Introduction
Traumatic brain injury (TBI) remains a serious
public health concern that ranks first among causes of
trauma-associated mortality and disability (1). It is estimated that ~69 million
individuals suffer TBI each year worldwide (2). The unfavorable mortality, disability
and the widespread prevalence of TBI brings a heavy economic burden
to society and to patients families (1). The severity of intracranial injury
and neurological complications increase the risk of an unfavorable
outcome after TBI. While non-neurological complications also
commonly occur after TBI and have been confirmed to be associated
with the adverse outcome of patients with TBI (3-5).
Occurring in 7.6-23% of patients with TBI, acute kidney injury
(AKI) is a common type of non-neurological organ dysfunction, which
has been verified to be correlated with increased mortality in
patients with TBI (6-11).
Therefore, evaluating renal function, predicting the possible
occurrence of AKI and sequentially adjusting treatment strategies
in early stage after initial brain injury is beneficial for
physicians to reduce the risk of AKI development and improve
prognosis of patients with TBI.
Encoded by SerpinC1, antithrombin III (ATIII) is a
serine protease inhibitor that plays an anticoagulant role in the
coagulation cascade (12). In
addition to the pivotal role involved in anticoagulation, ATIII has
been revealed to have anti-inflammation properties in previous
studies (13-16).
Previously, several studies explored the beneficial effects of
ATIII on renal protection and found that renal ischemia-reperfusion
injury in a rat model can be alleviated by ATIII through
coagulation-independent anti-inflammation effects (17-19).
In addition, the association between decreased ATIII level and the
occurrence of AKI has been confirmed in several clinical settings
including acute severe pancreatitis, sepsis and in patients
undergoing cardiac surgery (17,20,21).
However, to the best of our knowledge, the relationship between
ATIII level and the development of AKI in patients with TBI has not
been explored. Thus, the present study aimed to test the hypothesis
that ATIII level is associated with the occurrence of AKI after
TBI.
Materials and methods
Study population
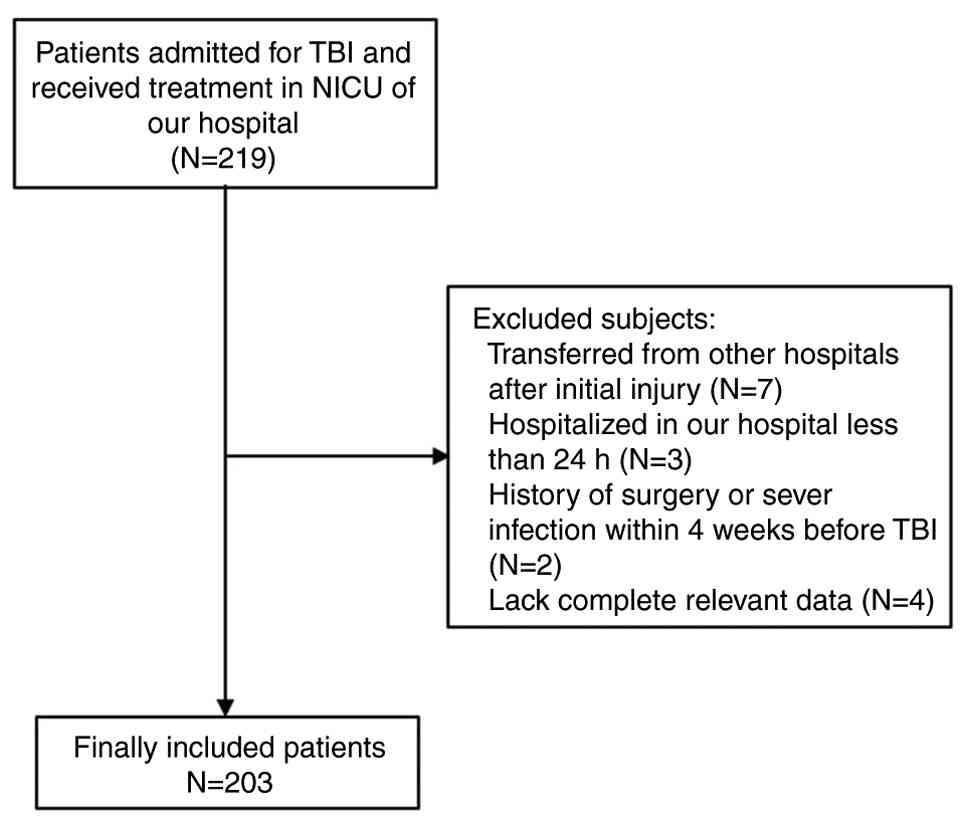
The present retrospective observational study was
performed in West China Hospital (Chengdu, China). Patients
admitted to West China Hospital for TBI and who then received
treatments in the neuro intensive care unit (NICU) between January
2015 and June 2019 were eligible for the present study. Patients
with low consciousness, unstable hemodynamics and intracranial
hypertension who required intensive monitoring and organ function
support were transferred from the emergency department to the NICU
within 24 h after admission. Therefore, the included patients were
mainly diagnosed with moderate to severe TBI. The present study
confirmed the diagnoses of TBI according to radiological signs of
computed tomography (CT) during hospitalization. There were four
exclusion criteria: i) Transferred from other hospitals after
initial injury; ii) hospitalized in West China Hospital for <24
h; iii) history of surgery or severe infection within 4 weeks
before TBI; and iv) lack of complete relevant data. The screening
diagram was shown as seen in Fig.
1. After screening, 203 patients with the median age of 44 and
male ratio of 80.3% were finally included in the present study.
This study was approved by the ethics committee of West China
Hospital and conducted according to the Declaration of Helsinki.
Informed consent form of each patient was routinely signed by
themselves or their authorized legal representatives.
Data collection
All clinical and laboratory variables were
retrospectively reviewed from the records of the electronic medical
record system. The present study included demographic
characteristics such as age and sex, injury mechanisms and vital
signs on admission including systolic blood pressure, diastolic
blood pressure, heart rate, body temperature and respiratory rate.
Occurrence of anisocoria, injury severity score (ISS) and Glasgow
Coma Scale (GCS) on admission were also recorded (22). Variables of laboratory test
including serum ATIII level were obtained by analyzing the first
blood samples collected when patients were admitted to the
emergency department of West China Hospital. Acute liver injury was
confirmed based on the liver function score of sequential organ
failure assessment score, namely total bilirubin ≥20.5 µmol/l
(23). Marshall CT score and
specified injury types, including subarachnoid hemorrhage and
intraventricular hemorrhage, were collected based on the findings
of the CT scans (24). AKI was
diagnosed according to the KDIGO criteria (25). As the aim of the present study was
to predict the development of AKI during hospitalizations, any AKIs
that occurred on the first day after admission were excluded and
only AKIs that occurred after the second day after admission were
selected as primary outcome (since the treatment of first day is
mainly focused on emergency treatment and maintaining vital signs).
