Introduction
Peripheral artery disease (PAD) is becoming a global
health concern, affecting >200 million individuals worldwide
(1) Despite the implementation of
antiplatelet therapy, patients with PAD remain at high risk of
atherosclerotic complications (2).
In particular, acute thromboembolic occlusions commonly occur in
this population. Current treatment strategies primarily focus on
restoring perfusion in the ischemic limb and mitigating ischemic
injury (3). Thromboembolism is
considered to be the primary etiology of acute limb ischemia, where
several clinical factors, including abdominal aortic surgery,
trauma, revascularization surgery and orthopedic procedures, can be
involved in ischemia-reperfusion (I/R) injury. I/R injury is a
well-documented complication in vascular surgery and contributes
markedly to postoperative morbidity and mortality (3).
In clinical practice, during revascularization,
tissues perfused by compromised arterial systems typically
experience varying durations of ischemia. The resulting burst of
oxidative and inflammatory mediators can induce both local and
systemic organ injury (4-6).
Experimental evidence has previously demonstrated that I/R can
activate oxidative and inflammatory molecular pathways, eventually
leading to the excessive generation of reactive oxygen species and
cellular damage, partly through dysregulation of the nuclear factor
erythroid 2-related factor 2 (Nrf2)-mediated antioxidant response
(7). Furthermore, I/R can disrupt
redox homeostasis, promote lipid peroxidation and deplete
endogenous antioxidants [such as glutathione (GSH)], as evidenced
in preclinical studies (3,8,9). As
the predominant tissue in the limb, the skeletal muscle exhibits
marked susceptibility to ischemic stress, underscoring the critical
need for prophylactic strategies to limit I/R-induced injury in
clinical settings such as acute limb ischemia, lower extremity
revascularization procedures and major vascular surgery (3).
Among the molecular hallmarks of I/R injury,
oxidative DNA damage is of particular relevance. The product of DNA
oxidation, 8-hydroxy-2'-deoxyguanosine (8-OHdG), is a
well-established biomarker of oxidative DNA modifications that has
been extensively studied in diseases associated with elevated
oxidative stress, including cardiovascular disorders and
malignancies (10). Elevated
8-OHdG levels indicate oxidative genomic instability, contributing
to mutagenesis and the progression of chronic diseases, including
cancer, atherosclerotic cardiovascular disease and
neurodegenerative disorders (10).
The key pathophysiological role of oxidative DNA damage in I/R
injury highlights the necessity of therapeutic interventions
targeting oxidative stress pathways.
Rivaroxaban is a direct oral anticoagulant that
selectively inhibits factor Xa and exhibits pleiotropic properties
beyond its antithrombotic action. Emerging evidence has suggested
that rivaroxaban can exert anti-inflammatory and antioxidative
effects by modulating protease-activated receptor signaling
(11). In addition to its
established role in thromboprophylaxis, rivaroxaban could reduce
ischemic complications in patients with PAD, thereby conferring
protective effects against I/R-induced tissue injury (4). Notably, rivaroxaban has been
investigated for its safety and efficacy in oncologic populations,
a cohort frequently predisposed to hypercoagulability and oxidative
stress-related complications (12). Its ability to mitigate oxidative
stress and DNA damage could therefore have broader clinical
implications, particularly in conditions characterized by
intertwined thrombo-inflammation mechanisms.
Therefore, the present study aimed to investigate
the effects of rivaroxaban on skeletal muscle I/R injury, with
emphasis on oxidative DNA damage. To comprehensively assess
oxidative stress and antioxidant defense mechanisms, the levels of
malondialdehyde (MDA), a marker of lipid peroxidation, in addition
to reduced GSH and oxidized GSH (GSSG) as key indicators of the
endogenous antioxidant defense system, were assessed (13,14).
Additionally, 8-OHdG levels were quantified in both tissue and
serum samples to determine the extent of oxidative DNA damage and
to reveal the potential role of rivaroxaban in I/R-induced
oxidative stress (10).
Materials and methods
Blinding and experimental design
The present study was a single-blind experimental
study. Although the investigator involved in treatment
administration was aware of the group allocations, all biochemical
analyses, including ELISA and high-performance liquid
chromatography (HPLC), were performed by an independent researcher
blinded to the experimental groups. All samples were anonymized and
coded prior to analysis to ensure objective data evaluation.
Animal ethics and study approval
The experimental protocol was approved by the Local
Ethics Committee for Animal Experiments of Dokuz Eylül University
(approval no. 12/2021; Izmir, Turkey). All animal procedures were
performed according to the National Institutes of Health Guide for
the Care and Use of Laboratory Animals (15). The total duration of the experiment
was 17 days, including a 7-day acclimatization period, 10 days of
drug administration and a 3-h I/R protocol.
