Introduction
Pulmonary sequestration (PS) is a rare pulmonary
dysplasia, accounting for 0.2-6.4% of congenital pulmonary
malformations (1,2). Radiologically, PS can manifest as a
solid mass, a cystic lesion, a cavity or a pneumonic consolidation,
with mass-like lesions being one of the most common presentations
(3). It often presents as
recurrent pulmonary infections; however, fungal infections are
rare. Pulmonary cryptococcosis (PC) is an invasive fungal infection
primarily caused by Cryptococcus neoformans or
Cryptococcus gattii (4),
which is widely distributed in nature and can be found in bird
droppings, soil and decaying wood (5). This infection invades the respiratory
system after inhalation of cryptococcal spores, followed by the
central nervous system (6).
Cryptococcal infections range from superficial, affecting the skin
and mucous membranes, to deep-seated or disseminated, involving
internal organs. PC falls into the latter category, representing an
invasive fungal disease. While pathogenic fungi including
Cryptococcus are ubiquitous in the environment, symptomatic
infection typically occurs in individuals harboring specific risk
factors. An immunocompromised state constitutes the primary risk
factor for developing invasive cryptococcosis. This includes
individuals living with HIV or acquired immunodeficiency syndrome,
solid organ transplant recipients, patients with hematologic
malignancies and those receiving immunosuppressive therapies such
as corticosteroids or biologic agents (4,7).
However, it is increasingly recognized that a marked proportion of
PC cases, <30-50% in certain studies, occur in immunocompetent
hosts who often exhibit a history of environmental exposure to
soil, decaying wood or bird droppings (8). In these immunocompetent individuals,
the clinical presentation is often more indolent and the disease is
frequently localized to the lungs. However, to the best of our
knowledge, the coexistence of PS and PC is exceedingly rare, with
only one prior case reported in the published English literature to
date (9). In this previously
reported case, the cryptococcal infection was located within the
sequestered lung segment. The concomitant occurrence of these two
entities, particularly when presenting in separate lobes, poses
unique diagnostic and therapeutic challenges. The non-specific
radiological features often lead to an initial misdiagnosis of lung
cancer, potentially resulting in inappropriate management (6,8).
Therefore, enhancing awareness and understanding of this rare
co-occurrence is of notable clinical importance. The present case
report outlines a unique case of intralobar PS in the right lower
lobe of the patient, coexisting with PC in the contralateral left
lower lobe, representing an alternative clinical scenario from the
previously reported, aforementioned case. Within the present case
report, the clinical presentation, imaging findings, diagnostic
workup and successful treatment strategy are described, followed by
a review of the relevant literature, with the aim to provide
insights that will aid clinicians in the timely diagnosis and
appropriate management of such complex cases.
Case report
In August 2021, a 52-year-old Han male patient was
admitted to Taihe Hospital (Shiyan, China), due to a cough,
expectoration and chest tightness for 2 weeks. The patient
complained of respiratory symptoms and denied any other discomfort.
However, the total leukocyte (white blood cell) count was elevated
at 11.2x109/l (normal range, 3.5-9.5x109/l).
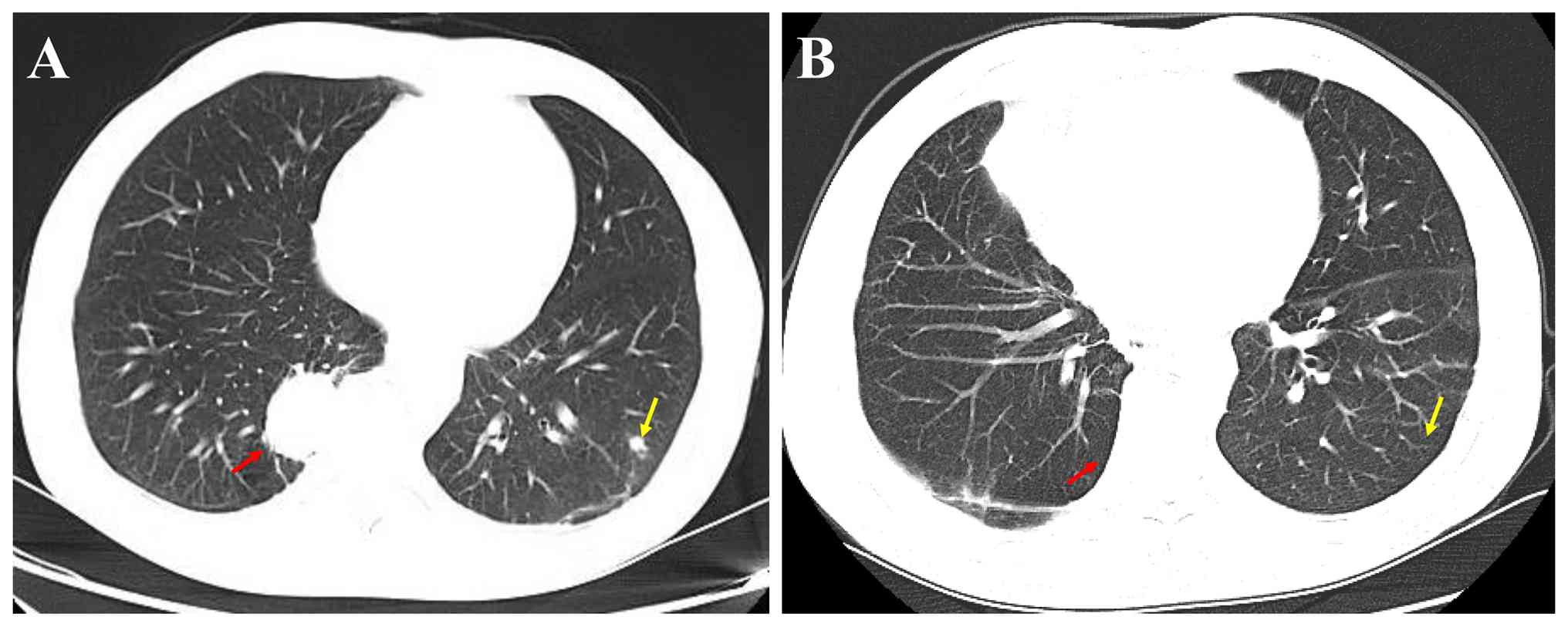
Contrast-enhanced chest CT scans showed an oval lesion measuring
3.5x2.7 cm in the right lower lobe of the lung (Fig. 1A). Notably, no definitive aberrant
systemic artery supplying the lesion was identified on the
contrast-enhanced CT scan due to institutional-specific practices.
