1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most
common and aggressive form of pancreatic cancer, accounting for 85%
of all PDAC cases and representing the seventh leading cause of
cancer globally (1,2). While PDAC predominantly arises from
the ductal epithelium of the pancreas, accumulating evidence
indicates that acinar cells can also undergo acinar-to-ductal
metaplasia and subsequently give rise to PDAC. It is characterized
by rapid progression and resistance to therapy, with a 5-year
survival rate of ~10% (3). PDAC is
frequently diagnosed too late due to heterogeneity in clinical
presentation with high metastatic potential, making PDAC the third
most common cause of cancer-associated mortality in the USA
(4,5).
PDAC is associated with poor prognosis due to a lack
of diagnostic biomarkers, a fibrotic tumor microenvironment,
desmoplasia, and resistance to chemotherapy and immunotherapy
(3,6). Additionally, variability in CA19-9,
including absent expression levels in a subset of patients,
elevation in benign conditions and inconsistent secretion by
tumors, reduces its reliability as a prognostic marker. These
limitations hinder early detection and accurate disease monitoring,
thereby contributing to delayed diagnosis and ultimately poor
prognosis, which restricts its clinical utility for screening
asymptomatic patients (7,8). PDAC management poses notable
challenges due to a stroma rich in hyaluronan, adhesion molecules,
cancer-associated fibroblasts (CAFs) and MMPs, which promote
disease progression and drug resistance (9).
A previous study has highlighted the role of cell
adhesion molecules, including CEA-related cell adhesion molecules
(CEACAMs), in cell-cell and cell-matrix interactions whilst
regulating tumor survival, invasion, metastasis, drug resistance
and progression of PDAC (10).
CEACAMs and integrins can modulate tumor progression through
epithelial-mesenchymal transition (EMT), immune evasion and anoikis
resistance (11). Understanding
the mechanisms and function of adhesion molecules in the fibrotic
and immunosuppressive microenvironment of PDAC is essential for the
development of novel therapeutic targets in PDAC management. The
present review provides an updated and PDAC-specific summary,
highlighting recent mechanistic insights and therapeutic
perspectives.
2. Adhesion molecules can facilitate PDAC
progression
Adhesion molecules, which are frequently upregulated
in cancer, enable tumor cell adhesion, invasion and migration by
activating signals that regulate differentiation, the cell cycle
and survival (12,13). These features render adhesion
molecules potential focal nodes for diagnosis and therapeutic
intervention.
Adhesion molecules typically exhibit unique
characteristics. Unlike numerous adhesion molecules that signal
through direct intracellular interactions through transmembrane and
cytoplasmic domains, CEACAM6, a glycosylphosphatidylinositol
(GPI)-anchored glycoprotein of the immunoglobulin (Ig) superfamily,
lacks both a transmembrane and cytoplasmic domain (14-16).
This enables CEACAM6 to mediate cell-cell and cell-matrix adhesion
through lateral associations with lipid rafts and co-receptors,
promoting PDAC progression (17).
Additionally, it can facilitate tumor cell invasion, motility and
signal transduction, thereby contributing to tumor growth and
metastasis processes in solid malignancies, including colorectal,
breast and gastric cancer (16-20).
CEACAM6 expression varies across different stages of
tumorigenesis, and is modulated by components of the tumor
microenvironment, including collagen, laminin and fibrin (14,16).
However, the dynamic and transient membrane association of CEACAM6,
particularly its localization within lipid rafts and its status as
a GPI-anchored protein lacking an intrinsic signaling domain,
hampers precise delineation of its downstream signaling mechanisms
(21). CEACAM6 is a key target in
PDAC due to its upregulation in the disease, promoting resistance
to anoikis, invasion, metastasis and therapy resistance, and it is
readily accessible as a GPI-anchored cell surface protein, making
it an attractive therapeutic target (17,22).
Nevertheless, it is also essential to understand the roles of
integrins and other adhesion molecules in the management of PDAC
(17-22).
Integrins serve a crucial role in cell
communication, tissue regulation and maintenance (23). In addition, integrins facilitate
tumor progression by mediating interactions between extracellular
matrix (ECM) proteins and cytoplasmic components, potentially
influencing the tumor microenvironment (13,16).
Therefore, understanding the role of integrins in PDAC is crucial
for developing targeted therapies, managing cancer spread, and
optimizing patient outcomes (Fig.
1).
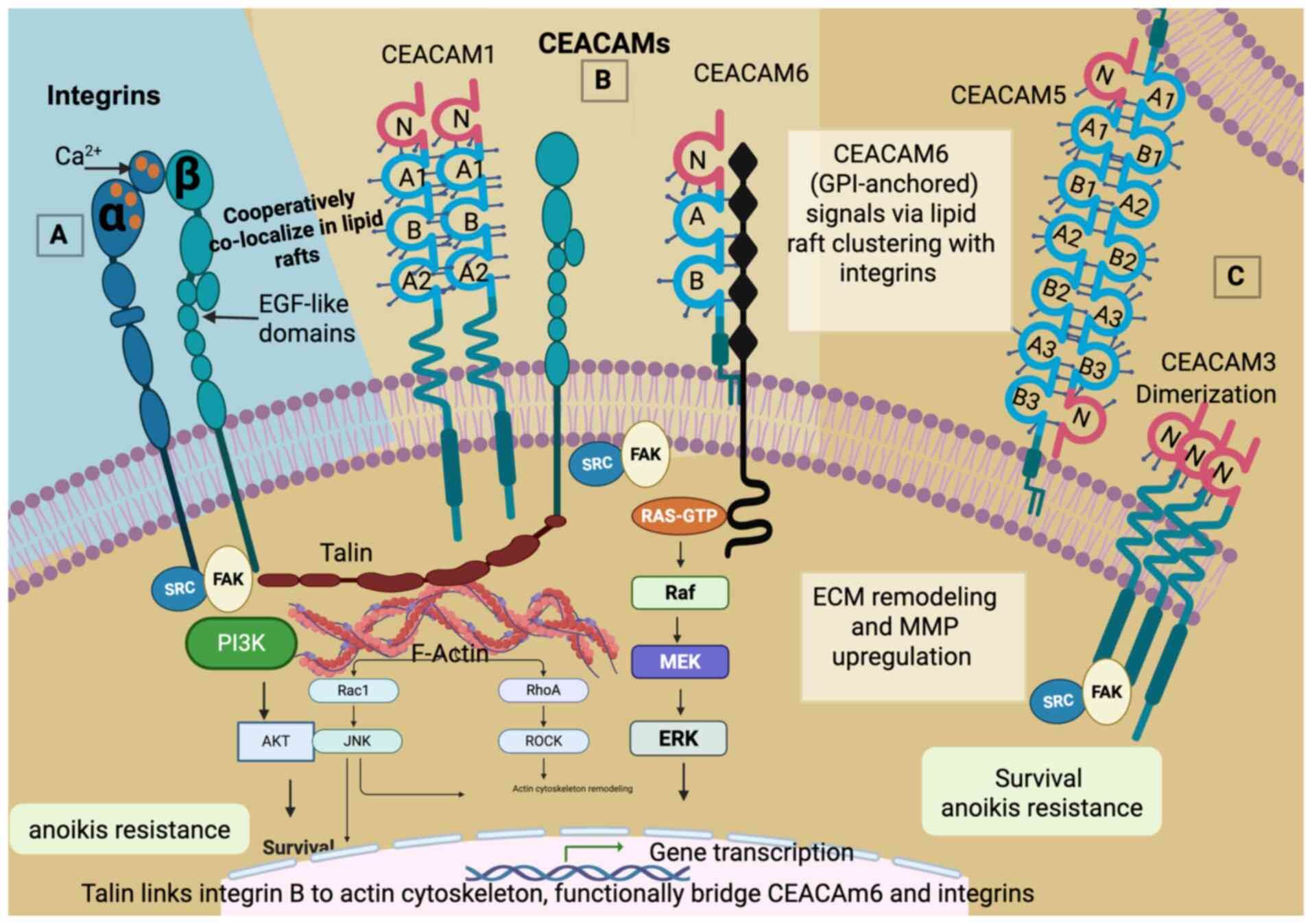 | Figure 1Integrin and CEACAM adhesion
receptors in pancreatic ductal adenocarcinoma. (A) Integrin (left)
receptors with a cytoplasmic domain contain α and β subunits with
EGF-like and calcium-binding domains, which link to intracellular
signaling via talin and focal adhesion kinase (FAK), and (B)
CEACAMs with either a transmembrane receptor anchored by
glycosylphosphatidylinositol on the cell surface, such as CEACAM6
and CEACAM5, or (C) CEACAM receptors with a cytoplasmic domain,
such as CEACAM1 and CEACAM3. Talin is illustrated as a cytoskeletal
adaptor protein that connects integrins to the actin cytoskeleton
and, functionally, to CEACAM-associated signaling hubs. While
direct binding of CEACAMs to talin has not been fully demonstrated,
CEACAMs and integrins co-localize in lipid rafts, enabling shared
downstream signaling via Src/FAK and PI3K/Akt pathways. CEACAM,
carcinoembryonic antigen-related cell adhesion molecule; FAK, focal
adhesion kinase; RhoA, Ras homolog family member A; ROCK, Rho
associated protein kinase. |
The present review aims to critically analyze the
oncogenic role of CEACAM6 in PDAC and the mechanisms by which
integrins influs to synthesize evidence on the association between
CEACAMs and integrins in PDAC, focusing on their co-expression,
cross-talk in signaling pathways and combined impact on tumor
progression, invasion and therapy resistance. The review further
discusses how this interplay highlights the potential for
developing targeted therapeutic strategies against these adhesion
molecules (Fig. 1).
3. CEACAMs contribute to PDAC
progression
Ig superfamily and CEACAMs in
PDAC
Ig cell adhesion molecules (IgCAMs), including
calcium-independent glycoproteins, such as CEA, constitute a large
family of cell surface glycoproteins specializing in cell-cell
adhesion (24). CEA, along with
other associated IgCAMs or proteins, are involved in homotypic and
heterotypic interactions, constituting essential diagnostic markers
for various solid tumors, such as colorectal cancer (25).
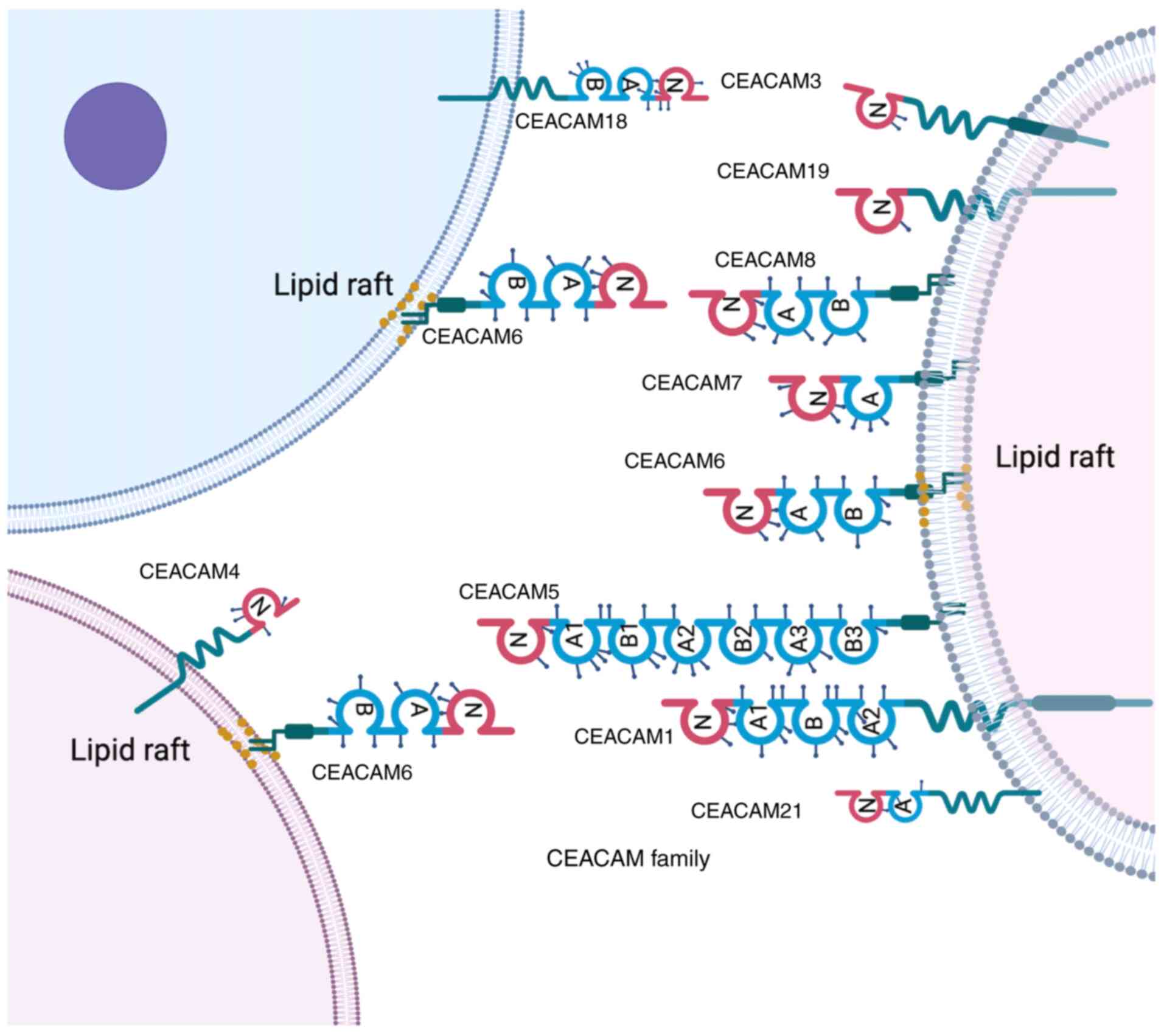
The human CEA family consists of 29 genes, 12 of
which are classified within the CEACAM subgroup (CEACAM1, CEACAM3,
CEACAM4, CEACAM5, CEACAM6, CEACAM7, CEACAM8, CEACAM16, CEACAM18,
CEACAM19, CEACAM20 and CEACAM21), which cluster on the long arm of
chromosome 19(26). Amongst these,
CEACAM1, CEACAM3, CEACAM4, CEACAM6, CEACAM7 and CEACAM8 differ in
their Ig-like domain composition, glycosylation patterns, membrane
anchoring mechanisms and tissue distribution (Fig. 2) (27,28).
