Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic lung condition marked by ongoing respiratory symptoms and
reduced airflow (1). Beyond the
immediate respiratory compromise, acute exacerbations of COPD
(AE-COPD) trigger a potent systemic inflammatory response, creating
a prothrombotic state that markedly elevates the risk of venous
thromboembolism (VTE), including deep vein thrombosis (DVT)
(2,3). The concurrence of DVT in patients
with AE-COPD is not a minor complication; it is a critical event
associated with a notable increase in short- and long-term
mortality. A study indicated that the 1-year mortality rate of
AE-COPD patients with VTE was significantly higher than that of
patients without VTE (12.9 vs. 4.5%) (2). In addition to acquired risk factors,
underlying hereditary thrombophilias may contribute to VTE risk, as
highlighted in patients with unprovoked pulmonary embolism
(4), highlighting the complexity
of thrombotic risk in inflammatory diseases such as AE-COPD,
thereby emphasizing the urgent need for effective risk
stratification.
Platelets play a crucial role not only in hemostasis
and thrombosis but also in immune and inflammatory responses; they
participate in the activation of the coagulation cascade and
interact with immune cells such as neutrophils, monocytes and
lymphocytes, promoting cellular activation (5-7).
During an AE-COPD episode, systemic inflammation leads to
widespread platelet activation, which in turn amplifies the
inflammatory cascade and promotes a hypercoagulable state. This
dual role of platelets provides a crucial mechanistic link between
the inflammatory surge of an exacerbation and the subsequent risk
of thrombosis. Consequently, abnormalities in platelet counts are
common in AE-COPD and serve as important prognostic indicators
(8). Thrombocytosis (platelet
count >400x109/l) is a well-documented phenomenon
associated with the severity of exacerbations and an independent
predictor of adverse outcomes (9),
consistent with its established role in promoting thrombosis.
However, a previous study found that a decrease in platelet count
was associated with an increased risk of AE-COPD over 2 years,
suggesting that platelet levels may serve as a predictive marker
for COPD exacerbation risk (10).
A more complex and clinically challenging scenario arises in a
marked subset of patients, particularly those with severe,
infection-driven exacerbations, who present with
thrombocytopenia.
The development of thrombocytopenia in this context
is not a passive event but rather a dynamic biomarker reflecting a
dysregulated host response to severe infection, the primary trigger
for most AE-COPD events. The occurrence of thrombocytopenia
signifies an advanced state of systemic inflammation where
accelerated platelet consumption, sequestration and impaired bone
marrow production occur. This leads to a critical clinical
conundrum known as the ‘thrombocytopenia-thrombosis paradox’
(8). While a low platelet count is
traditionally viewed as a risk factor for bleeding, in pathological
states of severe inflammation, such as sepsis or disseminated
intravascular coagulation, it is paradoxically associated with a
heightened risk of microvascular and macrovascular thrombosis
(11). This occurs as the low
platelet count is a direct consequence of the widespread activation
and consumption of platelets in the formation of thrombi, driven by
endothelial injury and systemic inflammation. This paradox presents
a formidable challenge for clinicians managing patients with
AE-COPD (12). The presence of
infection-induced thrombocytopenia obscures the true thrombotic
risk, creating a dilemma where the need to prevent potentially
fatal thrombotic events must be weighed against a perceived risk of
major bleeding.
Current risk assessment models for VTE are not
specifically designed for this patient population and often fail to
account for the complex interplay between inflammation, infection
severity and platelet dysregulation. Therefore, a clear gap exists
in the ability to accurately risk-stratify patients with AE-COPD,
particularly those with thrombocytopenia, for the development of
DVT. To address this, the aim of the current study was to explore
the intricate association between thrombocytopenia, the severity of
the underlying infection and the incidence of DVT in patients
hospitalized with AE-COPD. It was hypothesized that a composite
model that integrates biomarkers reflecting both the inflammatory
state, platelet-to-lymphocyte ratio (PLR) and downstream
coagulation activation (D-dimer), can more accurately predict DVT
risk in this vulnerable population. The development of such a model
would provide a valuable tool for clinical decision-making,
enabling targeted prophylactic strategies and ultimately improving
outcomes for patients with AE-COPD.
Patients and methods
Subjects
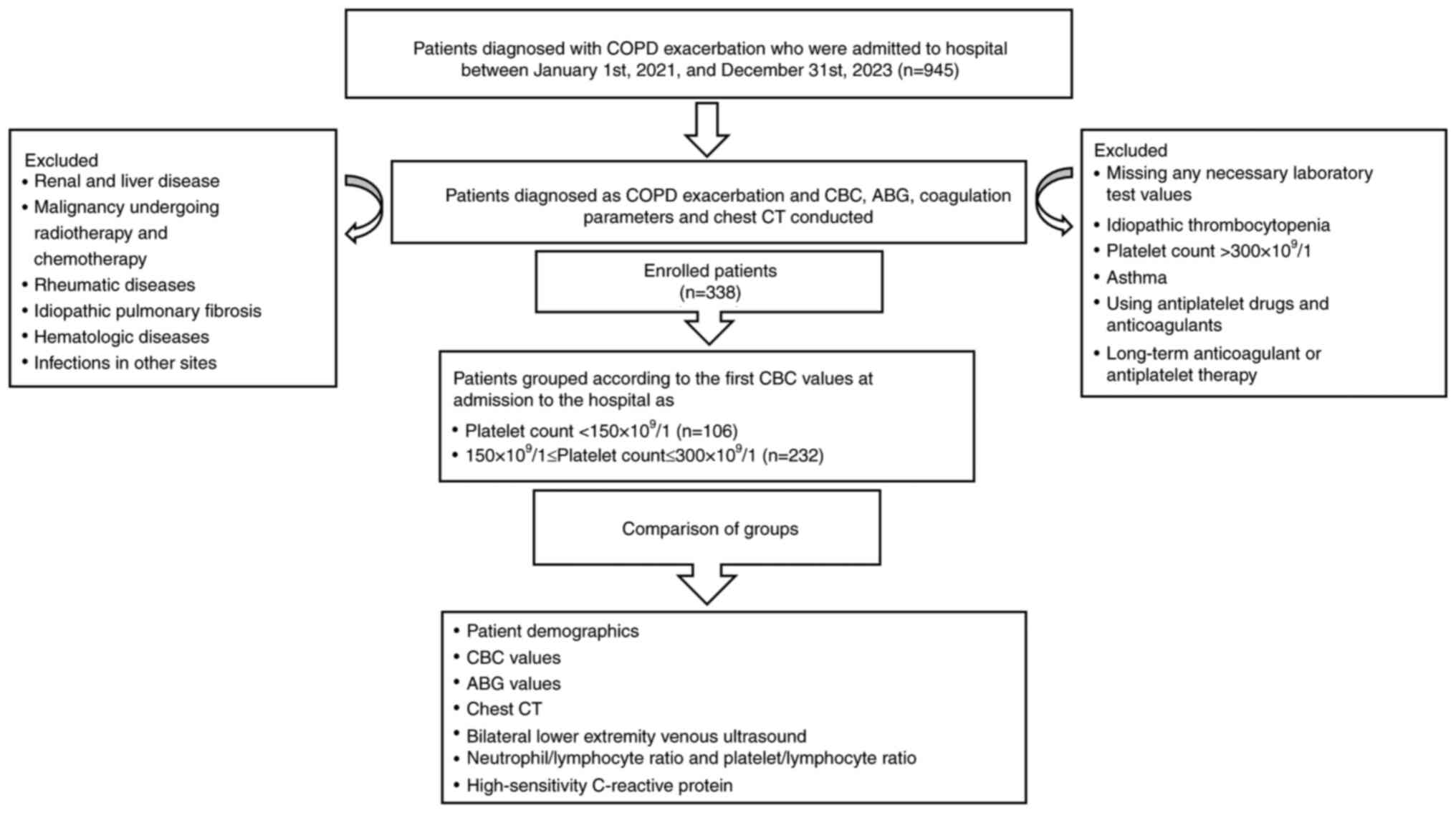
