Introduction
As rare benign lesions derived from vascular
endothelial cells, epithelioid angiomatous nodules (EANs), were
first reported in the English literature in 2004(1). EAN occurs in the head and neck
(including the face, nasal cavity, scalp, oral cavity and outer
ear), trunk (including the breast and penis) and limbs (2,3).
However, to the best of our knowledge, no case of EAN of the vocal
cord has been documented internationally to date. Moreover, single
lesions are more common than multifocal lesions. Microscopically,
the tumor has clear boundaries and comprises proliferating
epithelioid vascular endothelial cells, with no obvious atypia (or
mild to moderate atypia in some cases), large nucleoli, vacuolated
nuclei, eosinophilic or clear cytoplasm, and no or few mitotic
figures, but with 9/10 high-powered fields (HPF) reported in
individual cases (4). The tumor
cells form primitive blood vessels, and the lumen contains red
blood cells. The interstitium has minimal inflammatory cell
infiltration and no obvious chondromyxoid matrix. Moreover,
immunohistochemical results show positive expression of CD31 and
CD34, and an almost complete absence of cytokeratin (CK) and
epithelial membrane antigen (EMA) expression (5). Since a small number of biopsies have
resulted in a diagnosis of epithelioid hemangioendothelioma (EHE)
(3), differentiation from
angiosarcoma is required to avoid overtreatment. EAN is typically
treated by tumor resection to ensure negative margins and no
recurrence; however, hormones or immunosuppressants are other
treatment options (6-8).
The present study reports a case of an EAN in the vocal cords to
provide some reference value for clinical and pathological
doctors.
Case report
In February 2025, a 31-year-old man was admitted to
the Department of Ear, Nose and Throat of The First People's
Hospital of Xiaoshan District (Hangzhou, China) due to repeated
hoarseness for over a month, accompanied by a small amount of
coughing and sputum, and occasional hemoptysis with bright red
blood streaks, but no fever. Initial screening was negative for
human immunodeficiency virus antibodies. However, a fiber
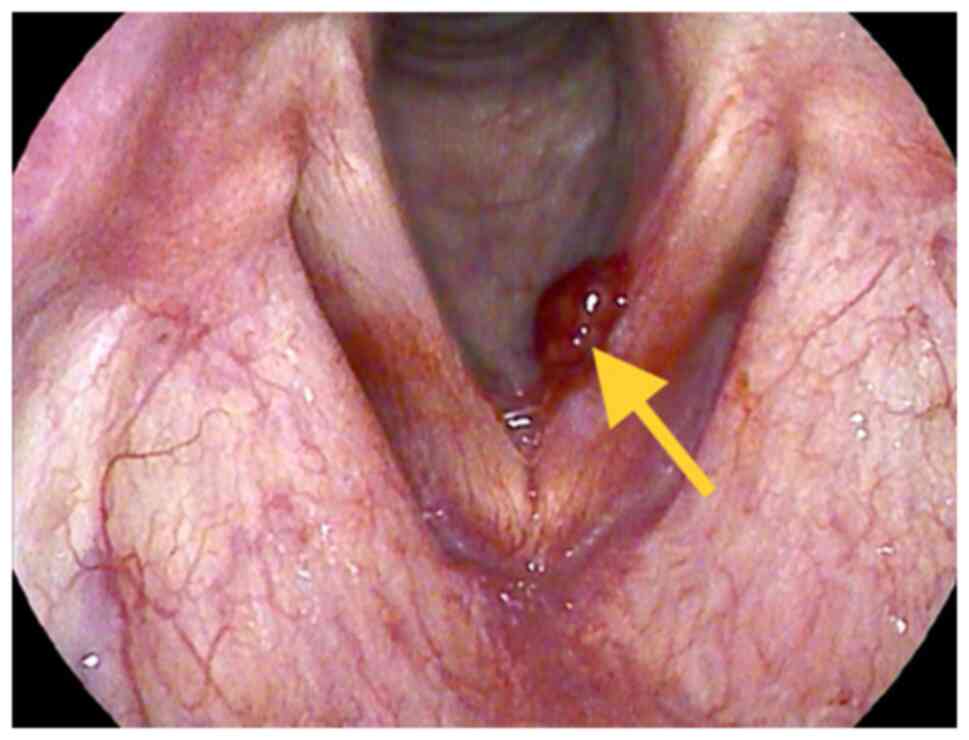
laryngoscopy showed a red polypoid mass with a diameter of 1 cm on
the left vocal cord (Fig. 1).
