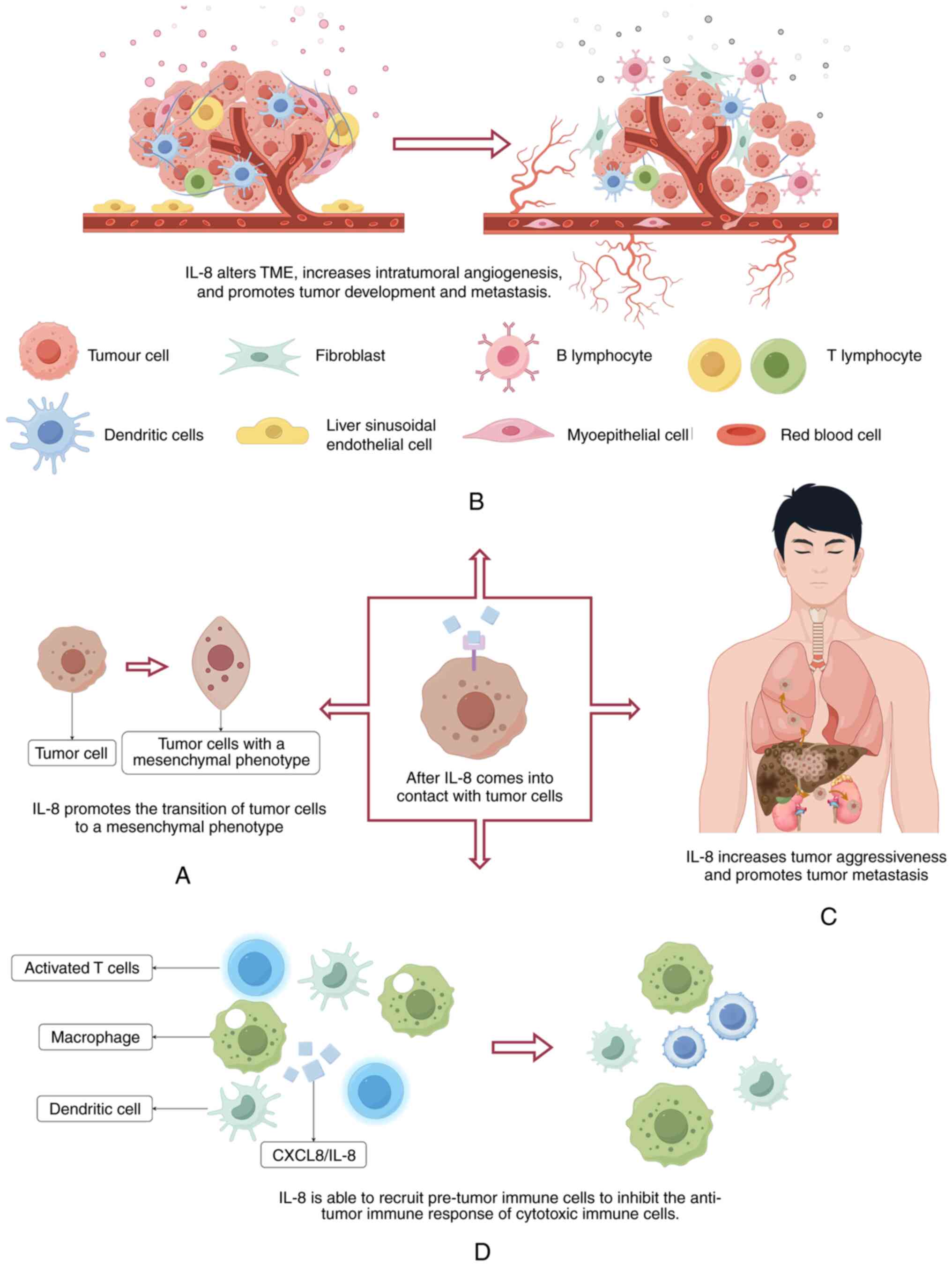
IL-8 has been shown to be associated with the
physiology of various cancers, including melanoma, prostate, colon,
pancreatic, breast and lung cancer (43). IL-8 can directly impact tumors by
altering the characteristics of tumor cells, inducing the
transition of tumor cells to a more mesenchymal phenotype,
consequently promoting migration and proliferation (44). This in turn also promotes the
occurrence of metastasis in vivo (45). By recruiting additional
infiltrating immunosuppressive cells, IL-8 increases angiogenesis
within tumors and alters the immune microenvironment, which further
promotes tumor growth and metastasis (46). The formation of tumors requires the
support of the surrounding normal matrix, which is referred to as
the tumor microenvironment (TME). IL-8 can not only influence the
cellular phenotypes of tumors and endothelial cells within the TME,
but can also alter the immune composition at the tumor site. IL-8
preferentially recruits pre-tumor immune cells (including
myeloid-derived suppressor cells, tumor-associated macrophages,
tumor-associated neutrophils and Natural Killer cells), thereby
suppressing the anti-tumor immune response of cytotoxic immune
cells (16). IL-8 gene
silencing has been shown to suppress the growth of ovarian cancer
through an anti-angiogenic mechanism (Fig. 2) (47). The aforementioned factors all
indicate that IL-8 serves a promotional role in the process of
tumorigenesis.
3. Mechanism of action for SCH527123
Since IL-8 and related chemokines (such as CCL2,
CXCL12, CCL5 and CX3CL1) have shown strong association in a variety
of inflammatory conditions and cancers, the research discovery of
small molecule antagonists of CXCR1/2 as novel targets has been
garnering attention. In 1998, the first potent and selective CXCR2
receptor antagonist SB225002 was reported (48). Subsequently, in 2003, PD0220245 was
demonstrated to be the most effective IL-8 antagonist at the time,
where compared with SB225002, PD0220245 was able to inhibit both
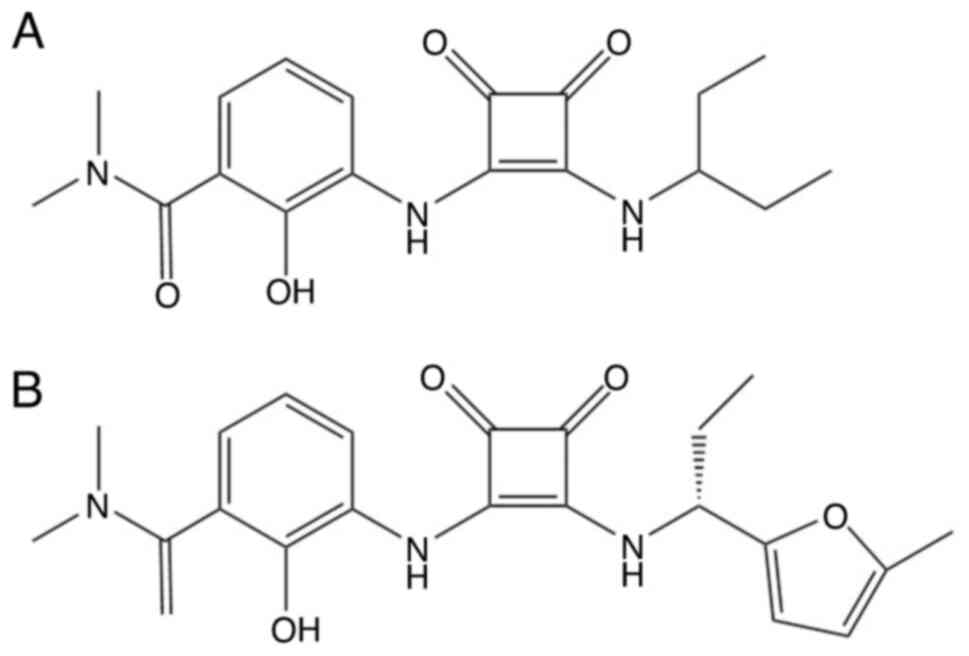
CXCR1 and CXCR2 as a dual inhibitor (49). In 2006, Dwyer et al
(50) discovered that the
furan-based derivative of lead (Fig.