The selection process and design of the present
study are shown in Fig. 1. The
medical records of patients with AE-COPD (n=945) who were
hospitalized in the Department of Respiratory and Critical Care
Medicine at The Affiliated Jiangning Hospital of Nanjing Medical
University (Nanjing, China) between January 2021 and December 2023
were retrospectively reviewed. All enrolled patients underwent a
chest computed tomography (CT) scan. The CT findings were utilized
to confirm the presence and assess the severity of pulmonary
infection, such as the pulmonary infiltrates of infection analyzed
in the manuscript. This provided objective radiological support for
the clinical diagnosis. To ensure that the present study focused
specifically on the systemic response driven by pulmonary
pathology, patients with definitive, active non-pulmonary
infections at the time of admission were excluded. This exclusion
process was based on a comprehensive review of each patient's
electronic medical record, which included, but was not limited to
the following: i) Reviewing the attending physician's diagnostic
records to rule out infections at other sites, such as urinary
tract infections, skin and soft tissue infections or
intra-abdominal infections; and ii) scrutinizing relevant
laboratory results (for example, urinalysis and procalcitonin) and
imaging studies from other body regions (for example, abdominal
ultrasound). All patients had peripheral blood drawn and underwent
a systematic vascular ultrasound of both lower extremities within
24 h of admission, before starting anticoagulant therapy. Bilateral
lower extremity vascular ultrasound screening is actually routinely
performed for all patients with AE-COPD within The Affiliated
Jiangning Hospital of Nanjing Medical University, regardless of
their symptoms. COPD was diagnosed based on clinical presentations,
pulmonary function tests and chest CT scans. The Padua Prediction
Score (including immobility status, recent surgery, prior VTE
history, history of cancer and corticosteroid use) was calculated
for each patient with AE-COPD (13). The specific diagnostic criteria
included: i) A previous pulmonary function test showing a
post-bronchodilator forced expiratory volume in 1 sec/forced vital
capacity value of <0.70, indicating irreversible airflow
limitation; ii) chest CT showing signs of emphysema; and iii)
AE-COPD defined as symptoms such as worsening dyspnea, increased
sputum volume and yellow sputum, requiring a change in the current
treatment regimen. The exclusion criteria were: i) Severe renal and
liver diseases; ii) a history of malignancy undergoing radiotherapy
or chemotherapy; iii) rheumatic diseases; iv) idiopathic pulmonary
fibrosis; v) bronchial asthma; vi) long-term use of oral
antiplatelet and anticoagulant drugs; vii) hematological diseases;
viii) infections in other sites; ix) missing essential laboratory
test results; and x) anticoagulant therapy initiation before blood
samples were obtained and a lower extremity venous ultrasound was
performed. Ultimately, 338 patients with AE-COPD were included in
the present study, with an age range of 57-93 years and a median
age of 75 years.
Data collection and assessment
Relevant clinical information was collected from the
patients' hospitalization records. If multiple repeated tests were
conducted during hospitalization, the results from the first test
upon admission were selected. Data on the demographic
characteristics, medical history, comorbidities, laboratory tests,
bilateral lower limb venous ultrasound examinations, chest CT scans
and mechanical ventilation treatment of patients with AE-COPD were
collected. The PLR was calculated as the platelet count divided by
the lymphocyte count. The neutrophil-to-lymphocyte ratio (NLR) was
calculated as the neutrophil count divided by the lymphocyte count.
The D-dimer-to-lymphocyte ratio (DLR) was calculated as the D-dimer
level divided by the lymphocyte count. Based on the platelet count
at the time of admission, patients were divided into the
thrombocytopenia group (<150x109 cells/l) and the
normal platelet count group (≥150 to <300x109
cells/l) (14).
Statistical analysis
All statistical analyses were conducted using SPSS
(version 26.0; IBM Corp.), GraphPad Prism (version 9.0; Dotmatics)
and the R (version 4.1.3; R Core Team). Normality of all continuous
variables was assessed using the Shapiro-Wilk test. Quantitative
data conforming to a normal distribution are presented as the mean
± standard deviation and were compared between groups using an
unpaired Student's t-test. Non-normally distributed continuous
variables are presented as median (interquartile range) and were
compared using the Mann-Whitney U test. Categorical variables are
described using frequencies (percentages) and were compared using
the χ2 test. Correlation analysis between two continuous
variables was conducted using Spearman's correlation coefficient.
