Invasive fungal disease (IFD) is a fungal infection
in which fungi invade the body, grow and multiply in tissues,
organs or the bloodstream, triggering both inflammatory cascades
and direct tissue damage (1,2). IFD
complications markedly elevate mortality in patients with
hematological malignancies (3). A
Chinese multicenter study (n=4,192) analyzing post-chemotherapy IFD
epidemiology revealed three key findings: i) Pediatric cases
accounted for 16.9% of the cohort, with acute myeloid leukemia
(AML; 28.5%), non-Hodgkin's lymphoma (26.3%) and acute
lymphoblastic leukemia (ALL; 20.2%) being predominant; ii) severe
neutropenia occurred in 33.4% of chemotherapy sessions; and iii)
while overall chemotherapy-related mortality was 1.5%, this
escalated to 11.7% when IFD was present (4). IFD also has a high prevalence in
patients with hematological malignancies and hematopoietic stem
cell transplantation (HSCT) (5).
Aspergillus, Candida and Trichosporon are the
major pathogens of IFD: Infections caused by these fungi usually
manifest as non-specific symptoms such as fever and dyspnea, making
early diagnosis challenging, particularly in children. Invasive
Candida infections tend to be candidemia and hepatic and
splenic infections; in patients with liver and spleen infections,
these infections also present with right upper abdominal tenderness
and abdominal pain, making early diagnosis challenging (6,7). In
conclusion, IFD not only increases mortality associated with
post-chemotherapy pediatric hematological malignancies but also
presents multiple diagnostic and therapeutic challenges. Previous
studies highlight that pediatric IFD diagnosis faces unique
challenges due to non-specific clinical manifestations and limited
validated biomarkers for children (8,9).
The diagnostic challenges specific to pediatric IFD
are multifaceted: Clinical manifestations such as persistent fever
and dyspnea lack specificity, overlapping with bacterial or viral
infections, which delays early recognition (10). Invasive candidiasis often presents
as candidemia or hepatosplenic lesions, yet abdominal pain and
right upper quadrant tenderness may be subtle in children, leading
to underdiagnosis (11).
Diagnostic biomarkers face validation gaps in pediatric
populations, while (1→3)-β-D-glucan (BDG) demonstrates
age-dependent diagnostic thresholds (80 pg/ml in children vs. 120
pg/ml in adults), its cross-reactivity with gut commensals limits
specificity (12). Similarly,
galactomannan (GM) assays require pediatric-specific cutoffs [0.7
optimal density index (ODI) for serum in children <12 years vs.
0.5 ODI in adults] (13). Emerging
non-culture techniques, such as metagenomic next-generation
sequencing (mNGS), show promise but lack standardized validation in
children (14-18).
A 2024 study demonstrated that plasma mNGS achieved 89% sensitivity
and 94% specificity for diagnosing IFD in immunocompromised
pediatric patients, although sample contamination risks necessitate
clinical correlation (19).
Furthermore, the absence of age-adjusted diagnostic criteria for
IFD in children contributes to delayed treatment initiation,
exacerbating mortality risks in this vulnerable population
(20,21).
Pediatric IFD exhibits distinct epidemiological
patterns across regions. A French multicenter study involving 2,721
patients and spanning 2015 to 2018 reported a 5.3% IFD incidence
rate, but the generalizability may be limited by the predominantly
Caucasian population (82% of participants were of European descent)
and exclusion of transplant recipients with graft-vs. -host disease
(22,23). While the French cohort provides
valuable multicenter data, its sample size lacks power for subgroup
analyses of rare fungi such as Fusarium. Comparatively,
Chinese cohort data (with 4,192 patients) showed higher mold
predominance (73 vs. 42% in the French cohort), highlighting
regional ecological variations in fungal epidemiology (4). Pediatric IFD exhibits distinct
epidemiological patterns, with patients with AML and allogeneic
HSCT (allo-HSCT) showing the highest susceptibility (12.9 and 4.3%
incidence, respectively). Breakthrough infections under prophylaxis
remain a concern, particularly with prolonged neutropenia lasting
>14 days (24). The prevalence
of IFD in patients with pediatric hematological malignancies is
generally high, and granulocyte deficiency persisting for >10
days after chemotherapy is an independent risk factor associated
with combined IFD in pediatric hematological malignancies, while
granulocyte deficiency persisting for >14 days and not receiving
antifungal prophylaxis (AP) is an independent risk factor
associated with combined IFD in pediatric hematological
malignancies after allo-HSCT. A retrospective analysis of the
incidence of IFD in hematological neoplasms in children showed that
IFD was diagnosed in 75 (7.2%) of 1,047 children, with 15 cases of
candidemia (60% of these being non-Albicans) and 60 cases of
mold infections (55% of these being non-Aspergillus spp.),
and the mortality rate of IFD was 21.7%. Among hematological
malignancies, AML and ALL showed the highest incidence of IFD: In
pediatric patients with these malignancies who developed IFD, 89%
had severe neutropenia and 73% received high-intensity therapy
(25). A previous study analyzing
the epidemiology of IFD occurring in children with hematological
malignancies showed that among 471 at-risk patients (median age,
9.8 years) with hematological malignancies, 27 children experienced
28 IFD episodes. These included 5 cases of candidemia and 23 cases
of bronchopulmonary mycosis; additionally, 20 patients developed
breakthrough infections, 8 required intensive care and six
succumbed to the disease (26).
Rosen et al (14) analyzed
the incidence, site of infection and mortality of IFD in pediatric
patients with hematological malignancies, and retrospectively
analyzed the treatment of 1,052 children with hematological
malignancies from 1991 to 2001. In the pediatric hematological
malignancies cohort study, the incidence of IFD was 4.9%, with the
IFD incidence increasing from 2.9 to 7.8% from 1996 to 2001. Acute
leukemia (AL) cases in children accounted for 36% of cases, but the
incidence of IFD infection was as high as 67%. Candida spp.
were the main pathogens of IFD, with a decrease in infections
caused by Candida and an increase in Aspergillus
infections over time. A total of 62% of all patients who developed
infections did so during the neutropenic phase of
post-chemotherapy, which was 2.6-fold higher in patients with IFD.
IFD is a common complication during chemotherapy for AL in
children, and relevant studies are shown in Table I (20,24,27-30).
The adoption of pharmacological prophylaxis against
IFD pathogens is an effective means of addressing the risk of IFD
infection. AP is preferentially recommended for pediatric patients
with hematological malignancies with a risk of developing IFD
>10% such as patients with AML, relapsed leukemia or undergoing
allo-HSCT. However, with the widespread use of AP in pediatric
patients with hematological malignancies, there is also a need to
address the issue of breakthrough IFD (bIFD), which is defined as
invasive fungal infection occurring during antifungal exposure, as
per the European Confederation of Medical Mycology guidelines
(2020) (31). bIFD has become an
issue in patients receiving systemic antifungal medications, with
time to bIFD defined as the first attributable clinical sign or
symptom, mycological evidence finding or imaging feature. The
duration of bIFD is dependent on pharmacokinetic properties and
lasts at least until one dosing interval after discontinuation
(32). Despite intensified AP
after chemotherapy or allo-HSCT, bIFD remains a common complication
and cause of mortality after hematological malignancy treatment
(33). Risk factors for bIFD
include severe neutropenia, use of corticosteroids and prolonged
use of broad-spectrum antibiotics. The immunosuppressive state of
the body in children with hematological neoplasms undergoing
chemotherapy or post-transplantation directly contributes to the
likelihood of an increased risk of developing bIFD. The occurrence
of a bIFD can be fatal and early intervention may improve the
outcome of IFD (Fig. 1).
Persistent IFD is defined as IFD that remains unchanged and
continues to require antifungal therapy since the initiation of
treatment, and is distinct from refractory IFD, which is defined as
disease progression. Recurrent IFD occurs after treatment and is
caused by the same pathogen at the same site, but transmission may
also occur (34). Fungemia is
predominantly caused by Candida, whereas pulmonary IFD is
primarily due to filamentous fungi, notably Aspergillus, and
the widespread use of AP has increased the proportion of
non-Aspergillus filamentous fungi (35,36).
IFD is a common and serious complication after
allo-HSCT for hematological malignancies in children. The incidence
of IFD is gradually increasing and has become one of the causes of
mortality after transplantation (37,38),
highlighting the need for IFD to be diagnosed and treated as early
as possible (39,40). The occurrence of IFD is related to
factors such as neutrophil deficiency, T-cell dysfunction,
prolonged application of hormones or high-dose chemotherapy
pretreatment during transplantation. The lack major of specificity
of clinical features makes early diagnosis of IFD difficult and
once it occurs the prognosis is notably poor (23,41).
A study has shown that the incidence of Aspergillus exceeded
that of Candida among pathogens causing IFD in transplant
patients, and that IFD occurs mainly in the early and
mid-transplantation periods (42).
In the early stages of transplantation, generally ≥1 month after
transplantation, in patients with neutropenia, broad-spectrum
application of antibiotics and impaired mucosal barrier are the
main risk factors for the development of IFD, with
Aspergillus and Candida being the most common
pathogenic organisms. The mid-transplantation period comprises 2-3
months after transplantation, and the main risk factor for the
occurrence of IFD in this period is the application of
anti-graft-vs. -host disease (GVHD) therapy such as glucocorticoids
(43-45).
Patients in this period may have a combination of GVHD or
cytomegalovirus infection, and the highest percentage of
Aspergillus infections occurs at this time, with a notable
decrease in the percentage of Candida infections (46).