Structurally, CEACAMs share an N-terminal Ig variable (IgV)-like
domain followed by constant-like domains (A and B), with extensive
glycosylation accounting for ≤50% of the molecular mass of the
protein (29).
In addition, these proteins are bound to the cell
membrane by either a GPI moiety (CEACAM5, CEACAM6, CEACAM7,
CEACAM8, CEACAM16 and CEACAM18) or a proteinaceous transmembrane
region (CEACAM1, CEACAM3, CEACAM4, CEACAM19, CEACAM20 and CEACAM21)
(Fig. 2) (25,30,31).
This structural diversity influences the functional roles of the
CEACAM subtypes in adhesion, migration and immune modulation in
cancer. However, limited expression of certain CEACAMs in animals
limits their mechanistic investigations (24,32,33).
For example, CEACAM5 and CEACAM6 are highly expressed in humans but
absent in mice, while CEACAM7 and CEACAM8 also lack murine
orthologs, thereby limiting their in vivo study potential
(24,32,33).
CEACAM1
CEACAM1 has a cytoplasmic tail that harbors
immunoreceptor tyrosine-based inhibition motifs (ITIMs), which are
critical for intracellular signaling (34), and has a molecular weight of ~90
kDa (35). Another feature of
CEACAM1 is the IgV domain that facilitates homophilic and
heterophilic adhesion through complementarity-determining regions
(CDR)-like loops, whilst ITIM phosphorylation recruits Src homology
region 2 domain-containing phosphatase (SHP)-1 (also known as
PTPN6) and SHP-2 (also known as PTPN11), thereby inhibiting T-cell
signaling and supporting immune evasion (34,36).
Previous studies have shown that CEACAM1 is involved in
bidirectional signaling, modulating immune checkpoint regulation
and tumor progression (28,37).
However, it remains under investigation as a prognostic marker
target in PDAC, melanoma, colorectal cancer and non-small cell lung
cancer, and as an emerging immunotherapeutic target across multiple
malignancies (28,37). However, its clinical relevance in
PDAC specifically remains under investigation (38,39).
CEACAM5
CEACAM5, has one IgV domain and six IgC2 domains
(arranged as A1-B1-A2-B2-A3-B3) and a GPI moiety anchored to the
cell membrane (40,41). Furthermore, it has a molecular
weight of 180-200 kDa with extensive N-linked glycosylation
(18,42). CEACAM5 mediates cell-cell adhesion
through its IgV domain and is likely involved in signaling cascades
due to its lipid raft localization (16). By associating with transmembrane
receptors, such as integrins (α5β1, αvβ3), CEACAM1 and EGFR,
CEACAM5 can influence downstream signaling, thereby supporting
metastatic spread and immune evasion (43,44).
Therefore, CEACAM5 has been proposed to be a possible serum
biomarker and target in PDAC immunotherapy in clinical settings
(20,43,45).
CEACAM6
CEACAM6 (also known as CD66c) comprises an
N-terminal IgV domain and two IgC2 domains (A1-B1), forming a
compact three-domain structure that is anchored to the membrane by
a GPI anchor (29). With a
molecular weight of 90-95 kDa, CEACAM6 is heavily glycosylated and
frequently localizes to lipid rafts (28,46).
CEACAM6 lacks a cytoplasmic domain but can facilitate signal
transduction through interactions with various integrin
co-receptors, such as α5β1 and αvβ3, to activate key pathways,
including the focal adhesion kinase (FAK), Src and PI3K/Akt
pathways (16,21,47).
Studies have previously demonstrated that CEACAM6 can promote tumor
cell survival, anoikis resistance and EMT, whilst also contributing
to chemoresistance in pancreatic, colorectal and breast cancer
(16,18-20,22,48).
Through indirect interactions with ECM components, such as
fibronectin and vitronectin, CEACAM6 can also serve a pivotal role
in PDAC invasion and progression (48).
CEACAM7
CEACAM7 consists of an N-terminal IgV domain and two
IgC2 domains (A1-B1), with a GPI anchor facilitating membrane
association (21), and has a
molecular weight of 75-85 kDa (43). Whilst CEACAM7 is generally
considered to be a tumor suppressor in normal epithelial tissues,
it displays oncogenic properties in PDAC (25,49,50).
CEACAM7 mediates both homophilic and heterophilic adhesion,
including interactions with CEACAM6 and integrins, and is involved
in lipid raft-associated signal transduction (49). In addition, CEACAM7 has been
implicated in peritoneal metastasis, is enriched in cancer stem
cell populations, and is a potential early diagnostic and
prognostic marker in PDAC (50).
However, the therapeutic relevance of CEACAM7 is currently under
investigation, including its applications in chimeric antigen
receptor (CAR)-T cell therapy (50).
Integrated structure-function
Within the CEACAM family, the IgV domain is a highly
conserved structural feature that provides adhesive functions
through β-sandwich folds and a CDR-like loop structure (51). However the type of membrane
anchoring varies, with CEACAM1 incorporating a transmembrane helix
allowing direct intracellular signaling, whereas CEACAM5, CEACAM6
and CEACAM7 are GPI-anchored, facilitating dynamic lipid raft
localization and co-receptor engagement (21,52).
By contrast, glycosylation of CEACAM family members influences
protein folding, stability and protease resistance, thereby
contributing to the functional plasticity in tumorigenesis
(29,53).
The IgV domain provides opportunities for
antibody-mediated blockade, since ITIM motifs can be exploited for
immune modulation, whereas GPI anchors may be targeted to disrupt
membrane clustering (54).
Furthermore, the unique structural attributes of each CEACAM
molecule are associated with their distinct roles in PDAC
pathobiology (17,29,53),
offering a framework for biomarker development and personalized
treatment strategies.
CEACAMs are upregulated in PDAC
Members of the CEACAM family, particularly CEACAM1,
CEACAM5, CEACAM6 and CEACAM7, have emerged as key markers of the
pathogenesis of PDAC, influencing diagnosis, prognosis and therapy
(14,53). CEACAM1 has a cytoplasmic tail
containing ITIMs (55) and is
frequently upregulated in PDAC, where it has been proposed as both
a clinical biomarker and a therapeutic target (38-58).
CEACAM1 modulates immune responses through co-inhibitory functions,
particularly by inhibiting T-cell receptor signaling through
SHP-1/2 recruitment, making CEACAM1 a promising target in
immunotherapy (28). However,
previous studies have reported conflicting findings, with CEACAM1
being expressed at lower levels in the tumors of patients with PDAC
compared with in normal pancreatic tissues (59,60).
Furthermore, the roles of CEACAM1 in tumorigenesis and cancer
progression have been inconclusively reported, highlighting the
need for further mechanistic studies (61). CEACAM1 is upregulated in PDAC
tissues and patient serum, and its increased levels are associated
with tumor stage and progression, supporting its potential as a
diagnostic and prognostic biomarker (58). In addition, it has been evaluated
as a non-invasive serum marker (58), with elevated CEACAM1 first reported
in the serum of patients with PDAC. Simeone et al (57) further demonstrated that combining
CEACAM1 with CA19-9 significantly improved sensitivity and
specificity for early-stage PDAC detection. Furthermore,
tissue-based assays revealed that CEACAM1 expression can
distinguish PDAC from chronic pancreatitis and normal pancreatic
tissue, supporting its role as both a serum and tissue biomarker
(57,62). By contrast, high-CEACAM1 expression
is associated with tumor grade, metastatic potential and patient
survival, with functional divergence between isoforms.
Specifically, isoform CEACAM1-L generally suppresses tumor
progression through ITIM-mediated signaling, whereas isoform
CEACAM1-S, lacking ITIM, is often upregulated and linked to
oncogenic behavior (39,62).
Through ITIM-mediated recruitment of SHP-1 and SHP-2
phosphatases, CEACAM1 can downregulate T-cell receptor signaling,
thereby facilitating tumor immune escape (63). In addition, CEACAM1 promotes can
tumor angiogenesis through VEGFR2 pathway activation, thereby
offering opportunities for anti-angiogenic combination therapies
with synergistic potential (59,64).
Monoclonal antibodies neutralizing the inhibitory
effects of CEACAM1 have been assessed, whereas bispecific
antibodies that can target CEACAM1 alongside other checkpoints,
such as TIM3, and small-molecule inhibitors of its downstream
signaling components, including SHP2 inhibitors, Src/FAK inhibitors
and PI3K/Akt or MAPK pathway inhibitors, are actively under
investigation (54,65). Emerging strategies utilizing
CEACAM1-directed CAR-T cell therapies that exploit its
tumor-specific expression profile to achieve targeted cytotoxicity
have also been explored (66,67).
Combinatorial approaches, pairing CEACAM1 inhibition with
chemotherapy, radiotherapy or co-targeting of integrins, may
further enhance therapeutic efficacy by disrupting survival
pathways and modifying the tumor microenvironment (54).
Methodological variability, including differences in
detection techniques, antibody specificity and scoring criteria,
result in heterogeneity in findings on CEACAM1 expression in PDAC
(68). Additionally, intratumoral
heterogeneity further adds to the complexity, with spatially
distinct expression patterns across tumor subclones and stromal
niches (53). The tumor
microenvironment, including immune cell infiltration, stromal
composition and hypoxia, can further influence CEACAM1 expression
and function, underscoring the need for context-specific evaluation
(69,70).
The multifaceted role of CEACAM1 in PDAC supports
its integration into current precision medicine strategies.
Understanding of its involvement in immune regulation, angiogenesis
and cell adhesion should inform the development of targeted
therapies and enable the predictive modeling of treatment outcomes.
Therefore, future research should aim to standardize CEACAM1
detection methods, validate its utility in large patient cohorts
and elucidate isoform-specific mechanisms of action (70).
Similarly, CEACAM5 is involved in cell adhesion,
intracellular signaling, tumor progression and metastasis (71-73).
CEACAM5 can mediate function through homophilic (with itself on
neighboring cells) and through heterophilic interactions with other
CEACAM family members, such as CEACAM6 and CEACAM1(53). CEACAM5 expression is frequently
upregulated in malignancies, including PDAC, where it is localized
to tumor cells and fibrotic or necrotic regions (71-75).
Associations have been reported between CEACAM5 and immune
checkpoint regulation and cancer stemness in PDAC, emphasizing its
potential role as a biomarker and therapeutic target (59).
CEACAM7 has been identified as a potential early
diagnostic and prognostic marker in PDAC (49). Although considered a tumor
suppressor due to its downregulation in several epithelial cancer
types, such as colorectal carcinoma, CEACAM7 expression is
frequently upregulated in PDAC, especially during early
tumorigenesis and in cancer stem cell-enriched populations
(20,75). CEACAM7 has also been implicated in
peritoneal metastasis through interactions with CEACAM6 and
integrin subunit αV (75). Its
dual expression profile and targeting in CAR-T cell study highlight
its complex but important therapeutic potential (50).
CEACAM6/CD66c is a GPI-anchored glycoprotein with a
variable IgV-like domain and two constant domains (A and B),
through which CEACAM6 can form both homo- and heterodimers.
Initially identified in hematological malignancies, CEACAM6 was
later identified as a biomarker for colorectal cancer (17,19,31).
CEACAM6 is expressed on epithelial and leukocyte surfaces (30).
In conclusion, CEACAMs exhibit diverse structural
features and biological functions that can each influence PDAC
development and progression in distinct manners. Their differential
expression patterns and interactions with the tumor
microenvironment offer potential for advancing early detection
strategies and developing targeted therapies.
Pathological role of CEACAM6 in PDAC
diagnosis and progression
CEACAM6 expression is upregulated in PDAC, with a
previous study showing a 20- to 25-fold increase in adenocarcinoma
cells compared with normal pancreatic ductal epithelial cells
(17). Upregulation typically
begins early in pancreatic tumorigenesis, including in pancreatic
intraepithelial neoplasia lesions (1,17),
where it is associated with metastasis and poor prognosis (14,16).
CEACAM6 has been identified to be a prognostic biomarker
independent of KRAS mutation status, and is associated with
aggressive PDAC subtypes (14,76).
