Introduction
Over the last decade, leucovorin (FOL) and
5-fluorouracil (5-FU) plus oxaliplatin (l-OHP) (FOLFOX) or
leucovorin and 5-FU plus irinotecan (SN-38) (FOLFIRI) have been
widely used as first-line chemotherapy in the treatment of advanced
colorectal cancer (CRC). Moreover, molecularly targeted drugs such
as bevacizumab and cetuximab have improved overall and
progression-free patient survival. In general, it is not important
whether FOLFOX or FOLFIRI is administered first. However, in order
to achieve improvements in terms of survival, it is crucial to
achieve full administration of the targeted dosages of all 3 drugs;
leucovorin and 5-FU plus l-OHP or SN-38.
Where possible, the most effective regimen should be
chosen as the first line of treatment. In an earlier study, we
reported that the area under the concentration curve (AUC) and
growth inhibition rate (IR) were combined to give the AUC-IR curve,
which was approximated to the logarithmic curve for 5-FU. Moreover,
our results from the collagen gel droplet embedded culture-drug
sensitivity test (CD-DST) indicated that the anti-tumor effect of
5-FU depended on the AUC in CRC (1). In another study, we reported that
individual 50% inhibitory AUCs could be calculated from individual
AUC-IR regression curves (2).
The aim of this study was to evaluate the effect of
the addition of l-OHP or SN-38 to 5-FU using the CD-DST to
establish whether FOLFOX or FOLFIRI should be chosen in
individualized chemotherapy for the first-line treatment of
advanced CRC.
Materials and methods
Patients
Specimens of primary tumors were obtained from 24
CRC patients who had received no preoperative chemotherapy between
March 2008 and April 2009. Informed consent for measuring drug
sensitivity was obtained from all patients.
Methods
Tumor tissue was excised from primary surgical
specimens and subjected to the CD-DST. The CD-DST was used to
evaluate the sensitivity of tumors to 5-FU and was performed as
described by Kobayashi et al (3,4).
Each specimen was washed 5 times with 50 ml saline, followed by
further washing 5 times with 50 ml antibiotic fluid containing 1.0
mg/ml piperacillin and 0.5 mg/ml kanamycin. The transport bottle
contained 1.0 mg/ml piperacillin, 0.5 mg/ml kanamycin and 2.5 μg/ml
amphotericin B.
Tissue (1 g) was treated with a dispersion enzyme
cocktail containing 1.0% collagenase for 2 h. Dispersed cell
suspensions were inoculated into pre-culture media on
collagen-coated flasks overnight, after which viable tumor cells
were recovered by 0.05% collagenase treatment. Recovered cells were
embedded in 30-μl collagen gel droplets. Embedded cells were
cultivated in culture media containing 5-FU at 10.0 μg for 24 h,
3.0 μg for 24 h, 1.0 μg for 120 h and 0.2 μg for 24 h; 5-FU +
l-OHP at 3.0 + 1.5 μg and 6.0 + 3.0 μg, respectively, for 24
h; and 5-FU + SN-38 at 3.0 + 0.1 μg and 6.0 + 0.2 μg, respectively,
for 24 h.
After removal of 5-FU-containing media, cells were
further cultured for 7 days in serum-free culture media to prevent
growth of fibroblasts. Viable cells were stained with neutral red
solution and counted by the imaging colorimetric quantification
method. Surviving cell number ratio between the drug-treated group
and the control group which received no drug treatment was
calculated. A growth rate in excess of 0.8 was considered
indicative of successful culture.
After converting drug concentration and contact time
to an AUC, an AUC-IR curve was plotted against the growth IR. The
effect of the individual growth IR on the AUC (144 μg*h/ml) of 5-FU
was calculated from the AUC-IR regression curve. The effect of the
addition of l-OHP or SN-38 on the same AUC was
evaluated.
Statistical analysis
Comparisons of the growth IR value between 5-FU and
5-FU + l-OHP or SN-38 were compiled using the paired t-test.