Although no abnormalities were observed on the right vocal cord,
blood oozing was observed during the vocal cord movement. The
clinical diagnosis was of a left vocal cord polyp. Dye to the
concern that a biopsy would cause significant bleeding and due to
the consideration of a benign lesion, the surgeon directly ordered
a surgical resection without biopsy to achieve a curative
effect.
The patient underwent endoscopically-assisted
laryngeal microsurgery of the vocal cord lesion. The mucosa was
incised along the base of the neoplasm, and the neoplasms were
removed with forceps in stages. The vocal cord wound was flattened
until the vocal cord's edge was flat, and the tissue was sent for
pathological examination.
The tissue was fixed with 10% neutral formalin (24 h
at 25˚C), embedded in paraffin, and serially sectioned at 3 µm,
before being subjected to hematoxylin and eosin staining (3 h at
25˚C) (all Shanghai Regal Biological Technology Development Co.,
Ltd.; Sinopharm Chemical Reagent Co., Ltd.). Observations were
performed under a Leica DM2000 light microscope (Leica Microsystems
GmbH). Macroexamination revealed a pile of soft gray-red tissue
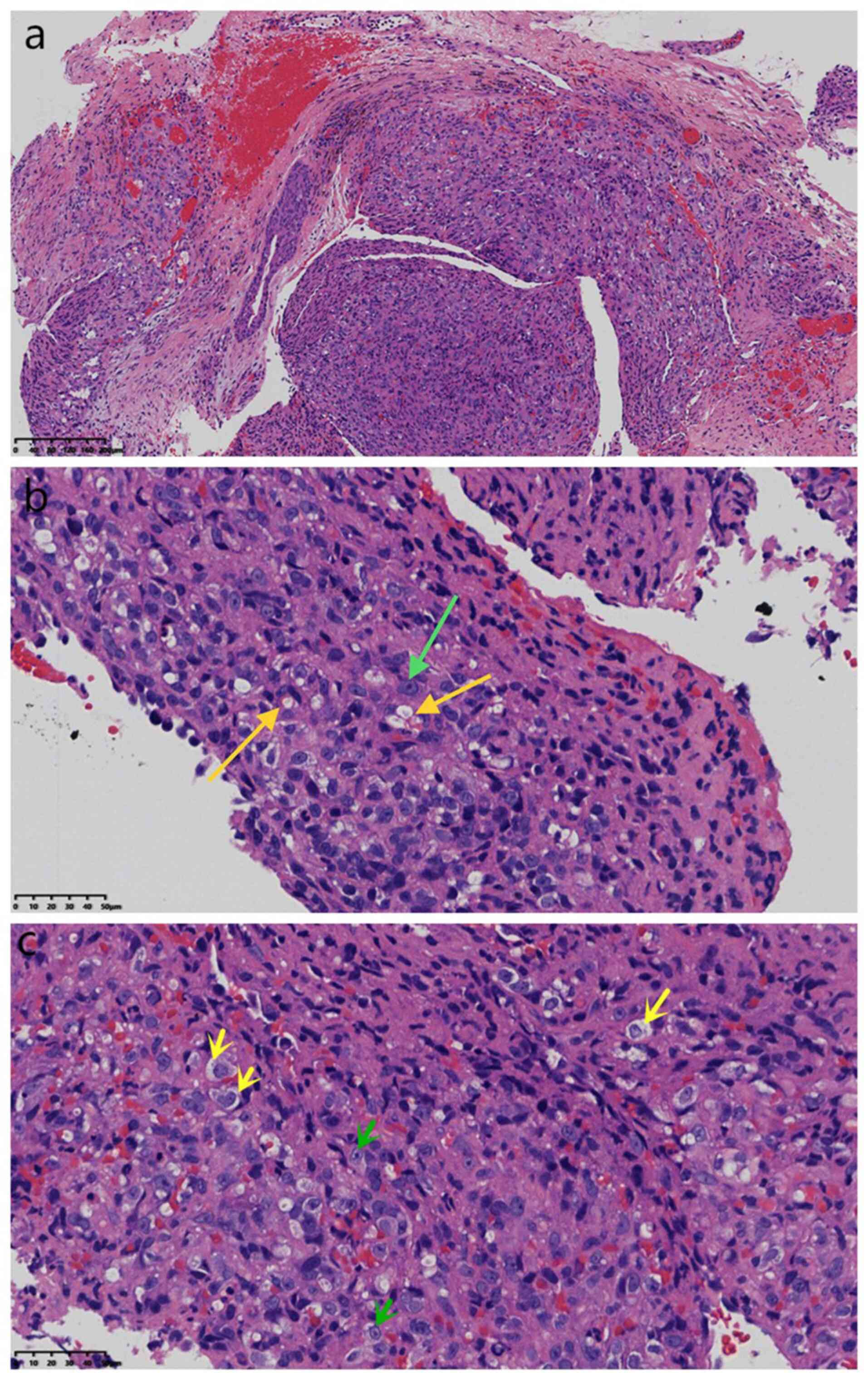
with a volume of 0.6x0.5x0.2 cm. Microscopically (Fig. 2, Fig.
3 and Fig. 4), the tissue was
fragmented. Furthermore, proliferative squamous subepithelial
epithelioid tumor cells showed solid lamellar hyperplasia with
clear boundaries, round or elliptic tumor cells, unequal nuclear
sizes, round or elliptic shapes, visible nucleoli, vacuolated
nuclei, eosinophilic or clear cytoplasm, partial vacuolation,
formation of primitive vascular lumina and no mitotic signs.
However, there was no cartilaginous mucoid matrix or suppurative
inflammatory cell infiltration on the surface. Diseased tissues
