Introduction
In recent years, RNA-based therapeutics, such as
small interfering RNA (siRNA) and messenger RNA (mRNA), have
garnered significant attention, offering new therapeutic approaches
that specifically modulate gene expression associated with various
diseases (1,2). The success of mRNA vaccines against
coronavirus disease 2019 (COVID-19) has accelerated the development
of mRNA-based pharmaceuticals. Consequently, mRNA therapeutics are
expected to have broad applications in fields such as cancer
immunotherapy, genome editing, genetic disorder treatments, and
regenerative medicine (3,4).
RNA therapeutics, including mRNA vaccines, require
the carriers for delivery to the targeted tissue. Various mRNA
carriers have been investigated, including cationic polymers,
lipoplexes, lipid-polymer hybrid nanoparticles, and lipid
nanoparticles (5,6). Among these, mRNA/cationic liposome
complexes (mRNA lipoplexes) have been extensively studied as
efficient delivery systems (7).
Lipoplexes are nanoparticles formed by complexation of mRNA and
cationic liposomes (8). Although
mRNA lipoplex-based products have been investigated for clinical
applications such as cancer immunotherapy (7,9),
successful cases remain limited, and further fundamental research
is needed on the lipid compositions and mRNA used in mRNA
lipoplexes.
To comprehensively investigate the efficacy of mRNA
lipoplexes, establishing analytical technologies is essential to
evaluate how differences in lipid composition influence the
efficiency of cellular delivery and gene expression. Achieving this
goal requires a reproducible, high-throughput mRNA transfection
method using multi-well plates in vitro. There are two major
approaches for in vitro mRNA transfection: forward and
reverse. For forward transfection, target cells are first seeded
and cultured, after which a suspension of pre-formed mRNA
lipoplexes is added. Notably, mRNA lipoplexes are typically
prepared immediately before use because mRNA and mRNA lipoplexes
can be unstable in aqueous suspensions and are unsuitable for
long-term storage (10,11). However, this method involves
multiple handling steps and is time-consuming. To address these
limitations, reverse transfection can be utilized to reduce the
labor and time required. In this method, mRNA lipoplexes are
directly mixed with the cell suspension, enabling simultaneous cell
seeding and mRNA transfection without prior cell culture. This
approach (commonly referred to as liquid-phase reverse
transfection) simplifies workflow. However, freshly prepared mRNA
lipoplexes are required, making them unsuitable for comprehensively
evaluating the effects of a wide variety of lipoplexes and mRNA. To
overcome these challenges, we propose the use of solid-phase
reverse transfection. In this method, mRNA lipoplexes are
pre-applied and lyophilized onto the surface of the culture plate
prior to cell seeding. The cell suspension is then directly added
to the dried mRNA lipoplexes, allowing for mRNA transfection upon
contact. The advantages of this approach include reduced manual
handling, compatibility with automation, and the ability to prepare
large batches of transfection-ready plates. Consequently, this
method facilitates the efficient and scalable screening of lipid
components for mRNA transfection into cells.
We previously reported a method for solid-phase
reverse transfection using lyophilized siRNA lipoplexes (12). Additionally, several studies have
investigated lyophilization conditions for plasmid DNA or siRNA
lipoplexes (13,14). However, little is known about the
effects of lyophilization on mRNA lipoplexes. In our previous
study, we demonstrated that the gene-silencing activity of siRNA
lipoplexes could be preserved regardless of the type of cationic
lipid used by employing disaccharides such as sucrose or trehalose
as cryoprotectants during lyophilization (15). However, whether the same strategy
can be applicable to mRNA lipoplexes remains unclear. To
investigate the impact of disaccharides and cationic lipid types on
the transfection efficiency of lyophilized mRNA lipoplexes,
cationic liposomes were prepared using five types of dialkyl or
trialkyl cationic lipids. Lyophilized mRNA lipoplexes were then
prepared in multi-well plates in the presence of trehalose or
sucrose solutions, and their transfection efficiency was evaluated,
in addition to the retention of transfection activity after
long-term storage.
Materials and methods
Materials
11-((1,3-Bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (cat. no. TC-1-12),
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride (cat. no. DC-6-14),
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide (cat. no. DC-1-16) and dimethyldioctadecylammonium bromide
(DDAB, cat. no. DC-1-18) were purchased from Sogo Pharmaceutical
Co., Ltd. (Tokyo, Japan). 1,2-Dioleoyl-3-trimethylammonium-propane
methyl sulfate salt (DOTAP) was purchased from Avanti Polar Lipids,
Inc. (Alabaster, AL, USA).
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE,
COATSOME® ME-8181) and polyethylene glycol-cholesteryl
ether (PEG-Chol, mean MW of PEG: 1600) were purchased from NOF Co.,
Ltd. (Tokyo, Japan).
mRNAs
CleanCap® firefly luciferase mRNA (FLuc
mRNA, 1922 nucleotides, cat. no. L-7602) and CleanCap®
enhanced green fluorescent protein mRNA (EGFP mRNA, 997
nucleotides, cat. no. L-7601) were purchased from TriLink
Biotechnologies (San Diego, CA, USA). EZ Cap™ Cy5 firefly
luciferase mRNA (5-moUTP) (Cy5-mRNA, 1921 nucleotides, cat. no.
R1010) was purchased from APExBIO Technology, LLC (Boston, MA,
USA).
Preparation of cationic liposomes and
mRNA lipoplexes
Liposomes were prepared using a mixture of cationic
lipid (DDAB, DOTAP, DC-1-16, DC-6-14, and TC-1-12), DOPE, and
PEG-Chol at a molar ratio of 49.5:49.5:1 via the thin-film
hydration method. Briefly, the lipids were dissolved in chloroform
and, subsequently, chloroform was removed using a rotary evaporator
set at 60˚C to obtain a thin lipid film. The lipid film was
hydrated with sterile water at 60˚C and sonicated to reduce the
particle size for 10 min at 100 W, 42 kHz, and room temperature in
a bath-type sonicator (Bransonic 2510J-MTH; Branson Ultrasonics
Corporation, Danbury, CT, USA). To prepare mRNA lipoplexes, each
mRNA was mixed with cationic liposomes at a charge ratio (+:-) of
4:1 and incubated at room temperature for 10 min.
Size measurement of liposomes and
lipoplexes
The particle size distribution and polydispersity
index (PDI) were measured using an ELS-Z2 light-scattering
photometer (Otsuka Electronics Co., Ltd., Osaka, Japan) at 25˚C
after diluting the particle dispersion to an appropriate
concentration with sterile water. The ζ-potential was measured
using electrophoretic light scattering with the same instrument at
25˚C after diluting the sample in the same manner.