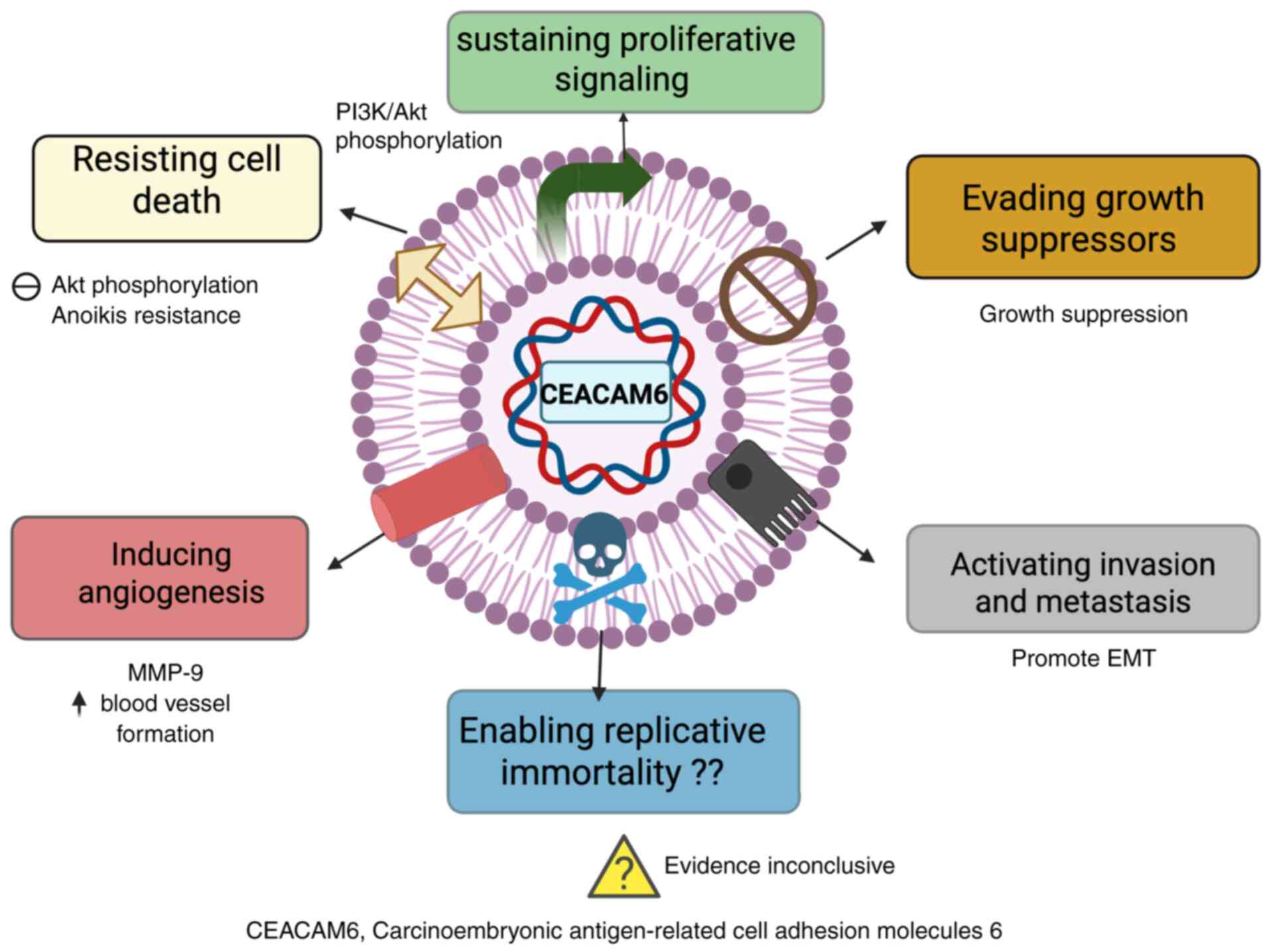
In addition, CEACAM6 can promote cell migration,
invasion and proliferation by upregulating MMPs and cyclin D1/CDK4
(53,77). CEACAM6 can facilitate EMT by
promoting loss of epithelial traits and acquisition of mesenchymal
characteristics, which is reflected in its inverse correlation with
E-cadherin expression and its association with increased expression
of mesenchymal markers such as vimentin (73). Mechanistically, miR29 a/b/c
directly targets CEACAM6 mRNA, and their downregulation leads to
CEACAM6 overexpression, thereby enhancing EMT and invasion
(73,74). In addition, CEACAM6 is associated
with anoikis (an apoptotic response triggered by detachment from
the ECM) resistance primarily through enhanced Akt phosphorylation,
which promotes cell survival (47,78).
Supporting this, CEACAM6 inhibition has been reported to increase
cell susceptibility to caspase-mediated anoikis by reducing Akt
phosphorylation (47).
CEACAM6 can support tumor angiogenesis by activating
FAK and paxillin signaling, promoting vasculogenic mimicry
(79). Anti-CEACAM6 antibodies
have been found to reduce MMP9 activity, invasion and angiogenesis
(32). Furthermore, upregulation
of CEACAM6 expression has been observed to contribute to
gemcitabine chemoresistance, whilst its inhibition sensitized cells
to gemcitabine-induced cytotoxicity (80).
Mechanistically, CEACAM6 interacts with ECM
components, such as fibronectin, through α5β1 and αvβ3 integrins,
thereby enhancing anchorage-independent survival (17,47).
These diverse roles, ranging from EMT and immune evasion to
chemoresistance and angiogenesis, highlight the role of CEACAM6 in
PDAC progression and as a promising therapeutic target (Fig. 3) (81,82).
Role of CEACAM6 in ECM component
interactions
CEACAM6 serves a key role in mediating interactions
with ECM components, influencing PDAC progression, therapeutic
resistance and tumor microenvironment dynamics. Although the
majority of the currently known functional insights stem from
studies in other malignancies, including lung adenocarcinoma, where
CEACAM6 promotes cisplatin resistance and is regulated by miR-146a
and miR-26a, the relevance of CEACAM6 to PDAC is becoming
increasingly evident (83,84).
In the tumor microenvironment, CEACAM6 can modulate
cell adhesion, migration and drug resistance through homotypic and
heterotypic cell-cell interactions involving integrin receptors
(14,17,45).
CEACAM6 activates integrin signaling cascades, particularly those
involving α5β1 and αvβ3 integrins, which are known to interact with
various ECM components, such as laminin, fibronectin, collagen and
hyaluronan (85). These
interactions contribute to anchorage-independent survival, EMT and
chemoresistance in PDAC (14,17).
A previous study has revealed that CEACAM6 can
regulate integrin-mediated signaling through PI3K/Akt activation,
which are mechanisms observed in lung and breast cancer and are
likely conserved in PDAC (16). By
contrast, CEACAM1 has been shown to require tyrosine
phosphorylation for interaction with β3 integrin in melanoma,
suggesting a broader CEACAM-integrin signaling axis (26,86,87).
Despite evidence of oncogenic roles, including
angiogenesis, invasion, resistance to apoptosis and immune evasion,
the molecular mechanisms underlying the interaction of CEACAM6 with
the ECM and the contribution to desmoplastic remodeling in PDAC
remain inadequately characterized (16,73,88-90).
CEACAM6 may influence the fibroinflammatory remodeling of the ECM,
but this desmoplastic interaction remains underexplored (91). Understanding how CEACAM6 interacts
with ECM receptors and regulates PDAC cell behavior is essential.
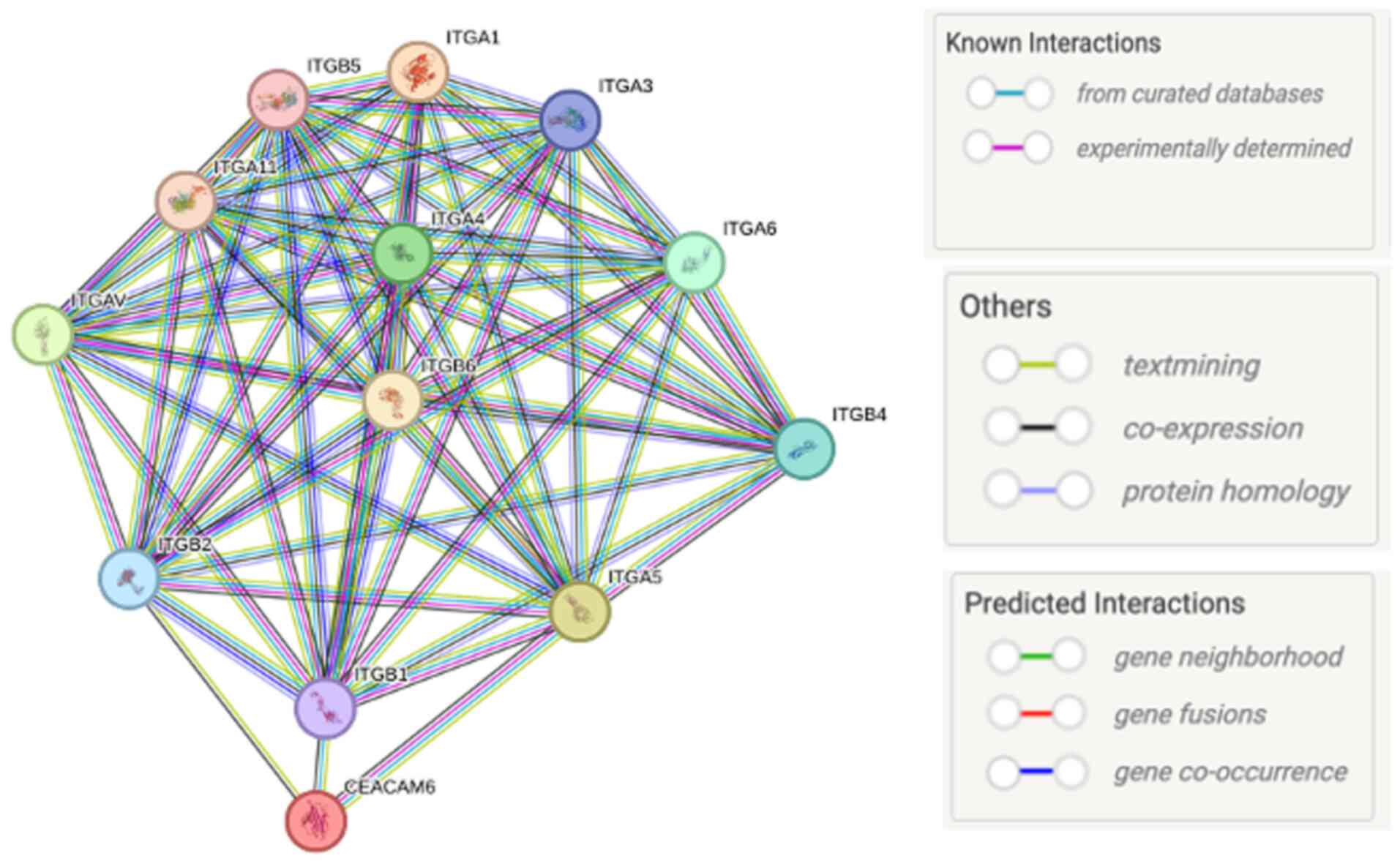
As shown in Fig. 4, STRING-based
protein-protein interaction analysis indicates that CEACAM6 is
connected with multiple integrin subunits [e.g. ITGA5, ITGA2,
ITGB3, integrin β (ITGB)4 and ITGA6], which are key ECM receptors.
These predicated and known associations suggest that CEACAM6 may
influence integrin-mediated adhesion, signaling and ECM remodeling,
thereby supporting its role as both a diagnostic marker and a
therapeutic target in PDAC (Fig.
4).
The desmoplastic reaction in PDAC, marked by dense
collagen deposition, activation of CAFs and altered ECM
composition, is influenced by CEACAM6(14). GPI-anchored localization to lipid
rafts facilitates clustering with integrins α5β1 and αvβ3,
promoting adhesion to fibronectin, collagen I and hyaluronan, and
forming signaling hubs that amplify matrix deposition and tumor
cell survival (16,21). In addition, upregulation of CEACAM6
expression has been found to enhance fibronectin assembly,
stimulate collagen cross-linking and stiffen the ECM (17).
CEACAM6 can also support CAF activation through
α5β1-mediated FAK/Src signaling and TGF-β feedback, thereby
sustaining myofibroblast differentiation (51,92,93).
CEACAM6 upregulates MMP-2 and MMP-9 through the NF-κβ pathway and
increases tissue inhibitors of MMP expression, thereby altering the
proteolytic balance toward ECM accumulation (91). Furthermore, CEACAM6 promotes
mechanotransduction through α5β1 and αvβ3 signaling, reinforcing
fibrosis through Yes-associated protein/WW domain-containing
transcription regulator 1 activation, whilst also driving
hyaluronan accumulation and proteoglycan remodeling, all of which
elevate interstitial fluid pressure and impair drug delivery
(94).
Clinically, CEACAM6-driven desmoplasia can limit
therapy efficacy by creating physical and pharmacological barriers
(14,95). Future studies should explore the
spatial heterogeneity of CEACAM6 expression, identify predictive
biomarkers and optimize anti-desmoplastic interventions for PDAC
management.
4. Integrin and CEACAM involvement in
signaling pathways in PDAC
Integrins are heterodimeric transmembrane receptors
consisting of 18 α and 18 β subunits that combine to form 24
distinct integrin receptors (96).
The structural architecture of integrin subtypes is directly
associated with their functional roles in PDAC progression
(75). Structurally, each subunit
includes a cytoplasmic tail, a transmembrane domain and a large
extracellular region (97). While
the β subunits anchor to the actomyosin cytoskeleton and transmit
intracellular signals, the α subunits mediate selective binding to
ECM proteins, including collagen and laminin (98,99).
