Introduction
Pearl powder is a Traditional Chinese Medicine with
a variety of active substrates, which can be applied for numerous
processes, such as wound healing and bone repair, rendering it a
promising biological agent. The nacre organic matrix, an active
component of pearl powder, mainly regulates the mineralization of
bivalve mollusks (1). The nacre is
an organic/inorganic complex that is not too dissimilar to human
bone tissues (2). In addition, the
organic matrix of pearl powder is the processed product of pearl
powder (3). The solvent capable of
dissolving the organic components of the medium is typically
combined with the pearl powder components, which are obtained by
centrifugation, freezing and other methods such as supercritical
CO2 extraction and enzyme-acid extraction. The insoluble
matrix components are then removed and the soluble organic matrix
is retained (4). Pearl powder has
been applied in traditional Chinese medicine and cosmetics for
>1,000 years (5). Chiu et
al (6) previously found that
pearl powder extract can prolong the life of Caenorhabditis
elegans. Additionally, in the same study, 20 subjects (aged
38-50 years) were administered pearl powder capsules and placebo.
After 10 weeks of treatment, antioxidant-related indices were found
to be increased in plasma samples, suggesting that pearl powder has
antioxidant activity (6). This
also suggests the potential of pearl powder for the treatment of
age-related degenerative diseases. Chen et al (7) conducted in vitro and in
vivo experiments with pearl powder with different particle
sizes, and found that the mixed preparation of pearl
powder/glycerol/normal saline could promote the proliferation and
migration of fibroblasts, accelerate wound closure and promote skin
angiogenesis in full-thickness skin excision wounds in
Sprague-Dawley rats and shaped dermal wounds in New Zealand
rabbits. In another study, Zhang et al (8) assessed the effects of pearl powder
and its extract on the motor ability and anticonvulsant ability of
mice, where the results showed that rearing was inhibited in
ordinary mice, whilst the convulsant latency of pentylenetetrazol
model mice was prolonged. These results provide the basis for the
development of sedative drugs based on pearl powder.
The bone tissue is comprised of a diverse range of
cell components, namely preosteoblasts, osteoblasts and
osteoclasts, which jointly modulate the growth and absorption of
bone tissues (9). Osteoblasts,
derived from bone marrow mesenchymal stem cells (MSCs), serve a
function in osteogenesis and serve a critical role during bone
remodeling. Specifically, osteoblasts promote bone tissue formation
by secreting organic matrix and regulating calcification (10). Certain cytokines, such as TNF-α,
have an important role in regulating bone metabolism (11). This cytokine, produced mainly by
activated macrophages, T lymphocytes, activated macrophages,
monocytes, CD4+ T cells, B cells, neutrophils and mast
cells, induces apoptosis of leukemia cell lines in vitro and
stimulates osteoclast activation, and TNFα promotes osteoblast
differentiation and bone formation by polarizing macrophages to
secrete insulin like 6 and Jagged1, while anti-TNF treatment may
indirectly affect osteoblast activity by inhibiting the expression
of these factors, which in turn promotes bone degradation (12,13).
Other factors, such as transcriptional coactivator Yes1-associated
transcriptional regulator (YAP), also serve important roles in
osteogenesis. YAP acts as a key molecular bridge connecting the
mechanical microenvironment (substrate size/spatial features) to
osteogenic differentiation, directly mediating the sequential
regulation of MSC and osteoblast differentiation through focal
adhesion-dependent cytoskeletal tension (regulated by F-actin and
phospho-myosin light chain 2) that drives its nuclear localization.
This mechanoregulatory role has further been confirmed in
cytoskeleton disruption assays (14).
As an active component of nacre pearls, nacre powder
organic matrix has been documented to regulate the mineralization
of bivalve mollusks such as Pinctada spp. and Crassostrea
gigas, where its effects on osteoblasts has been extensively
studied (1,15).
Pearl powder is similar to nacre powder. Pearl
powder and nacre powder differ in their origins: Natural pearls
form when foreign irritants (such as sand or parasites)
accidentally enter oysters and become progressively mineralized
under nacreous encapsulation (16). By contrast, the nacreous layer
constitutes a laminated composite material secreted by mantle
epithelial cells, characterized by alternating deposition of
calcium carbonate crystals and chitinous proteins (17). Both materials share similar
chemical compositions, containing 95% calcium carbonate and 5%
organic components, including diverse amino acids, trace elements,
vitamins and bioactive peptides (18,19).
Notably, their core bioactive constituents derive from the organic
matrix, which is a complex system comprising proteins,
polypeptides, glycoproteins, chitin, lipids and pigments (18,19).
A proteomic study has revealed compositional differences between
nacre from Pinctada maxima (Kamingi shell) and that of
cultured pearls in protein profiles (20). This structure confers
osteoinductive properties, enabling stimulation of osteoblast
differentiation via the bone morphogenetic protein 2/Smad signaling
pathway and biomimetic mineralization, making it suitable for bone
regeneration applications (21).
However, to the best of our knowledge, research on the effect of
the organic matrix extracted from pearl powder on osteoblasts
remains scarce. In the present study, hFOB1.19 cells were cultured
with the water-soluble matrix (WSM) derived from nanometer pearl
powder (NPP) to analyze its possible effects on the osteogenic
differentiation of these osteoblasts. It is hoped that the present
study will encourage the application of pearl powder for promoting
bone formation in the clinic. In particular, pearl powder WSM can
be applied in artificial bone materials or for the treatment of
osteoporosis.