3A) was part of the active structure of lead cyclobutene dione,
leading to the development of SCH527123 (Fig. 3B). This is a type of selective
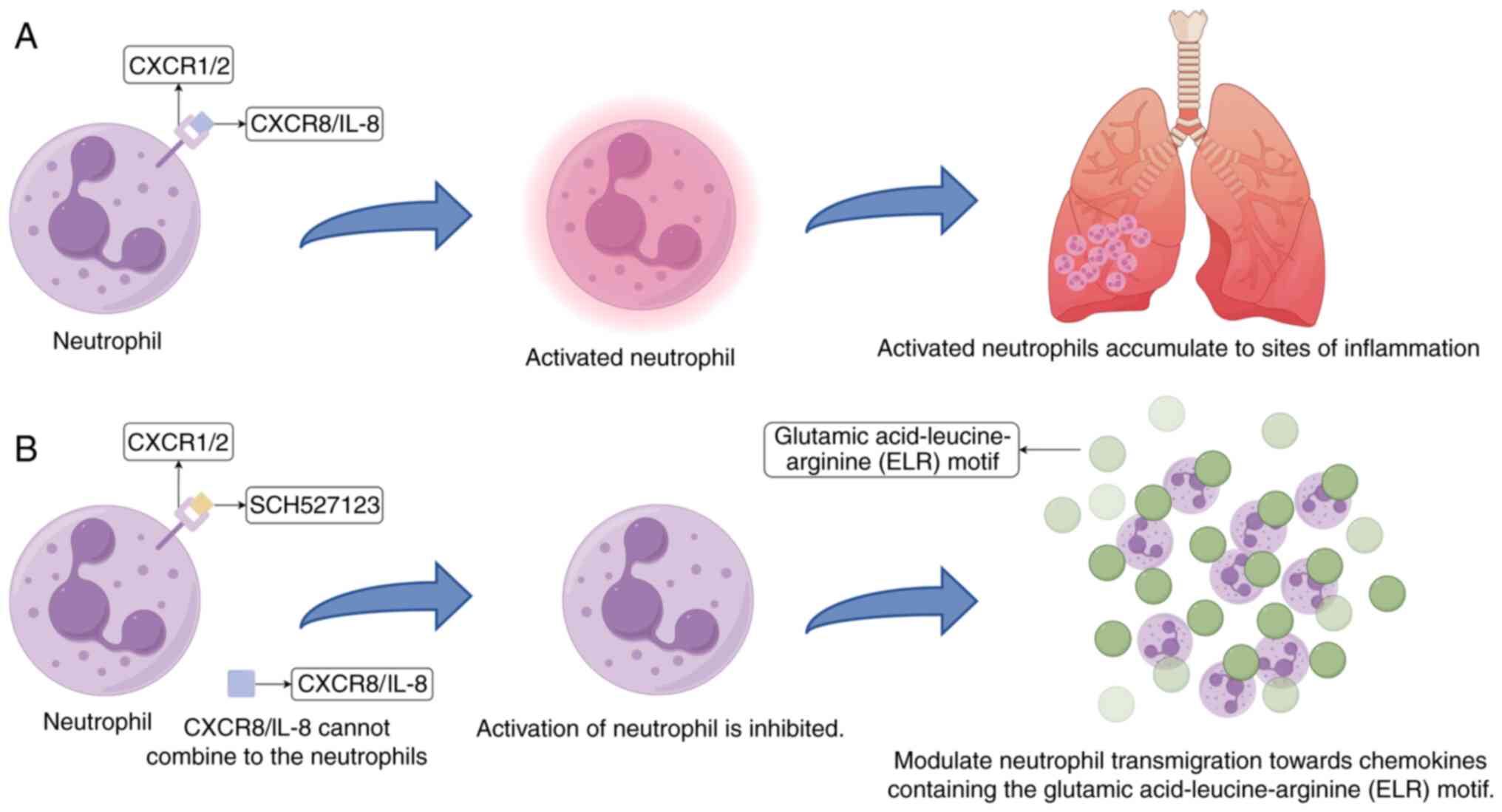
CXCR1/2 antagonist, also known as navarixin (20). CXCL8/IL-8 has been demonstrated to
induce the migration of neutrophils (51), which meant that CXCL8/IL-8 can
induce the recruitment of neutrophils to inflammatory sites by
interacting with CXCR1/2 expressed on the surfaces of these cells
(52). SCH527123 acts as a CXCR1/2
antagonist by blocking the binding of ELR-motif chemokines to
CXCR2, thereby inhibiting downstream signaling pathways (AKT, ERK,
STAT3 and S6 phosphorylation). This suppresses neutrophil
activation and migration toward chemokines, while also reducing
tumor cell proliferation and survival, resulting in comprehensive
modulation of immune cell function and tumor progression. These
chemokines include IL-8, growth homolog (Gro)-α, epithelial
cell-derived neutrophil attractant-78 and neutrophil-activating
peptide-2(53). SCH527123 can
directly block the binding of CXCL8/IL-8 to CXCR1/2, thereby
interrupting the subsequent series of harmful reactions that
CXCL8/IL-8 would otherwise trigger in the human body (Fig. 4). Although SCH527123 can bind to
CXCR1 and CXCR2, SCH527123 binds to CXCR1 with lower affinity
(Kd=3.9±0.3 nM), such that the compound is more
CXCR2-selective (Kd=0.049±0.004 nM). Therefore,
SCH527123 has a CXCR2-specific antagonistic effect, which also
suggests its potential therapeutic effect on inflammation.
4. Role of SCH527123 in relevant
diseases
SCH527123 is a novel small molecule antagonist that
serves as an effective allosteric inhibitor for both CXCR1 and
CXCR2, exhibiting high affinity towards both receptors (albeit with
a higher selectivity for CXCR2). It exerts its therapeutic effects
by suppressing the chemotactic activity of all chemokines on
CXCR1/2, thereby alleviating or treating diseases (25).
Asthma
Asthma is caused by chronic inflammation in the
lower respiratory tract. It is a relatively common disease and a
2024 analysis based on the Global Burden of Disease Study found
asthma prevalence rates of 9.1% in children, 11.0% in adolescents,
and 6.6% in adults worldwide. Variations across regions highlight
asthma as a common and significant public health concern (54). The main symptoms of asthma are
breathing difficulties, shortness of breath, cough and chest
tightness (54). During severe
asthma, neutrophils and eosinophils can be found in the lung
epithelium and mucus. The expression of ELR+ chemokine and its
receptor was found to be increased (ELR+ chemokines, including IL-8
and CXCL1-3) (55), where CXCR1/2
was observed to be expressed on airway smooth muscle cells and
mediated cell contraction and migration to enhance airway
responsiveness and remodeling, causing bronchoconstriction.
IL-8/CXCR2-dependent neutrophil recruitment is critical for the
development of asthma, where blocking this signaling pathway may
provide a novel approach for its treatment (56).
In 1996, a clinical trial on patients with asthma
with a mean age of 39 years found that their bronchoalveolar lavage
fluid contained higher levels of IL-8 compared with those from
healthy individuals, suggesting that non-specific inflammation may
be occurring in the airway (57).
