Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common human cancers associated with a poor patient prognosis.
Although previous studies have shown that chemotherapy improves
patient survival in completely resected NSCLC (1,2),
only an additional 5–15% of treated individuals ultimately benefit
with improvement in their long-term interval (3). On the other hand, recent molecular
biology studies have revealed that many molecules affect the
various biological behaviors of malignant tumors (4). Several molecules have been proven to
be associated with the responsiveness to chemotherapy, and the
selection of an effective chemotherapy based on an evaluation of
these molecules, namely ‘tailor-made chemotherapy’, may improve the
clinical outcome of NSCLC patients (5,6). In
fact, epidermal growth factor receptor (EGFR)-specific tyrosine
kinase inhibitors, such as gefitinib and erlotinib, have been
proven to be effective for NSCLCs with EGFR gene mutations
(7). In addition, 5-fluorouracil
(5-FU)-derived agents, such as S-1 and UFT, are effective for
NSCLCs with a low expression of thymidylate synthase (TS) (8). At present, we are utilizing
tailor-made chemotherapy based on the co-evaluation of EGFR
mutations and TS expression for NSCLC patients.
However, most NSCLCs with EGFR mutations or low TS
expression are adenocarcinomas. The remaining populations of NSCLCs
require chemotherapy using other drugs based on the evaluation of
other targeted molecules. Other anti-tumor agents are considered
based on evaluations of other chemotherapy-associated molecules.
For example, the intratumoral expression of excision repair
cross-complementing (ERCC)-1, a leading component of nucleotide
excision repair (NER), is associated with the responsiveness to
platinum-based chemotherapy, such as cisplatin and carboplatin
(9–12). In addition, the intratumoral
expression of class III β-tubulin is associated with the
responsiveness to taxanes, such as paclitaxel and docetaxel
(13–15). Therefore, a clinical study on the
expression of ERCC1 and class III β-tubulin was conducted in
resected advanced stage NSCLC patients treated with induction
chemoradiotherapy using carboplatin-taxane, in order to more widely
establish the treatment strategy of tailor-made chemotherapy.
Materials and methods
Clinical characteristics of the
patients
From January 2000 to April 2006, 41 patients with
bulky-cN2, N3 stage III NSCLC underwent surgery after induction
chemoradiotherapy at the Department of General Thoracic Surgery,
Breast and Endocrinological Surgery, Faculty of Medicine, Kagawa
University, as reported previously (16). This study was approved by the
Institutional Review Board of Kagawa University (14-7, a clinical
study of biological markers in non-small cell lung cancers).
Signed, written informed consent was obtained from all patients
before therapy was initiated. Patient clinical records and
histopathological diagnoses were fully documented.
The therapeutic schedule for induction
chemoradiotherapy was performed as follows (16): chemotherapy was conducted during
week 1, and concurrent radiotherapy with 30 Gy was conducted during
weeks 1, 2 and 3. After a 1-week withdrawal period, chemotherapy
was carried out during week 5, and concurrent radiotherapy with 20
Gy was carried out during weeks 5 and 6. The therapies were
discontinued for 1–4 weeks depending on the condition of the
patient, and a re-evaluation was carried out using chest CT,
abdominal CT, brain CT or magnetic resonance imaging and bone scan.
A routine re-evaluation of induction chemoradiotherapy was carried
out according to the ‘New guidelines to evaluate the response to
treatment in solid tumors’ (17).
A complete resolution of all targets was defined as complete
response and at least a 30% reduction (quantified as the longest
diameter) in the tumor size was defined as partial response. After
the re-evaluation of induction chemoradiotherapy, complete surgical
resections were performed for all patients.
Regarding chemotherapy, the patients were randomly
assigned to a carboplatin-paclitaxel (CP) arm and a
carboplatin-docetaxel (CD) arm (16). Patients in the CP arm received
carboplatin (area under the curve 6 mg/(ml,min), 30-min intravenous
infusion) and paclitaxel (180 mg/m2, 3-h intravenous
infusion) on day 1. The patients in the CD arm received carboplatin
(area under the curve 6 mg/(ml,min), 30-min intravenous infusion)
and docetaxel (60 mg/m2, 3-h intravenous infusion) on
day 1. Regarding radiotherapy, an area including the hilum of the
lung and mediastinum with a 1.5-cm margin from the periphery of the
primary lesion was irradiated with 2 Gy/day. The patients received
irradiation five times weekly, with 2 non-irradiation days set
up.