In addition, AKI on the first day is difficult to identify because
the AKI is diagnosed based on fluctuation of SCr and the level of
SCr may not be measured more than one time within the first day
after admission. Records of blood product transfusion, drugs
reducing intracranial pressure, nephrotoxic antibiotics and surgery
types were included in the present study. The indications for fresh
frozen plasma (FFP) in West China Hospital for trauma patients were
shown as follows: i) Acute massive hemorrhage (blood loss ≥70 ml/kg
within 24 h); and ii) prothrombin time (PT) or activated partial
thromboplastin time (APTT) prolongation >1.5 times. Records of
aforementioned medicine and operation for AKI group were collected
until AKI developed while those for non-AKI group were collected
during the whole hospitalization.
Statistical analysis
The Kolmogorov-Smirnov test was used to verify the
normality of the included variables. Normally distributed variables
were presented as mean ± standard deviation while non-normally
distributed variables were presented as median (interquartile
range). And categorical variables were presented as counts
(percentage). The present study conducted unpaired Student's t-test
to analyze the difference between two groups of normally
distributed variables. And the difference between two groups of
non-normally distributed variables were testified by utilizing
Mann-Whitney U test. χ2 test or Fisher's exact test were
performed to verify the difference between two groups of
categorical variables. To discover potential risk factors for AKI
in included patients with TBI, univariate logistic regression
analysis was first conducted to present the relationship without
adjusting confounding effects. Odds ratio (OR) and 95% confidence
intervals (CI) of each risk factors were calculated and shown.
Because of the numerous included variables and limited number of
outcomes, the least absolute shrinkage and selection operator
(LASSO) regression, which could minimize the collinearity of
selected risk factors and avoid overfitting of these factors, was
performed to identify predictors with non-zero coefficients.
Identifying the strongest predictors from plenty of potential risk
factors for targeted outcome with relatively small quantity is the
advantage of LASSO regression. Predictors with non-zero
coefficients were included in multivariate logistic regression
analysis to construct models for predicting AKI in included
patients with TBI. Finally, receiver operating characteristic (ROC)
curve was drawn and area under the ROC curve (AUC) value was
calculated to evaluate the predictive value of single ATIII, SCr
and constructed models. The corresponding sensitivity and
specificity of ATIII and constructed model were shown. Z test was
conducted to verify the difference of AUC value between single
ATIII and constructed models. Two-tailed P<0.05 was considered
to indicate a statistically significant difference. SPSS 22.0
Windows software (IBM Corp.) and R (version 3.6.1; R Foundation)
were used to carry out all statistical analyses.
Results
Baseline characteristics of included
patients with TBI
A total of 203 patients with TBI were finally
included in the present study, with 43 developing AKI 24 h after
admission (Table I). Among 43
confirmed patients with AKI, 30 patients were confirmed as stage 1,
7 patients confirmed as stage 2 and 6 patients confirmed as stage 3
according to KDIGO criteria. No patient received renal replacement
therapy among the included patients with TBI. The age and sex ratio
did not differ between the non-AKI group and AKI group (age, 43 vs.
47, P=0.057; sex, 78.8 vs. 86.0%, P=0.270). Among injury
mechanisms, traffic accident and high falling ranked the first and
the second with rate of 60.1 and 23.2%. Vital signs on admission
did not show any significant differences between the non-AKI group
and AKI group. The AKI group had significantly lower GCS (5 vs. 6;
P=0.002) and higher ISS (25 vs. 24; P=0.009) compared with the
non-AKI group. Records of laboratory test presented that AKI group
had higher blood glucose (12.68 vs. 8.94; P<0.001), prothrombin
time (14.6 vs. 13.4; P=0.002), blood urea nitrogen (BUN; 7.70 vs.
5.75; P=0.001) and SCr (98 vs. 68; P<0.001) compared with the
non-AKI group. While the platelet (100 vs. 122; P=0.031), albumin
(28.95 vs. 32.15; P=0.001), hemoglobin (79 vs. 92; P<0.001),
fibrinogen (1.94 vs. 2.81; P=0.006) and ATIII (62.1 vs. 81.0;
P<0.001) levels were significantly lower in the AKI group
compared with the non-AKI group. Radiological findings demonstrated
that Marshall CT score and incidence of subarachnoid hemorrhage and
intraventricular hemorrhage were not significantly different
between those two groups. As for blood product transfusion, AKI
group were more likely to receive FFP (55.8 vs. 17.5%; P<0.001),
hydroxyethyl starch (46.5 vs. 30.0%; P=0.045) and red blood cell
(55.8 vs. 32.5%; P=0.006). Considering the drugs reducing
intracranial pressure (ICP), hypertonic saline was less likely to
be used in the AKI group (18.6 vs. 43.8%; P=0.02). In addition,
vancomycin was also less likely to be used in the AKI group (11.6
vs. 28.1%; P=0.018). The AKI group had a significantly higher
in-hospital mortality rate compared with the non-AKI group (65.1
vs. 30.0%; P<0.001). Furthermore, the length of ICU stay (11 vs.
16; P=0.177) and length of hospital stay (13 vs. 26; P=0.001) were
both shorter in the AKI group due to the notable early mortalities
among the AKI group.
 | Table IBaseline characteristics of non-AKI
group and AKI group in included patients with TBI. |
Table I
Baseline characteristics of non-AKI
group and AKI group in included patients with TBI.