Correlations between the growth IRs of 5-FU and 5-FU + l-OHP
or SN-38 on the same AUC of 5-FU were analyzed by linear regression
analysis. Statistical tests were carried out using the SPSS package
(version II for Windows). P-values <0.05 were regarded as
statistically significant.
Results
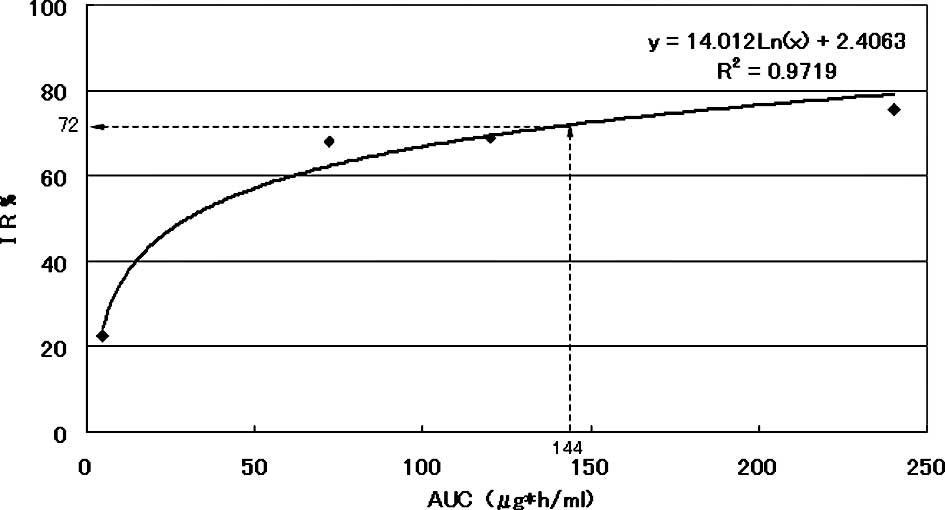
The AUC-IR curve of a representative patient is
shown in Fig. 1. Approximate
expression and correlation coefficients were y=14.012Ln(x)+2.4063
(R2=0.9719) for AUC and 72.0% for the individual growth
IR value on the AUC (144 μg*h/ml) of 5-FU calculated from the
regression curve of this patient.
The growth IR value on the AUC (144 μg*h/ml) of 5-FU
was calculated to give the AUC-IR curve for each patient (Table I).
 | Table I.Growth inhibition rates measured and
calculated for all of the studied patients. |
Table I.
Growth inhibition rates measured and
calculated for all of the studied patients.
| 5-FU concentration
(μg/ml)/contact times (h)
| Correlation
coefficient
| Calculated value
| Concentration
(5-FU/l-OHP μg/ml)/24 h
| Concentration
(5-FU/SN-38 μg/ml)/24 h
|
|---|
| Patient no. | 0.2/24 AUC 4.8 | 3/24 AUC
72a | 1/120 AUC 120 | 10/24 AUC 240 | y=aLn(x)+b | R2 | AUC
144b | 3.0/1.5 5-FU AUC
72c | 6.0/3.0 5-FU AUC
144d | 3.0/0.1 5-FU AUC
72e | 6.0/0.2 5-FU AUC
144f |
|---|
| 1 | 13.0 | 64.1 | 72.0 | 78.1 |
y=17.28Ln(x)-12.811 | 0.9889 | 73.1 | 67.9 | 80.1 | 71.9 | 82.9 |
| 2 | 14.7 | 59.6 | 61.4 | 66.0 |
y=13.766Ln(x)-5.0286 | 0.9673 | 63.4 | 63.8 | 71.3 | 69.5 | 79.2 |
| 3 | 15.0 | 44.4 | 48.2 | 60.9 |
y=11.272Ln(x)-3.2824 | 0.9889 | 52.7 | 47.6 | 60.0 | 53.9 | 68.7 |
| 4 | 4.9 | 19.0 | 21.1 | 31.1 |
y=6.121Ln(x)-5.6327 | 0.9428 | 24.8 | 28.4 | 29.9 | 44.8 | 66.5 |
| 5 | 11.4 | 61.1 | 61.8 | 71.7 |
y=15.706Ln(x)-11.771 | 0.9801 | 66.3 | 64.0 | 69.7 | 82.9 | 89.6 |
| 6 | 10.5 | 42.2 | 46.5 | 58.8 |
y=11.975Ln(x)-8.7382 | 0.9935 | 50.8 | 43.2 | 58.7 | 51.4 | 63.2 |