The pathological effect of induction therapy was
evaluated according the General Rules for Clinical and Pathological
Record of Lung Cancer, 6th edition (18); a pathologically complete response
(complete cancer cell death), a major response (fewer than
one-third of cancer cells viable) and a minor response (more than
one-third of cancer cells viable). Among them, 7 patients with a
pathologically complete response were excluded, since
immunohistochemical evaluations using surgically resected tumor
specimens were not possible. Finally, 34 patients with stage III
NSCLC were investigated as shown in Table I. They included 19 adenocarcinomas
and 15 squamous cell carcinomas. Regarding chemotherapy, 15
patients received the CP arm and 19 patients received the CD arm.
Regarding surgical method, a lobectomy or bilobectomy was performed
in 24 patients, and a pneumonectomy was performed in 10
patients.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | No. of patients | Percentage |
|---|
| Total no. of
patients | 34 | 100 |
| Age | | |
| Median | 63.5 | |
| Range | 46–75 | |
| Gender | | |
| Male | 28 | 82.4 |
| Female | 6 | 17.6 |
| Histology | | |
| Adenocarcinoma | 19 | 55.9 |
| Squamous cell
carcinoma | 15 | 44.1 |
| Pathological
stage | | |
| Stage IIIA | 24 | 70.6 |
| T1N2M0 | 8 | 23.5 |
| T2N2M0 | 15 | 44.1 |
| T3N2M0 | 1 | 2.9 |
| Stage IIIB | 10 | 29.4 |
| T2N3M0 | 3 | 8.8 |
| T4N2M0 | 6 | 17.6 |
| T4N3M0 | 1 | 2.9 |
| Chemotherapy | | |
| Carboplatin plus
paclitaxel | 15 | 44.1 |
| Carboplatin plus
docetaxel | 19 | 55.9 |
| Radiotherapy | 34 | 100 |
| Method of surgical
resection | | |
| Lobectomy or
bilobectomy | 24 | 70.6 |
| Pneumonectomy | 10 | 29.4 |
| Response to induction
therapy | | |
| Partial
response | 24 | 70.6 |
| Stable disease | 10 | 29.4 |
| Pathological effect
of induction therapy | | |
| Major response | 23 | 67.6 |
| Minor
response | 11 | 32.4 |
Immunohistochemistry
A mouse monoclonal antibody for ERCC1 (8F1; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) diluted at 1:300 and
a mouse monoclonal antibody for class III β-tubulin (TU-20; AbD
Serotec, Oxford, UK) diluted at 1:3 were used. Formalin-fixed
paraffin-embedded tissue was cut into 4-μm sections and mounted on
poly-L-lysine-coated slides. Sections were deparaffinized and
rehydrated. The slides were then heated in a microwave for 10 min
in a 10 μmol/l citrate buffer solution at pH 6.0. After quenching
the endogenous peroxidase activity with 0.3%
H2O2 (in absolute methanol) for 30 min, the
sections were treated for 2 h with 5% bovine serum albumin to block
non-specific staining. Duplicate sections were incubated overnight
with primary antibodies, respectively. Slides were then incubated
for 1 h with biotinylated anti-mouse IgG secondary antibodies
(Vector Laboratories Inc., Burlingame, CA, USA). The sections were
incubated with the avidin-biotin-peroxidase complex (Vector
Laboratories Inc.) for 1 h, and antibody binding was visualized
with 3,3′-diaminobenzidine tetrahydrochloride. Lastly, the sections
were lightly counterstained with Mayer's hematoxylin. All
immunostained sections were independently evaluated by two authors
(C.H. and K.K.), without knowledge of the patient characteristics.
Five areas were selected at random and scored in cases with
multiple areas of low intensity. Also, one random field was
selected in sections where all staining appeared intense. At least
200 cells were scored per ×40 field about tumor cells.
Statistical analysis
Since the distributions of two values, including the
percentages of ERCC1-positive tumor cells (P=0.8830) and class III
β-tubulin-positive tumor cells (P=0.5397), showed normal
distributions (Kolmogorov-Smirnov analysis), the statistical
significances of ERCC1 or class III β-tubulin expression in
relation to the clinical and pathological parameters were assessed
by the t-test or the χ2 test. The sample was classified
as an ERCC1-high tumor when >30% of the tumor cells had positive
staining of ERCC1 because of the greatest significance in relation
to patient survival. The sample was also classified as a class III
β-tubulin-high tumor when >30% of tumor cells had positive
staining of class III β-tubulin because of the greatest
significance in relation to patient survival. Overall survival was
defined as the time from treatment initiation (surgical resection,
chemotherapy or radiation) to the date of death from any cause. The
Kaplan-Meier method was used to estimate the probability of overall
survival as a function of time, and differences in the survival of
subgroups of patients were compared by using the Mantel's log-rank
test. A multivariate analysis was performed using the Cox
regression model to study the effects of different variables on
survival. All P-values were based on two-tailed statistical
analysis, and a P-value of <0.05 was considered to indicate
statistical significance.