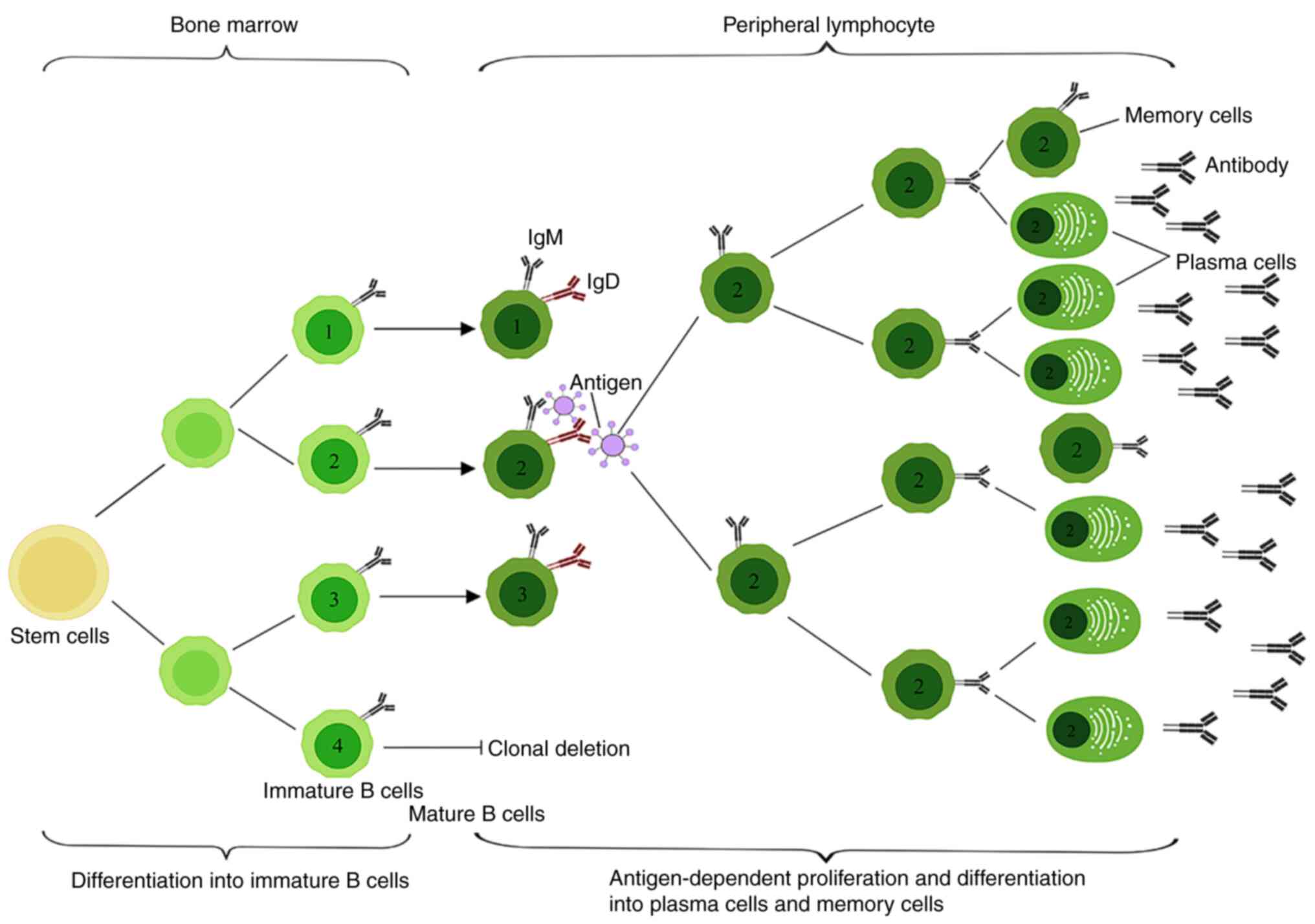
Late B-lymphocyte function after HSCT may take
months to years to fully return to normal; in this period, patients
are susceptible to infections and other complications during the
complex process of B-lymphocyte development, in which HTSCs
differentiate into lymphoid progenitor cells in the bone marrow,
further differentiate into pre-B and immature B cells and finally
develop into mature B cells (47,48).
Following HSCT, the recovery of myeloid lineage cells precedes the
reconstitution of lymphocytes, with B-cell development exhibiting a
delayed time course. B cells are characterized by their cytosolic
membrane surface expression of a variety of cytokine receptors
(such as IL-1, IL-2 and IL-4), which can be secreted by the
cytokines of T helper cells to produce a response (49,50).
Cytokines such as IL-2 and IL-4 promote B cell proliferation and
their subsequent differentiation into antibody-producing plasma
cells. Some of the proliferating B cells migrate to the medulla of
the lymphoid tissue and continue to proliferate and differentiate
to provide a defensive response to antibody production by plasma
cells, whereas some of the B cells and associated T cells migrate
to the nearby primary lymphoid follicle B cell region to continue
to proliferate and form secondary lymphoid follicles in the
germinal center (Fig. 2) (51,52).
The greater the intensity of transplantation preconditioning and
the more immunosuppressive agents applied, the greater the
possibility of IFD infection, and if a patient has a history of IFD
infection prior to transplantation, the risk of IFD infection
during transplantation is markedly increased (53).
In a prospective multicenter study analyzing
clinical data on combined IFD after chemotherapy or allo-HSCT for
hematological malignancies in children, a total of 304 children
were treated with chemotherapy or allo-HSCT, and 19 developed IFD,
including 10 cases of Aspergillus spp. and 5 cases of
Candida spp. that were confirmed. Among these patients, no
fatalities were attributed to IFD; however, in a subgroup of 8
patients who underwent allo-HSCT and developed IFD, 3 cases
resulted in mortality (54).
Invasive candidiasis is a common IFD in children
with hematological malignancies (55). This most commonly presents as
candidemia and liver and spleen infections, with the main clinical
symptoms being fever and other non-specific manifestations such as
fatigue and malaise or reduced appetite and weight loss. In
children with hematological malignancies and suspected invasive
candidiasis, if broad-spectrum antibiotics fail to improve clinical
symptoms (e.g., persistent fever), laboratory examinations for
fungal BDG should be performed to aid in the diagnosis (56). IFD occurring in the liver and
spleen sites upon fungal infections increase the incidence and
mortality after chemotherapy for hematological malignancies in
children (57,58). To the best of our knowledge, there
are insufficient data to support the optimal diagnosis of IFD at
the liver and spleen sites in children, and clinicians should be
alerted to the presence of persistent fever, back pain extending to
the shoulders, widespread muscle pain and elevated serum GM levels
in children with neutropenia after chemotherapy. In children with
prolonged neutropenic fever, early detection and diagnosis of IFD
in the liver and spleen should be recommended by abdominal
ultrasound and abdominal computed tomography (CT), even in the
absence of localized signs or symptoms (59). Invasive aspergillosis (IA) is the
most common type of infection in children after chemotherapy for
hematological malignancies, especially after allo-HSCT, and its
symptomatic presentation depends on the site of infection (60,61).
The most common site of infection in children with hematological
malignancies is the lower respiratory tract, which can cause
Aspergillus bronchitis and pneumonia, followed by nasal
infections and central nervous system (CNS) infections. Common
symptoms of respiratory Aspergillus infections include cough
and dyspnea, brownish-black mucus plugs in some patients and
coughing up blood in severe cases (62). CNS Aspergillus infections
may present with unusual headaches, seizures and severe loss of
consciousness. However, it can be difficult to recognize IA early
in children, as they may present initially with only non-specific
signs such as fever, which can obscure or precede the development
of more specific neurological symptoms. In CNS fungal infections,
Aspergillus spp. are the most common pathogens, and tests
such as magnetic resonance imaging are difficult to use for
diagnosis: By contrast, detecting fungal antigens such as GM or BDG
or early diagnosis by molecular detection of fungal nucleic acids
are preferred (63). In a
multicenter retrospective study, 51 children with AL combined with
CNS fungal infections were analyzed, of whom six patients underwent
HSCT and 17 were clinically diagnosed by combining typical imaging
manifestations of fungal infections of the CNS with positive
microbial results in the cerebrospinal fluid or a positive BDG or
GM assay. The proposed diagnosis was made in 34 cases with only
typical imaging manifestations of fungal infections of the CNS. The
median time from fever to diagnosis was 5 days for all patients,
and the most common fungal pathogen was Aspergillus spp. In
total, 16 patients received monotherapy and 35 received combination
antifungal therapy, whereas 23 patients underwent surgery. A total
of 22 patients eventually succumbed to the disease, and 10 other
patients had neurological sequelae. Early diagnosis and prompt
treatment of childhood AL combined with fungal infections of the
CNS, either antifungal therapy or surgery, are essential to improve
clinical outcomes (64).
Diagnosing pediatric IFD requires overcoming three
key barriers: i) Overlapping symptoms with bacterial infections;
ii) lower sensitivity of GM assays in children compared with
adults; and iii) invasive procedures being less feasible in young
patients. Emerging non-culture techniques such as mNGS show promise
but require pediatric-specific validation (67). Laboratory diagnostic methods for
IFD include traditional fungal tests such as microscopic smear
microscopy, culture and histopathological examination, as well as
laboratory diagnostic tools such as the BDG and GM assays. The BDG
assay detects cell wall components of fungi, such as the
polysaccharide component of the cell wall of yeast-like fungi. The
cell wall component, BDG is a component of numerous pathogenic
fungi, and it is widely used as a diagnostic tool in clinical
practice in assay form, including in children and neonates
(68,69).
The diagnosis of IFD can be categorized into four
levels: Confirmed, clinically diagnosed, proposed and undetermined.
When the condition of a patient is critical and histopathological
biopsy is limited, the diagnosis of IFD consists of host factors
(for example, severe immunodeficiency), clinical manifestations and
microbiological basis for confirmation of the diagnosis in addition
to host factors and identifying microorganisms in the
histopathology (73). The basis
for the proposed diagnosis requires a host factor and a major
clinical manifestations, especially typical imaging basis and a
laboratory basis for the diagnosis of IFD diagnostic grading to the
clinical diagnosis (74). For
instance, the BDG/GM assay demonstrates high sensitivity in
diagnosing pulmonary IFD; a positive BDG/GM result, combined with
definitive lung imaging findings, supports a clinical diagnosis of
IFD. Confirmatory diagnosis requires fiberoptic bronchoscopy with
lavage or tissue biopsy for pathological examination, when feasible
(75).
The consensus on IFD was revised and updated by the
European Organization for Research and Treatment of Cancer and the
Fungal Disease Research Group Education and Research Federation in
2020(31). This consensus suggests
that there has been a marked increase in evidence for using GM to
diagnose IFDs such as IA, and the detection of BDG assay should
also be expanded to a wider range of patients. At present, an
increasing number of fungal PCR have undergone considerable
standardization, coupled with the availability of commercial
analysis, external quality assessment schemes and a large quantity
of performance validation data, which can be widely used for
screening and diagnosing IFDs (76). Molecular diagnostics exhibit
pediatric-specific characteristics: Fungal PCR achieves 92%
sensitivity in children using whole blood samples (minimum volume,
3 ml), compared with 78% with serum samples, reflecting higher
fungal burdens in pediatric hematological malignancies (77). mNGS shows superior performance in
pediatric pulmonary IFD, with 15% higher detection rates compared
with adults, although environmental fungal DNA contamination may
cause false positives in 8-12% of cases (67,78).
