Introduction
Endothelial cells (ECs), which are derived from
vascular progenitor cells, are responsible for angiogenesis and the
events of wound healing (1). They
are characterized by the expression of vascular
endothelial-cadherin (VE-cadherin, CDH5) (2–4),
vascular endothelial growth factor receptor 2 (VEGFR2, KDR)
(5,6), and CD31 (also called platelet
endothelial cell adhesion molecule-1, PECAM-1) (7). Since a blood supply is crucial for
wound healing and tissue regeneration, it is important to determine
how the progenitors of ECs differentiate into vascular cells in
order to establish a practical strategy for regenerative therapy
(1).
The periodontal ligament (PDL) is located between
the tooth root and alveolar bone (8). Most PDL cells are fibroblasts with
relatively high alkaline phosphatase (ALP) activity (9,10).
Fibroblasts derived from the PDL have the ability to form bone-like
tissues in vitro, similar to osteoblasts (9,10),
and thus PDL cells function similarly to osteoblasts in hard tissue
formation. Recently, several studies have demonstrated that PDL
cells also differentiate into cementoblastic cells and adipogenic
cells in vitro (1,11,12).
Therefore, the PDL probably contains pluripotent progenitor cells
or putative stem cells.
For therapy involving PDL tissue, biologically
active soluble factors such as cytokines and growth factors are
being evaluated for clinical use in the regeneration of periodontal
tissue damaged or lost as a result of periodontitis. Of these
factors, basic fibroblast growth factor (FGF-2) is a
multifunctional growth factor that has a variety of effects,
including the induction of proliferation and differentiation in a
wide range of mesodermal and neuro-ectodermal cells. Moreover,
FGF-2 is one of the most potent angiogenesis inducers (13). Therefore, we investigated whether
FGF-2 induces EC-specific markers in cultured PDL cells in
vitro.
In this study, we demonstrated that the expression
of VE-cadherin, VEGFR2 and CD31 mRNA is induced in cultured PDL
cells by treatment with heparin alone or with FGF-2. We also
demonstrated CD31 protein expression in PDL cell cultures using
Western blot analysis. This is the first report on the inducible
expression of the endothelial cell phenotype by PDL cells derived
from human deciduous teeth.
Materials and methods
Reagents
FGF-2 was obtained from R&D Systems
(Minneapolis, MN, USA). Anti-CD31 monoclonal antibody for the
Western blot analysis was obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Cell culture
PDL tissues were obtained from the middle third of
the root surfaces of healthy human deciduous teeth (obtained from
three donors, aged 7–8 years), as described previously (14,15).
Informed consent was obtained from the donors' parents before tooth
extraction, which was carried out in our hospital during the course
of orthodontic treatment. The study protocol was approved by the
Ethics Committee of Iwate Medical University, School of Dentistry
(no. 01101).
The PDL tissues were cut into pieces using a
surgical blade and were digested with collagenase (2 mg/ml) at 37°C
for 30 min. Then, the tissues were washed with Dulbecco's
phosphate-buffered saline (PBS), placed on culture dishes, and
maintained in α-modified minimum essential medium (α-MEM; Gibco
BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco BRL). Fibroblastic cells that outgrew from the
PDL tissues were used as PDL cells. When the cells reached
confluence, they were detached with 0.2% trypsin and 0.02% EDTA
•4Na in PBS, and subcultured at a 1:4 split ratio. The experiments
were performed using 4th-passage cells cultured in α-MEM
supplemented with 10% FBS in the absence or presence of 15 ng/ml
heparin or 10 ng/ml FGF-2 for 2 days. The cultures were maintained
at 37°C in a humidified atmosphere of 5% CO2 in air.
Isolation of total RNA
Total RNA was extracted from the cultured PDL cells
by using Isogen (Nippon Gene, Tokyo, Japan) as described previously
(14,15). The pellet of total RNA was washed
briefly with 75% ethanol, resuspended in 30 μl of
diethylpyrocarbonate (DEPC)-treated water, and stored at −80°C. The
concentration of total RNA was determined spectrophotometrically by
measuring the optical density at 260 nm.
