Introduction
Chronic diarrhea, arthralgia and weight loss are the
hallmarks of Whipple's disease (WD), which is conventionally
associated with the fastidious Gram-positive actinobacterium
Tropheryma whipplei (T. whipplei) (1). WD is a chronic systemic infectious
disease, with an incidence of ~0.1 per 100,000 annually, that
mainly affects the gastrointestinal tract (1), whereas pulmonary parenchymal
involvement is particularly rare (2). Before the advent of antibiotics WD
was often fatal if untreated. With modern treatment, prognosis has
improved significantly, though untreated cases can still be
potentially fatal. WD mainly affects individuals with
immunocompromised states (for example, individuals with HIV or
recipients of organ transplants) and is transmitted via a
fecal-oral route (1).
Environmental exposure to T. whipplei is also a key risk
factor. Conventional microbiological techniques, such as bacterial
culture and serum analysis, frequently yield false-negative results
due to the slow growth, intracellular tropism and low antibody
titers of the organism (2).
Metagenomic next-generation sequencing (mNGS) is a
culture-independent diagnostic technique that can directly utilize
the patient specimen for pan-nucleic acid detection (3). In mNGS, all nucleic acids in the
specimen are extracted and sequenced in parallel, so as to obtain
the sequence of the host and microorganisms. Therefore, mNGS has
emerged as a notable tool for detecting unculturable or unexpected
pathogens (3). The present study
outlines a case of T. whipplei-associated pneumonia
diagnosed using mNGS, emphasizing its diagnostic utility in
atypical respiratory infections.
Case report
Following 4 days of wheezing, coughing and fever
(38.5˚C), with random blood glucose levels ranging from 8.0-18.8
mmol/l, a Chinese woman, aged 55, was admitted to Taizhou Second
People's hospital (Taizhou, China) in October 2023. The medical
history of this patient included well-controlled hypertension and
elevated blood glucose levels. The patient had a history of
elevated blood glucose levels, but did not undergo regular glycemic
monitoring or adhere to prescribed glucose-lowering medications. In
addition, the patient did not have any immunodeficiency disease and
had not been taking any immunosuppressive drugs. The patient was a
farmer without a history of travel and had never interacted with
any individual or animal known to be infected with T.
whipplei. The patient involved in the present study was
subjected to standard clinical practice and provided written
informed consent for the publication of medical data and
images.
A thorough physical examination was performed,
including assessment of vital signs (temperature, blood pressure,
heart rate and respiratory rate). Lung auscultation revealed clear
breath sounds in the left lung, while a few fine crackles were
audible in the right lung fields. No wheezes or rhonchi were noted.
No chest tenderness was noted on palpation. Cardiac examination
indicated normal heart sounds without murmurs, gallops or rubs and
no jugular vein distention was observed. Abdominal examination was
unremarkable, with a soft abdomen, lack of tenderness or rebound
tenderness, normal bowel sounds (4-5 times/min) and lack of
peripheral edema. Lymph node examination revealed no palpable
lymphadenopathy in the cervical, axillary or inguinal regions.