were identified at the surgical margins.
Immunohistochemical staining was conducted with the
EnVision Systems method using antibodies purchased from Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd., and Fuzhou Maixin
Biotechnology Development Co., Ltd. Blocking was performed with 5%
goat serum for 1 h at 25˚C. Incubation with primary antibodies was
for 1-2 h at 37˚C, while secondary antibody incubation was for 1 h
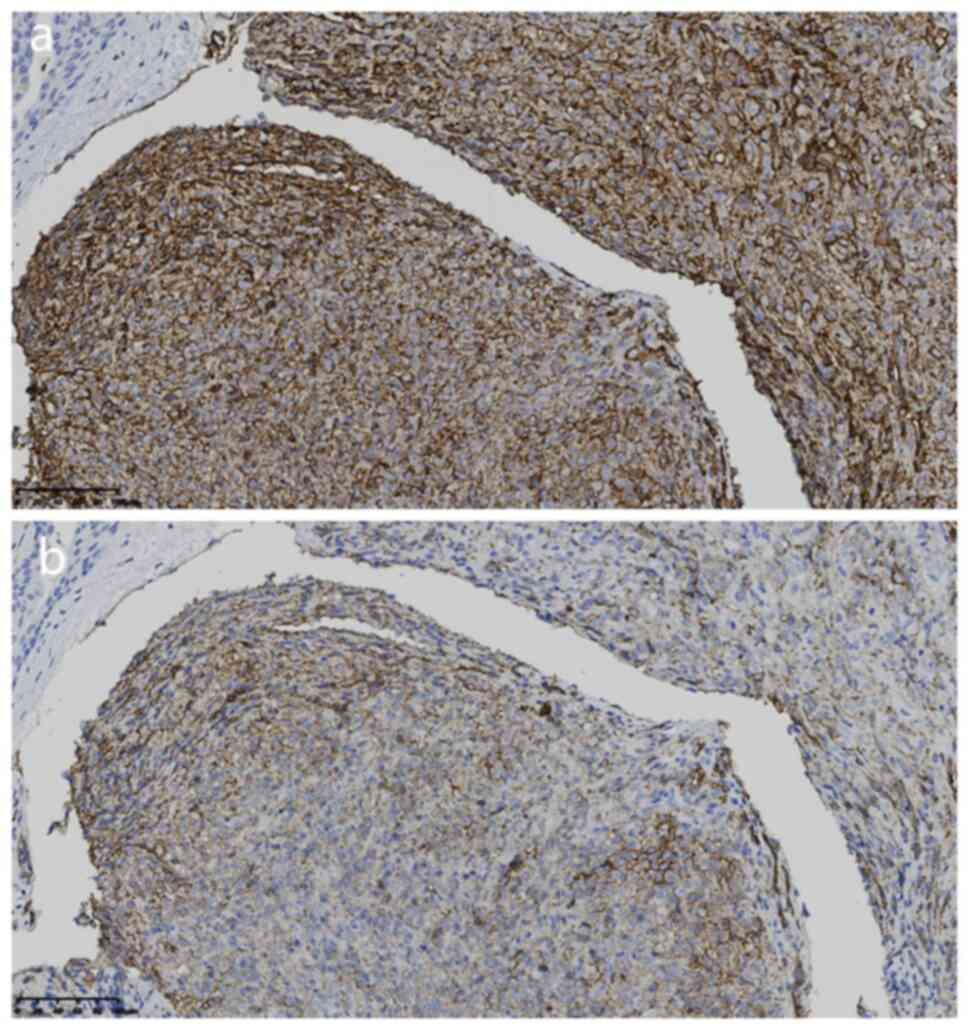
at 37˚C. The tumor cells were positive for CD31 (1:100; cat. no.
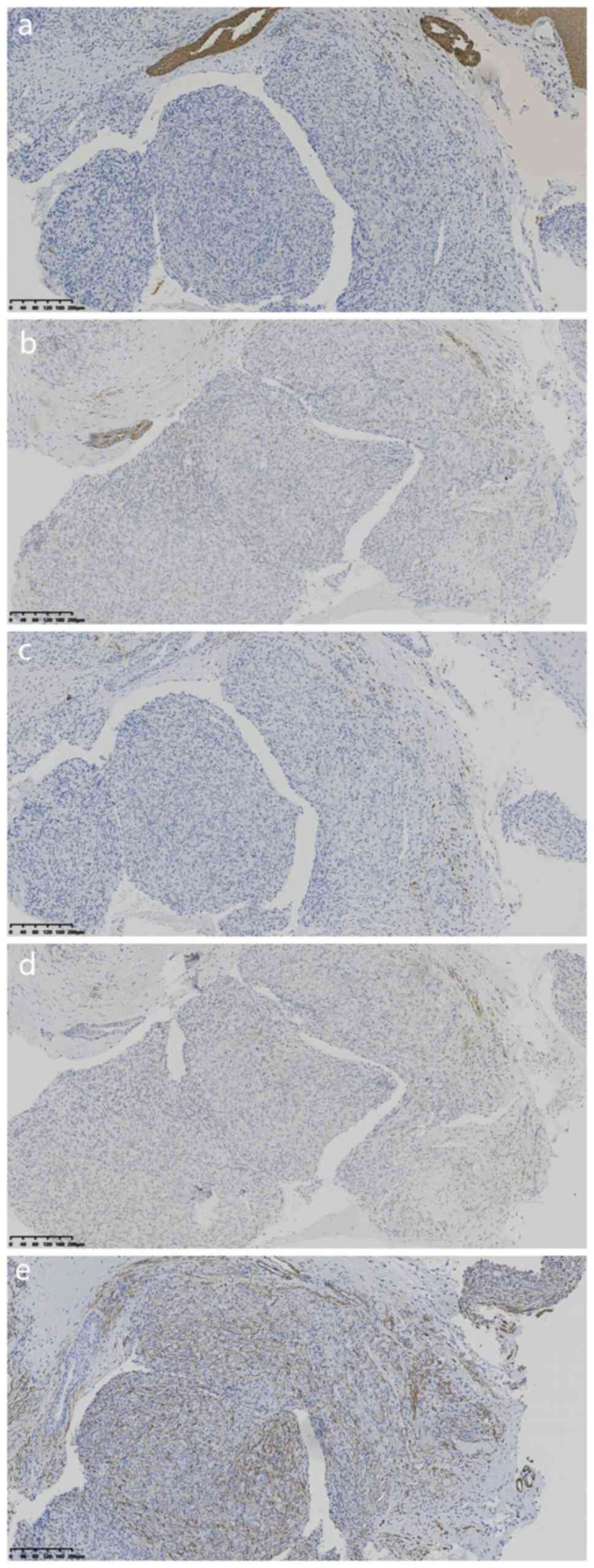
20073115), CD34 (1:100; cat. no. 21016826) (Fig. 3A and B) and negative for cytokeratin (1:100;
cat. no. 21061509), EMA (1:100; cat. no 21020730), desmin (1:200;
cat. no. 21011686), transcription factor E3 (TFE3; working fluid;
cat. no 2205110663c) and S-100 protein (1:100; cat. no.
