Introduction
Vascular cell adhesion molecule-1 (VCAM-1, CD106) is
a 90-kDa glycoprotein containing six or seven immunoglobulin
domains. VCAM-1 is a member of the immunoglobulin superfamily of
adhesion molecules and is constitutively expressed in many
different cell types, including activated endothelial cells, bone
marrow stromal cells, spleen stromal cells, thymic epithelial
cells, peripheral lymph nodes (LNs) and mesenteric LN high
endothelial venules (1,2).
It is possible that VCAM-1 is involved in mediating
tumor cell adhesion to vascular endothelial cells and promoting the
metastatic process (3).
VCAM-1-dependent adhesion is known to be responsible for lung
metastasis (4). VCAM-1 expression
has recently been demonstrated in gastric tumor cells, as well as
in tumor-associated endothelial cells, and its expression level is
related to invasiveness and advanced stage in gastric tumors
(5). However, the mechanism behind
the tumor-promoting function of VCAM-1 produced by carcinoma cells
is poorly understood. Integrin α4β1, a partner molecule of VCAM-1,
is strongly expressed in the endothelium (6–8), and
it is therefore conceivable that endothelial integrin α4β1 and
tumor cell-produced VCAM-1 may play roles in the adhesion of
carcinoma cells to the endothelium during the malignant progression
of tumors.
Other reports have suggested a mechanism of tumor
immune evasion, whereby tumor expression of VCAM-1 may promote
T-cell migration away from tumors, resulting in fewer T cells
accumulating in the tumor microenvironment (9,10).
This decreased accumulation of T cells around tumor cells
contributes to the ability of VCAM-1-expressing tumor cells to
escape immune attack. VCAM-1 is therefore thought to play a key
role in the process of malignant progression.
Soluble forms of VCAM-1 (sVCAM-1) have previously
been identified (11). It is
likely that the soluble products are derived from a range of
sources, including enzymatic cleavage from endothelial, leukocyte
and tumor cell surfaces, possibly influenced by the intra-tumoral
cytokine environment. Elevated serum levels of sVCAM-1 have
recently been shown to be associated with disease stage and
progression in patients with gastric (8), colorectal (12), breast (13), prostate (14) and bladder (15) cancers, and leukemia (16). In contrast, sVCAM-1 acts as a
chemoattractant (17) and
competitive inhibitor through interaction with α4 integrins in the
local microenvironment (18).
Although many studies have suggested the importance
of VCAM-1, the kinetics of the two forms of the molecule have not
been investigated. The aim of this study was to evaluate the tissue
concentrations of sVCAM-1 in colorectal cancer and to clarify its
relation to disease progression, with special reference to VCAM-1
expression in the tumor, especially in the cancer cell cytoplasm or
cancer stroma.
Patients and methods
A total of 150 patients undergoing surgery for
colorectal cancer from 1996 to 2002 at Mie University Hospital,
Japan, were enrolled in this study. Ninety-four of these patients
were male. The mean age was 64.9 years (range 37–86 years). No
perioperative mortalities were observed among these patients. No
patients had received chemotherapy or radiation therapy prior to
surgery.
The location of the tumors and distant metastases
were determined by barium enema, colonoscopy, computed tomography
(CT) and magnetic resonance image. The primary lesion was located
in the rectum in 66 patients, the sigmoid colon in 45 patients, the
ascending colon in 27 patients, the transverse colon in 7 patients,
and the descending colon in 5 patients. Twenty-eight patients were
diagnosed with synchronous liver metastasis and 3 patients with
both liver metastasis and peritoneal dissemination. All patients
underwent tumor resection and 15 patients underwent simultaneous
partial hepatectomy for liver metastasis. Other patients with stage
4 disease had synchronous lung metastasis or peritoneal
dissemination. No perioperative mortalities were observed among
these patients. Thirteen patients had poorly differentiated
adenocarcinomas, while 137 patients had well- or moderately
differentiated adenocarcinomas. All patients were classified
according to the Union Internationale Contre le Cancer (UICC)
classification system, based on resected specimens. There were 37
patients with UICC stage I (T1-2N0M0), 35 patients with UICC stage
II (T3-4N0M0), 39 patients with UICC stage III (TXN1-2M0) and 39
patients with UICC stage IV (TXNXM1) disease. Stage III and IV
patients received fluorouracil-based chemotherapy, whereas stage I
and II patients received no postoperative adjuvant therapy.
Patients were observed at 3-month intervals for 24 months after
completion of surgery, followed by every 6 months for 3 years, and
then yearly. A history was taken and a physical examination was
performed at each visit, and chest X-ray, colonoscopy and CT were
performed once a year. The median follow-up time was 60.6 months
(mean 51.6±28.4 months). Among the 150 patients studied, 47 died
due to primary or recurrent disease. The clinicopathologic
parameters studied for their possible prognostic value were T
classification, vessel involvement, lymphatic invasion, lymph node
metastases and distant metastasis.
Fresh, operative specimens of the primary colorectal
cancer and adjacent normal mucosa, taken ∼10 cm from the neoplasm,
were immediately frozen in liquid nitrogen after resection and
stored at −80°C until assay and DNA extraction.