Materials and methods
NPP WSM preparation
Micron-grade pearl powder (Hainan Jingrun Pearl Co.,
Ltd.) was first ground into nano-grade pearl powder. Briefly, a
CNB-T1L nanorstick needle mill (Dongguan Kangbo Machinery Co. Ltd.)
was used, sterile distilled water was used for cleaning to
eliminate potential contaminants, anhydrous ethanol was used as the
medium, the rotation speed of the main engine was set at 540 x g,
the feed pressure was 0.35 kPa, the mass/volume ratio was 1:15
(g/ml) to add pearl powder, and mechanical grinding was performed
for 1 h at room temperature (25˚C). A uniform nanosuspension was
obtained. The slurry was oven-dried at 60˚C for 24 h, manually
pulverized using a ceramic mortar in a clean workbench, sieved
through a 120 mesh, and stored as uniform nanopearl powder in
sterile containers. After freeze drying and irradiation
sterilization, the pearl powder was mixed with Milli-Q ultra-pure
water (MilliporeSigma) at a ratio of 1:2 (g/ml), before the
supernatant was collected by centrifugation (500 x g; 20 h) at room
temperature (25˚C). The sample was later filtered with a 0.22-µm
strainer and freeze-dried to obtain the WSM powder, which was then
dissolved in PBS. The stock WSM protein concentration was
determined using the BCA protein quantitative kit and the stock
solution was stored at 4˚C. The protein concentration of WSM
calculated by the BCA protein assay method was 2.689 mg/ml. It was
calculated that 134.47 mg protein could be extracted per 100 g of
nano-pearl powder. WSM was then diluted to 10, 20 and 40 µg/ml
before the subsequent experiments.
hFOB1.19 cell culture
hFOB1.19 cells (cat. no. CL-0353; Procell Life
Science & Technology Co., Ltd.) were cultivated in DMEM/F12
(cat. no. C11330500BT; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% FBS (cat. no. 10099141C; Gibco; Thermo Fisher
Scientific, Inc.) and 0.3 mg/ml G418 [cat. no. 1811031;
Geneticin™ Selective Antibiotic (G418 Sulfate); Gibco;
Thermo Fisher Scientific, Inc.] in a 5% CO2 atmosphere
at 34˚C. The medium was changed every 2 days. After reaching 80-90%
confluency, trypsin (Gibco; Thermo Fisher Scientific, Inc.) was
added to digest the cells and 1:2 passaging was performed. In the
experimental groups, solutions of 10, 20 and 40 µg/ml WSM were
added to the cells, whilst an equal amount of complete medium was
added to the control group. Co-cultures were incubated for 1, 4, 7
and 14 days as required. The cultures were incubated at 34˚C in a
5% CO2 atmosphere.
Cell differentiation detection based
on alkaline phosphatase (ALP) activity
Cells in passage 3 were selected and inoculated into
a 24-well plate at 2x105 cells/well. The cells were
grouped as aforementioned and three replicate wells were set up for
every group. The culture medium was discarded when the cells
attached to the well, before WSM was added. After intervention for
1, 4 and 7 days at 34˚C with 5% CO2, the medium was
discarded, and subsequent procedures were conducted according to
the ALP kit manufacturer's protocol (cat. no. A059-1-1; Nanjing
Jiancheng Bioengineering Institute). The absorbance was measured
using an absorbance reader (800TS; BioTek; Agilent Technologies,
Inc.).
Cell mineralization detection by
alizarin red staining
Cells in passage 3 were selected and inoculated into
6-well plates at 5x106 cells/well. Cell grouping and
culture were performed following the same method as those performed
for measuring ALP activity. Medium was removed after culturing for
7 and 14 days, before the cells were washed three times with PBS
for 5 min each and fixed with 4% paraformaldehyde for 30 min at
25˚C. After washing with PBS three times again, 4 ml 2% alizarin
red solution (cat. no. DF0563; Yingxin Biotechnology, Co., Ltd.)
was added to every well for 15 min at ambient temperature (25˚C)
for staining. Following further washing with PBS three times, the
calcified nodules formed were monitored using an LED light
microscope (Nikon TS100; Nikon Corporation), before images were
collected and the relative absorbance value (595 nm) of the
mineralized area (ImageJ Software 1.54a; National Institutes of
Health) was determined for statistical analysis.
Relevant osteogenic gene expression
levels measured by reverse transcription-quantitative PCR
(RT-qPCR)
Cells in passage 3 were selected and inoculated into
a 6-well plate at 5x106 cells/well. Cell grouping and
culture were conducted with the same method as those performed for
measuring ALP activity. The medium was removed following a 7-day
culture and TRNzol Universal Reagent (cat. no. DP424; Tiangen
Biotech Co., Ltd.) was utilized to extract the total cellular RNA.
The RNA concentration and purity were determined by an ultraviolet
spectrophotometer (BioTek; Agilent Technologies, Inc.).
Subsequently, 0.85 µg RNA was reverse transcribed using a
PrimeScript™ RT Master Mix (Perfect Real Time; cat. no.
RR036A; Takara Bio, Inc.) to synthesize cDNA at 37˚C for 15 min and
85˚C for 5 sec. The cDNA was used as the template and amplified
according to the instructions of the Hieff® qPCR SYBR
Green Master Mix (cat. no. 11201ES08; Shanghai Yeasen Biotechnology
Co., Ltd.). The relative mRNA expression levels of type I collagen
(Col-I), osteocalcin (OCN), osteopontin (OPN) and Runt-associated
transcription factor 2 (Runx-2) were detected by 2-ΔΔCq
method, with GAPDH as the reference gene (22). Table
I displays the primers. The qPCR conditions included
pre-denaturation at 95˚C for 5 min, followed by 40 cycles of
denaturation at 95˚C for 10 sec and annealing at 60˚C for 30
sec.
 | Table IPrimers designed for reverse
transcription-quantitative PCR. |
Table I
Primers designed for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| GAPDH |
CACCCACTCCTCCACCTTTGAC |
| |
GTCCACCACCCTGTTGCTGTAG |
| Collagen-I |
CCTGCCTGGTGAGAGAGGT |
| |
AGTAGCACCATCATTTCCACGA |
| Osteocalcin |
AGCAAAGGTGCAGCCTTTG |
| |
GCGCCTGGGTCTCTTCACT |
| Osteopontin |
GACCTGAACGCGCCTTCTGAT |
| |
ATCTGGACTGCTTGTGGCTGTG |
| Runt-associated
transcription factor 2 |
CCGCTTCTCCAACCCACGAAT |
| |
TGGCAGGTAGGTGTGGTAGTGA |
Relevant osteogenic protein expression
levels detected by western blotting
Western blotting was performed according to Jurisic
et al (23). Cells in
passage 3 were selected and inoculated into a 6-well plate at
5x106 cells/well. Cell grouping and culture were
conducted with the same method as those performed for measuring ALP
activity. The medium was removed following a 7-day culture, before
cellular proteins were extracted from every group using RIPA lysis
buffer (cat. no. BL504A; Biosharp Life Sciences). The protein
concentration was quantified using the BCA method. Equal amounts of
protein (50 µg per lane) were resolved by 12% SDS-PAGE and
electrophoretically transferred onto 0.22-µm PVDF membranes. The
proteins were transferred onto PVDF membranes, which were
subsequently blocked with 5% skimmed milk in TBS with 20% Tween 20
(cat. no. ST1726; Beyotime Institute of Biotechnology) for 1 h at
room temperature (25˚C). The membranes were then incubated with the
primary antibodies at 4˚C overnight. The primary antibodies (all
from Affinity Biosciences) were as follows: Rabbit polyclonal Col-I
(cat. no. AF7001; 1:1,000), rabbit polyclonal OCN (cat. no.