2012240585C8) (Fig. 4A-E).
Moreover, the Ki-67 proliferative index was 25% (1:200; cat. no.
21030436) (digital slice scanner; Ningbo Jiangfeng Biological
Information Technology Co. Ltd.). Additionally, a fluorescence
in situ hybridization molecular assay revealed no
rearrangement of the calmodulin-binding transcription activator 1
(CAMTA1) fusion gene (as performed by the Department of Pathology,
Shanghai Cancer Hospital, Shanghai, China). The pathological
diagnosis was an EAN of the left vocal cord.
The postoperative management included a single dose
of prophylactic intravenous methylprednisolone (80 mg) to mitigate
airway edema, and budesonide nebulization (2 mg twice a day for 3
days) for localized anti-inflammatory effects. However, there was
no hoarseness at the 3-month follow-up. At the 6-month follow-up, a
fiber laryngoscopy was performed, and no recurrence of vocal cord
lesions was observed. However, it was recommended that the patient
undergo regular fiber laryngoscopic examinations every 3 months due
to the diseased tissue at the surgical margin.
Discussion
EAN was first reported in 2004 by Brenn and Fletcher
(1). A total of 67 EAN cases have
been reported in the international literature between 2004 and 2024
(2,3) according to a search of the PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
Scopus (https://www.elsevier.com/products/scopus/) databases
using the following key words: ‘Epithelioid’, ‘angiomatous’,
‘nodule’, ‘vocal cords’, ‘angiosarcoma’ and ‘hemangioendothelioma’.
Inclusion criteria for this search were as follows: Research
related to one or more of these key words, covering the clinical
features, pathological diagnosis, differential diagnosis, treatment
methods and other aspects of these lesions. The exclusion criteria
were as follows: Studies unrelated to the key words, low quality of
literature and duplicate publications. Among the vascular neoplasms
of the larynx, hemangiomas are the most common tumors in infants
and are less common in adults; however, angiosarcomas are rarely
reported (9). To the best of our
knowledge, the present case report is the first in the
international literature to describe an EAN in a laryngeal
location. An extensive literature review may provide an
evidence-based framework of diagnosis and treatment for this
variant of EAN on the vocal cord.
EAN occurs in the head and neck (including the face,
nasal cavity, scalp, oral cavity and outer ear), the trunk
(including the breast and penis) and the limbs (2,3).
Based on the reviewed cases, the age range of such cases is 13-84
years. The mean age is 42 years, and the male-to-female ratio is
1.31:1 (38:29). The asymptomatic clinical symptoms vary by site;
some studies have also reported pain, bleeding and itching (25%;
17/67). Most cases are present on the body surface, with bluish-red
papules or nodules (mostly single and some multifocal) visible to
the naked eye, ranging from 0.2 to 2.5 cm; however, those in the
nasal cavity require an endoscopy to detect the lesions (10,11).