Experimental groups
A total of 21 female Wistar albino rats aged 10-12
weeks (weight, 200-250 g) were obtained from the Dokuz Eylül
University Multidisciplinary Animal Experimentation Laboratory.
Only female rats were used because the institutional breeding
colony available at the time of the experiment predominantly
contained female animals of required age and weight range. The
required sample size was calculated using the G*Power
software (version 3.1.9.7; http://www.gpower.hhu.de) for one-way ANOVA across
three independent groups. An effect size (f) of 0.45 was assumed
based on a previously published experimental study evaluating
8-OHdG as the primary oxidative stress outcome, with a significance
level (α) of 0.05 and a power (1-β) of 0.80(10). The calculated total sample size was
21 rats (n=7 per group). This rat model was selected based on its
availability, high reliability and reproducibility in I/R studies
(3,16,17).
Rats were maintained under controlled environmental conditions,
with a 12-h light/dark cycle, a temperature of 22±2˚C and a
relative humidity of 50±10% with ad libitum access to
standard pellet feed. All animals were housed in standard rat
cages, where all experiments were conducted under the same
laboratory conditions. Humane endpoints were predefined according
to ARRIVE guidelines and animals were to be euthanized if any of
the following occurred: >20% weight loss, severe hypoactivity or
inability to ambulate, respiratory distress, hypothermia
(<30˚C), self-mutilation or uncontrollable bleeding. No animals
reached these humane endpoints. A total of 21 animals entered the
study, where all completed the planned procedures and all were
euthanized at the scheduled experimental endpoint under deep
anesthesia. No unplanned deaths occurred and no animal died due to
procedural complications.
The primary outcome measured was the quantification
of 8-OHdG levels in serum and skeletal muscle tissues as a
biomarker of oxidative DNA damage following I/R and its modulation
by rivaroxaban (Xarelto®; Bayer AG) treatment. The rats
were randomly allocated into the following three groups (n=7 each):
i) Group 1 (sham group), where no I/R procedure was applied, but
rats received anesthesia, limb shaving and antiseptic skin
preparation to ensure comparable procedural stress; ii) group 2
(control group), where rats were subjected to I/R injury without
treatment; and iii) group 3 (rivaroxaban treatment group), where
rats received 3 mg/kg/day rivaroxaban by oral gavage for 10 days
prior to I/R induction.
The rivaroxaban dosing regimen was selected based on
previous studies employing similar animal models (17,18).
Rivaroxaban was diluted in distilled water to a final concentration
of 1 mg/ml and administered daily at a volume of 1 ml per 250 g
body weight to ensure accurate and consistent dosing across
animals. Following 10 days of treatment, rats were administered
ketamine (50 mg/kg; Ketalar®; Pfizer, Inc.) and xylazine
(10 mg/kg Xylazine®; Bayer AG) by intraperitoneal
injection to achieve anesthesia and analgesia. The left hind limb
was then shaved, disinfected and a tourniquet was applied at the
level of the hip joint using a standardized elastic tension.
Ischemia was induced by tightening the elastic band with three
turns and secured with consistent tension, where adequate occlusion
was verified by the absence of Doppler flow signals in the
posterior tibial artery using a portable Doppler ultrasound device
(Getinge AB). This form of ischemia was maintained for 1 h. The
tourniquet method was selected based on a previous experimental
study indicating that simple femoral artery ligation is
insufficient to induce lower extremity ischemia due to robust
collateral circulation in the rat hind limb (16). After 1 h of ischemia, the
tourniquet was removed, the animals were allowed to recover from
anesthesia and a reperfusion period of 2 h was allowed. At the end
of the reperfusion period, whilst under deep anesthesia (ketamine
50 mg/kg and xylazine 10 mg/kg), ~3-4 ml blood was collected by
intracardiac puncture. Euthanasia was subsequently completed by
exsanguination. Death was confirmed by cessation of cardiac and
respiratory activity. Skeletal tissue samples were obtained from
the medial head of the left gastrocnemius muscle at the central
portion, washed in 100 ml 0.9% NaCl and placed on ice for transport
to the biochemistry laboratory. The tissue samples were stored in
Eppendorf tubes at -80˚C for subsequent analysis. Blood samples
were centrifuged at 450 x g for 10 min at 4˚C to separate serum,
which was then stored in Eppendorf tubes at -80˚C for
preservation.
Tissue homogenization and HPLC
procedure
Skeletal muscle tissue samples were stored in
Eppendorf tubes at -80˚C until homogenization. Prior to
homogenization, samples were thawed on ice, weighed and homogenized
in chilled PBS at a tissue-to-buffer ratio of 1:10 (w/v; 100 mg
tissue in 1 ml PBS) to ensure consistent homogenate concentrations.
