Introduction
Dilated cardiomyopathy (DCM) is a primary myocardial
disorder characterized by dilation and impaired systolic function
of the left or both ventricles. In children, DCM is the most common
type of cardiomyopathy and a leading indication for heart
transplantation (1,2). Despite advances in diagnostic and
therapeutic strategies, DCM is associated with considerable
morbidity and mortality in the pediatric population (1).
The incidence of pediatric DCM is relatively low but
clinically significant, with reported annual rates ranging from
0.34 to 1.13 cases per 100,000 children, depending on geographic
region and study methodology (1,2).
Etiological factors are heterogeneous and include post-infectious
myocarditis, genetic mutations affecting cytoskeletal or sarcomeric
proteins, metabolic disorders, neuromuscular diseases,
chemotherapy-related cardiotoxicity and idiopathic forms. Several
studies have shown that more than one-third of pediatric cases
remain idiopathic, highlighting the diagnostic challenges
associated with this condition (2).
Clinical presentation is equally diverse. The
majority of children present with signs of congestive heart
failure, such as tachypnea, feeding difficulties or reduced
exercise tolerance, whereas others may present with arrhythmias,
syncope or even sudden cardiac death. The clinical course is highly
variable, with certain patients demonstrating partial recovery,
while others progress to end-stage heart failure, requiring
mechanical support or transplantation. Despite optimal management,
transplant-free survival remains limited, with 5-year survival
rates reported to be only 50-70% (2). Determining prognosis in pediatric DCM
remains a clinical challenge. Although echocardiographic parameters
such as left ventricular ejection fraction, fractional shortening
and left-ventricular end-diastolic diameter (LVEDD), and clinical
parameters such as New York Heart Association or Ross functional
class and heart failure-related hospitalization, are commonly used
to estimate outcomes, they may not fully reflect the complex
pathophysiology of disease progression (1,2).
These conventional measures are load-dependent and provide only a
snapshot of systolic performance and clinical status at a single
time-point, whereas pediatric DCM is influenced by heterogeneous
mechanisms, including genetic predisposition, myocardial
inflammation, remodeling and arrhythmic risk, which are not
adequately captured by baseline echocardiography or bedside
clinical scores (2). Previously
published evidence has suggested that inflammation may serve a role
in the pathogenesis and progression of DCM, raising interest in the
potential prognostic value of inflammatory markers (3-5).
Amongst the inflammatory markers, the
neutrophil-to-lymphocyte ratio (NLR) and the the systemic
inflammatory index (SII), calculated as platelet count x neutrophil
count/lymphocyte count, have been garnering attention as accessible
indicators of systemic inflammation. Previous studies in pediatric
DCM have demonstrated the prognostic utility of elevated NLR,
showing its association with poor clinical outcomes (4,5).
However, to the best of our knowledge, no study has evaluated the
prognostic significance of SII in children with DCM, and the
present study is the first to assess both NLR and SII
simultaneously in this patient population.
The present study aims to evaluate the prognostic
significance of clinical, echocardiographic and laboratory
parameters, particularly NLR and SII, in pediatric patients
diagnosed with DCM. By analyzing a long-term, single-center cohort,
the present study aims to contribute to the current understanding
of the prognostic value of systemic inflammatory markers in
pediatric DCM.
Patients and methods
Patient cohort
The present study included 52 pediatric patients
(age, 0-18 years) diagnosed with DCM who were followed at the
Department of Pediatric Cardiology of Marmara University Faculty of
Medicine (Istanbul, Turkey) between January 2000 and June 2021.
Inclusion criteria were a DCM phenotype defined by LV dilatation
with impaired systolic function on echocardiography, including
cases with various underlying causes, such as idiopathic,
post-myocarditis, anthracycline-related or genetic/metabolic.
Exclusion criteria were structural heart disease causing
ventricular dilatation/dysfunction (e.g., critical aortic stenosis,
aortic coarctation with LV dysfunction, coronary anomalies/ischemic
heart disease), and other primary cardiomyopathy phenotypes not
consistent with DCM (e.g., hypertrophic, LV noncompaction,
restrictive or arrhythmogenic cardiomyopathy).
Relevant clinical, diagnostic and outcome data were
retrospectively reviewed using patient charts and electronic
medical records. Data collected included age at diagnosis, sex,
presenting symptoms, etiological classification, echocardiographic
and rhythm monitoring findings (including Holter rhythm monitoring
and ECG), treatment modalities, duration of follow-up and survival
status. The NLR was calculated by dividing the absolute neutrophil
count by the absolute lymphocyte count. The SII was calculated
using the following formula: Platelet count x neutrophil
count/lymphocyte count. These values were obtained retrospectively
from complete blood count results derived from peripheral venous
blood samples collected at the time of diagnosis. All analyses were
performed using standardized automated hematology analyzers in the
Central Laboratory of Marmara University Hospital (Istanbul,
Turkey). Echocardiographic measurements were extracted from
standardized reports prepared by experienced pediatric
cardiologists at the time of diagnosis. Although formal
inter-observer variability testing was not performed due to the
retrospective nature of the study, all evaluations were conducted
using consistent institutional protocols.
The aim of the present study was to evaluate the
prognostic impact of various clinical and laboratory parameters,
particularly the NLR and the SII, on mortality.
Ethical approval for the present study was obtained
from the Marmara University Faculty of Medicine Clinical Research
Ethics Committee (approval no. 09.2022.463). The requirement for
informed consent was waived due to the retrospective design of the
study, in accordance with the approval granted by the ethics
committee.
Statistical analysis
Overall survival (OS) was defined as the time from
the diagnosis of DCM to either mortality or the last follow-up.
Statistical analyses were performed using SPSS 15.0 (SPSS, Inc.)
for Windows. Descriptive statistics are presented as numbers and
percentages for categorical variables, and as mean ± standard
deviation for numerical variables with normal distribution. Group
comparisons for categorical variables were made using the
χ2 test, with Fisher's exact test applied when expected
cell counts were <5. For continuous variables, the
independent-samples t-test was used when the assumption of
normality, as assessed by the Shapiro-Wilk test, was met;
otherwise, the Mann-Whitney U test was applied. Within-patient
comparisons (namely FS and EF from baseline to final follow-up)
were analyzed using the Wilcoxon signed-rank test. Survival rates
were analyzed using Kaplan-Meier analysis, whereas risk factors
were evaluated using Cox regression analysis. Cut-off values were
determined using receiver operating characteristic (ROC) curve
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic characteristics
A total of 52 pediatric patients diagnosed with DCM
were included in the present study. The mean age at diagnosis was
58.8±69.8 months (range, 1-216 months). Within the cohort, 48.1%
were female (n=25), and no statistically significant difference was
observed in the sex distribution between deceased (n=16) and
surviving patients. Although the mean age at diagnosis tended to be
higher among patients who succumbed (73.2±67.6 months) compared
with that in survivors (52.4±70.7 months), this difference did not
reach statistical significance (Table
I).
