Introduction
Photodynamic therapy (PDT) is a well-known procedure
in the field of clinical medicine for the treatment of cancer,
although there has also been research into its application for
non-malignant disorders, such as psoriasis or actinic keratosis and
for cases of choroidal neovascularization.
The treatment is currently under active
investigation for palliative or curative applications. PDT is an
evolving cancer treatment that depends on three known and variable
components: photosensitizer (PS), light and oxygen (1). PDT relies on selective accumulation
of a PS in tumor tissue, which on illumination with light of
appropriate wavelengths, generates reactive oxygen species,
particularly singlet oxygen, and destroys tumor tissue (2). In recent years, treatment of cancer
by PDT has gained considerable interest due to its intrinsic dual
selectivity. The PS localizes in the malignant tissue, and the
light is spatially focused on the lesion (3). Phthalocyanines (PCs) are PSs of the
dye family. PCs and their derivatives have been intensively
investigated as the second generation PSs for PDT (4). Most of the activity of clinical PSs
in the dye family comes from phthalocyanines and their relatives,
the naphthalocyanines. These structures are active in a range of
650–850 nm. Most dyes are hydrophobic requiring delivery agents,
such as a liposomal preparation, for clinical use. Linking dyes to
a variety of metals improves efficacy. Aluminum, zinc and silicon
appear to offer the best PDT activity (5).
The development of new compounds as potential PSs in
PDT is of great scientific interest. The advantage of this therapy
over available therapies is the high selectivity of tumor
destruction and the minimum damage to normal tissues. PDT kills
tumor cells by destruction of vascular endothelium and/or direct
tumor cell kill. This treatment destroys cells by either apoptotic
or necrotic cell death (6).
In this study, a comparative PDT analysis was
carried out administrating two phthalocyanine derivatives,
ZnPc(OCH3)4 and
ZnPc(CF3)4, in a mouse tumor model.
Materials and methods
Photosensitizers
Zinc (II) 2,9,16,23-tetrakis
(4-trifluoromethylbenzyloxy) phthalocyanine
[ZnPc(CF3)4] and zinc (II) 2,9,16,23-tetrakis
(methoxy) phthalocyanine [ZnPc(OCH3)4] were
synthesized as described by Yslas et al (7).
Animal model
Female Balb/c mice were obtained from the Fundación
Balseiro, Buenos Aires, Argentina. At the start of the experiments,
the mice were 7 to 8 weeks old, with an average body weight of
20–25 g. Three mice were housed per cage in a room with constant
temperature (24–26°C) and humidity (30–50%). The dark/light cycles
were 12/12 h. The animals were given free access to regular chow
pellets and water. All animals used in this study were handled in
strict adherence to ethical care according to the guidelines
established by the ANMAT Disposition N. 6344/96, pp1–7 for Human
Care of Experimental Animals. The mice were closely monitored daily
for signs of pain and distress by evaluating appetite, hydration
status and activity level.
Cells and tumor model
For the generation of the experimental tumors, mouse
mammary adenocarcinoma cell line LM2 (obtained from Hospital Roffo,
Buenos Aires, Argentina) was used. The LM2 cell line was maintained
in a humidified 5% CO2 atmosphere at 37°C using
Dulbecco’s modified Eagle’s medium (DMEM) suplemented with 10%
fetal bovine serum and 1% penicillin-streptomycin solution. The LM2
cells (1×106) were suspended in 0.1 ml of
phosphate-buffered saline (pH 7.0) and subcutaneously injected into
the right flanks in the depilated dorsal region of the mice.
When the tumor size reached 7 mm on the outer
diameter, tumoral propagation was carried out extracting 2 mm of
tumoral tissue which was subcutaneously re-implanted into the
dorsal region of the desired number of mice for each
experiment.
Tumor growth was documented regularly by external
measurements with electronic calipers.
No spontaneous regression of the tumor was observed
during our investigations.
When required, animals were anaesthetized using an
intraperitoneal (i.p.) injection of a mixture of ketamine
hydro-chloride [Ketaject; Phoenix Pharmaceutical, St. Joseph, MO,
USA; 50 mg/kg body weight (bw), Acedan (Holliday-Scott, SA, Buenos
Aires, Argentina; 17 mg/kg bw) and xylazine hydrochloride (Bayer,
Shawnee Mission, KS, USA; 5 mg/kg bw).
Irradiation
For the phototherapeutic studies, tumors were
irradiated employing a Kodak projector equipped with a 150-W lamp.