Subgroup analyses were performed to assess intergroup associations
and thrombosis risk within different subgroups. Univariate and
multivariate analyses were conducted using binary logistic
regression. Discrimination in the prediction model was assessed
using the area under the receiver operating characteristic curve
(AUC-ROC). Bootstrap ROC was used to evaluate the performance of
the prediction model. Two-sided P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics and clinical
characteristics
As shown in Table
I, 338 patients with AE-COPD were included, with 72.4% being
male and with an overall mean age of 75.60±7.42 years. Among these,
106 patients had platelet counts <150x109 cells/l,
and 232 patients had platelet counts of 150-300x109
cells/l. The body mass index (BMI) of patients in the
thrombocytopenia group was significantly lower than that of
patients in the normal platelet count group (P=0.039). There were
no significant statistical differences between the two groups
regarding age, smoking history sex and Padua prediction score.
 | Table ISociodemographics of patients with
acute exacerbations of chronic obstructive pulmonary disease. |
Table I
Sociodemographics of patients with
acute exacerbations of chronic obstructive pulmonary disease.
| Factors | Thrombocytopenia | Normal platelets | P-value |
|---|
| Total patients,
n | 106 | 232 | - |
| Age, years | 73.60±7.18 | 76.60±7.35 | 0.448 |
| Male, n (%) | 83 (78.3) | 162 (69.8) | 0.106 |
| Smoking history, n
(%) | 51 (48.1) | 86 (37.1) | 0.055 |
| BMI,
kg/m2 | 21.32±3.51 | 22.24±3.92 | 0.039a |
| Padua prediction
score | 3.43±1.86 | 3.71±1.81 | 0.196 |
Laboratory results (Table II) showed that the
thrombocytopenia group had a significantly lower white blood cell
count (WBC; P<0.001), neutrophil count (P<0.001) and monocyte
count (P<0.001), as well as lower PLR (P<0.001) and
plateletcrit (P=0.014), compared with the normal platelet group,
with no other results showing statistical significance.
 | Table IILaboratory data of patients with
acute exacerbations of chronic obstructive pulmonary disease. |
Table II
Laboratory data of patients with
acute exacerbations of chronic obstructive pulmonary disease.
| Factors | Reference
range |
Thrombocytopenia | Normal
platelets | P-value |
|---|
| WBC count,
x109/l | 3.5-9.5 | 5.8 (2.9) | 7.77 (4.33) |
<0.001a |
| Red blood cell
count, x1012/l | | | | |
|
Male | 4.3-5.8 | 4.20 (0.88) | 4.36 (0.89) | 0.258 |
|
Female | 3.8-5.1 | 4.03 (0.83) | 4.34 (0.68) | 0.252 |
| Neutrophil count,
x109/l | 1.8-6.3 | 4.4 (3.09) | 6.5 (4.22) |
<0.001a |
| Neutrophils, % | 40-75 | 78.9 (16.35) | 80.2 (15.17) | 0.231 |
| Lymphocyte count,
x109/l | 1.1-3.2 | 0.72 (0.59) | 0.9 (0.64) | 0.460 |
| Lymphocytes, % | 20-50 | 13.20 (13.4) | 11.2 (9.7) | 0.191 |
| Monocyte count,
x109/l | 0.1-0.6 | 0.40 (0.22) | 0.54 (0.45) |
<0.001a |
| Monocytes, % | 3-10 | 6.58±2.75 | 6.50±3.78 | 0.888 |
| PLR | - | 150.0 (116.6) | 230.97 (172.6) |
<0.001a |
| NLR | - | 6.16 (8.00) | 7.07 (7.13) | 0.164 |
| DLR | - | 0.85 (1.11) | 0.78 (1.44) | 0.528 |
| MPV, fl | 7.4-12.5 | 10.92±1.41 | 10.78±1.49 | 0.534 |
| PDW, % | 12-16.5 | 15.2 (3.6) | 14.4 (4.7) | 0.120 |
| Plateletcrit,
% | 0.11-0.28 | 0.19 (0.09) | 0.21 (0.08) | 0.014b |
| Hemoglobin,
mmol/l | | | | |
|
Male | 8.1-10.9 | 7.94±1.10 | 8.06±1.09 | 0.390 |
|
Female | 7.1-9.3 | 7.88±1.06 | 7.74±1.05 | 0.588 |
| Hs-CRP, mg/l | 0.95-95.2 | 84.1 (404.2) | 100.3 (583.4) | 0.285 |
| Procalcitonin,
pmol/l | 0.0-38.5 | 4.6 (10.8) | 5.4 (13.1) | 0.318 |
| D-dimer,
µmol/l | 0.0-3.06 | 3.50 (3.06) | 3.89 (5.78) | 0.052 |
| FBG-G, g/l | 5.9-11.8 | 10.47 (3.59) | 10.58 (5.38) | 0.823 |
| TT, sec | 14-21 | 14.3 (2.15) | 14.1 (1.9) | 0.408 |
| PT, sec | 9-15 | 11.9 (2.0) | 12 (1.7) | 0.943 |
| APTT, sec | 20-40 | 32.3 (4.1) | 31.8 (5.1) | 0.132 |
| INR, % | 0.8-1.2 | 1.1 (0.16) | 1.11 (0.16) | 0.764 |
| OI, mmHg | 300-500 | 313.8±78.0 | 313.2±98.11 | 0.960 |
| PaCO2,
mmHg | 35-45 | 46 (17.5) | 45 (19.9) | 0.241 |
| Pathogen positivity
rate of sputum, n (%) | Negative | 21 (19.8) | 61 (26.3) | 0.197 |
Comparison of comorbidities and
changes in clinical status upon admission and discharge in the two
groups
As shown in Table
III, there were no statistically significant differences in the
proportions of arrhythmias, cor pulmonale, respiratory failure,
assisted ventilation, hypertension and diabetes between the
thrombocytopenia group and the normal platelet group among patients
with AE-COPD. However, the ratio of patients with cardiovascular
diseases (CVDs) was 33.0% in the thrombocytopenia group compared
with 44.4% in the normal platelet group, a difference that was
statistically significant (P=0.048). The incidence of DVT during
hospitalization was significantly lower in the thrombocytopenia
group than in the normal platelet group (4.7 vs. 12.5%; P=0.027).
While there was no significant difference in the overall pulmonary
infiltrate rates between the two groups, the percentage of patients
with pulmonary confluent infiltrates infections was significantly
higher in the thrombocytopenia group than in the normal platelets
group (43.4 vs. 17.7%; P<0.001). The analysis revealed the
following results: None vs. patchy infiltrates, P=0.198; none vs.
confluent infiltrates, P<0.001; and patchy infiltrates vs.
confluent infiltrates, P<0.001.
 | Table IIIComparison of comorbidities and
changes in clinical status upon admission and discharge. |
Table III
Comparison of comorbidities and
changes in clinical status upon admission and discharge.