| 7 | 11.3 | 60.3 | 56.3 | 77.0 |
y=16.112Ln(x)-13.680 | 0.9651 | 66.4 | 68.1 | 73.0 | 76.1 | 85.2 |
| 8 | 10.1 | 47.9 | 51.9 | 62.0 |
y=13.242Ln(x)-10.367 | 0.9974 | 55.4 | 57.2 | 63.2 | 70.4 | 75.9 |
| 9 | 5.5 | 60.5 | 81.1 | 81.6 |
y=20.639Ln(x)-25.965 | 0.9732 | 76.6 | 70.6 | 77.9 | 76.6 | 85.5 |
| 10 | 19.0 | 71.0 | 67.2 | 82.6 |
y=16.126Ln(x)-5.0119 | 0.9675 | 75.1 | 75.1 | 76.3 | 78.1 | 85.6 |
| 11 | 5.1 | 45.8 | 53.8 | 56.7 |
y=13.869Ln(x)-15.521 | 0.9836 | 53.4 | 50.8 | 53.6 | 53.6 | 62.6 |
| 12 | 6.4 | 30.2 | 28.5 | 38.4 |
y=7.8614Ln(x)-5.7936 | 0.9679 | 33.3 | 35.4 | 41.9 | 50.6 | 60.7 |
| 13 | 6.3 | 66.8 | 71.8 | 80.2 |
y=19.559Ln(x)-22.517 | 0.9836 | 74.7 | 70.4 | 81.3 | 74.5 | 80.9 |
| 14 | 7.1 | 30.6 | 37.5 | 40.0 |
y=8.7177Ln(x)-6.3181 | 0.9901 | 37.0 | 36.8 | 42.3 | 50.5 | 70.2 |
| 15 | −1.5 | 68.3 | 79.2 | 84.6 |
y=23.147Ln(x)-35.596 | 0.9814 | 79.4 | 72.5 | 84.8 | 78.4 | 86.8 |
| 16 | 22.7 | 68.2 | 68.8 | 75.7 |
y=14.012Ln(x)+2.4063 | 0.9719 | 72.0 | 76.0 | 75.9 | 76.7 | 83.9 |
| 17 | 5.6 | 44.5 | 54.5 | 56.5 |
y=13.720Ln(x)-14.995 | 0.9824 | 53.2 | 53.8 | 59.2 | 64.3 | 76.4 |
| 18 | 4.2 | 49.8 | 47.6 | 63.4 |
y=14.805Ln(x)-18.390 | 0.9755 | 55.2 | 61.6 | 69.9 | 75.5 | 85.5 |
| 19 | 9.3 | 44.4 | 41.9 | 49.9 |
y=10.506Ln(x)-5.9474 | 0.9606 | 46.3 | 48.8 | 57.0 | 42.7 | 49.7 |
| 20 | 1.6 | 67.1 | 69.4 | 78.6 |
y=20.411Ln(x)-28.046 | 0.9748 | 73.4 | 76.1 | 79.2 | 83.5 | 83.0 |
| 21 | 5.7 | 74.0 | 90.1 | 85.7 |
y=22.417Ln(x)-26.430 | 0.9506 | 85.0 | 79.8 | 86.1 | 80.6 | 89.1 |
| 22 | 2.7 | 54.6 | 63.9 | 61.4 |
y=16.436Ln(x)-20.561 | 0.9484 | 61.1 | 58.6 | 67.3 | 73.3 | 74.4 |
| 23 | 6.2 | 72.7 | 74.2 | 84.0 |
y=20.588Ln(x)-23.661 | 0.9733 | 78.7 | 71.7 | 81.3 | 79.6 | 85.2 |
| 24 | 13.3 | 59.9 | 62.1 | 66.0 |
y=14.226Ln(x)-6.9829 | 0.9640 | 63.7 | 58.7 | 60.4 | 69.7 | 71.9 |
Use of l-OHP or SN-38 in combination with
5-FU yielded a significant increase in the growth IR value on the
AUC of 5-FU (Table I).