Within the institution, three-dimensional CT angiography
reconstruction is performed only as part of a dedicated vascular CT
angiography protocol, which was not included in contrast-enhanced
CT. Furthermore, a 0.9x0.6 cm nodule in the lower lobe of the left
lung (Fig. 1A) was also observed,
which was initially suspected to be peripheral lung cancer. Given
the non-specific imaging features and the primary differential
diagnosis of lung cancer, a CT-guided percutaneous biopsy of the
right lower lobe lesion was conducted to obtain a definitive
diagnosis. The patient was immunocompetent, had no history of
recent travel, no history of exposure to pigeon manure or soil, no
history of smoking or drinking and no extensive use of hormones and
antibiotics before presenting to Taihe Hospital (Shiyan, China).
The medical history of the patient included chronic gastritis for
>2 years. Upon admission, the vital signs were within normal
limits: Heart rate was 77 bpm (normal, 60-100 bpm), blood pressure
was 112/73 mmHg (normal, <120/80 mmHg), respiratory rate was 20
bpm (normal, 12-20 bpm) and body temperature was 36.6˚C (normal,
36.1-37.2˚C). Physical examination showed normal breath sounds.
There were no skin lesions, lymph node enlargement or splenomegaly
present. Laboratory results showed that the whole blood leukocyte
count was 6.82x109/l [neutrophils, 63.6% (normal range,
50-70%); lymphocytes, 27.9% (normal range, 20-50%); monocytes, 6.2%
(normal range, 3-10%); eosinophils, 1.7% (normal range, 0.4-8%);
and basophils, 0.6% (normal range, 0-1%)], the red blood cell count
was 5.1x1012/l (normal range, 4.3 to 5.8
x1012/l), hemoglobin was 157 g/l (normal range, 130-175
g/l) and the platelet count was 231x109/l (normal range,
125-350x109/l). Blood glucose was measured as 4.65
mmol/l (normal range, 3.9-6.1 mmol/l), total bilirubin was 21.85
µmol/l (normal range, 3.42-20.5 µmol/l), aspartate aminotransferase
was 18 U/l (normal range, 0-40 U/l), alanine aminotransferase was
16 U/l (normal range, 0-50 U/l), lactate dehydrogenase was 99 IU/l
(normal range, 100-240 IU/l) and highly sensitive C-reactive
protein was 3.05 mg/l (normal range, 0-5 mg/l). Urinalysis and
microscopic examination results appeared normal. Tumor marker
analysis demonstrated that neuron-specific enolase [15.6 ng/ml
(normal range, 0-16.3 ng/ml)], carcinoembryonic antigen [1.25 µg
(normal range, 0-5 µg/l)] and ferritin [68.1 ng/ml, (normal range,
30-400 ng/ml)] exhibited normal levels. However, the cytokeratin 19
fragment exhibited a value of 5.8 µg/l (normal range, 0-3.3 µg/l),
which exceeded the normal upper limit of 2.5 µg/l. The patient had
normal T-lymphocyte subsets and was negative for HIV antibodies.
Sputum gram stain and bacterial culture showed no microorganisms
present. No acid-fast bacteria were found in acid-fast staining and
sputum culture. Fiberbronchoscopy was performed and revealed no
endobronchial lesions in either the right or left main bronchi.