| Factors |
Thrombocytopenia | Normal
platelets | P-value |
|---|
| Patient number | 106 | 232 | - |
| Arrhythmia, n
(%) | 19 (17.9) | 78 (33.6) | 0.810 |
| Assisted
ventilation, n (%) | 19 (17.9) | 39 (16.8) | 0.951 |
| Respiratory
failure, n (%) | 62 (58.5) | 129 (55.6) | 0.619 |
| Hypertension, n
(%) | 53 (50.0) | 116 (50.0) | 1.000 |
| Diabetes, n
(%) | 16 (15.1) | 34 (14.7) | 0.916 |
| CVD, n (%) | 35 (33.0) | 103 (44.4) | 0.048a |
| Pulmonary
infection, n (%) | 86 (81.1) | 170 (74.6) | 0.118 |
| Cor pulmonale, n
(%) | 43 (40.6) | 79 (34.1) | 0.247 |
| DVT, n (%) | 5 (4.7) | 29 (12.5) | 0.027a |
| Pulmonary
infiltrates of infection, n (%)c | | |
<0.001b |
|
None | 20 (18.9) | 62 (26.7) | |
|
Patchy
infiltrates | 40 (37.7) | 129 (55.6) | |
|
Confluent
infiltrates | 46 (43.4) | 41 (17.7) | |
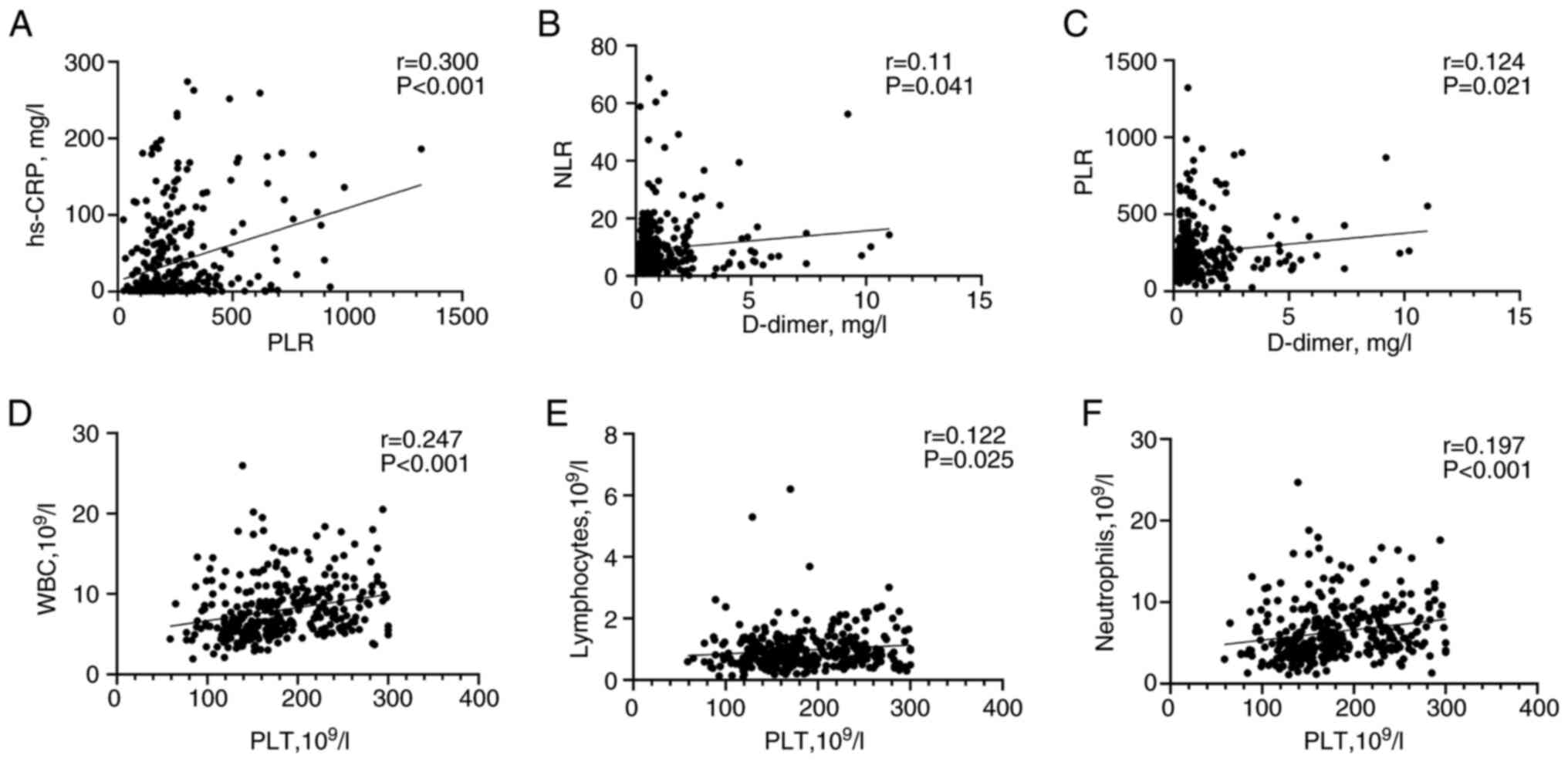
Correlation between laboratory
measurements in the two groups
The results of the correlation analysis between
platelet parameters, coagulation markers and infection indicators
in patients with AE-COPD are shown in Fig. 2. The correlations were weak, with
PLR being positively correlated with high sensitivity C-reactive
protein (r=0.300; P<0.001; Fig.
2A), and with D-dimer being positively correlated with NLR
(r=0.110; P=0.041; Fig. 2B) and
PLR (r=0.124; P=0.021; Fig. 2C),
all showing statistical significance. Additionally, platelet count
was positively significantly correlated with WBC (r=0.247;
P<0.001; Fig. 2D), lymphocyte
count (r=0.122; P<0.025; Fig.
2E) and neutrophil count (r=0.197; P<0.001; Fig. 2F).