Neutrophils are recruited to the lungs in response to various
chemokines, such as IL-8, GRO-α, MIP-2 and MIP-1 α, which then
accumulate in the airways of patients with asthma (58). The biological effects of IL-8 are
mediated through chemokine receptors CXCR1 and CXCR2(59). In 2012, a number of clinical
studies have reported that blocking CXCR1/2 was safe and
well-tolerated, which could reduce the numbers of sputum
neutrophils in patients with severe asthma (58). In 2016, 19 non-smoking patients
with mild allergic asthma were included in a clinical study. This
previous study demonstrated that CXCLs, including IL-8 and their
receptors CXCR1 and CXCR2, are upregulated in the airways during
asthma exacerbations (60).
Therefore, inhibiting neutrophil chemotaxis by blocking CXCR1 and
CXCR2 may provide an alternative therapeutic strategy for patients
with asthma complicated with neutrophilic inflammation. By
targeting cells that accumulate in airway tissues through
chemotaxis mediated by CXCR1 and CXCR2, the 2016 study conducted by
Todd et al (61) showed
that SCH527123 has emerged as a novel therapeutic agent that may
exert a specific therapeutic effect on inflammation.
COPD
COPD is an insidious but progressive chronic
inflammatory disease of the respiratory system, characterized by an
obstructive ventilation pattern, manifested as incompletely
reversible airflow limitation. In severe cases, it can lead to
chronic respiratory failure (53,62).
Due to the inhalation of harmful particles and gases (such as
chemicals in cigarette smoke) or aberrant innate immune responses,
epithelial cells and resident macrophages can become activated.
These cells in turn release cytokines and chemokines, thereby
recruiting neutrophils to the lungs. The number of activated
macrophages and neutrophils in the lungs then increases, where the
proteases they secrete causes alveolar damage, leading to the
spread of inflammation during COPD (53,63).
CXCR1/2 serves an important role in the chemotaxis
of neutrophils. By studying eight patients with COPD and eight
smokers without COPD, IL-8 was demonstrated to serve a major role
in neutrophil chemotaxis induced by alveolar macrophage-derived
conditioned medium, where blockade of CXCR1 and CXCR2 was observed
to inhibit this chemotaxis. Furthermore, the dual CXCR1/2
antagonist SCH527123 exerted a superior inhibitory effect on
neutrophils compared with that mediated by the single CXCR2
antagonist SB656933, whilst also demonstrating a greater role in
blocking the chemotaxis of IL-8(63). Another study conducted a
single-point, randomized, double-blind, multi-dose,
placebo-controlled, three-way cross-over study on healthy
non-smoking volunteers with normal airway reactivity, which found
that SCH527123 could inhibit the migration of neutrophils to CXCLs,
thereby reducing the number of neutrophils in the sputum of healthy
non-smoking volunteers exposed to ozone (53). Various stimuli, such as cigarette
smoke, air pollution and ozone, can activate lung macrophages,
leading to the secretion of several cell factors, such as IL-8,
TNF-α, IL-1β, IL-6, CCL2 and GM-CSF, which causes an inflammatory
response and the aggregation of neutrophils at the site of
inflation. Subsequently, the various enzymes released by
neutrophils and the mucus produced by hypertrophic goblet cells can
also contribute to the progression of COPD (64-67).
Therefore, controlling the number of neutrophils using medications
appears to be key to treating COPD. Therefore, it can be
hypothesized that SCH527123 can be a treatment option for COPD,
where its ability to reduce the number of neutrophils may serve a
role in the disease treatment process.
5. CXCL8/IL-8 and SCH527123 in cancers
Accumulating evidence has indicated that TME serves
a key role in promoting cancer progression and regulating the
response to standard treatments (68,69).
Various components can coexist and interact within the TME,
including tumor-associated macrophages, CD4+ and CD8+ T cells,
dendritic cells, natural killer cells, tumor-associated endothelial
cells, the abnormal tumor vasculature, cancer-associated
fibroblasts and myeloid-derived suppressor cells (70). The presence of inflammatory cells
and immune regulatory mediators in the TME polarizes the host
immune response towards specific phenotypes that affect the
progression of tumors (71).
Various studies have previously found that CXCLs are expressed at
increased levels through both autocrine and paracrine pathways in
different types of tumors, suggesting their involvement in tumor
growth and metastasis (72,73).
CXCR2 chemokine ligands, including CXCL1-8, have all been known to
be secreted and expressed in various types of cancers, including
solid tumors, such as melanoma, breast, lung, bladder, pancreatic,
liver, prostate and colorectal cancer, in addition to hematological
malignancies (43). This section
primarily focused on liver, pancreatic, ovarian and colon cancer,
as the CXCL/CXCR2 signaling pathway is highly active in these
tumors and closely linked to inflammatory tumor microenvironment,
tumor growth and metastasis. Moreover, these types of cancer are
clinically common with poor prognosis, making them ideal targets
for evaluating the potential efficacy of the CXCR2 antagonist
SCH527123 (Table I).
 | Table ISummary of the role of SCH527123 in
various cancers. |
Table I
Summary of the role of SCH527123 in
various cancers.
| First author/s,
year | Disease | Experimental
result | (Refs.) |
|---|
| Goral et al,
2015 | Liver cancer | CXCR1, CXCR2 and
IL-8 proteins were highly expressed in the cytoplasm of
hepatocellular carcinoma cells, where IL-8 induced the expression
of CXCR1/2. | (82) |
| Fu et al,
2018 | Pancreatic
cancer | SCH527123 can
inhibit the proliferation of PDAC cells by reducing the activity of
cancer cells, inhibiting cancer cell proliferation and blocking
signaling pathways, including the IL-8/CXCR1/2 signaling
pathway. | (26) |
| Ning et al,
2012 | | SCH527123 was found
to inhibit tumor growth in a mouse subcutaneous xenograft
model. | (87) |
| Fu et al,
2018 | | The combination of
SCH527123 and bazedoxifene showed a potent inhibitory effect on
PDAC cells. | (88) |
| Zhang et al,
2022 | Ovarian cancer | Bazedoxifene
hydrochloride and SCH527123 were used to inhibit the proliferation
of ovarian cancer cells in mice by blocking the IL-6 and IL-8
pathways. | (93) |
| Singh et al,
2011 | | IL-8 gene
silencing can inhibit the invasion of tumor cells. | (98) |
| Singh et al,
2011 | | Silencing
CXCR1/2 regulated endothelial cell growth, migration,
survival and new vessel formation, in addition to ERK1/2
phosphorylation by using shRNA. | (98) |
| Varney et
al, 2011 | Colon cancer | Oral administration
of SCH527123 inhibited CXCR1/2 signaling, malignant cell survival
and new vessel formation to inhibit liver metastasis by human colon
cancer in a mouse model. | (80) |
| Ning et al,
2012 | | IL-8/CXCR2 pathway
is the key to the development of colon cancer. SCH527123 has
anti-tumor effect through NF-κB/AKT/MAPK signaling pathway. | (87) |
Liver cancer
Liver cancer is one of the most common fatal cancers
worldwide. HCC ranks as the 6th most common cancer and the 3rd
leading cause of cancer-related death worldwide (74). Even after curative surgical
resection, hepatocellular carcinoma patients have a 5-year overall
survival rate of 30-50% and a median survival of 60 months. This
reflects high recurrence risk and poor long-term prognosis
post-resection, highlighting the malignancy's aggressiveness and
therapeutic challenges (75).
There are various risk factors for liver cancer, including
hepatitis virus infection, fatty liver disease, alcohol-related
cirrhosis, smoking, obesity, diabetes, iron overload and various
dietary exposures (aflatoxins, high-fat diet, red and processed
meats and alcohol consumption) (76). A definitive diagnosis is typically
made when patients are already in the advanced stages of the
disease, where due to the high frequency of recurrence and distant
metastasis after surgical excision, prognosis is poor (76,77).