Neurological and musculoskeletal evaluations showed no focal
deficits such as limb weakness and sensory disturbance or joint
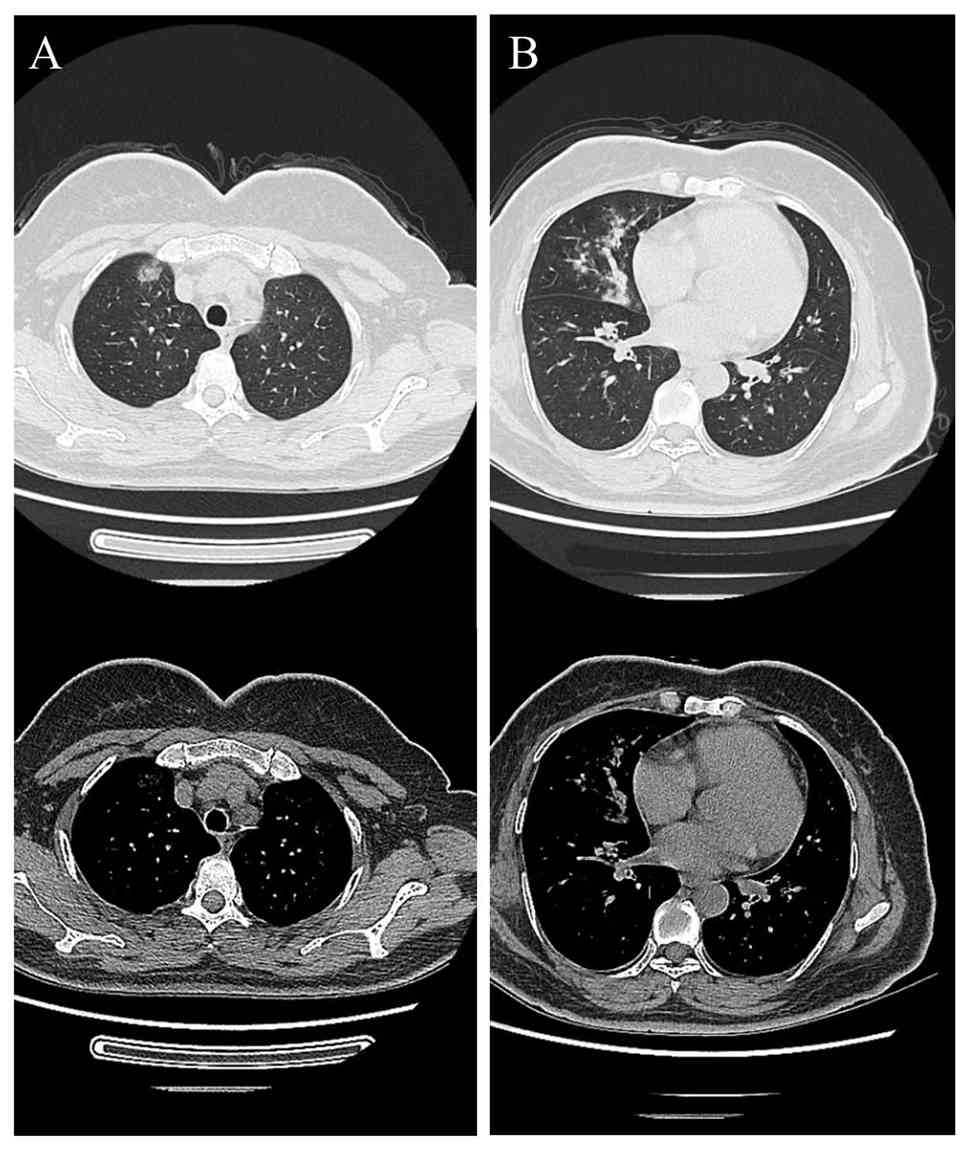
abnormalities such as swelling and tenderness. A chest CT scan
obtained in October 2023 (Fig. 1)
indicated patchy and nodular opacities with slightly increased
density and ill-defined borders. Test results for 1,3-β-D-glucan,
erythrocyte sedimentation rate, C-reactive protein, endotoxin and
procalcitonin were within normal ranges (Table I). Legionella pneumoniae,
Chlamydia pneumoniae, Mycoplasma pneumoniae,
respiratory viruses, coronavirus disease of 2019 and HIV were
negative in serological testing. The results for autoantibodies
were still negative. Diagnostic tests, including sputum smear and
bacterial culture yielded negative results. The patient was
diagnosed with community-acquired pneumonia (CAP) and empirical
treatment with moxifloxacin was initiated. During hospitalization,