Lyophilization process of mRNA
lipoplexes
The mRNA lipoplexes containing 0.5 µg mRNA were
dissolved in 125 µl of 50, 100 or 150 mM trehalose or sucrose
solution and placed in a 12-well plate for transfection assay. For
cytotoxicity assay, those containing 0.05 µg mRNA were dissolved in
12.5 µl of 150 mM trehalose or sucrose solution and placed in a
96-well plate. The mixture was first frozen at -80˚C and then dried
under high vacuum (10-20 Pa) using a freeze dryer (FDU-540, Tokyo
Rikakikai Co., Ltd. (EYELA), Tokyo, Japan) equipped with a drying
chamber (DRC-2L, EYELA). For characterization after long-term
storage, the freeze-dried mRNA lipoplexes were stored under vacuum
in a desiccator at room temperature before use.
Cell culture and reverse
transfection
HeLa cells (cat. no. 93021013; Cellosaurus;
CVCL_0030) were obtained from the European Collection of
Authenticated Cell Cultures. The cells were cultured under the
standard condition in Eagle's Minimum Essential Medium (EMEM;
FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) supplemented
with 10% heat-inactivated fetal bovine serum (FBS, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 100 µg/ml kanamycin in
humidified atmosphere with 5% CO2 at 37˚C. For
liquid-phase reverse transfection, mRNA lipoplex solution
containing 0.5 µg mRNA was mixed with 1x105 HeLa cells
seeded in 1 ml medium and transferred into the wells of a 12-well
plate. Then it was incubated at 37˚C with 5% CO2 for 24
h. For solid-phase reverse transfection, HeLa cells were seeded at
a density of 1x105 cells per well in a 12-well plate or
1x104 cells per well in a 96-well plate, both containing
freeze-dried mRNA lipoplexes, and incubated at 37˚C with 5%
CO2 for 24 h.
Luciferase activity
quantification
HeLa cells in 12-well plate were lysed 24 h
post-transfection by adding 125 µl of cell lysis buffer (Pierce™
Luciferase Cell Lysis Buffer, ThermoFisher Scientific Inc.). The
lysate was frozen at -80˚C, followed by thawing at 37˚C, and then
centrifuged at 13,000 x g for 10 sec. A 10 µl aliquot of the
supernatants were mixed with 50 µl of PicaGene MelioraStar-LT
Luminescence Reagent (Toyo Ink Mfg. Co. Ltd., Tokyo, Japan) and the
luminescence was measured as counts per sec (cps) with a
chemoluminometer (ARVO X2, PerkinElmer, inc., Waltham, MA, USA).
The protein concentration in the supernatant was measured using the
BCA reagent (Pierce™ BCA Protein Assay Kit, Thermo Fisher
Scientific, Inc.), with bovine serum albumin (BSA) as the standard
and then luciferase activity was calculated as cps/µg protein.
Cytotoxicity of lyophilized mRNA
lipoplexes on HeLa cells
In a 96-well plate containing freeze-dried mRNA
lipoplexes, HeLa cells were seeded at a density of 1x104
cells per well, and incubated at 37˚C with 5% CO2 for 24
h. After transfection, cell viability was evaluated using the WST-8
assay (Cell Counting Kit-8, Dojindo Laboratories, Tokyo, Japan),
according to the manufacturer's instructions.
Flow cytometry analysis for expression
analysis and cellular uptake evaluation
To evaluate the cellular uptake of mRNA lipoplexes,
HeLa cells were seeded at a density of 1x105 cells per
well in a 12-well plate containing freeze-dried Cy5-mRNA lipoplexes
(Cy5-mRNA: 0.5 µg/well), and incubated at 37˚C with 5%
CO2 for 3 h. On the other hand, for expression analysis,
HeLa cells were seeded at a density of 1x105 cells per
well in a 12-well plate containing freeze-dried EGFP mRNA
lipoplexes (EGFP mRNA: 0.5 µg/well), and incubated at 37˚C with 5%
CO2 for 24 h. After transfection, the cells were
harvested using TrypLE™ Express Enzyme (Gibco; Thermo Fisher
Scientific, Inc.) and resuspended in phosphate-buffered saline
containing with 0.1% BSA and 1 mM ethylenediaminetetraacetic acid.
To evaluate the Cy5 fluorescence intensity or EGFP expression in
the cells, 10,000 events per sample were processed using BD
FACSVerseTM (BD Biosciences, Franklin Lakes, NJ, USA)
and analyzed using BD FACSuite software ver. 1.0.3 (BD
Biosciences).
Intracellular co-localization of the
Cy5-mRNA and lysosome observed by confocal microscopy
HeLa cells were seeded at a density of
1x105 cells per well in a 35 mm dish containing
freeze-dried Cy5-mRNA lipoplexes (Cy5-mRNA: 0.5 µg/dish), and
incubated at 37˚C with 5% CO2 for 3 h. The cells were
stained with 75 nM Lysotracker® Red DND-99 (Life
Technologies, Carlsbad, CA, USA) for 30 min. After washing with
PBS, the cells were fixed with Mildform® 10N (FUJIFILM
Wako Pure Chemical Corporation) and then stained with 5 µg/ml
Hoechst 33342 (Thermo Fisher Scientific, Inc.) for 10 min.
Fluorescence images were acquired using a FLUOVIEW FV3000 confocal
laser scanning microscope (Olympus, Tokyo, Japan).
Statistical analysis
Data were collected from at least three independent
experiments. All results are presented as mean + standard deviation
(S.D.). Statistical analyses were performed using unpaired
Student's t-test or one-way ANOVA followed by Tukey's Multiple
Comparison Test. Statistical analyses were performed using GraphPad
Prism software (version 4.0; GraphPad Software Inc., San Diego, CA,
USA). Statistical significance was set at P<0.05.
Results
Characterization of mRNA lipoplexes
after lyophilization
When liposomes or lipoplexes are lyophilized,
cryoprotectants such as disaccharides are commonly used to enhance
their stability. Previously, we reported that freeze-drying siRNA
lipoplexes in trehalose or sucrose solution resulted in long-term
stability (1 month) without a significant loss of gene-silencing
activity, regardless of the type of cationic lipid used in the
cationic liposomes (15). In this
study, we investigated whether trehalose or sucrose solutions could
have similar effect as cryoprotectants for mRNA lipoplexes during
lyophilization and examined the effect of these disaccharides on
transfection activity after lyophilization. DOTAP, DDAB, DC-6-14,
DC-1-16, and TC-1-12 were used as cationic lipids (Fig. 1). Based on our previous report
(16), liposomes were prepared
with cationic lipids, DOPE, and PEG-Chol at a molar ratio of
49.5:49.5:1, using the thin-film hydration method. The sizes of the
prepared cationic liposomes were approximately 85-106 nm, and the
ζ-potentials were approximately 49-58 mV (Table I).
 | Figure 1Structures of the lipid components of
liposomes used for mRNA lipoplexes. DDAB,
dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt;
DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine;
PEG1600-Chol, polyethylene glycol-cholesteryl ether. |
 | Table IParticle size of cationic liposomes
and mRNA lipoplexes. |
Table I
Particle size of cationic liposomes
and mRNA lipoplexes.