Given the peripheral location of both the right lower lobe mass and
the left lower lobe nodule, a transbronchial biopsy was not
attempted. Bronchoalveolar lavage was performed in the right lower
lobe, targeting the region of the PS mass. The bacteriological,
cytological and pathological examinations of the bronchoalveolar
lavage fluid were negative. To obtain a definitive diagnosis, a
CT-guided percutaneous lung biopsy of the lesion in the lower lobe
of the right lung was performed, which was pathologically confirmed
to be PS. The histopathological examination of the biopsy specimens
revealed features highly suggestive of and consistent with PS,
including: i) Marked fibrotic thickening of the alveolar septa; ii)
chronic inflammation with lymphocyte infiltration; and iii)
dilated, cystically remodeled airspaces. These features, in
conjunction with the radiological presentation of a well-defined
mass in a typical location (posteromedial lower lobe), the absence
of an endobronchial lesion on bronchoscopy to suggest an
obstructive etiology, as well as the overall clinical context,
collectively supported a working diagnosis of PS. Malignancy was
ruled out by the absence of malignant cells. One week after
admission, a CT-guided percutaneous lung needle biopsy was
performed in the left lower lobe nodule, which was
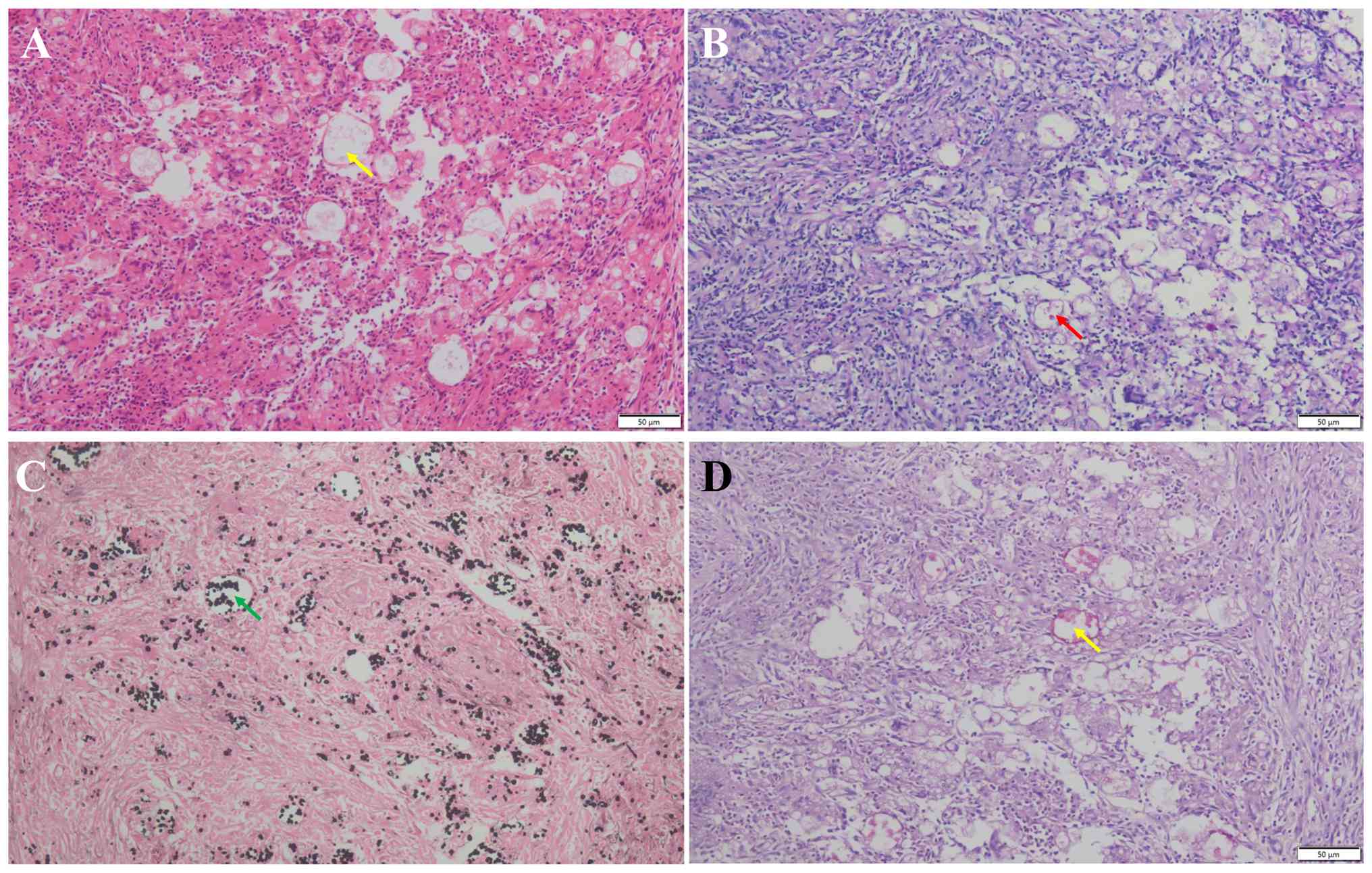
histopathologically confirmed to be a PC infection. Histologically,
hematoxylin and eosin (H&E) staining revealed granulomatous
inflammation with multinucleated giant cells containing a number of
round and oval yeast cells, which were identified as cryptococcus
through periodic acid-Schiff (PAS) stain, silver hexamethonium
(GMS) and mucocarsine staining (Fig.
2A-D). For histopathological examination, the percutaneous lung
biopsy specimens from the left lower lobe nodule were immediately
fixed in 10% neutral buffered formalin at room temperature for
24-48 h. After routine processing, tissues were embedded in
paraffin and serially sectioned at 4-µm thickness. Sections were
stained with H&E for initial assessment. For fungal
identification, special stains including PAS, GMS and mucicarmine
were performed according to the manufacturer's standard protocols
(all reagents from Baso Inc.). Staining incubation was performed at
room temperature with durations as follows: PAS (15 min), GMS (60
min) and mucicarmine (10 min). All stained slides were examined
under a light microscope (Olympus CX31; Olympus Corp.).