 | Table IDemographic and clinical
characteristics of patients with DCM according to survival
status. |
Table I
Demographic and clinical
characteristics of patients with DCM according to survival
status.
| Patient demographic
and clinical characteristics | Total (n=52) | Deceased (n=16) | Survived (n=36) | P-value |
|---|
| Sex, n (%) | | | | 0.677a |
|
Female | 25 (48.1) | 7 (43.8) | 18 (50.0) | |
|
Male | 27 (51.9) | 9 (56.3) | 18 (50.0) | |
| Age at diagnosis,
months (mean ± SD) | 58.8±69.8 | 73.2±67.6 | 52.4±70.7 | 0.051b |
| Weight, kg (mean ±
SD) | 19.5±16.9 | 23.3±17,1 | 17.8±16.8 | 0.111b |
| Height, cm (mean ±
SD) | 116.1±34.1 | 97.3±28.9 | 121.7±34.8 | 0.271b |
| Weight percentile
range, n (%) | | | | 0.831a |
|
<P3 | 19 (37.3) | 4(25) | 15 (42.9) | |
|
P3-P10 | 5 (9.8) | 2 (12.5) | 3 (8.6) | |
|
P10-P25 | 11 (21.6) | 4 (25.0) | 7 (20.0) | |
|
P25-P50 | 9 (17.6) | 4 (25.0) | 5 (14.3) | |
|
P50-P75 | 4 (7.8) | 1 (6.3) | 3 (8.6) | |
|
>P75 | 3 (5.9) | 1 (6.3) | 2 (5.7) | |
| Height percentile
range, n (%) | | | | 0.770a |
|
<P3 | 6 (46.2) | 1 (33.3) | 5 (50.0) | |
|
P3-P10 | 1 (7.7) | 0 (0.0) | 1 (10.0) | |
|
P10-P25 | 1 (7.7) | 0 (0.0) | 1 (10.0) | |
|
P25-P50 | 3 (23.1) | 1 (33.3) | 2 (20.0) | |
|
P50-P75 | 1 (7.7) | 0 (0.0) | 1 (10.0) | |
|
>P75 | 1 (7.7) | 1 (33.3) | 0 (0.0) | |
| Admission symptoms, n
(%) | | | | 0.773a |
|
Heart
failure symptoms | 31 (59.6) | 10 (62.5) | 21 (58.3) | |
|
Heart
failure symptoms + GI symptoms | 6 (11.5) | 3 (18.8) | 3 (8.3) | |
|
GI symptoms
(isolated) | 5 (9.6) | 1 (6.3) | 4 (11.1) | |
|
Isolated
murmur | 2 (3.8) | 0 (0.0) | 2 (5.6) | |
|
Incidentally
detected | 8 (15.4) | 2 (12.5) | 6 (16.7) | |
| Family history of
DCM, n (%) | | | | 0.116a |
|
Negative | 42 (80.8) | 10 (62.5) | 32 (88.9) | |
|
Positive | 5 (9.6) | 3 (18.8) | 2 (5.6) | |
|
Consanguinity,
n (%) | 21 (41.2) | 9 (60.0) | 12 (33.3) | 0.078a |
| Etiology, n
(%)c | | | | 0.469a |
|
Idiopathic | 36 (69.2) | 11 (30.6) | 25 (69.4) | |
|
Post-myocarditis | 15 (28.8) | 4 (26.7) | 11 (73.3) | |
|
Anthracycline-associated | 1 (1.9) | 1 (100.0) | 0 (0.0) | |
| Hospitalization
duration at diagnosis, days (mean ± SD) | 8.29±10.5 | 10.06±13.94 | 7.50±8.71 | 0.212b |
| Follow-up duration,
months (mean ± SD) | 25.3±30.2 | 17.6±24.1 | 28.7±32.2 | 0.264b |
Consanguinity was present in 41.2% of +patients
overall and was more frequent in the deceased group (60.0%)
compared with that in the survivor group (33.3%), though this was
not statistically significant. The mean weight and height at
diagnosis were 19.5±16.9 kg (range, 4-70 kg) and 116.1±34.1 cm
(range, 55-185 cm), respectively. However, there was no significant
difference in these anthropometric parameters between the two
groups. A total of 37.3% patients had weight in the <3rd
percentile and 46.2% had height in the <3rd percentile. No
significant difference was observed between the deceased and
surviving patient groups for either of the aforementioned
parameters. All baseline demographic and clinical characteristics
of the patients are summarized in Table I.
Etiological classification and
outcome
Among the 52 patients diagnosed with DCM, 15 (28.8%)
had a history of suspected post-myocarditis and 1 patient (1.9%)
had anthracycline-associated cardiomyopathy, whereas 36 patients
(69.2%) were classified as idiopathic in the absence of a clearly
identifiable secondary cause. Additionally, several patients in the
idiopathic group had identifiable genetic or metabolic syndromes,
including Duchenne muscular dystrophy (n=1), Pompe disease (n=2),
Prader-Willi syndrome (n=1) and propionic acidemia (n=2). However,
these syndromic cases were included into the idiopathic group as
they could not be considered separate categories due to their small
numbers. Etiological subgroup comparisons between deceased and
surviving patients showed no statistically significant differences
(Table I).
Clinical findings
Patients presented with a variety of symptoms at
diagnosis (Table I). The most
frequent presentation was typical signs of heart failure, observed
in 59.6% patients. This group included clinical findings, such as
reduced exercise tolerance, tachypnea and tachycardia.
A total of 21.1% patients reported gastrointestinal
(GI) symptoms, including nausea, vomiting or abdominal pain. Among
them, 6 patients (11.5%) had GI symptoms in combination with heart
failure signs, whereas 5 patients (9.6%) presented with isolated GI
complaints. An isolated murmur was the referral reason in 2
patients (3.8%), whilst 15.4% were referred after incidental
findings during routine pediatric evaluations. No statistically
significant difference was found in the presenting symptoms between
deceased and surviving patients.