Subsequently, metal beads were added and the tissues were
homogenized using the TissueLyser II QIAGEN® device
(Qiagen, Inc.). The homogenates were then centrifuged at 10,000 x g
for 15 min at 4˚C. Following centrifugation, the supernatant was
transferred into new Eppendorf tubes, whilst the remaining pellet
and beads were discarded. On the same day, a portion of the
supernatant was used to determine protein content with the Pierce
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions. The results for MDA, GSH/GSSG
and 8-OhdG assays were normalized to total tissue protein levels.
Tissue and serum levels of MDA and GSH/GSSG were quantified using
an HPLC system (Shimadzu Prominence HPLC system; Shimadzu
Corporation) with commercial assay kits (ImmuChrom GmbH). This
method was selected for its high analytical precision and
reproducibility in biochemical marker quantification (13). Chromatographic separation was
carried out using reversed-phase columns maintained at 30˚C, namely
Bischoff Prontosil Eurobond (5 µm; 125x4 mm; BİSCHOFF
Chromatography) for MDA analysis and MZ Inertsil ODS (5 µm; 125x4
mm; MZ-Analysentechnik GmbH) for GSH/GSSG analysis. The mobile
phase was delivered at a flow rate of 0.8 ml/min. Samples were
injected at a volume of 20 µl, and the total run time for each
analysis was 4 min. Fluorescence detection was performed with an
excitation wavelength of 515 nm and an emission wavelength of 553
nm for MDA and 385/515 nm for GSH/GSSG. All HPLC analyses were
performed by an external service (Delta Analiz Laboratuvar
Hizmetleri).
ELISA
Quantification of 8-OHdG levels in tissue and serum
samples was performed using the BT Lab Rat 8-Hydroxy-deoxyguanosine
ELISA® kit (cat. no. E0031Ra; BT LAB Bioassay Technology
Laboratory; Shanghai Korain Biotech Co., Ltd.) in strict accordance
with the manufacturer's protocol based on the sandwich ELISA
principle. Colorimetric detection was carried out at 450 nm using a
microplate reader. The amount of 8-OHdG in the samples was
determined by comparing the measured absorbance values to a
standard curve generated from known concentrations of 8-OHdG, where
the concentration of 8-OHdG is expressed in ng/ml. The measurement
range provided by the kit was 0.05-20 ng/ml, with a lower detection
limit of 0.027 ng/ml.
Statistical analysis
All statistical analyses were performed using IBM
SPSS version 29 (IBM Corp.). The data are expressed as the mean ±
standard deviation. The normality of distribution was assessed
using the Shapiro-Wilk test and serum GSH levels did not initially
show normal distribution (P<0.05). Therefore, log10
transformation was applied prior to analysis. Log10
transformation was chosen for serum GSH to reduce positive
skewness, stabilize variance and allow the use of parametric
models, which are more powerful in small experimental samples when
their assumptions are satisfied. These assumptions include
approximate normality of the data distribution and homogeneity of
variances. All other variables, including tissue parameters,
satisfied normality assumptions. Following transformation, all
variables met the criteria for normal distribution (Shapiro-Wilk
P>0.05), allowing for parametric analysis using one-way ANOVA.
Homogeneity of variances was assessed using Levene's homogeneity
test. For variables with homogeneous variances, one-way ANOVA
followed by Bonferroni's post-hoc test was performed. For variables
with heterogeneous variances, Welch's ANOVA was used together with
Tamhane's T2 post-hoc test, which is recommended for unequal
variances and small sample sizes. P<0.05 was considered to
indicate a statistically significant difference. To assess the
robustness of the findings, non-parametric analyses (Kruskal-Wallis
followed by Dunn's post-hoc test) were also performed for the
non-normally distributed serum biomarker (GSH), where this yielded
a significance pattern consistent with the parametric analyses.