Photomicrographs were captured using an attached digital camera
(Mshot MS60; Micro-shot Technology, Ltd.). The scale bars (50 µm)
and magnifications (x200) for each image are provided in Fig. 2A-D.
At two weeks after admission, the patient underwent
a video-assisted thoracoscopic surgery resection of PS. Notably,
the decision to perform a lobectomy, rather than a more limited
resection, was based on intraoperative findings and surgical
principles for PS, namely the sequestered lung tissue was diffusely
abnormal and involved multiple segments of the lower lobe.
Furthermore, the risk of injury to the often large, friable and
anomalous systemic vessels was deemed higher with a sublobar
resection, making lobectomy the safer and more definitive
procedure. Intraoperatively, careful dissection along the inferior
pulmonary ligament and the posteromedial aspect of the lesion
revealed a single aberrant systemic feeding artery. This vessel, ~6
mm in diameter, originated directly from the descending thoracic
aorta. It was meticulously isolated, doubly ligated with
non-absorbable suture and then divided prior to parenchymal
dissection. Securing this anomalous vessel was a step prioritized
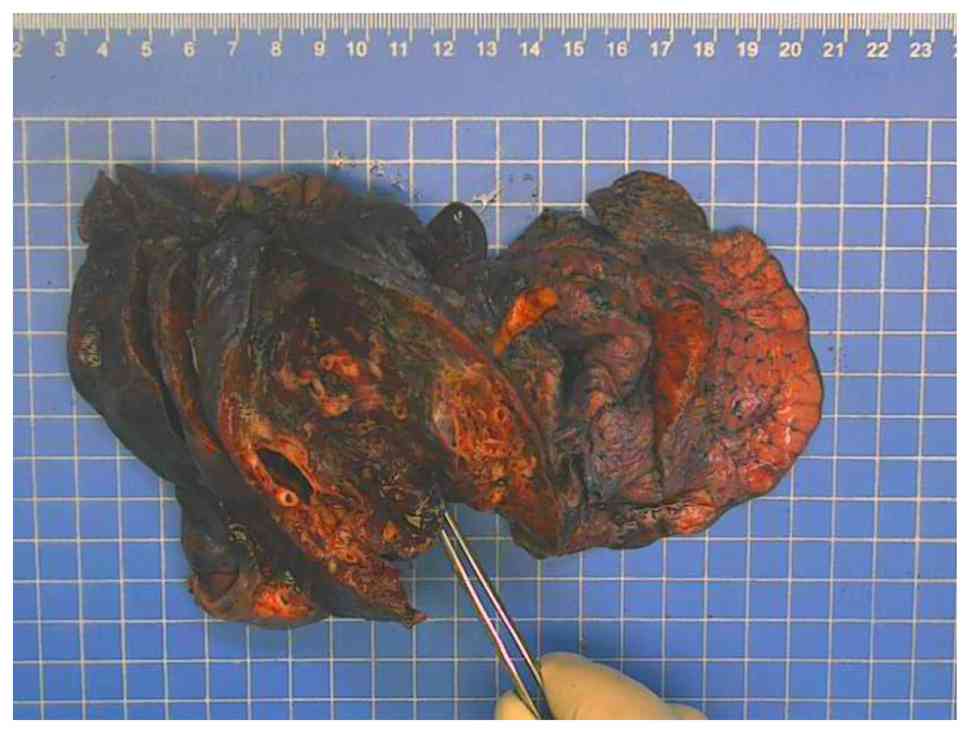
to prevent catastrophic hemorrhage. Macroscopically, the resected
lower lobe of the right lung was 17x11x3 cm and a capsule with a
size of 3.5x3x1.5 cm was observed in the section 3.5 cm from the
broken end of the bronchus (the contents had been lost; Fig. 3). The thickness of the capsule wall
was 0.1-0.2 cm.