DF12303; 1:1,000), rabbit polyclonal OPN (cat. no. AF0227;
1:1,000); rabbit polyclonal Runx-2 (cat. no. AF5186; 1:1,000) and
rabbit polyclonal GAPDH (cat. no. AF7021; 1:10,000). The membrane
was then incubated with goat anti-rabbit IgG (H + L) HRP secondary
antibody (cat. no. S0001; 1:10,000; Affinity Biosciences, Ltd.) at
room temperature for 1 h. The Affinity™ ECL kit
(femtogram) (cat. no. KF8003; Affinity Biosciences, Ltd.) was used
for band exposure development. Finally, ImageJ software
(version1.53e; National Institutes of Health) was used for the
semi-quantitative analysis of the protein bands, before the ratio
between the target protein and the internal reference protein
(GAPDH) was calculated for statistical analysis.
Statistical analysis
Each assay was conducted three times. The
experimental data are presented as the mean ± standard deviation.
Statistical analysis and mapping were performed using GraphPad
Prism 8.2.1 software (Dotmatics). Data among several groups were
compared by one-way analysis of variance. The Tukey post hoc test
was used for analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of different NPP WSM
concentrations on the early osteogenic differentiation of
cells
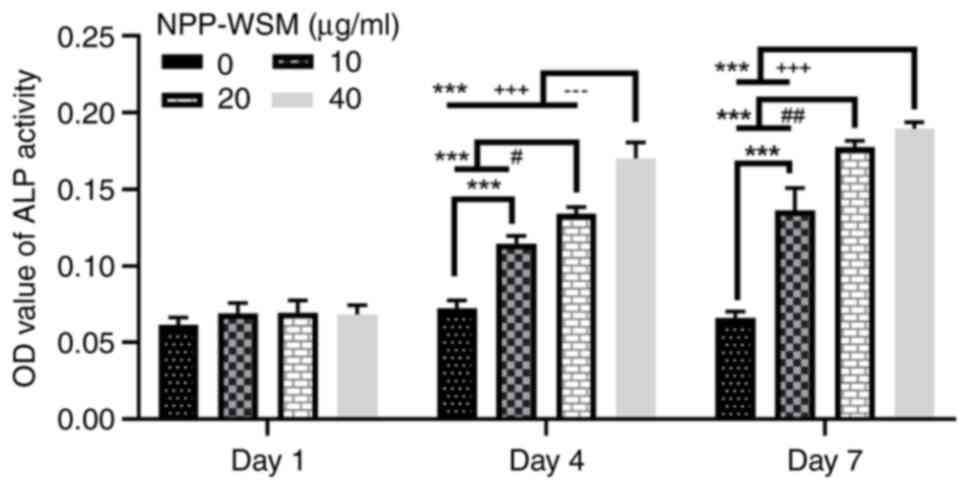
The ALP activity was not found to be significantly
different among all experimental groups after 1 day of treatment
with WSM (Fig. 1). Following WSM
treatment for 4 and 7 days, the ALP activity was enhanced as the
WSM concentration increased, as significant differences were
detected between the groups with different WSM concentrations and