Results
ERCC1 expression in NSCLCs
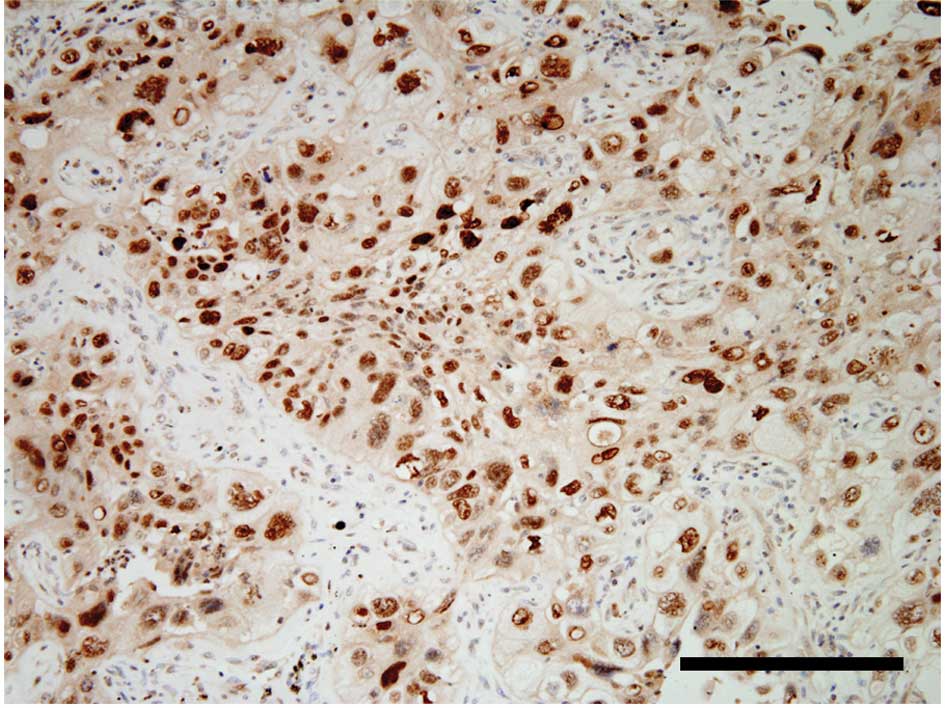
The intratumoral ERCC1 expression exhibited a
nuclear staining pattern (Fig.
1A). The percentage of ERCC1-positive tumor cells varied
greatly (median 30%; mean ± SD, 34.1±22.9%). Among 34 stage III
NSCLCs, 19 tumors (55.9%) were ERCC1-high (Table IIA). Seven of the 19
adenocarcinomas (36.8%) were ERCC1-high tumors. Twelve of the 15
squamous cell carcinomas (80.0%) were ERCC1-high tumors. The
percentage of ERCC1-high tumors was significantly higher in the
squamous cell carcinomas than in the adenocarcinomas (P=0.0119).
Furthermore, the percentage of ERCC1-positive tumor cells was
significantly higher in the squamous cell carcinomas than in the
adenocarcinomas (53.3±24.8 vs. 21.3±17.3%, P=0.0050).
 | Table II.ERCC1 and class III β-tubulin
expression in 34 non-small cell lung cancers. |
Table II.
ERCC1 and class III β-tubulin
expression in 34 non-small cell lung cancers.
| A, ERCC1
expression. |
|
| | Percentage of
ERCC1-positive cells
| |
| Histology | n | 0 | 1–30 | 31–50 | 51–100 | P-value |
|
| Adenocarcinoma | 19 | 9 | 3 | 4 | 3 | 0.0119 |
| Squamous cell
carcinoma | 15 | 3 | 0 | 5 | 7 | |
| Total | 34 | 12 | 3 | 9 | 10 | |
|
| B, Class III
β-tubulin expression. |
|
| | Percentage of class
III β-tubulin-positive cells
| |
| Histology | n | 0 | 1–30 | 31–50 | 51–100 | P-value |
|
| Adenocarcinoma | 19 | 8 | 4 | 3 | 4 | 0.4840 |
| Squamous cell
carcinoma | 15 | 10 | 1 | 2 | 2 | |
| Total | 34 | 18 | 5 | 5 | 6 | |
Class III β-tubulin expression in
NSCLCs
The intratumoral class III β-tubulin expression
exhibited a cytoplasmic staining pattern (Fig. 1C). The percentage of class III
β-tubulin-positive tumor cells varied greatly (median 0%; mean ±
SD, 15.5±12.1%). Among 34 stage III NSCLCs, 11 tumors (32.4%) were
class III β-tubulin-high (Table
IIB). Seven of the 19 adenocarcinomas (36.8%) were class III
β-tubulin-high tumors. Four of the 15 squamous cell carcinomas
(26.7%) were class III β-tubulin-high tumors. No significant
difference was observed in the percentage of class III
β-tubulin-positive tumor cells between the adenocarcinomas and the
squamous cell carcinomas (18.3±13.4 vs. 11.9±10.4%).