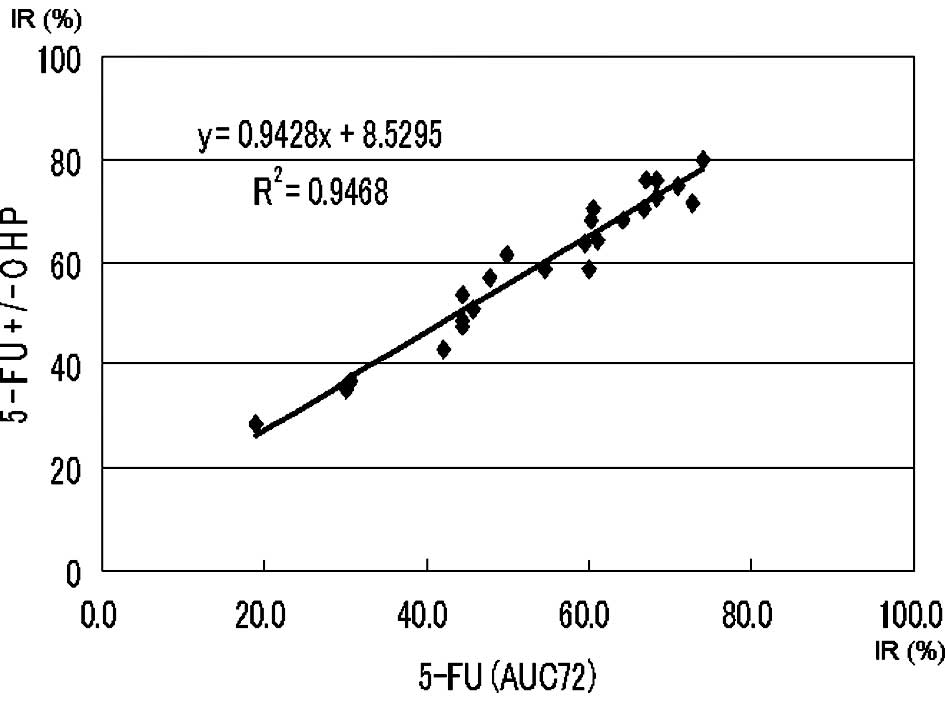
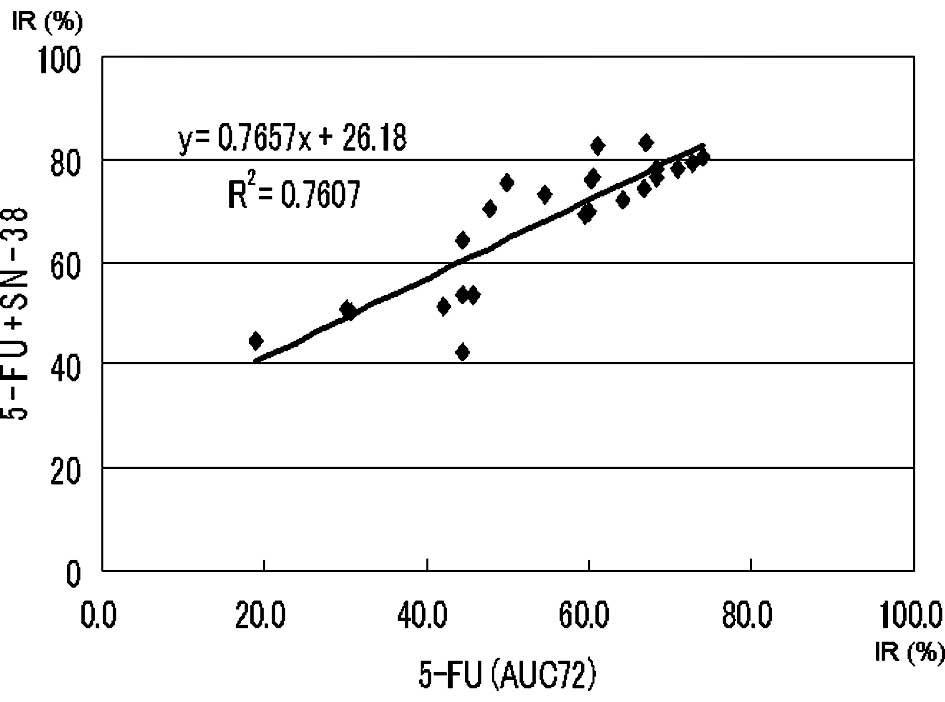
Approximate expression and correlation coefficients
on the AUC (72 μg*h/ml) of 5-FU (5-FU vs. 5-FU + l-OHP and