For infections caused by Aspergillus, Candida and
Pneumocystis jirovecii, PCR testing combined with
serological assays is recommended to enhance diagnostic accuracy
(77).
IFD often involves the lower respiratory tract and
fungemia, among which, lung infection is the most common (79). Chest CT performance is complex and
varied, generally manifested as nodular or a patchy shadow.
Ocassionally, a typical halo or crescent sign can be observed,
which is a relatively more characteristic change (80). Some patients may present
non-characteristic changes in the early stage of the disease, such
as bronchial dilatation sign, buds sign, hairy glass shadow, solid
shadow and tiny nodular shadow (81). Ground glass shadows are notable for
early diagnosis and are characteristic of Aspergillus airway
infiltration. It is also important to note that new signs of
infiltration on CT imaging should also be considered, and early use
of effective antifungal medications is a key factor in good
control. IFD is difficult to diagnose early due to the lack of
diagnostic indicators with high specificity and sensitivity
(82). In patients with
hematological malignancies, the presence of characteristic imaging
changes in the lungs combined with a positive specific BDG/GM assay
can clinically confirm the diagnosis. Since BDG is widely present
in fungal cell walls, the BDG assay is not effective in
differentiating Aspergillus from Candida (21), whereas the GM assay is mostly used
to carry out the diagnosis of Aspergillus infections
(70).
Invasive trichothecenes are common in children after
chemotherapy for hematological malignancies, especially after HSCT.
The clinical manifestations of trichothecenes are varied and depend
mainly on the site of infection, which is commonly skin, nasal and
lung infections (83). A previous
multicenter study was conducted to analyze the characteristics of
hematological malignancies involving trichothecenes in children. In
a cohort of 39 pediatric patients with combined trichothecenes, 92%
of trichothecene cases occurred in patients with AL, with a notable
association with high-risk ALL and advancing age, and a total of 15
patients (38%) succumbed to trichothecenes (84). Pulmonary mucormycosis may cause
non-specific segmental or lobar bronchopneumonia with cavitation
similar to aspergillosis and imaging may help to determine the
extent of mucormycosis infection (85,86).
Current serological testing for the diagnosis of trichophytosis is
limited and usually relies on clinical presentation, tissue biopsy
or culture results for diagnosis and PCR can assist in identifying
the pathogen (87).
IFD treatment using drugs can be categorized into
monotherapy and combination therapy according to the presence or
absence of clinical manifestations at the beginning of treatment in
patients with high-risk factors, as well as the type and outcome of
obtaining a diagnostic basis for IFD (88). The choice of therapeutic agents is
based on a combination of factors, including condition of the
patient, the local epidemiology of the fungus, previous antifungal
therapy, and drug metabolism and sensitization results. IFD risk
factors should be considered in clinical practice, and the
increasing age of children should also be taken into account when
assessing IFD risk (89). IFD
treatment strategies can be categorized as preventive, empirical,
diagnosis-driven or targeted. IFD needs to be treated aggressively
due to its diverse clinical manifestations and high mortality rate,
which notably affects the efficacy of chemotherapy (90). Common first-line antifungal agents
for treating IFD include voriconazole injection, itraconazole
injection, caspofungin and amphotericin B (91,92).
These agents may be administered as monotherapy or in combination
regimens, such as voriconazole with caspofungin, voriconazole with
amphotericin B or caspofungin with amphotericin B (93,94).
Previous studies on the treatment of pediatric hematological
malignancies combined with IFD are shown in Table II (95-99).
Prophylaxis includes primary prevention and
re-prophylaxis. Primary prophylaxis refers to the pre-application
of antifungal medications in patients with risk factors for IFD
before the patients develop symptoms of infection, with recommended
medications such as posaconazole, fluconazole, itraconazole and
voriconazole. Posaconazole, micafungin, fluconazole, itraconazole,
voriconazole and caspofungin are recommended for patients
undergoing allo-HSCT transplantation (100). Posaconazole is a triazole
antifungal drug that is available as an intravenous solution, oral
suspension and extended-release tablets, with the oral suspension
being the preferred formulation for pediatric use (101). Micafungin is an intravenous
echinocandin with activity against Candida and
Aspergillus spp; it has a favorable safety profile compared
with other antifungal drugs and is one of the more desirable
options for IFD prophylaxis of hematological malignancies in
children. Secondary prophylaxis refers to the administration of
antifungal medications to prevent recurrence of IFD in patients
with a prior history of confirmed or clinically diagnosed IFD who
are being treated again with chemotherapy or HSCT. Re-prophylaxis
recommended medications are preferred to those effective on
previous antifungal therapy, at the same dosage as for primary
prevention. This is due to the fact that the pathogen may have
developed resistance or tolerance to previously used antifungal
agents, and maintaining the same dosage ensures therapeutic
efficacy while minimizing the risk of suboptimal exposure that
could promote further resistance (102-104).
Additionally, consistent dosing simplifies clinical management and
reduces errors in high-risk populations such as allo-HSCT
recipients (105). The course of
prophylactic therapy is largely dependent on the improvement of the
risk factors of the patient for IFD and generally covers ≥3 months
post-transplantation in patients undergoing HSCT. In a prospective
study comparing caspofungin with fluconazole for the prevention of
IFD during post-chemotherapy neutropenia in children, adolescents
and young adults with AML, there were 23 cases of comorbid IFD in
517 patients, including 6 cases of caspofungin and 17 cases of
comorbid IFD after fluconazole prophylaxis and the pathogenic
organisms included 14 species of molds, seven species of yeasts and
two species of unclassified fungi. The cumulative incidence of IFD
was 0.5% in the caspofungin group and 3.1% in the fluconazole group
(106).
Empirical treatment is generally defined as
persistent granulocyte deficiency with fever after chemotherapy in
children and ineffective treatment with broad-spectrum antibiotics
for 4-7 days as the main criteria for initiating treatment
(107). Risk factors for IFD
after chemotherapy in children at high risk for hematological
malignancies should be one of the following: i) Expected absolute
neutrophil count <0.1x109/l for >7 days; ii)
development of hemodynamically unstable clinical comorbidities;
iii) oral or gastrointestinal mucositis and dysphagia; iv)
intravascular catheter infections; v) new-onset pulmonary
infiltrates or hypoxemia; and vi) hepatic insufficiency or renal
insufficiency (108,109). The pathogens of IFD in pediatric
hematological malignancies are predominantly Aspergillus;
thus, broad-spectrum antibiotics covering Aspergillus are
generally selected, and empirical treatment of IFD starting with
fever after chemotherapy for pediatric hematological malignancies
without any microbiological or imaging evidence is aimed at early
initiation of antifungal agents to reduce the morbidity and
mortality associated with IFD, this has become a standard of care
in the clinic (110). Empirical
treatment should be accompanied by an active search for infectious
lesions, microbiological and imaging tests, such as fungal
cultures, non-culture microbiological tests and chest CT, as well
as tests such as bronchoscopy or biopsy when the condition of the
patient permits, in order to facilitate the diagnosis of IFD and
the adjustment of empirical treatment (111). For febrile neutropenia after
chemotherapy for hematological malignancies in children, most
centers prefer empiric treatment, and the recommended drugs for
empiric treatment are itraconazole, caspofungin, micafungin,
liposomal amphotericin B, amphotericin B and voriconazole.
First-line treatment for candidemia includes fluconazole or
liposomal amphotericin B, while voriconazole is the first-line
treatment for IA (112,113).
Diagnosis-driven treatment refers to the combination
of clinical imaging markers of IFD such as the presence of
Aspergillus infection-related imaging changes on lung CT and
microbiological markers such as a positive BDG/GM assay, positive
fungal culture or microscopic examination of specimens obtained
from non-sterile sites or non-sterile manipulations in pediatric
hematological malignancies patients after chemotherapy; this is in
the absence of clinical symptoms of infection, or in the presence
of a persistent neutrophilic deficiency fever ineffective on
treatment with a broad-spectrum antibiotics. For low-risk patients
with IFD, empiric antifungal therapy, which is also
diagnosis-driven therapy, is recommended in the presence of a
diagnostic basis for IFD such as clinical imaging abnormalities or
a positive serum BDG/GM assay (114).
Comparative analyses reveal distinct
pathophysiological features between pediatric and adult IFD:
Pediatric patients exhibit increased serum BDG levels (median 120
vs. 80 pg/ml in adults) and delayed GM antigenemia positivity
(median 5 vs. 3 days post-symptom onset), contributing to
diagnostic challenges unique to children (21,70).
Notably, CNS involvement occurs in 38% of pediatric IFD cases vs.