Results
Model of oxidative stress
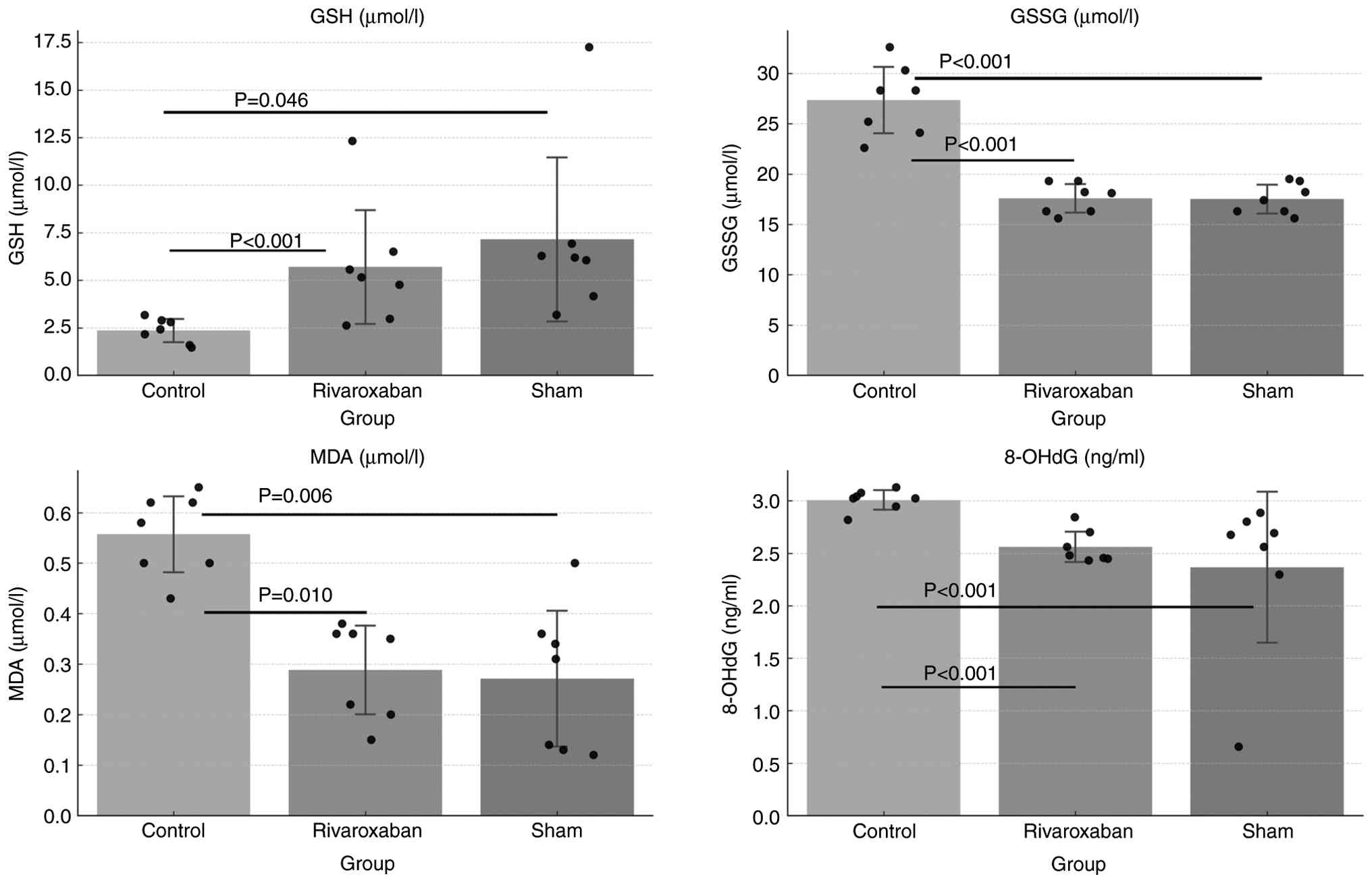
In the present study, GSH levels were significantly
reduced in blood samples in the control (I/R) group compared with
those in the sham group (P=0.046). By contrast, GSSG, MDA and
8-OHdG levels were significantly higher in the control (I/R) group
compared with those in the sham group (P<0.001, P=0.006 and
P<0.001, respectively; Fig. 1).
These findings suggest an increase in oxidative markers (GSSG, MDA
and 8-OHdG) and depletion of antioxidant markers (GSH) following
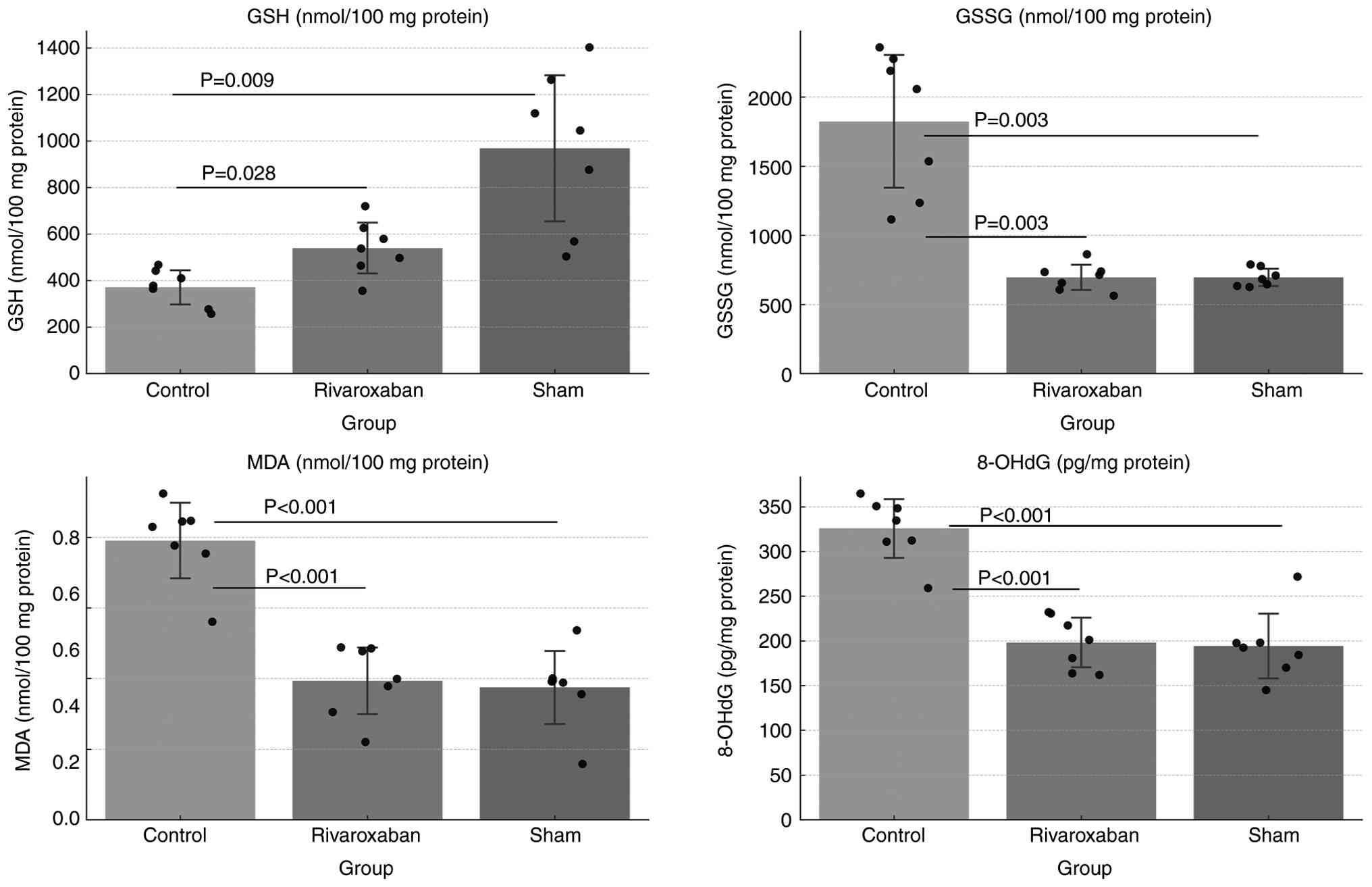
I/R injury. Consistently, in skeletal muscle tissues, GSH levels
were found to be significantly reduced in the control group
compared with those in the sham group (P=0.009), whereas GSSG, MDA
and 8-OHdG levels were significantly elevated (P=0.003, P<0.001
and P<0.001, respectively; Fig.
2). These results indicated that I/R-related oxidative stress
was successfully induced in the current experimental model.
Effect of rivaroxaban on circulating
oxidative biomarkers
Pairwise comparisons revealed that rivaroxaban
treatment significantly increased blood GSH levels compared with
those in the untreated control group (P<0.001). By contrast,
circulating GSSG, MDA and 8-OHdG levels were significantly lower in