Microscopically, EAN exhibits consistent histomorphological
patterns across various anatomical sites. The tumor is
well-circumscribed and consists of proliferating epithelioid
vascular endothelial cells. Epithelioid cells are round, oval or
polygonal, without atypia, and in individual cases with
immunosuppression, cells show mild to moderate atypia (4). These endothelial cells exhibit large
nuclei, distinct nucleoli, vacuolated nuclei and few or no mitotic
images, but with 9/10 HPF reported in individual cases (4). The cytoplasm is abundant,
eosinophilic or clear. Cytoplasmic vacuoles are observed, original
blood vessels have been formed and the lumen contains red blood
cells. The interstitium shows reduced inflammatory cell
infiltration (including eosinophils) and no chondromyxoid matrix.
Immunohistochemical expression of CD31, CD34 and coagulation factor
VIII is often positive, while that of CK and S-100 is negative
(1,2). Among the literature cases, 1 patient
showed positive EMA and estrogen receptor expression (5). Ki-67 was expressed by <30% of
tumor cells in 7 reported cases (2,3). To
the best of our knowledge, the present case reports the occurrence
of EAN on the vocal cord for the first time. The clinical
manifestations included repeated hoarseness for over a month, and
the symptoms were similar to those of vocal cord polyps. A fiber
laryngoscopy revealed a red polypoid mass on the left vocal cord.
The polypoid tumor displayed clear boundaries, and epithelioid
cells showed a solid pattern with the original blood vessels and
vacuolated structures. Immunohistochemically, the tumor cells
expressed CD31 and CD34. Ki-67 was expressed in 25% of the tumor
cells, indicating some proliferative capacity, but is within the
range reported in the literature (2,3). The
CAMTA1 fusion gene was not rearranged, and the pathological
diagnosis was consistent with EAN.
EAN has also been midiagnosed as EHE (3) and should be distinguished from other
tumors as follows: i) In EHE, epithelioid endothelial cells grow in
cords/nests in chondromyxoid tissue, with mildly eosinophilic
cytoplasm. Genetic testing often shows WW domain containing
transcription regulator 1-CAMTA1 or Yes-associated protein
1-transcription factor E3-TFE3 rearrangements. ii) In epithelioid
angiosarcoma, irregular vascular lumina are present, with
invasive/destructive growth, obvious cell atypia and prominent
mitotic figures. iii) Epithelioid hemangiomas (benign) are composed
of hyperplastic small vessels (few with dilated lumina), while
epithelioid endothelial cells (large, abundant cytoplasm) exhibit
round/oval nuclei and prominent nucleoli, and mild nuclear atypia
is present in some cases. iv) Epithelioid sarcoma mostly occurs in
the distal extremities, and its microscopic features include
obvious central tumor necrosis, significant pleomorphism of
epithelioid cells, numerous mitotic figures and no obvious
primitive blood vessels or cytoplasmic vacuolation. Molecular
detection of SWI/SNF related matrix associated actin-dependent
regulator of chromatin subfamily B1 deletion helps in the
diagnosis. v) Malignant melanomas may exhibit visible pigment,
marked cell atypia, discoverable mitotic figures and necrosis.
S-100 protein and human melanoma black-45 are positively expressed.
vi) Pyogenic granulomas contain lobulated proliferating capillaries
with mildly hyperplastic endothelial cells on microscopy. The
stroma shows fibromyxoid degeneration with edema. Additionally,
surface mucosal erosion is visible, as well as acute and chronic
inflammatory cell infiltration (12).
The primary treatment for EAN is surgical excision;
however, a few studies have reported regression after using topical
corticosteroids and cryotherapy (6,7). In
another patient with multiple-skin EAN, the lesions gradually
disappeared 16 months after discontinuing cyclosporine (8). However, 2 cases showed recurrence
after an incomplete resection (4),
and another case with multiple sites reported recurrence in a new
location post-resection (13). A
total of 67 non-metastatic cases have been reported in the
literature (100% benign), with follow-up periods ranging between 1
month and 7 years. In the present case, the patient underwent an
endoscopic resection, anti-inflammatory detumescence and
symptomatic treatment. Although the surgical margins showed the
presence of diseased tissue, the pathological results suggested
that the EAN was a benign lesion. If the lesion reoccurred on the
vocal cords, this might cause complications, such as hoarseness, if
treated again. Hence, a regular fiber laryngoscopy every 3 months
was recommended. The patient was followed up for 3 months without
hoarseness, and there was no recurrence at 6 months. Therefore,
regular postoperative follow-up may be used as a valuable
reference.