moxifloxacin was administered intravenously at a daily dose of 0.4
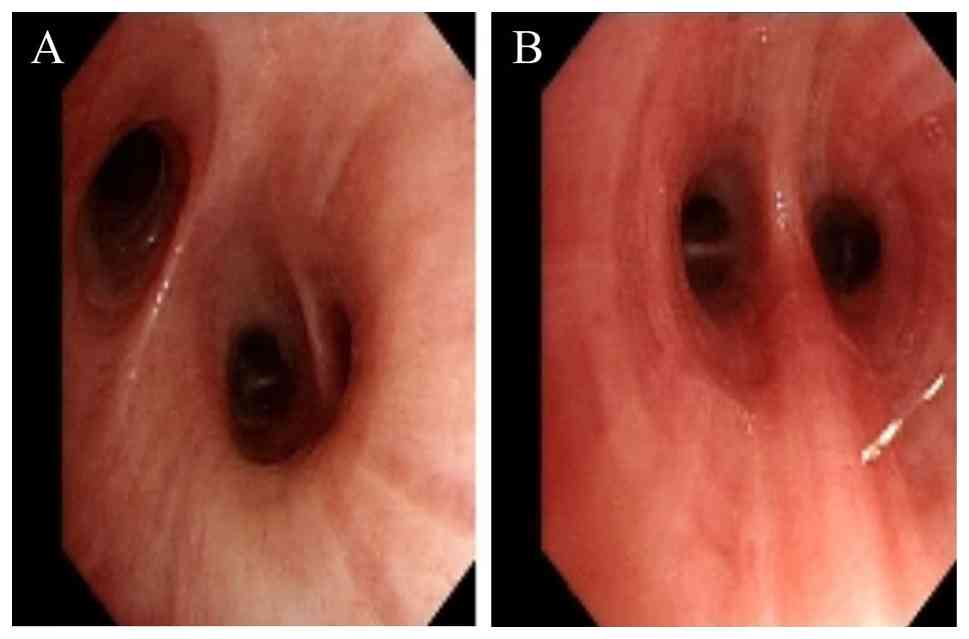
g. To identify the etiology of the illness, the patient underwent
bronchoscopy, which revealed edematous bronchial mucosa (Fig. 2). In addition, the bronchoalveolar
lavage fluid (BALF) was analyzed using mNGS. The sequencing of mNGS
was performed by Adicon Clinical Laboratories, Inc. (Hangzhou,
China). The service report indicated qualified internal and
negative controls, host read removal, nucleic acid extraction
concentration of 0.11 ng/µl, and library concentration of 29.60
ng/µl. Sequencing QC metrics included 17,699,043 total reads,
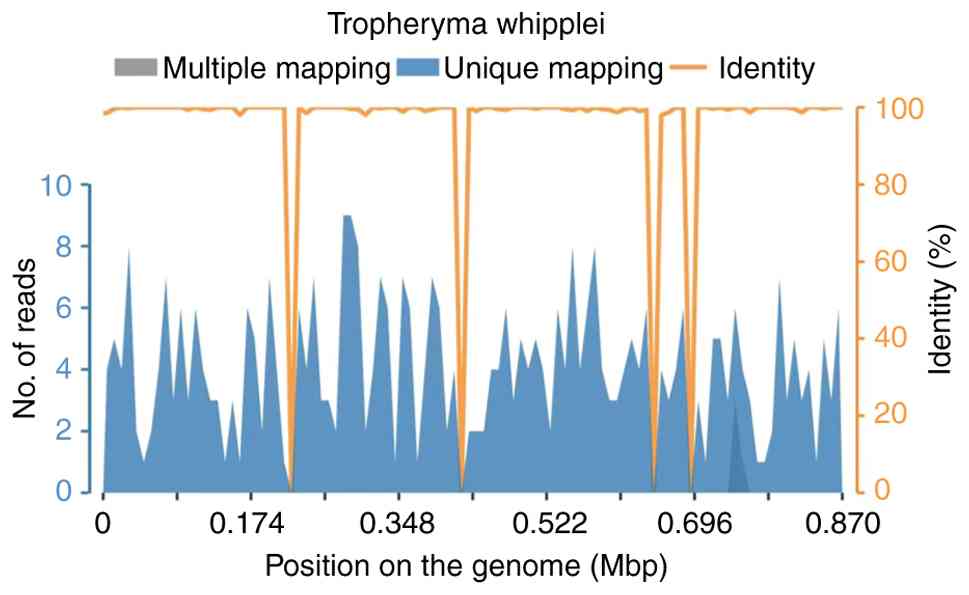
1,252,093 non-human reads, and 96.37% Q30. Microbial identification
was conducted by comparing sequencing reads to reference microbial
nucleic-acid databases, with T. whipplei detected at a total
of 19,731 base pairs (bp), a coverage of 2.26%, and an average