Tissue concentrations of sVCAM-1
Surgical specimens were stored in liquid nitrogen
until use. Three hundred specimens (150 cancer and 150 normal
colorectal mucosa) were prepared for analysis of tissue sVCAM-1
expression. These samples were thawed, quickly weighed, and placed
in 5 ml of phosphate-buffered saline (PBS). The tissues were
homogenized on ice in 1 ml extraction buffer per 100 mg wet weight
of tissue using a motor-driven Teflon pestle for 5 min. The tissue
extract obtained after centrifugation at 12,000 rpm for 15 min at
4°C was placed in a 200-μl vial and stored at −80°C. The
concentrations of sVCAM-1 and protein in these tissues were
measured in the supernatant, using a commercially available
enzyme-linked immunosorbent assay (ELISA) kit (BioSource
International, Camarillo, CA, USA). Protein concentrations were
measured using a BCA Protein Assay Kit (Pierce Chemical, Rockford,
IL, USA). Tissue concentrations were expressed as ng/mg protein.
This ELISA was specific for the measurement of sVCAM-1 and did not
detect membrane-bound VCAM-1. Informed consent was obtained from
each subject. The protocol was approved by the review board of our
institute.
Immunohistochemical analysis
Sixty patients were randomly selected for
immunohistochemical analysis. Thirty-eight of them were male. The
mean age was 63.6 years (range 37–83 years). There were 14 patients
with UICC stage I, 11 with UICC stage II, 19 with UICC stage III
and 16 patients with UICC stage IV disease. The median follow-up
time was 61.9 months (mean 53.0±28.3 months). Nineteen of the 60
patients died due to primary or recurrent disease.
Paraffin-embedded specimens were cut into 5-μm sections and
attached to glass slides with melted wax at 65°C. The sections were
then dewaxed, hydrated and incubated in 3% hydrogen peroxide for 30
min. The sections were washed in cold tap water, heated in a
microwave oven, and washed three times in PBS (pH 7.4) for 5 min.
After washing with PBS, sections were incubated with primary
antibodies overnight at 4°C. Non-specific binding was blocked by
incubation with blocking solution for 1 h at room temperature. The
sections were incubated with a mouse monoclonal antibody raised
against VCAM-1 (Santa Cruz, CA, USA) at a dilution of 1:100
overnight at 4°C in a moist chamber. The sections were washed and
incubated for 30 min at room temperature with biotinylated
anti-rabbit IgG diluted in PBS. The sections were then incubated
with avidin/biotin complex reagent for 3 h at room temperature. The
color was developed for 90 sec using a Vector DAB substrate kit and
counterstained with Meyer’s hematoxylin (Vector Laboratories,
Burlingame, CA, USA). The specificity of the immunoreaction was
verified by staining known positive and negative control tissue
sections, and by negative staining when the primary antibody was
replaced by normal mouse serum. The percentage of positively
stained tumor cells was evaluated for each tumor section after
counting 1,000 cells per high power field. VCAM-1 expression was
classified as positive when >30% of carcinoma and/or stromal
cells were stained. The slides were evaluated three times by three
independent investigators who were blinded to the nature of the
specimens and antibodies used. This immunohistochemical analysis
was specific for the measurement of membrane-bound VCAM-1 and did
not detect sVCAM-1.
Statistical analysis
Statistical analysis was performed using StatView
software (version 5; Abacus Concepts, Inc., Berkeley, CA, USA).
Results are expressed as mean ± standard deviation (SD).
Kruskal-Wallis analysis of variance (ANOVA) and the Mann-Whitney U
test were used to evaluate differences between multiple groups and
unpaired observations, respectively. The Spearman rank correlations
test was used to determine statistical correlations. Analyses of
nonparametric operating characteristics (ROC) were performed to
calculate the cut-off values according to the most accurate value
obtained using Medcalc 7.2 for Windows (Mariakerke, Belgium).
Actuarial survival curves were obtained using the Kaplan-Meier
method, and comparisons were made using log-rank tests. The Cox
proportional hazards regression model was used for multivariate
analysis, after the relevant prognostic variables had been defined
by univariate analysis. Contingency tables were analyzed by
χ2 tests with Yates’ correction. Two-sided p-values
<0.05 were considered to be statistically significant.