Quantitative real-time reverse
transcription-polymerase chain reaction (PCR)
The RNA sample (1 μg) was reverse-transcribed
to first-strand cDNA using a PrimeScript RT Reagent Kit (Takara
Shuzo, Kyoto, Japan) according to the manufacturer's protocol. A
Thermal Cycler Dice Real-time System (Takara Shuzo) was used for
the two-step reverse transcription-PCR. The cDNA was amplified with
SYBR Premix ExTaq and specific oligonucleotide primers for target
sequences encoding parts of VE-cadherin, VEGFR2 and CD31. The
primers (listed in Table I) were
designed based on the cDNA sequences of human mRNA for VE-cadherin,
VEGFR2, CD31 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Amplification conditions consisted of 10 sec at 95°C, followed by
40 cycles at 95°C for 5 sec and 60°C for 30 sec, with a final 15
sec at 95°C and 30 sec at 60°C in the Thermal Cycler Dice Real-time
System.
 | Table I.Primers used in the quantitative
real-time reverse transcription-polymerase chain reaction
(real-time PCR). |
Table I.
Primers used in the quantitative
real-time reverse transcription-polymerase chain reaction
(real-time PCR).
| Gene name | Primer | Oligonucleotide
sequence (5′-3′) |
|---|
| VE-cadherin | Forward: |
GAGACCTCATCAGCCTTGGGATAG |
| Reverse: |
CTGGATTTGCCAGCATTTGAGA |
| VEGFR2 | Forward: |
CCAGGCAACGTAAGTGTTCGAG |
| Reverse: |
GGGACCCACGTCCTAAACAAAG |
| CD31 | Forward: |
GACGTGCAGTACACGGAAGTTCA |
| Reverse: |
GTGCATCTGGCCTTGCTGTC |
| GAPDH | Forward: |
GCACCGTCAAGGCTGAGAAC |
| Reverse: |
TGGTGAAGACGCCAGTGGA |
Western blot analysis of cell surface
CD31 expression in PDL cells
After treatment with heparin and/or FGF-2 for 21
days, PDL cells were washed twice with PBS and then treated with
lysis buffer [10 mM HEPES-KOH (pH 7.5), 100 mM KCL and 0.1% NP-40].
The protein concentration in the cell lysate was measured using a
BioRad Protein Assay Kit (BioRad, Hercules, CA, USA). Each sample