the control group. On day 4, significant differences were observed
between all treatment groups and the control group, demonstrating a
concentration-dependent increase in ALP activity. By day 4, there
were statistically significant differences between each treatment
group and the control group, and between all treatment groups. By
day 7, statistically significant variations were noted not only
between each treatment group and the control group, but also
between the 10 µg/ml group and both the 20 and 40 µg/ml groups.
However, no significant difference was detected between the 20 and
40 µg/ml groups on day 7 (Fig. 1).
Therefore, these data suggest that WSM promoted the secretion and
activity of ALP in hFOB1.19 cells.
Effects of different NPP WSM
concentrations on cell mineralization
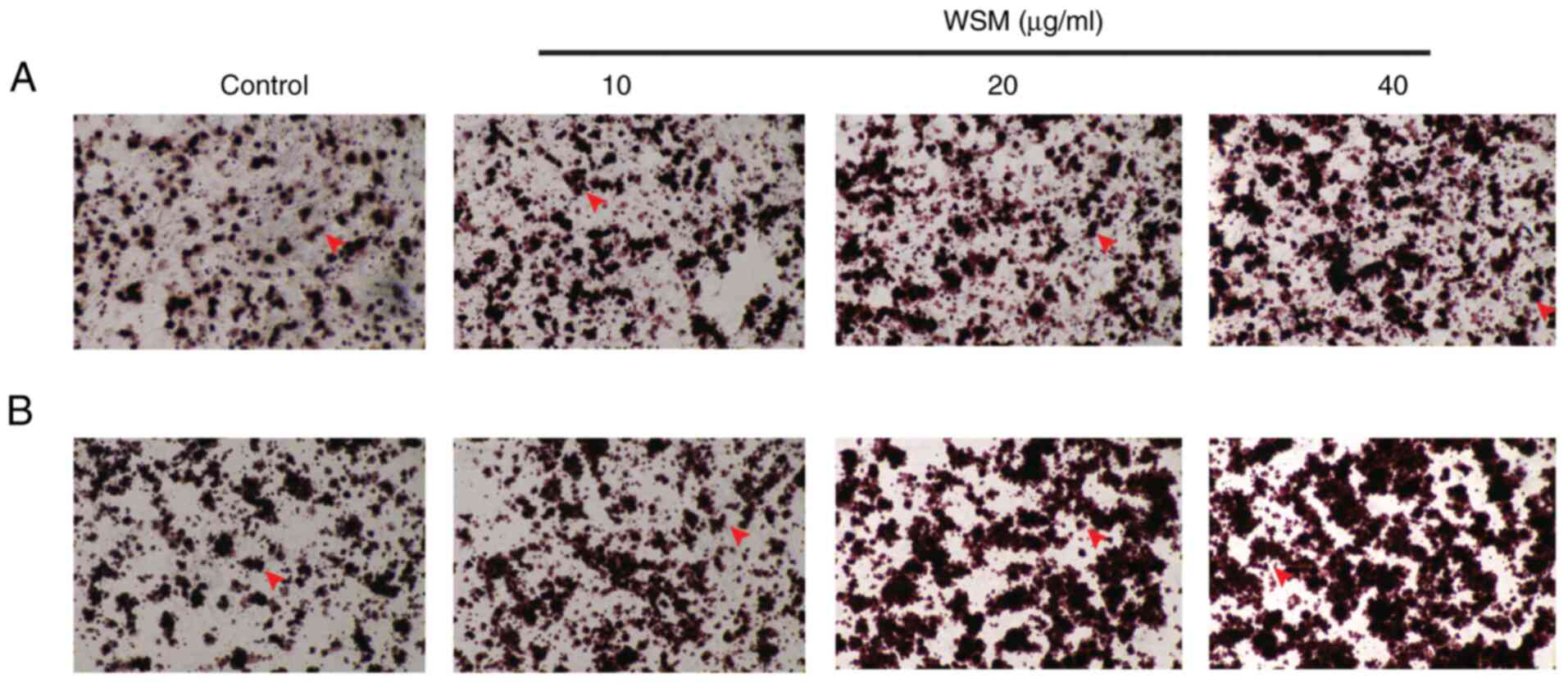
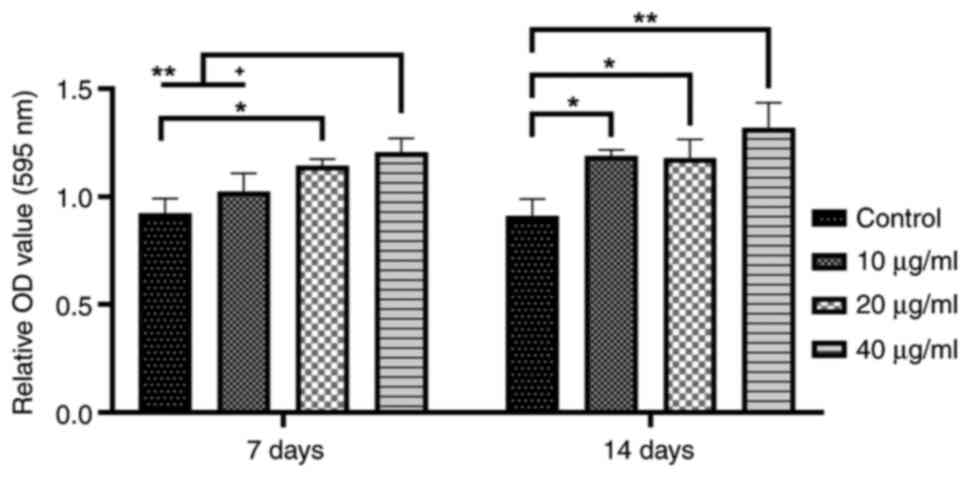
After 7 days of culture, mineralization in the 20
and 40 µg/ml WSM groups was found to be significantly increased
compared with that in the blank control group. Following a 14-day
culture, compared with that in the blank control group, the area of
mineralized nodules in the 10-40 µg/ml WSM groups was significantly
elevated, particularly in the 40 µg/ml WSM group. However, there
was no difference among the WSM treatment groups on day 14
(Figs. 2 and 3).
Effects of different NPP WSM
concentrations on osteogenic gene expression
As shown in Fig. 4,
after 7 days of culture, the expression of target genes (Col-I,
OCN, OPN and Runx-2) in the 10-40 µg/ml WSM groups was found to be