12% in adults, with Aspergillus predominating in both groups
but showing increased mucormycosis prevalence in pediatric AML (7.2
vs. 2.1%) (64,84). IFDs are rare in individuals with
intact immune systems; however, children who have relatively low
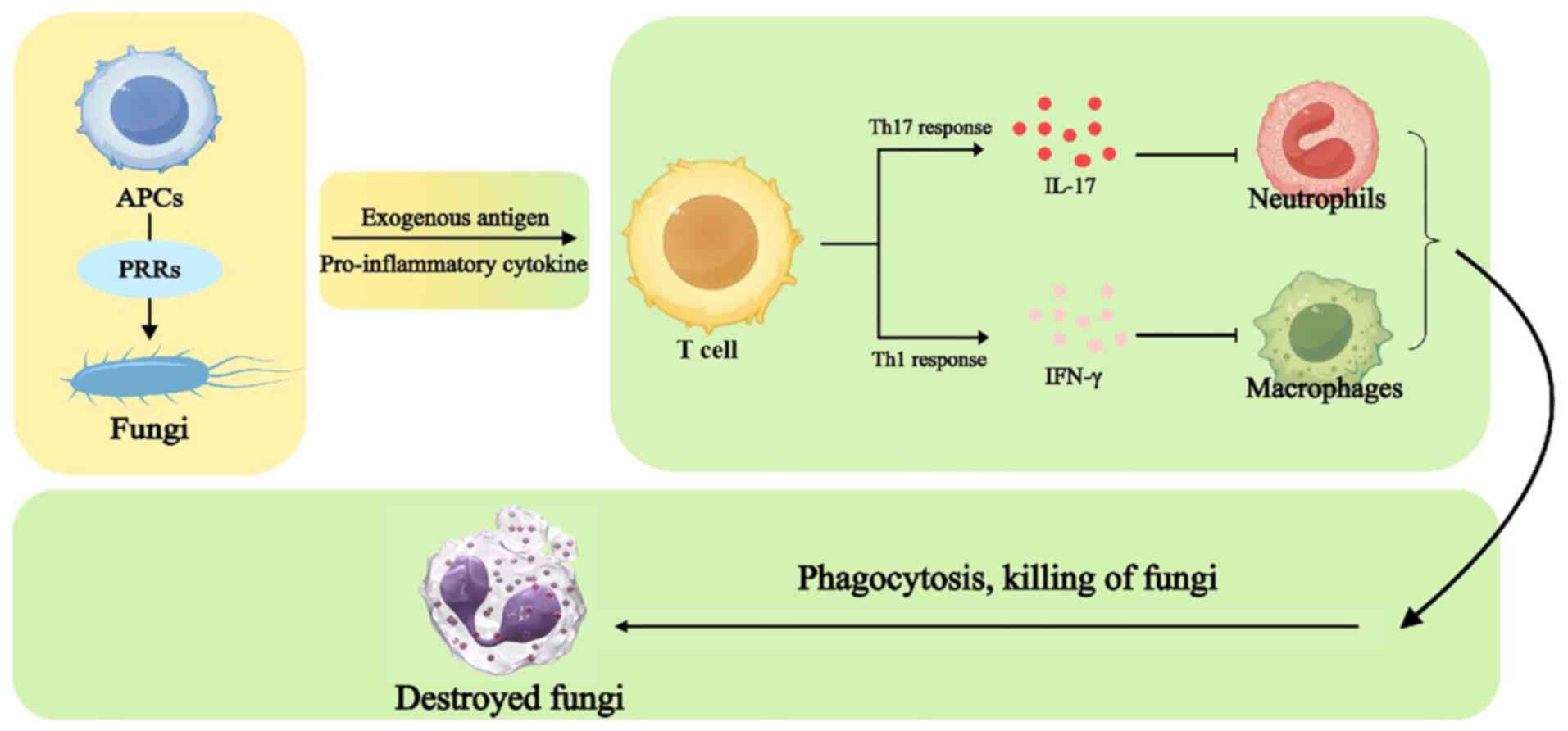
immunity are susceptible to IFD. In response to IFD innate and
adaptive immune responses, several pattern recognition receptors on
antigen-presenting cells (APCs) recognize the fungus. Exogenous
antigens and proinflammatory cytokines presented by APCs promote
T-cell activation; secretion of IL-17 by T helper (Th)17 cells
promote the production of chemokines recruited by neutrophils.
Interferon γ induced by Th1 cells activates macrophages, which,
together with neutrophils, phagocytose and kill the fungus. The
mechanisms of innate and adaptive immune responses in IFD are shown
in Fig. 3. This may be one of the
main reasons why children with low immune function are at risk of
IFD. The heightened vulnerability of immunocompromised children
stems from specific defects in both innate and adaptive immune
responses. For instance, neutrophils from pediatric patients
post-HSCT exhibit impaired phagocytic capacity and reduced
oxidative burst activity compared with healthy controls (118).
IFD in pediatric patients with hematological
malignancies undergoing chemotherapy or HSCT presents three major
clinical dilemmas: The higher prevalence of IFD in specific
subgroups, non-specific manifestations complicating early diagnosis
and the emergence of breakthrough infections during prophylaxis or
therapy. First, for the IFD prevalence profile, which is higher in
children with AML and allo-HSCT combined with IFD, this group of
patients requires further clinical attention and therapeutic
management. Second, the clinical presentation increases the
difficulty of diagnosing and treating IFD; common symptoms of IFD,
including fever, cough and dyspnea, exhibit non-specific
characteristics that overlap with bacterial or viral infections.
Furthermore, bIFD can develop during AP or therapy as a consequence
of pathogen profile shifts or resistance evolution (119). The management of bIFD in
immunocompromised children presents multifaceted challenges.
Diagnostic complexity is exacerbated under AP. Conventional
biomarkers such as BDG may lose specificity due to cross-reactivity
with gut commensals, while tissue sampling is often delayed by
thrombocytopenia and bleeding risks (22).
Therapeutic strategies diverge markedly between
populations: Pediatric IFD requires 30% higher voriconazole doses
compared with adult doses, to achieve therapeutic trough levels,
while echinocandin clearance is 1.5-fold faster in children
necessitating weight-adjusted dosing (101,116). Combination therapy shows superior
outcomes in pediatric cohorts compared with adults, particularly
for CNS infections where blood-brain barrier penetration differs
developmentally (122).
Specifically, voriconazole demonstrates non-linear pharmacokinetics
in children <12 years, requiring 30-50% increased
weight-adjusted doses than adults to achieve therapeutic trough
levels (2-6 mg/l) (101,123). Similarly, posaconazole exhibits
40% lower bioavailability in pediatric patients compared with
adults, necessitating therapeutic drug monitoring (13,19).
Implementation of first-line prophylaxis has yielded notable
protective effects, and early diagnosis and prompt treatment,
including antifungals and surgery, are key to improving patient
survival. The use of new diagnostic techniques has helped in the
rapid clinical identification of the causative fungus and the
timely development of therapeutic strategies. The role of early and
accurate diagnosis in the initial stages of active containment of
fungal infections has become critical in preventing the development
of life-threatening conditions. The growing clinical demands in
medical mycology have catalyzed a diagnostic evolution from
conventional microscopy and culture-based methods to advanced
non-culture platforms. A total of four cutting-edge approaches,
namely mNGS, novel PCR systems, next-generation biosensors and
nanotechnology-enhanced tools, collectively demonstrate superior
pathogen detection capabilities (124-126).
These innovations address critical limitations of traditional
isolation techniques, including suboptimal sensitivity and
culturability constraints (67).
Diagnostic advancements highlight age-specific considerations; mNGS
exhibits 92% sensitivity in pediatric pulmonary IFD vs. 78% in
adults, which is attributable to higher fungal burden in children
(67). Conversely, the specificity
of GM assay drops to 67% in children <5 years due to
cross-reacting dietary GMs, compared with 89% in older populations
(10). These differences
underscore the need for pediatric-specific diagnostic
algorithms.
In terms of antifungal regimen selection,
itraconazole, voriconazole, amphotericin B and caspofungin are
generally selected for patients in whom Candida is the
primary source of infection. For Aspergillus infections,
voriconazole continues to be used as the preferred regimen. When
comparing caspofungin monotherapy and voriconazole combination
therapy, voriconazole monotherapy or in combination with
caspofungin resulted in a markedly lower IFD-related mortality
compared with caspofungin monotherapy (122). The selection between monotherapy
and combination antifungal therapy requires consideration of
multiple factors, where infection characteristics carry out a key
role; for example, pulmonary involvement favors voriconazole
monotherapy (127).
Trichophytosis is a rare but emerging life-threatening fungal
disease with limited therapeutic options (128), and the novel antifungal agent
esaconazole, a new triazole, has demonstrated efficacy in both
initial and salvage treatment of trichophytosis in adults and
children, offering more effective therapeutic options for
trichophytosis and other fungal infections (129,130). There is an increasing number of
novel antifungal agents that may be used in the future for
pediatric hematological malignancies during chemotherapy or after
allo-HSCT, such as encochleated amphotericin B deoxycholate,
isavuconazole, olorofim, opelconazole, oteseconazole, fosmanogepix,
ibrexafungerp and rezafungin (13,19).
Early diagnosis and effective treatment of IFD after
chemotherapy and HSCT for pediatric hematological malignancies
requires multiple tools to overcome clinical challenges and improve
patient prognosis. Literature analysis shows that, compared with
bacterial and viral infections, chemotherapy for hematological
malignancies in children combined with IFD only accounts for a
minority of cases, but its impact may be much more severe,
especially in cases where long-term antifungal therapy or even
surgical treatment is required to eradicate colonization (27,131,132). A personalized approach is
recommended, as pediatric patients with hematological malignancies
usually present with different comorbidities that require
tailor-made treatments (133).
Pediatric hematological malignancies, particularly patients with
AML and relapsed patients, are prone to IFD, and the major
challenges facing physicians include the diversity of pathogenic
organisms, the difficulty of early identification and diagnosis,
and the efficacy and safety of drug therapy.
While the present review synthesizes current
evidence on IFD management in pediatric hematological malignancies,
several limitations warrant acknowledgment. First, the majority of
the included studies are retrospective, introducing potential
selection bias and heterogeneity in diagnostic criteria across
centers. Second, pediatric-specific pharmacokinetic data remain
scarce for newer antifungals such as isavuconazole and rezafungin
(13,19). Third, the diagnostic accuracy of
biomarkers (BDG/GM assay) shows notable inter-study variability in
pediatric cohorts (sensitivity range, 62-89%), reflecting unmet
standardization needs (70,72).
These gaps highlight the necessity for prospective multicenter
studies using harmonized protocols. Further research and clinical
practice are needed to advance multiple domains, including the
development of novel antifungal agents, enhancement of
pharmacological prophylaxis strategies, optimization of rapid
pathogen detection methods and exploration of more effective
infection control strategies.
The management of IFD in pediatric hematological
malignancies post-chemotherapy and allo-HSCT remains a key
challenge, necessitating a multifaceted approach to optimize
outcomes. The present review underscores that children with AML and
those undergoing allogeneic HSCT face increased susceptibility to
IFD due to severe immunosuppression, with innate and adaptive
immune dysregulation exacerbating fungal pathogenicity. The
non-specific clinical manifestations of IFD, overlapping with
bacterial or viral infections, coupled with the pathogen diversity
and frequent emergence of breakthrough infections, necessitate
advancements in diagnostic precision.