Laboratory parameters
All laboratory results were obtained at the time of
diagnosis, prior to the initiation of medical treatment (Table II).
 | Table IILaboratory parameters and prognostic
comparison. |
Table II
Laboratory parameters and prognostic
comparison.
| Parameter
(reference range) | Total (n=52) | Deceased
(n=16) | Survived
(n=36) | P-value |
|---|
| White blood cell,
x10³/µl (4.5-13.5) | 10.4±3.6 | 10.6±4.1 | 10.3±3.4 | 0.809a |
| Neutrophil, %
(40.0-75.0) | 59.3±20.8 | 71.7±14.7 | 52.1±20.7 | 0.003a |
| Lymphocyte, %
(20.0-45.0) | 31.6±17.3 | 22.0±13.2 | 37.2±17.1 | 0.005a |
| Hemoglobin, g/dl
(11.0-16.0) | 11.47±2.10 | 10.95±1.45 | 11.74±2.35 | 0.239a |
| Hematocrit, %
(33.0-45.0) | 34.81±6.06 | 33.7±4.12 | 35.38±6.82 | 0.391a |
| Platelet, x10³/µl
(150.0-450.0) | 319.0±137.5 | 310.5±155.1 | 323.6±129.9 | 0.980b |
|
Neutrophil-to-lymphocyte ratio | 2.96±2.49 | 4.53±2.72 | 2.06±1.85 | 0.003b |
| Systemic
inflammatory index |
929,393.6±972,589.4 |
1,532,880.4±1,312,699.9 |
581,228.2±451,576.3 | 0.016b |
| C-reactive protein,
mg/l (<5.0) | 6.24±9.11 | 10.66±16.21 | 4.72±4.39 | 0.176b |
| B-type natriuretic
peptide, pg/ml (<100.0) |
15,317.3±13,311.1 |
11,777.0±12,360.1 |
17,399.9±13,767.8 | 0.290b |
| Na, mmol/l
(135.0-145.0) | 136.7±2.6 | 134.3±2.4 | 137.6±2.1 |
<0.001b |
| Mg, mg/dl
(1.7-2.3) | 2.19±0.42 | 1.98±0.24 | 2.26±0.45 | 0.084a |
| K, mmol/l
(3.5-5.0) | 4.68±0.78 | 4.71±0.77 | 4.67±0.80 | 0.913b |
| Ca, mg/dl
(8.5-10.5) | 9.50±0.89 | 9.42±0.68 | 9.53±0.97 | 0.736a |
| Blood urea
nitrogen, mg/dl (7.0-20.0) | 14.45±8.89 | 16.77±7.22 | 13.46±9.47 | 0.069b |
| Urea, mg/dl
(10.0-50.0) | 30.9±19.9 | 37.7±16.4 | 28.61±20.71 | 0.050b |
| Creatinine, mg/dl
(0.3-1.0) | 0.67±1.50 | 0.39±0.16 | 0.78±1.76 | 0.174b |
| Aspartate
aminotransferase, U/l (5.0-40.0) | 77.3±103.7 | 100.9±163.4 | 67.4±66.8 | 0.842b |
| Alanine
aminotransferase, U/l (7.0-56.0) | 54.8±75.7 | 78.9±111.7 | 44.9±54.9 | 0.430b |
| Albumin,
g/dl(3.5-5.5) | 4.02±0.61 | 3.92±0.60 | 4.05±0.62 | 0.351b |
| CK,
U/l(20.0-200.0) | 599.1±1,390.8 | 249.3±341.8 | 721.6±1,596.6 | 0.472b |
| CK-MB, U/l
(0.0-24.0) | 7.37±6.75 | 10.94±9.80 | 5.81±4.45 | 0.216b |
| Troponin I,
ng/ml(<0.04) | 0.06±0.10 | 0.06±0.04 | 0.06±0.12 | 0.155b |
| Troponin T, ng/l
(<14.0) | 82.3±97.3 | 75.2±65.3 | 85.8±113.1 | 0.391b |
The mean white blood cell count was
10,420.9±3,629.7/mm³ (range, 2,800-19,600/mm³). Neutrophil and
lymphocyte percentages were 59.3±20.8% (range, 14-91%) and
31.6±17.3% (range, 3.5-77%), respectively. Hemoglobin level
averaged 11.47±2.10 g/dl (range, 6.3-16.1 g/dl) and hematocrit was
34.81±6.06% (range, 20.2-47.5%). Platelet count was
319,046.5±137,517.3/mm³ (range, 103,000-742,000/mm³).
The mean C-reactive protein level was 6.24±9.11 mg/l
(range, 0-80 mg/l). Electrolyte analysis revealed a mean sodium
level of 136.7±2.6 mmol/l (range, 129-143 mmol/l) and a mean
magnesium level of 2.19±0.42 mg/dl (range, 1.2-2.9 mg/dl). Serum
sodium levels were significantly lower in the deceased group
compared with the survivors (134.3±2.4 vs. 137.6±2.1 mmol/l;
P<0.001). Serum magnesium levels were also lower in the deceased
group (1.98±0.24 vs. 2.26±0.45 mg/dl), although this difference did
not reach statistical significance (P=0.084). Serum potassium and
calcium levels showed no significant differences between groups
(P=0.913 and P=0.736) and remained within normal limits, with mean
values of 4.68±0.78 mmol/l and 9.50±0.89 mg/dl, respectively
(Table II).
Cardiac biomarkers included B-type natriuretic
peptide (BNP), creatine kinase (CK), CK-MB and troponin I. They
were all markedly elevated compared with the reference range. BNP
levels were also markedly elevated, with a mean of
15,317.3±13,311.1 pg/ml (range, 100-57,000 pg/ml). The mean CK
level was 599.1±1,390.8 U/l (range, 70-5,000 U/l), the CK-MB mean
level was 7.37±6.75 U/l (range, 1.2-28 U/l) and the mean troponin I
level was 0.06±0.10 ng/ml (range, 0-0.50 ng/ml). No significant
differences were observed in cardiac biomarkers between the two
groups (Table II).
Renal function markers measured included blood urea
nitrogen, urea and creatinine, with mean values of 14.45±8.89 mg/dl
(range, 3.0-45.0 mg/dl), 30.9±19.9 mg/dl (range, 10-80 mg/dl) and
0.67±1.50 mg/dl (range, 0.2-6.0 mg/dl), respectively. Liver
function tests showed mean aspartate aminotransferase (AST) and
alanine aminotransferase (ALT) levels of 77.3±103.7 and 54.8±75.7
U/l, respectively. Although these means were above the reference
ranges, the elevation was driven by a few patients with markedly
high transaminase levels, while the majority of the cohort had
values within normal limits. These findings are summarized in
Table II.
Echocardiographic findings
Echocardiographic evaluation at the time of
diagnosis demonstrated varying degrees of left ventricular
dilatation and systolic dysfunction, consistent with the clinical
profile of DCM. The mean LVEDD was 4.38±1.10 cm. Although LVEDD
values were higher in the deceased group of patients compared with
those in the survivors, the difference did not reach statistical
significance (Table III).
 | Table IIIEchocardiographic findings at
diagnosis, Holter rhythm monitoring and electrocardiographic
abnormalities according to survival status. |
Table III
Echocardiographic findings at
diagnosis, Holter rhythm monitoring and electrocardiographic
abnormalities according to survival status.