5-FU vs. 5-FU + SN-38) were y=0.94x+8.53 (p<0.0004) and
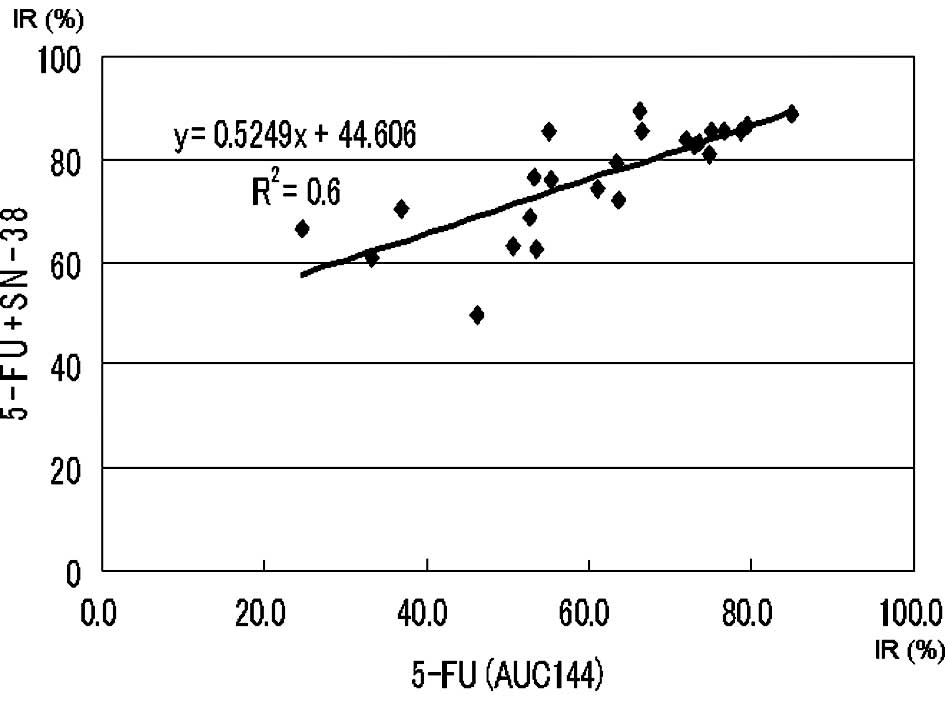
y=0.77x+26.18 (p<0.0004), respectively (Figs. 2 and 3).