Relationship between ERCC1 and class III
β-tubulin expression in NSCLCs
No correlation was observed between ERCC1 and class
III β-tubulin expression (r=0.208, P=0.2385). Among the 34 stage
III NSCLCs, 12 tumors (35.3%) were both ERCC1-low and class III
β-tubulin-low, 11 tumors (32.4%) were ERCC1-high but class III
β-tubulin-low, 3 tumors (8.8%) were ERCC1-low but class III
β-tubulin-high and 8 tumors (23.5%) were both ERCC1-high and class
III β-tubulin-high.
Response to induction therapy in relation
to ERCC1 or class III β-tubulin expression in NSCLCs
Regarding the radiological evaluation of response to
induction therapy, 24 tumors had a partial response to induction
therapy and 10 exhibited stable disease (Table I). Regarding the pathological
effect of induction therapy, a major response was observed in 23
patients and a minor response was observed in 11 patients.
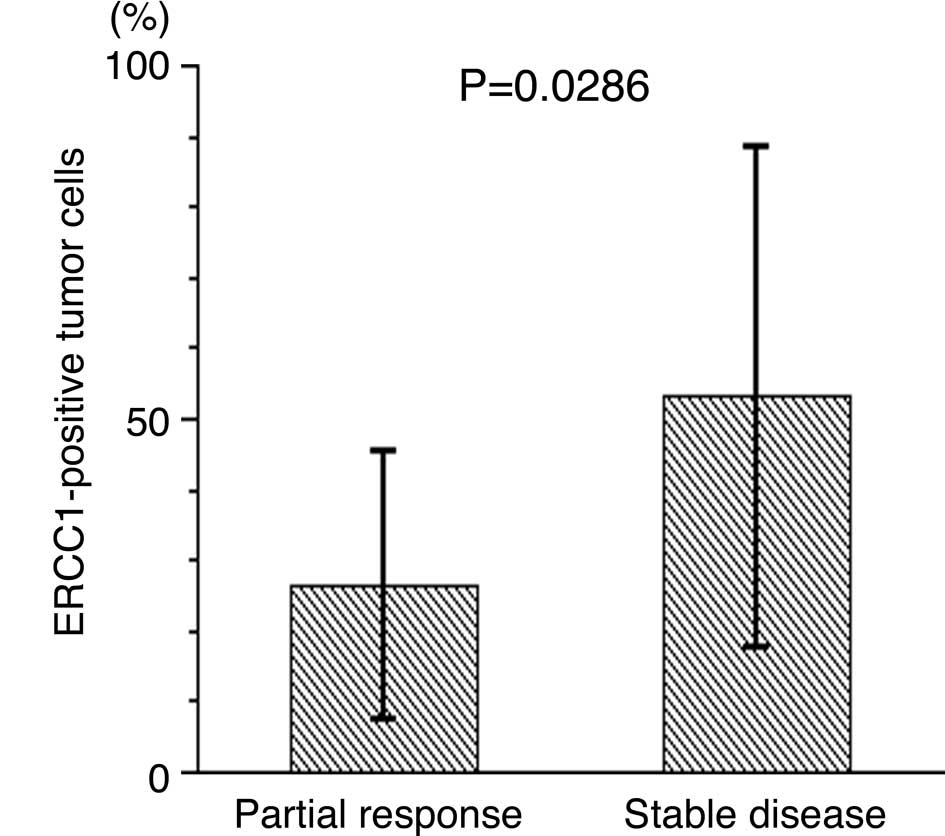
Concerning ERCC1 expression, the percentage of
ERCC1-positive tumor cells was significantly lower in tumors with a
partial response than in tumors with stable disease (26.3±19.0 vs.
53.0±35.6%, P=0.0286, Fig. 2A).