| Parameter | Total (n=52) | Deceased
(n=16) | Survived
(n=36) | P-value |
|---|
| Interventricular
septal thickness in diastole, cm | 0.63±0.17 | 0.69±0.19 | 0.60±0.15 | 0.076a |
| Left ventricular
end-diastolic diameter, cm | 4.38±1.10 | 4.74±1.01 | 4.23±1.11 | 0.121a |
| Left ventricular
end-systolic diameter, cm | 3.37±1.30 | 3.64±1.33 | 3.26±1.29 | 0.393a |
| Left ventricular
posterior wall thickness in diastole, cm | 0.68±0.48 | 0.69±0.18 | 0.68±0.56 | 0.054b |
| Fractional
shortening, % | 19.1±7.97 | 16.6±5.6 | 20.3±8.7 | 0.132a |
| Ejection fraction,
% | 39.3±13.8 | 34.8±10.1 | 41.3±14.9 | 0.187b |
| Left atrial
diameter, cm | 2.44±0.87 | 2.63±0.91 | 2.37±0.85 | 0.362a |
| Aortic root
diameter, cm | 1.63±0.60 | 1.98±0.76 | 1.50±0.49 | 0.017a |
| Mitral E,
m/sec | 0.89±0.22 | 0.78±0.18 | 0.94±0.22 | 0.031a |
| Mitral A,
m/sec | 0.64±0.19 | 0.59±0.18 | 0.66±0.19 | 0.240a |
| Deceleration time,
msec | 96.6±39.9 | 92.9±34.4 | 98.2±42.5 | 0.695a |
| Isovolumic
relaxation time, msec | 64.8±18.5 | 66.9±23.7 | 63.9±16.3 | 0.639b |
| Aortic root
velocity, m/sec | 1.14±0.32 | 1.05±0.22 | 1.17±0.35 | 0.219b |
| Pulmonary artery
velocity, m/sec | 1.09±0.29 | 0.98±0.24 | 1.13±0.30 | 0.105a |
| Descending Aortic
root velocity, m/sec | 1.17±0.33 | 1.08±0.28 | 1.20±0.35 | 0.219b |
| Holter rhythm
monitoring findings, n (%) | | | |
>0.999c |
|
Supraventricular
ectopic activity | 1 (1.9) | 0(0) | 1 (2.8) | |
|
Ventricular
ectopic activity | 7 (13.7) | 2 (13.3) | 5 (13.9) | |
|
Supraventricular
tachycardia | 5 (9.8) | 1 (6.7) | 4 (11.1) | |
|
Ventricular
tachycardia | 0 (0) | 0 (0) | 0 (0) | |
|
Electrocardiographic findings, n (%) | | | | |
|
No
additional ECG finding | 16 (31.4) | 6 (40.0) | 10 (27.8) | 0.967c |
|
Left
ventricular hypertrophy | 9 (17.6) | 3 (20.0) | 6 (16.7) | |
|
Left
ventricular hypertrophy + inverted T in V5-V6 | 5 (9.8) | 1 (6.7) | 4 (11.1) | |
|
Left
ventricular hypertrophy + left axis deviation | 5 (9.8) | 1 (6.7) | 4 (11.1) | |
|
Left
ventricular hypertrophy + RBBB | 1(2) | 1 (6.7) | 0 (0) | |
|
Frequent
ventricular ectopic activity | 3 (5.9) | 1 (6.7) | 2 (5.6) | |
|
Left axis
deviation | 1(2) | 0 (0) | 1 (2.8) | |
|
Left axis
deviation + left atrial enlargement | 1(2) | 0 (0) | 1 (2.8) | |
|
Right axis
deviation + ST depression | 2 (3.9) | 0 (0) | 2 (5.6) | |
|
Right axis
deviation + biventricular hypertrophy | 1 (2.0) | 0 (0) | 1 (2.8) | |
|
Low QRS
voltage | 3 (5.9) | 1 (6.7) | 2 (5.6) | |
|
Low QRS
voltage + ST elevation | 1 (2.0) | 0 (0) | 1 (2.8) | |
|
Peaked T
wave | 1 (2.0) | 1 (6.7) | 0 (0) | |
|
Supraventricular
tachycardia episode | 1 (2.0) | 0 (0) | 1 (2.8) | |
Left ventricular systolic function, as measured by
FS and EF, was reduced in both groups compared with the expected
normal pediatric ranges (FS <28% and EF <55% indicating
systolic dysfunction). The mean FS was 19.1±7.97% and the mean EF
was 39.3±13.8%. Although deceased patients had lower values for
both FS and EF compared with those in the survivor group (FS:
16.6±5.6 vs. 20.3±8.7%; EF: 34.8±10.1 vs. 41.3±14.9%), the
differences were not statistically significant. Mitral E wave
velocity was, however, found to be significantly lower in the
deceased group (0.78±0.18 vs. 0.94±0.22 m/sec; P=0.031).
Although the mean aortic root diameter was
statistically larger in deceased patients (1.98±0.76 cm) compared
with that in the survivor group (1.50±0.49 cm; P=0.017), all
measurements fell within the normal Z-score range for age (-2 to
+2). This difference was likely attributable to the older mean age
of patients in the deceased group and was not considered clinically
significant.
Other parameters, including left atrial diameter,
mitral A wave velocity, deceleration time, isovolumic relaxation
time and flow velocities across the aortic valve, pulmonary valve
and descending aorta, showed no statistically significant
differences between groups (Table
III).
Rhythm monitoring findings
Electrocardiograph and Holter rhythm monitoring data
were available for all patients. Ventricular ectopic activity (VEA)
was detected in 7 patients (13.5%) and supraventricular VEA in 1
patient (1.9%). In these cases, the ectopic beat burden was <1%
on 24-h Holter rhythm recordings. Therefore, these arrhythmias were
considered rare and clinically insignificant. Supraventricular
tachycardia was observed in 5 patients (9.6%) and no episodes of
ventricular tachycardia were recorded.
Additional electrocardiographic findings included
left axis deviation, low-voltage QRS complexes, ST-T wave
abnormalities and biventricular or left ventricular hypertrophy. No
statistically significant association was found between any
electrocardiographic or Holter rhythm abnormality and mortality.
All Holter rhythm and electrocardiographic findings are summarized
in Table III.
Heart failure management and follow-up
echocardiography
All patients received standard heart failure
therapy. The most commonly prescribed medications were diuretics
(82.7%), angiotensin-converting enzyme inhibitors (80.8%) and
digoxin (69.2%), followed by beta-blockers (19.2%). In addition,
supportive treatments, such as carnitine (50%) and coenzyme Q10
(48.1%), were frequently utilized. Follow-up echocardiographic
evaluations, performed after a mean follow-up duration of 25.3±30.2
months (range, 1-108 months), demonstrated significant improvements
in both EF and FS (Table IV). The
mean EF increased from 39.3% at diagnosis to 49.8% at the final
follow-up (P<0.001), whilst FS improved from 19.1 to 25.6%
(P<0.001). Final echocardiographic measurements revealed that
deceased patients had significantly lower EF and FS values compared
with the survivors group (both P=0.002). These final post-treatment
follow-up measurements according to survival status are summarized
in Table IV.
 | Table IVBaseline and final echocardiographic
measurements according to survival status. |
Table IV
Baseline and final echocardiographic
measurements according to survival status.
| | All patients | Current status |
|---|
| Parameter | Baseline (mean ±
SD) | Final (mean ±
SD) |
P-valuea | Deceased group
(mean ± SD) | Surviving group
(mean ± SD) |
P-valueb |
|---|
| Fractional
shortening, % | 19.1±7.97 | 25.6±8.9 | <0.001 | 19.4±8.1 | 28.4±7.9 | 0.002 |
| Ejection fraction,
% | 39.3±13.8 | 49.8±15.3 | <0.001 | 38.5±14.5 | 54.7±13.1 | 0.002 |
Inflammatory markers and prognostic
indices
At the time of diagnosis, inflammatory indices,
including the NLR and SII, were calculated from complete blood
count parameters (Table II). Both
NLR and SII values were found to be significantly higher in
deceased patients compared with those in the survivors group.