| | Liposome | Lipoplex |
|---|
| Cationic lipid | Size, nm | PDI | ζ-potential,
mV | Size, nm | PDI | ζ-potential,
mV |
|---|
| DDAB | 96.0±0.6 | 0.22±0.00 | 50.8±0.9 | 358.8±12.2 | 0.17±0.00 | 34.7±2.1 |
| DOTAP | 85.4±0.5 | 0.22±0.00 | 53.4±1.9 | 367.6±13.4 | 0.17±0.01 | 14.3±0.4 |
| DC-1-16 | 103.5±3.6 | 0.24±0.02 | 58.4±1.4 | 306.6±40.0 | 0.18±0.04 | 20.8±0.4 |
| DC-6-14 | 106.4±0.8 | 0.25±0.01 | 48.9±2.2 | 203.2±14.5 | 0.15±0.07 | 39.9±2.0 |
| TC-1-12 | 85.8±1.5 | 0.22±0.00 | 50.9±1.0 | 278.0±6.4 | 0.14±0.01 | 29.6±0.8 |
Based on our previous report that the optimal charge
ratio (+:-) for mRNA lipoplexes composed of dialkyl or trialkyl
cationic lipids is 4:1(17), we
used the same charge ratio for preparation of mRNA lipoplexes in
subsequent experiments. The mRNA lipoplexes composed of DDAB,
DOTAP, and DC-1-16 formed relatively large particles with diameters
of approximately 310-370 nm. In contrast, the mRNA lipoplexes
containing DC-6-14 were approximately 200 nm in size, whereas those
containing TC-1-12 were approximately 280 nm in size, forming
relatively smaller particles than the aforementioned lipoplexes.
All lipoplexes exhibited a monodisperse distribution (PDI:
0.14-0.18) and the ζ-potentials were approximately 14-40 mV
(Table I).
Effect of lyophilization on the
transfection activity of the mRNA lipoplexes
To investigate the effect of lyophilization on the
transfection activity of mRNA lipoplexes, lyophilized FLuc mRNA
lipoplexes containing 0.5 µg of mRNA per well in 12-well plate,
were reverse-transfected into HeLa cells and luciferase activity
was measured 24 h after transfection, because our previous results
showed that a dose of 0.5 µg mRNA with an incubation time of 24 h
resulted in the highest transfection activity in HeLa cells after
forward transfection with mRNA lipoplexes composed of cationic
lipid, neutral lipid and, PEG-Chol at a molar ratio of
49.5:49.5:1(16). Initially,
liquid-phase reverse transfection was performed using mRNA
lipoplexes containing DDAB, DOTAP, DC-1-16, DC-6-14, or TC-1-12
without lyophilization. As previously reported (16), the transfection of mRNA lipoplexes
showed that those containing DC-1-16 or TC-1-12 exhibited high
luciferase activity (Fig. 2A).
FLuc mRNA lipoplexes were lyophilized in 50, 100, or 150 mM
trehalose or sucrose solutions, followed by reverse transfection
into HeLa cells (Fig. 2B).
Regardless of the cationic lipid type used to prepare the
lipoplexes, higher disaccharide concentrations tended to correlate
with increased luciferase activity. Furthermore, in mRNA lipoplexes
containing DDAB, DC-6-14, or TC-1-12, the use of sucrose as a
cryoprotectant resulted in significantly higher activity than
trehalose. Although lyophilization tended to reduce luciferase
activity compared to non-lyophilized conditions, this reduction was
minimized when lipoplexes were lyophilized in 150 mM sucrose
solution. Therefore, in subsequent experiments, a disaccharide
solution with a concentration of 150 mM was used as the
cryoprotectant.
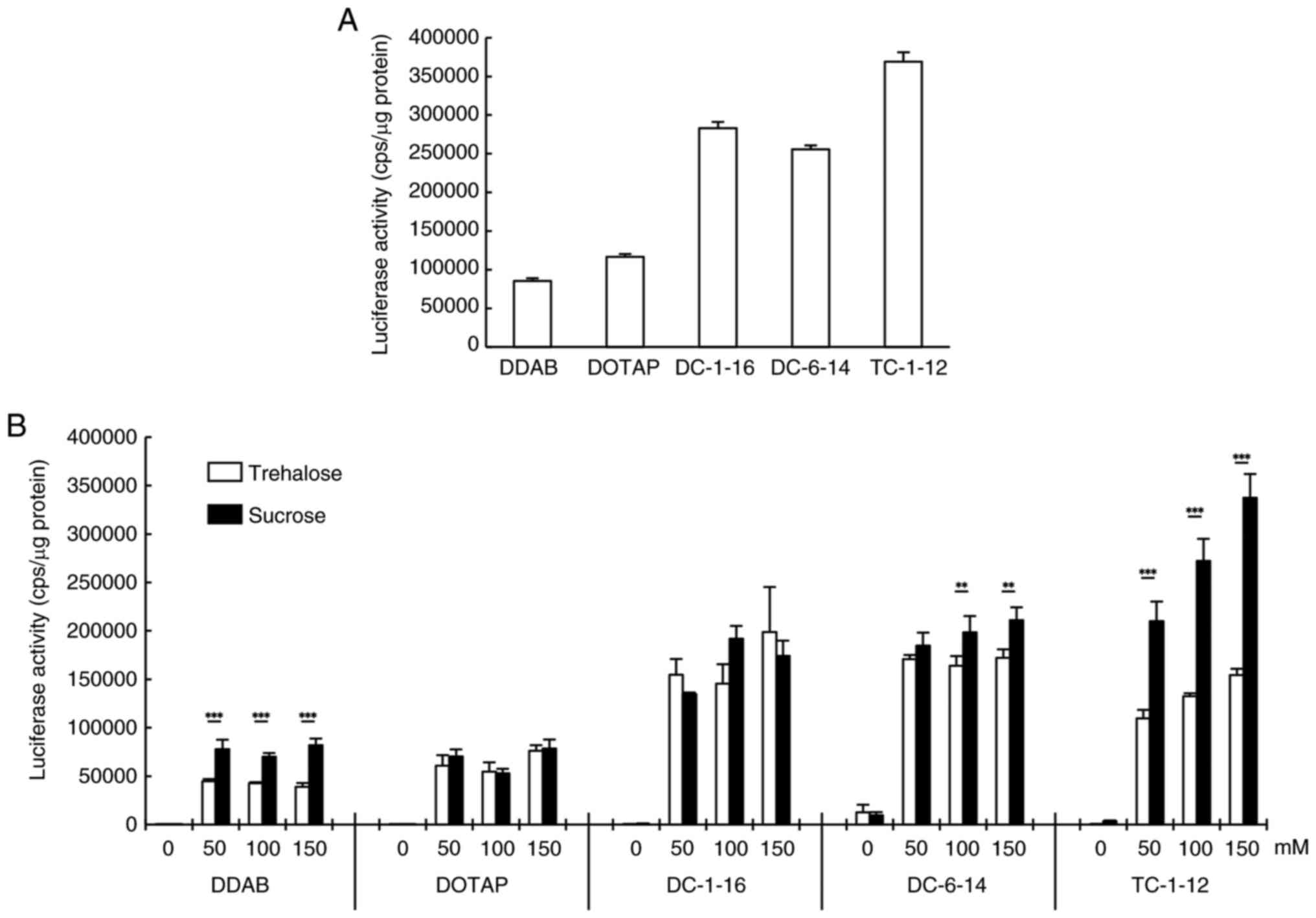 | Figure 2Luciferase activity following reverse
transfection with FLuc mRNA lipoplexes. (A) non-lyophilized FLuc
mRNA lipoplexes containing each cationic lipid were reverse
transfected into HeLa cells and luciferase activity was measured 24
h after the transfection. (B) Lyophilized FLuc mRNA lipoplexes with
0-150 mM trehalose or sucrose were reverse transfected into HeLa
cells. Luciferase activity was measured 24 h after the
transfection. **P<0.01 and ***P<0.001
(trehalose vs. sucrose). Data are presented as mean ± standard
deviation (n=3 in each group). DDAB, dimethyldioctadecylammonium
bromide; DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane methyl
sulfate salt; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; cps, count per sec. |
Effect of lyophilization on the
particle size and cytotoxicity of the mRNA lipoplexes
To investigate whether lyophilization affects the
physical properties of mRNA lipoplexes, mRNA lipoplexes were
lyophilized on a plate using 150 mM trehalose or sucrose solution
as a cryoprotectant. Particle size measurements after rehydration
of lyophilized mRNA lipoplexes revealed that regardless of the type
of cryoprotectant, mRNA lipoplexes containing DDAB, DOTAP, or
DC-1-16, which exhibited a tendency for larger particle sizes
before lyophilization, showed a reduction in size to approximately
250-280 nm (Table II). In
contrast, the particle size of the mRNA lipoplexes containing
DC-6-14, which were relatively small in diameter before
lyophilization, tended to increase slightly upon lyophilization,
reaching approximately 230 nm. In addition, the particle size of
the mRNA lipoplexes containing TC-1-12 increased upon
lyophilization, reaching approximately 350-380 nm. The ζ-potentials
after lyophilization were approximately 23-40 mV (Table II).