Regarding the pathological effect of induction therapy, a major
response was observed in 11 of the 15 ERCC1-low tumors (73.3%). A
major response was observed in 12 of the 19 ERCC1-high tumors
(63.2%). The percentage of ERCC1-positive tumor cells was lower in
tumors with a major response than in tumors with a minor response
(27.4±19.1 vs. 48.2±37.4%, P=0.0851, Fig. 2B).
Concerning class III β-tubulin expression, the
percentage of class III β-tubulin-positive tumor cells was
significantly lower in tumors with a partial response than in
tumors with stable disease (8.8±8.1 vs. 31.5±23.3%, P=0.0045,
Fig. 2C). Regarding the
pathological effect of induction therapy, a major response was
observed in 19 of the 23 class III β-tubulin-low tumors (82.6%). A
major response was observed in 4 of the 11 class III β-tubulin-high
tumors (36.4%). The percentage of class III β-tubulin-positive
tumor cells was significantly lower in tumors with a major response
than in tumors with a minor response (9.0±8.5 vs. 29.1±23.5%,
P=0.0105, Fig. 2D).
Overall survival of stage III NSCLC
patients treated with carboplatin-taxane in relation to ERCC1 and
class III β-tubulin expression
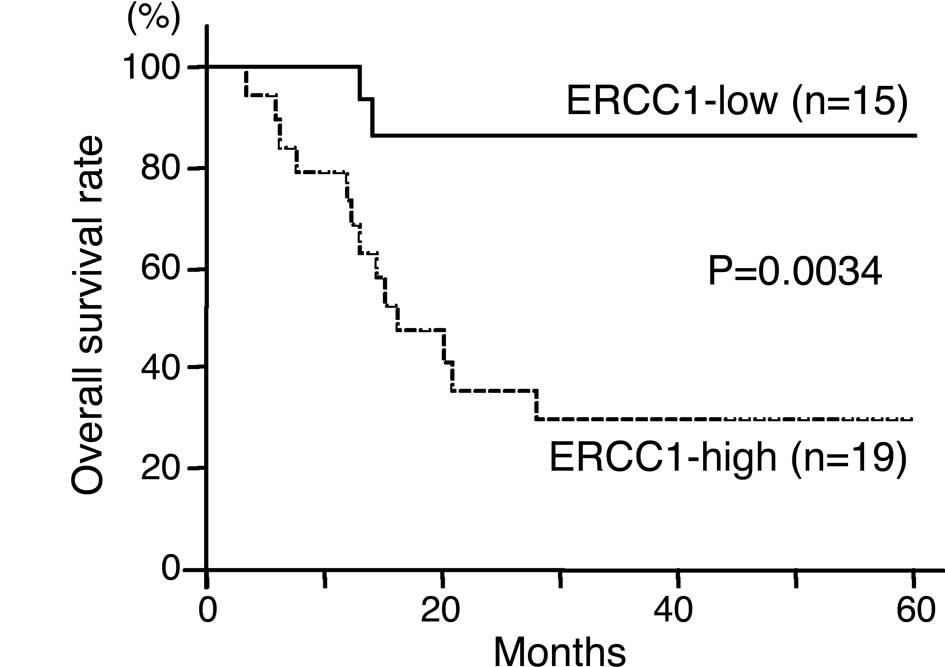
Regarding ERCC1 expression, the 3-year survival rate
was 86.7% in patients with ERCC1-low tumors and 29.6% in patients
with ERCC1-high tumors (Fig. 3A).
The overall survival was significantly higher in patients with
ERCC1-low tumors than in those with ERCC1-high tumors
(P=0.0034).
Regarding class III β-tubulin expression, the 3-year
survival rate was 65.8% in patients with class III β-tubulin-low
tumors and 27.3% in patients with class III β-tubulin-high tumors
(Fig. 3B). The overall survival
was significantly higher in patients with class III β-tubulin-low
tumors than in those with class III β-tubulin-high tumors
(P=0.0185).
Regarding expression of ERCC1 and class III
β-tubulin, the 3-year survival rate was 100.0% in 12 patients with
both ERCC1- and class III β-tubulin-low tumors, 31.2% in 14
patients with either a ERCC1- or class III β-tubulin-high tumor and
25.0% in 8 patients with both ERCC1- and class III β-tubulin-high
tumors (Fig. 3C). The overall
survival of the patients with both ERCC1- and class III
β-tubulin-low tumors was significantly the highest among the three
groups (P=0.0014).