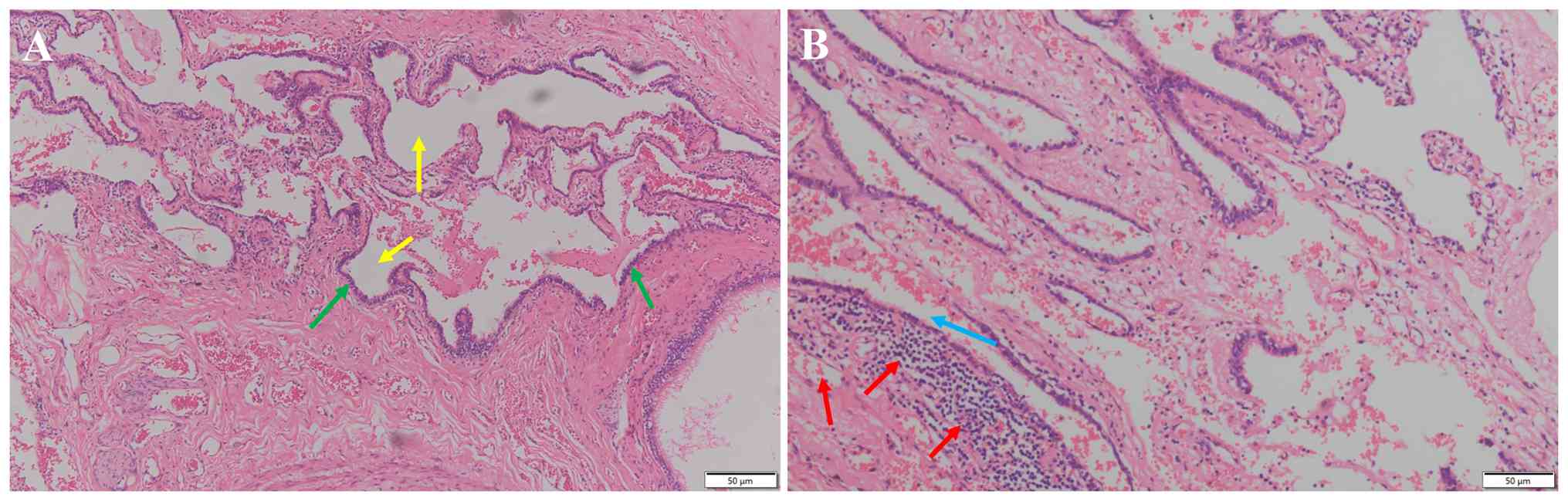
Histologically, compared to the normal lung
architecture, the alveolar septa were widened, interstitial fibrous
tissue was proliferated and there was increased lymphocyte
infiltration, along with hemorrhage, cystic change and histiocyte
aggregation-findings consistent with pulmonary sequestration
(Fig. 4). Notably, a thorough
histopathological examination of the entire resected PS specimen,
including specific staining (PAS and GMS) for fungi, revealed no
evidence of cryptococcal yeast forms or any other fungal elements
within the sequestered lung tissue. Finally, PS in the right lower
lobe and cryptococcal infection in the left lower lobe were
confirmed. Fluconazole (200 mg/day) was administered after surgery
for 6 months. Furthermore, for a total of 3 years after surgery,
the patient was regularly followed up in the Department of
Pulmonary and Critical Care Medicine (Taihe Hospital; Shiyan,
China) and shown to be in good condition. In July 2024,
contrast-enhanced CT showed that there was no recurrence of
cryptococcal infection in the left lower lung and that the right
lower lung appeared stable after surgery (Fig. 1B).
Literature review and discussion
PC is an invasive lung fungal disease caused by
Cryptococcus neoformans or Cryptococcus gattii
(4), which typically presents in
immunosuppressed patients (10,11),
often as a solitary nodule mimicking malignancy (10). PS, on the other hand, is a
congenital malformation prone to recurrent infections (12). The concomitant occurrence of PS and
PC is exceedingly rare. The established standard for the diagnosis
of PS has previously been the demonstration of an aberrant systemic
artery on angiography. While contrast-enhanced CT with
three-dimensional reconstruction is highly sensitive and often
diagnostic by visualizing this vascular anomaly (13,14),
it is important to recognize that a subset of cases may present
without clear identification of the feeding vessel on standard
imaging (14-16).
The present case exemplifies this challenging scenario. The absence
of a definitively visualized aberrant artery on contrast-enhanced
CT, compounded by the patient's decision to forgo three-dimensional
reconstruction, shifted the diagnostic pathway towards tissue
confirmation. This underscores a key clinical point, that in the
presence of a radiologically ambiguous mass, particularly when
malignancy is a concern, percutaneous biopsy remains an
indispensable tool. The diagnosis of PS on biopsy is established by
identifying characteristic histopathological features of dysplastic
lung tissue, which in the present case included interstitial
fibrosis, chronic inflammation and cystic changes, findings that
are well-documented in the pathology literature on PS (17). The histopathological findings of
characteristic dysplastic lung tissue with cystic changes, fibrosis
and chronic inflammation can establish the diagnosis of PS even in
the absence of the classic angiographic finding of an aberrant
systemic feeding artery. Therefore, while non-invasive imaging is
still a first-line investigation tool, biopsy remains a key method
within ambiguous cases, in order to rule out malignancy and obtain
tissue supporting the clinical and radiological suspicion of PS.
Histopathological findings can support the diagnosis of PS when
interpreted in conjunction with characteristic imaging findings and
after excluding other causes of localized chronic lung pathology,
such as obstruction (1,12,17).
The present report acknowledges that biopsy findings
themselves are not specific. The definitive diagnosis of PS in the
present case was therefore not based on pathology alone, but on the
integration of the following: i) Typical radiological location and
appearance; ii) histopathology ruling out malignancy and showing
changes compatible with chronic sequestration/recurrent infection;
iii) bronchoscopic exclusion of an endobronchial obstructing lesion
(which would favor a diagnosis of obstructive pneumonia); and iv)
the subsequent surgical resection and pathological examination of
the entire specimen, which demonstrated the absence of a bronchial
communication, a key feature of PS.
The majority of PSs often present as solid masses,
with or without cavity lesions. Wei and Li (3) retrospectively analyzed the chest CT
characteristics of 1,106 PS cases and found that there were four
main types of PS, namely mass lesions (49.01%), cystic lesions
(28.57%), cavitary lesions (11.57%) and pneumonic lesions (7.96%).
Yue et al (13)
demonstrated that the accuracy (>95%) and sensitivity (>95%)
of CT angiography in diagnosing PS were relatively high, which was
considered to be comparable to the established standard, digital
subtraction angiography. In this case, PS exhibited a solid mass
shadow upon chest CT scans. PC is primarily observed in patients
who are immunosuppressed or those with a history of exposure to
soil, dust or pigeon droppings (4,8,18).