the rivaroxaban group compared with those in the control group
(P<0.001, P=0.010 and P<0.001, respectively). These findings
indicated that rivaroxaban could exhibit antioxidant and potential
cytoprotective effects against I/R-induced oxidative stress,
highlighting its ability to mitigate oxidative stress (Fig. 1; Table
I).
 | Table ISerum levels of oxidative stress
markers. |
Table I
Serum levels of oxidative stress
markers.
| Group | GSH, µM/l
(log10 transformed) | GSSG, µM/l | MDA, µM/l | 8-OHdG, ng/ml |
|---|
| Control | 0.34±0.11 | 27.3±3.5 | 0.55±0.08 | 3.00±0.10 |
| Sham | 0.79±0.22 | 17.5±1.5 | 0.27±0.14 | 2.36±0.77 |
| Rivaroxaban | 0.66±0.15 | 17.5±1.5 | 0.28±0.09 | 2.55±0.15 |
Skeletal muscle oxidative stress
markers
In skeletal muscle tissues, GSH levels were
significantly higher in the rivaroxaban group compared with those
in the control group (P=0.028), whereas GSSG (P=0.003), MDA
(P<0.001) and 8-OHdG (P<0.001) levels were significantly
lower (Fig. 2; Table II), thus verifying that
rivaroxaban not only attenuated oxidative damage but it was also
involved in maintaining redox homeostasis at the tissue level. The
observed reduction in oxidative products and restoration of
antioxidant defenses in both serum and tissue samples supported
that rivaroxaban could exert a protective effect against
I/R-induced oxidative stress.