The light was filtered through a 3-cm water layer to absorb the
heat. A wavelength of range 350–800 nm was selected with the aid of
optical filters. The diameter of the light in the treatment site
was 1 cm. This area was obtained by making a hole in a Tergopol
layer, which finally isolated the mouse body. Light intensity at
the treatment site was 210 J/cm2 (Radiometer Laser
Mate-Q, Coherent, Hilton, Australia).
Hepatic and renal function
In order to determine the toxicity of
ZnPc(CF3)4 and
ZnPc(OCH3)4, physiological tests were
performed employing the following diagnostic kits (obtained from
Weiner Laboratories, SAIC, Rosario, Argentina): direct creatinine,
uremia and transaminase GPT 200. Mice were sacrificed by cervical
dislocation at 1 (n=5), 7 (n=5) and 30 days (n=5) after injection
of the phthalocyanine derivatives ZnPc(CF3)4
and ZnPc(OCH3)4 (0.2 mg/kg bw), and blood was
extracted to obtain the serum for the functionality tests. To
evaluate hepatic function, the levels of serum enzyme
glutamic-pyruvic transaminase (GPT) were measured, since high
levels in serum of GPT generally are associated with
hepatotoxicity. Kidney function was monitored by measuring the
serum levels of creatinine and urea.
Dark toxicity and histopathology
examination
ZnPc(CF3)4 and
ZnPc(OCH3)4 in D,L-α-dipalmitoyl
phosphatidylethanolamina liposome (0.2 mg/kg bw) were administered
by i.p. injection. The animals were placed in metabolic cages in
the dark, and 5 mice were sacrificed after
ZnPc(CF3)4 or
ZnPc(OCH3)4 administration for histological
examination. Seven days after injection, internal organs, such as
the liver, kidney and spleen, were excised, fixed in 4%
formaldehyde and embedded in paraffin. Blocks were sectioned (3-μm)
and stained with H&E for microscopical analysis.
The histopathological analysis was carried out in
the Animal Pathology Department of Agronomy and Veterinary Faculty,
UNRC, under the supervision of Silvia Romanini.
Phototherapeutic studies
Tumor regression
After PDT, tumor regression analysis was performed.
Tumor growth was documented regularly by external measurements with
electronic calipers. The tumor size was assessed by taking three
caliper measurements at right angles to each other and by applying
the following formula: V = (L x W x H x 0.5636), where L is the
length, W is the width and H is the height of the tumor (8,9).
The effectiveness of the treatment was evaluated by
comparing the rate of tumor growth of the mice treated with
ZnPc(CF3)4 or
ZnPc(OCH3)4 and irradiated with that observed
for the control mice treated only with
ZnPc(CF3)4 or
ZnPc(OCH3)4, the control-light [without
ZnPc(CF3)4 or
ZnPc(OCH3)4 but with tumor irradiation] and
the control-control [without ZnPc(CF3)4 or
ZnPc(OCH3)4 and without tumor irradiation]
mice.
Degree of tumor necrosis. The degree of tumor
necrosis was measured utilizing the vital stain Evan’s blue
(10). This dye reflects the
mechanism of tumor destruction (11). To determine the phototherapeutic
effect of the phthalocyanine derivatives, the degree of tumor
necrosis was assessed after PDT following this technique (10). Vital stain was performed by i.p.
injection of 0.4 ml 1% solution. This was injected into the mice of
the different groups: control-control (n=4), control-light (n=4),
ZnPc(CF3)4-dark [with
ZnPc(CF3)4, but without tumor irradiation]
(n=4), ZnPc(OCH3)4-dark [with
ZnPc(OCH3)4, but without tumor irradiation]
(n=4), ZnPc(CF3)4-light [with
ZnPc(CF3)4 and tumor irradiation] (n=7) and
ZnPc(OCH3)4- light [with
ZnPc(OCH3)4 and tumor irradiation] (n=6).
After 1, 4 and 10 days post-PDT, the animals were
injected with Evan’s blue, and then, 6 h post-administration (to
permit distribution of the dye) the animals were sacrificed. The
tumors were excised, and 2- to 3-mm cross-section slices were cut.
The tumors were examined macroscopically and photographed using a
magnification glass (x4) and analyzed using an image analyzer
(Motic Images Plus). The tumor sections were examined
microscopically in planes corresponding to the image plane. The
unstained area was considered to be necrotic tissue, whereas the
stained area was tissue with a preserved blood supply. In addition,
the histological sections of the tumors were obtained as described
above.