depth of 1.01X. The report also described a machine learning-based
pipeline for error correction, denoising, and exact sequence
inference. Antibiotic resistance gene interpretation referenced the
CARD database. The results identified T. whipplei (sequence
number: 399; relative abundance: 0.08%) as the only pathogen
(Fig. 3). After treatment for 8
days, the patient's symptoms improved, with cough resolution and
normalization of body temperature. Following discharge, the patient
continued oral moxifloxacin at a daily dose of 0.4 g for 1 week and
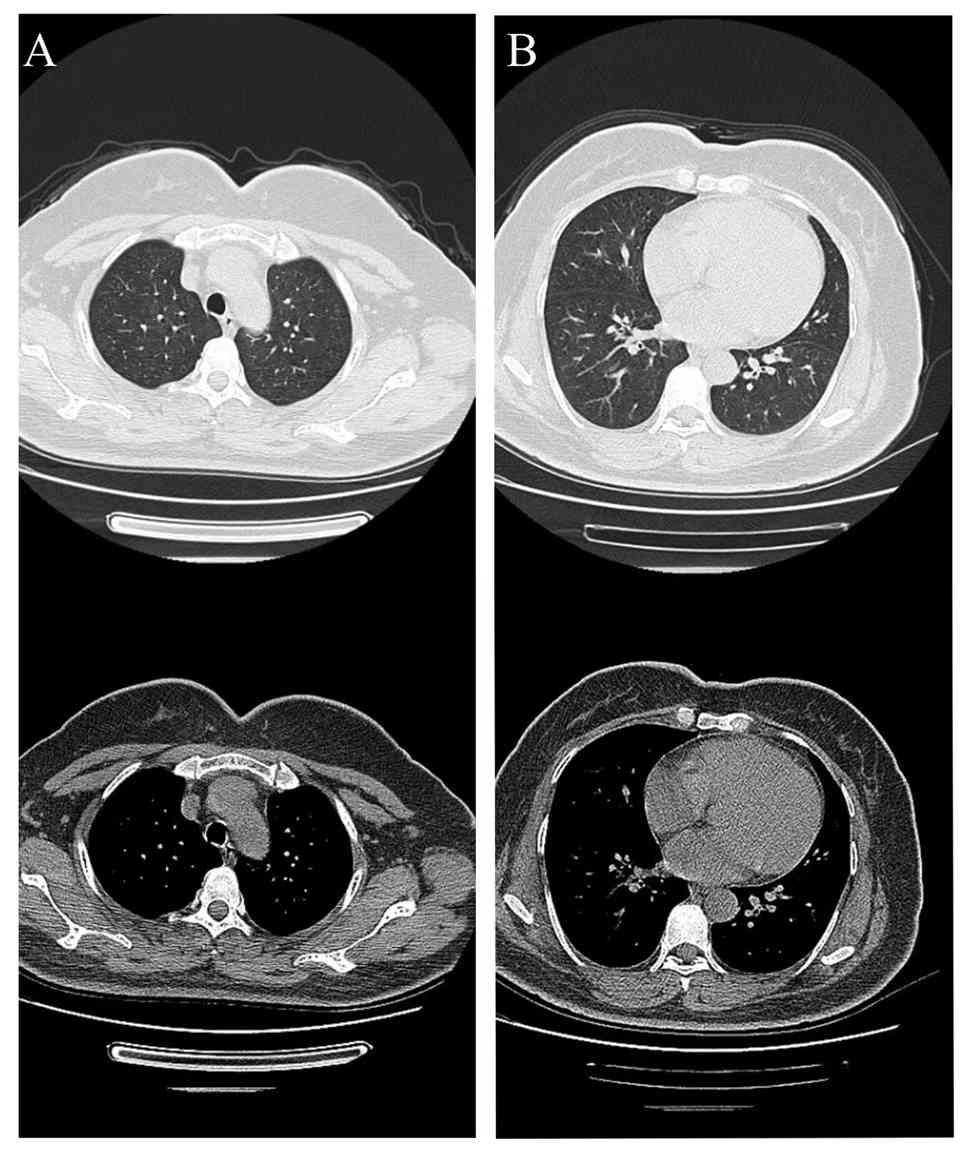
remained in good health. A follow-up chest CT scan in November 2023
(Fig. 4) indicated complete
resolution of the bilateral patchy/nodular opacities. A telephone
follow-up was conducted after discharge. The patient reported that
she was in good health and had not experienced any further
complications. The patient declined to visit the hospital for
follow-up examinations, including a chest CT scan.