Results
Tissue levels of sVCAM-1 in cancer and
normal mucosa, and their relation to clinicopathological
parameters
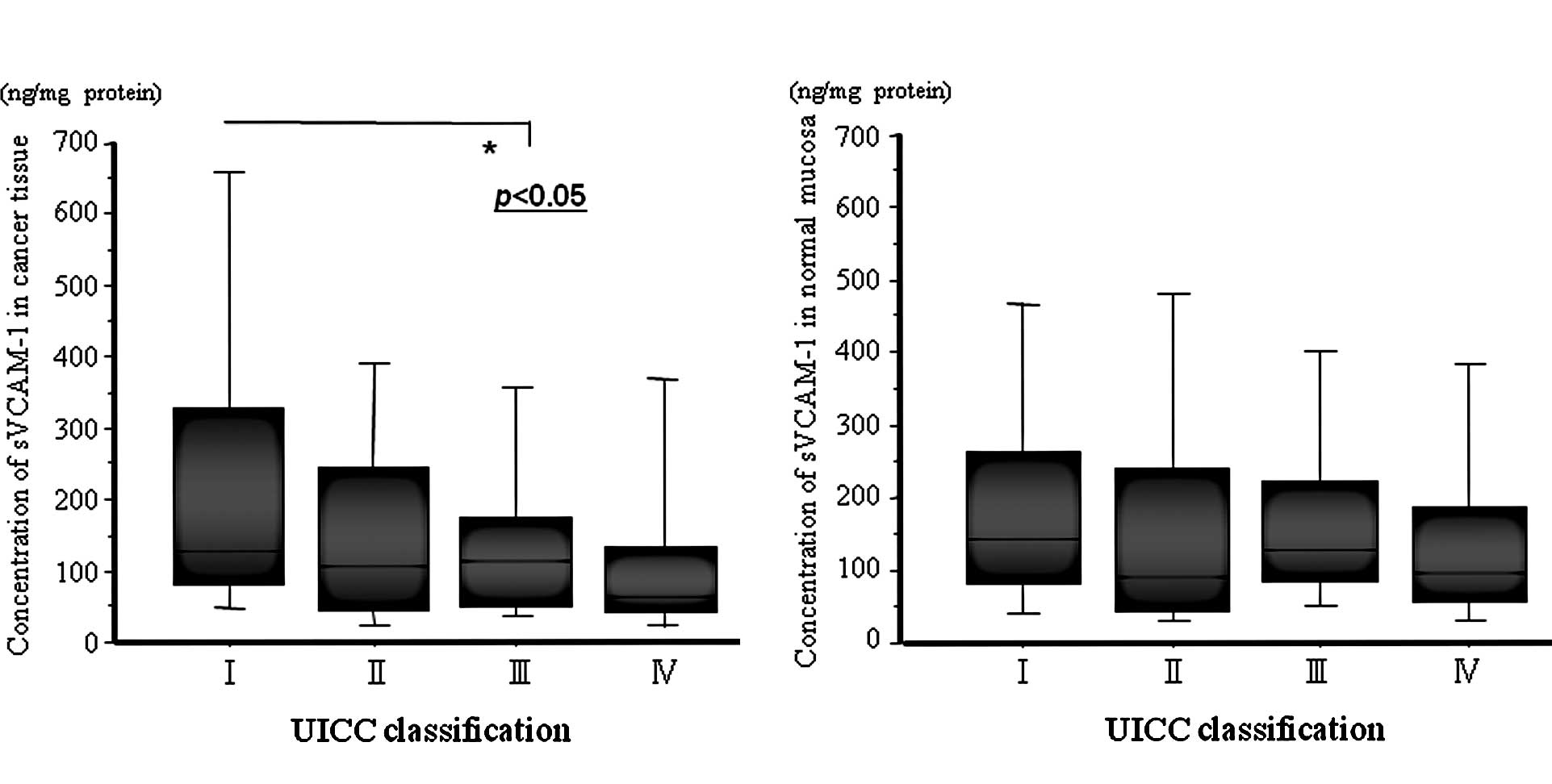
The concentrations of sVCAM-1 in cancer tissues
ranged from 8.8 to 2,611.8 ng/mg protein, with a mean of
201.4±326.1 ng/mg protein. The concentrations of sVCAM-1 in normal
mucosa ranged from 18.2 to 3,061.8 ng/mg protein, with a mean of
204.8±325.0 ng/mg protein. The mean sVCAM-1 level in cancer tissue
from stage I patients was significantly higher than that from stage
III patients (Fig. 1). Table I shows the relationship between
tissue concentrations of sVCAM-1 and the clinicopathological
findings. The decrease in the concentration of sVCAM-1 in cancer
tissues was associated with factors reflecting disease progression,
such as T classification, vessel involvement, lymphatic vessel
involvement, lymph node metastasis and the presence of distant
metastasis. In addition, the cancer tissue level of sVCAM-1
decreased significantly in accordance with the progression of UICC
classification. In contrast, there was no significant relationship
between tissue levels of sVCAM-1 in normal mucosa and disease
progression factors, except for vessel involvement.
 | Table I.Relationships between sVCAM-1 level in
cancer tissue and normal mucosa, and clinicopathological factors in
150 colorectal patients. |
Table I.
Relationships between sVCAM-1 level in
cancer tissue and normal mucosa, and clinicopathological factors in
150 colorectal patients.