Specifically, the mean NLR value for the entire
cohort was 2.96±2.49. NLR was significantly elevated in the
deceased group (4.53±2.72) compared with that in the survivors
group (2.06±1.85; P=0.003). Similarly, the mean SII was
929,393.6±972,589.4 in the overall cohort, with patients in the
deceased group having a significantly higher SII
(1,532,880.4±1,312,699.9) compared with that in the survivors group
(581,228.2±451,576.3; P=0.016).
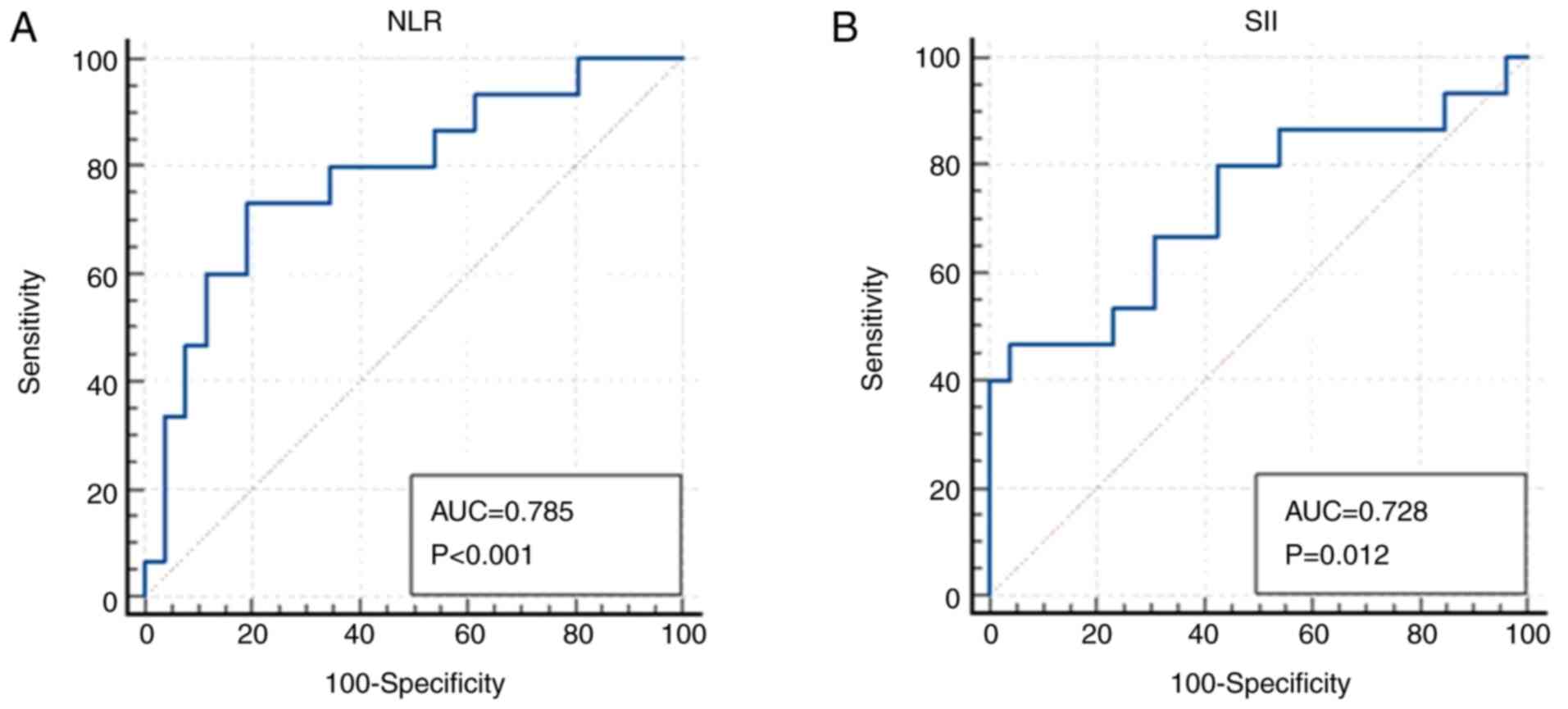
ROC curve analyses were performed separately for NLR
and SII to predict mortality (deceased vs. surviving). The AUC for
NLR was 0.785 (P<0.001) and for SII, it was 0.728 (P=0.012)
(Fig. 1). Based on ROC-derived
thresholds, a cut-off value of >2.75 for NLR and >1,428,898
for SII was associated with increased mortality risk. The AUC
values and corresponding P-values were calculated using the DeLong
test, 95% confidence intervals were computed with the binomial
exact method and optimal cut-off values were determined according
to the Youden index. These findings are summarized in Table V and illustrated in Fig. 1.
 | Table VROC curve analysis parameters for NLR
and SII. |
Table V
ROC curve analysis parameters for NLR
and SII.