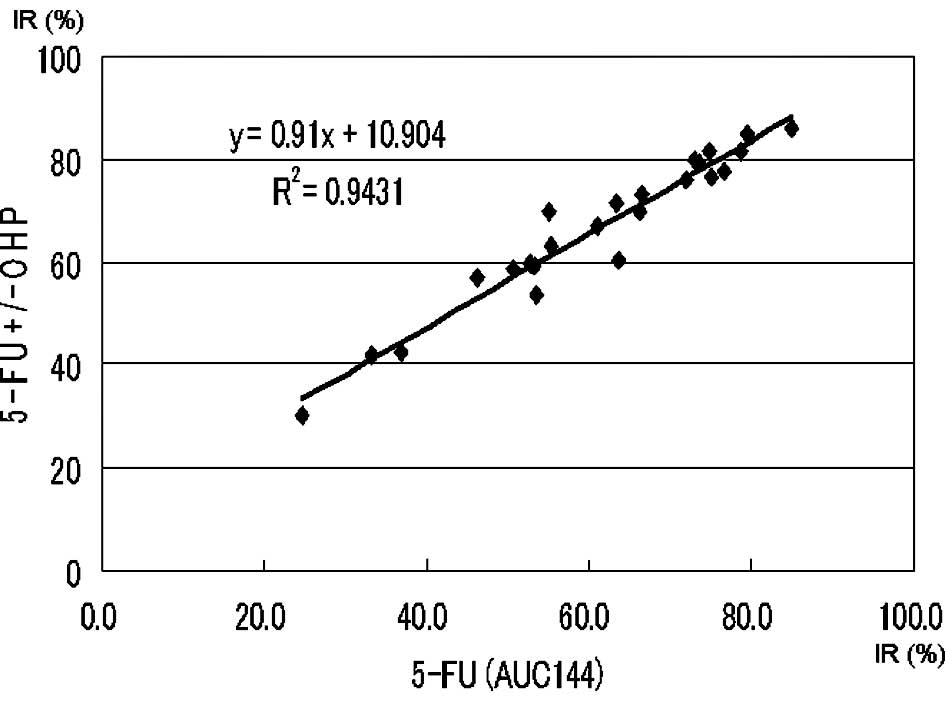
Approximate expression and correlation coefficients
on the AUC (144 μg*h/ml) of 5-FU (5-FU vs. 5-FU + l-OHP and
5-FU vs. 5-FU + SN-38) were y=0.91x+10.90 (p<0.0004) and
y=0.52x+44.61 (p<0.0004), respectively (Figs. 4 and 5).
Discussion
In several randomized controlled trials, both FOLFOX
and FOLFIRI have demonstrated improved patient survival as a
first-line therapy in the treatment of advanced CRC (5–10).
To obtain the same level of efficacy as that observed in these
earlier trials, full administration of the targeted dosages of all
three drugs (leucovorin and 5-FU + l-OHP or SN-38) is
crucial (11). However, the
pharmacokinetics of these drug combinations sometimes varies in CRC
patients. Moreover, patients with a poorer performance status and,
therefore, shorter life expectancy may have to be excluded from
oxaliplatin- or irinotecan-based second-line chemotherapy.
Therefore, since the best treatment for such patients may be either
FOLFOX or FOLFIRI as first-line therapy, it is essential to be able
to select which will be the most effective in each individual
case.
In planning individualized chemotherapy, the drug
sensitivity of the tumor cells is a key issue in assessing the
anti-tumor effect of the anticancer drugs to be used. The CD-DST is
a method of evaluating drug sensitivity using isolated,
3-dimensionally cultured tumor cells in a small collagen gel
droplet (12). This method offers
the following advantages: i) a high success rate in testing due to
the micro-3-dimensional culture; ii) the ability to work with a
small quantity of specimen; iii) the ability to evaluate the
anti-tumor effect of drugs in clinically equivalent doses; and iv)
the ability to accurately evaluate anti-cancer effects using an
image analysis device when fibroblast contamination is less than
67% (4). However, it is critical
that the sample being examined is obtained from the soft part of
the tumor tissue in order to prevent potential contamination by the
fibroblast component. Determination of 5-FU exposure in this study
was based on the report of Kobayashi (13). Determination of SN-38 and
l-OHP exposure was based on the AUCs of 3 and 6 courses of
FOLFOX or FOLFIRI. Using the CD-DST, we were able to calculate the
individual growth IR value on the AUC (144 μg*h/ml) of 5-FU from
the individual AUC-IR curve in each patient.