| Variable | No. | sVCAM-1 level in
cancer tissue (ng/mg protein) | p-value | sVCAM-1 level in
normal mucosa (ng/mg protein) | p-value |
|---|
| Gender | | | | | |
| Male | 94 | 211.020±351.170 | 0.3612a | 208.091±345.779 | 0.8521a |
| Female | 56 | 185.181±281.170 | | 199.249±289.710 | |
| Age (years) | | | | | |
| <66 | 69 | 183.910±258.153 | 0.7216a | 202.385±266.340 | 0.4317a |
| ≥66 | 81 | 216.150±375.327 | | 206.839±369.356 | |
| T classification | | | | | |
| 1 | 14 | 458.792±669.456 | 0.0086b | 204.790±208.870 | 0.1769b |
| 2 | 31 | 191.458±234.389 | | 181.049±162.751 | |
| 3 | 92 | 165.976±244.823 | | 219.171±392.835 | |
| 4 | 13 | 198.229±394.276 | | 121.283±125.353 | |
| Vessel
involvement | | | | | |
| + | 79 | 130.717±238.577 | <0.0001a | 148.024±347.232 | <0.0001a |
| − | 71 | 279.991±388.540 | | 267.953±287.751 | |
| Lymphatic vessel
involvement | | | | | |
| + | 134 | 178.877±263.666 | 0.0070a | 199.585±336.079 | 0.0536a |
| − | 16 | 389.782±630.259 | | 248.385±213.239 | |
| Lymph node
metastasis | | | | | |
| N0 | 81 | 256.608±395.142 | 0.0091a | 226.239±369.880 | 0.4819a |
| N1 | 69 | 136.532±203.358 | | 179.612±263.251 | |
| Distant
metastasis | | | | | |
| M0 | 111 | 213.457±327.157 | 0.0130a | 200.187±246.763 | 0.2361a |
| M1 | 39 | 166.983±324.626 | |
217.893±487.506 | |
| UICC stage
classification | | | | | |
| 1 | 37 |
299.903±467.795 | 0.0187b |
211.157±186.171 | 0.2264b |
| 2 | 35 |
202.431±286.849 | |
176.424±203.953 | |
| 3 | 39 |
141.338±127.147 | |
211.104±324.736 | |
| 4 | 39 |
166.983±324.626 | |
217.893±487.506 | |
Relationship between the sVCAM-1
expression level in cancer tissue and tumor size
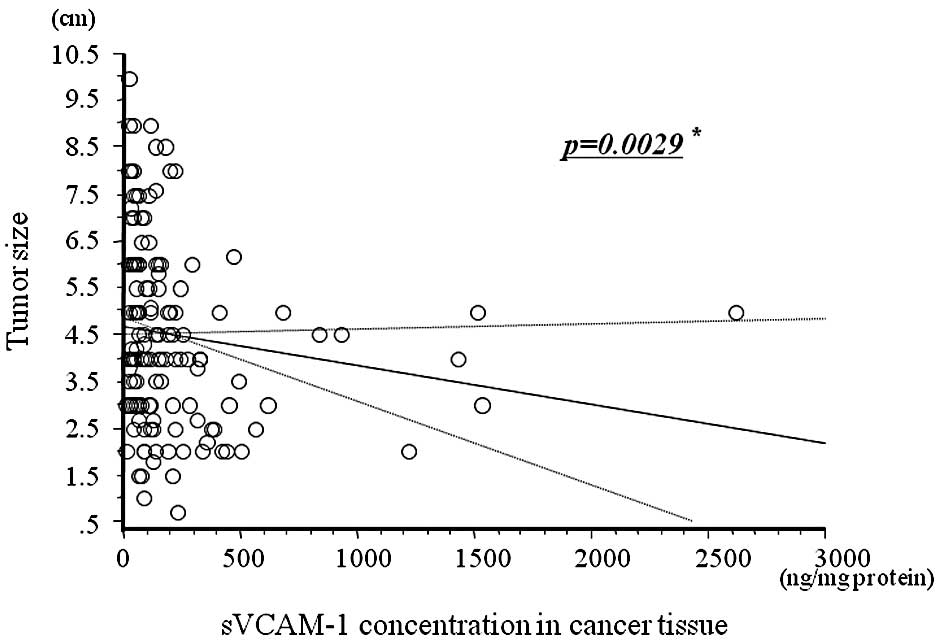
Fig. 2 shows the
relationship between cancer tissue concentrations of sVCAM-1 and
tumor size. There was a negative correlation between sVCAM-1 levels
in cancer tissues and tumor size (r=−0.145, p=0.0029).
Relationship between sVCAM-1 expression
in cancer tissue and the 5-year patient survival
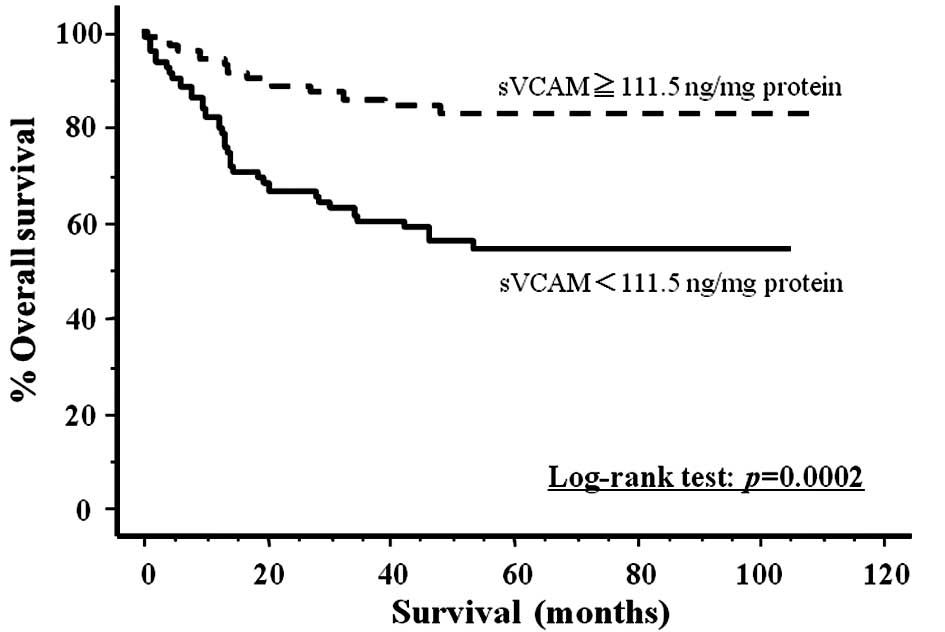
Cut-off points for the tissue concentrations of
sVCAM-1 in cancer and normal mucosa were determined using the best
pair of values for high sensitivity and high specificity (cancer
tissue 111.5 ng/ml protein, sensitivity 76.6%, specificity 56.3%;
normal mucosa 227.3 ng/ml protein, sensitivity 85.1%, specificity
31.1%). Fig. 3 shows the actual
survival curves for all colorectal carcinoma patients, subdivided
by their tissue levels of sVCAM-1. The patients with lower levels
of sVCAM-1 expression had significantly poorer prognoses than those
with levels above the cut-off value (log-rank test, p=0.0002).