| Parameter | NLR | SII |
|---|
| Area under the ROC
curve, area under the curve | 0.785 | 0.728 |
| Standard
errora | 0.0777 | 0.0904 |
| 95% Confidence
intervalb | 0.628-0.897 | 0.567-0.855 |
| z statistic | 3.661 | 2.524 |
| Significance level
P-value (area=0.5) | 0.0003 | 0.0116 |
| Youden index J | 0.5410 | 0.4282 |
| Associated
criterion | >2.75 | >1,428,898 |
| Sensitivity | 73.33 | 46.67 |
| Specificity | 80.77 | 96.15 |
Survival and regression analysis
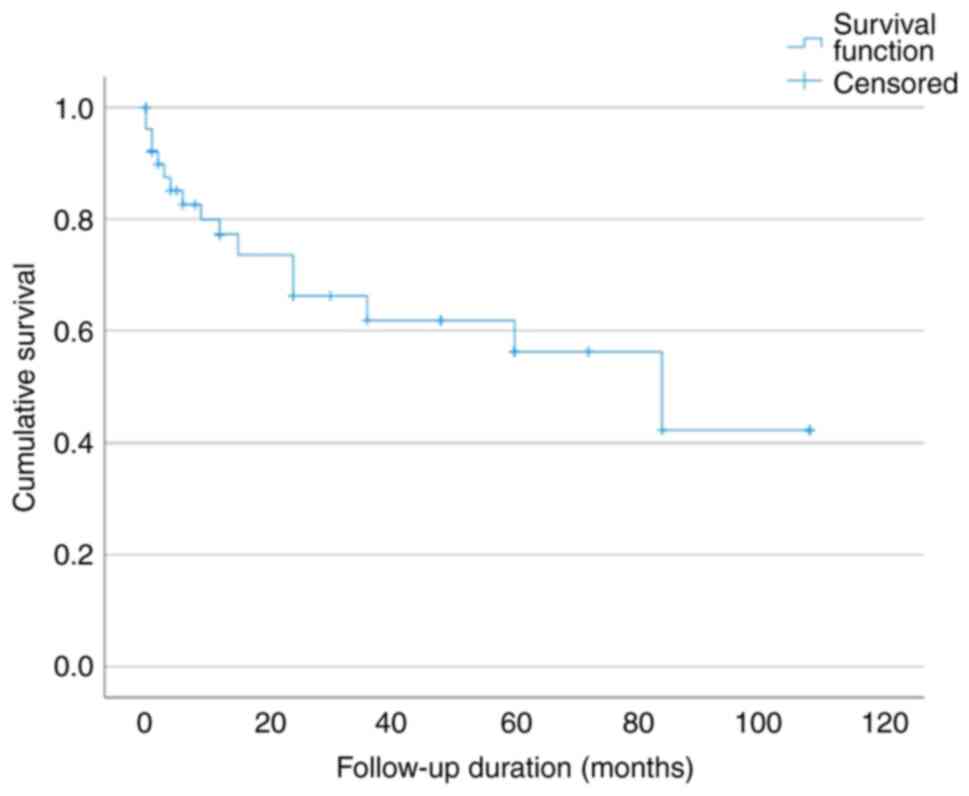
During the follow-up period, 16 of the 52 patients
(30.8%) succumbed. Survival rates were therefore analyzed using
Kaplan-Meier analysis. The OS rates at 1, 2, 3, 5 and 7 years were
77.3, 66.2, 61.8, 56.2 and 42.2%, respectively (Fig. 2). At the end of the follow-up
period, 36 of the 52 patients (69.2%) were alive. This crude
observed survival reflects the survival status at a median
follow-up time of 25 months (mean, 25.3±30.2 months), whereas the
Kaplan-Meier estimate at 7 years (42.2%) was based on a much
smaller number of patients remaining under observation. Although
the median follow-up duration was shorter in deceased patients
(17.6 months) compared with that in the survivors group (28.7
months), this difference was not statistically significant.
Univariate Cox regression analysis (Table VI) identified several variables
that were significantly associated with mortality, including low
serum sodium levels, elevated NLR, higher SII values, increased
aortic root diameter, decreased mitral E wave velocity, low
magnesium levels (within the normal reference range), elevated
neutrophil percentage, decreased lymphocyte percentage and a
positive family history of DCM (the per-unit HR for SII
approximated 1.0 due to its large numeric scale, which limits
interpretability per single-unit increase; however, both group
comparisons and ROC analysis confirmed a significant association
with mortality). Serum sodium levels emerged as one of the most
consistently associated parameters with mortality among all
variables analyzed (P<0.001 for group comparison; HR=0.669, 95%
CI 0.527-0.850; P=0.001 in Cox regression).
 | Table VIUnivariate Cox regression analysis of
risk factors for mortality. |
Table VI
Univariate Cox regression analysis of
risk factors for mortality.
| | | 95% CI |
|---|
| Patient
characteristics | P-value | Hazard ratio | Min | Max |
|---|
| Sex (ref:
Female) | 0.953 | 1.031 | 0.380 | 2.793 |
| Age at diagnosis,
months | 0.134 | 1.005 | 0.998 | 1.011 |
| Medical history
present (ref: Absent) | 0.287 | 1.800 | 0.609 | 5.317 |
| Family history
positive (ref: Negative) | 0.049 | 2.808 | 1.006 | 7.838 |
| Consanguinity
present (ref: Absent) | 0.120 | 2.274 | 0.806 | 6.411 |
| Left ventricular
end-diastolic diameter, cm | 0.164 | 1.404 | 0.871 | 2.265 |
| Left ventricular
end-systolic diameter, cm | 0.335 | 1.258 | 0.789 | 2.006 |
| Fractional
shortening, % | 0.618 | 0.980 | 0.905 | 1.061 |
| Ejection fraction,
% | 0.510 | 0.985 | 0.942 | 1.030 |
| Left atrial
diameter, cm | 0.182 | 1.467 | 0.836 | 2.574 |
| Ao diameter,
cm | 0.006 | 2.746 | 1.344 | 5.612 |
| Mitral E,
m/sec | 0.034 | 0.018 | 0.000 | 0.741 |
| Mitral A,
m/sec | 0.210 | 0.126 | 0.005 | 3.221 |
| Ao velocity,
m/sec | 0.511 | 0.471 | 0.050 | 4.441 |
| Pulmonary artery
velocity, m/sec | 0.176 | 0.266 | 0.039 | 1.815 |
| Descending Ao
velocity, m/sec | 0.476 | 0.529 | 0.092 | 3.045 |
| CK, U/l | 0.432 | 0.999 | 0.998 | 1.001 |
| CK-MB, U/l | 0.229 | 1.048 | 0.971 | 1.132 |
| Troponin I,
ng/ml | 0.811 | 0.299 | 0.000 | 5.942 |
| Troponin T,
ng/l | 0.579 | 1.003 | 0.992 | 1.015 |
| B-type natriuretic
peptide, pg/ml | 0.215 | 1.000 | 1.000 | 1,000 |
| White blood cells,
x10³/µl | 0.645 | 1.000 | 1.000 | 1.000 |
| Neutrophil, % | 0.017 | 1.047 | 1.008 | 1.088 |
| Lymphocyte, % | 0.025 | 0.953 | 0.913 | 0.994 |
| Hemoglobin,
g/dl | 0.268 | 0.828 | 0.593 | 1.156 |
| Hematocrit, % | 0.446 | 0.957 | 0.854 | 1.072 |
| Platelet,
x10³/µl | 0.801 | 1.000 | 1.000 | 1.000 |
|
Neutrophil-to-lymphocyte ratio | 0.006 | 1.303 | 1.077 | 1.575 |
| Systemic
inflammation index | 0.001 | 1.000 | 1.000 | 1.000 |
| C-reactive protein,
mg/l | 0.905 | 1.003 | 0.959 | 1.048 |
| Blood urea
nitrogen, mg/dl | 0.100 | 1.045 | 0.992 | 1.102 |
| Urea, mg/dl | 0.182 | 1.021 | 0.990 | 1.053 |
| Creatinine,
mg/dl | 0.679 | 0.801 | 0.280 | 2.293 |
| Albumin, g/dl | 0.359 | 0.549 | 0.152 | 1.979 |
| Aspartate
aminotransferase, U/l | 0.199 | 1.003 | 0.999 | 1.007 |
| Alanine
aminotransferase, U/l | 0.285 | 1.003 | 0.997 | 1.010 |
| Na, mmol/l | 0.001 | 0.669 | 0.527 | 0.850 |
| K, mmol/l | 0.631 | 0.773 | 0.270 | 2.211 |
| Ca, mg/dl | 0.463 | 0.768 | 0.381 | 1.552 |
| Mg, mg/dl | 0.019 | 0.026 | 0.001 | 0.547 |
Discussion
DCM in childhood is a rare condition characterized
by impaired ventricular function and progressive heart failure. The
annual incidence of pediatric DCM ranges between 0.57 and 1.13
cases per 100,000 children, with an estimated prevalence of ~1 per
250,000(2). DCM remains one of the
leading indications for cardiac transplantation in the pediatric
population. Despite advances in medical therapy and heart failure
management, predicting outcomes in pediatric DCM continues to be a
major clinical challenge (6). This
is largely due to the disease's heterogeneous etiology, variable
clinical course and the limited data regarding prognostic
indicators in children (2,6).