These subunits coordinate to form functional integrin receptors
that engage various ECM glycoproteins, such as collagen, laminins
and fibronectin, thereby facilitating bidirectional signaling
between tumor cells and their microenvironment (100).
In PDAC, integrin-mediated signaling can activate
several oncogenic pathways, including the Ras/MAPK, PI3K/Akt and
Rho-GTPase cascades (101,102).
ITGB5 expression, which is upregulated by TGF-β signaling in CAFs,
has been documented to contribute to tumor growth and metastasis.
By contrast, inhibition of TGF-β using LY2157299 in murine models
could reduce ITGB5 expression, leading to smaller tumors and
improved survival (103,104).
Other integrin subtypes also serve distinct roles in
PDAC. Integrin α3 can upregulate the EMT transcription factor zinc
finger E-box binding homeobox 1 (ZEB1) and activate JNK, thereby
promoting chemoresistance and metastasis (105). ITGB4, which is elevated in PDAC,
can also enhance local invasion and metastasis through the
MEK1/ERK1/2 cascade and FAK binding (105-108).
Although ITGB4 is known to facilitate cell migration and invasion,
its precise mechanistic role in PDAC remains to be fully
elucidated. The structural basis for ITGB4 activation involves
tyrosine phosphorylation at Y1510, which induces conformational
changes that facilitate downstream oncogenic signaling and
crosstalk with EGFR pathways. ITGB4 engages in crosstalk with EGFR,
promoting tumor cell proliferation, drug resistance and signaling
interplay central to PDAC pathogenesis (83,109,110).
Beyond the classical integrin subunits, other CEACAM
family members, such as CEACAM1, can contribute to
integrin-associated signaling in PDAC through their roles in immune
modulation, angiogenesis and adhesion regulation (86). CEACAM1 is a valuable clinical
biomarker and a promising therapeutic target in pancreatic cancer
due to its N-terminal IgV-like domain and ≤3 constant C2-like
domains, which enable diverse functional roles in tumor biology
(53). CEACAM1 has been found to
exhibit immunomodulatory functions, where the structural domains
facilitate co-inhibitory activities by inhibiting T-cell receptor
signaling through the recruitment of SHP-1 and SHP-2(87). Importantly, CEACAM1 can also
interact with integrins, such as β2 integrins on immune cells and
β3 integrins on endothelial cells, thereby influencing adhesion and
co-inhibitory signaling (87).
CEACAM1 can promote angiogenesis through the VEGFR2 pathway, which
may inform the development of anti-angiogenic therapeutic
strategies (111).
Despite advances in structural biology, the complete
3D architecture of integrin receptors remains to be fully resolved,
particularly the structural basis for integrin signaling. Further
research into integrin-CEACAM interactions and downstream signaling
mechanisms is essential to understand their role in PDAC
progression and therapeutic resistance.
Integrins as facilitators with other
proteins in PDAC
Integrins are essential mediators of cell-ECM
interactions in PDAC, influencing cell adhesion, migration and
intracellular signaling. By binding to specific ECM proteins,
integrins initiate signal transduction pathways that regulate
cancer progression and therapeutic resistance (112). Certain integrins can also serve
as prognostic biomarkers in PDAC, being associated with disease
severity and patient outcomes (92,93,111,112). For example, high ITGA2 expression
correlates with shorter progression-free and overall survival
times, as well as chemoresistance in PDA (113,114).
Integrins engage in signaling crosstalk with
numerous growth factors and cytokines, including EGF, insulin
growth factor and TGF-β, thereby influencing cellular behavior and
ECM remodeling (115,116). ITGB1 can form heterodimers with
α2, α4 and α5 subunits to mediate focal adhesions between tumor
cells and the ECM (117). By
contrast, cytoplasmic proteins, such as talin and kindlin, can
regulate ligand affinity and integrin activation by linking
integrins to the actin cytoskeleton (113-118).
In addition, as illustrated in Fig.
1, talin may also functionally bridge integrins and CEACAMs
within adhesion complexes. While direct physical binding between
CEACAMs and talin has not been fully demonstrated, experimental
evidence supports integrin-CEACAM co-localization and clustering in
lipid rafts, which facilitates shared downstream signaling through
Src/FAK pathways (16,19,47).
This suggests that integrins and CEACAMs may operate with the same
adhesion and signaling hubs. Integrins, including ITGB3, can be
internalized through clathrin-mediated endocytosis when they are
not under high mechanical tension (119,120).
Within the tumor microenvironment, CAFs can drive
integrin-mediated desmoplasia (121). Integrin αvβ5 regulates the
endocytosis and recycling of active α5β1, which sustains
fibronectin matrix assembly and focal adhesion turnover (122). These processes enhance actomyosin
contractility and ECM stiffening, thereby activating a fibroblast
into myofibroblastic CAF phenotype that maintains ECM remodeling
and promotes disease recurrence (121). Similarly, integrin α11β1 can
mediate CAF-fibronectin interactions, enhancing PDAC cell migration
(122). Integrin α3β1 binds to
laminin-111, collagen I and laminin-332 in the ECM, to facilitate
PDAC cell motility (123).
Targeting integrins in vitro has shown
therapeutic potential. Monoclonal antibodies against α11 integrin
have been developed to disrupt CAF adhesion to collagen and impair
cancer cell invasion (123,124). In 3D models, PDAC cells migrated
along fibroblast extensions using α5β1, which adhered to
fibronectin deposited by CAFs (124). Integrins αvβ3, α5β1 and αvβ5 are
involved in directing tumor invasion and ECM deposition (125). However, the molecular crosstalk
between α11β1 and other integrins in PDAC remains poorly
characterized.
Integrins can also interact with CEACAMs,
co-localizing in lipid rafts where they cluster and modulate
signaling. Specifically, CEACAM1 and CEACAM6 have been reported to
associate with β1 and β4 integrins, enhancing adhesion and survival
pathways in epithelial cancers (19). In PDAC, ITGB4, which influences
tumorigenesis, is activated through tyrosine phosphorylation at
Y1510, regulating downstream MEK1-ERK1/2 signaling (19,108). In addition, ITGB4 expression has
been found to be upregulated in PDAC compared with in normal or
inflamed pancreatic tissues (126). Its activation promotes EMT,
metastasis and chemoresistance, making ITGB4 a potential prognostic
marker (102,127). In gastric cancer, ECM protein 1
promotes metastasis and glycolytic activity through the
ITGB4/FAK/SOX2/hypoxia-inducible factor-1α axis, suggesting a
conserved oncogenic mechanism (126,127).
Additionally, ZEB1 can directly upregulate ITGA3
and ITGB1 by binding to their promoters, contributing to tumor cell
proliferation and migration in PDAC (128). The integration of
integrin-mediated signaling with EMT programs and fibroinflammatory
remodeling emphasizes their role in tumor aggressiveness.
Understanding the function of integrins and their molecular
partners in PDAC is essential for the identification of novel
therapeutic targets. Table I
(74,105,111,129-134).
summarizes the various integrins associated with PDAC pathogenesis
and their functional roles in PDAC.
 | Table IDifferent integrins in PDAC and the
distinct functional roles. |
Table I
Different integrins in PDAC and the
distinct functional roles.
| First author/s,
year | Integrin
subtype | Mechanism of
action | Functional
role | (Refs.) |
|---|
| Kemper et
al, 2021 | ITGAV | Mediated by E and P
selectin; high expression of ITGAV activates latent TGF-β and
upregulates TGF-βR1 through the activity of SMAD4. | Upregulation in
selectin deficiency enhances intraperitoneal and pulmonary
metastatic PDAC and drives EMT. | (74) |
| Mia et al,
2021 | ITGβ1 | Binds to TGF-β
receptor 2; signals through focal adhesion molecules and
upregulates pro-fibrotic signaling. | Promotes tumor
growth of PDAC and metastasis through an increase in the expression
of fibronectin or vitronectin; associated with poor patient
survival. | (131) |
| Kuninty et
al, 2019 | ITGA5 | Modulates
TGF-β1/SMAD pathways via focal adhesion kinase pathways. | High ITGA5 in PDAC
is associated with poor overall survival; increases desmoplasia,
decreases tumor perfusion and contributes to gemcitabine
resistance. | (132) |
| Li et al,
2015 | ITGβ6 | Cytoplasmic domain
of ITGβ6 directly binds to ERK2 and increases MAPK activity
(101); ITGB6 induces ETS1
phosphorylation in the ETS-ERK-signaling pathway in the
upregulation of MMP-9(102). | Promotes
proliferation. | (133) |
| Schnittert et
al, 2019 | ITGA11 | Binds to collagen
on the cell surface; Integrin α11 is a receptor of collagen type I
and is upregulated by CAFs; the positive correlation between TGF-β
indicates that the upregulation of ITGA11 may be attributed to
TGF-β, with no clear mechanistic activity. | Promotes cell
metastasis and invasion; regulates pancreatic stellate cell
differentiation into CAFs. | (129) |
| Chen et al,
2022 | ITGA3 | Upregulates
cytokeratin-19 suppresses T-cell activity. ITGA3 interacts with
collagen I and upregulates EGFR signaling via LRIGI induction. | High ITGA3
expression promotes cell invasion and aids tumor cells in evading
apoptosis, and its high expression is associated with a poor
prognosis in PDAC. | (130) |
| Humphries et
al, 2022 | α6β4
(ITGA6/ITGB4) | Interacts with ECM
components, such as fibronectin and laminin. | Formation of
hemidesmosomes is inhibited, allowing α6β4 to act as a signaling
integrin that stimulates tumor progression. | (134) |
| Masugi et
al, 2015 | ITGβ4 | Combines with
several oncogenic receptor tyrosine kinases, including c-Met, ErbB1
and ErbB2, to amplify the signaling pathways that accelerate cancer
invasion. | Involved in
promoting EMT and regulating cancer invasion. | (105) |
| Cavaco et
al, 2018 | α2β1
(ITGA2/ITGB1) | Mediates cancer
cell adhesion to ECM components. | Promotes strong
adhesion of tumor cells to ECM components (e.g., collagen), thereby
supporting PDAC progression. | (111) |
Association between integrins and
CEACAM6 in PDAC
The interplay between CEACAM6 and integrins
contributes to PDAC progression by regulating adhesion, migration
and signaling dynamics within the tumor microenvironment (14,17).
CEACAM6 mediates both homophilic interactions with other CEA family
members and heterophilic interactions with integrins, particularly
αvβ3 and α5β1 (16,17). These interactions facilitate cancer
cell adhesion, invasion, EMT and resistance to anoikis (73,135,136). These interaction between CEACAM
family members and various integrins, supported by both
experimental and predictive evidence, are summarized in Table II (50,74,103,129,137,144-167).
 | Table IIAssociation between different CEACAM
members and integrin proteins. |
Table II
Association between different CEACAM
members and integrin proteins.
| First author/s,
year | Association | Integrin
protein | CEACAM member | (Refs.) |
|---|
| Brümmer et
al, 2001 | Interaction between
integrin β3 and a fusion protein containing the
cytoplasmic domain of CEACAM1; cis associations between
integrin β3 and CEACAM1. | β3 | CEACAM1 | (166) |
| Vuijk et al,
2020 | Co-localization at
the cell membrane and highly expressed in pancreatic ductal
adenocarcinoma; co-expressed at the cell membrane and diffusely
expressed in tumor cells and surrounding fibrosis; co-localization
and activation via lipid rafts. | αvβ6; α5β1 | CEACAM5 | (167) |
| Kemper et
al, 2021 | CEACAM7
co-localizes with integrin αV in pancreatic ductal adenocarcinoma,
facilitating lipid raft–mediated signaling. This interaction
enhances ECM remodeling and supports fibrotic progression. | αV | CEACAM7 | (74) |
| Schnittert et
al, 2018 | Integrin ανβ3
expression is upregulated through CEACAM6 cross-links with
extracellular matrix proteins, fibronectin and vitronectin. In
addition, CEACAM6 co-localizes with integrin α5β1 within the lipid
rafts, where Src kinase-mediated activation of α5β1 enhances
downstream oncogenic signaling | α5β1; ανβ3 | CEACAM6 | (129) |
| Raj et al,
2021 | Fusion of
extracellular Ig variable domain of CEACAM3 and intracellular
domain of integrin β1. | β1 | CEACAM3 | (50) |
CEACAM6 has been implicated in modulating
integrin-dependent signaling, particularly through the activation
of FAK and c-Src kinase pathways, which enhance integrin affinity
for ECM proteins, such as fibronectin and vitronectin (17). In PDAC, CEACAM6 overexpression
upregulates the activation state of α5β1 integrins without altering
receptor abundance, promoting increased fibronectin binding, matrix
assembly, and disruption of normal differentiation and survival
mechanisms (139). Furthermore,
CEACAM6-integrin crosstalk is not limited to cancer but also can
regulate inflammatory and infectious diseases, including the role
of CEACAM6 as a receptor for adherent-invasive E. coli in
Crohn's disease (126),
suggesting its functional versatility across pathologies (20,140). CEACAM6 has also been shown to
facilitate lipid raft-mediated signaling, supporting uptake
mechanisms and the phosphorylation of key downstream effectors,
such as Akt and MAPK, through integrin-linked kinase (ILK)
activation (141,142).