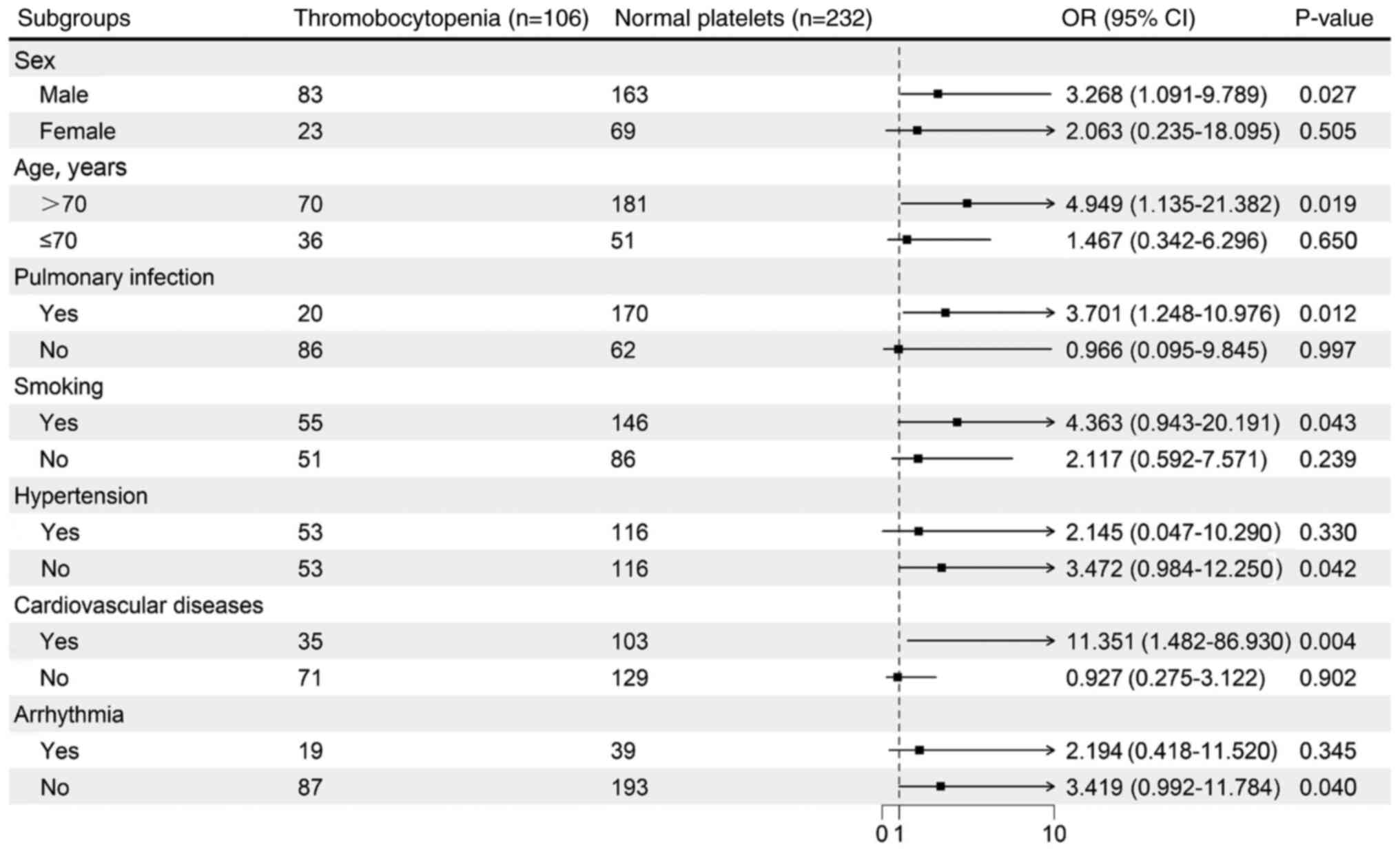
Subgroup analysis
A forest plot was used to perform stratified
analysis of DVT risk. In most subgroups, the risk of DVT was lower
in the thrombocytopenia group compared with that in the normal
platelet group. Notably, in the subgroup of males who were >70
years old with CVD and concurrent pulmonary infection, patients
with AE-COPD in the normal platelet group had a higher risk of DVT
(Fig. 3).
Risk factors associated with DVT
development in patients with AE-COPD
Initially, a univariate logistic regression analysis
was performed to identify the variables influencing thrombus
formation during hospitalization in patients with AE-COPD.
Significant indicators identified include platelet count (P=0.130),
normal platelets (P=0.875), lymphocyte count (P=0.845), lymphocyte
percentage (P=0.723), monocyte percentage (P=0.366), NLR (P=0.894),
PLR (P=0.012), DLR (P=0.720) and D-dimer (P=0.024) (Table IV). Subsequent multivariate
logistic regression analysis revealed that PLR [odds ratio (OR),
1.004; 95% confidence interval (CI), 1.001-1.007; P=0.012] and
D-dimer (OR, 1.454; 95% CI, 1.051-2.011; P=0.024) were independent
risk factors for thrombus formation during hospitalization of
patients with AE-COPD (Table IV).
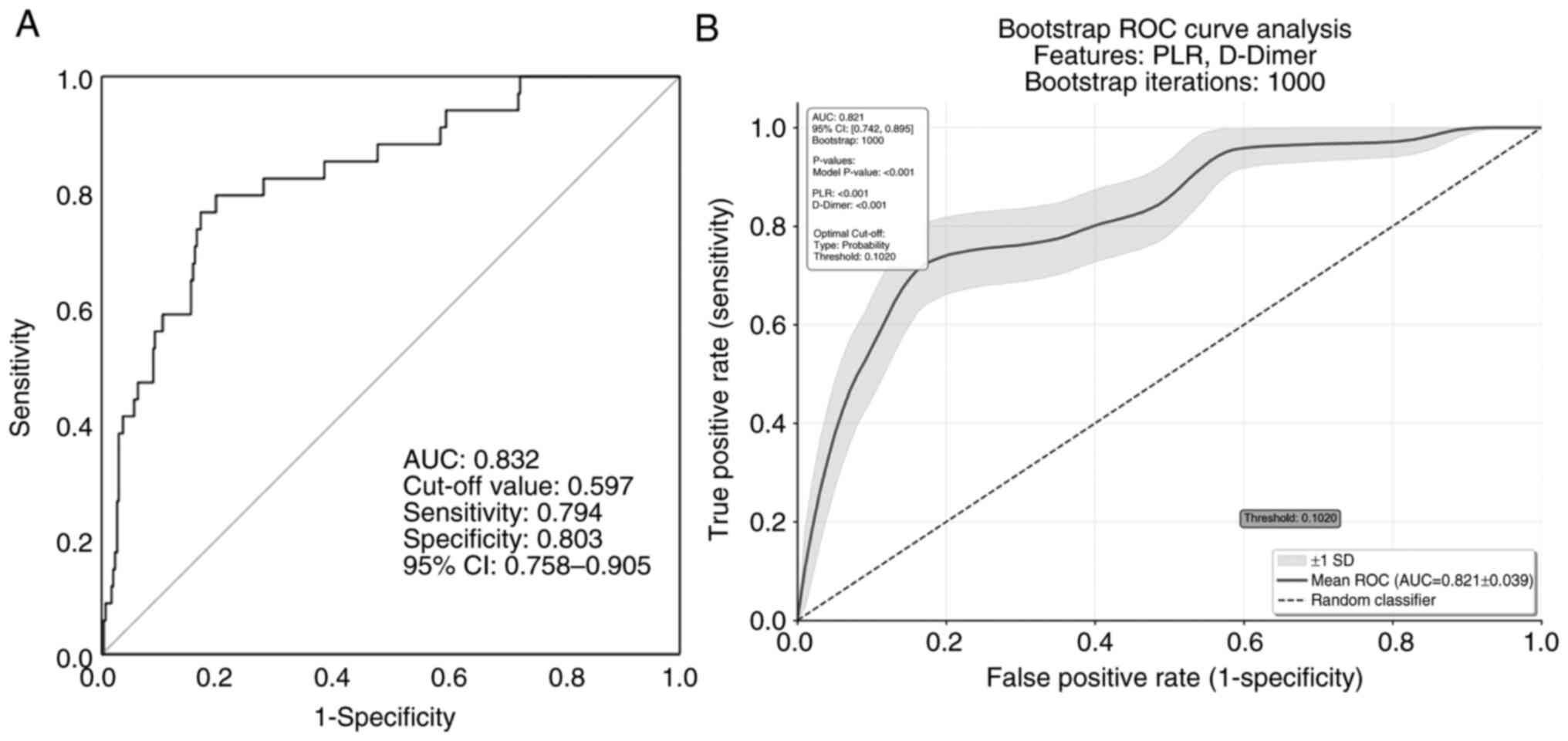
The AUC-ROC values of D-dimer and PLR were 0.802 (95% CI,
0.733-0.971; cut-off, 0.520; P=0.012) and 0.745 (95% CI,
0.649-0.841; cut-off, 408.51; P=0.024), respectively (Table V). The AUC-ROC in the model of PLR
and D-dimer in the present study was 0.832 (95% CI, 0.758-0.905;
P<0.001; Fig. 4A). Given the
small sample size, the bootstrap method was used to validate the
prediction model. After 1,000 repeated samplings, the AUC-ROC was
0.821 and the optimal cut-off was 0.1 (95% CI, 0.742-0.895;
P<0.001; Fig. 4B).