In this study, approximate expression of 5-FU vs.
5-FU + l-OHP almost fit the regression line
(y=x+b1). This suggests that addition of l-OHP
yields a constant effect, independent of the IR of 5-FU. This may
be explained by the fact that the mechanisms of 5-FU and
oxaliplatin function independently of each other. 5-FU is an
S-phase-specific drug, and is only active during certain cell
cycles. Three enzymes, in particular, are of great importance in
the metabolism of 5-FU; orotate phosphoribosyl transferase (OPRT),
thymidylate synthase (TS) and dihydropyrimidine dehydrogenase
(DPD). OPRT is the most important phosphorylation enzyme of 5-FU;
DPD is the degradation enzyme of 5-FU; and TS is the main enzyme of
DNA synthesis (14). In general,
poor efficacy for 5-FU is correlated with high expression of TS,
whereas good efficacy for 5-FU is correlated with low expression of
TS. Oxaliplatin is a cell-cycle non-specific antineoplastic agent
(16). Therefore, addition of
oxaliplatin yields a constant additive effect, independent of the
IR of 5-FU.
On the other hand, irinotecan is an S-phase-specific
drug like 5-FU and is only active during certain cell cycles
(15). Approximate expression of
5-FU vs. 5-FU + SN-38 fit the regression line (y=ax+b2,
a<1, b2≥b1). This suggests that addition
of SN-38 yields a greater effect due to the lower growth IR of
5-FU. Therefore, there is a marked synergetic efficacy between 5-FU
and irinotecan due to the lower growth IR of 5-FU. This synergy
between irinotecan and 5-FU may be explained by prolonged
inhibition of TS, down-regulation of TS mRNA expression and
increased incorporation of 5-FU metabolites into DNA. There are
several reports of the down regression of TS caused by irinotecan
(17–22).
In conclusion, the results of this study suggest
that FOLFIRI should be selected as first-line therapy in the
treatment of poor responders to 5-FU. Moreover, it is suggested
that, in planning individualized chemotherapy, it is vital to
evaluate the efficacy of 5-FU when making the choice between FOLFOX
or FOLFIRI as first-line treatment. In addition, it may be
necessary to consider non-5-FU-based chemotherapy (i.e., irinotecan
or oxaliplatin and molecularly targeted drugs) as the first-line
treatment in cases where response to 5-FU is extremely poor.
Accurate evaluation of the efficacy of 5-FU is of the utmost
importance in the establishment of individualized 5-FU-based
chemotherapy.
References
|
1.
|
Ochiai T, Nishimura K, Noguchi H, Kitajima
M, Tsuruoka Y and Takahashi Y: Evaluation of 5-fluorouracil
applicability by multi-point collagen gel droplet embedded drug
sensitivity test. Oncol Rep. 14:201–205. 2005.PubMed/NCBI
|
|
2.
|
Ochiai T, Nishimura K, Noguchi H, et al:
Evaluation of 5-fluorouracil applicability by the collagen gel
droplet embedded drug sensitivity test with area under the curve
analysis. Anticancer Drugs. 18:17–21. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kobayashi H, Tanisaka K, Doi O, et al: An
in vitro chemosensitivity test for soid human tumors using
collagen gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.
|
|
4.
|
Kobayashi H, Higashiyama M, Minamigawa K,
et al: Examination of in vitro chemosensitivity test using collagen
droplet culture method with colorimetric endpoint quantification.