A previous study has demonstrated that clustering
of CEACAM6 on the membrane could promote co-localization with
integrin α5β1, which in turn activated the Akt and MAPK signaling
cascades. This integration of CEA family proteins with integrin
signaling reinforced malignant behaviors, such as enhanced cell-ECM
adhesion, increased invasive capacity and survival under
anchorage-independent conditions (141). Furthermore, STRING network
analysis (Fig. 4) illustrated the
functional protein-protein interaction landscape between CEACAM6
and key integrins. Protein-protein interactions were analyzed using
the STRING database (version 11.5; https://string-db.org/). The query was performed
using Homo sapiens as the organism. Interaction sources included
experimental data, curated databases, co-expression, text mining,
co-occurrence and homology. The confidence score threshold was set
at 0.700 (high confidence). The resulting network was visualized in
STRING, and interaction lines were color-coded based on the
evidence channel (e.g., experimental, co-expression and text
mining).
This bioinformatics representation consolidates
experimental evidence and predictive modeling, reinforcing the
functional relevance of CEACAM6-integrin networks in PDAC
progression.
Mechanistic analysis of
CEACAM6-integrin interactions in PDAC
CEACAM6, a GPI-anchored adhesion molecule, clusters
within lipid rafts and engages integrin receptors, particularly
α5β1 and αvβ3(47). These
interactions enhance integrin avidity for ECM ligands, such as
fibronectin and vitronectin, facilitating stable cell-matrix
adhesion and initiating downstream signal transduction (17). Upon co-localization with integrins,
CEACAM6 promotes the activation of FAK and c-Src, leading to the
phosphorylation of ILK and the subsequent activation of the
PI3K/Akt and MAPK signaling pathways. This dual activation supports
key malignant phenotypes, including sustained proliferation,
survival and invasion (47).
CEACAM6 engages integrins, particularly αvβ3 and
α5β1, to activate Akt signaling, conferring anoikis resistance
under anchorage-independent conditions, a survival effect supported
in multiple models, including colorectal, breast, lung and gastric
cancer, where CEACAM6-driven upregulation of anti-apoptotic
proteins, such as Bcl-2 and survivin (16,51,80,94,136,139). Additionally, CEACAM6 can modulate
ECM remodeling by enhancing the expression and activity of MMP-2
and MMP-9, primarily through FAK- and NF-κB-dependent
transcriptional regulation. This effect is mediated via its
interactions with integrins, particularly α5β1 and αvβ3, which
cluster with CEACAM6 in lipid rafts to activate FAK/Src signaling.
The resulting upregulation of MMPs facilitates the degradation of
the basement membrane, thereby promoting local invasion and
metastatic dissemination (17,142).
CEACAM6-integrin signaling also contributes to EMT
by downregulating E-cadherin and inducing mesenchymal markers, such
as Snail and ZEB1, through the sustained activation of the Akt and
MAPK pathways (143,144). These signaling cascades result in
resistance to gemcitabine and other chemotherapeutic agents
(103,143,144). In addition, CEACAM6-mediated
integrin signaling can increase drug efflux and reinforce survival
pathways, suppressing apoptotic responses to cytotoxic stress
(103). The tumor
microenvironment then further modulates CEACAM6-integrin dynamics.
Increased matrix stiffness, cytokine gradients and altered ECM
composition increase integrin signaling, reinforcing
CEACAM6-mediated oncogenic programs in PDAC (103).
5. Therapeutic targeting of CEACAM6 and
integrins in PDAC
Current therapeutic strategies
CEACAM6 and integrins are promising targets for the
treatment of PDAC due to their roles in tumor adhesion, survival,
invasion and chemoresistance (41). Monoclonal antibodies targeting
CEACAM6 have exhibited preclinical efficacy by disrupting integrin
interactions and downstream signaling pathways, thereby reducing
invasion and metastasis (67).
Similarly, integrin-specific strategies using α5β1 and αvβ3
inhibitors have been shown to impair tumor-ECM interactions and
enhance chemosensitivity (145).
CAR-T therapies targeting CEACAM6 and associated
CEACAM family members, such as CEACAM7, represent an emerging
immunotherapeutic avenue that can overcome the immunosuppressive
microenvironment found in PDAC (146). Combination strategies, pairing
CEACAM6/integrin inhibitors with chemotherapy (gemcitabine) or
matrix-modulating agents, have exhibited synergistic effects and
offer promise in overcoming drug resistance (147).
Clinical trial status and biomarker
validation
Early-phase trials of anti-CEACAM6 antibodies and
antibody-drug conjugates have reported favorable safety and
preliminary efficacy, particularly in patients with high
CEACAM6-urpregulated malignancies, such as colorectal and gastric
cancers. In PDAC, these agents remain at the preclinical stage,
where dual-specificity constructs such as CT109-SN-38 have
demonstrated potent cytotoxicity in a pancreatic cancer model
(148). Integrin inhibitors,
particularly those targeting αvβ3 and α5β1, are currently
undergoing phase I/II evaluation in several solid tumors, including
melanoma, and lung cancer, where combination regimens generally
outperforming monotherapy (149,150). Although clinical data in PDAC
remain limited, a preclinical study suggested that the CEACAM6 and
integrin expression levels are associated with treatment response
(60). Additionally,
CEACAM6-integrin expression profiles may predict chemotherapy
sensitivity, supporting the development of personalized treatment
approaches (17).
Challenges and limitations
Therapeutic resistance remains an important
barrier, mediated by pathway redundancy, tumor heterogeneity and
microenvironmental adaptation (151,152). Subclonal variation in
CEACAM6/integrin expression may lead to incomplete responses,
whilst upregulation of alternative adhesion molecules can bypass
targeted inhibition (153). By
contrast, the desmoplastic and immunosuppressive microenvironment
of PDAC limits drug delivery and the efficacy of immunotherapy
(154,155). Furthermore, variable expression
levels and a lack of standardized cut-off values complicate the
development of biomarkers (60).
6. Clinical translational applications and
future perspectives
Clinical relevance of CEACAM6-integrin
interactions
Although several CEACAM family members have been
implicated in cancer biology, CEACAM6-integrin interactions hold
significant clinical value across early diagnosis, prognosis,
treatment selection and disease monitoring in PDAC (17,57).
CEACAM6 expression is frequently upregulated, by ≤25-fold, in PDAC
tissues and precursor lesions, making it a promising biomarker for
early detection (57,75). High CEACAM6 expression levels are
also associated with aggressive tumor subtypes and poor survival,
serving as an independent prognostic marker regardless of KRAS
status (14,60). This enables risk stratification and
informed decisions on the use of intensified or experimental
therapies and predicts treatment response. Elevated CEACAM6
expression is associated with gemcitabine resistance, whilst
specific integrin expression patterns influence chemotherapy
outcomes, supporting personalized therapeutic strategies (17,156).
Monitoring of circulating CEACAM6 and
integrin-related proteins enables real-time assessment of treatment
efficacy, providing a dynamic alternative to traditional imaging
methods (17,157). Additionally, the strong
association of CEACAM6/integrins with metastasis risk can inform
post-treatment surveillance and the planning of adjuvant therapy
(60).
Translational challenges and
limitations
Translating CEACAM6-integrin research into clinical
application faces several key barriers. A major challenge is the
lack of assay standardization, with varying methodologies across
laboratories hindering reproducibility and cross-study comparison
(158,159). Tumor heterogeneity further
complicates clinical translation, since the expression of CEACAM6
and integrins varies within and between tumors (14,75).
Therefore, single-site biopsies may misrepresent the full molecular
landscape, limiting the accuracy of treatment decisions (160).
The dynamic expression of these molecules during
disease progression and therapy adds complexity, requiring
longitudinal monitoring that may be impractical in routine settings
due to cost and infrastructure constraints (47,161,162). Additionally, unfavorable
regulatory environments, particularly for advanced therapies, such
as CAR-T cell therapies, necessitate specialized clinical trial
designs that can prolong the approval process (163,164).
Future clinical applications
CEACAM6-integrin research is essential in
transforming PDAC management through precision diagnostics,
targeted therapies and real-time monitoring. Molecular profiling of
CEACAM6-integrin expression will enable personalized treatment
selection, guiding choices of chemotherapy, targeted agents or
immunotherapies based on individual tumor characteristics. By
contrast, the emergence of liquid biopsy platforms incorporating
CEACAM6-integrin biomarkers, such as circulating tumor cells,
extracellular vesicles and soluble proteins, offers a non-invasive
method for monitoring disease progression and treatment response,
allowing dynamic, real-time clinical decision-making (166,167).
Integrating CEACAM6-integrin targeting with
immunotherapy or oncolytic virus platforms represents a compelling
strategy to enhance antitumor immune responses by modulating the
tumor microenvironment. In addition, the relevance of
CEACAM6-integrin signaling across multiple tumor types suggests the
potential for pan-cancer therapeutic strategies.
7. Discussion
The present review aimed to highlight the crucial
role of CEACAM6-integrin crosstalk in the pathogenesis of PDAC.
CEACAM6, a GPI-anchored adhesion molecule, is frequently
upregulated in PDAC, which contributes to tumor cell survival,
immune evasion, chemoresistance and desmoplastic remodeling
(14,17). Through lipid raft-mediated
clustering, CEACAM6 co-localizes with integrins, particularly α5β1
and αvβ3, amplifying downstream oncogenic signaling through the
FAK, Src, PI3K/Akt and MAPK pathways (47,48).
The CEACAM6-integrin axis facilitates
anchorage-independent survival, EMT, matrix stiffening and CAF
activation, which are hallmarks of PDAC aggressiveness. These
interactions also contribute to resistance to chemotherapy by
altering drug uptake and activating anti-apoptotic signaling
pathways. CEACAM6-integrin co-expression serves as a prognostic
marker and may guide personalized therapy. High CEACAM6 levels are
associated with poor survival and gemcitabine resistance, whilst
integrin profiles predict responsiveness to combination therapies.
Despite promising therapeutic targets, pathway redundancy, tumor
heterogeneity and assay standardization make PDAC management
challenging.
Therapeutic strategies combining anti-CEACAM6
agents with integrin inhibitors, matrix-degrading enzymes or
immunotherapies show synergistic potential. Additionally, there is
a need to consider integrating CEACAM6-integrin biomarkers into
clinical workflows through liquid biopsy approaches, such as
blood-based assays of circulating tumor cells, extracellular
vesicles or circulating tumor DNA (156-160).
Future studies should explore isoform-specific functions,
protein-protein interactions and temporal expression changes across
disease stages. Mechanistic insights into CEACAM6-integrin
signaling and its impact on ECM remodeling will be crucial for
designing next-generation precision therapies in PDAC.
In conclusion, CEACAM6 and integrins represent
critical regulators of PDAC progression through their influence on
adhesion, survival signaling, desmoplasia and immune modulation.
Their interactions activate oncogenic pathways, such as the FAK,
PI3K/Akt and MAPK pathways, thereby enhancing tumor invasion,
metastasis and therapeutic resistance. The lipid raft-mediated
clustering of CEACAM6 with integrins, such as α5β1 and αvβ3,
reinforces ECM remodeling, chemoresistance and
anchorage-independent survival, whilst integrins themselves
contribute to EMT, angiogenesis and immune evasion. These molecules
shape a tumor microenvironment that is conducive to malignancy and
hinders the effectiveness of therapy.
CEACAM6-integrin dynamics are essential in
precision oncology in PDAC, ranging from biomarker-driven
diagnostics to targeted therapies. Integrating molecular profiling
with emerging treatments, such as CAR-T cells, bispecific
antibodies and matrix-disruptive strategies, may overcome current
limitations posed by tumor heterogeneity, signaling redundancy, and
stromal barriers.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AAK was responsible for conceptualization,
literature review, drafting, writing, critical revision and final
approval of the manuscript. Data authentication is not applicable.
The author has read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares they have no competing
interests.
References
|
1
|
Zińczuk J, Zaręba K, Romaniuk W, Kamińska
D, Nizioł M, Baszun M, Kędra B, Guzińska-Ustymowicz K and
Pryczynicz A: Expression of chosen carcinoembryonic-related cell
adhesion molecules in pancreatic intraepithelial neoplasia (PanIN)
associated with chronic pancreatitis and pancreatic ductal
adenocarcinoma (PDAC). Int J Med Sci. 16:583–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu JX, Zhao CF, Chen WB, Liu QC, Li QW,
Lin YY and Gao F: Pancreatic cancer: A review of epidemiology,
trend, and risk factors. World J Gastroenterol. 27:4298–4321.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pereira SP, Oldfield L, Ney A, Hart PA,
Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, et al:
Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol.
5:698–710. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH
and Neoptolemos JP: Pancreatic cancer. Nat Rev Dis Primers 2:
16022. 2016.
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schober M, Jesenofsky R, Faissner R,
Weidenauer C, Hagmann W, Michl P, Heuchel RL, Haas SL and Löhr JM:
Desmoplasia and chemoresistance in pancreatic cancer. Cancers
(Basel). 6:2137–2154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Azizian A, Rühlmann F, Krause T, Bernhardt
M, Jo P, König A, Kleiß M, Leha A, Ghadimi M and Gaedcke J: CA19-9
for detecting recurrence of pancreatic cancer. Sci Rep.