| Variables | Overall patients
(n=203) | Non-AKI group
(n=160; 80.3%) | AKI group (n=43;
19.7%) | P-value |
|---|
| Age, years | 44 (28-58) | 43 (27-55) | 47 (33-64) | 0.057 |
| Male sex, n
(%) | 163 (80.3%) | 126 (78.8%) | 37 (86.0%) | 0.270 |
| Injury
mechanism | | | | 0.152 |
|
Traffic
accident | 122 (60.1%) | 100 (62.5%) | 22 (51.2%) | |
|
High
fall | 47 (23.2%) | 34 (21.3%) | 13 (30.2%) | |
|
Stumble | 21 (10.3%) | 14 (8.8%) | 7 (16.3%) | |
|
Others | 13 (6.4%) | 12 (7.5%) | 1 (2.3%) | |
| Vital signs on
admission | | | | |
|
Systolic
blood pressure, mmHg | 123 (108-138) | 123 (110-138) | 117 (101-145) | 0.324 |
|
Diastolic
blood pressure, mmHg | 73.5±16.3 | 74.2±14.5 | 71.1±21.6 | 0.384 |
|
Heart rate,
min-1 | 98 (80-120) | 98 (80-120) | 100 (80-125) | 0.901 |
|
Body
temperature,˚C | 36.8
(36.5-37.2) | 36.8
(36.5-37.3) | 36.8
(36.5-37.0) | 0.520 |
|
Respiratory
rate, min-1 | 20 (17-24) | 20 (17-24) | 20 (17-22) | 0.588 |
| Anisocoria | 76 (37.4%) | 60 (37.5%) | 16 (37.2%) | 0.972 |
| GCS | 6 (5-7) | 6 (5-8) | 5 (3-7) | 0.002 |
| ISS | 25 (16-25) | 24 (16-25) | 25 (16-29) | 0.009 |
| Laboratory
tests | | | | |
|
Glucose,
mmol/l | 9.70
(7.23-13.31) | 8.94
(6.77-12.61) | 12.68
(8.83-15.45) | <0.001 |
|
White blood
cell, 109/l | 15.45
(11.27-20.43) | 15.38
(11.33-20.39) | 15.65
(10.05-20.73) | 0.974 |
|
Neutrophil,
109/l | 11.75
(8.70-15.81) | 11.81
(9.00-15.76) | 11.63
(7.34-16.9) | 0.713 |
|
Lymphocyte,
109/l | 0.80
(0.53-1.12) | 0.79
(0.53-1.18) | 0.80
(0.50-1.05) | 0.358 |
|
Platelet,
109/l | 113 (80-169) | 122 (86-178) | 100 (58-161) | 0.031 |
|
Albumin,
g/l | 31.47±5.63 | 32.15±5.22 | 28.95±6.39 | 0.001 |
|
Hemoglobin,
g/l | 88 (77-106) | 92 (79-109) | 79 (72-88) | <0.001 |
|
Fibrinogen,
mg/l | 2.53
(1.63-4.07) | 2.81
(1.71-4.49) | 1.94
(1.15-3.31) | 0.006 |
|
D-dimmer,
mg/l | 15.24
(7.37-34.99) | 14.76
(6.10-33.96) | 17.27
(10.38-38.00) | 0.124 |
|
Prothrombin
time, sec | 13.6
(12.2-15.4) | 13.4
(12.0-15.0) | 14.6
(13.3-18.2) | 0.002 |
|
APTT,
sec | 27.4
(26.6-28.9) | 27.2
(26.5-27.9) | 31.3
(28.8-33.2) | |
|
ATIII,
% | 77.0±19.9 | 81.0±18.4 | 62.1±18.3 | <0.001 |
|
Blood urea
nitrogen, mmol/l | 5.93
(4.70-8.51) | 5.75
(4.46-7.82) | 7.70
(5.41-11.44) | 0.001 |
|
Serum
creatinine, µmol/l | 71 (55-98) | 68 (52-86) | 98 (76-131) | <0.001 |
| Acute liver
dysfunction | 58 (28.6%) | 47 (29.4%) | 11 (25.6%) | 0.622 |
| Radiological
findings | | | | |
|
Marshall CT
score | 4 (3-6) | 4 (3-6) | 6 (3-6) | 0.280 |
|
Subarachnoid
hemorrhage, n (%) | 87 (42.9%) | 68 (42.5%) | 19 (44.2%) | 0.843 |
|
Intraventricular
hemorrhage, n (%) | 8 (3.9%) | 8 (5.0%) | 0 | 0.207 |
| Blood product
transfusion, n (%) | | | | |
|
Fresh frozen
plasma | 52 (25.6%) | 28 (17.5%) | 24 (55.8%) | <0.001 |
|
Fibrinogen
transfusion | 26 (12.8%) | 18 (11.3%) | 8 (18.6%) | 0.218 |
|
Hydroxyethyl
starch | 68 (33.5%) | 48 (30.0%) | 20 (46.5%) | 0.045 |
|
Red blood
cell transfusion | 76 (37.4%) | 52 (32.5%) | 24 (55.8%) | 0.006 |
| Drugs reducing ICP,
n (%) | | | | |
|
Mannitol | 158 (77.8%) | 124 (77.5%) | 34 (79.1%) | 0.825 |
|
Fructose
glycerol | 21 (10.3%) | 15 (9.4%) | 6 (14.0%) | 0.401 |
|
Hypertonic
saline | 78 (38.4%) | 70 (43.8%) | 8 (18.6%) | 0.002 |
|
Furosemide | 70 (34.5%) | 53 (33.1%) | 17 (39.5%) | 0.436 |
| Nephrotoxic
antibiotics, n (%) | | | | |
|
Vancomycin | 50 (24.6%) | 45 (28.1%) | 5 (11.6%) | 0.018 |
|
Meropenem | 27 (13.3%) | 22 (13.8%) | 5 (11.6%) | 0.712 |
| Surgical
operations | | | | |
|
Decompressive
craniectomy, n (%) | 74 (36.5%) | 53 (33.1%) | 21 (48.8%) | 0.061 |
|
Hematoma
evacuation, n (%) | 87 (42.9%) | 66 (41.3%) | 21 (48.8%) | 0.374 |
| Length of ICU stay,
days | 15 (5-27) | 16 (6-27) | 11 (2-27) | 0.177 |
| Length of hospital
stay, days | 24 (10-41) | 26 (13-42) | 13 (3-35) | 0.001 |
| In-hospital
mortality, n (%) | 76 (37.4%) | 48 (30.0%) | 28 (65.1%) | <0.001 |
Univariate logistic regression
analysis of risk factors for AKI in patients with TBI
Univariate logistic regression analysis showed that
ISS (P=0.005), glucose (P=0.001), BUN (P=0.035), prothrombin time
(P=0.001), SCr (P<0.001) and transfusion of FFP (P<0.001),
hydroxyethyl starch (P=0.044) and RBC (P=0.006) were potential risk
factors for AKI after TBI (Table
II). Whereas GCS (P=0.004), platelet (P=0.035), albumin
(P=0.001), hemoglobin (P=0.002), fibrinogen (P=0.007), ATIII
(P<0.001) and usage of hypertonic saline (P=0.004) and
vancomycin (P=0.032) were negatively associated with occurrence of
AKI after TBI.
 | Table IIUnivariate logistic regression
analysis of risk factors for acute kidney injury in included
patients with traumatic brain injury. |
Table II
Univariate logistic regression
analysis of risk factors for acute kidney injury in included
patients with traumatic brain injury.