 | Table IIParticle size of lyophilized and
rehydrated mRNA lipoplexes. |
Table II
Particle size of lyophilized and
rehydrated mRNA lipoplexes.
| | Trehalose | Sucrose |
|---|
| Cationic lipid | Size, nm | PDI | ζ-potential,
mV | Size, nm | PDI | ζ-potential,
mV |
|---|
| DDAB | 250.6±38.0 | 0.17±0.08 | 28.1±2.5 | 253.1±7.0 | 0.12±0.00 | 26.6±1.6 |
| DOTAP | 251.2±30.1 | 0.18±0.05 | 24.2±2.7 | 282.3±15.5 | 0.13±0.01 | 25.4±0.2 |
| DC-1-16 | 279.1±9.1 | 0.13±0.01 | 30.4±2.1 | 283.0±17.3 | 0.13±0.01 | 29.8±0.6 |
| DC-6-14 | 230.3±16.0 | 0.19±0.07 | 22.8±0.4 | 234.4±7.1 | 0.18±0.05 | 24.7±0.6 |
| TC-1-12 | 350.7±16.3 | 0.16±0.01 | 40.0±0.4 | 379.1±37.1 | 0.17±0.02 | 36.3±1.3 |
Furthermore, HeLa cell viability was assessed 24 h
after reverse transfection with lyophilized mRNA lipoplexes
(Fig. 3). When lyophilized in the
presence of a 150 mM trehalose or sucrose solution, the mRNA
lipoplexes containing DC-1-16 exhibited a slightly lower cell
viability of approximately 60%. In contrast, the mRNA lipoplexes
containing DOTAP showed cell viability of approximately 90%. For
mRNA lipoplexes containing other cationic lipids, cell viability
was approximately 70%. No significant effect on cell viability was
owing due to the differences in disaccharides.
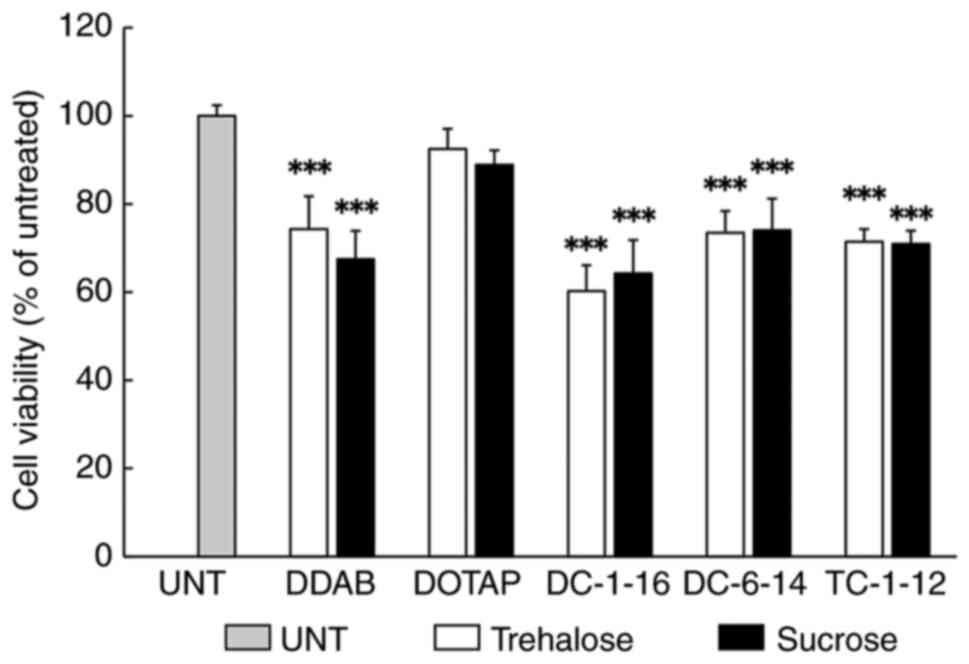 | Figure 3Toxicity test of lyophilized mRNA
lipoplexes. FLuc mRNA lipoplexes containing each cationic lipid
were lyophilized with 150 mM trehalose or sucrose solution. The
lipoplexes were reverse transfected into HeLa cells and the cell
viability was measured using WST-8 assay 24 h after the
transfection. ***P<0.001 vs. UNT. Data are presented
as mean ± standard deviation (n=5 in each group). UNT, untreated
group; DDAB, dimethyldioctadecylammonium bromide; DOTAP,
1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate salt;
DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide. |
Effect of lyophilization on the
transfection efficiency and cellular uptake
To further investigate the effects of lyophilization
on the transfection activity of mRNA lipoplexes, we examined the
transfection efficiency using mRNA lipoplexes containing DC-1-16,
DC-6-14 or TC-1-12, which exhibited high transfection activity in
the experiments using Luc mRNA. EGFP mRNA lipoplexes were
lyophilized and reverse transfected into HeLa cells, and 24 h after
transfection, flow cytometry showed the appearance of a new peak on
the higher fluorescence intensity side of the histogram compared to
untreated cells, indicating the presence of EGFP-expressing cells
(Fig. S1). Based on these
results, the percentage of EGFP-positive cells was calculated
(Fig. 4A). When mRNA lipoplexes
containing DC-6-14 were lyophilized in a trehalose solution, the
proportion of EGFP-expressed cells decreased compared to that in
the non-lyophilized lipoplexes. However, unexpectedly, when the
mRNA lipoplexes containing DC-1-16 were lyophilized in a sucrose
solution, the proportion of them was increased. In other mRNA
lipoplexes lyophilized in sucrose or trehalose solution, no
significant difference in percentage was observed between
lyophilized and non-lyophilized lipoplexes.