Emerging non-culture-based diagnostic modalities,
including mNGS and nanotechnology-enhanced assays, offer high
resolution in pathogen identification, enabling timely and targeted
therapeutic interventions; however, challenges such as lack of
standardization, high costs and complex result interpretation
persist (134). For instance,
mNGS may yield false positives due to sample contamination in
immunocompromised hosts, necessitating integration with
conventional culture and clinical context. While voriconazole
retains its primacy in treating Aspergillus infections,
combination therapies (such as voriconazole with caspofungin)
demonstrate marked mortality reduction compared with monotherapy.
Novel antifungals, including esaconazole and rezafungin, expand the
therapeutic arsenal, particularly for refractory cases such as
trichophytosis, although their pediatric-specific safety and
efficacy profiles warrant further validation (135,136).
The present review has several limitations: First,
the heterogeneity in study designs (namely, high proportion of
retrospective studies) may affect result consistency; second, some
data derive from single-center studies with limited sample
representativeness; and third, rare pathogens or special
populations (such as congenital immunodeficiency) were not deeply
analyzed. Therefore, future multicenter prospective studies are
warranted to validate conclusions. Future efforts should prioritize
the development of pediatric-optimized antifungals, enhanced
pharmacovigilance frameworks and scalable rapid diagnostics to
address the persistent gaps in managing this life-threatening
complication. Ultimately, linking basic research, translational
medicine and clinical practice is essential to redefine the
diagnostic and therapeutic criteria for IFD in pediatric
haemato-oncology. A key unmet need is pediatric-focused
pharmacokinetic studies on antifungal agents. Current dosing
regimens for novel antifungals such as isavuconazole and rezafungin
are primarily extrapolated from adult data, despite documented
age-dependent variations in drug metabolism. Future research should
establish age-stratified dosing guidelines through prospective
multicenter trials incorporating population pharmacokinetic
modeling.
Not applicable.
Funding: The present review was supported by the Municipal
Financial Subsidy of Nanshan District Medical Key Discipline
Construction, Shenzhen Nanshan District Health System Science and
Technology Major Project (grant no. NSZD2023018) and the National
Health Commission Key Laboratory of Nuclear Technology Medical
Transformation (Mianyang Central Hospital; grant no.
2023HYX033).
Not applicable.
The present review was conceptualized by MH, FC and
XX. The original draft was written by MH. Writing, reviewing and
editing of the manuscript content was performed by ZG. The present
work was supervised by ZG, who also acquired funding. All authors
have read and approved the final manuscript. Data authentication is
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Adzic-Vukicevic T, Mladenovic M, Jovanovic
S, Soldatović I and Radovanovic-Spurnic A: Invasive fungal disease
in COVID-19 patients: A single-center prospective observational
study. Front Med (Lausanne). 10(1084666)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu Q, Chen P, Xin L, Zhang J and Jiang M:
A rare intestinal mucormycosis caused by Lichtheimia ramosa in a
patient with diabetes: A case report. Front Med (Lausanne).
11(1435239)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mori G, Diotallevi S, Farina F, Lolatto R,
Galli L, Chiurlo M, Acerbis A, Xue E, Clerici D, Mastaglio S, et
al: High-Risk neutropenic fever and invasive fungal diseases in
patients with hematological malignancies. Microorganisms.
12(117)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun Y, Huang H, Chen J, Li J, Ma J, Li J,
Liang Y, Wang J, Li Y, Yu K, et al: Invasive fungal infection in
patients receiving chemotherapy for hematological malignancy: A
multicenter, prospective, observational study in China. Tumour
Biol. 36:757–767. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li C, Zhu DP, Chen J, Zhu XY, Li NN, Cao
WJ, Zhang ZM, Tan YH, Hu XX, Yuan HL, et al: Invasive fungal
disease in patients undergoing allogeneic hematopoietic stem cell
transplantation in China: A multicenter epidemiological study
(CAESAR 2.0). Clin Infect Dis. 80:807–816. 2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schmiedel Y and Zimmerli S: Common
invasive fungal diseases: An overview of invasive candidiasis,
aspergillosis, cryptococcosis, and Pneumocystis pneumonia.
Swiss Med Wkly. 146(w14281)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Richardson M and Lass-Flörl C: Changing
epidemiology of systemic fungal infections. Clin Microbiol Infect.
14 (Suppl 4):S5–S24. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zabolinejad N, Naseri A, Davoudi Y, Joudi
M and Aelami MH: Colonic basidiobolomycosis in a child: Report of a
culture-proven case. Int J Infect Dis. 22:41–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huppler AR, Fisher BT, Lehrnbecher T,
Walsh TJ and Steinbach WJ: Role of molecular biomarkers in the
diagnosis of invasive fungal diseases in children. J Pediatric
Infect Dis Soc. 6 (Suppl 1):S32–S44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fisher BT, Westling T, Boge CLK, Zaoutis
TE, Dvorak CC, Nieder M, Zerr DM, Wingard JR, Villaluna D,
Esbenshade AJ, et al: Prospective evaluation of galactomannan and
(1→3) β-d-glucan assays as diagnostic tools for invasive fungal
disease in children, adolescents, and young adults with acute
myeloid leukemia receiving fungal prophylaxis. J Pediatric Infect
Dis Soc. 10:864–871. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang J, Liu C and Zheng X: Clinical
features of invasive fungal disease in children with no underlying
disease. Sci Rep. 12(208)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lehrnbecher T, Hassler A, Groll AH and
Bochennek K: Diagnostic approaches for invasive
aspergillosis-specific considerations in the pediatric population.
Front Microbiol. 9(518)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zimmermann P, Brethon B, Roupret-Serzec J,
Caseris M, Goldwirt L, Baruchel A and de Tersant M: Isavuconazole
treatment for invasive fungal infections in pediatric patients.
Pharmaceuticals (Basel). 15(375)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rosen GP, Nielsen K, Glenn S, Abelson J,
Deville J and Moore TB: Invasive fungal infections in pediatric
oncology patients: 11-year experience at a single institution. J
Pediatr Hematol Oncol. 27:135–140. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blauwkamp TA, Thair S, Rosen MJ, Blair L,
Lindner MS, Vilfan ID, Kawli T, Christians FC, Venkatasubrahmanyam
S, Wall GD, et al: Analytical and clinical validation of a
microbial cell-free DNA sequencing test for infectious disease. Nat
Microbiol. 4:663–674. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schlaberg R, Chiu CY, Miller S, Procop GW
and Weinstock G: Professional Practice Committee and Committee on
Laboratory Practices of the American Society for Microbiology;
Microbiology Resource Committee of the College of American
Pathologists. Validation of metagenomic next-generation sequencing
tests for universal pathogen detection. Arch Pathol Lab Med.
141:776–786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Agudelo-Pérez S, Fernández-Sarmiento J,
Rivera León D and Peláez RG: Metagenomics by next-generation
sequencing (mNGS) in the etiological characterization of neonatal
and pediatric sepsis: A systematic review. Front Pediatr.
11(1011723)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wilke J, Ramchandar N, Cannavino C, Pong
A, Tremoulet A, Padua LT, Harvey H, Foley J, Farnaes L and Coufal
NG: Clinical application of cell-free next-generation sequencing
for infectious diseases at a tertiary children's hospital. BMC
Infect Dis. 21(552)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hsu AJ, Hanisch BR, Fisher BT and Huppler
AR: Pipeline of novel antifungals for invasive fungal disease in
transplant recipients: A pediatric perspective. J Pediatric Infect
Dis Soc. 13 (Suppl 1):S68–S79. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ávila Montiel D, Saucedo Campos A, Avilés
Robles M, Murillo Maldonado MA, Jiménez Juárez R, Silva Dirzo M and
Dorantes Acosta E: Fungal infections in pediatric patients with
acute myeloid leukemia in a tertiary hospital. Front Public Health.
11(1056489)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Otto WR, Dvorak CC, Boge CLK,
Ostrosky-Zeichner L, Esbenshade AJ, Nieder ML, Alexander S,
Steinbach WJ, Dang H, Villaluna D, et al: Prospective evaluation of
the fungitell® (1→3) beta-D-glucan assay as a diagnostic
tool for invasive fungal disease in pediatric allogeneic
hematopoietic cell transplantation: A report from the children's
oncology group. Pediatr Transplant. 27(e14399)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Olivier-Gougenheim L, Rama N, Dupont D,
Saultier P, Leverger G, AbouChahla W, Paillard C, Gandemer V,
Theron A, Freycon C, et al: Invasive fungal infections in
immunocompromised children: Novel insight following a national
study. J Pediatr. 236:204–210. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fisher BT, Robinson PD, Lehrnbecher T,
Steinbach WJ, Zaoutis TE, Phillips B and Sung L: Risk factors for
invasive fungal disease in pediatric cancer and hematopoietic stem
cell transplantation: A systematic review. J Pediatric Infect Dis
Soc. 7:191–198. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yeoh DK, Moore AS, Kotecha RS, Bartlett
AW, Ryan AL, Cann MP, McMullan BJ, Thursky K, Slavin M, Blyth CC,
et al: Invasive fungal disease in children with acute myeloid
leukaemia: An Australian multicentre 10-year review. Pediatr Blood
Cancer. 68(e29275)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mor M, Gilad G, Kornreich L, Fisher S,
Yaniv I and Levy I: Invasive fungal infections in pediatric
oncology. Pediatr Blood Cancer. 56:1092–1097. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Calle-Miguel L, Garrido-Colino C,
Santiago-García B, Moreno Santos MP, Gonzalo Pascual H, Ponce Salas
B, Beléndez Bieler C, Navarro Gómez M, Guinea Ortega J and
Rincón-López EM: Changes in the epidemiology of invasive fungal
disease in a Pediatric hematology and oncology unit: The relevance
of breakthrough infections. BMC Infect Dis. 23(348)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lin GL, Chang HH, Lu CY, Chen CM, Lu MY,
Lee PI, Jou ST, Yang YL, Huang LM and Chang LY: Clinical
characteristics and outcome of invasive fungal infections in
pediatric acute myeloid leukemia patients in a medical center in
Taiwan. J Microbiol Immunol Infect. 51:251–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sezgin Evim M, Tüfekçi Ö, Baytan B, Ören
H, Çelebi S, Ener B, Üstün Elmas K, Yılmaz Ş, Erdem M,
Hacımustafaoğlu MK and Güneş AM: Invasive fungal infections in
children with leukemia: Clinical features and prognosis. Turk J
Haematol. 39:94–102. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tüfekçi Ö, Yılmaz Bengoa Ş, Demir
Yenigürbüz F, Şimşek E, Karapınar TH, İrken G and Ören H:
Management of invasive fungal infections in pediatric acute
leukemia and the appropriate time for restarting chemotherapy. Turk
J Haematol. 32:329–337. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lehrnbecher T, Groll AH, Cesaro S, Alten
J, Attarbaschi A, Barbaric D, Bodmer N, Conter V, Izraeli S, Mann
G, et al: Invasive fungal diseases impact on outcome of childhood
ALL-an analysis of the international trial AIEOP-BFM ALL 2009.