containing equal amounts of protein was separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred
to a polyvinylidene difluoride membrane (Millipore, Bedford, MA,
USA). After being blocked with 5% skim milk in Tris-buffered saline
containing 0.1% Tween-20 (TBST), the membrane was incubated with
mouse anti-human CD31 antibodies and subsequently with anti-mouse
secondary antibodies (Zymed Laboratories Inc., San Francisco, CA,
USA). Specific protein bands on the membrane were detected by using
an enhanced AP Conjugate Substrate Kit (BioRad) as described
previously (14,15).
Statistical analysis
The results are expressed as the mean ± SEM.
Statistical significance was determined by one-way analysis of
variance Bonferroni comparisons between pairs of groups. Data with
a P-value <0.01 were considered statistically significant.
Results
PDL cells derived from deciduous teeth in
primary culture show various cell morphologies
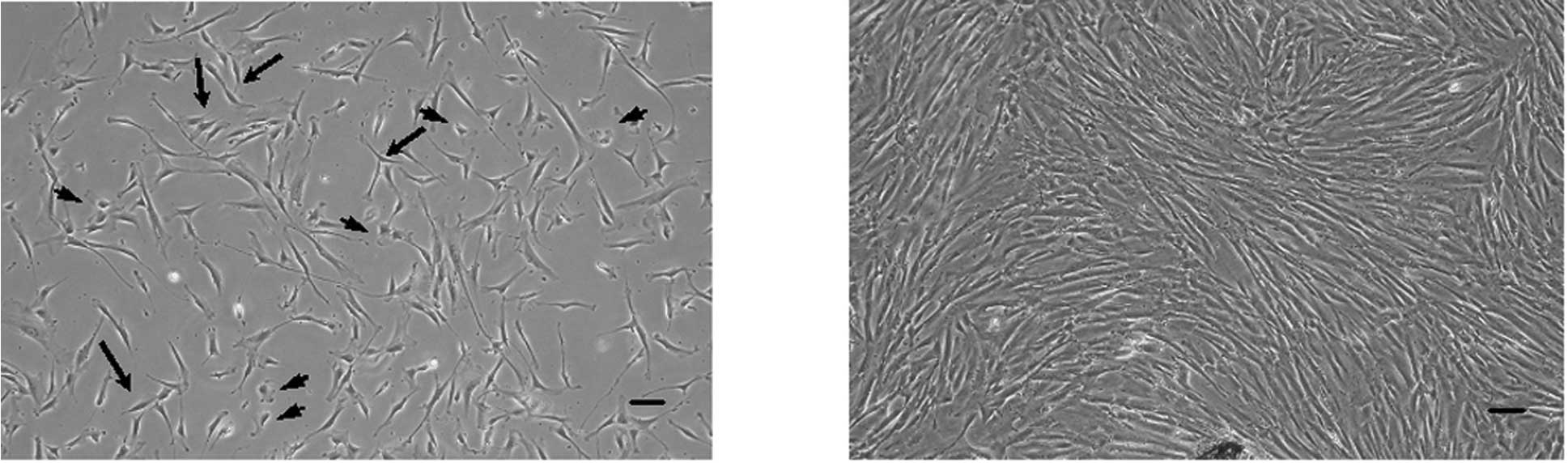
As shown in Fig. 1,
PDL cells exhibited various cell types at 10 days after isolation
from PDL tissues using phase-contrast microscopy. Most of the PDL
cells derived from deciduous teeth were fibroblastic cells
(Fig. 1A, arrows). Some cells were
polygonal shape, similar to epithelial cells and mature osteoblasts
(Fig. 1A, arrowheads). A few cells
were senescent fibroblastic cells (Fig. 1A, arrowheads). After the cells
reached confluence and subculture, it was not possibe to
distinguish between the cell morphologies (Fig. 1B).
Morphological changes in PDL cells are
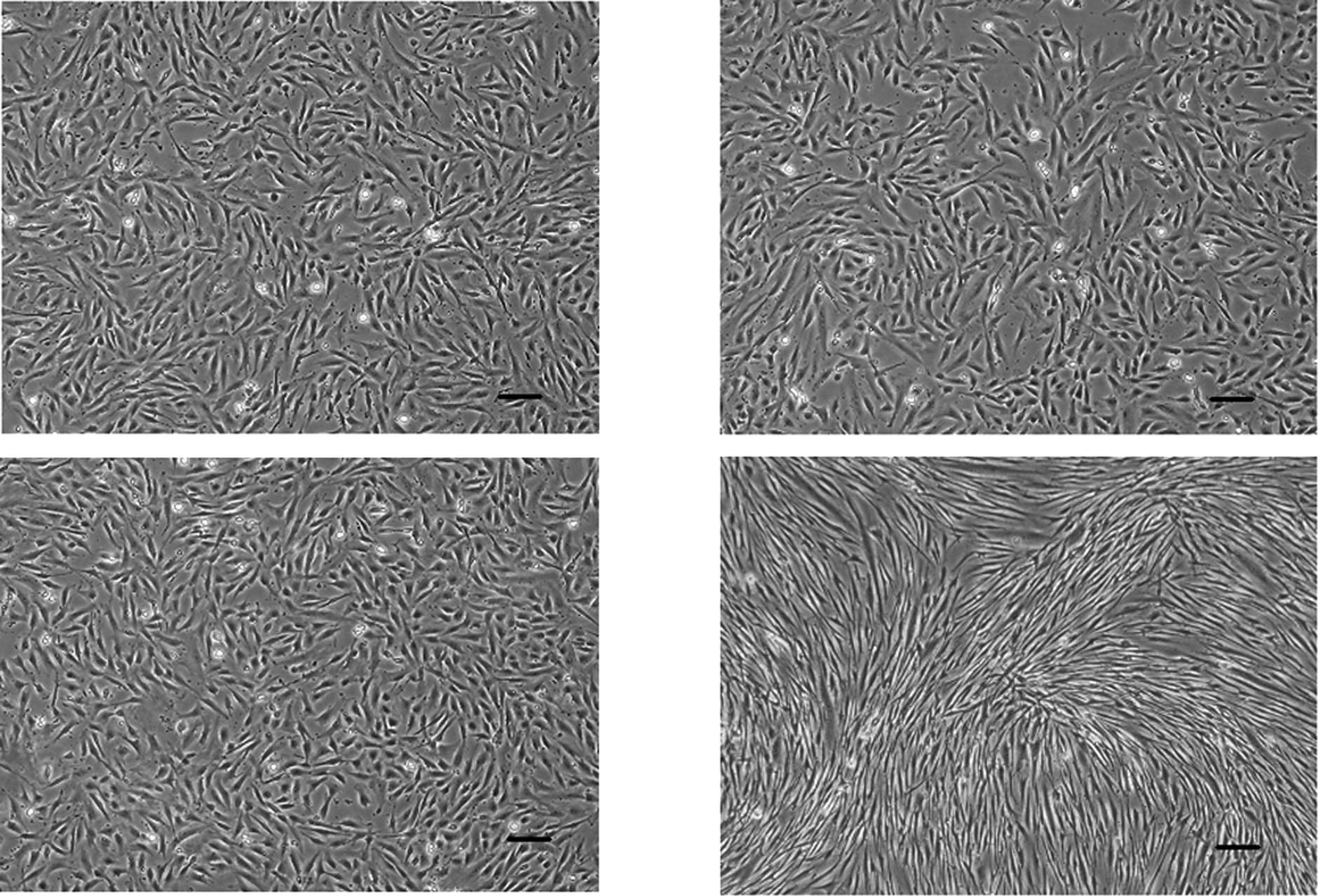
induced by treatment with both heparin and FGF-2 for 2 days
After culturing for 2 days, PDL cells were
subconfluent in the control media and the presence of heparin or
FGF-2 (Fig. 2). Upon treatment
with both heparin and FGF-2, PDL cells reached confluence, and
their morphology was altered into long and thin spindle-shaped
fibroblasts (Fig. 2D).