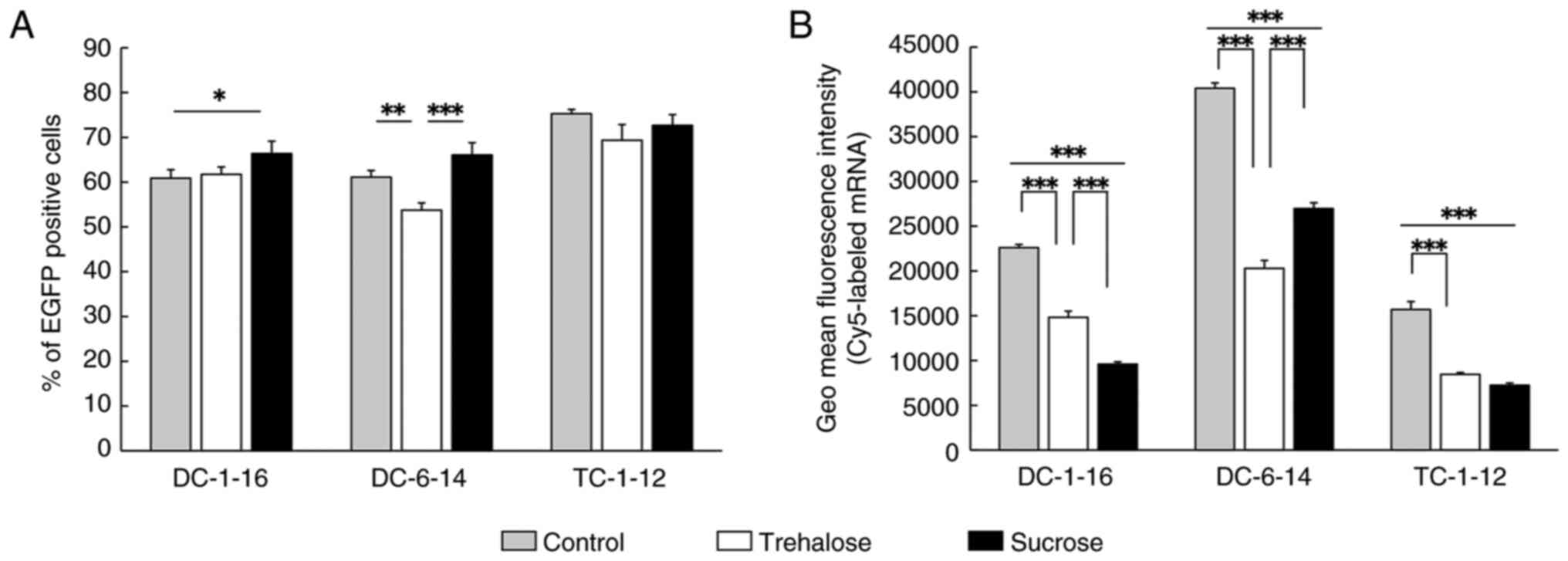 | Figure 4Analysis of transfection efficiency
and cellular uptake of the lyophilized mRNA lipoplexes. (A) EGFP
mRNA lipoplexes containing DC-1-16, DC-6-14 or TC-1-12 were reverse
transfected into HeLa cells. The percentage of EGFP positive cells
was measured 24 h after transfection. (B) Cy5-mRNA lipoplexes
containing DC-1-16, DC-6-14 or TC-1-12 were reverse transfected
into HeLa cells. The fluorescence intensity was measured 3 h after
transfection. Control, Group subjected to reverse transfection with
non-lyophilized mRNA lipoplexes. *P<0.05,
**P<0.01 and ***P<0.001. Data are
presented as mean ± standard deviation (n=3 in each group). EGFP,
enhanced green fluorescent protein; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide. |
These differences in transfection efficiency could
be influenced by the efficiency of the intracellular uptake of mRNA
lipoplexes. Therefore, to investigate this question, we examined
cellular uptake using the same liposome as in the aforementioned
experiment. Lyophilized Cy5-mRNA lipoplexes were reverse
transfected into HeLa cells and the fluorescence intensity of the
internalized Cy5-mRNA was measured using flow cytometry 3 h after
transfection. Compared to untreated cells, the cells transfected
with Cy5-mRNA lipoplexes showed a rightward shift in the histogram
peak, indicating successful uptake of Cy5-mRNA (Fig. S2). The mean fluorescence intensity
of Cy5-mRNA internalized cells was measured, lyophilization led to
a reduction in the Cy5 fluorescence intensity in the cells,
regardless of the type of cationic lipid used (Fig. 4B). Focusing on the differences in
cationic lipids within the lipoplexes, mRNA lipoplexes containing
DC-6-14 exhibited the highest cellular uptake of mRNA regardless of
whether lyophilization was performed with trehalose or sucrose as
the cryoprotectant. Moreover, a similar tendency was observed in
liquid-phase reverse transfection without lyophilization (Fig. 4B). In contrast, focusing on the
differences in the disaccharides used as cryoprotectants, mRNA
lipoplexes containing DC-6-14 lyophilized in sucrose exhibited
higher cellular uptake than those lyophilized in trehalose. In
contrast, those containing DC-1-16 lyophilized in sucrose showed
lower fluorescence intensity than those in trehalose. However, for
those containing TC-1-12, no significant difference in cellular
uptake was observed between lyophilization with sucrose and
trehalose solutions (Fig. 4B).
These results indicate that the cellular uptake of mRNA lipoplexes
may have a limited impact on transfection efficiency.
On the other hand, endosomal escape is also an
important factor for protein expression from transfected mRNA.
Therefore, we additionally examined the intracellular localization
of mRNA and lysosomes. As a result, although partial colocalization
of mRNA with lysosomes was detected regardless of the type of
lipoplex used (Fig. S3), no
noticeable differences in colocalization were observed among the
types of cationic lipid contained or between the disaccharides used
during lyophilization.
Assessment of long-term stability of
lyophilized mRNA lipoplexes
Since we confirmed that lyophilization does not
necessarily lead to a reduction in the transfection activity of
mRNA lipoplexes, depending on the cationic lipid of the liposome
and the type of cryoprotectant, we evaluated the long-term
stability of mRNA lipoplexes after lyophilization. mRNA lipoplexes
containing DC-1-16, DC-6-14, or TC-1-12 were lyophilized under the
same conditions as in the previous experiments and stored in a
desiccator under vacuum at room temperature for 1 month.