Tumor histological examination. The animals
were sacrificed after 10 days. Tumor samples from the
tumor-regressed mice and control tumor-bearing mice (tumor samples
before PDT) were excisioned and fixed in 10% formalin for routine
histological preparation. The representative tissues were
dehydrated in ascending grades of alcohol, embedded in paraffin
wax, and sections (3- to 4-μm) were obtained. The tissue
sections were stained with H&E and examined under a microscope
(Axiovert 135; Zeiss, Germany). The images were recorded using a
digital color camera (Axiocam; Zeiss) and Axiovision 4.3
software.
Tumor sections were stained with H&E to identify
the areas of viable and necrotic tissue. All experiments were
repeated at least three times.
Statistics
Statistical comparisons were performed using ANOVA.
The Duncan test was used when appropriate post hoc
comparison was possible, and a value of p=0.05 was considered
significant. Data are expressed as mean ± SEM, and differences
between means were considered statistically significant at
p<0.05.
Results
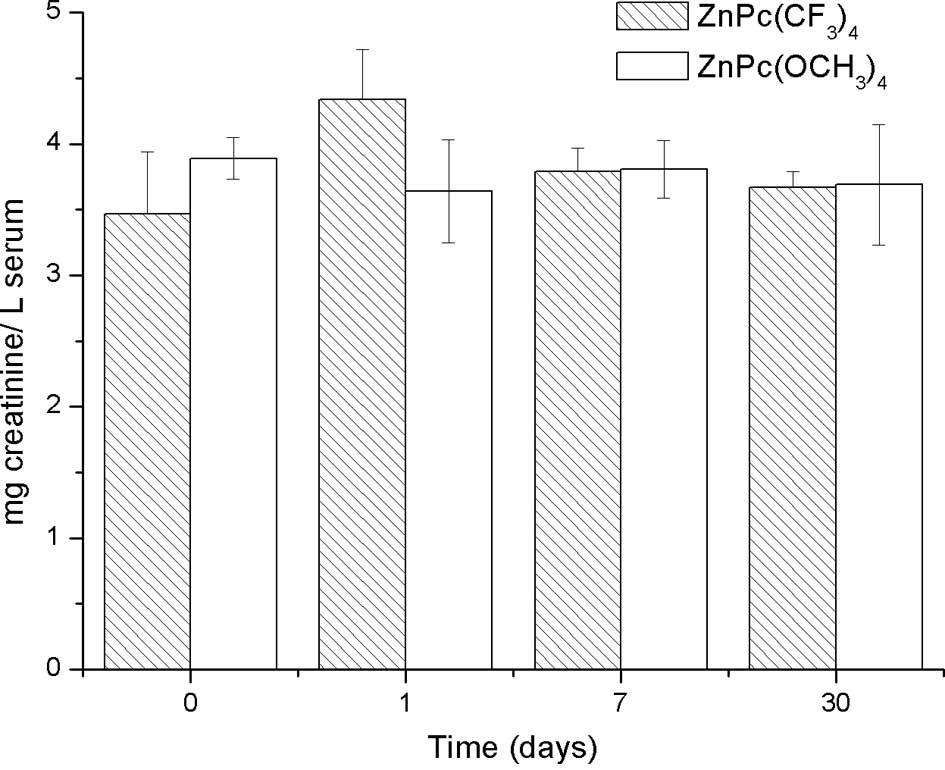
Hepatic and renal function
No pathological damage was observed in the liver and
kidney of mice treated with ZnPc(OCH3)4 (0.2
mg/kg bw). Also, there were no differences in creatinine, urea and
GPT serum concentration among the control mice, 1, 7 and 30 days
post-injection of ZnPc(OCH3)4 (0.2 mg/kg bw)
(Figs. 1, 2 and 3,
respectively). These results suggest that
ZnPc(OCH3)4 does not cause adverse effects in
mice. In contrast, statistical analysis did not show a significant
difference in the creatinine serum concentration among the control
mice, 1, 7 and 30 days post-injection of
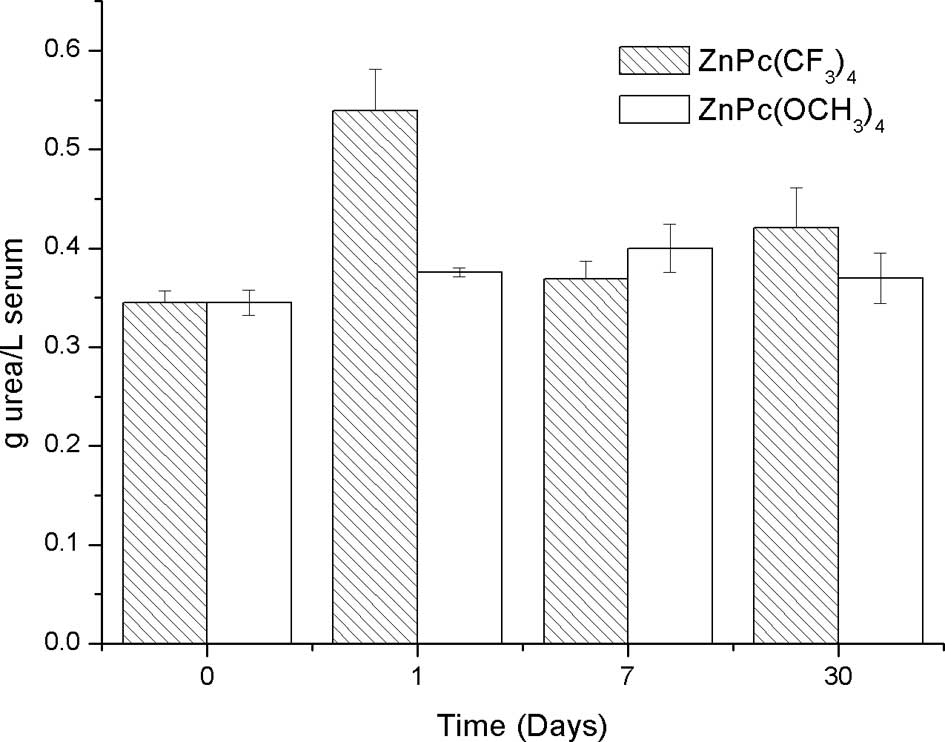
ZnPc(CF3)4 (0.2 mg/kg bw) (Fig. 1). Nevertheless, significantly high
levels of uremia in mice on the first day post-injection with
respect to the controls were observed. There were no significant
differences 7 and 30 days post-injection compared to the controls
(Fig. 2).
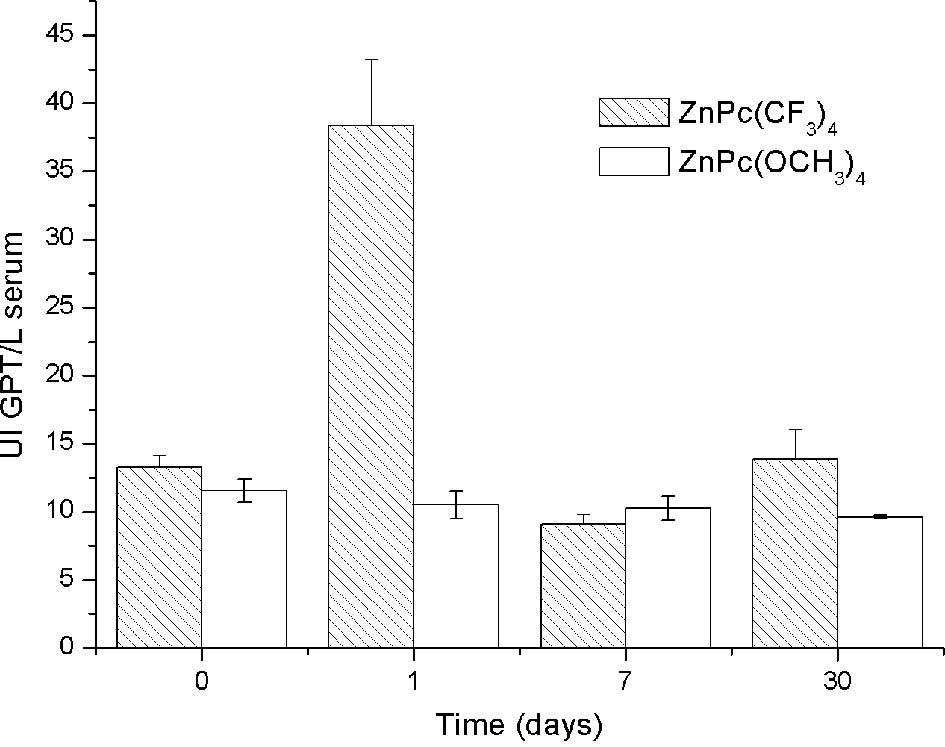
Significantly high levels of GPT serum concentration
were observed 1 day post-injection in relation to the controls.