Leukemia. 37:72–78. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Donnelly JP, Chen SC, Kauffman CA,
Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR,
Lockhart SR, Groll AH, et al: Revision and update of the consensus
definitions of invasive fungal disease from the european
organization for research and treatment of cancer and the mycoses
study group education and research consortium. Clin Infect Dis.
71:1367–1376. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jenks JD, Cornely OA, Chen SC, Thompson GR
III and Hoenigl M: Breakthrough invasive fungal infections: Who is
at risk? Mycoses. 63:1021–1032. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liberatore C, Farina F, Greco R, Giglio F,
Clerici D, Oltolini C, Lupo Stanghellini MT, Barzaghi F, Vezzulli
P, Orsenigo E, et al: Breakthrough invasive fungal infections in
allogeneic hematopoietic stem cell transplantation. J Fungi
(Basel). 7(347)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cornely OA, Hoenigl M, Lass-Flörl C, Chen
SC, Kontoyiannis DP, Morrissey CO and Thompson GR III: Mycoses
Study Group Education and Research Consortium (MSG-ERC) and the
European Confederation of Medical Mycology (ECMM). Defining
breakthrough invasive fungal infection-position paper of the
mycoses study group education and research consortium and the
European confederation of medical mycology. Mycoses. 62:716–729.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Arendrup MC, Arikan-Akdagli S, Jørgensen
KM, Barac A, Steinmann J, Toscano C, Arsenijevic VA, Sartor A,
Lass-Flörl C, Hamprecht A, et al: European candidaemia is
characterised by notable differential epidemiology and
susceptibility pattern: Results from the ECMM Candida III
study. J Infect. 87:428–437. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Marín Martínez EM, Aller García AI and
Martín-Mazuelos E: Epidemiology, risk factors and in vitro
susceptibility in candidaemia due to non-Candida albicans
species. Rev Iberoam Micol. 33:248–252. 2016.(In Spanish).
|
|
37
|
Castagnola E, Mariani M, Ricci E, Russo C,
Saffioti C and Mesini A: Fungal infections in pediatric patients:
Challenges and considerations in treatment. Expert Rev Anti Infect
Ther: Oct 12, 2025 (Epub ahead of print).
|
|
38
|
Popova M and Rogacheva Y: Epidemiology of
invasive fungal diseases in patients with hematological
malignancies and haematopoietic cell transplantation recipients:
Systematic review and meta-analysis of trends over time. J Infect
Public Health. 18(102804)2025.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lehrnbecher T: The clinical management of
invasive mold infection in children with cancer or undergoing
hematopoietic stem cell transplantation. Expert Rev Anti Infect
Ther. 17:489–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Groll AH, Pana D, Lanternier F, Mesini A,
Ammann RA, Averbuch D, Castagnola E, Cesaro S, Engelhard D,
Garcia-Vidal C, et al: 8th European conference on infections in
leukaemia: 2020 Guidelines for the diagnosis, prevention, and
treatment of invasive fungal diseases in paediatric patients with
cancer or post-haematopoietic cell transplantation. Lancet Oncol.
22:e254–e269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang Q, Lei Y, Wang J, Xu X, Wang L, Zhou
H and Guo Z: Tumor and microecology committee of China anti-cancer
association. Expert consensus on the relevance of intestinal
microecology and hematopoietic stem cell transplantation. Clin
Transplant. 38(e15186)2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Elhaj Mahmoud D, Hérivaux A, Morio F,
Briard B, Vigneau C, Desoubeaux G, Bouchara JP, Gangneux JP, Nevez
G, Le Gal S and Papon N: The epidemiology of invasive fungal
infections in transplant recipients. Biomed J.
47(100719)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pana ZD, Roilides E, Warris A, Groll AH
and Zaoutis T: Epidemiology of invasive fungal disease in children.
J Pediatric Infect Dis Soc. 6 (Suppl 1):S3–S11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Steinbach WJ and Fisher BT: International
collaborative on contemporary epidemiology and diagnosis of
invasive fungal disease in children. J Pediatric Infect Dis Soc. 6
(Suppl 1):S1–S2. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Czyżewski K, Gałązka P, Frączkiewicz J,
Salamonowicz M, Szmydki-Baran A, Zając-Spychała O,
Gryniewicz-Kwiatkowska O, Zalas-Więcek P, Chełmecka-Wiktorczyk L,
Irga-Jaworska N, et al: Epidemiology and outcome of invasive fungal
disease in children after hematopoietic cell transplantation or
treated for malignancy: Impact of national programme of antifungal
prophylaxis. Mycoses. 62:990–998. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wu X, Ma X, Song T, Liu J, Sun Y and Wu D:
The indirect effects of CMV reactivation on patients following
allogeneic hematopoietic stem cell transplantation: An evidence
mapping. Ann Hematol. 103:917–933. 2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
van der Maas NG, von Asmuth EGJ, Berghuis
D, van Schouwenburg PA, Putter H, van der Burg M and Lankester AC:
Modeling influencing factors in B-cell reconstitution after
hematopoietic stem cell transplantation in children. Front Immunol.
12(684147)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Marie-Cardine A, Divay F, Dutot I, Green
A, Perdrix A, Boyer O, Contentin N, Tilly H, Tron F, Vannier JP and
Jacquot S: Transitional B cells in humans: characterization and
insight from B lymphocyte reconstitution after hematopoietic stem
cell transplantation. Clin Immunol. 127:14–25. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Maliszewski CR, Sato TA, Vanden Bos T,
Waugh S, Dower SK, Slack J, Beckmann MP and Grabstein KH: Cytokine
receptors and B cell functions. I. Recombinant soluble receptors
specifically inhibit IL-1- and IL-4-induced B cell activities in
vitro. J Immunol. 144:3028–3033. 1990.PubMed/NCBI
|
|
50
|
Pan L, Sato S, Frederick JP, Sun XH and
Zhuang Y: Impaired immune responses and B-cell proliferation in
mice lacking the Id3 gene. Mol Cell Biol. 19:5969–5980.
1999.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Inaba A, Tuong ZK, Zhao TX, Stewart AP,
Mathews R, Truman L, Sriranjan R, Kennet J, Saeb-Parsy K, Wicker L,
et al: Low-dose IL-2 enhances the generation of IL-10-producing
immunoregulatory B cells. Nat Commun. 14(2071)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Roy K, Chakraborty M, Kumar A, Manna AK
and Roy NS: The NFκB signaling system in the generation of B-cell
subsets: from germinal center B cells to memory B cells and plasma
cells. Front Immunol. 14(1185597)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Puerta-Alcalde P and Garcia-Vidal C:
Changing epidemiology of invasive fungal disease in allogeneic
hematopoietic stem cell transplantation. J Fungi (Basel).
7(848)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lehrnbecher T, Schöning S, Poyer F, Georg
J, Becker A, Gordon K, Attarbaschi A and Groll AH: Incidence and
outcome of invasive fungal diseases in children with hematological
malignancies and/or allogeneic hematopoietic stem cell
transplantation: Results of a prospective multicenter study. Front
Microbiol. 10(681)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Said AM, Afridi F, Redell MS, Vrana C,
O'Farrell C, Scheurer ME, Dailey Garnes NJ, Gramatges MM and Dutta
A: Invasive candidiasis in pediatric hematologic malignancy:
Increased risk of dissemination with Candida tropicalis.
Pediatr Infect Dis J. 44:58–63. 2025.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Barantsevich N and Barantsevich E:
Diagnosis and treatment of invasive candidiasis. Antibiotics
(Basel). 11(718)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang L, Wang Y, Hu J, Sun Y, Huang H, Chen
J, Li J, Ma J, Li J, Liang Y, et al: Clinical risk score for
invasive fungal diseases in patients with hematological
malignancies undergoing chemotherapy: China assessment of
antifungal therapy in hematological diseases (CAESAR) study. Front
Med. 13:365–377. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Sun Y, Meng F, Han M, Zhang X, Yu L, Huang
H, Wu D, Ren H, Wang C, Shen Z, et al: Epidemiology, management,
and outcome of invasive fungal disease in patients undergoing
hematopoietic stem cell transplantation in China: A multicenter
prospective observational study. Biol Blood Marrow Transplant.
21:1117–1126. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Celkan T, Kizilocak H, Evim M, Meral Güneş
A, Özbek NY, Yarali N, Ünal E, Patiroğlu T, Yilmaz Karapinar D,
Sarper N, et al: Hepatosplenic fungal infections in children with
leukemia-risk factors and outcome: A multicentric study. J Pediatr
Hematol Oncol. 41:256–260. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Machado M, Fortún J and Muñoz P: Invasive
aspergillosis: A comprehensive review. Med Clin (Barc).
163:189–198. 2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Boyer J, Feys S, Zsifkovits I, Hoenigl M
and Egger M: Treatment of invasive aspergillosis: How it's going,
where it's heading. Mycopathologia. 188:667–681. 2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Duréault A, Tcherakian C, Poiree S,
Catherinot E, Danion F, Jouvion G, Bougnoux ME, Mahlaoui N, Givel
C, Castelle M, et al: Spectrum of pulmonary aspergillosis in
hyper-IgE syndrome with autosomal-dominant STAT3 deficiency. J
Allergy Clin Immunol Pract. 7:1986–1995.e3. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Luckowitsch M, Rudolph H, Bochennek K,
Porto L and Lehrnbecher T: Central nervous system mold infections
in children with hematological malignancies: Advances in diagnosis
and treatment. J Fungi (Basel). 7(168)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Karaman S, Kebudi R, Kizilocak H, Karakas
Z, Demirag B, Evim MS, Yarali N, Kaya Z, Karagun BS, Aydogdu S, et
al: Central nervous system fungal infections in children with
leukemia and undergoing hematopoietic stem cell transplantation: A
retrospective multicenter study. J Pediatr Hematol Oncol.