Previous studies have discovered that the expression of IL-8 is
upregulated and involved in the progression of various human
cancers, including liver cancer (78,79).
In in vitro cultured human liver cells, IL-8 has been shown
to serve a role in promoting cell migration and/or invasion
(77). Therefore, inhibiting the
function of IL-8 may suppress liver cancer malignancy.
Although there is no direct in vivo evidence
that CXCR1 and CXCR2 are expressed in liver cancer, according to an
analysis of immunohistochemical results in China (78) by Bi et al (77), IL-8, CXCR1 and CXCR2 levels were
elevated in peripheral blood mononuclear cells of liver cancer
patients and immunohistochemistry showed higher CXCR1 and CXCR2
expression in tumor tissues compared with adjacent non-cancerous
tissues. CXCR1/CXCR2 expression correlated with clinical stage and
metastasis. In vitro, IL-8 promoted liver cancer cell
migration and invasion, which was attenuated by CXCR1/CXCR2
blockade. These findings indicate that high IL-8 secretion induces
CXCR1 and CXCR2 overexpression on both tumor and inflammatory
cells, contributing to liver cancer progression. This can further
confirm that the high level of IL-8 secretion by liver cancer cells
induces the overexpression of CXCR1 and CXCR2 on both liver cancer
and inflammatory cells, which in turn participate in the
occurrence, invasion and metastasis of liver cancer (77). The study by Bi et al
(77) provides experimental
evidence that IL-8 promotes liver cancer cell migration via CXCR1
and CXCR2. Immunohistochemical and RT-qPCR analyses showed that
IL-8, CXCR1, and CXCR2 were significantly upregulated in liver
cancer tissues compared with normal liver tissues, with IL-8 mRNA
expression positively correlated with CXCR1 (r=0.618, P<0.01)
and CXCR2 (r=0.569, P<0.01). In vitro, treatment of Huh-7
and HepG2 cells with 50 ng/ml IL-8 for 24 h increased wound closure
from 35-78 and 32-72%, respectively in wound healing assays, and
significantly enhanced Transwell migration and invasion
(P<0.01). Pre-treatment with 5 µM CXCR1 or CXCR2 antagonists for
30 min reduced IL-8-induced migration by ~60% (P<0.01),
indicating that the effect is mediated through these receptors.
These results demonstrate that IL-8 facilitates liver cancer cell
migration and invasion in a CXCR1/2-dependent manner. SCH527123 can
inhibit the binding of IL-8 with CXCR1 and CXCR2, thereby
suppressing the chemotactic migration and angiogenic response of
malignant human colon cancer cells (80), slowing down or preventing the
progression of liver cancer, which has development potential for
treating liver cancer or extending the survival cycle of patients
with advanced liver cancer.
Pancreatic cancer (PC)
PC is a deadly cancer among the most insidious
cancers at early stages; typically asymptomatic and hard to detect
early, leading to late diagnosis and poor outcomes, with 90% cases
being of the ductal adenocarcinoma (PDAC) subtype (81). Because the pancreas is located in
the retroperitoneum (82) and its
deep position, its malignancies cannot be readily found during the
early stages clinically, where a variety of inherent factors (such
as age, sex, environment, blood type and family history) and
lifestyle factors (such as smoking, drinking, eating habits and
obesity) have all been reported to be risk factors for PC (83). All of these factors further
exacerbate the already high mortality rate of PC. The traditional
treatment method of PC includes surgery, chemotherapy, radiotherapy
and palliative treatment (84).
Over the previous decade, research on the immunotherapy of this
cancer has been gradually expanding (82).
Although the etiology of PC remains unclear,
accumulating evidence has shown that IL-8 and its receptors CXCR1
and CXCR2 are involved in various tumor initiation and
developmental stages (85,86). Inhibition of the CXCR1/2 signaling
pathway has been reported to reduce the activation of downstream
effectors AKT, ERK, STAT3 and S6, inhibiting the proliferation of
PDAC cells (27). SCH527123 has
also been shown to inhibit tumor growth in a xenograft model
(87). Fu and Lin (88) previously found a potent inhibitory
effect of SCH527123 in combination with bazedoxifene on PDAC cells
(88). Taken together, these
results suggest that SCH527123 may serve be a novel targeted
therapy drug that can be used for the treatment of PC
clinically.
Ovarian cancer
Ovarian cancer has one of the poorest survival rates
of all cancers of the female reproductive system (89), which also ranks as the seventh most
common female cancer in the world, 10th in China (90). Between 1990 and 2019, the incidence
of ovarian cancer in China increased by an average of 2.03%
annually and the mortality rate by 1.58% annually. This
three-decade data change shows the growing disease burden of
ovarian cancer in China, with rising new cases and deaths
continuously threatening women's health (90). A number of factors, such as older
age, genetic factors, family history, history of other cancers,
smoking and high fat diet, are risk factors of ovarian cancer
(91). Despite the recognition of
ovarian cancer, treatment and survival trends have not changed
significantly, because the means of early diagnosis are not
accurate, where >50% patients are diagnosed already at advanced
stages. In addition, ~75% patients will relapse due to intrinsic
and acquired chemotherapy resistance, contributing to cancer
recurrence. These are the two main reasons for the low 5-year
overall survival rate (92).
Therefore, the development of targeted therapy with a precise site
of action and few side effects is crucial for the treatment of
ovarian cancer.
Previous mouse experiments have used bazedoxifene
hydrochloride and SCH527123 combined to block IL-6 and IL-8
pathways to inhibit ovarian cancer cell proliferation (93,94).
It has also been previously found that targeted therapy with IL-8
siRNA-1,2-dioleoyl-sn-glycero-3-phosphocholine combined with
chemotherapy effectively reduced tumor growth in both
chemotherapy-sensitive and chemotherapy-resistant ovarian cancer
models, by observing human ovarian cancer section specimens and
female athymic mouse orthotopic cancer models (48,95,96).
To the best of our knowledge, no study has reported targeting IL-8
as a treatment strategy for ovarian cancer, but it has been
previously found that silencing the IL-8 gene can inhibit
ovarian tumor cell invasion (97).
Furthermore, it has been demonstrated that silencing CXCR1
or CXCR2 can modulate endothelial cell proliferation,
migration, survival and new vessel formation, in addition to ERK1/2
phosphorylation (98). Silencing
CXCR1 or CXCR2 has been shown to modulate endothelial
cell proliferation, migration, survival, and angiogenesis through
pathways including ERK1/2 phosphorylation. CXCL1 and CXCL2, as
ligands of CXCR2, further regulate endothelial cell proliferation
by activating ERK1/2 and PI3K/AKT signaling, promoting cell cycle
progression and survival (99). In
ovarian cancer, the IL-8/CXCR1/2 axis plays a critical role in
tumor progression by promoting tumor-associated angiogenesis, which
supplies nutrients and oxygen to support tumor growth. Activation
of this pathway also enhances tumor cell migration and invasion,
facilitating metastasis, particularly within the peritoneal cavity.
Furthermore, CXCR1/2 signaling contributes to chemoresistance and
shapes an immunosuppressive tumor microenvironment by recruiting
myeloid-derived suppressor cells. Therefore, targeting CXCR1/2 not
only inhibits neovascularization but also suppresses ovarian cancer
cell survival and invasiveness, highlighting its potential as a
therapeutic strategy (95,100,101). Therefore, it can be speculated
that blocking the binding of IL-8 to CXCR1/2 using SCH527123 can
inhibit the proliferation of ovarian cancer cells, which has
prospects for the control of invasion and metastasis of ovarian
cancer cells clinically.