However, normal values of GPT were noted 7 and 30 days
post-injection (Fig. 3). This
indicates that adverse effects were significantly observed at 24 h
post-administration in levels of uremia and GPT, but these values
returned to normal. The dose of 0.2 mg/kg bw of
ZnPc(CF3)4 did not produce time-persistent
toxicity, thus it is suitable for administration in PDT.
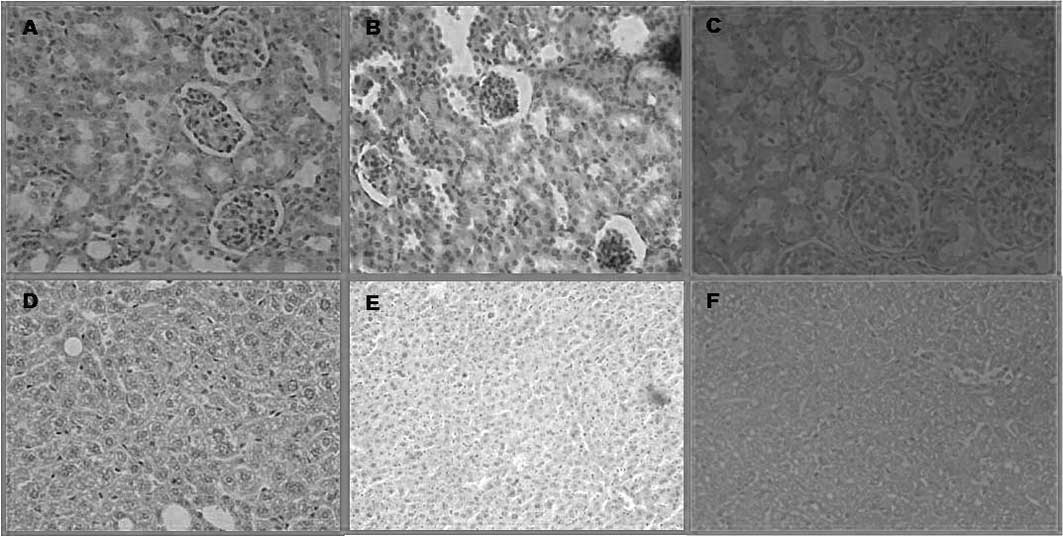
Histopathological examination
Tissues 10 days after PDT were obtained immediately
after euthanasia for histopathologic examination. On the other
hand, the tumors were removed 10 days after PDT using
ZnPc(OCH3)4 and
ZnPc(CF3)4.
On the basis of the pharmacokinetic data,
histological examinations were performed in the liver and kidney.
In these organs, ZnPc(CF3)4 administration
resulted in a slight acute toxicity. Its adverse affects were
reverted at 7 days post-injection. Histological damage was not
found 10 days after the administration of
ZnPc(OCH3)4 using the same dose as
ZnPc(CF3)4. This result indicates that both
drugs are suitable for use in PDT at the dose of 0.2 mg/kg bw, as
they do not produce irreversible damage (Fig. 4).
Significant differences were not found at the
histological level of the kidney, liver and spleen between control
mice and mice sacrificed 7 days after injection of
ZnPc(CF3)4 or
ZnPc(OCH3)4 (0.2 mg/kg bw).