 | Table IVUnivariate and multivariate logistic
regression analysis of deep vein thrombosis formation during
hospitalization in patients with acute exacerbations of chronic
obstructive pulmonary disease. |
Table IV
Univariate and multivariate logistic
regression analysis of deep vein thrombosis formation during
hospitalization in patients with acute exacerbations of chronic
obstructive pulmonary disease.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Platelet count,
x109/l | 1.012
(1.005-1.019) |
<0.001a | 1.009
(0.997-1.020) | 0.130 |
| Normal
platelets | 2.886
(1.085-7.678) | 0.034b | 0.891
(0.211-3.765) | 0.875 |
| Lymphocyte count,
x109/l | 0.286
(0.109-0.747) | 0.011b | 0.845
(0.183-4.014) | 0.845 |
| Lymphocytes, % | 0.934
(0.882-0.989) | 0.019b | 1.081
(0.923-1.122) | 0.723 |
| Monocytes, % | 0.867
(0.776-0.967) | 0.011b | 0.945
(0.837-1.068) | 0.366 |
| NLR | 1.043
(1.016-1.071) | 0.032b | 0.995
(0.944-1.049) | 0.894 |
| PLR | 1.004
(1.003-1.006) |
<0.001a | 1.004
(1.001-1.007) | 0.012b |
| DLR | 1.212
(1.089-1.349) |
<0.001a | 0.975
(0.846-1.123) | 0.720 |
| D-dimer, mg/l | 1.423
(1.203-1.683) |
<0.001a | 1.454
(1.051-2.011) | 0.024b |
 | Table VROC curve analysis for D-dimer and
PLR. |
Table V
ROC curve analysis for D-dimer and
PLR.
| Factors | AUC-ROC | 95% CI | Cut-off | P-value |
|---|
| D-dimer, mg/l | 0.802 | 0.733-0.971 | 0.520 | 0.012b |
| PLR | 0.745 | 0.649-0.841 | 408.510 | 0.024b |
Discussion
The current study demonstrated that a diagnostic
model combining D-dimer level and PLR exhibited excellent
performance in detecting DVT in patients with AE-COPD.
Additionally, it was shown that males >70 years old with
pulmonary infections and CVDs are at higher risk of DVT.
Furthermore, thrombocytopenia may be associated with more severe
pulmonary infections, as the results confirmed that patients with
AE-COPD and thrombocytopenia were more likely to develop extensive
infiltrative infections. These findings can assist clinicians in
risk-stratifying patients upon admission to rapidly identify those
at high risk for developing thrombocytopenia.
The interplay between inflammation, platelet
activation and coagulation function has been extensively studied
(15,16). The present study found that the
incidence of DVT was significantly lower in the thrombocytopenia
group compared with that in patients with normal platelet counts
(4.7 vs. 12.5%). We speculate that this phenomenon may be related
to consumptive thrombocytopenia (17). A previous study found that 20% of
patients with AE-COPD experience thrombocytopenia, which is closely
associated with systemic infections (18). In the context of infection and
inflammation, platelets may be excessively consumed or destroyed.
Infection can cause abnormal aggregation and consumption of
platelets within the body, especially at sites of pulmonary
inflammation, further exacerbating thrombocytopenia (19). The severe inflammatory response and
infection in AE-COPD can activate the coagulation system, leading
to the formation of microthrombi within the pulmonary and systemic
microvasculature (20). This
process consumes a large number of platelets, resulting in a
decreased peripheral platelet count.
D-dimer level and PLR reflect different yet related
pathological processes. While the continuous OR from the current
regression analysis quantified the statistical risk associated with
rising D-dimer levels, a more clinically actionable insight arose
from the ROC curve analysis. This analysis identified an optimal
D-dimer cut-off of 0.520 for predicting DVT. This finding
reinforces the utility of using a standard D-dimer threshold to
identify patients with high-risk AE-COPD who may require further
diagnostic evaluation, such as lower limb venous ultrasound, even
when other traditional risk factors are not prominent. PLR
represents the inflammation-platelet activation state (an upstream
event), whereas D-dimer reflects the ultimate outcome of fibrin
formation and degradation (a downstream event) (21). The weak correlation suggests that
PLR and D-dimer may provide complementary, rather than redundant,
risk information. For instance, a high PLR might indicate that a
patient is in an intense inflammatory storm with high thrombotic
potential, even while their D-dimer level has not yet become
significantly elevated (22).
Combining these two markers may therefore offer a more
comprehensive assessment of a patient's thrombosis risk than using
either one alone. After internal validation, the optimal cut-off
value for the combined prediction model was determined to be 0.10.
In clinical practice, this model can be developed into a tool where
clinicians input a patient's D-dimer and PLR values to calculate a
predictive score. A score >0.10 would indicate a higher risk for
DVT, thereby prompting the early initiation of pharmacological and
mechanical anticoagulant therapies.
A key finding of the current study is the
discordance between absolute platelet count and thrombotic risk.
Logistic regression analysis identified D-dimer level and PLR as
independent prognostic factors for in-hospital thrombosis in
patients with AE-COPD, but it did not confirm platelet count as an
independent factor influencing DVT. In acute inflammatory diseases
such as AE-COPD, the risk of thrombosis depends not only on the
quantity of procoagulant cells but, more importantly, on the
complex interplay between the systemic inflammatory state and the
coagulation system, a concept known as immuno-thrombosis (23). The PLR, as a composite biomarker,
aptly captures the essence of this interaction; it reflects
platelet-driven prothrombotic activity (the numerator) occurring
within the context of severe systemic inflammation and
immunosuppression (the denominator). By contrast, a simple platelet
count fails to capture this critical inflammatory background, thus
limiting its predictive power. Therefore, in the context of
AE-COPD, the driving force behind DVT risk is not the absolute
number of platelets, but rather their activation state and the
host's inflammatory microenvironment, as reflected by
lymphocytopenia (24).