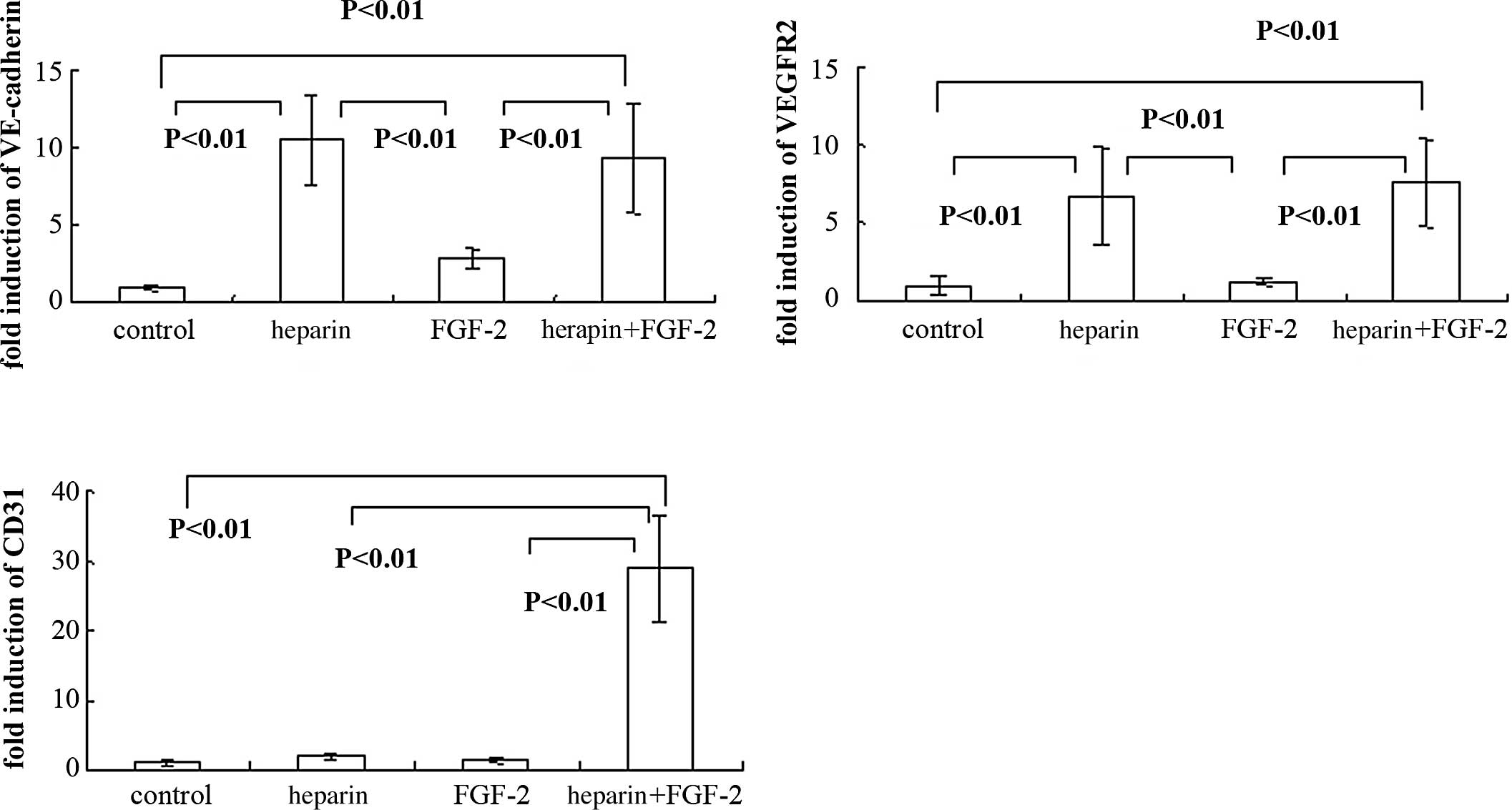
EC-specific markers are induced in PDL
cells cultured in the presence of heparin alone or with FGF-2 for 2
days
As shown in Fig. 3A and
B, when PDL cells were cultured in the presence of heparin
alone, VE-cadherin and VEGFR2 mRNA expression was markedly
increased. Treatment with both heparin and FGF-2 also increased
both VE-cadherin and VEGFR2 expression in PDL cells (Fig. 3A and B). However, upon treatment
with FGF-2 alone, VE-cadherin and VEGFR2 mRNA expression was not
induced in cultured PDL cells (Fig. 3A
and B). In contrast, CD31 expression was significantly induced
by treatment with both heparin and FGF-2 (Fig. 3C), but not with heparin or FGF-2
alone.
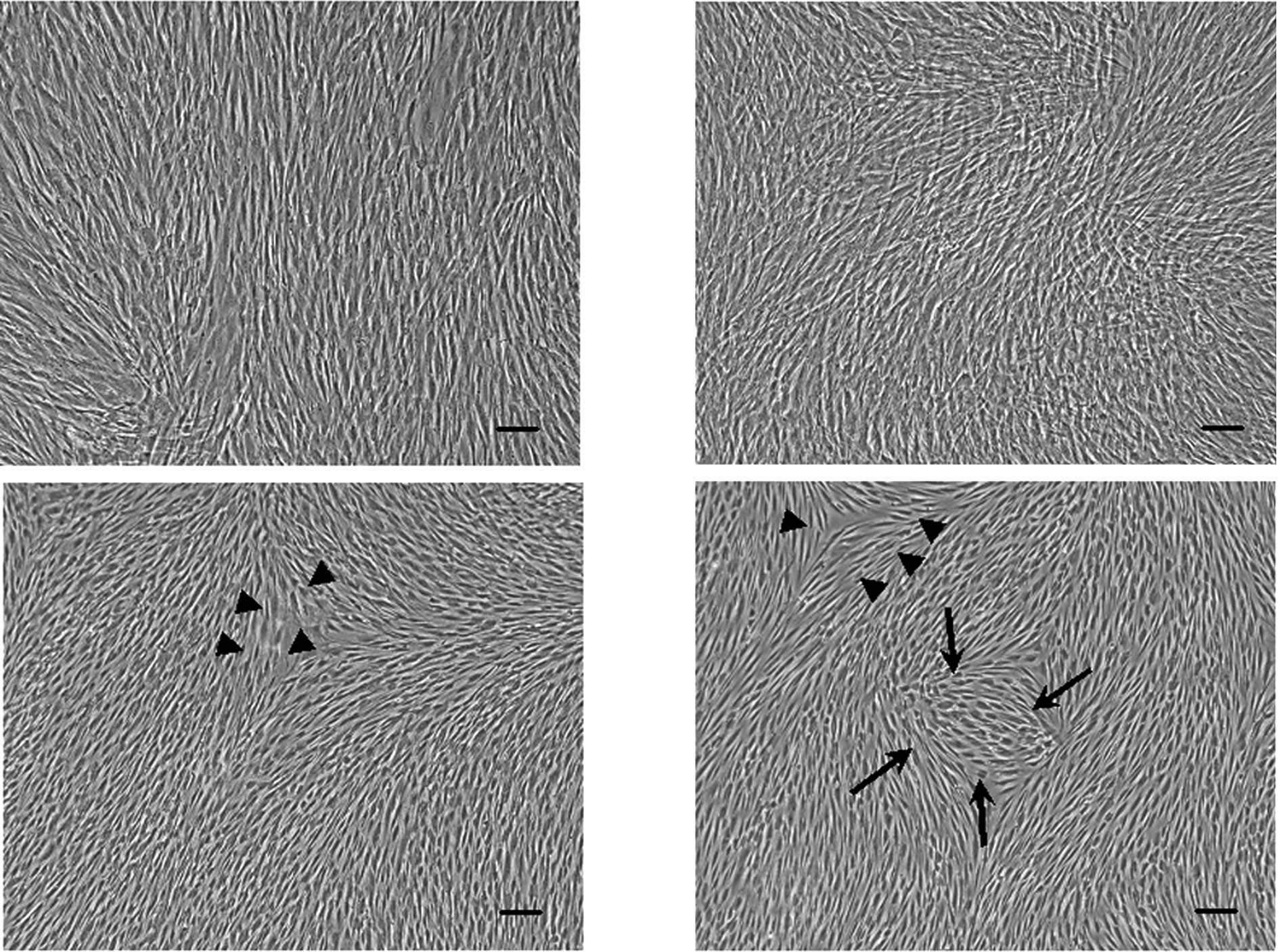
Morphological changes in PDL cells are
induced by treatment with FGF-2 alone and both heparin and FGF-2
for 3 weeks
As shown in Fig. 4,
PDL cells treated with FGF-2 alone and/or heparin reached confluent
multilayers when culturing for 3 weeks. Due to confluence, there
were no large differences in the morphology between the control and
the cells treated with heparin alone (Fig. 4A and B). Upon culturing in the
presence of FGF-2 alone and both heparin and FGF-2, PDL cells