 | Table IAdmission laboratory test results of
the patient. |
Table I
Admission laboratory test results of
the patient.
| A, Blood glucose
markers |
|---|
| Specific test
item | Result | Normal range |
|---|
| Random blood glucose,
mmol/l | 8.0-18.8 | 3.9-6.1 (fasting);
7.8 (2 h postprandial) |
| Fasting blood
glucose, mmol/l | 9.1 | 3.9-6.1 |
| Glycated hemoglobin,
% | 7.8 | 4.0-6.0 |
| B, Inflammatory
markers |
| Specific test
item | Result | Normal range |
| 1,3-β-D-glucan,
pg/ml | <30 | <60 |
| Erythrocyte
sedimentation rate, mm/h | 15 | 0-20 (females) |
| C-reactive protein,
mg/l | 8.2 | 0-10 |
| Endotoxin, EU/ml | <0.05 | <0.1 |
| Procalcitonin,
ng/ml | 0.12 | <0.5 |
| C, Routine blood
test |
| Specific test
item | Result | Normal range |
| White blood cell
count, x109/l | 6.8 | 4.0-10.0 |
| Neutrophil % | 62 | 50-70 |
| Lymphocyte % | 30 | 20-40 |
| Hemoglobin, g/l | 125 | 115-150
(females) |
| Platelet count,
x109/l | 148 | 100-300 |
| D, Biochemical
indicators |
| Specific test
item | Result | Normal range |
| Serum alanine
aminotransferase, U/l | 22 | 7-40 |
| Serum aspartate
aminotransferase, U/l | 23 | 13-35 |
| Serum creatinine,
µmol/l | 51 | 44-97 µmol/l |
| Blood urea nitrogen,
mmol/l | 5.17 | 2.9-8.2 |
| Serum sodium,
mmol/l | 138 | 137-147 |
| Serum potassium,
mmol/l | 4.1 | 3.5-5.3 |
| E,
Autoantibodies |
| Specific test
item | Result | Normal range |
| Antinuclear
antibody | Negative | Titer <1:40 |
| Anti-double-stranded
DNA antibody | Negative | Negative |
| Anti-neutrophil
cytoplasmic antibody | Negative | Negative |
| Anti-cyclic
citrullinated peptide antibody | Negative | Negative |
| Rheumatoid factor,
IU/ml | 10 | 0-20 |
Discussion
T. whipplei is a potentially harmful
commensal organism that is naturally found in 1.5-7% of the general
population, without exhibiting any symptoms (4). Some cases reported WD had
immunocompromised conditions or immunopathologic abnormalities,
suggesting impaired host immunity may be a predisposing factor
(5). These individuals typically
acquire the infection through exposure to contaminated water or
soil. Notably, it is the fecal-oral pathway that leads to the
spread of T. whipplei (1,6) and
pneumonia can result from the subject inhaling T. whipplei
orally (7). In addition, saliva
from asymptomatic patients contains T. whipplei (8). The digestive tract, nervous system,
skin and heart are the primary organs affected by this condition,
with the lungs being less commonly affected (9). To the best of our knowledge, only a
limited number of case reports have described respiratory
infections, despite the advanced detection and treatment of
Whipple's disease in the digestive tract (1,10).