 | Table IISkeletal muscle tissue levels of
oxidative stress markers. |
Table II
Skeletal muscle tissue levels of
oxidative stress markers.
| Group | GSH, nM/100 mg
protein | GSSG, nM/100 mg
protein | MDA, nM/100 mg
protein | 8-OHdG, pg/mg
protein |
|---|
| Control | 370.2±79.0 | 1,823.0±518.0 | 0.79±0.11 | 325±35 |
| Sham | 967.5±338.0 | 694.0±67.0 | 0.37±0.11 | 194±39 |
| Rivaroxaban | 539.4±117.0 | 695.0±98.0 | 0.39±0.10 | 198±29 |
Discussion
PAD remains a major cause of morbidity and mortality
worldwide, where the optimal medical therapeutic strategy following
revascularization continues to be a subject of debate. I/R injury
is a critical concern in PAD management, since it exacerbates
tissue injury and contributes to both local and systemic
complications (1,19). The present study aimed to
investigate the potential effects of rivaroxaban, a direct factor
Xa inhibitor, on skeletal muscles subjected to I/R injury. The
results demonstrated that rivaroxaban could significantly attenuate
oxidative stress and DNA damage in both serum and skeletal muscles,
thereby supporting its potential therapeutic role in mitigating
I/R-related complications.
To the best of our knowledge, only a few
experimental studies have evaluated 8-OHdG as a primary biomarker
of oxidative DNA damage in lower extremity I/R models. In the
present study, the results showed that even a brief period of I/R
could induce a significant increase in 8-OHdG levels, reinforcing
its value as a sensitive and quantifiable indicator of oxidative
DNA injury. Previous experimental studies have demonstrated that
I/R injury is closely associated with oxidative stress-mediated
inflammation, apoptosis and functional tissue impairment using
alternative oxidative stress markers and experimental endpoints
(9,20). Furthermore, the significant
reduction in 8-OHdG levels observed in the rivaroxaban-treated
group in the present study suggested that rivaroxaban could
mitigate DNA oxidative damage, potentially contributing to the
preservation of cellular integrity under oxidative stress
conditions.
The antioxidant properties of rivaroxaban observed
in the present study were further supported by the decrease in MDA
and GSSG levels, accompanied by the preservation of GSH
concentrations. These markers reflect lipid peroxidation and redox
balance, respectively, and are commonly used to quantify oxidative
stress in experimental models (7,8). The
consistent improvements detected in both serum and skeletal muscle
tissues indicated that rivaroxaban could exert systemic protective
effects against I/R-induced oxidative stress.
Although the present study did not directly
investigate molecular signaling mechanisms, prior evidence
indicated that rivaroxaban can modulate key oxidative
stress-related pathways. Specifically, a previous study
demonstrated that inhibition of the protease-activated receptor-2
and NF-κB pathways was associated with attenuated inflammation and
oxidative stress in experimental cellular and ex vivo tissue
models (21). Additionally,
oxidative stress responses are regulated by the Nrf2 pathway, which
regulates the expression of antioxidant enzymes and may be relevant
in the context of reduced oxidative stress following factor Xa
inhibition (22). However, these
pleiotropic effects warrant further investigation in mechanistic
studies.
While rivaroxaban was chosen based on its documented
non-anticoagulant properties, other oral anticoagulants, including
apixaban and dabigatran, have also been studied for their potential
antioxidant or endothelial-protective effects. These effects were
demonstrated in various in vitro endothelial cell models
exposed to inflammatory or oxidative stimuli, highlighting the
shared endothelial-protective and antioxidant actions of these
agents (23). However, comparative
evidence remains limited, where rivaroxaban continues to be the
most extensively investigated agent in both clinical and
experimental models of PAD, likely due to its earlier approval,
extensive evaluation in large-scale trials and its dual capacity to
inhibit both factor Xa and thrombin generation, mechanisms that are
particularly relevant to thrombosis and vascular inflammation in
PAD (4,5,11,17,21,24).
Results of the present study are in line with
clinical evidence from various trials, such as VOYAGER-PAD and
COMPASS, which demonstrated that low-dose rivaroxaban reduced
limb-related events (such as acute limb ischemia, major amputation
and repeat revascularization) in patients with PAD undergoing
revascularization (5,25). However, it should be noted that in
the present study an acute I/R injury model was employed in healthy
rats, which differs markedly from the chronic and comorbid
conditions observed in human PAD. Consequently, the translational
relevance of these findings should be interpreted with caution.
Future studies involving chronic ischemia models or animals with
comorbid models could provide additional insights.
The present study there were several limitations.