Colon cancer
Colorectal cancer is a leading cause of mortality
from gastrointestinal malignancies and the third most commonly
diagnosed cancer, of which >50% patients succumb due to its
associated complications (102).
Modifiable risk factors with clear environmental components include
the lack of activity, sedentary behavior, obesity, smoking, alcohol
consumption, excessive intake of red meat and processed meat. In
addition, hereditary colorectal cancer syndrome, inflammatory bowel
disease and history of colorectal adenoma are also reported risk
factors for colon cancer (103).
SCH527123 was previously reported to inhibit colon
cancer cell survival and new blood vessel formation by inhibiting
CXCR2 and possibly CXCR1 signaling. This in turn inhibited liver
metastasis by human colon cancer cells in a mouse model (77). It has also been demonstrated that
the IL-8/CXCR2 pathway can serve a key role in mediating the
development of colorectal cancer. Specifically, it was found that
SCH527123 can inhibit cancer cell proliferation, motility and
angiogenesis through NF-κB/AKT/MAPK signaling pathway (87). Oxaliplatin is used as an adjuvant
therapy for colon cancer (104).
When it is combined with SCH527123, the NF-κB/AKT/MAPK signal
transduction pathway was found to be further inhibited (87). Based on the results of the
aforementioned previous studies, CXCR2 may be a novel therapeutic
target for colon cancer, where SCH527123 as an antagonist of CXCR2
pathway may provide an idea for the subsequent drug treatment of
colon cancer.
6. Discussion
In the context of increasing refractory inflammation
and rising tumors incidence, in the present review, the
relationship between IL-8, CXCR1/CXCR2 and inflammation and cancers
were discussed, with focus on the CXCR1 and CXCR2 antagonist
SCH527123 to illustrate its possible application for the treatment
of inflammatory diseases and tumors.
Starting in 2007, cytological experiments were
implemented on a cellular level to report that SCH527123 represents
a novel and specific CXCR2 antagonist, with clinical potential in a
variety of inflammatory diseases (21,25,53,62).
In subsequent animal experiments, inhibition of CXCR1- and
CXCR2-mediated chemotaxis by SCH527123 has been observed in rodents
and cynomolgus monkey models, where it was speculated that
SCH527123 may prove beneficial in the treatment of inflammatory
lung diseases, by monitoring bronchoalveolar lavage mucin content
in cynomolgus monkeys (21). To
further confirm the clinical safety of SCH527123 in the human body,
two clinical trials were conducted in 2007 and 2012, which also
yielded certain clinical curative effects (53,58).
In a 2007 trial, oral SCH527123 significantly reduced ozone-induced
airway neutrophilia in healthy subjects, demonstrating good
tolerability and inhibition of neutrophil recruitment (53). In a 2012 multicenter study in
patients with severe refractory asthma, it reduced exacerbation
rates, improved lung function, and decreased airway neutrophil
levels, indicating potential clinical value in chronic inflammatory
diseases (58). In 2011, gene
silencing experiments found that by silencing the CXCR1/2
gene in human microvascular endothelial cells (HMEC-1)
significantly inhibited IL-8-dependent proliferation, survival,
migration and angiogenesis, and attenuated ERK phosphorylation and
cytoskeletal reorganization; these findings suggest that targeting
CXCR1/2 may offer a novel anti-angiogenic strategy for cancer
treatment (98). In 2018, a
cytological experiment inhibiting the IL-6/IL-8 pathway achieved
inhibitory effects on triple-negative breast cancer and PDAC. In
particular, it also showed that SCH527123 was more effective in
inhibiting the progression of triple-negative breast cancer and
PDAC when combined with bazedoxifene or rempacine (88). In addition, when combined with
bazedoxifene hydrochloride, SCH527123 was found to block the IL-6
and IL-8 pathways and inhibited ovarian cancer cell proliferation
in a mouse model (93).
As information on SCH527123 accumulates further,
clinical trials are becoming a possibility to assess the role of
SCH527123 for disease treatment. In 2016, a randomized,
double-blind, placebo-controlled, multicenter crossover trial
involving 19 non-smoking subjects with mild atopic asthma was
performed (60). For patients with
mild asthma, SCH5271213 was found to effectively reduce the number
of blood neutrophils migrating to the airway, thereby relieving
symptoms without causing adverse effects on bone marrow cells
(60). In 2017, another clinical
trial used SCH527123 in 58 patients with acute exacerbations of
chronic liver failure (ACLF), which found that blocking CXCR1/2
could significantly suppress hepatocyte cell death, which may
alleviate ACLF (23). It has also
been observed that the CXCR1/2 antagonist SCH527123 can inhibit
melanoma, lung, breast, pancreatic and liver tumor cell
proliferation and metastasis (22-24,105-107),
in addition to inhibiting chemotaxis of neutrophils to control the
development of COPD and asthma (Table
I) (58,62).
However, there are also limitations to the clinical
application of SCH527123 at present. SCH527123 research is
relatively mature only at the preclinical stages. Clinical trials
have only begun over the past decade, where the focus has been
mainly on tumors and chronic refractory diseases in the respiratory
system. The role of SCH527123 in inflammation in other organs has
not been tested. In addition, its safety for the treatment of
chronic refractory inflammation and tumors remains to be proven,
such that the biosafety of SCH527123 will become a major testing
avenue for future clinical studies. It has been previously reported
that SCH527123 is generally well-tolerated, where neutropenia is
the most common adverse event, with an incidence rate of 9%
(58). Although SCH527123 has
conferred inhibitory effects on the metastasis of colon cancer and
melanoma cells, neutrophils also serve a dual role in tumor immune
surveillance (108). Therefore,
its long-term use may weaken anti-tumor immunity.
7. Conclusion
Although research on SCH527123 has only been limited
to pre-clinical trials, the existing experimental data suggest that
SCH527123 is more reliable compared with other small molecule
antagonists. Oral administration of SCH527123 is generally safe and
well tolerated, with few adverse reactions. SCH527123 has shown
advantages in combating inflammation and inhibiting tumor growth,
especially with particular potential for the treatment of
refractory inflammation and cancers. However, current experimental
evidence is not sufficient to support the large-scale clinical use
of SCH527123, though it provides a ideas for clinical treatment of
inflammation and cancers. In the future, with the continuous
development of novel therapeutic strategies, SCH527123 is expected
to bring hope to patients.
Acknowledgements
The authors express their sincere gratitude to
Figdraw 2.0 (https://www.figdraw.com) for
providing the drawing platform.
Funding
Funding: The present review was supported by Scientific Research
Project of Luzhou Medical Association (grant no.
2024-YXXM-071).
Availability of data and materials
Not applicable.
Authors' contributions
JMZ and JWH made major contribution to manuscript
writing and figures. YX contributed to the conception of the
manuscript and performed literature searches. MZL participated in
writing and revising the manuscript. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Serhan CN and Sulciner ML: Resolution
medicine in cancer, infection, pain and inflammation: Are we on
track to address the next Pandemic? Cancer Metastasis Rev.
42:13–17. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Furman D, Campisi J, Verdin E,
Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW,
Fasano A, Miller GW, et al: Chronic inflammation in the etiology of
disease across the life span. Nat Med. 25:1822–1832.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lee KE, Tu VY and Faye AS: Optimal
management of refractory Crohn's disease: Current landscape and
future direction. Clin Exp Gastroenterol. 17:75–86. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hofman ZLM, Roodenrijs NMT, Nikiphorou E,
Kent AL, Nagy G, Welsing PMJ and van Laar JM: Difficult-to-treat
rheumatoid arthritis: What have we learned and what do we still
need to learn? Rheumatology (Oxford). 64:65–73. 2025.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pelaia C, Giacalone A, Ippolito G, Pastore
D, Maglio A, Piazzetta GL, Lobello N, Lombardo N, Vatrella A and
Pelaia G: Difficult-to-treat and severe asthma: Can real-world
studies on effectiveness of biological treatments change the lives
of patients? Pragmat Obs Res. 15:45–51. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bertin L, Crepaldi M, Zanconato M,
Lorenzon G, Maniero D, De Barba C, Bonazzi E, Facchin S, Scarpa M,
Ruffolo C, et al: Refractory Crohn's disease: Perspectives, unmet
needs and innovations. Clin Exp Gastroenterol. 17:261–315.