A multivariate analyses using the Cox regression
model demonstrated that ERCC1 (hazard ratio, 4.686; P=0.0467) and
the class III β-tubulin expression levels (hazard ratio, 5.224;
P=0.0237) were significant prognostic factors for completely
resected stage III NSCLC patients treated with induction
chemoradiotherapy using carboplatin-taxane (Table III).
 | Table III.Multivariate regression analysis in
predicting the survival of resected stage III NSCLC patients
treated with induction chemoradiotherapy using
carboplatin-taxane. |
Table III.
Multivariate regression analysis in
predicting the survival of resected stage III NSCLC patients
treated with induction chemoradiotherapy using
carboplatin-taxane.
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| ERCC1
expression | 4.686 | (1.022–21.475) | 0.0467 |
| Class III β-tubulin
expression | 5.224 | (1.247–21.879) | 0.0237 |
| Age (≤65 vs. >65
years) | 4.253 | (1.015–17.830) | 0.0477 |
| Method of surgical
resection | 3.231 | (0.510–20.479) | 0.2132 |
| Chemotherapy (CP
vs. CD) | 2.025 | (0.585–7.006) | 0.2653 |
Discussion
Selection of the effective chemotherapy treatment
based on the evaluation of tumor-associated molecules, tailor-made
chemotherapy, may improve the clinical outcome of NSCLC patients
(5,6). At present, we are utilizing
tailor-made chemotherapy based on the co-evaluation of EGFR
mutations and TS expression for NSCLC patients. NSCLCs with EGFR
mutations may be successfully treated with EGFR-specific tyrosine
kinase inhibitors, such as gefitinib or erlotinib (7). Furthermore, tumors with low
expression of thymidylate synthase can be successfully treated with
5-FU-derived agents, such as S-1 and UFT (8). However, most NSCLCs with EGFR
mutations or low TS expression are adenocarcinomas, and the
remaining populations of NSCLCs require chemotherapy using other
drugs based on an evaluation of other targeted molecules.
Therefore, the present study on the expression of ERCC1 against
carboplatin (9–12) and class III β-tubulin against
taxanes (13–15) in resected advanced stage NSCLC
patients treated with carboplatin-taxane was conducted in order to
expand the treatment strategy of the tailor-made chemotherapy.
Platinum-based chemotherapy, such as cisplatin and
carboplatin, is still the scaffolding of combination chemotherapy
in NSCLC patients (1,2). Platinum-based chemotherapy exerts its
cytotoxic effect by disrupting the DNA macromolecule, mainly
through the formation of intrastrand adducts and interstrand
cross-links that could be repaired through the NER pathway
(19). The NER pathway involves
several steps, and approximately 30 proteins participate in this
repair process. Among them, ERCC1 has a crucial role in the
incision step, which is the rate-limiting step of the NER pathway.
ERCC1 forms a heterodimer with XPF, and the ERCC1/XPF complex is
responsible for the incision to cleave the damaged DNA strand.
Therefore, functional ERCC1 is considered to play an important role
in the repair of cisplatin DNA adducts and in cisplatin sensitivity
(11). In fact, previous clinical
studies have found a low ERCC1 expression to be associated with a
better response to platinum-based chemotherapy and a more favorable
survival of cancer patients receiving combination chemotherapy
using platinum-based chemotherapy, for NSCLC, gastric cancer and
colorectal cancer (9–12). The present study also demonstrated
the survival to be significantly higher in patients with ERCC1-low
tumors than in patients with ERCC1-high tumors, among resected
stage III NSCLC patients treated with induction chemoradiotherapy
using carboplatin.
On the other hand, taxanes, including paclitaxel and
docetaxel, are also antitumor agents that are widely used for the
treatment of NSCLC patients (20,21).
Taxanes bind to β-tubulin, one of the major components of
microtubules, and exert their growth-inhibitory effects through the
inhibition of microtubule dynamics, resulting in the growth arrest
of tumor cells at the G2-M phase (22). There are at least six distinct
β-tubulin isotypes (classes I, II, III, IVa, IVb and VI) in humans
(23). Among them, class III
β-tubulin differs from the other tubulin isotypes in its amino acid
sequence and post-translational modifications (24). Experimental studies have revealed
that the interaction of class III β-tubulin with taxanes is
different from that of other isotypes (25–27).
Class III β-tubulin reduces the polymerization rate of
microtubules, thereby overcoming microtubule polymerization by
taxanes (28). In fact, high
levels of class III β-tubulin expression have been reported to be
associated with resistance to taxanes in many human cancer cell
lines, including lung, breast and ovarian cancer (22,29,30).