10(1332)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goh SK, Gold G, Christophi C and
Muralidharan V: Serum carbohydrate antigen 19-9 in pancreatic
adenocarcinoma: A mini review for surgeons. ANZ J Surg. 87:987–992.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moffitt RA, Marayati R, Flate EL, Volmar
KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung
AH, et al: Virtual microdissection identifies distinct tumor- and
stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat
Genet. 47:1168–1178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tanase CP, Neagu M, Albulescu R and
Hinescu ME: Advances in pancreatic cancer detection. Adv Clin Chem.
51:145–180. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang D, Li Y, Ge H, Ghadban T, Reeh M and
Güngör C: The extracellular matrix: A key accomplice of cancer stem
cell migration, metastasis formation, and drug resistance in PDAC.
Cancers (Basel). 14(3998)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Janiszewska M, Primi MC and Izard T: Cell
adhesion in cancer: Beyond the migration of single cells. J Biol
Chem. 295:2495–2505. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cox TR: The matrix in cancer. Nat Rev
Cancer. 21:217–238. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pandey R, Zhou M, Islam S, Chen B, Barker
NK, Langlais P, Srivastava A, Luo M, Cooke LS, Weterings E and
Mahadevan D: Carcinoembryonic antigen cell adhesion molecule 6
(CEACAM6) in pancreatic ductal adenocarcinoma (PDA): An integrative
analysis of a novel therapeutic target. Sci Rep.
9(18347)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Han ZW, Lyv ZW, Cui B, Wang YY, Cheng JT,
Zhang Y, Cai WQ, Zhou Y, Ma ZW, Wang XW, et al: The old CEACAMs
find their new role in tumor immunotherapy. Invest New Drugs.
38:1888–1898. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johnson B and Mahadevan D: Emerging role
and targeting of carcinoembryonic antigen-related cell adhesion
molecule 6 (CEACAM6) in human malignancies. Clin Cancer Drugs.
2:100–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Duxbury MS, Ito H, Ashley SW and Whang EE:
c-Src-dependent cross-talk between CEACAM6 and alphavbeta3 integrin
enhances pancreatic adenocarcinoma cell adhesion to extracellular
matrix components. Biochem Biophys Res Commun. 317:133–141.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hatakeyama K, Wakabayashi-Nakao K, Ohshima
K, Sakura N, Yamaguchi K and Mochizuki T: Novel protein isoforms of
carcinoembryonic antigen are secreted from pancreatic, gastric and
colorectal cancer cells. BMC Res Notes. 6(381)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Blumenthal RD, Leon E, Hansen HJ and
Goldenberg DM: Expression patterns of CEACAM5 and CEACAM6 in
primary and metastatic cancers. BMC Cancer. 7(2)2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Y, Zang M, Li J, Ji J, Zhang J, Liu
X, Qu Y, Su L, Li C, Yu Y, et al: CEACAM6 promotes tumor migration,
invasion, and metastasis in gastric cancer. Acta Biochim Biophys
Sin (Shanghai). 46:283–290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tchoupa AK, Schuhmacher T and Hauck CR:
Signaling by epithelial members of the CEACAM family-mucosal
docking sites for pathogenic bacteria. Cell Commun Signal.
12(27)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu G, Wang D, Xiong F, Wang Q, Liu W, Chen
J and Chen Y: The emerging roles of CEACAM6 in human cancer
(Review). Int J Oncol. 64(27)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Valdembri D and Serini G: The roles of
integrins in cancer. Fac Rev. 10(45)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kuespert K, Pils S and Hauck CR: CEACAMs:
Their role in physiology and pathophysiology. Curr Opin Cell Biol.
18:565–571. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schölzel S, Zimmermann W, Schwarzkopf G,
Grunert F, Rogaczewski B and Thompson J: Carcinoembryonic antigen
family members CEACAM6 and CEACAM7 are differentially expressed in
normal tissues and oppositely deregulated in hyperplastic
colorectal polyps and early adenomas. Am J Pathol. 156:595–605.
2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zimmermann W and Kammerer R:
Carcinoembryonic antigen. Tumor-Associated Antigens:
Identification, Characterization, and Clinical Applications,
pp201-218, 2009.
|
|
27
|
Gold P and Freedman SO: Specific
carcinoembryonic antigens of the human digestive system. J Exp Med.
122:467–481. 1965.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gray-Owen SD and Blumberg RS: CEACAM1:
Contact-dependent control of immunity. Nat Rev Immunol. 6:433–446.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bonsor DA, Günther S, Beadenkopf R,
Beckett D and Sundberg EJ: Diverse oligomeric states of CEACAM IgV
domains. Proc Natl Acad Sci USA. 112:13561–13566. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Noworolska A, Harłozińska A, Richter R and
Brodzka W: Non-specific cross-reacting antigen (NCA) in individual
maturation stages of myelocytic cell series. Br J Cancer.
51:371–377. 1985.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hanenberg H, Baumann M, Quentin I, Nagel
G, Grosse-Wilde H, von Kleist S, Göbel U, Burdach S and Grunert F:
Expression of the CEA gene family members NCA-50/90 and NCA-160
(CD66) in childhood acute lymphoblastic leukemias (ALLs) and in
cell lines of B-cell origin. Leukemia. 8:2127–2133. 1994.PubMed/NCBI
|
|
32
|
Chan CHF and Stanners CP: Novel mouse
model for carcinoembryonic antigen-based therapy. Mol Ther.
9:775–785. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Blumenthal RD, Hansen HJ and Goldenberg
DM: Inhibition of adhesion, invasion, and metastasis by antibodies
targeting CEACAM6 (NCA-90) and CEACAM5 (carcinoembryonic antigen).
Cancer Res. 65:8809–8817. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Skubitz KM: The role of CEACAM s in
neutrophil function. Eur J Clin Invest. 54 (Suppl
2)(e14349)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schumann D, Huang J, Clarke PE, Kirshner
J, Tsai SW, Schumaker VN and Shively JE: Characterization of
recombinant soluble carcinoembryonic antigen cell adhesion molecule
1. Biochem Biophys Res Commun. 318:227–233. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lu R, Pan H and Shively JE: CEACAM1
negatively regulates IL-1β production in LPS activated neutrophils
by recruiting SHP-1 to a SYK-TLR4-CEACAM1 complex. PLoS Pathog.
8(e1002597)2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Klaile E, Vorontsova O, Sigmundsson K,
Müller MM, Singer BB, Ofverstedt LG, Svensson S, Skoglund U and
Obrink B: The CEACAM1 N-terminal Ig domain mediates cis- and
trans-binding and is essential for allosteric rearrangements of
CEACAM1 microclusters. J Cell Biol. 187:553–567. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Markel G, Achdout H, Katz G, Ling KL,
Salio M, Gruda R, Gazit R, Mizrahi S, Hanna J, Gonen-Gross T, et
al: Biological function of the soluble CEACAM1 protein and
implications in TAP2-deficient patients. Eur J Immunol.
34:2138–2148. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kiriyama S, Yokoyama S, Ueno M, Hayami S,
Ieda J, Yamamoto N, Yamaguchi S, Mitani Y, Nakamura Y, Tani M, et
al: CEACAM1 long cytoplasmic domain isoform is associated with
invasion and recurrence of hepatocellular carcinoma. Ann Surg
Oncol. 21 (Suppl 4):S505–S514. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shamsuddin SHB: Biosensors for detection
of colorectal cancer. University of Leeds, 2018.
|
|
41
|
Chan CHF and Stanners CP: Recent advances
in the tumour biology of the GPI-anchored carcinoembryonic antigen
family members CEACAM5 and CEACAM6. Curr Oncol. 14:70–73.
2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chiang WF, Cheng TM, Chang CC, Pan SH,
Changou CA, Chang TH, Lee KH, Wu SY, Chen YF, Chuang KH, et al:
Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6)
promotes EGF receptor signaling of oral squamous cell carcinoma
metastasis via the complex N-glycosylation. Oncogene. 37:116–127.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Swords DS, Firpo MA, Scaife CL and
Mulvihill SJ: Biomarkers in pancreatic adenocarcinoma: Current
perspectives. Onco Targets Ther. 9:7459–7467. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Thomas J, Klebanov A, John S, Miller LS,
Vegesna A, Amdur RL, Bhowmick K and Mishra L: CEACAMS 1, 5, and 6
in disease and cancer: Interactions with pathogens. Genes Cancer.
14:12–29. 2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ordonez C, Zhai AB, Camacho-Leal P,
Demarte L, Fan MM and Stanners CP: GPI-anchored CEA family
glycoproteins CEA and CEACAM6 mediate their biological effects
through enhanced integrin alpha5beta1-fibronectin interaction. J
Cell Physiol. 210:757–765. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kelleher M, Singh R, O'Driscoll CM and
Melgar S: Carcinoembryonic antigen (CEACAM) family members and
inflammatory bowel disease. Cytokine Growth Factor Rev. 47:21–31.
2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Al-Khinji A: 122 CEACAM6 molecules mediate
cell adhesion and signaling by modifying integrins in human solid
tumors. J Clin Transl Sci. 9 (Suppl 1)(S35)2025.
|
|
48
|
Zhao D, Cai F, Liu X, Li T, Zhao E, Wang X
and Zheng Z: CEACAM6 expression and function in tumor biology: A
comprehensive review. Discov Oncol. 15(186)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dhasmana A, Dhasmana S, Kotnala S, Laskar
P, Khan S, Haque S, Jaggi M, Yallapu MM and Chauhan SC: CEACAM7
expression contributes to early events of pancreatic cancer. J Adv
Res. 55:61–72. 2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Raj D, Nikolaidi M, Garces I, Lorizio D,
Castro NM, Caiafa SG, Moore K, Brown NF, Kocher HM, Duan X, et al:
CEACAM7 is an effective target for CAR T-cell therapy of pancreatic
ductal adenocarcinoma. Clin Cancer Res. 27:1538–1552.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pavlopoulou A and Scorilas A: A
comprehensive phylogenetic and structural analysis of the
carcinoembryonic antigen (CEA) gene family. Genome Biol Evol.
6:1314–1326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rizeq B, Zakaria Z and Ouhtit A: Towards
understanding the mechanisms of actions of carcinoembryonic
antigen-related cell adhesion molecule 6 in cancer progression.
Cancer Sci. 109:33–42. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Beauchemin N and Arabzadeh A:
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs)
in cancer progression and metastasis. Cancer Metastasis Rev.
32:643–671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhao L, Li T, Zhou Y, Wang P and Luo L:
Monoclonal antibody targeting CEACAM1 enhanced the response to
anti-PD1 immunotherapy in non-small cell lung cancer. Int
Immunopharmacol. 143(113395)2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Singer BB: CEACAMs. In Encyclopedia of
Signaling Molecules. Springer, pp1-9, 2016.
|
|
56
|
Tilki D, Singer BB, Shariat SF, Behrend A,
Fernando M, Irmak S, Buchner A, Hooper AT, Stief CG, Reich O and
Ergün S: CEACAM1: A novel urinary marker for bladder cancer
detection. Eur Urol. 57:648–654. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Simeone DM, Ji B, Banerjee M, Arumugam T,
Li D, Anderson MA, Bamberger AM, Greenson J, Brand RE, Ramachandran
V and Logsdon CD: CEACAM1, a novel serum biomarker for pancreatic
cancer. Pancreas. 34:436–443. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ji B, Simeone D, Ramachandran V, Arumugam
T, Hanash S, Giordano T, Greenson J, Taylor J and Logsdon CD:
CEACAM1 is a biomarker for pancreatic cancer. Pancreas. 29:336–337.
2004.
|
|
59
|
Shi JF, Xu SX, He P and Xi ZH: Expression
of carcinoembryonic antigen-related cell adhesion molecule
1(CEACAM1) and its correlation with angiogenesis in gastric cancer.
Pathol Res Pract. 210:473–476. 2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kurlinkus B, Ger M, Kaupinis A, Jasiunas
E, Valius M and Sileikis A: CEACAM6's role as a chemoresistance and
prognostic biomarker for pancreatic cancer: A comparison of
CEACAM6's diagnostic and prognostic capabilities with those of
CA19-9 and CEA. Life (Basel). 11(542)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Qian W, Huang P, Liang X, Chen Y and Guan
B: High expression of carcinoembryonic antigen-associated cell
adhesion molecule 1 is associated with microangiogenesis in
esophageal squamous cell carcinoma. Transl Cancer Res. 9:4762–4769.
2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhou M, Jin Z, Liu Y, He Y, Du Y, Yang C,
Wang Y, Hu J, Cui L, Gao F and Cao M: Up-regulation of
carcinoembryonic antigen-related cell adhesion molecule 1 in
gastrointestinal cancer and its clinical relevance. Acta Biochim
Biophys Sin (Shanghai). 49:737–743. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kim WM, Huang YH, Gandhi A and Blumberg
RS: CEACAM1 structure and function in immunity and its therapeutic
implications. Semin Immunol. 42(101296)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lu R, Kujawski M, Pan H and Shively JE:
Tumor angiogenesis mediated by myeloid cells is negatively
regulated by CEACAM1. Cancer Res. 72:2239–2250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Dong J, Wang B, Wang D, Ni J, Bu T, Wang
H, Nayak B, Wang B, Dong X and Zhang Y: Abstract 2732: NB203: A
novel therapeutic bispecific antibody targeting CEACAM1/VEGF for
tumor immunity and angiogenesis modulation in the tumor
microenvironment. Cancer Res. 84 (6 Suppl)(S2732)2024.
|
|
66
|
Zuo J, Wang Y, Chen Z, Li H, Yang Y, Zhang
Y, Zhang L and Chen J: Development of CEACAM1-specific CAR-T cells
for the treatment of malignant melanoma. Oncol Rep. 49(72)2023.
|
|
67
|
Huang Y, Wang H, Lichtenberger J, Zhang J,
Kappes J and Wu Y: CEACAM1 as a potential therapeutic target in
melanoma and other malignancies. Front Oncol. 9(1072)2019.
|
|
68
|
Partyka K, Maupin KA, Brand RE and Haab
BB: Diverse monoclonal antibodies against the CA 19-9 antigen show
variation in binding specificity with consequences for clinical
interpretation. Proteomics. 12:2212–2220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yang L, Liu Y, Zhang B, Yu M, Huang F,
Zeng J, Lu Y and Yang C: CEACAM1 is a prognostic biomarker and
correlated with immune cell infiltration in clear cell renal cell
carcinoma. Dis Markers. 2023(3606362)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ligorio M, Sil S, Malagon-Lopez J, Nieman
LT, Misale S, Di Pilato M, Ebright RY, Karabacak MN, Kulkarni AS,
Liu A, et al: Stromal microenvironment shapes the intratumoral
architecture of pancreatic cancer. Cell. 178:160–175.e27.