showed long and thin spindle-shaped fibroblastic morphology
(Fig. 4C and D, arrowheads) and
polygonal morphology (Fig. 4D,
arrows).
CD31 expression was induced in PDL cells
cultured in the presence of FGF-2 and/or heparin
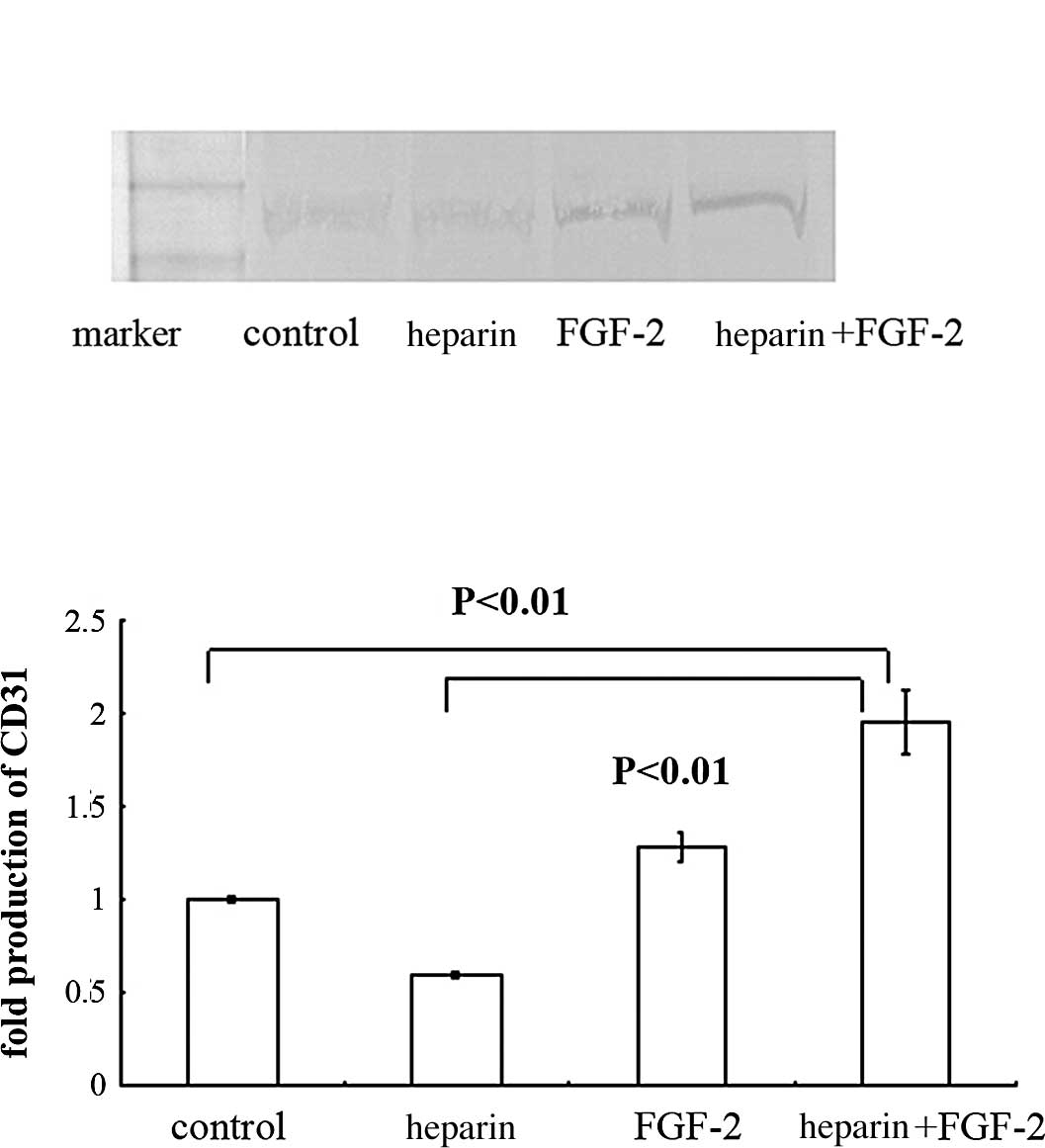
To determine whether CD31 protein was induced in PDL
cells, they were cultured in the presence of heparin alone, FGF-2
alone or both heparin and FGF-2 (Fig.
5). Upon treatment with heparin alone or with FGF-2 for 2 days,
CD31 expression was not detected by using Western blot analysis
(data not shown). Until 2 weeks of culture, CD31 protein expression
was not detected in PDL cells. After treatment with FGF-2 alone and
both heparin and FGF-2 for 3 weeks, CD31 expression was detected by
Western blotting (Fig. 5A).
Compared with the control, the production of CD31 was induced in
PDL cells by treatment with both FGF-2 and heparin (Fig. 5B).
Discussion
Tissue regeneration and homeostasis in response to
pathological and environmental changes such as periodontal disease,
wounding and tooth movement with orthodontic treatment are thought
to depend in large part upon angiogenesis in the periodontal