The present case report indicated that, despite a history of raised
blood glucose levels, the patient did not undergo regular checkups
or adhere to prescribed medications, which may have contributed to
T. whipplei infection.
The established method for diagnosing T.
whipplei infection is histopathology combined with PCR
(8). However, for T.
whipplei-related pneumonia, lung biopsies are invasive and not
routinely performed. With this, the use of BALF exhibits lower
sensitivity due to the presence of a low bacterial load.
Conventional bacterial cultures are impractical due to the slow
proliferation of T. whipplei (9). In the present case report, mNGS
analysis of BALF identified T. whipplei as the only pathogen
present. mNGS, while not well established as a technique in this
context, detected the pathogen without prior suspicion and
exhibited results that aligned with the clinical response of the
patient to moxifloxacin. Other pathogens were excluded through
negative sputum culture and serological tests. Histopathology was
not performed due to the stable condition of the patient and the
invasiveness of conducting a lung biopsy. BALF PCR for T.
whipplei was not available at the time of diagnosis; therefore,
mNGS served as an alternative to detect pathogen nucleic acid,
which aligns with guidelines suggesting mNGS as a valuable tool for
rare or unculturable pathogens (3,9). In
conclusion, mNGS was shown to be a practical and effective method,
particularly for rare manifestations, such as pneumonia, where
standard tests are inaccessible.
Since the initial effective treatment of T.
whipplei infection in 1952, a number of antibiotic combinations
have been utilized (11). This
includes penicillin, tetracycline, streptomycin, meropenem and
doxycycline, trimoxazole, hydroxychloroquine and ceftriaxone.
Treatment procedures for typical Whipple's disease, including a
combination of oral doxycycline with trimethoprim-sulfamethoxazole
or hydroxychloroquine for 1 year, have been described in previous
case reports (12,13).
However, large-scale clinical trials have not been
conducted and previous studies have contained small sample sizes
(10,14). To the best of our knowledge,
currently there are no guidelines for the management of T.
whipplei infection (12). Zhou
et al reported (13)
optimal short-term clinical results using amoxicillin/clavulanic
acid for the management of T. whipplei-induced lung
abscesses. Furthermore, the efficacy of moxifloxacin monotherapy in
treating co-infection of Chlamydia psittaci and flagellates
has been reported (15). Although
these findings suggest that both amoxicillin/clavulanic acid and
moxifloxacin may be effective short-term treatment options for
T. whipplei infection, further research is needed to confirm
their efficacy.
In the present case report, insulin was administered
to maintain optimal blood glucose control. While awaiting
diagnostic results, empirical moxifloxacin therapy was initiated
for CAP. Once mNGS identified T. whipplei as the causative
pathogen, the patient indicated notable clinical improvement within
1 week and moxifloxacin was continued as a short-term treatment.
Following discharge, the patient completed a 1-week course of oral
moxifloxacin and remained stable. A follow-up chest CT scan 1 month
later indicated complete resolution of the bilateral opacities. The
patient's condition continued to improve and the patient declined
further chest CT scans. Although moxifloxacin is not a
guideline-recommended treatment for T. whipplei infection,
its short-term use in the present case was reasonable given the
acute presentation of the patient, the absence of other pathogens
and the requirement for prompt therapy. Despite the favorable
response, evidence supporting fluoroquinolone monotherapy for T.
whipplei infection remains limited (16,17).
Further studies are needed to evaluate the efficacy and durability
of such regimens and to establish optimal treatment strategies for
T. whipplei infections.
In conclusion, the present case report underscores
the utility of mNGS in diagnosing atypical infections and
highlights T. whipplei as a potential cause of CAP in
immunocompetent hosts. While moxifloxacin was used empirically and
indicated efficacy, additional research is required to clarify its
role in the management of T. whipplei infection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. mNGS data were deposited
in the National Center for Biotechnology Information under the
BioProject number PRJNA1390448 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1390448).