Based on Cox univariate proportional hazards analysis, advanced T
classification (T3,T4), vessel involvement, lymph node metastasis,
distant metastasis and decreased tissue levels of sVCAM-1 were
associated with poor prognosis (Table
II). Upon multivariate analysis, distant metastasis and
decreased tissue sVCAM-1 levels were independent risk factors
predicting a poor prognosis (Table
III).
 | Table II.Univariate analysis for predictors of
survival in 150 colorectal cancer patients. |
Table II.
Univariate analysis for predictors of
survival in 150 colorectal cancer patients.
| Variables | HR | 95% CI | p-value |
|---|
| Gender (male vs.
female) | 0.682 | 0.384–1.212 | 0.1920 |
| Age (<66 vs.
≥66) | 0.855 | 0.479–1.525 | 0.5958 |
| T classification
(T1, 2 vs. T3, 4) | 0.174 | 0.063–0.486 | 0.0008a |
| Lymphatic vessel
involvement (yes vs. no) | 3.067 | 0.743–12.66 | 0.1213 |
| Vessel involvement
(yes vs. no)+ | 2.398 | 1.282–4.484 | 0.0062a |
| Node involvement
(yes vs. no) | 2.874 | 1.567–5.263 | 0.0006a |
| Distant metastasis
(yes or no) | 15.150 | 7.874–28.57 | <0.0001a |
| Level of sVCAM-1 in
cancer tissue (≥111.5 vs. <111.5) | 0.309 | 0.160–0.597 | 0.0005a |
| Level of sVCAM-1 in
normal mucosa (≥227.3 vs. <227.3) | 0.491 | 0.229–1.050 | 0.0666 |
 | Table III.Multivariate analysis for predictors
of survival in 150 colorectal cancer patients. |
Table III.
Multivariate analysis for predictors
of survival in 150 colorectal cancer patients.
| Vaiables | HR | 95% CI | p-value |
|---|
| T classification
(T1, 2 vs. T3, 4) | 0.396 | 0.134–1.171 | 0.0940 |
| Vessel involvement
(yes vs. no) | 0.583 | 0.263–1.294 | 0.1846 |
| Node involvement
(yes vs. no) | 0.931 | 0.472–1.838 | 0.8378 |
| Distant metastasis
(yes or no) | 12.820 | 5.917–27.78 | <0.0001a |
| Cancer tissue
sVCAM-1 level (≥111.5 vs. <111.5) | 0.352 | 0.159–0.780 | 0.0101a |
Immunohistochemical staining of VCAM-1 in
colorectal cancer tissues and its relation to sVCAM-1 levels
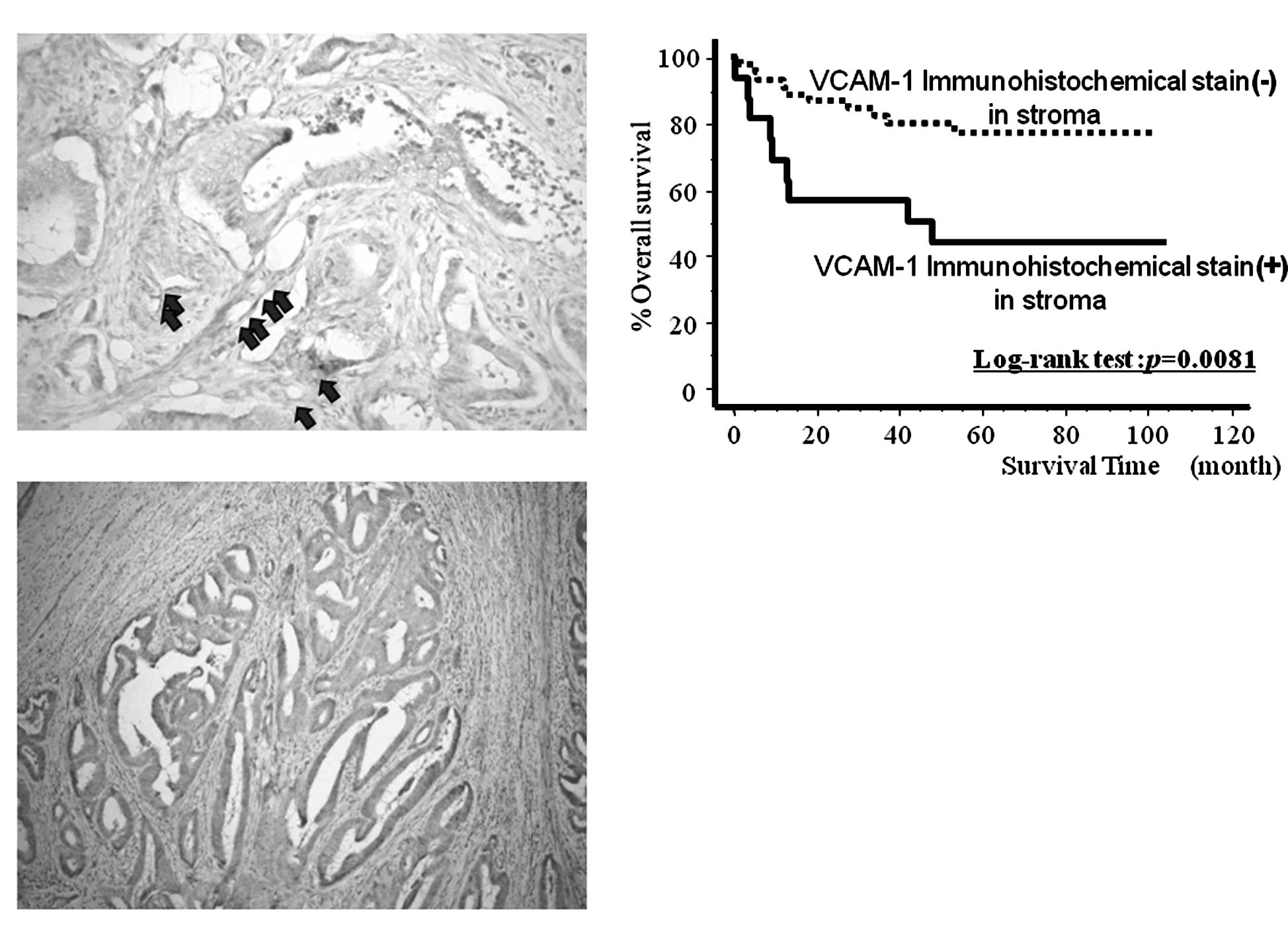
Fig. 4A and B shows
the results of the immunohistochemical staining of VCAM-1 in the
cancer tissues. VCAM-1 was localized in the cancer stroma (Fig. 4A) and/or the cancer cell cytoplasm
(Fig. 4B), especially in vascular
endothelial cells. VCAM-1 immunoreactivity in stromal cells was
positive in 16 of 60 cases (26.7%), whereas VCAM-1 immunostaining
in cancer cells was observed in only 9 (15%) cases. Fig. 4C shows the survival curves for the
60 randomly selected patients, subdivided according to their cancer
stroma VCAM-1 expression levels determined by immunohistochemical
analysis. The patients with intense VCAM-1 expression in the stroma