| Variables | Odds ratio | 95% Confidence
interval | P-value |
|---|
| Age, years | 1.019 | 1.001-1.038 | 0.041 |
| Male, n (%) | 1.664 | 0.649-4.269 | 0.289 |
|
Systolic
blood pressure, mmHg | 0.995 | 0.982-1.009 | 0.514 |
|
Diastolic
blood pressure, mmHg | 0.989 | 0.969-1.009 | 0.274 |
|
Heart rate,
min-1 | 1.002 | 0.989-1.014 | 0.790 |
|
Body
temperature,˚C | 0.947 | 0.651-1.377 | 0.774 |
|
Respiratory
rate, min-1 | 1.000 | 0.942-1.063 | 0.991 |
| Anisocoria | 0.988 | 0.492-1.982 | 0.972 |
| GCS | 0.798 | 0.684-0.932 | 0.004 |
| ISS | 1.053 | 1.016-1.091 | 0.005 |
|
Glucose,
mmol/l | 1.130 | 1.050-1.215 | 0.001 |
|
White blood
cell, 109/l | 1.011 | 0.964-1.060 | 0.655 |
|
Neutrophil,
109/l | 0.995 | 0.968-1.023 | 0.716 |
|
Lymphocyte,
109/l | 0.642 | 0.328-1.255 | 0.195 |
|
Platelet,
109/l | 0.994 | 0.989-1.000 | 0.035 |
|
Albumin,
g/l | 0.896 | 0.838-0.959 | 0.001 |
|
Hemoglobin,
g/l | 0.971 | 0.953-0.989 | 0.002 |
|
Fibrinogen,
mg/l | 0.738 | 0.592-0.921 | 0.007 |
|
D-dimmer,
mg/l | 1.016 | 0.991-1.042 | 0.218 |
|
Prothrombin
time, sec | 1.149 | 1.057-1.250 | 0.001 |
|
APTT,
sec | 1.600 | 1.357-1.886 | <0.001 |
|
ATIII,
% | 0.943 | 0.922-0.965 | <0.001 |
|
Blood urea
nitrogen, mmol/l | 1.056 | 1.004-1.112 | 0.035 |
|
Serum
creatinine, µmol/l | 1.011 | 1.005-1.017 | <0.001 |
| Acute liver
dysfunction, n (%) | 0.826 | 0.385-1.776 | 0.625 |
| Marshall CT
score | 1.139 | 0.923-1.405 | 0.225 |
| Subarachnoid
hemorrhage, n (%) | 1.071 | 0.543-2.111 | 0.843 |
| Intraventricular
hemorrhage, n (%) | 0 | 0 | 0.999 |
| Fresh frozen
plasma, n (%) | 5.955 | 2.878-12.32 | <0.001 |
| Fibrinogen
transfusion, n (%) | 1.803 | 0.725-4.485 | 0.205 |
| Hydroxyethyl
starch, n (%) | 2.029 | 1.020-4.037 | 0.044 |
| Red blood cell
transfusion, n (%) | 2.623 | 1.320-5.214 | 0.006 |
| Mannitol, n
(%) | 1.097 | 0.482-2.498 | 0.826 |
| Fructose glycerol,
n (%) | 1.568 | 0.569-4.318 | 0.385 |
| Hypertonic saline,
n (%) | 0.294 | 0.128-0.673 | 0.004 |
| Furosemide, n
(%) | 1.320 | 0.659-2.643 | 0.433 |
| Vancomycin, n
(%) | 0.336 | 0.124-0.909 | 0.032 |
| Meropenem, n
(%) | 0.825 | 0.293-2.324 | 0.716 |
| Decompressive
craniectomy, n (%) | 1.927 | 0.974-3.814 | 0.060 |
| Hematoma
evacuation, n (%) | 1.360 | 0.692-2.672 | 0.373 |
Value of ATIII and constructed
predictive models for predicting AKI after TBI
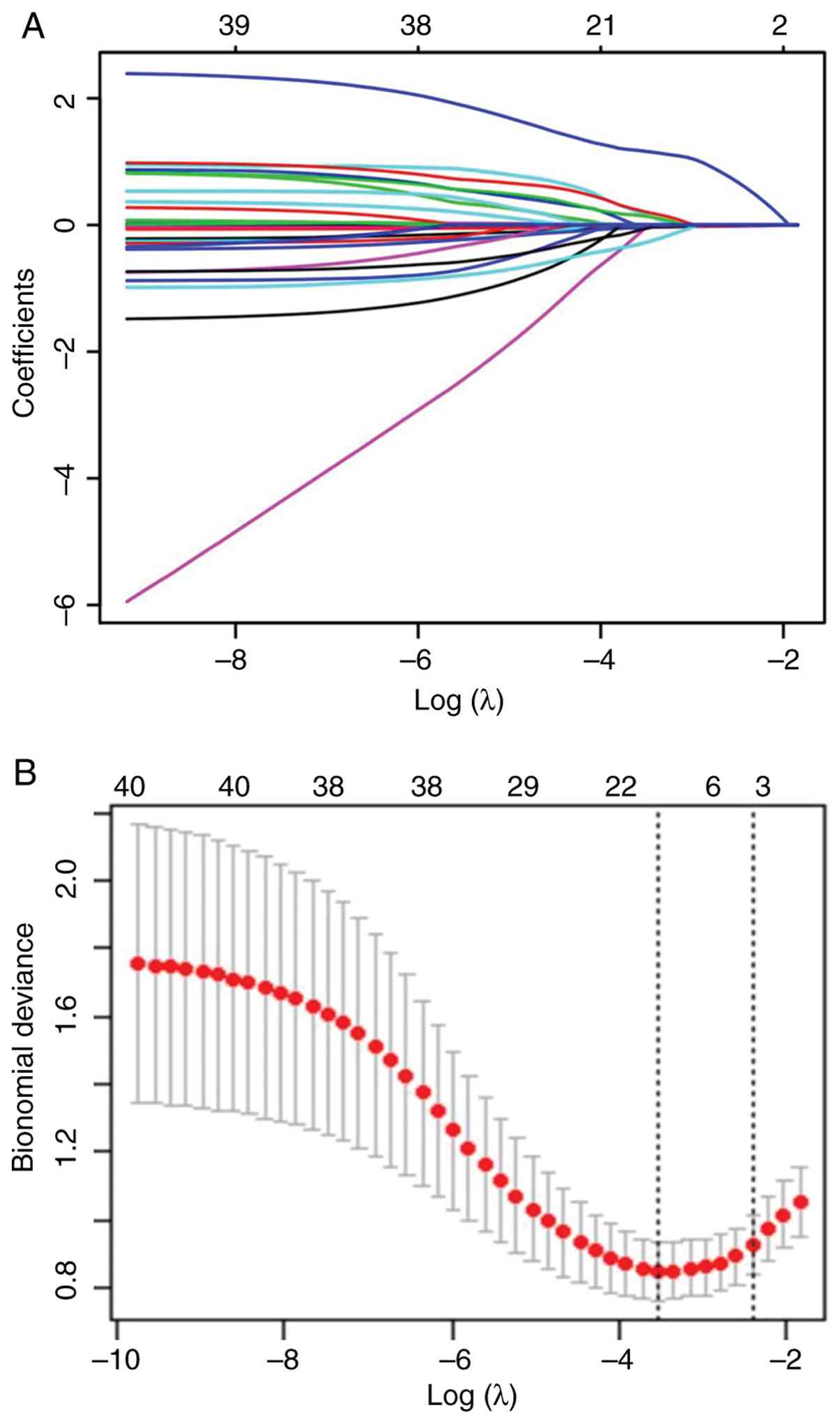
Utilizing LASSO regression, SCr, ATIII and
transfusion of FFP were finally recognized as the most potent
predictors of AKI in included patients with TBI (Table III; Fig. 2A and B). To enhance predictive accuracy,
multivariate logistic regression was performed to construct
predictive models incorporating these three factors. The AUC value
of single ATIII and SCr was 0.759 and 0.760, respectively (Table IV; Fig. 3). And the AUC value of
three-factors predictive model was 0.850. After removing ATIII,
two-factors model composed of SCr and transfusion of FFP had lower
AUC, but without statistical significance (0.831 vs. 0.850;
Z=0.4404; P>0.05). The two-factors model had a higher AUC
compared with single ATIII (0.831 vs. 0.759; Z=1.3582; P>0.05)
without statistical significance while the three-factors model had
significantly higher AUC compared with single ATIII (0.850 vs.