In conclusion, EAN of the vocal cord is a rare
benign lesion. Since its diagnosis relies on pathological analysis,
the differentiation between malignant epithelioid vascular tumors
is essential. Although surgical resection is the conventional
treatment, regular postoperative follow-ups should be conducted due
to the risk of recurrence. Owing to the limited literature on such
lesions, this case report may have positive effects on the
diagnosis and treatment of vocal cord tumors.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KL and BH drafted the manuscript and conceived the
study. LC and JS were responsible for the collection and analysis
of the case data and literature. BH, YG and KL revised the
manuscript and interpreted the data. MJ and MH obtained medical
images. NQ carried out the immunohistochemical analysis. LC and JS
confirmed the authenticity of the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Ethical approval was provided by the Ethics
Committee of Xiaoshan District First People's Hospital (Hangzhou,
China; approval no. 2024-07).
Patient consent for publication
The patient provided written informed consent for
the publication of the case report and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brenn T and Fletcher CDM: Cutaneous
Epithelioid angiomatous nodule: A distinct lesion in the
morphologic spectrum of epithelioid vascular tumors. Am J
Dermatopathol. 26:14–21. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dubus M and Kanitakis J: Cutaneous
epithelioid angiomatous nodule: Report of a new case and literature
review. Dermatopathology (Basel). 10:112–119. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Özer E, Bingöl UA and Sav MA: Multifocal
penile epithelioid angiomatous nodule: A rare tumor of penis. Turk
J Plast Surg. 32:32–34. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chetty R, Kamil ZS, Wang A, Al Habeeb A
and Ghazarian D: Cutaneous epithelioid angiomatous nodule: A report
of a series including a case with moderate cytologic atypia and
immunosuppression. Diagn Pathol. 13(50)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McLemore MS, Huo L, Deavers MT, Curry JL,
Torres-Cabala CA, Wang WL and Prieto VG: Cutaneous epithelioid
angiomatous nodule of the chest wall with expression of estrogen
receptor: A mimic of carcinoma and a potential diagnostic pitfall.
J Cutan Pathol. 38:818–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dastgheib L, Aslani FS, Sepaskhah M, Saki
N and Motevalli D: A young woman with multiple cutaneous
epithelioid angiomatous nodules (CEAN) on her forearm: A case
report and follow-up of therapeutic intervention. Dermatol Online
J. 19(1)2013.PubMed/NCBI
|
|
7
|
Sangüeza OP, Walsh SN, Sheehan DJ, Orland
AF, Llombart B and Requena L: Cutaneous epithelioid angiomatous
nodule: A case series and proposed classification. Am J
Dermatopathol. 30:16–20. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng DJ, Zheng XY and Tang SF: Large
cutaneous epithelioid angiomatous nodules in a patient with
nephrotic syndrome: A case report. World J Clin Cases. 8:600–605.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Katna R, Deshmukh A, Sridhar E, Chaukar D
and D'Cruz A: Primary angiosarcoma of the larynx: A rare entity.
Ann R Coll Surg Engl. 94:e146–e148. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leroy X, Mortuaire G, Chevalier D and
Aubert S: Epithelioid angiomatous nodule of the nasal cavity.
Pathol Res Pract. 204:929–932. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wong WK, Lim DH and Ong CW: Epithelioid
angiomatous nodule of the nasal cavity: Report of 2 cases. Auris
Nasus Larynx. 42:341–344. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dube U, Corliss M, Bowling KM, Heusel JW
and Coughlin CC: Age, sex, and anatomical location patterns in
cutaneous pyogenic granuloma cases. JAMA Dermatol. 161:305–309.
2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pavlidakey PG, Burroughs C, Karrs T and
Somach SC: Cutaneous epithelioid angiomatous nodule: A case with
metachronous lesions. Am J Dermatopathol. 33:831–834.
2011.PubMed/NCBI View Article : Google Scholar
|