had significantly poorer prognoses than those without expression of
VCAM-1 (log-rank test, p=0.0081). By contrast, there was no
correlation between VCAM-1 expression in cancer cell cytoplasm and
prognosis (log-rank test, p=0.2246, data not shown). In addition,
the mean concentration of sVCAM-1 in the cancer tissue with intense
stromal VCAM-1 immunoreactivity was significantly lower than that
in the cancer tissue without VCAM-1 expression (108.7±67.2 vs.
219.9±212.1; p=0.0251), and there was no significant relationship
between cancer tissue levels of sVCAM-1 and VCAM-1 immunoreactivity
in the cancer cell cytoplasm (Table
IV).
 | Table IV.Relationship between VCAM-1
immunoreactivity and the level of sVCAM-1 in tissue in cancer cell
cytoplasm and stroma. |
Table IV.
Relationship between VCAM-1
immunoreactivity and the level of sVCAM-1 in tissue in cancer cell
cytoplasm and stroma.
| VCAM-1
immunoreactivity | No. | sVCAM-1 level in
cancer tissue | p-value |
|---|
| Cancer cell
cytoplasm | + | 9 |
136.448±111.072 | 0.2421 |
| – | 51 |
199.839±200.978 | |
| Cancer stroma | + | 16 |
108.774±67.2830 | 0.0251a |
| – | 44 |
219.998±212.182 | |
Discussion
In the present study, we determined a strong
correlation between decreased sVCAM-1 concentrations in colorectal
cancer tissues and disease progression.
Cell adhesion molecules are thought to play an
important role in the process of metastasis, since each step
requires cell-cell and cell-extracellular matrix interactions
(19–21). VCAM-1, one of the most important
adhesion molecules, exists as a membrane-bound and a soluble form,
and plays a crucial role in this process (3,4).
Other reports have suggested a mechanism of tumor immune evasion
whereby tumor expression of VCAM-1 might promote T-cell migration
away from tumors, therefore allowing them to escape immune attack
(9,10). VCAM-1 is thus considered to play a
key role in the process of malignant progression. A previous study
using Northern blotting revealed that VCAM-1 mRNA was overexpressed
in colorectal cancer, in comparison to normal controls (22). It has also been demonstrated that
VCAM-1 expression in gastric cancers is highly related to
invasiveness and advanced stage (5).
In contrast, sVCAM-1 has been found in the culture
supernatants of cytokine-treated endothelial cells in human serum
and in the synovial fluid of patients with rheumatoid arthritis
(23,24). The origin of sVCAM-1 is not
completely understood, but it is possible that it is released from
the cell surface by proteolytic cleavage (25). sVCAM-1 could act as a
chemoattractant (17) and
competitive inhibitor through interaction with α4 integrins in the
local microenvironment (18).