In previous years, systemic inflammation has been
increasingly recognized to be a contributing factor in various
cardiovascular conditions (7).
Inflammatory responses may influence myocardial function through
cytokine-mediated pathways, microvascular dysfunction and
myocardial remodeling (7,8). Among the accessible inflammatory
markers, NLR and SII have gained attention as simple cost-effective
indicators reflecting the balance between proinflammatory and
regulatory immune responses (9,10).
In pediatric DCM, data on the prognostic role of
inflammatory markers remain limited. The majority of studies to
date have focused solely on the NLR. Ahmed et al (5) demonstrated that elevated NLR levels
were significantly associated with disease severity and adverse
outcomes in children with DCM-related acute heart failure.
Similarly, da Rocha Araújo et al (4) reported that elevated NLR was
associated with poor clinical progression, increased mortality and
the necessity for cardiac transplantation. To the best of our
knowledge, no study to date has evaluated the prognostic role of
the SII or investigated both NLR and SII in the same pediatric DCM
population. By introducing SII into the pediatric cardiology
literature, the present study provides a contribution to the
understanding of inflammation-based risk assessment in this
population.
NLR and SII have both been studied as prognostic
markers in adult patients with heart failure and cardiomyopathies,
where it has been determined that these markers are associated with
poor outcomes and increased mortality (11-14).
By evaluating the prognostic value of NLR and SII simultaneously in
a pediatric DCM cohort, the present study offers data and
contributes to addressing the current knowledge gap in this
field.
In present study, both the NLR and the SII were
significantly higher in patients who succumbed compared with those
in the survivors group. ROC curve analysis confirmed that both
markers had prognostic value for mortality, with NLR showing an AUC
of 0.785 and SII exhibiting an AUC of 0.728. The identified cut-off
values (>2.75 for NLR and >1,428,898 for SII), as determined
by ROC analysis, were statistically significant predictors of
mortality. Specifically, the NLR cut-off demonstrated a sensitivity
of 73.3% and a specificity of 80.8%, indicating balanced diagnostic
accuracy. By contrast, the SII cut-off yielded a lower sensitivity
(46.7%) but a notably high specificity (96.2%), suggesting that
patients exceeding this threshold could be identified as truly
high-risk with greater confidence. These findings were further
supported by univariate Cox regression analysis, in which both
markers showed significant associations with mortality. Notably,
low serum sodium levels also demonstrated the strongest statistical
association with mortality among all variables included in the
analysis. This observation aligns with previous reports from both
adult and pediatric populations, where hyponatremia has been
independently associated with adverse outcomes in heart failure
(15,16).
In addition to systemic inflammatory markers and
serum sodium, several other parameters were also found to be
significantly associated with mortality in the present study. These
included low serum magnesium levels, reduced mitral E wave velocity
and a positive family history of DCM. These findings are consistent
with previous studies demonstrating that hypomagnesemia may
increase cardiovascular mortality risk, whereas reduced mitral E
velocity may indicate diastolic dysfunction and worse outcomes, and
that a positive family history is associated with disease
progression and poorer prognosis in pediatric DCM (17-19).
Notably, although mitral E wave velocity has been
previously studied as a diastolic function parameter, its
prognostic role in pediatric DCM remains underexplored.
Bressieux-Degueldre et al (20) did not observe a significant
association between mitral E velocity and mortality in their
pediatric cohort. By contrast, the present study identified a
statistically significant difference in mitral E velocity, with
patients in the deceased group showing lower E wave velocities
compared with survivors (0.78±0.18 vs. 0.94±0.22 m/sec; P=0.031).
This reduction reflects impaired left ventricular relaxation and
diastolic filling in patients with more advanced myocardial
dysfunction, suggesting that this parameter may warrant further
evaluation as a potential prognostic marker in this population.
It is also worth noting that although aortic root
diameter was found to be statistically larger in patients who
succumbed, all measurements were within the normal Z-score range
for age. This difference likely reflects the older mean age of
deceased patients, instead of an independent prognostic
relationship. Since absolute diameters were used in the analysis
without adjustment for age or body surface area, this finding most
likely reflects age-related anatomical variation rather than
clinically significant aortic dilatation. Therefore, this finding
was not interpreted as clinically meaningful.
To the best of our knowledge, the present study was
the first to evaluate both NLR and SII as prognostic indicators in
pediatric DCM. By integrating two distinct inflammatory markers
derived from complete blood count parameters, the present findings
highlight the potential value of systemic inflammation-based
indices in early risk stratification. Given that NLR and SII are
inexpensive, readily accessible and routinely obtained in clinical
practice, their incorporation into the initial assessment of
children with DCM may enhance prognostic evaluation and inform
closer monitoring strategies. These markers may serve as useful
adjuncts to echocardiographic and clinical data, particularly in
resource-limited settings where advanced biomarkers are not readily
available.
Whilst the present study provides important
preliminary insights, several limitations should be acknowledged.
The present study was a retrospective, single-center analysis with
a relatively small cohort, which may limit the generalizability of
the findings. Systemic inflammatory markers were evaluated only at
the time of diagnosis. Therefore, longitudinal trends and the
potential impact of concurrent infections or other inflammatory
conditions could not be assessed. Additionally, due to the limited
number of events, multivariate analysis could not be performed,
restricting the evaluation of independent associations. The
etiological heterogeneity of the cohort, encompassing idiopathic,
post-infectious, genetic and chemotherapy-related cases, may also
have influenced inflammatory responses and outcomes. Despite these
limitations, the present study introduces novel findings and
highlights the need for prospective multicenter research to further
clarify the prognostic role of inflammatory markers in pediatric
DCM. The findings of the present study may also help inform
clinical decision-making by supporting early risk stratification
and identifying children who may benefit from closer monitoring,
timely intensification of therapy or earlier referral to transplant
centers.