tissue. PDL cells exist surrounding tooth roots and thus are likely
to play an important role in periodontal tissue maintenance.
Recently, PDL cells were shown to have biological characteristics
in common with bone marrow mesenchymal cells, suggesting that
multipotent stem cells are present in the PDL tissue (11). However, it remains unclear whether
PDL cells derived from human deciduous teeth can give rise to the
endothelial cell (EC) lineage in vitro. To investigate PDL
tissue regeneration and homeostasis, it is crucial to determine
whether PDL cells have the ability to become ECs.
In this study, we used PDL cells derived from human
deciduous teeth to investigate the effects of heparin and FGF-2 on
the expression of markers specific for mature ECs: VE-cadherin
(2–4), VEGFR2 (5,6) and
CD31 (7). Previous studies have
found that PDL cells, which are not stimulated with FGF-2, do not
express EC markers such as CD31 (12,16).
We also found that PDL cells do not express VE-cadherin, VEGFR2, or
CD31 without treatment with FGF-2. Surprisingly, PDL cells
increased the expression of VE-cadherin and VEGFR2 when cultured in
the presence of heparin alone or with FGF-2. The expression of CD31
was also significantly increased in PDL cells cultured with both
heparin and FGF-2. This discrepancy might have been due to the
presence of heparin in the cell culture conditions. Heparin, a
soluble derivative of heparin sulfate and a well known cofactor for
FGF-2, substantially enhances the activity of FGF-2 (17). Our observation that the addition of
heparin in the absence of FGF-2 stimulated the expression of
VE-cadherin and VEGFR2 suggests that heparin enhanced the activity
of endogenously produced FGF-2. Nevertheless, other mechanisms
cannot be excluded. For example, heparin might have enhanced the
activity of growth factors present in the fetal bovine serum,
resulting in up-regulated expression of VE-cadherin and VEGFR2 in
PDL cells.
Compared with mRNA expression, CD31 protein
production showed relatively small changes in the PDL cells.
However, the treatment with FGF-2 was sufficient to induce CD31
protein. As shown in Fig. 5, when
PDL cells were treated with heparin and FGF-2, CD31 production was
increased approximately 2-fold compared with the control group. The
amount of protein, which is determined, not only by the mRNA level,
but also by multiple processes of protein synthesis and
degradation, may be a critical factor.