However, no history of immunosuppression or exposure was found in
the present case.
Currently, to the best of our knowledge, there is no
consensus on whether there is an association between PS and PC.
Only one previous case by Guan et al (9) has reported this coexistence, whereby
the cryptococcal infection was located within the sequestered lung
segment. Despite aggressive antifungal therapy, the pulmonary
infection relapsed, necessitating a lobectomy to eradicate the
source of persistent infection within the PS. By contrast, the
present case presents a novel pattern of ‘separated coexistence’,
whereby the PS was situated in the right lower lobe and the
cryptococcal nodule was identified as a distinct entity in the
contralateral left lower lobe; therefore, a ‘split-and-conquer’
strategy was employed, involving surgical resection of the PS
combined with targeted antifungal therapy for the PC. The surgery
was primarily aimed at addressing the PS itself, rather than
controlling a drug-refractory infection. The coexistence of PS and
PC in the present case, albeit in different lobes, raises the
question of a potential pathophysiological association beyond just
a coincidence. While the patient in the present case was
immunocompetent systemically, the local immune environment within a
PS may be altered, predisposing it to infections. A PS is
characterized by impaired bronchial communication and defective
drainage, leading to the stagnation of secretions (19,20).
This stagnant environment can compromise local mucociliary
clearance and create a niche susceptible to colonization by a
number of pathogens, including fungi. Furthermore, the chronic
inflammation and structural damage within the sequestered lung
tissue, as seen in the present case with lymphocyte infiltration
and fibrosis, could disrupt the normal local immune surveillance.
This concept of ‘local immunosuppression’ is supported by the
well-documented higher incidence of aspergillosis in patients with
PS compared with the general population (19). Although the cryptococcal infection
in the present case was in a separate lobe, it is plausible that
the patient had a generalized, subclinical susceptibility to
respiratory infections, with the PS and the left lower lobe nodule
representing two different manifestations of this susceptibility.
The route of cryptococcal spread to the sequestration, as suggested
in the literature, could be hematogenous, lymphatic or through the
pores of Kohn (9). Therefore,
while a causal relationship cannot be established from the present
case, the presence of PS may indicate a regional lung environment
that is more permissive to fungal establishment and proliferation.
The management of coexisting PS and PC should be tailored based on
the anatomical relationship between the two conditions (9,19).
The present case underscores the importance of a nuanced treatment
strategy. When PC is located within the sequestered lung segment,
surgical resection of the PS serves a dual purpose. It is both an
established treatment for the congenital malformation and an
effective means of eradicating the entrenched fungal infection. By
contrast, when PC presents as a separate lesion in a different
lobe, as seen in the present patient, a staged approach is
warranted. This involves surgical resection of the PS to address
the source of recurrent infection and potential complications,
combined with preoperative or postoperative antifungal therapy
targeted at the distinct PC nodule. This bifurcated strategy
optimizes outcomes by matching the intervention to the specific
pathological context.
Surgical resection is the optimal choice in the
treatment of PS. At present, PS accounts for 0.15-6.45% of
congenital lung malformations, 1.1-1.8% of lung resections and the
surgical detection rate is ~1.5% (21). A key procedure during this is
complete resection of the diseased lung tissue and ligation of the
blood supply vessels to prevent severe bleeding. The conventional
treatment consists of surgical resection of the PS, but in previous
years, endovascular embolization has been proposed as a valid
therapeutic alternative (22-27).
Deng et al (28) reported a
PS case presented with haemoptysis, coil embolization was performed
instead of surgery and no complications were found after the
operation. In addition, Bi et al (23) conducted a single-center,
retrospective study and assessed the safety and efficacy of
transarterial embolization for PS, showing a clinical success rate
of 90.9%. Borzelli et al (24) demonstrated that arterial
embolization was a valid and effective therapeutic alternative to
surgical resection in the treatment of PS. Based on previous
literature, endovascular embolization concurs the advantages of
less trauma, lower risk and fewer complications and may be the best
alternative to surgery for PS treatment.
According to 2010 updated clinical practice
guidelines from the Infectious Diseases Society of America
(4), amphotericin B combined with
fluconazole is recommended as a primary therapy regimen, followed
by fluconazole as consolidation therapy (4). To avoid the occurrence of reinfection
that destroys the adjacent lung parenchyma, resection of the
malformation is recommended in the presence of complications such
as persistent or recurrent infections, hemoptysis as well as in the
absence of antifungal agents. In the previously reported case
(9), the patient underwent right
lower lobectomy and received liposomal amphotericin B for 3 months,
after which the patient was stable. In the present case, PS was
removed and cryptococcal lesions were treated with anti-infectives
and 200 mg/day fluconazole was administered for 6 months.
Therefore, the accurate differentiation between PS, PC and lung
malignancy is of notable clinical importance, as it directly
determines the treatment strategy and spares patients from
potentially harmful and unnecessary therapies. In the present case,
the initial suspicion of peripheral lung cancer could have led to
more aggressive surgical intervention or even chemotherapy being
performed. Notably, the definitive diagnosis of PC guided targeted
antifungal therapy. The first-line treatment for cryptococcosis,
fluconazole, while generally well-tolerated, is not without risks.