Jpn J Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Tournigand C, André T, Achille E, et al:
FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: a randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Falcone A, Ricci S, Brunetti I, et al:
Gruppo Oncologico Nord Ovest. Phase III trial of infusional
fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXIRI)
compared with infusional fluorouracil, leucovorin and irinotecan
(FOLFIRI) as first-line treatment for metastatic colorectal cancer:
the Gruppo Oncologico Nord Ovest. J Clin Oncol. 25:1670–1676.
2007.
|
|
7.
|
Saltz LB, Clarke S, Díaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kabbinavar FF, Hambleton J, Mass RD, et
al: Combined analysis of efficacy: the addition of bevacizumab to
fluorouracil/leucovorin improves survival for patients with
metastatic colorectal cancer. J Clin Oncol. 23:3706–3712. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
André T, Louvet C, Maindrault-Goebel F, et
al: CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin
and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for
pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer.
35:1343–1347. 1999.PubMed/NCBI
|
|
10.
|
Maindrault-Goebel F, Louvet C, André T, et
al: Oxaliplatin added to the simplified bimonthly leucovorin and
5-fluorouracil regimen as second-line therapy for metastatic
colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer. 35:1338–1342.
1999. View Article : Google Scholar
|
|
11.
|
Grothey A, Sargent D, Goldberg RM, et al:
Survival of patients with advanced colorectal cancer improves with
the availability of fluorouracil-leucovorin, irinotecan and
oxaliplatin in the course of treatment. J Clin Oncol. 22:1209–1214.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Matsuo A, Watanabe A, Takahashi T, et al:
A simple method for classification of cell death by use of thin
layer collagen gel for the detection of apoptosis and/or necrosis
after cancer chemotherapy. Jpn J Cancer Res. 92:813–819. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet culture and image
analysis for clinical usefulness. Recent Results Cancer Res.
161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ochiai T, Nishimura K, Noguchi H, et al:
Prognotic impact of orotate phosphoribosyl transferase among
5-fluorouracil metabolic enzymes in resectable colorectal cancers
treated by oral 5-fluorouracil-based adjuvant chemotherapy. Int J
Cancer. 118:3084–3088. 2006. View Article : Google Scholar
|
|
15.
|
Minderman H, Conroy JM, O'Loughlin KL, et
al: In vitro and in vivo irinotecan-induced changes in expression
profiles of cell cycle and apoptosis-associated genes in acute
myeloid leukemia cells. Mol Cancer Ther. 4:885–900. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lévi F, Metzger G, Massari C, et al:
Oxaliplatin: pharmacokinetics and chronopharmacological aspects.
Clin Pharmacokinet. 38:1–21. 2000.
|
|
17.
|
Guichard S, Hennebelle I, Bugat R, et al:
Cellular interactions of 5-fluorouracil and the camptothecin
analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell
line. Biochem Pharmacol. 55:667–676. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Pavillard V, Formento P, Rostagno O, et
al: Combination of irinotecan (CPT-11) and 5-fluorouracil with an
analysis of cellular determinants of drug activity. Biochem
Pharmacol. 56:1315–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Banerjee D, Schnieders B, Fu JZ, Adhikari
D, Zhao SC and Bertino JR: Role of E2F-1 in chemosensitivity.
Cancer Res. 58:4292–4296. 1998.PubMed/NCBI
|
|
20.
|
Fukushima M, Uchida J, Sakamoto K, Ohshima
H and Taguchi T: Molecular mechanism of down-regulation by CPT-11
of thymidylate synthase highly expressing in gastrointestinal
cancer xenografts during combined treatment with fluoropyrimidines.
Eur J Cancer. 1:S622003. View Article : Google Scholar
|
|
21.
|
Ichikawa W, Takahashi T, Suto K, et al:
Thymidylate synthase predictive power is overcome by irinotecan
combination therapy with S-1 for gastric cancer. Br J Cancer.
91:1245–1250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fukushima M: S-1 review from preclinical
pharmacology. Gastric Cancer. 12:3–9. 2009. View Article : Google Scholar
|