44:e1039–e1045. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alexander BD, Lamoth F, Heussel CP, Prokop
CS, Desai SR, Morrissey CO and Baddley JW: Guidance on imaging for
invasive pulmonary aspergillosis and mucormycosis: From the imaging
working group for the revision and update of the consensus
definitions of fungal disease from the EORTC/MSGERC. Clin Infect
Dis. 72 (Suppl 2):S79–S88. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wu Y, Yan L, Wang H, et al: Clinical study
on empirical and diagnostic-driven (pre-emptive) therapy of
voriconazole in severe aplastic anaemia patients with invasive
fungal disease after intensive immunosuppressive therapy. Eur J
Clin Microbiol Infect Dis. 40:949–954. 2021.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Fang W, Wu J, Cheng M, Zhu X, Du M, Chen
C, Liao W, Zhi K and Pan W: Diagnosis of invasive fungal
infections: challenges and recent developments. J Biomed Sci.
30(42)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Lamoth F, Cruciani M, Mengoli C,
Castagnola E, Lortholary O, Richardson M and Marchetti O: Third
European Conference on Infections in Leukemia (ECIL-3). β-Glucan
antigenemia assay for the diagnosis of invasive fungal infections
in patients with hematological malignancies: A systematic review
and meta-analysis of cohort studies from the third European
conference on infections in leukemia (ECIL-3). Clin Infect Dis.
54:633–643. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chen M, Xu Y, Hong N, Yang Y, Lei W, Du L,
Zhao J, Lei X, Xiong L, Cai L, et al: Epidemiology of fungal
infections in China. Front Med. 12:58–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Çağlar İ, Özkerim D, Tahta N, Düzgöl M,
Bayram N, Demirağ B, Karapinar TH, Sorguç Y, Gözmen S, Dursun V, et
al: Assessment of serum galactomannan test results of pediatric
patients with hematologic malignancies according to consecutive
positivity and threshold level in terms of invasive aspergillosis
diagnosis: Cross-sectional research in a tertiary care hospital. J
Pediatr Hematol Oncol. 42:e271–e276. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Springer J, Held J, Mengoli C, Schlegel
PG, Gamon F, Träger J, Kurzai O, Einsele H, Loeffler J and Eyrich
M: Diagnostic performance of (1→3)-β-D-glucan alone and in
combination with Aspergillus PCR and galactomannan in serum
of pediatric patients after allogeneic hematopoietic stem cell
transplantation. J Fungi (Basel). 7(238)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Ferreras-Antolin L, Borman A, Diederichs
A, Warris A and Lehrnbecher T: Serum beta-D-glucan in the diagnosis
of invasive fungal disease in neonates, children and adolescents: A
critical analysis of current data. J Fungi (Basel).
8(1262)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Saffioti C, Mesini A, Bandettini R and
Castagnola E: Diagnosis of invasive fungal disease in children: A
narrative review. Expert Rev Anti Infect Ther. 17:895–909.
2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Warris A and Lehrnbecher T: Progress in
the diagnosis of invasive fungal disease in children. Curr Fungal
Infect Rep. 11:35–44. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Otto WR and Green AM: Fungal infections in
children with haematologic malignancies and stem cell transplant
recipients. Br J Haematol. 189:607–624. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cao GJ, Xing ZF, Hua L, Ji YH, Sun JB and
Zhao Z: Evaluation of the diagnostic performance of panfungal
polymerase chain reaction assay in invasive fungal diseases. Exp
Ther Med. 14:4208–4214. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
White PL, Alanio A, Brown L, Cruciani M,
Hagen F, Gorton R, Lackner M, Millon L, Morton CO,
Rautemaa-Richardson R, et al: An overview of using fungal DNA for
the diagnosis of invasive mycoses. Expert Rev Mol Diagn.
22:169–184. 2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Singh S and Singh M, Verma N, Sharma M,
Pradhan P, Chauhan A, Jaiswal N, Chakrabarti A and Singh M:
Comparative accuracy of 1,3 beta-D glucan and galactomannan for
diagnosis of invasive fungal infections in pediatric patients: A
systematic review with meta-analysis. Med Mycol. 59:139–148.
2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wang Z, Pan M and Zhu J: Global burden of
reported lower respiratory system fungal infection. Front Cell
Infect Microbiol. 15(1542922)2025.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Srivali N, Permpalung N, Ammannagari N,
Cheungpasitporn W and Bischof EF: Significance of halo, reversed
halo and air crescent signs in lymphomatoid granulomatosis and
pulmonary fungal infections. Thorax. 68:1070–1071. 2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lamoth F, Prakash K, Beigelman-Aubry C and
Baddley JW: Lung and sinus fungal infection imaging in
immunocompromised patients. Clin Microbiol Infect. 30:296–305.
2024.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Alamdaran SA, Bagheri R, Darvari SF,
Bakhtiari E and Ghasemi A: Pulmonary invasive fungal disease:
Ultrasound and computed tomography scan findings. Thorac Res Pract.
24:292–297. 2023.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Perez P, Patiño J, Franco AA, Rosso F,
Beltran E, Manzi E, Castro A, Estacio M and Valencia DM:
Prophylaxis for invasive fungal infection in pediatric patients
with allogeneic hematopoietic stem cell transplantation. Blood Res.
57:34–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Elitzur S, Arad-Cohen N, Barg A,
Litichever N, Bielorai B, Elhasid R, Fischer S, Fruchtman Y, Gilad
G, Kapelushnik J, et al: Mucormycosis in children with
haematological malignancies is a salvageable disease: A report from
the israeli study group of childhood leukemia. Br J Haematol.
189:339–350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bonifaz A, Tirado-Sánchez A,
Hernández-Medel ML, Araiza J, Kassack JJ, Del Angel-Arenas T,
Moisés-Hernández JF, Paredes-Farrera F, Gómez-Apo E, Treviño-Rangel
RJ and González GM: Mucormycosis at a tertiary-care center in
Mexico. A 35-year retrospective study of 214 cases. Mycoses.
64:372–380. 2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Pagano L, Dragonetti G, De Carolis E,
Veltri G, Del Principe MI and Busca A: Developments in identifying
and managing mucormycosis in hematologic cancer patients. Expert
Rev Hematol. 13:895–905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Antoniadi K, Iosifidis E, Vasileiou E,
Tsipou C, Lialias I, Papakonstantinou E, Kattamis A,
Polychronopoulou S, Roilides E and Tragiannidis A: Invasive
mucormycosis in children with malignancies: Report from the
infection working group of the hellenic society of pediatric
hematology-oncology. J Pediatr Hematol Oncol. 43:176–179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Zhang Z, Bills GF and An Z: Advances in
the treatment of invasive fungal disease. PLoS Pathog.
19(e1011322)2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Sun Y, Hu J, Huang H, Chen J, Li J, Ma J,
Li J, Liang Y, Wang J, Li Y, et al: Clinical risk score for
predicting invasive fungal disease after allogeneic hematopoietic
stem cell transplantation: Analysis of the China assessment of
antifungal therapy in hematological diseases (CAESAR) study.
Transpl Infect Dis. 23(e13611)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhang T, Shen Y and Feng S: Clinical
research advances of isavuconazole in the treatment of invasive
fungal diseases. Front Cell Infect Microbiol.
12(1049959)2022.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Herbrecht R, Denning DW, Patterson TF,
Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P,
Lortholary O, et al: Voriconazole versus amphotericin B for primary
therapy of invasive aspergillosis. N Engl J Med. 347:408–415.
2002.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Turkova A, Roilides E and Sharland M:
Amphotericin B in neonates: Deoxycholate or lipid formulation as
first-line therapy-is there a ‘right’ choice? Curr Opin Infect Dis.
24:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Tissot F, Agrawal S, Pagano L, Petrikkos
G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C and
Herbrecht R: ECIL-6 guidelines for the treatment of invasive
candidiasis, aspergillosis and mucormycosis in leukemia and
hematopoietic stem cell transplant patients. Haematologica.
102:433–444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ruhnke M, Cornely OA, Schmidt-Hieber M,
Alakel N, Boell B, Buchheidt D, Christopeit M, Hasenkamp J, Heinz
WJ, Hentrich M, et al: Treatment of invasive fungal diseases in
cancer patients-Revised 2019 recommendations of the infectious
diseases working party (AGIHO) of the German society of hematology
and oncology (DGHO). Mycoses. 63:653–682. 2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Tu S, Zhang K, Wang N, Chu J, Yang L and
Xie Z: Comparative study of posaconazole and voriconazole for
primary antifungal prophylaxis in patients with pediatric acute
leukemia. Sci Rep. 13(18789)2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lee KH, Lim YT, Hah JO, Kim YK, Lee CH and
Lee JM: Voriconazole plus caspofungin for treatment of invasive
fungal infection in children with acute leukemia. Blood Res.
52:167–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Qiu KY, Liao XY, Fang JP, Xu HG, Li Y,
Huang K and Zhou DH: Combination antifungal treatment for invasive
fungal disease after hematopoietic stem cell transplantation in
children with hematological disorders. Transpl Infect Dis.
21(e13066)2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Goscicki BK, Yan SQ, Mathew S, Mauguen A
and Cohen N: A retrospective analysis of micafungin prophylaxis in
children under 12 years undergoing chemotherapy or hematopoietic
stem cell transplantation. J Pediatr Pharmacol Ther. 29:379–384.
2024.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kazakou N, Vyzantiadis TA, Gambeta A,
Vasileiou E, Tsotridou E, Kotsos D, Giantsidi A, Saranti A,
Palabougiouki M, Ioannidou M, et al: Invasive fungal infections in
a pediatric hematology-oncology department: A 16-year retrospective
study. Curr Med Mycol. 6:37–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Itsaradisaikul S, Pakakasama S,
Boonsathorn S, Techasaensiri C, Rattanasiri S and Apiwattanakul N:
Invasive fungal disease among pediatric and adolescent patients
undergoing itraconazole prophylaxis after hematopoietic stem cell
transplantation. Transplant Proc. 53:2021–2028. 2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
McCann S, Sinha J, Wilson WS, McKinzie CJ,
Garner LM and Gonzalez D: Population pharmacokinetics of
posaconazole in immune-compromised children and assessment of
target attainment in invasive fungal disease. Clin Pharmacokinet.