Here, we demonstrated for the first time that PDL
cells derived from human deciduous teeth inducibly express
EC-specific markers, such as VE-cadherin, VEGFR2 and CD31 upon
treatment with heparin alone or with FGF-2 in vitro. These
findings are useful to understand how to regenerate PDL tissue by
inducing angiogenesis.
Acknowledgements
This work was supported, in part, by a
Grant-in-Aid for Scientific Research (no. 18592026 to A.I., no.
19791370 to N.C., and no. 18592239 to T.H.) from the Ministry of
Education, Culture, Sports, Science, and Technology of Japan; the
Open Research Project and High-Tech Research Project from the
Ministry of Education, Culture, Sports, Science, and Technology of
Japan; the Akiyama Foundation (to T.H., 2005); and a grant from the
Keiryokai Research Foundation (no. 100 to N.C., 2008, and no. 106
to T.H., 2009).
References
|
1.
|
Bartold PM, Shi S and Gronthos S: Stem
cells and periodontal regeneration. Periodontol 2000. 40:164–172.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Dejana E: Endothelial cell-cell junctions:
happy together. Nat Rev Mol Cell Biol. 5:261–270. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Cavallaro U, Liebner S and Dejana E:
Endothelial cadherins and tumor angiogenesis. Exp Cell Res.
312:659–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Carmeliet P, Lampugnani MG, Moons L, et
al: Targeted deficiency or cytosolic truncation of VE-cadherin gene
in mice impairs VEGF-mediated endothelial survival and
angiogenesis. Cell. 98:147–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lampugnani MG, Orsenigo F, Gagliani MC,
Tacchetti C and Dejana E: Vascular endothelial cadherin controls
VEGFR-2 internalization and signaling from intracellular
compartments. J Cell Biol. 174:593–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Yamaguchi TP, Dumont DJ, Conlon RA,
Breitman ML and Rossant J: flk-1, an flt-related receptor tyrosine
kinase, is an early marker for endothelial cell precursors.
Development. 118:489–498. 1993.PubMed/NCBI
|
|
7.
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: mobilization, differentiation and homing.
Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Freeman E: Periodontium. Oral Histology:
Development, Structure and Junction. Ten Cate AR: Mosby, St. Louis:
pp. 276–312. 1994
|
|
9.
|
Groeneveld MC, Everts V and Beertsen W:
Alkaline phosphatase activity in the periodontal ligament and
gingiva of the rat molar: its relation to cementum formation. J
Dent Res. 74:1374–1381. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Beertsen W and van den Bos T: Alkaline
phosphatase induces the mineralization of sheets of collagen
implanted subcutaneously in the rat. J Clin Invest. 89:1974–1980.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang C and Shi S:
Investigation of multi-potent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nagatomo K, Komaki M, Sekiya I, Sakaguchi
Y, Noguchi K, Oda S, Muneta T and Ishikawa I: Stem cell properties
of human periodontal ligament cells. J Periodontal Res. 41:303–310.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor (Review). Endocr Rev.
18:4–25. 1997. View Article : Google Scholar
|
|
14.
|
Hasegawa T, Yoshimura Y, Kikuiri T, Yawaka
Y, Takeyama S, Matsumoto A, Oguchi H and Shirakawa T: Expression of
receptor activator of NF-kappa B ligand and osteoprotegerin in
culture of human periodontal ligament cells. J Periodont Res.
37:405–411. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Hasegawa T, Kikuiri T, Takeyama S,
Yoshimura Y, Mitome M, Oguchi H and Shirakawa T: Human periodontal
ligament cells derived from deciduous teeth induce
osteoclastogenesis in vitro. Tissue Cell. 34:44–51. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Trubiani O, Isgro A, Zini N, Antonucci I,
Aiuti F, Di Primio R, Nanci A, Caputi S and Paganelli R: Functional
interleukin-7/interleukin-7Ralpha, and SDF-1alpha/CXCR4 are
expressed by human periodontal ligament derived mesenchymal stem
cells. J Cell Physiol. 214:706–713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Furue MK, Na J, Jackson JP, Okamoto T,
Jones M, Baker D, Hata R, Moore HD, Sato JD and Andrews PW: Heparin
promotes the growth of human embryonic stem cells in a defined
serum-free medium. Proc Natl Acad Sci USA. 105:13409–13414. 2008.
View Article : Google Scholar : PubMed/NCBI
|