significantly increased compared with that in the blank control
group. Additionally, the target gene mRNA levels in the 40 µg/ml
WSM group were significantly elevated compared with those in the 10
and 20 µg/ml WSM groups.
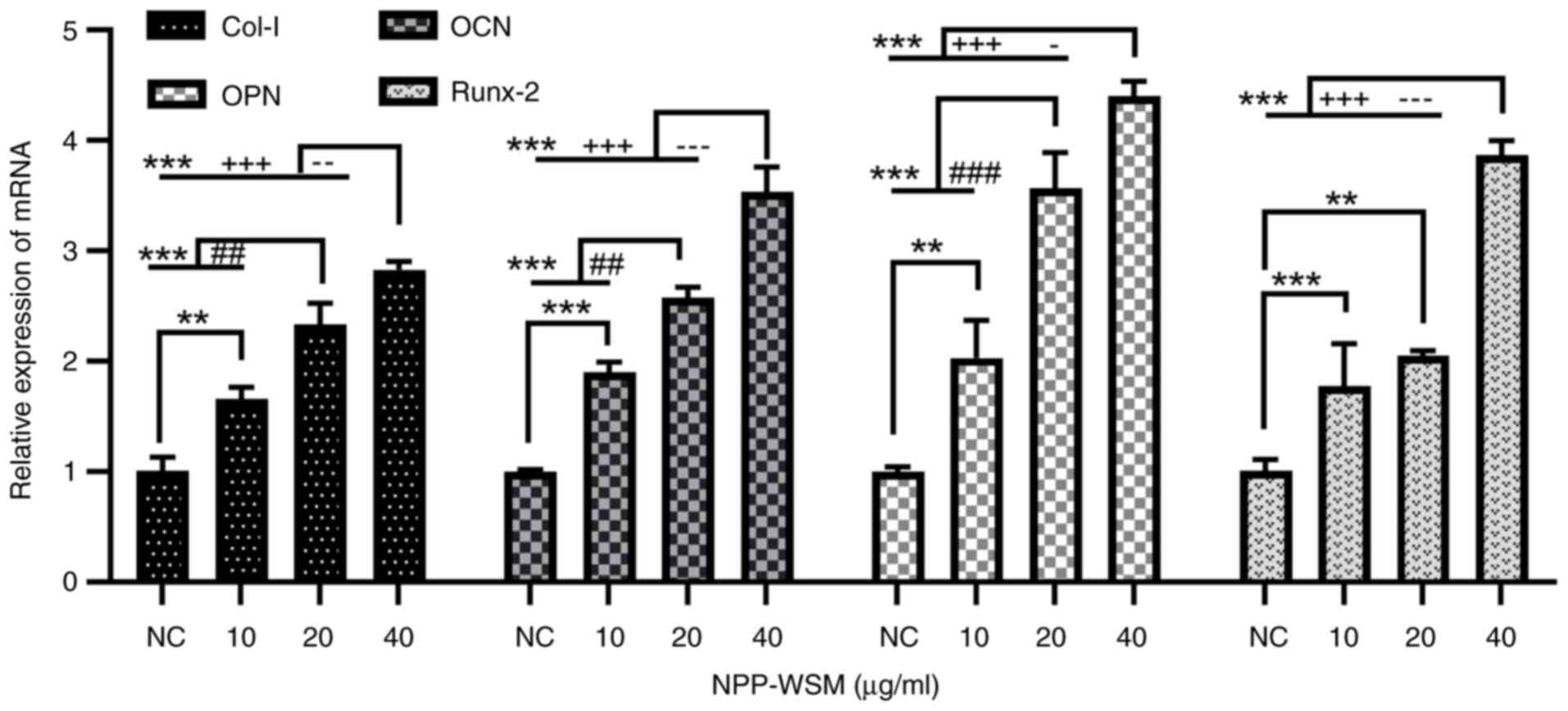 | Figure 4mRNA expression of osteogenic genes
in each WSM treatment group (0, 10, 20 and 40 µg/ml) measured
through reverse transcription-quantitative PCR. Data are presented
as the means ± standard deviation, n=4; **P<0.01 and
***P<0.001 (0 µg/ml vs. all other groups);
##P<0.01 and ###P<0.001 (10 vs. 20
µg/ml); -P<0.05, --P<0.01 and
---P<0.001 (20 vs. 40 µg/ml);
+++P<0.001 (10 vs. 40 µg/ml). WSM, water-soluble
matrix; Col-I, type I collagen; OCN, osteocalcin; OPN, osteopontin;
Runx-2, Runt-associated transcription factor 2; NC, negative
control. |
Effects of different NPP WSM
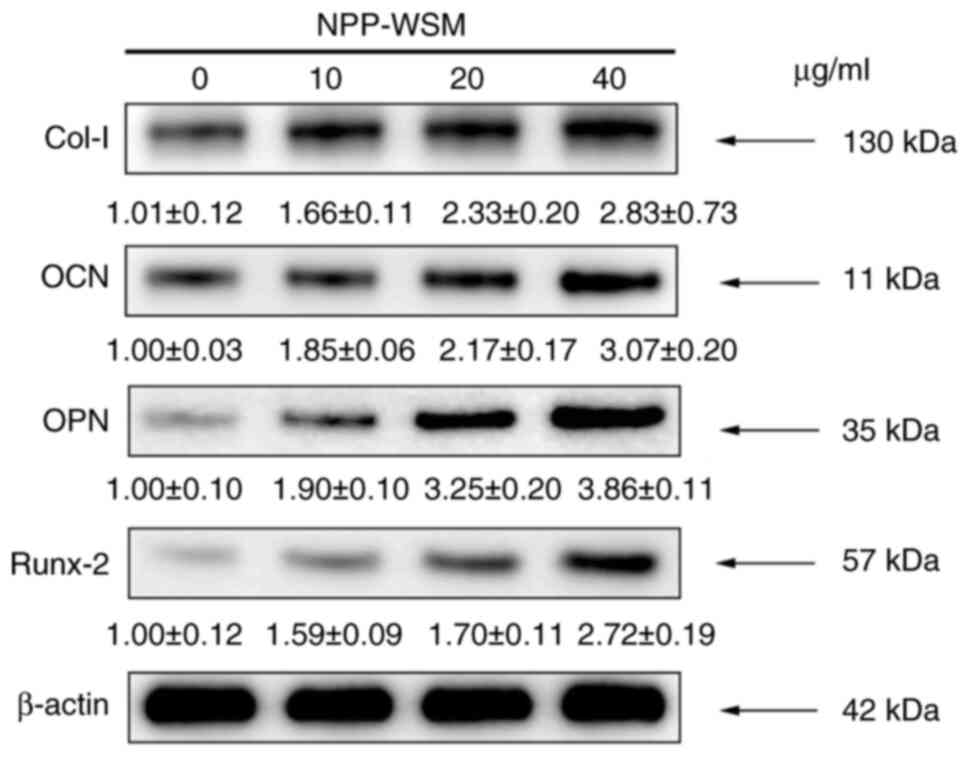
concentrations on osteogenic protein expression
As shown in Figs. 5
and 6, target protein (Col-I, OCN,
OPN and Runx-2) expression in the 10, 20 and 40 µg/ml WSM groups
was found to be increased following 7 days of culture compared with
that in blank control group. However, the protein expression level
in the 40 µg/ml group was not significantly different compared with
that in the 20 µg/ml group.
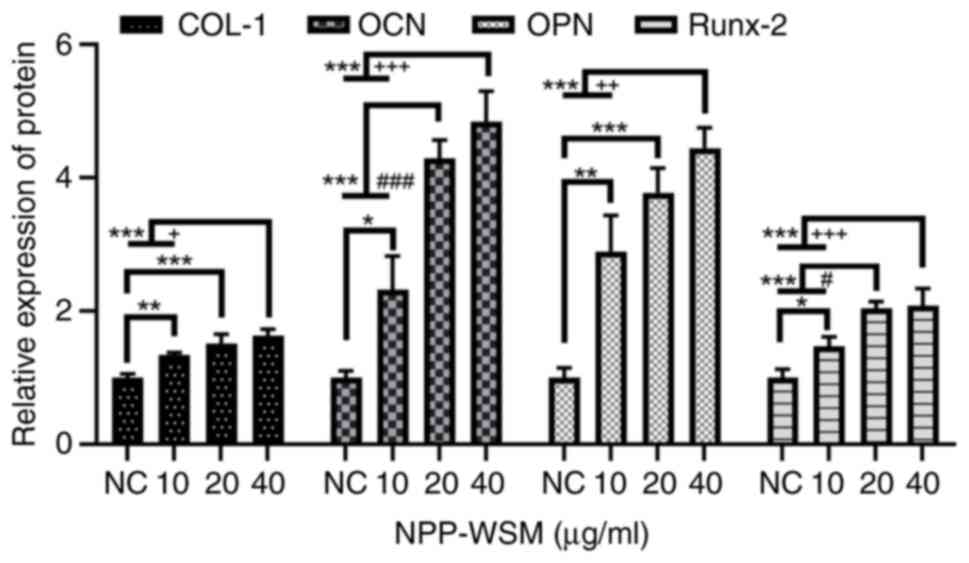 | Figure 6Osteogenic protein expression
detected in each group following WSM treatment (0, 10, 20 and 40
µg/ml) by western blotting. There were significant differences in
the expression of bone-related proteins following WSM treatment.