2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gisina A, Novikova S, Kim Y, Sidorov D,
Bykasov S, Volchenko N, Kaprin A, Zgoda V, Yarygin K and Lupatov A:
CEACAM5 overexpression is a reliable characteristic of
CD133-positive colorectal cancer stem cells. Cancer Biomark.
32:85–98. 2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chen J, Li Q, An Y, Lv N, Xue X, Wei J,
Jiang K, Wu J, Gao W, Qian Z, et al: CEACAM6 induces
epithelial-mesenchymal transition and mediates invasion and
metastasis in pancreatic cancer. Int J Oncol. 43:877–885.
2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Witzens-Harig M, Hose D, Jünger S,
Pfirschke C, Khandelwal N, Umansky L, Seckinger A, Conrad H,
Brackertz B, Rème T, et al: Tumor cells in multiple myeloma
patients inhibit myeloma-reactive T cells through carcinoembryonic
antigen-related cell adhesion molecule-6. Blood. 121:4493–4503.
2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kemper M, Schiecke A, Maar H, Nikulin S,
Poloznikov A, Galatenko V, Tachezy M, Gebauer F, Lange T, Riecken
K, et al: Integrin alpha-V is an important driver in pancreatic
adenocarcinoma progression. J Exp Clin Cancer Res.
40(214)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mahadevan D, Pandey R, Chen Y, Essif J and
Al-Khinji A: Oncogenic roles of CEACAM6 in pancreatic ductal
adenocarcinoma. J Clin Oncol. 38 (15 Suppl)(e16744)2020.
|
|
76
|
Kim H, Woo CG, Son SM, Lee YP, Kim HK,
Yang Y, Kwon J, Lee KH, Lee HC, Lee OJ and Han HS: Targeted
suppression of CEACAM6 via pHLIP-delivered rnas in pancreatic
ductal adenocarcinoma. Medicina (Kaunas). 61(598)2025.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wu J, Pan TH, Xu S, Jia LT, Zhu LL, Mao
JS, Zhu YL and Cai JT: The virus-induced protein APOBEC3G inhibits
anoikis by activation of Akt kinase in pancreatic cancer cells. Sci
Rep. 5(12230)2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zang M, Zhang Y, Zhang B, Hu L, Li J, Fan
Z, Wang H, Su L, Zhu Z, Li C, et al: CEACAM6 promotes tumor
angiogenesis and vasculogenic mimicry in gastric cancer via FAK
signaling. Biochim Biophys Acta. 1852:1020–1028. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Niu G, Murad YM, Gao H, Hu S, Guo N,
Jacobson O, Nguyen TD, Zhang J and Chen X: Molecular targeting of
CEACAM6 using antibody probes of different sizes. J Control
Release. 161:18–24. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ma RX, Wei JR and Hu YW: Characteristics
of carcinoembryonic antigen related cell adhesion molecules and
their relationship to cancer. Mol Cancer Ther. 23:939–948.
2024.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Te Molder L, Kreft M, Heemskerk N,
Schuring J, de Pereda JM, Wilhelmsen K and Sonnenberg A:
EGFR-dependent tyrosine phosphorylation of integrin β4 is not
required for downstream signaling events in cancer cell lines. Sci
Rep. 11(8675)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Du H, Li Y, Sun R, Yuan Y, Sun S and Zhang
Y: CEACAM6 promotes cisplatin resistance in lung adenocarcinoma and
is regulated by microRNA-146a and microRNA-26a. Thorac Cancer.
11:2473–2482. 2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ambrosi C, Scribano D, Sarshar M, Zagaglia
C, Singer BB and Palamara AT: Acinetobacter baumannii targets human
carcinoembryonic antigen-related cell adhesion molecules (CEACAMs)
for invasion of pneumocytes. mSystems. 5:e00604–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Al Khinji A: Carcinoembryonic antigen cell
adhesion molecule 6 enhances invasiveness and immune suppression in
pancreatic adenocarcinoma, 2022.
|
|
86
|
Chen Z, Chen L, Qiao SW, Nagaishi T and
Blumberg RS: Carcinoembryonic antigen-related cell adhesion
molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J
Immunol. 180:6085–6093. 2008.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Götz L, Rueckschloss U, Ergün S and
Kleefeldt F: CEACAM1 in vascular homeostasis and inflammation. Eur
J Clin Invest. 54 (Suppl 2)(e14345)2024.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Lee EH, Kim HT, Chun SY, Chung JW, Choi
SH, Lee JN, Kim BS, Yoo ES, Kwon TG, Kim TH and Ha YS: Role of the
JNK pathway in bladder cancer. Onco Targets Ther. 15:963–971.
2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Liao J, Chen R, Lin B, Deng R, Liang Y,
Zeng J, Ma S and Qiu X: Cross-Talk between the TGF-β and cell
adhesion signaling pathways in cancer. Int J Med Sci. 21:1307–1320.
2024.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kim EY, Cha YJ, Jeong S and Chang YS:
Overexpression of CEACAM6 activates Src-FAK signaling and inhibits
anoikis, through homophilic interactions in lung adenocarcinomas.
Transl Oncol. 20(101402)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Do CTP, Prochnau JY, Dominguez A, Wang P
and Rao MK: The road ahead in pancreatic cancer: Emerging trends
and therapeutic prospects. Biomedicines. 12(1979)2024.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Chen K, Li Y, Guo Z, Zeng Y and Zhang W:
Identification of CEACAM6 as a biomarker for circulating tumor
cells and its clinical significance in pancreatic cancer. Front
Oncol. 11(659133)2021.
|
|
93
|
Primac I, Maquoi E, Blacher S, Heljasvaara
R, Van Deun J, Smeland HY, Canale A, Louis T, Stuhr L, Sounni NE,
et al: Stromal integrin α11 regulates PDGFR-β signaling and
promotes breast cancer progression. J Clin Invest. 129:4609–4628.
2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Hartupee C, Nagalo BM, Chabu CY, Tesfay
MZ, Coleman-Barnett J, West JT and Moaven O: Pancreatic cancer
tumor microenvironment is a major therapeutic barrier and target.
Front Immunol. 15(1287459)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
González-Amaro R and Sánchez-Madrid F:
Cell adhesion molecules: Selectins and integrins. rit Rev Immunol.
19:389–429. 1999.PubMed/NCBI
|
|
96
|
Aplin AE, Howe A, Alahari SK and Juliano
RL: Signal transduction and signal modulation by cell adhesion
receptors: The role of integrins, cadherins, immunoglobulin-cell
adhesion molecules, and selectins. Pharmacol Rev. 50:197–263.
1998.PubMed/NCBI
|
|
97
|
Gao J and Nakamura F: Actin-associated
proteins and small molecules targeting the actin cytoskeleton. Int
J Mol Sci. 23(2118)2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Nishiuchi R, Takagi J, Hayashi M, Ido H,
Yagi Y, Sanzen N, Tsuji T, Yamada M and Sekiguchi K: Ligand-binding
specificities of laminin-binding integrins: A comprehensive survey
of laminin-integrin interactions using recombinant alpha3beta1,
alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol.
25:189–197. 2006.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Brassart-Pasco S, Brézillon S, Brassart B,
Ramont L, Oudart JB and Monboisse JC: Tumor microenvironment:
Extracellular matrix alterations influence tumor progression. Front
Oncol. 10(397)2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Giancotti FG and Tarone G: Positional
control of cell fate through joint integrin/receptor protein kinase
signaling. Annu Rev Cell Dev Biol. 19:173–206. 2003.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Ivaska J and Heino J: Cooperation between
integrins and growth factor receptors in signaling and endocytosis.
Annu Rev Cell Dev Biol. 27:291–320. 2011.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Cabodi S, Moro L, Bergatto E, Boeri Erba
E, Di Stefano P, Turco E, Tarone G and Defilippi P: Integrin
regulation of epidermal growth factor (EGF) receptor and of
EGF-dependent responses. Biochem Soc Trans. 32:438–442.
2004.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Hurtado de Mendoza T, Mose ES, Botta GP,
Braun GB, Kotamraju VR, French RP, Suzuki K, Miyamura N, Teesalu T,
Ruoslahti E, et al: Tumor-penetrating therapy for β5 integrin-rich
pancreas cancer. Nat Commun. 12(1541)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Tsai YT, Wu AC, Yang WB, Kao TJ, Chuang
JY, Chang WC and Hsu TI: ANGPTL4 induces TMZ resistance of
glioblastoma by promoting cancer stemness enrichment via the
EGFR/AKT/4E-BP1 cascade. Int J Mol Sci. 20(5625)2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Masugi Y, Yamazaki K, Emoto K, Effendi K,
Tsujikawa H, Kitago M, Itano O, Kitagawa Y and Sakamoto M:
Upregulation of integrin β4 promotes epithelial-mesenchymal
transition and is a novel prognostic marker in pancreatic ductal
adenocarcinoma. Lab Invest. 95:308–319. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Weinel RJ, Rosendahl A, Pinschmidt E,
Kisker O, Simon B and Santoso S: The alpha 6-integrin receptor in
pancreatic carcinoma. Gastroenterology. 108:523–532.
1995.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Meng X, Liu P, Wu Y, Liu X, Huang Y, Yu B,
Han J, Jin H and Tan X: Integrin beta 4 (ITGB4) and its
tyrosine-1510 phosphorylation promote pancreatic tumorigenesis and
regulate the MEK1-ERK1/2 signaling pathway. Bosn J Basic Med Sci.
20:106–116. 2020.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Jeong BY, Cho KH, Jeong KJ, Park YY, Kim
JM, Rha SY, Park CG, Mills GB, Cheong JH and Lee HY: Rab25 augments
cancer cell invasiveness through a β1 integrin/EGFR/VEGF-A/Snail
signaling axis and expression of fascin. Exp Mol Med.
50(e435)2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Schinke H, Shi E, Lin Z, Quadt T, Kranz G,
Zhou J, Wang H, Hess J, Heuer S, Belka C, et al: A transcriptomic
map of EGFR-induced epithelial-to-mesenchymal transition identifies
prognostic and therapeutic targets for head and neck cancer. Mol
Cancer. 21(178)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Calinescu A, Turcu G, Nedelcu RI, Brinzea
A, Hodorogea A, Antohe M, Diaconu C, Bleotu C, Pirici D, Jilaveanu
LB, et al: On the dual role of carcinoembryonic antigen-related
cell adhesion molecule 1 (CEACAM1) in human malignancies. J Immunol
Res. 2018(7169081)2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Cavaco ACM, Rezaei M, Caliandro MF, Lima
AM, Stehling M, Dhayat SA, Haier J, Brakebusch C and Eble JA: The
interaction between laminin-332 and α3β1 integrin determines
differentiation and maintenance of CAFs, and supports invasion of
pancreatic duct adenocarcinoma cells. Cancers (Basel).
11(14)2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Aziz MH, Saida L, van Eijck CHJ and
Mustafa DAM: Overexpression of the adhesion signaling pathway is
linked to short-term survival in pancreatic ductal adenocarcinoma.
Pancreatology. 24:62–65. 2024.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Calderwood DA: Talin controls integrin
activation. Biochem Soc Trans. 32:434–437. 2004.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Moser M, Legate KR, Zent R and Fässler R:
The tail of integrins, talin, and kindlins. Science. 324:895–899.
2009.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Baig MMFA, Zhang QW, Younis MR and Xia XH:
A DNA nanodevice simultaneously activating the EGFR and integrin
for enhancing cytoskeletal activity and cancer cell treatment. Nano
Lett. 19:7503–7513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Liu M, Zhang Y, Yang J, Cui X, Zhou Z,
Zhan H, Ding K, Tian X, Yang Z, Fung KMA, et al: ZIP4 increases
expression of transcription factor ZEB1 to promote Integrin α3β1
signaling and inhibit expression of the gemcitabine transporter
ENT1 in pancreatic cancer cells. Gastroenterology. 158:679–692.e1.
2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Yu CH, Rafiq NB, Cao F, Zhou Y,
Krishnasamy A, Biswas KH, Ravasio A, Chen Z, Wang YH, Kawauchi K,
et al: Integrin-beta3 clusters recruit clathrin-mediated endocytic
machinery in the absence of traction force. Nat Commun.