2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chadda KR and Puthucheary Z: Persistent
inflammation, immunosuppression, and catabolism syndrome (PICS): A
review of definitions, potential therapies, and research
priorities. Br J Anaesth. 132:507–518. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Halpin DM, Miravitlles M, Metzdorf N and
Celli B: Impact and prevention of severe exacerbations of COPD: A
review of the evidence. Int J Chron Obstruct Pulmon Dis.
12:2891–2908. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Papi A, Brightling C, Pedersen SE and
Reddel HK: Asthma. Lancet. 391:783–800. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Raeber ME, Sahin D and Boyman O:
Interleukin-2-based therapies in cancer. Sci Transl Med.
14(eabo5409)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins
JJ, Wagner ES, Gabaldon TA and Zaharoff DA: Localized
interleukin-12 for cancer immunotherapy. Front Immunol.
11(575597)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ye C, Yano H, Workman CJ and Vignali DAA:
Interleukin-35: Structure, function and its impact on
immune-related diseases. J Interferon Cytokine Res. 41:391–406.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Salkeni MA and Naing A: Interleukin-10 in
cancer immunotherapy: From bench to bedside. Trends Cancer.
9:716–725. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen L, Fan J, Chen H, Meng Z, Chen Z,
Wang P and Liu L: The IL-8/CXCR1 axis is associated with cancer
stem cell-like properties and correlates with clinical prognosis in
human pancreatic cancer cases. Sci Rep. 4(5911)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ji Z, Tian W, Gao W, Zang R, Wang H and
Yang G: Cancer-associated fibroblast-derived interleukin-8 promotes
ovarian cancer cell stemness and malignancy through the
notch3-mediated signaling. Front Cell Dev Biol.
9(684505)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Uwagboe I, Adcock IM, Lo Bello F, Caramori
G and Mumby S: New drugs under development for COPD. Minerva Med.
113:471–496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ghallab AM, Eissa RA and El Tayebi HM:
CXCR2 small-molecule antagonist combats chemoresistance and
eenhances immunotherapy in triple-negative breast cancer. Front
Pharmacol. 13(862125)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME,
Teijeira Á, Oñate C, González Á, Ponz M, Schalper KA, Pérez-Gracia
JL and Melero I: Interleukin-8 in cancer pathogenesis, treatment
and follow-up. Cancer Treat Rev. 60:24–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chapman RW, Minnicozzi M, Celly CS,
Phillips JE, Kung TT, Hipkin RW, Fan X, Rindgen D, Deno G, Bond R,
et al: A novel, orally active CXCR1/2 receptor antagonist,
Sch527123, inhibits neutrophil recruitment, mucus production, and
goblet cell hyperplasia in animal models of pulmonary inflammation.
J Pharmacol Exp Ther. 322:486–493. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh S, Sadanandam A, Nannuru KC, Varney
ML, Mayer-Ezell R, Bond R and Singh RK: Small-molecule antagonists
for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing
tumor cell proliferation, survival, and angiogenesis. Clin Cancer
Res. 15:2380–2386. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khanam A, Trehanpati N, Riese P, Rastogi
A, Guzman CA and Sarin SK: Blockade of Neutrophil's chemokine
receptors CXCR1/2 abrogate liver damage in acute-on-chronic liver
failure. Front Immunol. 8(464)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Baggiolini M and Clark-Lewis I:
Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett.
307:97–101. 1992.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gonsiorek W, Fan X, Hesk D, Fossetta J,
Qiu H, Jakway J, Billah M, Dwyer M, Chao J, Deno G, et al:
Pharmacological characterization of Sch527123, a potent allosteric
CXCR1/CXCR2 antagonist. J Pharmacol Exp Ther. 322:477–485.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fu S, Chen X, Lin HJ and Lin J: Inhibition
of interleukin 8/C-X-C chemokine receptor 1,/2 signaling reduces
malignant features in human pancreatic cancer cells. Int J Oncol.
53:349–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zinkernagel AS, Timmer AM, Pence MA, Locke
JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E and
Nizet V: The IL-8 protease SpyCEP/ScpC of group A Streptococcus
promotes resistance to neutrophil killing. Cell Host Microbe.
4:170–178. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simpson S, Kaislasuo J, Guller S and Pal
L: Thermal stability of cytokines: A review. Cytokine.
125(154829)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsushima K, Yang D and Oppenheim JJ:
Interleukin-8: An evolving chemokine. Cytokine.
153(155828)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kofanova O, Henry E, Aguilar Quesada R,
Bulla A, Navarro Linares H, Lescuyer P, Shea K, Stone M, Tybring G,
Bellora C and Betsou F: IL8 and IL16 levels indicate serum and
plasma quality. Clin Chem Lab Med. 56:1054–1062. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shkundin A and Halaris A: IL-8 (CXCL8)
correlations with psychoneuroimmunological processes and
neuropsychiatric conditions. J Pers Med. 14(488)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kunkel SL, Standiford T, Kasahara K and
Strieter RM: Interleukin-8 (IL-8): The major neutrophil chemotactic
factor in the lung. Exp Lung Res. 17:17–23. 1991.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harada A, Sekido N, Akahoshi T, Wada T,
Mukaida N and Matsushima K: Essential involvement of interleukin-8
(IL-8) in acute inflammation. J Leukoc Biol. 56:559–564.
1994.PubMed/NCBI
|
|
34
|
Rampart M, Van Damme J, Zonnekeyn L and
Herman AG: Granulocyte chemotactic protein/interleukin-8 induces
plasma leakage and neutrophil accumulation in rabbit skin. Am J
Pathol. 135:21–25. 1989.PubMed/NCBI
|
|
35
|
Brennan FM, Zachariae CO, Chantry D,
Larsen CG, Turner M, Maini RN, Matsushima K and Feldmann M:
Detection of interleukin 8 biological activity in synovial fluids
from patients with rheumatoid arthritis and production of
interleukin 8 mRNA by isolated synovial cells. Eur J Immunol.
20:2141–2144. 1990.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Scheibenbogen C, Möhler T, Haefele J,
Hunstein W and Keilholz U: Serum interleukin-8 (IL-8) is elevated
in patients with metastatic melanoma and correlates with tumour
load. Melanoma Res. 5:179–181. 1995.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Waugh DJJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luppi F, Longo AM, de Boer WI, Rabe KF and
Hiemstra PS: Interleukin-8 stimulates cell proliferation in
non-small cell lung cancer through epidermal growth factor receptor
transactivation. Lung Cancer. 56:25–33. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Singh N, Baby D, Rajguru JP, Patil PB,
Thakkannavar SS and Pujari VB: Inflammation and cancer. Ann Afr
Med. 18:121–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li X, Li J, Zhang Y and Zhang L: The role
of IL-8 in the chronic airway inflammation and its research
progress. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
35:1144–1148. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
42
|
Ghasemi H, Ghazanfari T, Yaraee R,
Faghihzadeh S and Hassan ZM: Roles of IL-8 in ocular inflammations:
A review. Ocul Immunol Inflamm. 19:401–412. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cheng Y, Ma XL, Wei YQ and Wei XW:
Potential roles and targeted therapy of the CXCLs/CXCR2 axis in
cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer.