The particle size, PDI, and ζ-potential of mRNA
lipoplexes rehydrated after one month of storage are shown in
Table III. The particle size of
the mRNA lipoplexes containing DC-1-16 or TC-1-12 remained
unchanged. However, those containing DC-6-14 exhibited an increase
in particle size to approximately 300-320 nm after one month of
storage. For the ζ-potential, mRNA lipoplexes containing DC-1-16
exhibited values of approximately 25-26 mV, while those containing
DC-6-14 showed approximately 27-35 mV, and those with TC-1-12 had
approximately 40-44 mV.
 | Table IIIParticle size of rehydrated mRNA
lipoplexes after 1 month of storage. |
Table III
Particle size of rehydrated mRNA
lipoplexes after 1 month of storage.
| | Trehalose | Sucrose |
|---|
| Cationic lipid | Size, nm | PDI | ζ-potential,
mV | Size, nm | PDI | ζ-potential,
mV |
|---|
| DC-1-16 | 264.4±14.4 | 0.13±0.01 | 26.0±1.3 | 270.1±4.2 | 0.13±0.00 | 24.6±2.5 |
| DC-6-14 | 321.0± 8.3 | 0.24±0.08 | 35.4±0.3 | 308.0±4.2 | 0.18±0.07 | 27.3±1.4 |
| TC-1-12 | 342.6±18.2 | 0.15±0.01 | 43.5±1.5 | 351.0±13.1 | 0.16±0.01 | 40.2±1.5 |
To investigate whether these changes also affected
transfection activity, we performed reverse transfection of HeLa
cells with lyophilized Luc mRNA lipoplexes after one month of
storage. Compared with the results of reverse transfection
performed immediately after lyophilization (Fig. 2B), the luciferase activity of mRNA
lipoplexes containing DC-1-16 or DC-6-14 showed little to no
decrease (Fig. 5). In contrast,
mRNA lipoplexes containing TC-1-12 exhibited a substantial
reduction in luciferase activity after one month of storage
(Fig. 5). Consistent with the
trend observed immediately after lyophilization (Fig. 2B), the use of sucrose solution as a
cryoprotectant resulted in significantly higher luciferase activity
than trehalose solution for all lipoplexes (Fig. 5). From the above results, although
dependent on the type of lipid, it was suggested that the long-term
storage of mRNA lipoplexes is feasible through lyophilization in a
disaccharide solution.
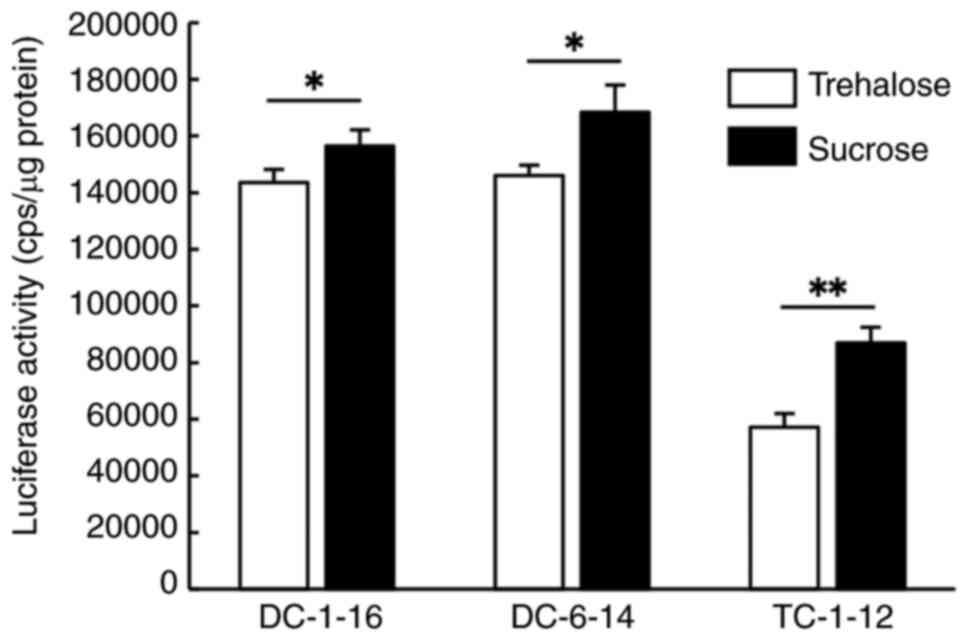 | Figure 5Luciferase activity following reverse
transfection with lyophilized FLuc mRNA lipoplexes after 1 month of
storage. FLuc mRNA lipoplexes containing each cationic lipid were
lyophilized with 150 mM trehalose or sucrose and stored for 1
month. The lipoplexes were reverse transfected into HeLa cells and
luciferase activity was measured 24 h after transfection.
*P<0.05 and **P<0.01. Data are
presented as mean ± standard deviation (n=3 in each group).
DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; cps, count per sec. |
Discussion
Lyophilization removes water via sublimation under
low temperature and vacuum conditions, and is considered an
effective method for maintaining the long-term stability of
lipoplexes (18,19). However, lyophilization imposes
significant stress on the liposomes. Therefore, in the absence of
appropriate cryoprotectants such as sugars, liposomes may be
damaged (13,20). Several studies have reported that,
with certain modifications or optimizations, the lyophilization of
various lipid nanoparticles complexed with nucleic acids, such as
mRNA, can enhance their long-term stability (21,22).
In this study, cationic liposomes were prepared using five types of
cationic lipids in combination with DOPE and PEG-Chol and used as
mRNA carriers for solid-phase reverse transfection. First, we
investigated the effect of lyophilization in the presence of
disaccharide solutions on the particle size of lipoplexes prepared
using these liposomes. The results showed that the mRNA lipoplexes
formulated with cationic lipids with relatively long carbon chains
exhibited a decrease in particle size after lyophilization and
rehydration. In contrast, lipoplexes formulated with cationic
lipids having relatively short carbon chains showed a slight
increase in particle size after lyophilization and rehydration.
Furthermore, the particle size of lyophilized plasmid DNA-based
lipoplexes containing DOTAP decreased after rehydration (23). In contrast, it has also been
reported that the particle size of siRNA lipoplexes containing DDAB
or DOTAP can increase depending on the type of disaccharide
solution used during lyophilization (12). These findings, together with our
results, suggest that both the type of cationic lipid and the
disaccharide solution influence particle size during the
lyophilization process of mRNA lipoplexes.
To evaluate the effect of lyophilization on the
transfection activity, transfection experiments using Luc mRNA were
performed on cultured cells. Initially, we conducted transfection
experiments using the liquid-phase reverse transfection. As
previously reported (16), mRNA
lipoplexes containing cationic lipids with relatively short carbon
chains exhibited higher luciferase activity than those with longer
carbon chains. To examine whether the activity trend due to lipid
differences changes upon lyophilization, solid-phase reverse
transfection was performed, and similar trends were observed. These
results suggest that the lyophilization process does not
significantly affect the variation in luciferase activity owing to
the type of cationic lipid.