0.759; Z=1.7356; P<0.05). However, the sensitivity of single
ATIII was 0.869, which was higher compared with sensitivity of SCr
and constructed models. And adding ATIII could improve the
sensitivity of constructed model, which was beneficial for
physicians to evaluate risk of developing AKI in early stage.
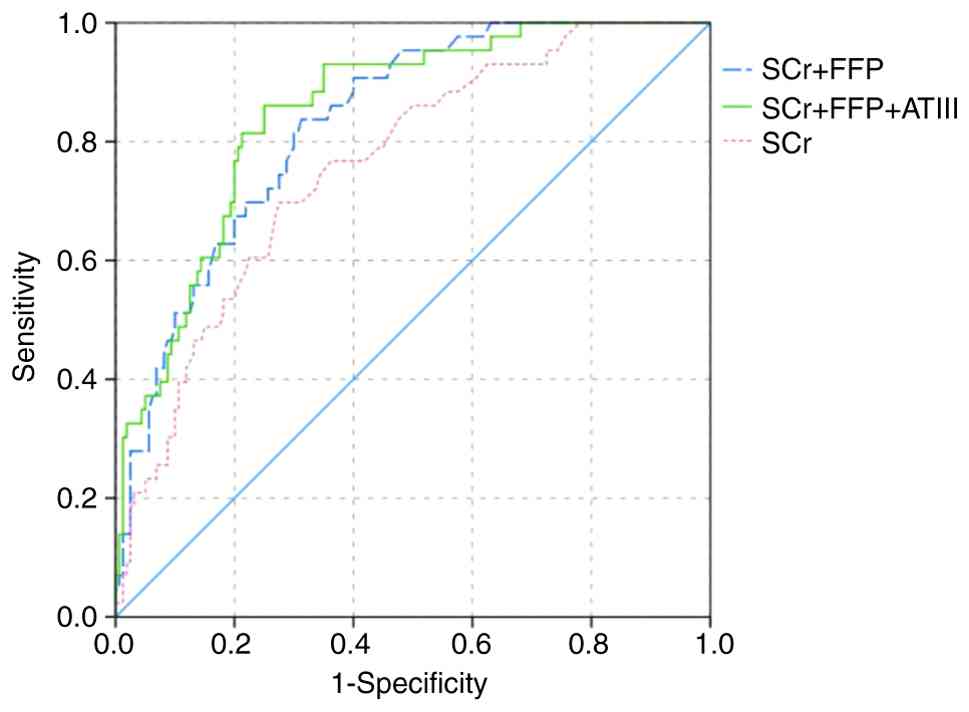 | Figure 3Receiver operating characteristic
curve of single ATIII and constructed predictive models for
predicting AKI in included TBI patients. The AUC value of ATIII,
serum creatinine, two-factors predictive model and three-factors
predictive model was 0.759 (0.674-0.844), 0.760 (0.684-0.836),
0.831 (0.770-0.891) and 0.850 (0.792-0.909), respectively. ATIII,
antithrombin III; AKI, acute kidney injury; TBI, traumatic brain
injury; AUC, area under the receiver operating characteristic
curve; Scr, serum creatinine; FFP, fresh frozen plasma. |
 | Table IIILASSO regression for selecting the
strongest predictors for acute kidney injury in included patients
with traumatic brain injury. |
Table III
LASSO regression for selecting the
strongest predictors for acute kidney injury in included patients
with traumatic brain injury.
| Variables | LASSO
coefficient | Regression
coefficient (β) |
|---|
| Serum
creatinine | 0.002 | -0.244 |
| ATIII | -0.017 | 0.224 |
| Transfusion of
FFP | 0.586 | 0.309 |
 | Table IVPredictive value of ATIII and
constructed models for predicting acute kidney injury after
traumatic brain injury. |
Table IV
Predictive value of ATIII and
constructed models for predicting acute kidney injury after
traumatic brain injury.
| Variable | AUC | 95% CI | SE | P-value | Sensitivity | Specificity | Best cut-off |
|---|
| SCr | 0.760 | 0.684-0.836 | 0.039 | <0.001 | 0.698 | 0.725 | 82.500 |
| ATIII | 0.759 | 0.674-0.844 | 0.043 | <0.001 | 0.869 | 0.581 | 60.650 |
| SCr + FFP | 0.831 | 0.770-0.891 | 0.031 | <0.001 | 0.837 | 0.687 | 0.127 |
| SCr + FFP +
ATIII | 0.850 | 0.792-0.909 | 0.030 | <0.001 | 0.860 | 0.750 | 0.170 |
Discussion
The present study found that ATIII level, SCr and
transfusion of FFP were independently associated with the
development of AKI after TBI. The logistic regression model
constructed using these three factors was effective in predicting
the development of AKI in early stages after TBI. Compared with the
non-AKI group, AKI group had lower level of ATIII on admission.
Based on findings of previous studies, the present study could
conclude that a lower level of ATIII on admission was detrimental
to maintenance of normal renal function and consequently promoted
the development of AKI in patients with TBI.
The results of the present study showed that ATIII
decreased after TBI and the decrease of ATIII was greater in the
AKI group than the non-AKI group. It was demonstrated that the
prevalence of coagulopathy ranged from 12.5 to 45.7% in patients
with TBI (26-29).
Through initiating the extrinsic coagulation pathway, the tissue
factor released from local injured brain tissue can promote the
development of disseminated intravascular coagulation, which
manifests as early hypercoagulation and subsequent fibrinolysis
(30-33).
As a crucial component of natural anticoagulation, ATIII can
neutralize increased production of factor Xa and coagulation
enzymes, including thrombin (factor IIa) and plasmin (34,35).
The reactive center of ATIII is cleaved by thrombin and then
irreversibly combined with thrombin to form an enzyme-inhibitor
complex which is quickly cleared from the circulation (35). In addition to increased
consumption, reduced synthesis due to the impaired liver function
may also lead to the decreased ATIII level. The impaired liver
function in patients with TBI can be attributable to the commonly
existing systemic inflammatory response with elevated inflammatory
cytokines level that promoted the hepato-cellular injury (36,37).
The renal-protective role of ATIII has been
investigated through inhibiting local renal inflammation, oxidative
stress activity and cell apoptosis (20). Both renal local inflammation and
systemic inflammation response take part in the progress of AKI
(38-41).