Data are presented as the means ± standard deviation, n=4.
*P<0.05, **P<0.01 and
***P<0.001 (0 µg/ml vs. all other groups);
#P<0.05 and ###P<0.001 (10 vs. 20
µg/ml); +P<0.05, ++P<0.01 and
+++P<0.001 (10 vs. 40 µg/ml). Col-I, type I collagen;
OCN, osteocalcin; OPN, osteopontin; Runx-2, Runt-associated
transcription factor 2; NC, negative control; WSM, water-soluble
matrix. |
Discussion
Remodeling of the bone tissue is regulated by a
variety of cytokines, where the key to maintaining the integrity of
bone tissue is to regulate the homeostasis between osteoblasts and
osteoclasts (24). Pro-osteoclast
cytokines include TNF-α and the IL family, whereas anti-osteoclast
cytokines mainly include IFN-α, IFN-β and IFN-γ (25). These aforementioned factors serve a
key role in osteoclast formation through the interaction between
the bone and the immune system (26). Lan et al (27) previously studied the regulatory
effect of hydrolyzed seawater pearl tablet (HSPT) on Th1/Th2
imbalance in immunosuppressed mice induced by cyclosporin A. The
results showed that the protein and mRNA expression levels of Th1
cytokines IL-2 and IFN-γ were increased in mice treated with HSPT.
HSPT may ameliorate Th1/Th2 imbalance under immunosuppressive
conditions by upregulating the expression of Th1 cytokines, IL-2
and IFN-γ. Future studies should further explore whether these
immunomodulatory effects indirectly influence bone metabolism or
the balance between osteoblast and osteoclast activity.
As a natural polymer, pearl powder is currently a
topic of intense research in the field of bone tissue engineering
(28-30).
The composition of pearl powder is similar to that of nacre powder
(31). Previous studies have shown
that pearl powder and artificial bone materials containing pearl
powder have osteogenic effects both in in vivo and in
vitro (32,33). However, the majority of recent
studies have focused on the osteogenic application of nacre powder
matrix (15,34), whilst to the best of our knowledge,
studies on the effect of pearl powder matrix on osteoblasts
remained scarce. The nacre organic matrix is a key substance in
regulating the mineralization of bivalve mollusks (1). The invertebrate mineralization
process and mammalian bone calcification have similar signaling
pathways where the signaling molecules have similar protein
domains. Previous studies have shown that the NPP organic matrix
can promote the differentiation of osteoblasts (35,36).
Pearl powder has also been shown to promote collagen production and
serve a role in wound healing following skin injuries (7,37).
In particular, pearl powder and composite materials constructed
with pearl powder have been documented to promote osteogenesis in a
variety of cell lines, such as mouse bone marrow MSCs (38) and rat bone MSCs (39).
Nanomaterials have properties that materials of
general size (>1 µm) do not have. Nano-treatment of pearl powder
or pearl layer powder not only retains the effective components of
pearl powder, but also results in an improved osteogenic effects
(40). A previous review examined
the effects of four inorganic or metallic nanoparticles widely used
as biomaterials, namely hydroxyapatite, silica, silver and calcium
carbonate, on the osteogenic and lipogenic differentiation of MSCs
(41). The size of the materials
can affect cell function, revealing that these materials have rich
application prospects on the nanoscale (41). There have been a number of studies
on the use of NPP and its scaffold materials. Chen et al
(7) previously found that NPP can
promote the proliferation and migration of skin cells, accelerate
wound healing and improve the biomechanical strength of healed skin
in both in vivo and in vitro experiments. In
addition, Yang et al (42)
constructed a NPP/poly (lactide-co-glycolide) biocomposite
scaffold, which promoted the uniform seeding, adhesion and
proliferation of MC3T3-E1 cells, which is a pre-osteoblastic cell
line derived from mouse calvaria, possessing osteogenic
differentiation potential. Therefore, NPP is relatively
reproducible and provides structural features that can enhance the
formation of bone tissues. A previous study also demonstrated that
scaffolds constructed with NPP had osteogenic effects (30).
The pearl powder matrix can be obtained according to
the different media, where the finished products are termed ‘WSM’,
‘water-insoluble matrix’ and ‘acid-soluble protein’ (31). WSM has a high water solubility and
can be widely used in cell assays and skin wound healing
experiments. The active ingredients of WSM are rapidly absorbed by
cells and skin, making it the pearl powder-related product that is
closest to clinical application (7,32,37,38,43).
Liu et al (44) previously
obtained the WSM of pearl powder using a CO2
supercritical extraction system, where the subsequent product was
found to promote the migration and proliferation of fibroblasts. In
rats with skin defects, 5 and 10 mg/ml WSM had a marked effect on
wound healing (the healing area of the wound was higher than that
in the control group), whilst 15 mg/ml had a poor effect (the wound
healing area was not as good as in other groups). It was speculated
that a high WSM concentration may have toxic effects on the skin,
since the Masson's staining of tissues has also shown abundant
collagen hyperplasia (38). In
another study, Dai et al (45) extracted the WSM from pearl powder
and further fractionated it into MR14 (>14 kDa), MR3-14 (3-14
kDa) and MR3 (<3 kDa) based on molecular weight. The authors
found that WSM and MR14 promoted proliferation, while all fractions
enhanced collagen deposition in primary oral fibroblasts from
BALB/c mice.