Furthermore, the effects of the type and
concentration of disaccharides used during lyophilization were
investigated. When Luc mRNA lipoplexes were lyophilized in
trehalose or sucrose solutions at concentrations ranging from 0 to
150 mM, luciferase activity increased in a concentration-dependent
manner. Additionally, lipoplexes lyophilized in sucrose solution
retained luciferase activity better than those lyophilized in
trehalose solution. These results suggest that, at least for the
liposomes containing cationic lipids used in this study, sucrose is
more suitable for maintaining transfection activity during
lyophilization. In recent years, various saccharides, including
monosaccharides such as glucose and mannitol, and disaccharides
such as sucrose and trehalose, have been used as cryoprotectants
for mRNA lipid nanoparticles (24-26);
however, limited knowledge regarding mRNA lipoplexes exist. Further
research is required to clarify the effects of other saccharide
solutions on mRNA lipoplexes.
To investigate whether the differences in luciferase
activity due to the lipid formulation were attributable to
differences in transfection efficiency, the percentage of
EGFP-expressing cells after reverse transfection with lyophilized
EGFP mRNA lipoplexes was evaluated by flow cytometry. The results
showed that although there were slight increases and decreases
depending on the lipid, similar to the luciferase activity results,
transfection efficiency with EGFP mRNA lipoplexes did not show a
significant difference between liquid- and solid-phase reverse
transfection methods. However, the lipid-dependent differences in
activity observed in the luciferase assay were not observed in the
transfection efficiency of EGFP mRNA lipoplexes. Therefore, to
investigate whether the differences in luciferase activity due to
the lipid formulation resulted from differences in cellular uptake,
the intracellular uptake of Cy5-mRNA was examined. As a result,
unexpectedly, both in the lyophilized and non-lyophilized
conditions, luciferase activity followed the order TC-1-12 >
DC-1-16 ≥ DC-6-14, whereas intracellular uptake followed the
reverse order DC-6-14 > DC-1-16 > TC-1-12, although the
differences were not large. Protein expression following mRNA
transfection may not always correlate with cellular uptake
(27,28). Considering that there were no
remarkable differences observed in endosomal escape, the
differences in transfection activity caused by the lipid
composition may be attributed to changes in mRNA stability or
translation efficiency and so on. While these factors were not
fully explored in this study, identifying the underlying causes is
a key challenge for future studies.
Finally, to evaluate the long-term stability of
these lipoplexes, the lyophilized mRNA lipoplexes were stored under
vacuum at room temperature in a desiccator for one month. No
significant changes in particle size were observed in the mRNA
lipoplexes containing DC-1-16 or TC-1-12. However, those containing
DC-6-14 exhibited an increase in particle size. This suggests that
long-term storage after lyophilization may cause structural changes
in mRNA lipoplexes, depending on the type of cationic lipid used.
However, the changes in luciferase activity after long-term storage
did not necessarily correlate with the changes in particle size.
Although no significant change in particle size was observed in
mRNA lipoplexes containing TC-1-12, luciferase activity was
significantly decreased; the underlying causes remain unclear.
However, we previously reported that siRNA lipoplexes containing
TC-1-12 were unstable following lyophilization (15). Although no clear signs of
aggregation were observed in this study, mRNA lipoplexes containing
TC-1-12 are likely to become unstable over time, which may
ultimately affect the transfection efficiency and luciferase
activity.
In this study, we investigated the effects of
cationic lipids in liposomes and disaccharides used as
cryoprotectants during lyophilization on the transfection
efficiency of lyophilized mRNA lipoplexes. These results suggested
that lyophilization in 150 mM sucrose solution could stabilize
various types of mRNA lipoplexes for at least one month while
minimizing the impact on particle size and transfection activity.
However, certain cationic lipids may not be suitable for long-term
storage, highlighting the need for further investigations into the
relationship between the type of disaccharide cryoprotectant,
selection of cationic lipids, and the long-term stability of
lyophilized mRNA lipoplexes. Furthermore, it remains to be
investigated whether this approach is applicable not only to HeLa
cells but also to other cell types, including primary cultured
cells. This will be an important subject for future studies. Among
the cationic lipids and disaccharide solutions examined, it was
confirmed that lyophilization of mRNA lipoplexes containing DC-6-14
in sucrose solution provided optimal conditions for long-term
storage without significant loss of transfection activity. Based on
the findings of this study, the use of lyophilized mRNA lipoplexes
in reverse transfection strategies suggests their potential for the
development of mRNA-based therapies and gene function screening
methods for various diseases. Specifically, in this platform, by
fixing candidate therapeutic mRNA lipoplexes in multi-well plates,
we can simultaneously test them against multiple types of cancer
cells to identify which types are effective. Additionally, the
preloading of multiple types of therapeutic mRNA as lipoplexes into
multi-well plates enables determination of the most effective
therapeutic mRNA for cancer cells. These approaches enable rapid
identification of therapeutic mRNA for effective cancer therapy,
thereby informing the design of personalized therapies.
In this study, all experiments were conducted in
HeLa cells. Future studies will need to confirm the transfection
efficacy of mRNA lipoplexes across various cancer types. In
particular, to advance toward clinical application, it will be
essential to assess the feasibility of implementing this platform
with patient-derived cancer cells.
Supplementary Material
Representative histograms and gating
areas for the flow cytometry analysis of EGFP positive cells. EGFP
mRNA lipoplexes containing DC-1-16, DC-6-14 or TC-1-12 were reverse
transfected to HeLa cells. The percentage of EGFP positive cells
was measured 24 h after the transfection. Control: Group subjected
to reverse transfection with non-lyophilized mRNA lipoplexes. EGFP,
enhanced green fluorescent protein; DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14,
2-(bis(2-(tetradecanoyloxy)ethyl)amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12, 11-((1,3-bis(dode
canoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide.
Representative histograms and gating
areas for the flow cytometry analysis of Cy5 positive cells.
Cy5-mRNA lipoplexes containing DC-1-16, DC-6-14 or TC-1-12 were
reverse transfected to HeLa cells. The fluorescence intensity was
measured 3 h after the transfection. Control: Group subjected to
reverse transfection with non-lyophilized mRNA lipoplexes. DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14, 2-(bis(2-(tetradecanoyloxy)ethyl)
amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide.
Localization of mRNAs and lysosomes in
HeLa cells after reverse transfection with lyophilized mRNA
lipoplexes. Cy5-mRNA lipoplexes containing DC-1-16, DC-6-14 or
TC-1-12 were reverse transfected to HeLa cells. Lysosomes were
stained by Lysotracker 3 h after the transfection. Blue indicates
cell nuclei stained with Hoechst 33342, green indicates lysosomes
labeled with LysoTracker Red, and red indicates the localization of
Cy5-mRNA. Scale bar, 20 μm. DC-1-16,
N-hexadecyl-N,N-dimethylhexadecan-1-aminium
bromide; DC-6-14, 2-(bis(2-(tetradecanoyloxy)ethyl)
amino)-N,N,N-trimethyl-2-oxoethan-1-aminium
chloride; TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl) propan-2-yl)
amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide.