ATIII can exert anti-inflammatory action in a renal
ischemia-reperfusion injury model by inhibiting the release of
proinflammatory cytokines from endothelial cells and subsequent
chemokine mediated leukocytes infiltration, promoting the
production of prostacyclin (PGI2) (42,43).
Previous studies showed that supplement of ATIII can reduce serum
TNF-α concentration and consequently inhibit the expression levels
of monocyte chemotactic protein 1 and intercellular cell adhesion
molecule 1 in renal tissues (14,20).
In addition, ATIII can exert anti-inflammatory effects by inducing
the synthesis of PGI2, which not only suppresses the
proinflammatory effects of platelets, neutrophils and cytokines,
but also restores renal cortical blood flow as an effective
vasodilator (14,16,17,44,45).
TBI can initiate the systemic inflammatory response syndrome and
the release of various cytokines into the circulation, which is
associated with non-neurological organ failure, such as AKI
(46-48).
Therefore, the present study considered that ATIII can also
alleviate the progress of AKI in patients with TBI by mitigating
the detrimental effects of systemic inflammation and renal local
inflammation.
Imbalanced oxidative stress has been confirmed as a
critical pathogenetic factor which plays an important role in the
development and progress of AKI (49-51).
Therapeutic strategies targeting oxidative stress have attracted
considerable attention (49).
Previous studies have demonstrated that ATIII can alleviate the
aggressive status of oxidative stress in renal tissue of rat models
with AKI (14,17,20).
The mitigation of increased malondialdehyde and decreased
superoxide dismutase in injured renal tissue has been observed in
rats that received a supplement of ATIII (52). The apoptosis of renal tubular
epithelial cells is another key mechanism of AKI (53-55).
It has been verified that ATIII are capable of reducing caspase-3
expression and increasing bcl-2 expression in rat models with AKI
(20). Thus, the present study
hypothesized the association between low ATIII level and AKI
following TBI may also be mediated by oxidative stress and
apoptosis.
Another possible explanation for the role of ATIII
on alleviating renal injury may be the anticoagulative action.
Previous studies hypothesized that thrombin generation can cause
impairment to renal microcirculation and damage to tubular cells
(53-55).
In summary, the present study demonstrated that low ATIII level is
independently related with the occurrence of AKI after TBI through
the aforementioned mechanisms. Maintaining the appropriate ATIII
level may be beneficial to prevent or lessen AKI after TBI.
However, the specific and precise maintaining level of ATIII should
be explored further. In addition, future studies involving animal
models is worthwhile to design to confirm the potential
aforementioned mechanism. In addition to ATIII, SCr and transfusion
of FFP were independent risk factors of AKI after TBI in the
present study. The clinical significance of SCr as an indication of
glomerular filtration function has been acknowledged. The AUC value
of only ATIII and SCr for predicting AKI in the present study was
0.759 and 0.760, respectively. Although the AUC value was
comparable between ATIII and SCr, the sensitivity of ATIII was
higher compared with SCr (0.869 vs. 0.698), which was beneficial
for physicians to quickly identify patients with TBI and high risk
of AKI. The SCr level may be influenced by blood loss, fluid
dilution, muscle mass, nutritional status, age and sex and
therefore may not reflect the acute change of estimated glomerular
filtration rate sensitively. Additionally, the accuracy of BUN
reflecting the renal function may also be disturbed by multiple
factors including gastrointestinal bleeding, dehydration,
inflammation and protein intake (56). The transfusion of FFP was also
confirmed to be a risk factor for AKI in various types of patients,
including those receiving liver transplantation (57-60).
It has been demonstrated that FFP transfusion can trigger and even
exacerbate an inflammatory, immunological and allergic reaction,
which might aggravate the renal injury (61). The results of the present study
showed that combining these factors together was more effective in
predicting AKI compared with ATIII alone in patients with TBI.
The significant differences in blood glucose,
prothrombin time, platelets, albumin, hemoglobin and fibrinogen do
exist but are not present in the predictive model. This
contradiction is due to the present study using the LASSO
regression to develop the predictive model. The LASSO regression
performs well in identifying predictors among a dataset that has
numerous variables and a limited number of outcomes, and it can
minimize the collinearity of selected risk factors and avoid
overfitting of these factors. The clinical outcome of the present
study that 43 TBI patients were identified with the AKI. Therefore,
the present study did not use a traditional multivariate logistic
regression model to explore the risk factors of AKI, but used LASSO
to discover the strongest predictors of AKI from numerous potential
risk factors. The blood glucose, prothrombin time, platelet,
albumin, hemoglobin and fibrinogen levels showed significant
differences; however, they did not show stronger predictive value
compared with the three factors the present study revealed,
including SCr, antithrombin III and FFP.
There were several limitations in the present study.
Firstly, the present study was conducted in a single medical center
and mainly included moderate to severe patients with TBI treated in
NICU. Patients with history of surgery or severe infection within 4
weeks before TBI were also excluded. Therefore, selection bias may
not be avoided. The findings of the present study should be further
verified in future studies with larger sample sizes and more
generalized patients with TBI. Secondly, the detailed dosage and
infusion rate of drugs reducing ICP and blood products were not
collected in the present study. The real confounding effects of
these drugs may be weakened. Thirdly, underlying diseases, such as
cardiovascular diseases, renal diseases and malignant tumor, were
not collected as variables due to the low prevalence of them in the
included patients. Finally, only levels of ATIII on admission but
not subsequent fluctuation of ATIII were recorded; therefore, the
present study could not evaluate the influence of ATIII changes on
AKI development. Therefore, the conclusion drawn from the present
study should be verified in future studies.
In conclusion, ATIII is an easily obtained indicator
of AKI after TBI. Decreased ATIII is associated with higher
possibilities of AKI. Evaluating and avoiding decreased ATIII level
may be helpful for clinicians to avoid possible occurrence of AKI
in patients with TBI.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the 1·3·5 project for
disciplines of excellence - Clinical Research Incubation Project,
West China Hospital, Sichuan University (grant no. 19HXFH012).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RRW and JGX conceived and designed the study,
performed the statistical analysis and drafted the manuscript for
intellectual content. RRW and MH acquired, analyzed and interpreted
the data. RRW and MH confirm the authenticity of all the raw data.
JGX reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
West China hospital, Sichuan University (approval number2021-1598)
and has been performed in accordance with the ethical standards
laid down in the Declaration of Helsinki. Informed consent forms of
each patients were legally obtained from themselves or their
authorized families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rubiano AM, Carney N, Chesnut R and Puyana
JC: Global neurotrauma research challenges and opportunities.
Nature. 527:S193–S197. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dewan MC, Rattani A, Gupta S, Baticulon
RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano
AM, et al: Estimating the global incidence of traumatic brain
injury. J Neurosurg. 130:1080–1097. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zygun DA, Kortbeek JB, Fick GH, Laupland
KB and Doig CJ: Non-neurologic organ dysfunction in severe
traumatic brain injury. Crit Care Med. 33:654–660. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zygun D: Non-neurological organ
dysfunction in neurocritical care: Impact on outcome and
etiological considerations. Curr Opin Crit Care. 11:139–143.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hanna K, Hamidi M, Vartanyan P, Henry M,
Castanon L, Tang A, Zeeshan M, Kulvatunyou N and Joseph B:
Non-neurologic organ dysfunction plays a major role in predicting
outcomes in pediatric traumatic brain injury. J Pediatr Surg.