Common side effects include gastrointestinal disturbances (nausea,
vomiting and abdominal pain), a skin rash and headache. More marked
adverse effects involve hepatotoxicity, evidenced by elevated liver
enzymes and rare but serious conditions such as QT interval
prolongation and alopecia (29,30).
Long-term administration, as required for consolidation therapy
(for example, 6-12 months), necessitates regular monitoring of
liver function. Had the pulmonary nodule been misdiagnosed as a
common bacterial infection, prolonged courses of broad-spectrum
antibiotics could have been prescribed, increasing the risk of
Clostridium difficile infection, fostering antimicrobial
resistance and causing dysbiosis without any clinical benefit
(30). Therefore, the combination
of contrast-enhanced CT and histopathological examination was key
in the present case, not only securing the correct diagnoses but
also ensuring that the patient received the most appropriate and
least harmful treatment. This manifested as surgical resection for
the PS and targeted, time-limited antifungal therapy for the PC,
thereby avoiding the consequences of misdirected and potentially
toxic medical or surgical interventions.
This present study exhibits a number of limitations.
Firstly, the spatial separation of the PS and PC lesions makes it
challenging to definitively establish a direct pathophysiological
association between the two conditions. A case with cryptococcal
infection within the sequestered segment itself would provide a
stronger model for investigating such a relationship. Second,
advanced immunological profiling could not be performed on the
resected lung tissue to characterize the local immune cell
populations and cytokine environment, which could have provided
mechanistic insights. Future research involving such cases should
aim to incorporate detailed microbiological and immunological
analyses of the sequestered tissue to improve the understanding of
the local factors that might predispose to specific infections such
as cryptococcosis.
The diagnostic challenge in the present case was
two-fold. First, the solid mass appearance of the PS in the right
lower lobe and the nodular presentation of PC in the left lower
lobe are both radiological mimics of primary lung malignancy, as
was initially suspected in the patient. This often leads to
unnecessary anxiety and could prompt more invasive procedures if
not correctly characterized. Second, the coexistence of two
different pathologies in one immunocompetent patient is uncommon.
There is a cognitive bias in clinical practice to seek a unifying
diagnosis. Upon identifying one pathology (PS), there is a risk of
attributing all abnormalities to this risk and overlooking a
second, independent entity (PC). Therefore, meticulous evaluation
of each lesion with appropriate diagnostic tools, such as
image-guided biopsy for histopathological and microbiological
confirmation, is key in such complex presentations to avoid missed
diagnosis and ensure both conditions are adequately addressed.
Overall, the unique present case of intralobar
pulmonary sequestration with a contralateral pulmonary
cryptococcosis nodule underscores that in the absence of a classic
systemic feeding artery on imaging, percutaneous biopsy is a key
step towards diagnosing PS and excluding malignancy. It
demonstrates the necessity of an anatomy-driven management
strategy, whereby spatially separated pathologies warrant a
combined approach of surgical resection for the PS and targeted
antifungal therapy for the PC. The definitive exclusion of
cryptococcal infection within the resected PS tissue argues for a
coincidental coexistence in this immunocompetent host, highlighting
the importance of evaluating each lesion independently rather than
seeking a unifying diagnosis in complex pulmonary
presentations.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LH and JW conceived and designed the clinical study.
LH, DC, YueW, and DY were responsible for the acquisition,
analysis, and interpretation of patient data. YueW, DY and YunW
performed the histopathological and surgical data collection and
interpretation. JL and JF contributed to the radiological and
microbiological data interpretation. All authors participated in
drafting the manuscript and critically revising it for important
intellectual content. LH and JW supervised the study and
coordinated the follow-up. JW and DC confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present report was approved by the ethics
committee of Taihe Hospital (approval no. 2024KS02) and performed
in accordance with the principles of Good Clinical Practice
following the Tri-Council guidelines.
Patient consent for publication
Written informed consent was obtained from the
patient for anonymized information (including medical records, case
information and images) to be published in the present report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savic B, Birtel FJ, Tholen W, Funke HD and
Knoche R: Lung sequestration: Report of seven cases and review of
540 published cases. Thorax. 34:96–101. 1979.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Montjoy C, Hadique S, Graeber G and
Ghamande S: Intralobar bronchopulmonary sequestra in adults over
age 50: Case series and review. W V Med J. 108:8–13.
2012.PubMed/NCBI
|
|
3
|
Wei Y and Li F: Pulmonary sequestration: A
retrospective analysis of 2625 cases in China. Eur J Cardiothorac
Surg. 40:e39–e42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Perfect JR, Dismukes WE, Dromer F, Goldman
DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O,
Nguyen MH, et al: Clinical practice guidelines for the management
of cryptococcal disease: 2010 Update by the infectious diseases
society of america. Clin Infect Dis. 50:291–322. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Swinne D, Deppner M, Laroche R, Floch JJ
and Kadende P: Isolation of Cryptococcus neoformans from
houses of AIDS-associated cryptococcosis patients in Bujumbura
(Burundi). AIDS. 3:389–390. 1989.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang R, Yan Y, Wang Y, Liu X and Su X:
Plain and contrast-enhanced chest computed tomography scan findings
of pulmonary cryptococcosis in immunocompetent patients. Exp Ther
Med. 14:4417–4424. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhou Y, Lin PC, Ye JR, Su SS, Dong L, Wu
Q, Xu HY, Xie YP and Li YP: The performance of serum cryptococcal
capsular polysaccharide antigen test, histopathology and culture of
the lung tissue for diagnosis of pulmonary cryptococcosis in
patients without HIV infection. Infect Drug Resist. 11:2483–2490.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Y, Li N, Zhang Y, Li H, Chen X, Wang
S, Zhang X, Zhang R, Xu J, Shi J and Yung RC: Clinical analysis of
76 patients pathologically diagnosed with pulmonary cryptococcosis.