6(8672)2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Ye F, Petrich BG, Anekal P, Lefort CT,
Kasirer-Friede A, Shattil SJ, Ruppert R, Moser M, Fässler R and
Ginsberg MH: The mechanism of kindlin-mediated activation of
integrin αIIbβ3. Curr Biol. 23:2288–2295. 2013.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Baschieri F, Dayot S, Elkhatib N, Ly N,
Capmany A, Schauer K, Betz T, Vignjevic DM, Poincloux R and
Montagnac G: Frustrated endocytosis controls
contractility-independent mechanotransduction at clathrin-coated
structures. Nat Commun. 9(3825)2018.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Franco-Barraza J, Francescone R, Luong T,
Shah N, Madhani R, Cukierman G, Dulaimi E, Devarajan K, Egleston
BL, Nicolas E, et al: Matrix-regulated integrin
αvβ5 maintains
α5β1-dependent desmoplastic traits prognostic
of neoplastic recurrence. Elife. 6(e20600)2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Harryman WL, Marr KD, Nagle RB and Cress
AE: Integrins and epithelial-mesenchymal cooperation in the tumor
microenvironment of muscle-invasive lethal cancers. Front Cell Dev
Biol. 10(837585)2022.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Myo Min KK, Ffrench CB, Jessup CF,
Shepherdson M, Barreto SG and Bonder CS: Overcoming the fibrotic
fortress in pancreatic ductal adenocarcinoma: Challenges and
opportunities. Cancers (Basel). 15(2354)2023.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Belhabib I, Zaghdoudi S, Lac C, Bousquet C
and Jean C: Extracellular matrices and cancer-associated
fibroblasts: Targets for cancer diagnosis and therapy? Cancers
(Basel). 13(3466)2021.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Schaffner F, Ray AM and Dontenwill M:
Integrin α5β1, the fibronectin receptor, as a pertinent therapeutic
target in solid tumors. Cancers (Basel). 5:27–47. 2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Gan L, Meng J, Xu M, Liu M, Qi Y, Tan C,
Wang Y, Zhang P, Weng W, Sheng W, et al: Extracellular matrix
protein 1 promotes cell metastasis and glucose metabolism by
inducing integrin β4/FAK/SOX2/HIF-1α signaling pathway in gastric
cancer. Oncogene. 37:744–755. 2018.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Yang H, Xu Z, Peng Y, Wang J and Xiang Y:
Integrin β4 as a potential diagnostic and therapeutic tumor marker.
Biomolecules. 11(1197)2021.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Liu F, Wu Q, Dong Z and Liu K: Integrins
in cancer: Emerging mechanisms and therapeutic opportunities.
Pharmacol Ther. 247(108458)2023.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Liu C, Wang M, Lv H, Liu B, Ya X, Zhao W
and Wang W: CEACAM6 promotes cholangiocarcinoma migration and
invasion by inducing epithelial-mesenchymal transition through
inhibition of the SRC/PI3K/AKT signaling pathway. Oncol Lett.
23(39)2022.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Schnittert J, Bansal R, Mardhian DF, van
Baarlen J, Östman A and Prakash J: Integrin α11 in pancreatic
stellate cells regulates tumor-stroma interactions in pancreatic
ductal adenocarcinoma. Cancers. 11(14)2019.DOI:
10.3390/cancers11010014.
|
|
130
|
Chen X, Liu P, Wu Y, Liu X, Huang Y, Yu B,
Han J, Jin H and Tan X: Integrin α3 promotes pancreatic cancer cell
invasion by activating EGFR signaling and cytoskeletal remodeling.
Bosn J Basic Med Sci. 22:106–114. 2022.DOI:
10.17305/bjbms.2022.6411.
|
|
131
|
Mia MM, Boersema M and Bank RA: αvβ1
Integrin-mediated activation of TGF-β controls αvβ6 integrin
expression to promote pancreatic carcinoma progression. J Pathol.
253:367–382. 2021.DOI: 10.1002/path.5597.
|
|
132
|
Kuninty PR, Bansal R, De Geus SWL,
Mardhian DF, Schnittert J, van Baarlen J, Storm G, Bijlsma MF, van
Laarhoven HW, Metselaar JM, et al: ITGA5 inhibition in pancreatic
stellate cells attenuates desmoplasia and potentiates efficacy of
chemotherapy in pancreatic cancer. Sci Adv.
5(eaax2770)2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Li X, Placencio V, Iturregui JM, Uwamariya
C, Sharif-Afshar AR, Koyama T, Hayward SW and Bhowmick NA: Prostate
fibroblast αvβ6 integrin expression drives prostate cancer
progression via epithelial-mesenchymal transition. Oncogene.
34:881–890. 2015.DOI: 10.1038/onc.2014.19.
|
|
134
|
Humphries JD, Chastney MR, Askari JA and
Humphries MJ: Signal transduction via integrin adhesion complexes.
Curr Opin Cell Biol. 56:14–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Zhu R, Ge J, Ma J and Zheng J:
Carcinoembryonic antigen related cell adhesion molecule 6 promotes
the proliferation and migration of renal cancer cells through the
ERK/AKT signaling pathway. Transl Androl Urol. 8:457–466.
2019.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Barnich N, Carvalho FA, Glasser AL, Darcha
C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P,
Colombel JF, et al: CEACAM6 acts as a receptor for
adherent-invasive E. coli, supporting ileal mucosa colonization in
Crohn disease. J Clin Invest. 117:1566–1574. 2007.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Fechter P, Cruz Da Silva E, Mercier MC,
Noulet F, Etienne-Seloum N, Guenot D, Lehmann M, Vauchelles R,
Martin S, Lelong-Rebel I, et al: RNA aptamers targeting integrin
α5β1 as probes for Cyto- and histofluorescence in glioblastoma. Mol
Ther Nucleic Acids. 17:63–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Schmitter T, Pils S, Weibel S, Agerer F,
Peterson L, Buntru A, Kopp K and Hauck CR: Opa proteins of
pathogenic neisseriae initiate Src kinase-dependent or lipid
raft-mediated uptake via distinct human carcinoembryonic
antigen-related cell adhesion molecule isoforms. Infect Immun.
75:4116–4126. 2007.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Zhang ZC, Zhao HF, Sun Z, Li Y, Zhong ML,
Wang BH and Jiang XZ: Tripartite motif-containing 9 promoted
proliferation and migration of bladder cancer cells through
CEACAM6-Smad2/3 axis. J Cell Commun Signal. 17:1323–1333.
2023.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Monkman JH, Thompson EW and Nagaraj SH:
Targeting epithelial mesenchymal plasticity in pancreatic cancer: A
compendium of preclinical discovery in a heterogeneous disease.
Cancers (Basel). 11(1745)2019.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Zhou R, Wu H, You H, Wang X, Yuan X, Sun
Z, Zhou D, Jiang Y and Shen Y: ESPN activates ZEB1-mediated EMT
through the PI3K/AKT/mTOR axis to promote osteosarcoma metastasis.
J Transl Med. 23(527)2025.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Rice AJ, Cortes E, Lachowski D, Cheung
BCH, Karim SA, Morton JP and Del Río Hernández A: Matrix stiffness
induces epithelial-mesenchymal transition and promotes
chemoresistance in pancreatic cancer cells. Oncogenesis.
6(e352)2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Debreli Coskun M, Sudha T, Bharali DJ,
Celikler S, Davis PJ and Mousa SA: αvβ3 integrin antagonists
enhance chemotherapy response in an orthotopic pancreatic cancer
model. Front Pharmacol. 11(95)2020.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Farhangnia P, Khorramdelazad H, Nickho H
and Delbandi AA: Current and future immunotherapeutic approaches in
pancreatic cancer treatment. J Hematol Oncol. 17(40)2024.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Cardenas KCA, Enos CW, Spear MR, Austin
DE, Almofeez R, Kortchak S, Pincus L, Guo HB, Dolezal S, Pierce JM,
et al: CT109-SN-38, a novel antibody-drug conjugate with dual
specificity for CEACAM5 and 6, elicits potent killing of pancreatic
cancer cells. Curr Cancer Drug Targets. 24:720–732. 2024.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Topalovski M and Brekken RA: Matrix
control of pancreatic cancer: New insights into fibronectin
signaling. Cancer Lett. 381:252–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Belvisi L, Riccioni T, Marcellini M, Vesci
L, Chiarucci I, Efrati D, Potenza D, Scolastico C, Manzoni L,
Lombardo K, et al: Biological and molecular properties of a new
alpha(v)beta3/alpha(v)beta5 integrin antagonist. Mol Cancer Ther.
4:1670–1680. 2005.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Sherman MH and Beatty GL: Tumor
microenvironment in pancreatic cancer pathogenesis and therapeutic
resistance. Annu Rev Pathol. 18:123–148. 2023.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Palma AM, Vudatha V, Peixoto ML and Madan
E: Tumor heterogeneity: An oncogenic driver of PDAC progression and
therapy resistance under stress conditions. Adv Cancer Res.
159:203–249. 2023.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Singer BB, Scheffrahn I, Kammerer R,
Suttorp N, Ergun S and Slevogt H: Deregulation of the CEACAM
expression pattern causes undifferentiated cell growth in human
lung adenocarcinoma cells. PLoS One. 5(e8747)2010.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Ju Y, Xu D, Liao MM, Sun Y, Bao WD, Yao F
and Ma L: Barriers and opportunities in pancreatic cancer
immunotherapy. NPJ Precis Oncol. 8(199)2024.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Olaoba OT, Yang M, Adelusi TI, Maidens T,
Kimchi ET, Staveley-O'Carroll KF and Li G: Targeted therapy for
highly desmoplastic and immunosuppressive tumor microenvironment of
pancreatic ductal adenocarcinoma. Cancers (Basel).
16(1470)2024.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Reader CS, Vallath S, Steele CW, Haider S,
Brentnall A, Desai A, Moore KM, Jamieson NB, Chang D, Bailey P, et
al: The integrin αvβ6 drives pancreatic cancer through diverse
mechanisms and represents an effective target for therapy. J
Pathol. 249:332–342. 2019.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Yang D, Tang Y, Fu H, Xu J, Hu Z, Zhang Y
and Cai Q: Integrin β1 promotes gemcitabine resistance in
pancreatic cancer through Cdc42 activation of PI3K p110β signaling.
Biochem Biophys Res Commun. 505:215–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Kim H, Huh S, Park J, Han Y, Ahn KG, Noh
Y, Lee SJ, Chu H, Kim SS, Jung HS, et al: Development of a
fit-for-purpose multi-marker panel for early diagnosis of
pancreatic ductal adenocarcinoma. Mol Cell Proteomics.
23(100824)2024.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Bukys T, Kurlinkus B, Sileikis A and
Vitkus D: The prospect of improving pancreatic cancer diagnostic
capabilities by implementing blood biomarkers: A study of
evaluating properties of a single IL-8 and in conjunction with
CA19-9, CEA, and CEACAM6. Biomedicines. 12(2344)2024.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Sivapalan L, Kocher HM, Ross-Adams H and
Chelala C: The molecular landscape of pancreatic ductal
adenocarcinoma. Pancreatology. 22:925–936. 2022.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Sivapalan L, Thorn GJ, Gadaleta E, Kocher
HM, Ross-Adams H and Chelala C: Longitudinal profiling of
circulating tumour DNA for tracking tumour dynamics in pancreatic
cancer. BMC Cancer. 22(369)2022.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Davidson C, Taggart D, Sims AH, Lonergan
DW, Canel M and Serrels A: FAK promotes stromal PD-L2 expression
associated with poor survival in pancreatic cancer. Br J Cancer.
127:1893–1905. 2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
S S KD, Joga R, Srivastava S, Nagpal K,
Dhamija I, Grover P and Kumar S: Regulatory landscape and
challenges in CAR-T cell therapy development in the US, EU, Japan,
and India. Eur J Pharm Biopharm. 201(114361)2024.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Sainatham C, Yadav D, Dilli Babu A,
Tallapalli JR, Kanagala SG, Filippov E, Murillo Chavez F, Ahmed N
and Lutfi F: The current socioeconomic and regulatory landscape of
immune effector cell therapies. Front Med (Lausanne).
11(1462307)2024.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Singhi AD, Koay EJ, Chari ST and Maitra A:
Early detection of pancreatic cancer: Opportunities and challenges.
Gastroenterology. 156:2024–2040. 2019.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Gebauer F, Wicklein D, Horst J, Sundermann
P, Maar H, Streichert T, Tachezy M, Izbicki JR and Bockhorn M:
Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6)
in pancreatic adenocarcinoma: an independent prognostic marker and
potential target for immunotherapy. Ann Surg Oncol. 21:2329–2337.
2014.
|
|
165
|
Zeltz C, Lu N and Gullberg D: Integrin
α11β1: A major collagen receptor on fibroblastic cells. Adv Exp Med
Biol. 819:73–83. 2014.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Brümmer J, Ebrahimnejad A, Flayeh R,
Schumacher U, Löning T, Bamberger AM and Wagener C: cis Interaction
of the cell adhesion molecule CEACAM1 with integrin beta(3). Am J
Pathol. 159:537–546. 2001.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Vuijk FA, van Beijnum JR, Cleuren ACA,
Bovenschen N, van Hinsbergh VWM and Griffioen AW: Co-expression of
CEACAM5 with integrins in pancreatic ductal adenocarcinoma and
surrounding stroma: Association with fibrosis and lipid raft
signaling. Cancers. 12(2857)2020.DOI: 10.3390/cancers12102857.
|