It is well known that PLR reflects the inflammatory
and immune status of the body, and research has shown that PLR is a
good predictor of VTE incidence, poor prognosis and mortality
(25,26). The NLR is a recognized indicator of
subclinical inflammation, with a high NLR suggesting increased
systemic inflammation and being considered a negative prognostic
factor for various arterial diseases (27,28).
Elevated NLR is markedly linked to mesenteric artery embolism and
venous thrombosis in patients with coronary artery disease
(29,30). The results of the present study
align with the findings of the study by Selvaggio et al
(31), which observed that
patients with concurrent DVT show a heightened inflammatory-immune
response, exhibiting higher NLR and PLR levels compared with those
without DVT. After adjusting for confounding factors, PLR, but not
NLR, remained notably associated with DVT (OR, 3.379). This
suggests that in acute inpatient settings, the interplay between
platelets and the immune response, captured by PLR, is a robust
indicator of thrombotic events. This conclusion somewhat
contradicts the results in the study by Artoni et al
(32), which determined that high
PLR and NLR were not generally linked to an increased risk of VTE
or cerebral venous thrombosis (CVT). However, this discrepancy is
likely attributable to fundamental differences in the study
populations. The study by Artoni et al (32) included non-acute outpatients
referred for thrombophilia screening after their first objectively
confirmed VTE or CVT episode. These patients were in a stable,
non-acute phase, whereas the current study focused exclusively on
patients during an active, inflammatory state of AE-COPD. It is
likely that the predictive value of inflammatory markers such as
PLR is most prominent during acute systemic inflammation, which
acts as a potent trigger for thrombosis. Therefore, the differing
results are not necessarily conflicting but rather highlight that
the utility of these biomarkers is context-dependent, being
particularly relevant in acutely ill, hospitalized patients.
Further research is still needed to fully elucidate these
associations.
Additionally, in the present study, it was observed
that the patients in the thrombocytopenia group had a significantly
lower BMI compared with the patients in the normal platelet count
group. COPD, being a chronic disease, is often accompanied by
malnutrition, which can result in a deficiency of key hematopoietic
nutrients, such as vitamin B12 and folic acid (33), potentially impairing bone marrow
function and resulting in reduced platelet production. This may, in
turn, lead to relatively lower D-dimer levels upon hospital
admission, which might explain the observations in the present
study. While the present study did not include specific nutritional
markers, such as serum albumin, the lower BMI in the
thrombocytopenia group suggests that poor nutritional status may be
a contributing factor, potentially leading to reduced platelet
production and relatively lower D-dimer levels upon hospital
admission. Future studies should incorporate these nutritional
assessments to further clarify their role.
However, as the present study is a retrospective
analysis, the results primarily reveal associations rather than
causal relationships. Future research should focus on validating
these findings through large-scale, prospective cohort studies and
further investigate the underlying pathophysiological mechanisms by
which these risk factors lead to thrombocytopenia, in order to
provide a scientific basis for more precise clinical interventions.
Limitations of the study include the fact that the number of DVT
cases in the thrombocytopenia group was small, which could lead to
increased variance in the statistical analysis. Additionally, the
potential influence of various hematological parameters should be
recognized. For example, protein-energy malnutrition and specific
micronutrient deficiencies, such as vitamin B12 or folate, are
known factors that can cause or exacerbate thrombocytopenia and
anemia (34). However, specific
nutritional markers other than hemoglobin were not systematically
gathered or recorded. Subsequent studies should build upon the
current results by incorporating protocols that include distinct
groups with and without the use of anticoagulants, intravenous
corticosteroids and other agents; these will allow for a more
detailed investigation into how these variables affect the
association between platelets and DVT incidence.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the Nanjing
Science and Technology Project (grant no. ZKX22062) and the
Affiliated Jiangning Hospital of Nanjing Medical University Youth
Innovation Scientific Research Project (grant nos. JNYYZXKY202305
and JNYYZXKY202205).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YG and LW were responsible for data collection and
the writing of the draft manuscript. ZW, YW and QL were responsible
for statistical analysis and editing the presentation of data and
figures. XZ analyzed the data. LW and BW were responsible for
designing and planning the entire research project, as well as
reviewing and revising the manuscript. LW and BW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee of The Affiliated Jiangning Hospital of Nanjing Medical
University (Nanjing, China; approval no. 2024-03-037-K01). Informed
consent was not required for this study, as it is a retrospective
analysis utilizing de-identified clinical data without active
patient recruitment. The study adheres to the principles outlined
in The Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim V and Aaron SD: What is a COPD
exacerbation? Current definitions, pitfalls, challenges and
opportunities for improvement. Eur Respir J.
52(1801261)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu X, Jiao X, Gong X, Nie Q, Li Y, Zhen
G, Cheng M, He J, Yuan Y and Yang Y: Prevalence, risk factor and
clinical characteristics of venous thrombus embolism in patients
with acute exacerbation of COPD: A prospective multicenter study.
Int J Chron Obstruct Pulmon Dis. 18:907–917. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song YJ, Zhou ZH, Liu YK, Rao SM and Huang
YJ: Prothrombotic state in senile patients with acute exacerbations
of chronic obstructive pulmonary disease combined with respiratory
failure. Exp Ther Med. 5:1184–1188. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gegin S, Karakaya T, Aksu EA, Özdemir B,
Özdemir L and Pazarlı AC: The distribution of hereditary risk
factors in patients with pulmonary thromboembolism without
identifiable acquired risk factors. Duzce Med J. 27:69–74.
2025.
|
|
5
|
Yan C, Wu H, Fang X, He J and Zhu F:
Platelet, a key regulator of innate and adaptive immunity. Front
Med (Lausanne). 10(1074878)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bakogiannis C, Sachse M, Stamatelopoulos K
and Stellos K: Platelet-derived chemokines in inflammation and
atherosclerosis. Cytokine. 122(154157)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rawish E, Nording H, Münte T and Langer
HF: Platelets as mediators of neuroinflammation and thrombosis.