Experiments were conducted in a healthy rat model using an acute
I/R protocol, which may not fully reproduce the complex
pathophysiology of chronic PAD in human. The I/R period was
relatively short, where long-term outcomes, such as tissue
regeneration or functional recovery, were not evaluated. In
addition, the analysis was limited to oxidative stress markers,
whereby histological or molecular analyses were not performed to
verify tissue-level damage or repair. Future studies in chronic I/R
models and clinical settings are warranted to validate the
translational relevance of these findings. Furthermore, only female
rats were used, which may introduce sex-related biological bias and
limit the generalizability of the findings to male subjects.
Current guidelines for PAD management remain
inconclusive regarding the optimal medical therapy following
revascularization. The 2016 American College of Cardiology and
American Heart Association guidelines recommend monotherapy with
either aspirin or clopidogrel, whereas the 2021 Turkish National
Guidelines for Peripheral Artery and Venous Disease suggest that
low-dose rivaroxaban combined with aspirin could provide additional
benefit in high-risk patients (6,26)
Despite these recommendations, the use of dual antiplatelet therapy
or anticoagulant-antiplatelet combinations remains common in
clinical practice.
Overall, the findings of the present study
contributed to the growing body of preclinical evidence supporting
the potential role of rivaroxaban in the management of PAD,
particularly under experimental conditions of acute I/R injury,
which simulate a high ischemic risk state. The observed reduction
in oxidative stress and DNA damage suggests that rivaroxaban may
confer additional protective effects beyond its anticoagulant
action, potentially improving long-term outcomes following
revascularization.
Acknowledgements
The authors would like to thank the staff of the
Multidisciplinary Animal Experimentation Laboratory at Dokuz Eylül
University (Izmir, Turkey) for their valuable assistance in the
care and handling of the experimental animals. The authors also
thank the Biochemistry Laboratory team at Dokuz Eylül University
for their support with the ELISA and HPLC analyses.
Funding
Funding: The present study was supported by the Dokuz Eylül
University Scientific Research Project (DEU-BAP; grant no.
TSA-2022-2647).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS conceived and designed the study, conducted the
animal experiments and drafted the manuscript. TG assisted with the
animal experiments and sample collection. ÖGD and TK performed the
biochemical analyses. ACE contributed to statistical analysis and
interpretation of data. CS and ACE confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Local
Ethics Committee for Animal Experiments of the Dokuz Eylül
University (approval no. 12/2021; Izmir, Turkey) and were performed
in accordance with the National Institutes of Health Guide for the
care and use of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript.
Specifically, ChatGPT (OpenAI-GPT 5.1) was used for text
refinement. Subsequently, the authors revised and edited the
content refined by the AI tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Fowkes FG, Rudan D, Rudan I, Aboyans V,
Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ,
Mensah GA and Criqui MH: Comparison of global estimates of
prevalence and risk factors for peripheral artery disease in 2000
and 2010: A systematic review and analysis. Lancet. 382:1329–1340.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sigvant B, Hasvold P, Kragsterman B,
Falkenberg M, Johansson S, Thuresson M and Nordanstig J:
Cardiovascular outcomes in patients with peripheral arterial
disease as an initial or subsequent manifestation of
atherosclerotic disease: Results from a Swedish nationwide study. J
Vasc Surg. 66:507–514.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blaisdell FW: The pathophysiology of
skeletal muscle ischemia and the reperfusion syndrome: A review.
Cardiovasc Surg. 10:620–630. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hess CN, Debus ES, Nehler MR, Anand SS,
Patel MR, Szarek M, Capell WH, Hsia J, Beckman JA, Brodmann M, et
al: Reduction in acute limb ıschemia with rivaroxaban versus
placebo in peripheral artery disease after lower extremity
revascularization: Insights from VOYAGER PAD. Circulation.
144:1831–1841. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Capell WH, Bonaca MP, Nehler MR, Chen E,
Kittelson JM, Anand SS, Berkowitz SD, Debus ES, Fanelli F, Haskell
L, et al: Rationale and design for the Vascular Outcomes study of
ASA along with rivaroxaban in endovascular or surgical limb
revascularization for peripheral artery disease (VOYAGER PAD). Am
Heart J. 199:83–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gerhard-Herman MD, Gornik HL, Barrett C,
Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG,
Hamburg NM, Kinlay S, et al: 2016 AHA/ACC Guideline on the
management of patients with lower extremity peripheral artery
disease: Executive summary: A report of the American College of
Cardiology/American Heart Association Task Force on clinical
practice guidelines. Circulation. 135:e686–e725. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qin Z and Xu Y: Dexmedetomidine alleviates
brain ıschemia/reperfusion ınjury by regulating
metastasis-associated lung adenocarcinoma transcript
1/MicroRNA-140-5p/nuclear factor erythroid-derived 2-like 2 axis.