55:1590–1595. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Corral L, Javierre CF, Ventura JL, Marcos
P, Herrero JI and Manez R: Impact of non-neurological complications
in severe traumatic brain injury outcome. Crit Care.
16(R44)2012.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Moore EM, Bellomo R, Nichol A, Harley N,
Macisaac C and Cooper DJ: The incidence of acute kidney injury in
patients with traumatic brain injury. Ren Fail. 32:1060–1065.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li N, Zhao WG and Zhang WF: Acute kidney
injury in patients with severe traumatic brain injury:
Implementation of the acute kidney injury network stage system.
Neurocrit Care. 14:377–381. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Skrifvars MB, Moore E, Martensson J,
Bailey M, French C, Presneill J, Nichol A, Little L, Duranteau J,
Huet O, et al: Erythropoietin in traumatic brain injury associated
acute kidney injury: A randomized controlled trial. Acta
Anaesthesiol Scand. 63:200–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Barea-Mendoza JA, Chico-Fernández M,
Quintana-Díaz M, Serviá-Goixart L, Fernández-Cuervo A,
Bringas-Bollada M, Ballesteros-Sanz MÁ, García-Sáez Í,
Pérez-Bárcena J and Llompart-Pou JA: Neurointensive Care and Trauma
Working Group of the Spanish Society of Intensive Care Medicine
(SEMICYUC). Traumatic brain injury and acute kidney injury-outcomes
and associated risk factors. J Clin Med. 11(7216)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maragkos GA, Cho LD, Legome E, Wedderburn
R and Margetis K: Prognostic factors for stage 3 acute kidney
injury in isolated serious traumatic brain injury. World Neurosurg.
161:e710–e722. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Caspers M, Pavlova A, Driesen J, Harbrecht
U, Klamroth R, Kadar J, Fischer R, Kemkes-Matthes B and Oldenburg
J: Deficiencies of antithrombin, protein C and protein S-practical
experience in genetic analysis of a large patient cohort. Thromb
Haemost. 108:247–257. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Levy JH, Sniecinski RM, Welsby IJ and Levi
M: Antithrombin: Anti-inflammatory properties and clinical
applications. Thromb Haemost. 115:712–728. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III Protects Against
Contrast-Induced Nephropathy. EBioMedicine. 17:101–107.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Opal SM: Interactions between coagulation
and inflammation. Scand J Infect Dis. 35:545–554. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Roemisch J, Gray E, Hoffmann JN and
Wiedermann CJ: Antithrombin: A new look at the actions of a serine
protease inhibitor. Blood Coagul Fibrinolysis. 13:657–670.
2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang F, Zhang G, Lu Z, Geurts AM, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ozden A, Sarioglu A, Demirkan NC, Bilgihan
A and Düzcan E: Antithrombin III reduces renal ischemia-reperfusion
injury in rats. Res Exp Med (Berl). 200:195–203. 2001.PubMed/NCBI
|
|
19
|
Yin J, Wang F, Kong Y, Wu R, Zhang G, Wang
N, Wang L, Lu Z and Liang M: Antithrombin III prevents progression
of chronic kidney disease following experimental
ischaemic-reperfusion injury. J Cell Mol Med. 21:3506–3514.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kong Y, Yin J, Cheng D, Lu Z, Wang N, Wang
F and Liang M: Antithrombin III Attenuates AKI Following Acute
Severe Pancreatitis. Shock. 49:572–579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xie Y, Tian R, Jin W, Xie H, Du J, Zhou Z
and Wang R: Antithrombin III expression predicts acute kidney
injury in elderly patients with sepsis. Exp Ther Med. 19:1024–1032.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Foreman BP, Caesar RR, Parks J, Madden C,
Gentilello LM, Shafi S, Carlile MC, Harper CR and Diaz-Arrastia RR:
Usefulness of the abbreviated injury score and the injury severity
score in comparison to the Glasgow Coma Scale in predicting outcome
after traumatic brain injury. J Trauma. 62:946–950. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vincent JL, Moreno R, Takala J, Willatts
S, De Mendonça A, Bruining H, Reinhart CK, Suter PM and Thijs LG:
The SOFA (Sepsis-related Organ Failure Assessment) score to
describe organ dysfunction/failure. On behalf of the Working Group
on Sepsis-Related Problems of the European Society of Intensive
Care Medicine. Intensive Care Med. 22:707–710. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Marshall LF, Marshall SB, Klauber MR,
Clark MvB, Eisenberg HM, Jane JA, Luerssen TG, Marmarou A and
Foulkes MA: A new classification of head injury based on
computerized tomography. J Neurosurg. 75 (Suppl):S14–S20. 1991.
|
|
25
|
Khwaja A: KDIGO clinical practice
guidelines for acute kidney injury. Nephron Clin Pract.
120:c179–c184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lustenberger T, Talving P, Kobayashi L,
Inaba K, Lam L, Plurad D and Demetriades D: Time course of
coagulopathy in isolated severe traumatic brain injury. Injury.
41:924–928. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Oliveira Manoel AL, Neto AC, Veigas PV
and Rizoli S: Traumatic brain injury associated coagulopathy.
Neurocrit Care. 22:34–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alexiou GA, Lianos G, Fotakopoulos G,
Michos E, Pachatouridis D and Voulgaris S: Admission glucose and
coagulopathy occurrence in patients with traumatic brain injury.
Brain Inj. 28:438–441. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wafaisade A, Lefering R, Tjardes T,
Wutzler S, Simanski C, Paffrath T, Fischer P, Bouillon B and
Maegele M: Trauma Registry of DGU. Acute coagulopathy in isolated
blunt traumatic brain injury. Neurocrit Care. 12:211–219.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Joseph B, Aziz H, Zangbar B, Kulvatunyou
N, Pandit V, O'Keeffe T, Tang A, Wynne J, Friese RS and Rhee P:
Acquired coagulopathy of traumatic brain injury defined by routine
laboratory tests: Which laboratory values matter? J Trauma Acute
Care Surg. 76:121–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kurland D, Hong C, Aarabi B, Gerzanich V
and Simard JM: Hemorrhagic progression of a contusion after
traumatic brain injury: A review. J Neurotrauma. 29:19–31.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Laroche M, Kutcher ME, Huang MC, Cohen MJ
and Manley GT: Coagulopathy after traumatic brain injury.
Neurosurgery. 70:1334–1345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Stein SC and Smith DH: Coagulopathy in
traumatic brain injury. Neurocrit Care. 1:479–488. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bucur SZ, Levy JH, Despotis GJ, Spiess BD
and Hillyer CD: Uses of antithrombin III concentrate in congenital
and acquired deficiency states. Transfusion. 38:481–498.