Although it is possible that sVCAM-1 itself may inhibit cancer
growth, there have been no reports on the function of sVCAM-1
itself in the colorectal cancer microenvironment.
In the present study, reduced levels of sVCAM-1 in
cancer tissues were significantly correlated with well-known
prognostic factors such as T classification, lymphatic duct and
vessel involvement, nodal metastasis and distant metastasis. By
contrast, there was no significant relationship between the levels
of sVCAM-1 in normal mucosa and clinicopathological factors, except
for vascular involvement. Patients with decreased cancer tissue
levels of sVCAM-1 had significantly poorer prognoses than those
with higher levels. Furthermore, a decreased cancer tissue level of
sVCAM-1 and distant metastasis were found to be independent
prognostic factors for colorectal carcinomas. These data strongly
suggest that decreased cancer tissue levels of sVCAM-1 are
intimately involved in the process of tumor progression. The
negative correlation between sVCAM-1 expression in cancer tissues
and tumor size may also be explained by the promotion of local
cancer growth by sVCAM-1 itself.
Notably, the present study showed that decreased
sVCAM-1 cancer tissue concentrations were associated with positive
expression of VCAM-1 in the cancer stroma, but not in the cancer
cell cytoplasm, suggesting that concentrations of sVCAM-1 in cancer
tissue mainly reflect the shedding of membranous VCAM-1 from tumor
stromal cells.
The shedding of VCAM-1 from cytokine-stimulated
endothelial cell surfaces is regulated by tissue inhibitor of
metalloprotease-3, which is mediated by tumor necrosis
factor-α-converting enzyme (ADAM17) (26,27).
Blanchot-Jossic et al found that ADAM17 was present in an
active form and was up-regulated at the mRNA level in primary colon
carcinomas compared with paired normal colonic mucosa. In addition,
ADAM17 was expressed by stroma cells, i.e. some immune cells,
vascular and non-vascular myocytes, and by some endothelial cells,
but not by cancer cells (28). The
association between the expression of VCAM-1 in the cancer stroma
and the concentration of sVCAM-1 found in our study might suggest
that shedding, resulting from proteolytic cleavage of the cancer
membrane-bound molecule, occurs in the cancer microenvironment. It
is therefore conceivable that shedding of VCAM-1 into the cancer
microenvironment as a result of proteolytic cleavage could
influence cancer progression and prognosis both by suppressing the
VCAM-1 pathway and by the competitive activity of sVCAM-1
itself.
In conclusion, we demonstrated that decreased tissue
concentrations of sVCAM-1 in colorectal cancer patients were
significantly correlated with clinicopathological parameters and
prognosis. Although the regulatory mechanisms of the membranous and
soluble forms of VCAM-1 are currently unknown, the present results
suggest that the induction of proteolytic cleavage could provide a
potential immunological basis for possible future cancer treatment
strategies.
Acknowledgements
The authors would like to thank Hiromi
Ueeda and Yuka Kato for their excellent technical assistance.
References
|
1.
|
Norris P, Poston RN, Thomas DS, Thornhill
M, Hawk J and Haskard DO: The expression of ELAM-1, ICAM-1 and
VCAM-1 in experimental cutaneous inflammation: a comparison of UVb
erythema and delayed hypersensitivity. J Invest Dermatol.
96:763–970. 1991.PubMed/NCBI
|
|
2.
|
Yoo NC, Chung HC, Chung HC, et al:
Synchronous elevation of soluble intracellular adhesion molecule-1
(ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) correlates
with gastric cancer progression. Yonsei Med J. 39:27–36. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Shin J, Kim J, Ryu B, Chi SG and Park H:
Caveolin-1 associated with VCAM-1 dependent adhesion of gastric
cancer cells to endothelial cells. Cell Physiol Biochem.
17:211–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Higashiyama A, Watanabe H, Okumura K and
Yagita H: Involvement of tumor necrosis factor alpha and very late
activation antigen 4/vascular cell adhesion molecule 1 interaction
in surgical-stress enhanced experimental metastasis. Cancer Immunol
Immunother. 42:231–236. 1996. View Article : Google Scholar
|
|
5.
|
Ding YB, Chen GY, Xia JG, Zang XW, Yang HY
and Yang L: Association of VCAM-1 overexpression with oncogenesis,
tumor angiogenesis and metastasis of gastric carcinoma. World J
Gastroenterol. 9:1409–1414. 2003.PubMed/NCBI
|
|
6.
|
Velikova G, Banks RE, Gearing A, et al:
Circulating soluble adhesion molecules E-cadherin, E-selectin,
intracellular adhesion molecule-1 (ICAM-1) and vascular cell
adhesion molecule-1 (VCAM-1) in patients with gastric cancer. Br J
Cancer. 76:1398–1404. 1997. View Article : Google Scholar
|
|
7.
|
Gulubova MV: Expression of cell adhesion
molecules, their ligands and tumor necrosis factor alpha in the
liver of patients with metastatic gastrointestinal carcinomas.
Histochem J. 34:672002. View Article : Google Scholar
|
|
8.