Front Immunol. 11(548631)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van der Vorm LN, Li L, Huskens D, Hulstein
JJJ, Roest M, de Groot PG, Ten Cate H, de Laat B, Remijn JA and
Simons SO: Acute exacerbations of COPD are associated with a
prothrombotic state through platelet-monocyte complexes,
endothelial activation and increased thrombin generation. Respir
Med. 171(106094)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Harrison MT, Short P, Williamson PA,
Singanayagam A, Chalmers JD and Schembri S: Thrombocytosis is
associated with increased short and long term mortality after
exacerbation of chronic obstructive pulmonary disease: A role for
antiplatelet therapy? Thorax. 69:609–615. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nieri D, Morani C, De Francesco M, Gaeta
R, Niceforo M, De Santis M, Giusti I, Dolo V, Daniele M, Papi A, et
al: Enhanced prothrombotic and proinflammatory activity of
circulating extracellular vesicles in acute exacerbations of
chronic obstructive pulmonary disease. Respir Med.
223(107563)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiménez-Zarazúa O, González-Carrillo PL,
Vélez-Ramírez LN, Vélez-Ramírez LN, Alcocer-León M, Salceda-Muñoz
PAT, Palomares-Anda P, Nava-Quirino OA, Escalante-Martínez N,
Sánchez-Guzmán S and Mondragón JD: Survival in septic shock
associated with thrombocytopenia. Heart Lung. 50:268–276.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiang M, Wu X, Jing H, Liu L, Wang C, Wang
Y, Novakovic VA and Shi J: The impact of platelets on pulmonary
microcirculation throughout COVID-19 and its persistent activating
factors. Front Immunol. 13(955654)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barbar S, Noventa F, Rossetto V, Ferrari
A, Brandolin B, Perlati M, De Bon E, Tormene D, Pagnan A and
Prandoni P: A risk assessment model for the identification of
hospitalized medical patients at risk for venous thromboembolism:
The Padua Prediction Score. J Thromb Haemost. 8:2450–2457.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fawzy A, Anderson JA, Cowans NJ, Crim C,
Wise R, Yates JC and Hansel NN: Association of platelet count with
all-cause mortality and risk of cardiovascular and respiratory
morbidity in stable COPD. Respir Res. 20(86)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Semple JW, Italiano JE Jr and Freedman J:
Platelets and the immune continuum. Nat Rev Immunol. 11:264–274.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Nicolai L and Massberg S: Platelets as key
players in inflammation and infection. Curr Opin Hematol. 27:34–40.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamada S and Asakura H: How we interpret
thrombosis with thrombocytopenia syndrome? Int J Mol Sci.
25(4956)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rahimi-Rad MH, Soltani S, Rabieepour M and
Rahimirad S: Thrombocytopenia as a marker of outcome in patients
with acute exacerbation of chronic obstructive pulmonary disease.
Pneumonol Alergol Pol. 83:348–351. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thomas MR and Storey RF: The role of
platelets in inflammation. Thromb Haemost. 114:449–458.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iba T, Umemura Y, Wada H and Levy JH:
Roles of coagulation abnormalities and microthrombosis in sepsis:
Pathophysiology, diagnosis, and treatment. Arch Med Res.
52:788–797. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mandel J, Casari M, Stepanyan M, Martyanov
A and Deppermann C: Beyond hemostasis: Platelet Innate immune
interactions and thromboinflammation. Int J Mol Sci.
23(3868)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wen H and Chen Y: The predictive value of
platelet to lymphocyte ratio and D-dimer to fibrinogen ratio
combined with WELLS score on lower extremity deep vein thrombosis
in young patients with cerebral hemorrhage. Neurol Sci.
42:3715–3721. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zaid Y and Merhi Y: Implication of
platelets in immuno-thrombosis and thrombo-inflammation. Front
Cardiovasc Med. 9(863846)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gasparyan AY, Ayvazyan L, Mukanova U,
Yessirkepov M and Kitas GD: The platelet-to-lymphocyte ratio as an
inflammatory marker in rheumatic diseases. Ann Lab Med. 39:345–357.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xue J, Ma D, Jiang J and Liu Y: Diagnostic
and prognostic value of immune/inflammation biomarkers for venous
thromboembolism: Is it reliable for clinical practice? J Inflamm
Res. 14:5059–5077. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yao C, Zhang Z, Yao Y, Xu X, Jiang Q and
Shi D: Predictive value of neutrophil to lymphocyte ratio and
platelet to lymphocyte ratio for acute deep vein thrombosis after
total joint arthroplasty: A retrospective study. J Orthop Surg Res.
13(40)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim NY, Chun DH, Kim SY, Kim NK, Baik SH,
Hong JH, Kim KS and Shin CS: Prognostic value of systemic
inflammatory indices, NLR, PLR, and MPV, for predicting 1-year
survival of patients undergoing cytoreductive surgery with HIPEC. J
Clin Med. 8(589)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yüksel M, Yıldız A, Oylumlu M, Akyüz A,
Aydın M, Kaya H, Acet H, Polat N, Bilik MZ and Alan S: The
association between platelet/lymphocyte ratio and coronary artery
disease severity. Anatol J Cardiol. 15:640–647. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang S, Liu H, Wang Q, Cheng Z, Sun S,
Zhang Y, Sun X, Wang Z and Ren L: Neutrophil-to-lymphocyte ratio
and platelet-to-lymphocyte ratio are effective predictors of
prognosis in patients with acute mesenteric arterial embolism and
thrombosis. Ann Vasc Surg. 49:115–122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo Q, Li X, Zhao Z, Zhao Q, Liu Z and
Yang W: Nomogram for hospital-acquired venous thromboembolism among
patients with cardiovascular diseases. Thromb J.
22(15)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Selvaggio S, Brugaletta G, Abate A, Musso
C, Romano M, Di Raimondo D, Pirera E, Dattilo G and Signorelli SS:
Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and
monocyte-to-HDL cholesterol ratio as helpful biomarkers for
patients hospitalized for deep vein thrombosis. Int J Mol Med.
51(52)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Artoni A, Abbattista M, Bucciarelli P,
Gianniello F, Scalambrino E, Pappalardo E, Peyvandi F and
Martinelli I: Platelet to lymphocyte ratio and neutrophil to
lymphocyte ratio as risk factors for venous thrombosis. Clin Appl
Thromb Hemost. 24:808–814. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Robalo Nunes A and Tátá M: The impact of
anaemia and iron deficiency in chronic obstructive pulmonary
disease: A clinical overview. Rev Port Pneumol (2006). 23:146–155.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lederer AK, Maul-Pavicic A, Hannibal L,
Hettich M, Steinborn C, Gründemann C, Zimmermann-Klemd AM, Müller
A, Sehnert B, Salzer U, et al: Vegan diet reduces neutrophils,
monocytes and platelets related to branched-chain amino acids-A
randomized, controlled trial. Clin Nutr. 39:3241–3250.
2020.PubMed/NCBI View Article : Google Scholar
|