Protein Pept Lett. 31:116–127. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bagheri Y, Ahmadian E, Hejazian SM, Raeesi
M, Zununi Vahed S and Ardalan M: The effect of fingolimod on renal
ıschemia/reperfusion ınjury in a rat model. Curr Mol Pharmacol: Oct
23, 2024 (Epub ahead of print).
|
|
9
|
Perrelli MG, Pagliaro P and Penna C:
Ischemia/reperfusion injury and cardioprotective mechanisms: Role
of mitochondria and reactive oxygen species. World J Cardiol.
3:186–200. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Di Minno A, Turnu L, Porro B, Squellerio
I, Cavalca V, Tremoli E and Di Minno MND:
8-Hydroxy-2-deoxyguanosine levels and cardiovascular disease: a
systematic review and meta-analysis of the literature. Antioxid
Redox Signal. 24:548–555. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ishibashi Y, Matsui T, Fukami K, Ueda S,
Okuda S and Yamagishi S: Rivaroxaban inhibits oxidative and
inflammatory reactions in advanced glycation end product-exposed
tubular cells by blocking thrombin/protease-activated receptor-2
system. Thromb Res. 135:770–773. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Costa OS, Kohn CG, Kuderer NM, Lyman GH,
Bunz TJ and Coleman CI: Effectiveness and safety of rivaroxaban
compared with low-molecular-weight heparin in cancer-associated
thromboembolism. Blood Adv. 4:4045–4051. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee SG, Yim J, Lim Y and Kim JH:
Validation of a liquid chromatography tandem mass spectrometry
method to measure oxidized and reduced forms of glutathione in
whole blood and verification in a mouse model as an indicator of
oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci.
1019:45–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dalle-Donne I, Rossi R, Colombo R,
Giustarini D and Milzani A: Biomarkers of oxidative damage in human
disease. Clin Chem. 52:601–623. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guide for the Care and Use of Laboratory
Animals. National Academies Press, Washington, DC, 2011.
|
|
16
|
Rosero O, Németh K, Turóczi Z, Fülöp A,
Garbaisz D, Győrffy A, Szuák A, Dorogi B, Kiss M, Nemeskéri Á, et
al: Collateral circulation of the rat lower limb and its
significance in ischemia-reperfusion studies. Surg Today.
44:2345–2353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Durmaz S, Kurtoğlu T, Rahman ÖF, Tataroğlu
C, Yılmaz M, Barbarus E and Erkan MH: Direct oral anticoagulant
agents attenuate temporary aortic occlusion-induced renal oxidative
and inflammatory responses in rats. Turk Gogus Kalp Damar Cerrahisi
Derg. 30:184–191. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Heitzer M, Winnand P, Bock A, Ooms M, Katz
MS, Kniha K, Grottke O, Hölzle F and Modabber A: Evaluation of the
hemostatic effect of an ınnovative tissue adhesive during
extraction therapy under rivaroxaban in a rodent model. J Funct
Biomater. 14(333)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Norgren L, Hiatt WR, Dormandy JA, Nehler
MR, Harris KA and Fowkes FGR: TASC II Working Group. Inter-society
consensus for the management of peripheral arterial disease (TASC
II). J Vasc Surg. 45 (Suppl 1):S5–S67. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ıschemia and reperfusion ınjury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bukowska A, Zacharias I, Weinert S, Skopp
K, Hartmann C, Huth C and Goette A: Coagulation factor Xa induces
an inflammatory signalling by activation of protease-activated
receptors in human atrial tissue. Eur J Pharmacol. 718:114–123.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Fan C, Wang J and Jiang M:
Rivaroxaban combined with atorvastatin ınhibits acute pulmonary
embolism by promoting the expression of NRF2/NQO1. Cardiovasc Drugs
Ther. 38:1271–1287. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Atzemian N, Kareli D, Ragia G and
Manolopoulos VG: Distinct pleiotropic effects of direct oral
anticoagulants on cultured endothelial cells: A comprehensive
review. Front Pharmacol. 14(1244098)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bonaca MP, Bauersachs RM, Anand SS, Debus
ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, et
al: Rivaroxaban in Peripheral Artery Disease after
Revascularization. N Engl J Med. 382:1994–2004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eikelboom JW, Connolly SJ, Bosch J,
Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM,
Anand SS, et al: Rivaroxaban with or without aspirin in stable
cardiovascular disease. N Engl J Med. 377:1319–1330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bozkurt AK: National Guideline for
Peripheral Arterial and Venous Diseases. Turkish Society of
Cardiovascular Surgery, Turkish Society of Vascular and
Endovascular Surgery, Turkish Phlebology Society; 2021. Available
at: https://www.tkdcd.org/uploads/content/pdf/kilavuzlar/Ulusal_Tedavi_Kilavuzu_2021.pdf.
|