1998.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Blajchman MA: An overview of the mechanism
of action of antithrombin and its inherited deficiency states.
Blood Coagul Fibrinolysis. 5 (Suppl 1):S5–S11; discussion S59-S64.
1994.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jacome T and Tatum D: Systemic
inflammatory response Syndrome (SIRS) Score independently predicts
poor outcome in isolated traumatic brain injury. Neurocrit Care.
28:110–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Szabo G, Romics L Jr and Frendl G: Liver
in sepsis and systemic inflammatory response syndrome. Clin Liver
Dis. 6:1045–1066, x. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mulay SR, Holderied A, Kumar SV and Anders
HJ: Targeting inflammation in so-called acute kidney injury. Semin
Nephrol. 6:17–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gomez H, Ince C, De Backer D, Pickkers P,
Payen D, Hotchkiss J and Kellum JA: A unified theory of
sepsis-induced acute kidney injury: Inflammation, microcirculatory
dysfunction, bioenergetics, and the tubular cell adaptation to
injury. Shock. 41:3–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Maiwall R, Chandel SS, Wani Z, Kumar S and
Sarin SK: SIRS at Admission Is a Predictor of AKI Development and
Mortality in Hospitalized Patients with Severe Alcoholic Hepatitis.
Dig Dis Sci. 61:920–929. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Toyonaga Y, Asayama K and Maehara Y:
Impact of systemic inflammatory response syndrome and surgical
Apgar score on post-operative acute kidney injury. Acta
Anaesthesiol Scand. 61:1253–1261. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Souter PJ, Thomas S, Hubbard AR, Poole S,
Römisch J and Gray E: Antithrombin inhibits
lipopolysaccharide-induced tissue factor and interleukin-6
production by mononuclear cells, human umbilical vein endothelial
cells, and whole blood. Crit Care Med. 29:134–139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ostrovsky L, Woodman RC, Payne D, Teoh D
and Kubes P: Antithrombin III prevents and rapidly reverses
leukocyte recruitment in ischemia/reperfusion. Circulation.
96:2302–2310. 1997.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Horie S, Ishii H and Kazama M:
Heparin-like glycosaminoglycan is a receptor for antithrombin
III-dependent but not for thrombin-dependent prostacyclin
production in human endothelial cells. Thromb Res. 59:895–904.
1990.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Harada N, Okajima K, Kushimoto S, Isobe H
and Tanaka K: Antithrombin reduces ischemia/reperfusion injury of
rat liver by increasing the hepatic level of prostacyclin. Blood.
93:157–164. 1999.PubMed/NCBI
|
|
46
|
Shein SL, Shellington DK, Exo JL, Jackson
TC, Wisniewski SR, Jackson EK, Vagni VA, Bayır H, Clark RS, Dixon
CE, et al: Hemorrhagic shock shifts the serum cytokine profile from
pro- to anti-inflammatory after experimental traumatic brain injury
in mice. J Neurotrauma. 31:1386–1395. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Maier B, Lefering R, Lehnert M, Laurer HL,
Steudel WI, Neugebauer EA and Marzi I: Early versus late onset of
multiple organ failure is associated with differing patterns of
plasma cytokine biomarker expression and outcome after severe
trauma. Shock. 28:668–674. 2007.PubMed/NCBI
|
|
48
|
Chaikittisilpa N, Krishnamoorthy V, Lele
AV, Qiu Q and Vavilala MS: Characterizing the relationship between
systemic inflammatory response syndrome and early cardiac
dysfunction in traumatic brain injury. J Neurosci Res. 96:661–670.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Tomsa AM, Alexa AL, Junie ML, Rachisan AL
and Ciumarnean L: Oxidative stress as a potential target in acute
kidney injury. PeerJ. 7(e8046)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lee KH, Tseng WC, Yang CY and Tarng DC:
The Anti-Inflammatory, Anti-Oxidative, and anti-apoptotic benefits
of stem cells in acute ischemic kidney injury. Int J Mol Sci.
20(3529)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gyurászová M, Kovalčíková AG, Renczés E,
Kmeťová K, Celec P, Bábíčková J and Tóthová Ľ: Oxidative stress in
animal models of acute and chronic renal failure. Dis Markers.
2019(8690805)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Erman T, Yildiz MS, Göçer AI, Zorludemir
S, Demirhindi H and Tuna M: Effects of antithrombin III on
myeloperoxidase activity, superoxide dismutase activity, and
malondialdehyde levels and histopathological findings after spinal
cord injury in the rat. Neurosurgery. 56:828–835. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Havasi A and Borkan SC: Apoptosis and
acute kidney injury. Kidney Int. 80:29–40. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang X, Yan X and Yang D, Zhou J, Song J
and Yang D: Rapamycin attenuates mitochondrial injury and renal
tubular cell apoptosis in experimental contrast-induced acute
kidney injury in rats. Biosci Rep. 38(BSR20180876)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Arai S, Kitada K, Yamazaki T, Takai R,
Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y,
et al: Apoptosis inhibitor of macrophage protein enhances
intraluminal debris clearance and ameliorates acute kidney injury
in mice. Nat Med. 22:183–193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Beier K, Eppanapally S, Bazick HS, Chang
D, Mahadevappa K, Gibbons FK and Christopher KB: Elevation of blood
urea nitrogen is predictive of long-term mortality in critically
ill patients independent of ‘normal’ creatinine. Crit Care Med.
39:305–313. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yu JH, Kwon Y, Kim J, Yang SM, Kim WH,
Jung CW, Suh KS and Lee KH: Influence of Transfusion on the Risk of
Acute Kidney Injury: ABO-Compatible versus ABO-Incompatible Liver
Transplantation. J Clin Med. 8(1785)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kalisvaart M, Schlegel A, Umbro I, de Haan
JE, Polak WG, IJzermans JN, Mirza DF, Perera MTP, Isaac JR,
Ferguson J, et al: The AKI Prediction Score: A new prediction model
for acute kidney injury after liver transplantation. HPB (Oxford).
21:1707–1758. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kim WH, Park MH, Kim HJ, Lim HY, Shim HS,
Sohn JT, Kim CS and Lee SM: Potentially modifiable risk factors for
acute kidney injury after surgery on the thoracic aorta: A
propensity score matched case-control study. Medicine (Baltimore).
94(e273)2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Aksu Erdost H, Ozkardesler S, Ocmen E,
Avkan-Oguz V, Akan M, Iyilikci L, Unek T, Ozbilgin M, Meseri Dalak
R and Astarcioglu I: Acute renal injury evaluation after liver
transplantation: With RIFLE Criteria. Transplant Proc.
47:1482–1487. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pandey S and Vyas GN: Adverse effects of
plasma transfusion. Transfusion. 52 (Suppl 1):65s–79s.
2012.PubMed/NCBI View Article : Google Scholar
|