Authors' contributions
GX and JP conceived and designed the present study.
YL and HD analyzed and summarized the data and wrote the
manuscript. YL, HD and GX collected the laboratory examination data
and CT images of the case. GX critically revised the manuscript. YL
and HD confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical principles of the Declaration of Helsinki.
Patient consent for publication
The patient involved in the present study provided
written informed consent for the publication of medical data and
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boumaza A, Azzouz EB, Arrindell J, Lepidi
H, Mezouar S and Desnues B: Whipple's disease and Tropheryma
whipplei infections: From bench to bedside. Lancet Infect Dis.
22:e280–e291. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang WM and Xu L: Pulmonary parenchymal
involvement caused by Tropheryma whipplei. Open Med (Wars).
16:843–846. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zheng Y, Qiu X, Wang T and Zhang J: The
diagnostic value of metagenomic next-generation sequencing in lower
respiratory tract infection. Front Cell Infect Microbiol.
11(694756)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhu B, Tang J, Fang R, Fei X and Wang Q,
Wang W, Wu X, Liu C and Wang Q: Pulmonary coinfection of
mycobacterium tuberculosis and Tropheryma whipplei: A case report.
J Med Case Rep. 15(359)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng Y, Zhang H, Lu J, Zhou Z, Zhang T and
Cui X: Whipple's disease of the respiratory system: A case report.
Exp Ther Med. 27(133)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carvalho N, Miguelote S, Pimenta J,
Trindade I and Cotter J: Fading away: A case report on Whipple's
disease. Cureus. 17(e78423)2025.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shen Y, Cui SS, Teng XB and Han MF: Acute
pneumonia due to Tropheryma whipplei diagnosed by metagenomic
next-generation sequencing and pathology: A case report. Heliyon.
10(e26747)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rolain JM, Fenollar F and Raoult D: False
positive PCR detection of Tropheryma whipplei in the saliva of
healthy people. BMC Microbiol. 7(48)2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin M, Wang K, Qiu L, Liang Y, Tu C, Chen
M, Wang Z, Wu J, Huang Y, Tan C, et al: Tropheryma whipplei
detection by metagenomic next-generation sequencing in
bronchoalveolar lavage fluid: A cross-sectional study. Front Cell
Infect Microbiol. 12(961297)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi J, Liu R, Qiu J, Wei C, Pan D, Xiang T
and Cheng N: Pulmonary infection caused by Tropheryma whipplei: A
case report and review of the literature. J Med Case Rep.
18(613)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lu Z, Zhang A, Guo J and Ni H: An unusual
case of severe pneumonia caused by Tropheryma whipplei combined
with Legionella pneumophila. World J Emerg Med. 14:492–494.
2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dolmans RA, Boel CH, Lacle MM and Kusters
JG: Clinical manifestations, treatment, and diagnosis of tropheryma
whipplei infections. Clin Microbiol Rev. 30:529–555.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou H and Zhang J: Short-Term amoxicillin
clavulanate in the treatment of pulmonary abscess caused by
tropheryma whipplei infection diagnosed by targeted next-generation
sequencing: A case report and literature review. Infect Drug
Resist. 17:4607–4616. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen Q, Gao B, Guo W, Liu H and Guo J:
Clinical features of patients with tropheryma whipplei detected in
lower respiratory tract samples in China. Infect Drug Resist.
18:3439–3448. 2025.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Du ZM and Chen P: Co-infection of
Chlamydia psittaci and Tropheryma whipplei: A case report. World J
Clin Cases. 11:7144–7149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lv Z, Chen Y, Zhou H, Chen Z, Yao Q, Ren
J, Liu X, Liu S, Deng X, Pang Y, et al: Genomic characterization of
two metagenome-assembled genomes of Tropheryma whipplei from China.
Front Cell Infect Microbiol. 12(947486)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Boulos A, Rolain JM and Raoult D:
Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells.
Antimicrob Agents Chemother. 48:747–752. 2004.PubMed/NCBI View Article : Google Scholar
|