62:997–1009. 2023.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Delarze E and Sanglard D: Defining the
frontiers between antifungal resistance, tolerance and the concept
of persistence. Drug Resist Updat. 23:12–19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kadariswantiningsih IN, Empitu MA, Santosa
TI and Alimu Y: Antifungal resistance: Emerging mechanisms and
implications (Review). Mol Med Rep. 32(247)2025.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Berman J and Krysan DJ: Drug resistance
and tolerance in fungi. Nat Rev Microbiol. 18:319–331.
2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Okada A, Kariya M, Irie K, Okada Y,
Hiramoto N, Hashimoto H, Kajioka R, Maruyama C, Kasai H, Hamori M,
et al: Population pharmacokinetics of vancomycin in patients
undergoing allogeneic hematopoietic stem-cell transplantation. J
Clin Pharmacol. 58:1140–1149. 2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Fisher BT, Zaoutis T, Dvorak CC, Nieder M,
Zerr D, Wingard JR, Callahan C, Villaluna D, Chen L, Dang H, et al:
Effect of caspofungin vs fluconazole prophylaxis on invasive fungal
disease among children and young adults with acute myeloid
leukemia: A randomized clinical trial. JAMA. 322:1673–1681.
2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Boeriu E, Borda A, Vulcanescu DD, Sarbu V,
Arghirescu ST, Ciorica O, Bratosin F, Marincu I and Horhat FG:
Diagnosis and management of febrile neutropenia in pediatric
oncology patients-a systematic review. Diagnostics (Basel).
12(1800)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Fisher BT: Fungal diagnostic testing and
therapy: Navigating the neutropenic period in children with
high-risk leukemia. Hematology Am Soc Hematol Educ Program.
2021:361–367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Villarroel M, Avilés CL, Silva P, Guzmán
AM, Poggi H, Alvarez AM, Becker A, O'ryan M, Salgado C, Topelberg
S, et al: Risk factors associated with invasive fungal disease in
children with cancer and febrile neutropenia: A prospective
multicenter evaluation. Pediatr Infect Dis J. 29:816–821.
2010.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Wang J, Liang J, He M, Xie Q, Wu Q, Shen
G, Zhu B, Yu J, Yu L, Tan X, et al: Chinese expert consensus on
intestinal microecology and management of digestive tract
complications related to tumor treatment (version 2022). J Cancer
Res Ther. 18:1835–1844. 2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Wang Q, He M, Liang J, Tan X, Wu Q, Wang
J, Li X, Qiao M, Huang Z, Xie Q, et al: Chinese guidelines for
integrated diagnosis and treatment of intestinal microecology
technologies in tumor application (2024 edition). J Cancer Res
Ther. 20:1130–1140. 2024.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Ferreras-Antolín L, Sharland M and Warris
A: Management of invasive fungal disease in neonates and children.
Pediatr Infect Dis J. 38 (6S Suppl 1):S2–S6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Bochennek K, Simon A, Laws HJ, Groll AH
and Lehrnbecher T: Febrile neutropenia in pediatric and adolescent
cancer patients. Monatsschr Kinderheilkd. 169:443–450.
2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
114
|
Ko BS, Chen WT, Kung HC, Wu UI, Tang JL,
Yao M, Chen YC, Tien HF, Chang SC, Chuang YC, et al: 2016 Guideline
strategies for the use of antifungal agents in patients with
hematological malignancies or hematopoietic stem cell
transplantation recipients in Taiwan. J Microbiol Immunol Infect.
51:287–301. 2018.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Hon KLE, Chan VP, Leung AK, Leung KKY and
Hui WF: Invasive fungal infections in critically ill children:
Epidemiology, risk factors and antifungal drugs. Drugs Context.
13(2023-9-2)2024.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Goldman JL and Abdel-Rahman SM:
Pharmacokinetic considerations in treating invasive pediatric
fungal infections. Expert Opin Drug Metab Toxicol. 12:645–655.
2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Maertens J, Pagano L, Azoulay E and Warris
A: Liposomal amphotericin B-the present. J Antimicrob Chemother. 77
(Suppl 2):ii11–ii20. 2022.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Härtel C, Scholz T, Kuhn M, Bendiks M,
Göpel W, Lauten M and Herting E: Innate immune responses to
Stenotrophomonas maltophilia in immunocompromised pediatric
patients and the effect of taurolidine. J Microbiol Immunol Infect.
46:115–120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Ruijters VJ, Oosterom N, Wolfs TFW, van
den Heuvel-Eibrink MM and van Grotel M: Frequency and determinants
of invasive fungal infections in children with solid and
hematologic malignancies in a nonallogeneic stem cell
transplantation setting: A narrative review. J Pediatr Hematol
Oncol. 41:345–354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Giannella M, Lanternier F, Dellière S,
Groll AH, Mueller NJ, Alastruey-Izquierdo A and Slavin MA: ECCMID
study groups on Invasive Fungal Infection and Infection in
Immunocompromised Hosts. Invasive fungal disease in the
immunocompromised host: changing epidemiology, new antifungal
therapies, and management challenges. Clin Microbiol Infect.
31:29–36. 2025.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Jia M, Zhang Q, Qin Z, Wang D, Liu P, Yang
J and Zhang X: Dose optimisation of posaconazole and therapeutic
drug monitoring in pediatric patients. Front Pharmacol.
13(833303)2022.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Raad II, Zakhem AE, Helou GE, Jiang Y,
Kontoyiannis DP and Hachem R: Clinical experience of the use of
voriconazole, caspofungin or the combination in primary and salvage
therapy of invasive aspergillosis in haematological malignancies.
Int J Antimicrob Agents. 45:283–288. 2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
John J, Loo A, Mazur S and Walsh TJ:
Therapeutic drug monitoring of systemic antifungal agents: A
pragmatic approach for adult and pediatric patients. Expert Opin
Drug Metab Toxicol. 15:881–895. 2019.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Kanaujia R, Biswal M, Angrup A and Ray P:
Diagnostic accuracy of the metagenomic next-generation sequencing
(mNGS) for detection of bacterial meningoencephalitis: A systematic
review and meta-analysis. Eur J Clin Microbiol Infect Dis.
41:881–891. 2022.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Gutiérrez-García L, Esteban-Cantos A,
Rodríguez-Centeno J, Marcelo-Calvo C, Arribas JR and Rodés B:
Duplex digital PCR assay on microfluidic chamber arrays for total
HIV DNA reservoir quantification in persons with HIV. Sci Rep.
15(31658)2025.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Fan C, He N and Yuan J: Cascaded
amplifying circuit enables sensitive detection of fungal pathogens.
Biosens Bioelectron. 250(116058)2024.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Hoenigl M, Salmanton-García J, Walsh TJ,
Nucci M, Neoh CF, Jenks JD, Lackner M, Sprute R, Al-Hatmi AMS,
Bassetti M, et al: Global guideline for the diagnosis and
management of rare mould infections: An initiative of the European
confederation of medical mycology in cooperation with the
international society for human and animal mycology and the
American society for microbiology. Lancet Infect Dis. 21:e246–e257.
2021.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Sonego B, Corio A, Mazzoletti V, Zerbato
V, Benini A, di Meo N, Zalaudek I, Stinco G, Errichetti E and Zelin
E: Trichophyton indotineae, an emerging drug-resistant
dermatophyte: A review of the treatment options. J Clin Med.
13(3558)2024.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Ashkenazi-Hoffnung L, Bilavsky E, Levy I,
Grisaru G, Sadot E, Ben-Ami R, Novikov A, Fischer S, Nahum E and
Scheuerman O: Isavuconazole as successful salvage therapy for
mucormycosis in pediatric patients. Pediatr Infect Dis J.
39:718–724. 2020.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Fernández Ledesma B, Mendoza-Palomar N,
Melendo Pérez S, Fernández-Polo A, Renedo Miró B, Pau Parra A,
Luque Pardos S, Grau Cerrato S, Vima Bofarull J, Martín-Gómez MT,
et al: Isavuconazole use and TDM in real-world pediatric practice.
Antimicrob Agents Chemother. 67(e0082923)2023.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Eissa S, Khedr R, Romeih M, Halaby L,
Elanany M and Madney Y: Clinical characteristics and outcome of
invasive fungal sinusitis in children with hematological
malignancies. Med Mycol. 60(myac010)2022.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Egan G, Robinson PD, Martinez JPD,
Alexander S, Ammann RA, Dupuis LL, Fisher BT, Lehrnbecher T,
Phillips B, Cabral S, et al: Efficacy of antibiotic prophylaxis in
patients with cancer and hematopoietic stem cell transplantation
recipients: A systematic review of randomized trials. Cancer Med.
8:4536–4546. 2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Bossù G, Di Sario R, Muratore E, Leardini
D, Pession A, Esposito S and Masetti R: Novel insights into fungal
infections prophylaxis and treatment in pediatric patients with
cancer. Antibiotics (Basel). 11(1316)2022.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Chen SC, Perfect J, Colombo AL, Cornely
OA, Groll AH, Seidel D, Albus K, de Almedia JN Jr, Garcia-Effron G,
Gilroy N, et al: Global guideline for the diagnosis and management
of rare yeast infections: An initiative of the ECMM in cooperation
with ISHAM and ASM. Lancet Infect Dis. 21:e375–e386.
2021.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Marty FM, Cornely OA, Mullane KM,
Ostrosky-Zeichner L, Maher RM, Croos-Dabrera R, Lu Q, Lademacher C,
Oren I, Schmitt-Hoffmann AH, et al: Isavuconazole for treatment of
invasive fungal diseases caused by more than one fungal species.
Mycoses. 61:485–497. 2018.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Thompson GR, Huang H, Feng S, Yu Y,
Soriano A, Cornely OA, Kullberg BJ, Pappas PG, Kollef M, Vazquez
JA, et al: Rezafungin versus caspofungin for the treatment of
candidemia and invasive candidiasis: results from the double-blind,
randomized, phase 3 ReSTORE trial including the China extension
study. Open Forum Infect Dis. 12(ofaf555)2025.PubMed/NCBI View Article : Google Scholar
|