Eur Respir J. 40:1191–1200. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guan C, Chen H, Shao C, He L and Song Y:
Intralobar pulmonary sequestration complicating with cryptococcal
infection. Clin Respir J. 9:22–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Setianingrum F, Rautemaa-Richardson R and
Denning DW: Pulmonary cryptococcosis: A review of pathobiology and
clinical aspects. Med Mycol. 57:133–150. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Smith JA and Kauffman CA: Pulmonary fungal
infections. Respirology. 17:913–926. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qian X, Sun Y, Liu D, Wu X, Wang Z and
Tang Y: Pulmonary sequestration: A case report and literature
review. Int J Clin Exp Med. 8:21822–21825. 2015.PubMed/NCBI
|
|
13
|
Yue SW, Guo H, Zhang YG, Gao JB, Ma XX and
Ding PX: The clinical value of computer tomographic angiography for
the diagnosis and therapeutic planning of patients with pulmonary
sequestration. Eur J Cardiothorac Surg. 43:946–951. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hertzenberg C, Daon E and Kramer J:
Intralobar pulmonary sequestration in adults: Three case reports. J
Thorac Dis. 4:516–519. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Corbett HJ and Humphrey GME: Pulmonary
sequestration. Paediatr Respir Rev. 5:59–68. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Büyükoğlan H, Mavili E, Tutar N, Kanbay A,
Bilgin M, Oymak FS, Gülmez I and Demir R: Evaluation of diagnostic
accuracy of computed tomography to assess the angioarchitecture of
pulmonary sequestration. Tuberk Toraks. 59:242–247. 2011.PubMed/NCBI
|
|
17
|
Gezer S, Taştepe I, Sirmali M, Findik G,
Türüt H, Kaya S, Karaoğlanoğlu N and Cetin G: Pulmonary
sequestration: A single-institutional series composed of 27 cases.
J Thorac Cardiovasc Surg. 133:955–959. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu XP, Wang RY, Wang X, Cao YH, Chen YQ,
Zhao HZ, Wu JQ, Weng XH, Gao XH, Sun RH and Zhu LP: Dectin-2
polymorphism associated with pulmonary cryptococcosis in
HIV-uninfected Chinese patients. Med Mycol. 53:810–816.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Morikawa H, Tanaka T, Hamaji M and Ueno Y:
A case of aspergillosis associated with intralobar pulmonary
sequestration. Asian Cardiovasc Thorac Ann. 19:66–68.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun X and Xiao Y: Pulmonary sequestration
in adult patients: A retrospective study. Eur J Cardiothorac Surg.
48:279–282. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kestenholz PB, Schneiter D, Hillinger S,
Lardinois D and Weder W: Thoracoscopic treatment of pulmonary
sequestration. Eur J Cardiothorac Surg. 29:815–818. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Belczak SQ, da Silva IT, Bernardes JC, de
Macedo FB, Lucato LL, Rodrigues B and Zeque BS: Pulmonary
sequestration and endovascular treatment: A case report. J Vasc
Bras. 18(e20180110)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bi Y, Li J, Yi M, Ren J and Han X:
Clinical outcomes of transarterial embolization in the treatment of
pulmonary sequestration. Cardiovasc Intervent Radiol. 44:1491–1496.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Borzelli A, Paladini A, Giurazza F, Tecame
S, Giordano F, Cavaglià E, Amodio F, Corvino F, Beomonte Zobel D,
Frauenfelder G, et al: Successful endovascular embolization of an
intralobar pulmonary sequestration. Radiol Case Rep. 13:125–129.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Szmygin M, Pyra K, Sojka M and Jargiełło
T: Successful endovascular treatment of intralobar pulmonary
sequestration-an effective alternative to surgery. Pol J Radiol.
86:e112–e114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ellis J, Brahmbhatt S, Desmond D, Ching B
and Hostler J: Coil embolization of intralobar pulmonary
sequestration-an alternative to surgery: A case report. J Med Case
Rep. 12(375)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roman S, Millet C, Mekheal N, Mekheal E
and Manickam R: Endovascular embolization of pulmonary
sequestration presenting with hemoptysis: A promising alternative
to surgery. Cureus. 13(e17399)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Deng Y, Fang X and Wu B: Coil embolization
to treat pulmonary sequestration in the right upper lobe. Interact
Cardiovasc Thorac Surg. 35(ivac178)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Egunsola O, Adefurin A, Fakis A,
Jacqz-Aigrain E, Choonara I and Sammons H: Safety of fluconazole in
paediatrics: A systematic review. Eur J Clin Pharmacol.
69:1211–1221. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Patangia DV, Anthony Ryan C, Dempsey E,
Paul Ross R and Stanton C: Impact of antibiotics on the human
microbiome and consequences for host health. Microbiologyopen.
11(e1260)2022.PubMed/NCBI View Article : Google Scholar
|