Furthermore, clinical studies have shown class III β-tubulin
overexpression to be associated with resistance to taxanes and the
survival of cancer patients treated with taxane-containing
chemotherapy (13–15). The present study also demonstrated
intratumoral class III β-tubulin expression to be associated with
the response to induction therapy using taxanes. Consequently, in
the present study, the survival was significantly higher in
patients with class III β-tubulin-low tumors than in patients with
class III β-tubulin-high tumors.
The present study simultaneously evaluated the
intratumoral expression of ERCC1 and class III β-tubulin to
demonstrate that expression of these proteins is independent in
advanced stage NSCLCs. In addition, both ERCC1 and class III
β-tubulin expression was associated with survival of patients
pre-operatively treated with chemotherapy using carboplatin-taxane.
In particular, the 3-year survival rate of patients with ERCC1-low
and class III β-tubulin-low tumors was 100%. Considering these
results, the co-evaluations of the intratumoral expression of ERCC1
and class III β-tubulin are thought to be clinically useful for
identifying patient populations that will effectively respond to
chemotherapy using carboplatin-taxane. Regarding chemotherapy, no
difference was observed in the survival between patients treated
with carboplatin-paclitaxel and patients treated with
carboplatin-docetaxel, as shown in Table III. In addition, no difference was
observed in the patient survival according to the method of
surgical resection in the present study, as shown in Table III.
The present study, nevertheless, has several
limitations. It was a retrospective study using a relatively small
number of patients. Furthermore, the immunohistochemistry was
performed using surgically resected tumor tissues after treatment
with induction chemoradiotherapy. Therefore, the
immunohistochemical evaluations in the present study were affected
by the induction therapy. In particular, cases with a
pathologically complete response (complete cancer cell death) could
not be investigated in the present study. A further prospective
study should be performed to evaluate the intratumoral expression
of these chemotherapy-associated biomarkers using tumor tissues
obtained before the initial administration of
chemoradiotherapy.
The results from the present and previous studies
suggest that platinum-base chemotherapy may be successfully used
for tumors with low ERCC1 expression; taxanes for tumors with low
class III β-tubulin expression; EGFR-specific TKIs for tumors with
EGFR mutations; 5-FU-derived agents for tumors with low TS
expression. In total, the simultaneous evaluation of these four
biomarkers enables the selection of effective chemotherapy for
approximately 80% of NSCLC patients (data not shown). Therefore,
tailor-made chemotherapy based on the evaluation of biomarkers may
improve the clinical outcome of NSCLC patients and decrease
treatment toxicity and costs by avoiding the administration of
ineffective therapy to patients destined not to benefit.
Acknowledgements
This study was supported by
Grants-in-Aid for Scientific Research from the Japanese Society for
Promotion of Science, grant no. 18390379 (C.H.).
References
|
1.
|
Arriagada R, Bergman B, Dunant A, et al:
Cisplatin-based chemotherapy in patients with completely resected
non-small cell lung cancer. N Engl J Med. 350:351–360. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Winton T, Livingston R, Johnson D, et al:
Vinorelbine plus cisplatin vs. observation in resected non-small
cell lung cancer. N Engl J Med. 352:2589–2597. 2005. View Article : Google Scholar
|
|
3.
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Role of adjuvant chemotherapy in patients
with resected non-small cell lung cancer: reappraisal with a
meta-analysis of randomized controlled trials. J Clin Oncol.
22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Huang C, Liu D, Masuya D, Nakashima T,
Kameyama K, Ishikawa S, Ueno M, Haba R and Yokomise H: Clinical
application of biological markers for treatments of resectable
non-small cell lung cancers. Br J Cancer. 92:1231–1239. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Bepler G: Using translational research to
tailor the use of chemotherapy in the treatment of NSCLC. Lung
Cancer. 50:S13–S14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Huang C, Yokomise H, Fukushima M and
Kinoshita M: Tailor-made chemotherapy for non-small cell lung
cancer patients. Future Oncol. 2:289–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mitsudomi T, Kosaka T, Endoh H, Horio Y,
Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T and Yatabe Y:
Mutations of the epidermal growth factor receptor gene predict
prolonged survival after gefitinib treatment in patients with
non-small cell lung cancer with postoperative recurrence. J Clin
Oncol. 23:2513–2520. 2005. View Article : Google Scholar
|
|
8.
|
Nakano J, Huang C, Liu D, Masuya D,
Nakashima T, Yokomise H, Ueno M, Wada H and Fukushima M:
Evaluations of biomarkers associated with 5-FU sensitivity for
non-small cell lung cancer patients postoperatively treated with
UFT. Br J Cancer. 95:607–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lord RV, Brabender J, Gandara D, et al:
Low ERCC1 expression correlates with prolonged survival after
cisplatin plus gemcitabine chemotherapy in non-small cell lung
cancer. Clin Cancer Res. 8:2286–2291. 2002.PubMed/NCBI
|
|
10.