Acknowledgements
The authors thank Ms. Rio Beppu and Ms. Yuino Mimura
(Department of Molecular Pharmaceutics, Hoshi University, Tokyo,
Japan) for their valuable technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH conceived the study and designed the project. RS
conducted the experiments. Both YH and RS confirm the authenticity
of the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sufian MA and Ilies MA: Lipid-based
nucleic acid therapeutics with in vivo efficacy. Wiley Interdiscip
Rev Nanomed Nanobiotechnol. 15(e1856)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hou X, Zaks T, Langer R and Dong Y: Lipid
nanoparticles for mRNA delivery. Nat Rev Mater. 6:1078–1094.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhao W, Hou X, Vick OG and Dong Y: RNA
delivery biomaterials for the treatment of genetic and rare
diseases. Biomaterials. 217(119291)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Inagaki M: Cell reprogramming and
differentiation utilizing messenger RNA for regenerative medicine.
J Dev Biol. 12(1)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Choudry MW, Riaz R, Raza MH, Nawaz P,
Ahmad B, Jahan N, Rafique S, Afzal S, Amin I and Shahid M:
Development of non-viral targeted RNA delivery vehicles-A key
factor in success of therapeutic RNA. J Drug Target. 33:171–184.
2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang J, Cai L, Li N, Luo Z, Ren H, Zhang B
and Zhao Y: Developing mRNA nanomedicines with advanced targeting
functions. Nanomicro Lett. 17(155)2025.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X, Qi J, Wang J, Hu W, Zhou W, Wang Y
and Li T: Nanoparticle technology for mRNA: Delivery strategy,
clinical application and developmental landscape. Theranostics.
14:738–760. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Audouy S and Hoekstra D: Cationic
lipid-mediated transfection in vitro and in vivo (review). Mol
Membr Biol. 18:129–143. 2001.PubMed/NCBI
|
|
9
|
Grabbe S, Haas H, Diken M, Kranz LM,
Langguth P and Sahin U: Translating nanoparticulate-personalized
cancer vaccines into clinical applications: Case study with
RNA-lipoplexes for the treatment of melanoma. Nanomedicine (Lond).
11:2723–2734. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lai E and Van Zanten JH: Evidence of
lipoplex dissociation in liquid formulations. J Pharm Sci.
91:1225–1232. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xian H, Zhang Y, Yu C and Wang Y:
Nanobiotechnology-enabled mRNA stabilization. Pharmaceutics.
15(620)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang M, Hu S and Hattori Y: Effect of
prefreezing and saccharide types in freeze-drying of siRNA
lipoplexes on genesilencing effects in the cells by reverse
transfection. Mol Med Rep. 22:3233–3244. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu J and Anchordoquy TJ: Synergistic
effects of surfactants and sugars on lipoplex stability during
freeze-drying and rehydration. J Pharm Sci. 98:3319–3328.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang M and Hattori Y: Effect of using
amino acids in the freeze-drying of sirna lipoplexes on gene
knockdown in cells after reverse transfection. Biomed Rep.
15(72)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hattori Y, Hu S and Onishi H: Effects of
cationic lipids in cationic liposomes and disaccharides in the
freeze-drying of siRNA lipoplexes on gene silencing in cells by
reverse transfection. J Liposome Res. 30:235–245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang M, Sagawa A, Inoue N, Torii S, Tomita
K and Hattori Y: Efficient mRNA delivery with mRNA lipoplexes
prepared using a modified ethanol injection method. Pharmaceutics.
15(1141)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hattori Y and Shimizu R: Effective mRNA
transfection of tumor cells using cationic triacyl lipid-based mRNA
lipoplexes. Biomed Rep. 22(25)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
del Pozo-Rodríguez A, Solinís MA, Gascón
AR and Pedraz JL: Short- and long-term stability study of
lyophilized solid lipid nanoparticles for gene therapy. Eur J Pharm
Biopharm. 71:181–189. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mohammed-Saeid W, Michel D, El-Aneed A,
Verrall RE, Low NH and Badea I: Development of lyophilized Gemini
surfactant-based gene delivery systems: Influence of lyophilization
on the structure, activity and stability of the lipoplexes. J Pharm
Pharm Sci. 15:548–567. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Arora S, Dash SK, Dhawan D, Sahoo PK,
Jindal A and Gugulothu D: Freeze-drying revolution: Unleashing the
potential of lyophilization in advancing drug delivery systems.
Drug Deliv Transl Res. 14:1111–1153. 2024.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Arte KS, Chen M, Patil CD, Huang Y, Qu L
and Zhou Q: Recent advances in drying and development of solid
formulations for stable mRNA and siRNA lipid nanoparticles. J Pharm
Sci. 114:805–815. 2025.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun Q, Zhang H, Ding F, Gao X, Zhu Z and
Yang C: Development of ionizable lipid nanoparticles and a
lyophilized formulation for potent CRISPR-Cas9 delivery and genome
editing. Int J Pharm. 652(123845)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rasoulianboroujeni M, Kupgan G, Moghadam
F, Tahriri M, Boughdachi A, Khoshkenar P, Ambrose JJ, Kiaie N,
Vashaee D, Ramsey JD and Tayebi L: Development of a DNA-liposome
complex for gene delivery applications. Mater Sci Eng C Mater Biol
Appl. 75:191–197. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ruppl A, Kiesewetter D, Köll-Weber M,
Lemazurier T, Süss R and Allmendinger A: Formulation screening of
lyophilized mRNA-lipid nanoparticles. Int J Pharm.
671(125272)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao P, Hou X, Yan J, Du S, Xue Y, Li W,
Xiang G and Dong Y: Long-term storage of lipid-like nanoparticles
for mRNA delivery. Bioact Mater. 5:358–363. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang T, Sung TC, Yu T, Lin HY, Chen YH,
Zhu ZW, Gong J, Pan J and Higuchi A: Next-generation materials for
RNA-lipid nanoparticles: Lyophilization and targeted transfection.
J Mater Chem B. 11:5083–5093. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Blakney AK, Deletic P, McKay PF, Bouton
CR, Ashford M, Shattock RJ and Sabirsh A: Effect of complexing
lipids on cellular uptake and expression of messenger RNA in human
skin explants. J Control Release. 330:1250–1261. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sayers EJ, Peel SE, Schantz A, England RM,
Beano M, Bates SM, Desai AS, Puri S, Ashford MB and Jones AT:
Endocytic profiling of cancer cell models reveals critical factors
influencing LNP-mediated mRNA delivery and protein expression. Mol
Ther. 27:1950–1962. 2019.PubMed/NCBI View Article : Google Scholar
|



