In conclusion, the present study demonstrates that
elevated NLR and SII at the time of diagnosis are significantly
associated with increased mortality in pediatric patients with DCM.
These easily accessible and inexpensive inflammatory markers may
serve as complementary tools to traditional clinical assessments,
particularly in settings where advanced laboratory testing is not
readily available. Although further prospective and multicenter
studies are warranted to validate these findings, the present
results highlight the prognostic relevance of systemic inflammation
in pediatric DCM and suggest a potential role for NLR and SII in
early risk stratification. However, since multivariate analysis
could not be performed due to the limited number of events, these
findings should be interpreted as associations rather than evidence
of independent prognostic value. Further prospective multicenter
studies are needed to confirm their utility before they can be
incorporated into routine clinical decision-making.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SA contributed to study conception, data collection,
statistical analysis, interpretation of results and manuscript
drafting. FA contributed to study design, interpretation of data
and critical manuscript revision. Both authors read and approved
the final manuscript. SA and FA confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee of Marmara University Faculty of Medicine (Istanbul,
Turkey; approval no. 09.2022.463). The requirement for informed
consent was waived due to the retrospective design of the study, in
accordance with the approval granted by the ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mallavarapu A and Taksande A: Dilated
cardiomyopathy in children: Early detection and treatment. Cureus.
14(e31111)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lipshultz SE, Cochran TR, Briston DA,
Brown SR, Sambatakos PJ, Miller TL, Carrillo AA, Corcia L, Sanchez
JE, Diamond MB, et al: Pediatric cardiomyopathies: Causes,
epidemiology, clinical course, preventive strategies and therapies.
Future Cardiol. 9:817–848. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Amorim S, Campelo M, Moura B, Martins E,
Rodrigues J, Barroso I, Faria M, Guimarães T, Macedo F,
Silva-Cardoso J and Maciel MJ: The role of biomarkers in dilated
cardiomyopathy: Assessment of clinical severity and reverse
remodeling. Rev Port Cardiol. 36:709–716. 2017.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
4
|
da Rocha Araújo FD, da Lisboa Silva RMF,
Oliveira CAL and Meira ZMA: Neutrophil-to-lymphocyte ratio used as
prognostic factor marker for dilated cardiomyopathy in childhood
and adolescence. Ann Pediatr Cardiol. 12:18–24. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ahmed M, El Amrousy D, Hodeib H and Elnemr
S: Neutrophil-to-lymphocyte ratio as a predictive and prognostic
marker in children with dilated cardiomyopathy. Cardiol Young.
33:2493–2497. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Malinow I, Fong DC, Miyamoto M, Badran S
and Hong CC: Pediatric dilated cardiomyopathy: A review of current
clinical approaches and pathogenesis. Front Pediatr.
12(1404942)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Murphy SP, Kakkar R, McCarthy CP and
Januzzi JL Jr: Inflammation in heart failure: JACC state-of-the-art
review. J Am Coll Cardiol. 75:1324–1340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boulet J, Sridhar VS, Bouabdallaoui N,
Tardif JC and White M: Inflammation in heart failure:
Pathophysiology and therapeutic strategies. Inflamm Res.
73:709–723. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fu X, Li X, Gao X, Zuo Q, Wang L, Peng H
and Wu J: The association between systemic inflammation markers and
the risk of incident dilated cardiomyopathy: A prospective study of
351,148 participants. Biomarkers. 30:192–199. 2025.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ye Z, Hu T, Wang J, Xiao R, Liao X, Liu M
and Sun Z: Systemic immune-inflammation index as a potential
biomarker of cardiovascular diseases: A systematic review and
meta-analysis. Front Cardiovasc Med. 9(933913)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Uthamalingam S, Patvardhan EA, Subramanian
S, Ahmed W, Martin W, Daley M and Capodilupo R: Utility of the
neutrophil to lymphocyte ratio in predicting long-term outcomes in
acute decompensated heart failure. Am J Cardiol. 107:433–438.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cho JH, Cho HJ, Lee HY, Ki YJ, Jeon ES,
Hwang KK, Chae SC, Baek SH, Kang SM, Choi DJ, et al:
Neutrophil-lymphocyte ratio in patients with acute heart failure
predicts in-hospital and long-term mortality. J Clin Med.
9(557)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qiu J, Huang X, Kuang M, Wang C, Yu C, He
S, Xie G, Wu Z, Sheng G and Zou Y: Evaluating the prognostic value
of systemic immune-inflammatory index in patients with acute
decompensated heart failure. ESC Heart Fail. 11:3133–3145.
2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang Y, Zeng X, Feng Y, Chen Q, Liu Z, Luo
H, Zha L and Yu Z: Association of systemic immune-inflammation
index with short-term mortality of congestive heart failure: A
retrospective cohort study. Front Cardiovasc Med.
8(753133)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Price JF, Kantor PF, Shaddy RE, Rossano
JW, Goldberg JF, Hagan J, Humlicek TJ, Cabrera AG, Jeewa A,
Denfield SW, et al: Incidence, severity, and association with
adverse outcome of hyponatremia in children hospitalized with heart
failure. Am J Cardiol. 118:1006–1010. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoo BS, Park JJ, Choi DJ, Kang SM, Hwang
JJ, Lin SJ, Wen MS, Zhang J and Ge J: COAST investigators.
Prognostic value of hyponatremia in heart failure patients: An
analysis of the clinical characteristics and outcomes in the
relation with serum sodium level in asian patients hospitalized for
heart failure (COAST) study. Korean J Intern Med. 30:460–470.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Adamopoulos C, Pitt B, Sui X, Love TE,
Zannad F and Ahmed A: Low serum magnesium and cardiovascular
mortality in chronic heart failure: A propensity-matched study. Int
J Cardiol. 136:270–277. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Playford D, Strange G, Celermajer DS,
Evans G, Scalia GM, Stewart S and Prior D: NEDA Investigators.
Diastolic dysfunction and mortality in 436 360 men and women: The
national echo database Australia (NEDA). Eur Heart J Cardiovasc
Imaging. 22:505–515. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khan RS, Pahl E, Dellefave-Castillo L,
Rychlik K, Ing A, Yap KL, Brew C, Johnston JR, McNally EM and
Webster G: Genotype and cardiac outcomes in pediatric dilated
cardiomyopathy. J Am Heart Assoc. 11(e022854)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bressieux-Degueldre S, Fenton M, Dominguez
T and Burch M: Exploring the possible impact of echocardiographic
diastolic function parameters on outcome in paediatric dilated
cardiomyopathy. Children (Basel). 9(1500)2022.PubMed/NCBI View Article : Google Scholar
|