1871:289–312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang J, Shao N, Yang X, Xie C, Shi Y and
Lin Y: Interleukin-8 promotes epithelial-to-mesenchymal ransition
via downregulation of mir-200 family in breast cancer cells.
Technol Cancer Res Treat: Dec 7, 2020 (Epub ahead of print). doi:
10.1177/1533033820979672.
|
|
45
|
Li XJ, Peng LX, Shao JY, Lu WH, Zhang JX,
Chen S, Chen ZY, Xiang YQ, Bao YN, Zheng FJ, et al: As an
independent unfavorable prognostic factor, IL-8 promotes metastasis
of nasopharyngeal carcinoma through induction of
epithelial-mesenchymal transition and activation of AKT signaling.
Carcinogenesis. 33:1302–1309. 2012.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Park SY, Han J, Kim JB, Yang MG, Kim YJ,
Lim HJ, An SY and Kim JH: Interleukin-8 is related to poor
chemotherapeutic response and tumourigenicity in hepatocellular
carcinoma. Eur J Cancer. 50:341–350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Merritt WM, Lin YG, Spannuth WA, Fletcher
MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley
RR, et al: Effect of interleukin-8 gene silencing with
liposome-encapsulated small interfering RNA on ovarian cancer cell
growth. J Natl Cancer Inst. 100:359–372. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
White JR, Lee JM, Young PR, Hertzberg RP,
Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold
DE and Sarau HM: Identification of a potent, selective non-peptide
CXCR2 antagonist that inhibits interleukin-8-induced neutrophil
migration. J Biol Chem. 273:10095–10098. 1998.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li JJ, Carson KG, Trivedi BK, Yue WS, Ye
Q, Glynn RA, Miller SR, Connor DT, Roth BD, Luly JR, et al:
Synthesis and structure-activity relationship of
2-amino-3-heteroaryl-quinoxalines as non-peptide, small-molecule
antagonists for interleukin-8 receptor. Bioorg Med Chem.
11:3777–3790. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dwyer MP, Yu Y, Chao J, Aki C, Chao J,
Biju P, Girijavallabhan V, Rindgen D, Bond R, Mayer-Ezel R, et al:
Discovery of 2-hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5-
methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide
(SCH 527123): A potent, orally bioavailable CXCR2/CXCR1 receptor
antagonist. J Med Chem. 49:7603–7606. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Russo RC, Garcia CC, Teixeira MM and
Amaral FA: The CXCL8/IL-8 chemokine family and its receptors in
inflammatory diseases. Expert Rev Clin Immunol. 10:593–619.
2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sitaru S, Budke A, Bertini R and Sperandio
M: Therapeutic inhibition of CXCR1/2: Where do we stand? Intern
Emerg Med. 18:1647–1664. 2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Holz O, Khalilieh S, Ludwig-Sengpiel A,
Watz H, Stryszak P, Soni P, Tsai M, Sadeh J and Magnussen H:
SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced
neutrophilia in healthy subjects. Eur Respir J. 35:564–570.
2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mims JW: Asthma: Definitions and
pathophysiology. Int Forum Allergy Rhinol. 5 (Suppl 1):S2–S6.
2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bizzarri C, Beccari AR, Bertini R,
Cavicchia MR, Giorgini S and Allegretti M: ELR+ CXC chemokines and
their receptors (CXC chemokine receptor 1 and CXC chemokine
receptor 2) as new therapeutic targets. Pharmacol Ther.
112:139–149. 2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ha H, Debnath B and Neamati N: Role of the
CXCL8-CXCR1/2 axis in cancer and inflammatory diseases.
Theranostics. 7:1543–1588. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nocker RE, Schoonbrood DF, van de Graaf
EA, Hack CE, Lutter R, Jansen HM and Out TA: Interleukin-8 in
airway inflammation in patients with asthma and chronic obstructive
pulmonary disease. Int Arch Allergy Immunol. 109:183–191.
1996.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nair P, Gaga M, Zervas E, Alagha K,
Hargreave FE, O'Byrne PM, Stryszak P, Gann L, Sadeh J and Chanez P:
Study Investigators. Safety and efficacy of a CXCR2 antagonist in
patients with severe asthma and sputum neutrophils: A randomized,
placebo-controlled clinical trial. Clin Exp Allergy. 42:1097–1103.
2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ordoñez CL, Shaughnessy TE, Matthay MA and
Fahy JV: Increased neutrophil numbers and IL-8 levels in airway
secretions in acute severe asthma: Clinical and biologic
significance. Am J Respir Crit Care Med. 161:1185–1190.
2000.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yan Q, Zhang X, Xie Y, Yang J, Liu C,
Zhang M, Zheng W, Lin X, Huang HT, Liu X, et al: Bronchial
epithelial transcriptomics and experimental validation reveal
asthma severity-related neutrophilc signatures and potential
treatments. Commun Biol. 7(181)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Todd CM, Salter BM, Murphy DM, Watson RM,
Howie KJ, Milot J, Sadeh J, Boulet LP, O'Byrne PM and Gauvreau GM:
The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in
mild atopic asthmatic subjects. Pulm Pharmacol Ther. 41:34–39.
2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Raherison C and Girodet PO: Epidemiology
of COPD. Eur Respir Rev. 18:213–221. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kaur M and Singh D: Neutrophil chemotaxis
caused by chronic obstructive pulmonary disease alveolar
macrophages: The role of CXCL8 and the receptors CXCR1/CXCR2. J
Pharmacol Exp Ther. 347:173–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Demkow U and van Overveld FJ: Role of
elastases in the pathogenesis of chronic obstructive pulmonary
disease: Implications for treatment. Eur J Med Res. 15 (Suppl
2):S27–S35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim S and Nadel JA: Role of neutrophils in
mucus hypersecretion in COPD and implications for therapy. Treat
Respir Med. 3:147–159. 2004.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Arai N, Kondo M, Izumo T, Tamaoki J and
Nagai A: Inhibition of neutrophil elastase-induced goblet cell
metaplasia by tiotropium in mice. Eur Respir J. 35:1164–1171.
2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Voynow JA, Fischer BM, Malarkey DE, Burch
LH, Wong T, Longphre M, Ho SB and Foster WM: Neutrophil elastase
induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell
Mol Physiol. 287:L1293–L1302. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wang Q, Shao X, Zhang Y, Zhu M, Wang FXC,
Mu J, Li J, Yao H and Chen K: Role of tumor microenvironment in
cancer progression and therapeutic strategy. Cancer Med.
12:11149–11165. 2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Desai SA, Patel VP, Bhosle KP, Nagare SD
and Thombare KC: The tumor microenvironment: Shaping cancer
progression and treatment response. J Chemother. 37:15–44.
2025.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Cheng K, Cai N, Zhu J, Yang X, Liang H and
Zhang W: Tumor-associated macrophages in liver cancer: From
mechanisms to therapy. Cancer Commun (Lond). 42:1112–1140.
2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lorusso G and Rüegg C: The tumor
microenvironment and its contribution to tumor evolution toward
metastasis. Histochem Cell Biol. 130:1091–1103. 2008.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chao CC, Lee CW, Chang TM, Chen PC and Liu
JF: CXCL1/CXCR2 paracrine axis contributes to lung metastasis in
osteosarcoma. Cancers (Basel). 12(459)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wu T, Yang W, Sun A, Wei Z and Lin Q: The
role of CXC chemokines in cancer progression. Cancers (Basel).
15(167)2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ganesan P and Kulik LM: Hepatocellular
carcinoma: New developments. Clini Liver Dis. 27:85–102.