In a previous study, NPP WSM was found to enhance
autophagy in MC3T3-E1 cell lines to promote their differentiation
through the MEK/ERK signaling pathway (46). In the present study, NPP WSM was
applied to human cells. Specifically, NPP WSM was further applied
to hFOB1.19 cells, where the results demonstrated that there was no
significant difference in ALP activity among all experimental
groups after 1 day of incubation, which may be due to the
insufficient time allowed for osteoblast differentiation. On days 3
and 7, the ALP activity of the treated hFOB1.19 cells was
significantly enhanced, where the effect of WSM on the osteogenic
differentiation of hFOB1.19 cells on day 7 was stronger compared
with that on day 3. These results suggest that 10, 20 and 40 µg/ml
NPP WSM can promote the osteogenesis of hFOB1.19 cells. Col-I, OCN,
OPN and Runx-2 are biomarkers commonly used in biological
experiments and clinical examinations to reflect the effects of
bone formation (43). In the
present study, the expression levels of Col-I, OCN, OPN and Runx-2
were found to be significantly increased in hFOB1.19 cells treated
with NPP WSM, suggesting that NPP WSM may achieve osteogenic
effects by regulating the expression of these proteins. In two
other previous studies on skin wound healing, Col-I expression was
observed to be increased in mice fibroblasts treated with pearl
powder WSM (44,45), whereas in another previous study
Col-I and Runx-2 expression levels were also reported to be
increased in MC3T3-E1 cells treated with NPP WSM (46). These results are consistent with
those in the present study.
However, there are a number of limitations in the
present study. The signaling pathways associated with NPP WSM
osteogenesis were not investigated further. In addition, a positive
control group with a known substance that can definitively induce
osteogenesis was not included.
In conclusion, the results of the present study
suggest that NPP WSM may promote the osteogenic differentiation of
hFOB1.19 cells, by regulating the expression of the osteogenic
factors, Col-I, OCN, OPN and Runx-2, in a dose-dependent manner.
These results suggest that NPP WSM could be further explored in
future studies for its potential application in oral implants
addressing insufficient alveolar ridge bone mass. In follow-up
studies, a bone defect model in animals is required, with
implantation surgeries, to explore the effective concentration and
therapeutic effect of NPP WSM in vivo, with aims of eventual
clinical studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the China National
Natural Science Foundation Project (grant no. 82060194), Hainan
Province Key Research and Development Project (grant no.
ZDYF2022SHFZ119).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The experiments were conceived and designed by LL
and PX. The experiments and analysis were performed by XX and LL.
The data were analyzed by WZ and LL. The manuscript was written and
revised by XX and PX. XX and PX confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cuif JP, Dauphin Y, Luquet G, Medjoubi K,
Somogyi A and Perez-Huerta A: Revisiting the organic template model
through the microstructural study of shell development in Pinctada
margaritifera, the polynesian pearl oyster. Minerals.
8(370)2018.
|
|
2
|
Farre B, Brunelle A, Laprévote O, Cuif JP,
Williams CT and Dauphin Y: Shell layers of the black-lip pearl
oyster Pinctada margaritifera: Matching microstructure and
composition. Comp Biochem Physiol B Biochem Mol Biol. 159:131–139.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ma Y, Qiao L and Feng Q: In-vitro study on
calcium carbonate crystal growth mediated by organic matrix
extracted from fresh water pearls. Mater Sci Eng C Mater Biol Appl.
32:1963–1970. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Almeida MJ, Milet C, Peduzzi J, Pereira L,
Haigle J, Barthelemy M and Lopez E: Effect of water-soluble matrix
fraction extracted from the nacre of Pinctada maxima on the
alkaline phosphatase activity of cultured fibroblasts. J Exp Zool.
288:327–334. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang J, Li S, Yao S, Si W, Cai L, Pan H,
Hou J, Yang W, Da J, Jiang B, et al: Ultra-performance liquid
chromatography of amino acids for the quality assessment of pearl
powder. J Sep Sci. 38:1552–1560. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chiu HF, Hsiao SC, Lu YY, Han YC, Shen YC,
Venkatakrishnan K and Wang CK: Efficacy of protein rich pearl
powder on antioxidant status in a randomized placebo-controlled
trial. J Food Drug Anal. 26:309–317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen X, Peng LH, Chee SS, Shan YH, Liang
WQ and Gao JQ: Nanoscaled pearl powder accelerates wound repair and
regeneration in vitro and in vivo. Drug Dev Ind Pharm.
45:1009–1016. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang JX, Li SR, Yao S, Bi QR, Hou JJ, Cai
LY, Han SM, Wu WY and Guo DA: Anticonvulsant and sedative-hypnotic
activity screening of pearl and nacre (mother of pearl). J
Ethnopharmacol. 181:229–235. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ansari N and Sims NA: The cells of bone
and their interactions. Handb Exp Pharmacol. 262:1–25.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dai R, Liu M, Xiang X, Xi Z and Xu H:
Osteoblasts and osteoclasts: An important switch of tumour cell
dormancy during bone metastasis. J Exp Clin Cancer Res.
41(316)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jurisic V, Terzic T, Pavlovic S, Colovic N
and Colovic M: Elevated TNF-alpha and LDH without parathormone
disturbance is associated with diffuse osteolytic lesions in
leukemic transformation of myelofibrosis. Pathol Res Pract.
204:129–132. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yao Z, Getting SJ and Locke IC: Regulation
of TNF-induced osteoclast differentiation. Cells.
11(132)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Akdis M, Aab A, Altunbulakli C, Azkur K,
Costa RA, Crameri R, Duan S, Eiwegger T, Eljaszewicz A, Ferstl R,
et al: Interleukins (from IL-1 to IL-38), interferons, transforming
growth factor β, and TNF-α: Receptors, functions, and roles in
diseases. J Allergy Clin Immunol. 138:984–1010. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhao Y, Sun Q and Huo B: Focal adhesion
regulates osteogenic differentiation of mesenchymal stem cells and
osteoblasts. Biomater Transl. 2:312–322. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nahle S, Lutet-Toti C, Namikawa Y, Piet
MH, Brion A, Peyroche S, Suzuki M, Marin F and Rousseau M: Organic
matrices of calcium carbonate biominerals improve osteoblastic
mineralization. Mar Biotechnol (NY). 26:539–549. 2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin C, Zhao JY, Liu XJ and Li JL:
Expressions of shell matrix protein genes in the pearl sac and its
correlation with pearl weight in the first 6 months of pearl
formation in Hyriopsis cumingii. Mar Biotechnol (NY). 21:240–249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Commission CP and Commission SP:
Pharmacopoeia of the people's Republic of China 2015. (No Title),
2015.
|
|
18
|
Huang F, Deng CM and Fu Z: GC-MS
determination of microamounts of organic chemical compositions in
nacre powder of Hyriopsis cumingii Lea. Phs Test Chem Anal.