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
et al: Clinical significance of serum levels of E-selectin,
intracellular adhesion molecule-1 and vascular cell adhesion
molecule-1 in gastric cancer patients. Am J Gastroenterol.
98:478–485. 2003. View Article : Google Scholar
|
|
9.
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Wu TC: The role of vascular cell adhesion
molecule-1 in tumor immune evasion. Cancer Res. 67:6003–6006. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gearing AJ, Hemingway I, Pigott R, Hughes
J, Rees AJ and Cashman SJ: Soluble forms of vascular adhesion
molecules, E-selectin, ICAM-1 and VCAM-1: pathological
significance. Ann NY Acad Sci. 667:324–331. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Alexiou D, Karayiannakis AJ, Syrigos KN,
Zbar A, Kremmyda A, Bramis I and Tsigris C: Serum levels of
E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients:
correlations with clinicopathological features, patient survival
and tumor surgery. Eur J Cancer. 37:2392–2397. 2001. View Article : Google Scholar
|
|
13.
|
O’Hanlon DM, Fitzsimons H, Lynch J, Tormey
S, Malone C and Given HF: Soluble adhesion molecules (E-selectin,
ICAM-1 and VCAM-1) in breast carcinoma. Eur J Cancer. 38:2252–2257.
2002.
|
|
14.
|
De Cicco C, Ravasi L, Zorzino L, et al:
Circulating levels of VCAM and MMP-2 may help identify patients
with more aggressive prostate cancer. Curr Cancer Drug Targets.
8:199–206. 2008.PubMed/NCBI
|
|
15.
|
Coskun U, Sancak B, Sen I, Bukan N, Tufan
MA, Gülbahar O and Sozen S: Serum P-selectin, soluble vascular cell
adhesion molecule-I (s-VCAM-I) and soluble intercellular adhesion
molecule-I (s-ICAM-I) levels in bladder carcinoma patients with
different stages. Int Immunopharmacol. 6:672–677. 2006. View Article : Google Scholar
|
|
16.
|
Christiansen I, Sundstrom C and Totterman
TH: Elevated serum levels of soluble vascular cell adhesion
molecule-1 (sVCAM-1) closely reflect tumour burden in chronic
B-lymphocytic leukemia. Br J Haematol. 103:1129–1137. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Shimizu Y, Newman W, Gopal TV, et al: Four
molecular pathways of T cell adhesion to endothelial cells: roles
of LFA-1, VCAM-1 and ELAM-1 and changes in pathway hierarchy under
different activation conditions. J Cell Biol. 113:1203–1212. 1991.
View Article : Google Scholar
|
|
18.
|
Makarem R, Newham P, Askari JA, et al:
Competitive binding of vascular cell adhesion molecule-1 and the
HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J
Biol Chem. 269:4005–4011. 1994.PubMed/NCBI
|
|
19.
|
Albelda SM: Role of integrins and other
cell adhesion molecules in tumor progression and metastasis. Lab
Invest. 68:4–17. 1993.PubMed/NCBI
|
|
20.
|
Ponta H, Sleeman J and Herrlich P: Tumor
metastasis formation: cell-surface proteins confer
metastasis-promoting or -suppressing properties. Biochem Biophys
Acta. 1198:1–10. 1994.PubMed/NCBI
|
|
21.
|
Ohene-Abuakwa Y and Pignatelli M: Adhesion
molecules as diagnostic tools in tumor pathology. Int J Surg
Pathol. 8:191–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Maurer CA, Friess H, Kretschmann B, et al:
Overexpression of ICAM-1, VCAM-1 and ELAM-1 might influence tumor
progression in colorectal cancer. Int J Cancer. 79:76–81. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Lloyd AR, Oppenheim JJ, Kelvin DJ and Taub
DD: Chemokines regulate T cell adherence to recombinant adhesion
molecules and extracellular matrix proteins. J Immunol.
156:932–938. 1996.PubMed/NCBI
|
|
24.
|
Kitani A, Nakashima N, Matsuda T, Xu B, Yu
S, Nakamura T and Matsuyama T: T cells bound by vascular cell
adhesion molecule-1/CD106 in synovial fluid in rheumatoid
arthritis: inhibitory role of soluble vascular cell adhesion
molecule-1 in T cell activation. J Immunol. 156:2300–2308.
1996.PubMed/NCBI
|
|
25.
|
Masumoto A and Hemler ME: Multiple
activation states of VLA-4. Mechanistic differences between
adhesion to CS1/fibronectin and to vascular cell adhesion
molecule-1. J Biol Chem. 268:228–234. 1993.PubMed/NCBI
|
|
26.
|
Garton KJ, Gough PJ, Philalay J, et al:
Stimulated shedding of vascular cell adhesion molecule 1 (VCAM-1)
is mediated by tumor necrosis factor-alpha-converting enzyme (ADAM
17). J Biol Chem. 278:37459–37464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Singh RJ, Mason JC, Lidington EA, et al:
Cytokine stimulated vascular cell adhesion molecule-1 (VCAM-1)
ectodomain release is regulated by TIMP-3. Cardiovasc Res.
67:39–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Blanchot-Jossic F, Jarry A, Masson D, et
al: Up-regulated expression of ADAM17 in human colon carcinoma:
co-expression with EGFR in neoplastic and endothelial cells. J
Pathol. 207:156–163. 2005. View Article : Google Scholar : PubMed/NCBI
|