2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lee JG, Kang CM, Park JS, Kim KS, Yoon DS,
Choi JS, Lee WJ and Kim BR: The actual five-year survival rate of
hepatocellular carcinoma patients after curative resection. Yonsei
Med J. 47:105–112. 2006.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Anwanwan D, Singh SK, Singh S, Saikam V
and Singh R: Challenges in liver cancer and possible treatment
approaches. Biochim Biophys Acta Rev Cancer.
1873(188314)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Bi H, Zhang Y, Wang S, Fang W, He W, Yin
L, Xue Y, Cheng Z, Yang M and Shen J: Interleukin-8 promotes cell
migration via CXCR1 and CXCR2 in liver cancer. Oncol Lett.
18:4176–4184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Yang S, Wang H, Qin C, Sun H and Han Y:
Up-regulation of CXCL8 expression is associated with a poor
prognosis and enhances tumor cell malignant behaviors in liver
cancer. Biosci Rep. 40(BSR20201169)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sun F, Wang J, Sun Q, Li F, Gao H, Xu L,
Zhang J, Sun X, Tian Y, Zhao Q, et al: Interleukin-8 promotes
integrin β3 upregulation and cell invasion through PI3K/Akt pathway
in hepatocellular carcinoma. J Exp Clin Cancer Res.
38(449)2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Varney ML, Singh S, Li A, Mayer-Ezell R,
Bond R and Singh RK: Small molecule antagonists for CXCR2 and CXCR1
inhibit human colon cancer liver metastases. Cancer Lett.
300:180–188. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620.
2011.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Goral V: Pancreatic cancer: Pathogenesis
and diagnosis. Asian Pac J Cancer Prev. 16:5619–5624.
2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhao Z and Liu W: Pancreatic cancer: A
review of risk factors, diagnosis, and treatment. Technol Cancer
Res Treat: Dec 24, 2020 (Epub ahead of print). doi:
10.1177/1533033820962117.
|
|
84
|
National Cancer Institute: Pancreatic
cancer treatment (PDQ®)-Health Professional Version.
National Cancer Institute, Bethesda, MD, 2025.
|
|
85
|
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T,
Chen Y, Han X and Wu K: The CXCL8-CXCR1/2 pathways in cancer.
Cytokine Growth Factor Rev. 31:61–71. 2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Xiong X, Liao X, Qiu S, Xu H, Zhang S,
Wang S, Ai J and Yang L: CXCL8 in tumor biology and its
implications for clinical translation. Front Mol Biosci.
9(723846)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Ning Y, Labonte MJ, Zhang W, Bohanes PO,
Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO, Nagulapalli
Venkata KC, et al: The CXCR2 antagonist, SCH-527123, shows
antitumor activity and sensitizes cells to oxaliplatin in
preclinical colon cancer models. Mol Cancer Ther. 11:1353–1364.
2012.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Fu S and Lin J: Blocking interleukin-6 and
interleukin-8 signaling inhibits cell viability, colony-forming
activity, and cell migration in human triple-negative breast cancer
and pancreatic cancer cells. Anticancer Res. 38:6271–6279.
2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Feng J, Xu L, Chen Y, Lin R, Li H and He
H: Trends in incidence and mortality for ovarian cancer in China
from 1990 to 2019 and its forecasted levels in 30 years. J Ovarian
Res. 16(139)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ali AT, Al-Ani O and Al-Ani F:
Epidemiology and risk factors for ovarian cancer. Prz Menopauzalny.
22:93–104. 2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zhang R, Roque DM, Reader J and Lin J:
Combined inhibition of IL-6 and IL-8 pathways suppresses ovarian
cancer cell viability and migration and tumor growth. Int J Oncol.
60(50)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Rašková M, Lacina L, Kejík Z, Venhauerová
A, Skaličková M, Kolář M, Jakubek M, Rosel D, Smetana K Jr and
Brábek J: The role of IL-6 in cancer cell invasiveness and
metastasis-overview and therapeutic opportunities. Cells.
11(3698)2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Shahzad MM, Arevalo JM, Armaiz-Pena GN, Lu
C, Stone RL, Moreno-Smith M, Nishimura M, Lee JW, Jennings NB,
Bottsford-Miller J, et al: Stress effects on FosB- and
interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J
Biol Chem. 285:35462–35470. 2010.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Son JS, Chow R, Kim H, Lieu T, Xiao M, Kim
S, Matuszewska K, Pereira M, Nguyen DL and Petrik J: Liposomal
delivery of gene therapy for ovarian cancer: A systematic review.
Reprod Biol Endocrinol. 21(75)2023.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Li Y, Liu L, Yin Z, Xu H, Li S, Tao W,
Cheng H, Du L, Zhou X and Zhang B: Effect of targeted silencing of
IL-8 on in vitro migration and invasion of SKOV3 ovarian
cancer cells. Oncol Lett. 13:567–572. 2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Singh S, Wu S, Varney M, Singh AP and
Singh RK: CXCR1 and CXCR2 silencing modulates CXCL8-dependent
endothelial cell proliferation, migration and capillary-like
structure formation. Microvasc Res. 82:318–325. 2011.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Park GY, Pathak HB, Godwin AK and Kwon Y:
Epithelial-stromal communication via CXCL1-CXCR2 interaction
stimulates growth of ovarian cancer cells through p38 activation.
Cell Oncol (Dordr). 44:77–92. 2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Yang G, Rosen DG, Liu G, Yang F, Guo X,
Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH, et al: CXCR2
promotes ovarian cancer growth through dysregulated cell cycle,
diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res.
16:3875–3886. 2010.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Li A, Dubey S, Varney ML, Dave BJ and
Singh RK: IL-8 directly enhanced endothelial cell survival,
proliferation, and matrix metalloproteinases production and
regulated angiogenesis. J Immunol. 170:3369–3376. 2003.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li
D, Zhang XY, Liu JL and Li JL: Combination of chemotherapy and
immunotherapy for colon cancer in China: A meta-analysis. World J
Gastroenterol. 20:1095–1106. 2014.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Katsaounou K, Nicolaou E, Vogazianos P,
Brown C, Stavrou M, Teloni S, Hatzis P, Agapiou A, Fragkou E,
Tsiaoussis G, et al: Colon cancer: From epidemiology to prevention.
Metabolites. 12(499)2022.PubMed/NCBI View Article : Google Scholar
|
|
104
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Singh JK, Farnie G, Bundred NJ, Simões BM,
Shergill A, Landberg G, Howell SJ and Clarke RB: Targeting CXCR1/2
significantly reduces breast cancer stem cell activity and
increases the efficacy of inhibiting HER2 via HER2-dependent and
-independent mechanisms. Clin Cancer Res. 19:643–656.
2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Khan MN, Wang B, Wei J, Zhang Y, Li Q,
Luan X, Cheng JW, Gordon JR, Li F and Liu H: CXCR1/2 antagonism
with CXCL8/Interleukin-8 analogue CXCL8(3-72)K11R/G31P restricts
lung cancer growth by inhibiting tumor cell proliferation and
suppressing angiogenesis. Oncotarget. 6:21315–21327.
2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Hertzer KM, Donald GW and Hines OJ: CXCR2:
A target for pancreatic cancer treatment? Expert Opin Ther Targets.
17:667–680. 2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Gungabeesoon J, Gort-Freitas NA, Kiss M,
Bolli E, Messemaker M, Siwicki M, Hicham M, Bill R, Koch P,
Cianciaruso C, et al: A neutrophil response linked to tumor control
in immunotherapy. Cell. 186:1448–1464.e20. 2023.PubMed/NCBI View Article : Google Scholar
|