46:300–302. 2010.
|
|
19
|
He JF, Deng Q, Pu YH, Liao B and Zeng M:
Amino acids composition analysis of pearl powder and pearl layer
powder. Food Ind. 37:270–273. 2016.
|
|
20
|
Berland S, Ma Y, Marie A, Andrieu JP,
Bedouet L and Feng Q: Proteomic and profile analysis of the
proteins laced with aragonite and vaterite in the freshwater mussel
Hyriopsis cumingii shell biominerals. Protein Pept Lett.
20:1170–1180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Green DW, Kwon HJ and Jung HS: Osteogenic
potency of nacre on human mesenchymal stem cells. Mol Cells.
38:267–272. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF-α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim JM, Lin C, Stavre Z, Greenblatt MB and
Shim JH: Osteoblast-osteoclast communication and bone homeostasis.
Cells. 9(2073)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li S, Liu G and Hu S: Osteoporosis:
Interferon-gamma-mediated bone remodeling in osteoimmunology. Front
Immunol. 15(1396122)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lan TJ, Luo X, Mo MY, Chen ZX, Luo F, Han
SY, Liu P, Liang ZX, Zhang T, Li TY, et al: Hydrolyzed seawater
pearl tablet modulates the immunity via attenuating Th1/Th2
imbalance in an immunosuppressed mouse model. J Tradit Chin Med.
41:397–405. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Loh XJ, Young DJ, Guo H, Tang L, Wu Y,
Zhang G, Tang C and Ruan H: Pearl powder-an emerging material for
biomedical applications: A review. Materials (Basel).
14(2797)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo W, Chen Y, Chen C and Ren G: The
proteomics of the freshwater pearl powder: Insights from
biomineralization to biomedical application. J Proteomics.
265(104665)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang W, Xu P, Cheng Y, Yang Y, Mao Q and
Chen Z: Preparation of a nanopearl powder/C-HA (chitosan-hyaluronic
acid)/rhBMP-2 (recombinant human bone morphogenetic protein-2)
composite artificial bone material and a preliminary study of its
effects on MC3T3-E1 cells. Bioengineered. 13:14368–14381.
2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pei J, Wang Y, Zou X, Ruan H, Tang C, Liao
J, Si G and Sun P: Extraction, purification, bioactivities and
application of matrix proteins from pearl powder and nacre powder:
A review. Front Bioeng Biotechnol. 9(649665)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li X, Xu P, Cheng Y, Zhang W, Zheng B and
Wang Q: Nano-pearl powder/chitosan-hyaluronic acid porous composite
scaffold and preliminary study of its osteogenesis mechanism. Mater
Sci Eng C Mater Biol Appl. 111(110749)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang X, Du X, Li D, Ao R and Yu B and Yu
B: Three dimensionally printed pearl powder/poly-caprolactone
composite scaffolds for bone regeneration. J Biomater Sci Polym Ed.
29:1686–1700. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li SG, Guo ZL, Tao SY, Han T, Zhou J, Lin
WY, Guo X, Li CX, Diwas S and Hu XW: In vivo study on osteogenic
efficiency of nHA/gel porous scaffold with nacre water-soluble
matrix. Tissue Cell. 88(102347)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
de Muizon CJ, Iandolo D, Nguyen DK,
Al-Mourabit A and Rousseau M: Organic matrix and secondary
metabolites in nacre. Mar Biotechnol (NY). 24:831–842.
2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Evans JS: The biomineralization proteome:
Protein complexity for a complex bioceramic assembly process.
Proteomics. 19(e1900036)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Han S, Huang D, Lan T, Wu Y, Wang Y, Wei
J, Zhang W, Ou Y, Yan Q, Liu P, et al: Therapeutic effect of
seawater pearl powder on UV-induced photoaging in mouse skin. Evid
Based Complement Alternat Med. 2021(9516427)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu Y, Ding Z, Ren H, Ji M and Yan Y:
Preparation, characterization and in vitro biological evaluation of
a novel pearl powder/poly-amino acid composite as a potential
substitute for bone repair and reconstruction. Polymers (Basel).
11(831)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Du X, Yu B, Pei P, Ding H, Yu B and Zhu Y:
3D printing of pearl/CaSO4 composite scaffolds for bone
regeneration. J Mater Chem B. 6:499–509. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qiu-Hua M and Pu X: Research progress of
pearl/nacer in bone tissue repair. J Oral Sci Res. 32:311–333.
2016.
|
|
41
|
Yang X, Li Y, Liu X, He W, Huang Q and
Feng Q: Nanoparticles and their effects on differentiation of
mesenchymal stem cells. Biomater Transl. 1:58–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang YL, Chang CH, Huang CC, Kao WMW, Liu
WC and Liu HW: Osteogenic activity of nanonized pearl powder/poly
(lactide-co-glycolide) composite scaffolds for bone tissue
engineering. Biomed Mater Eng. 24:979–985. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rico-Llanos GA, Borrego-González S,
Moncayo-Donoso M, Becerra J and Visser R: Collagen type I
biomaterials as scaffolds for bone tissue engineering. Polymers
(Basel). 13(588)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu M, Tao J, Guo H, Tang L, Zhang G, Tang
C, Zhou H, Wu Y, Ruan H and Loh XJ: Efficacy of water-soluble pearl
powder components extracted by a CO2 supercritical
extraction system in promoting wound healing. Materials (Basel).
14(4458)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dai J.P, Chen J, Bei Y.F, Han B.X, Guo S.B
and Jiang L.L: Effects of pearl powder extract and its fractions on
fibroblast function relevant to wound repair. Pharm Biol.
48:122–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cheng Y, Zhang W, Fan H and Xu P:
Water-soluble nano-pearl powder promotes MC3T3-E1 cell
differentiation by enhancing autophagy via the MEK/ERK signaling
pathway. Mol Med Rep. 18:993–1000. 2018.PubMed/NCBI View Article : Google Scholar
|