|
Olaussen KA, Dunant A, Fouret P, et al:
DNA repair by ERCC1 in non-small cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Metzger R, Leichman CG, Danenberg KD, et
al: ERCC1 mRNA levels complement thymidylate synthase mRNA levels
in predicting response and survival for gastric cancer patients
receiving combination cisplatin and fluorouracil chemotherapy. J
Clin Oncol. 16:309–316. 1998.
|
|
12.
|
Shirota Y, Stoehlmacher J, Brabender J,
Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg
PV and Lenz HJ: ERCC1 and thymidylate synthase mRNA levels predict
survival for colorectal cancer patients receiving combination
oxaliplatin and fluorouracil chemotherapy. J Clin Oncol.
19:4298–4304. 2001.
|
|
13.
|
Seve P, Mackey J, Isaac S, Tredan O,
Souquet PJ, Perol M, Lai R, Voloch A and Dumontet C: Class III
β-tubulin expression in tumor cells predicts response and outcome
in patients with non-small cell lung cancer receiving paclitaxel.
Mol Cancer Ther. 4:2001–2007. 2005.
|
|
14.
|
Mozzetti S, Ferlini C, Concolino P, et al:
Class III β-tubulin overexpression is a prominent mechanism of
paclitaxel resistance in ovarian cancer patients. Clin Cancer Res.
11:298–305. 2005.
|
|
15.
|
Paradiso A, Mangia A, Chiriatti A, Tommasi
S, Zito A, Latorre A, Schittulli F and Lorusso V: Biomarkers
predictive for clinical efficacy of taxol-based chemotherapy in
advanced breast cancer. Ann Oncol. 16(Suppl 4): 14–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Yokomise H, Gotoh M, Okamoto T, Yamamoto
Y, Ishikawa S, Nakashima T, Masuya D, Liu D and Huang CL: Induction
chemoradiotherapy (carboplatin-taxane and concurrent 50-Gy
radiation) for bulky cN2,N3 non-small cell lung cancer. J Thorac
Cardiovasc Surg. 133:1179–1185. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
18.
|
The Japanese Lung Cancer Society. Rule for
Clinical and Pathological Record of Lung Cancer. 6th edition.
Kanehara; Tokyo: pp. 168–169. 2003
|
|
19.
|
Rosell R, Taron M, Barnadas A, Scagliotti
G, Sarries C and Roig B: Nucleotide excision repair pathways
involved in cisplatin resistance in non-small cell lung cancer.
Cancer Control. 10:297–305. 2003.PubMed/NCBI
|
|
20.
|
Rigas JR: Taxane-platinum combinations in
advanced non-small cell lung cancer: a review. Oncologist. 9:16–23.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non-small cell lung cancer: a Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
22.
|
Burkhart CA, Kavallaris M and Horwitz SB:
The role of β-tubulin isotypes in resistance to antimitotic drugs.
Biochim Biophys Acta. 1471:1–9. 2001.
|
|
23.
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Katsetos CD, Legido A, Perentes E and Mork
SJ: Class III β-tubulin isotype: a key cytoskeletal protein at the
crossroads of developmental neurobiology and tumor neuropathology.
J Child Neurol. 18:851–866. 2003.
|
|
25.
|
Lu Q and Luduena RF: Removal of β-III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993.
|
|
26.
|
Kamath K, Wilson L, Cabral F and Jordan
MA: β III-tubulin induces paclitaxel resistance in association with
reduced effects on microtubule dynamic instability. J Biol Chem.
280:12902–12907. 2005.
|
|
27.
|
Derry WB, Wilson L, Khan IA, Luduena RF
and Jordan MA: Taxol differentially modulates the dynamics of
microtubules assembled from unfractionated and purified β-tubulin
isotypes. Biochemistry. 36:3554–3562. 1997.PubMed/NCBI
|
|
28.
|
Hari M, Yang H, Zeng C, Canizales M and
Cabral F: Expression of class III β-tubulin reduces microtubule
assembly and confers resistance to paclitaxel. Cell Motil
Cytoskeleton. 56:45–56. 2003.
|
|
29.
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
β-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.PubMed/NCBI
|
|
30.
|
Ranganathan S, Benetatos CA, Colarusso PJ,
Dexter DW and Hudes GR: Altered β-tubulin isotype expression in
paclitaxel-resistant human prostate carcinoma cells. Br J Cancer.
77:562–566. 1998.
|