Phototherapeutic study
Tumor regression
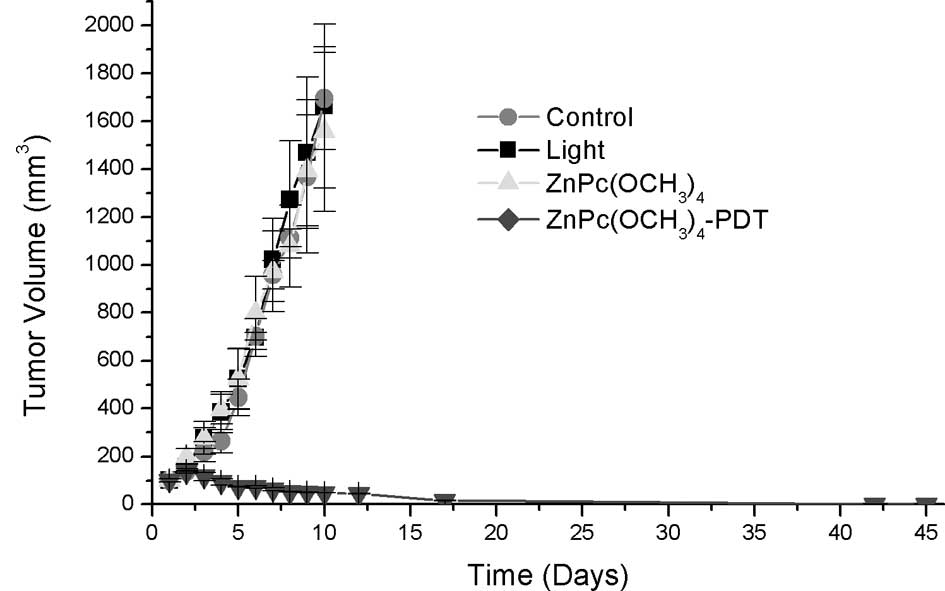
No differences in tumor volume were noted between
the control and control irradiated with 210 J/cm2 and
the mice treated with ZnPc(OCH3)4 or
ZnPc(CF3)4 in the dark. Light alone or
sensitizers alone had no effect on the growth of tumors. Compared
to the control group, the growth of the implanted tumors was
significantly inhibited with reduced tumor volumes after PDT. On
the other hand, there were obvious differences between
ZnPc(OCH3)4-PDT or
ZnPc(CF3)4-PDT and the control groups
regarding tumor size throughout the observation period (Fig. 5A and B, respectively).
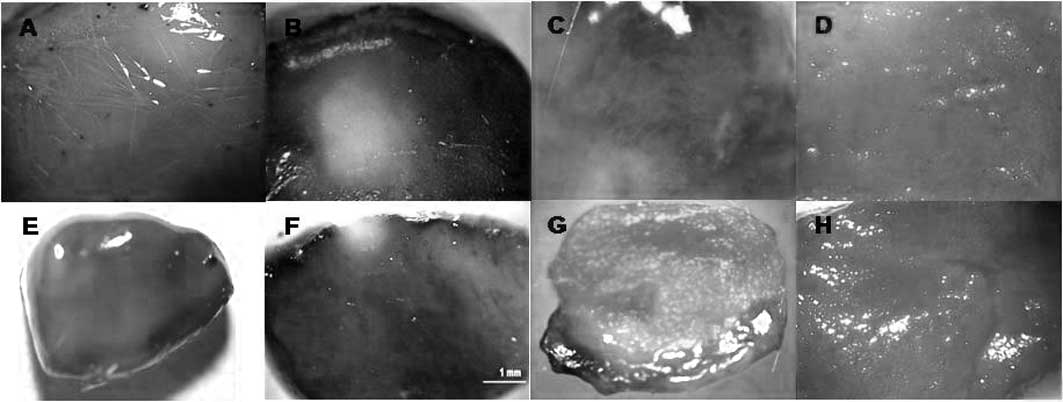
Degree of tumor necrosis. In tumors of the
control-control, control-light,
ZnPc(CF3)4-dark and
ZnPc(OCH3)4-dark groups, total staining with
Evan’s blue was observed, indicating that tumor death did not occur
(Fig. 6A, B and C,
respectively).
The area of tumor necrosis was measured by Evan’s
blue dye staining at 1, 4 and 10 days post-PDT.
Mice administered Evan’s blue 1 day after PDT using
ZnPc(CF3)4 exhibited a percentage of tumor
death of 12%, but when ZnPc(OCH3)4 was used,
the tumor death percentage was 8.4% with the same dose of
irradiation (210 J/cm2) and drug concentration (0.2
mg/kg bw) (Fig. 6D and E). Four
days post-PDT, the tumor death percentage was 89.4% when
ZnPc(CF3)4 was used, while this percentage
decreased to 71.5% using ZnPc(OCH3)4
(Fig. 6D and E). Ten days
post-PDT, the tumor death percentage was 72.2% when
ZnPc(CF3)4 was used, while this percentage
increased to 72.8% with ZnPc(OCH3)4 (Fig. 6F and G). After 10 days, the death
percentage was the same for both drugs (72%).
Thus, the untreated viable tumors stained blue,
while the tumors post-PDT exhibited white areas and showed no
evidence of stain uptake. This regression of necrosis was also
supported by the drastic reduction in Evans blue incorporation,
which was clearly evident as an unstained area.
Four of 10 (40%) tumors exhibited complete
regression 4 days post ZnPc(CF3)4-PDT, while
6 of 8 (75%) tumors showed total regression 10 days post
ZnPc(OCH3)4-PDT.
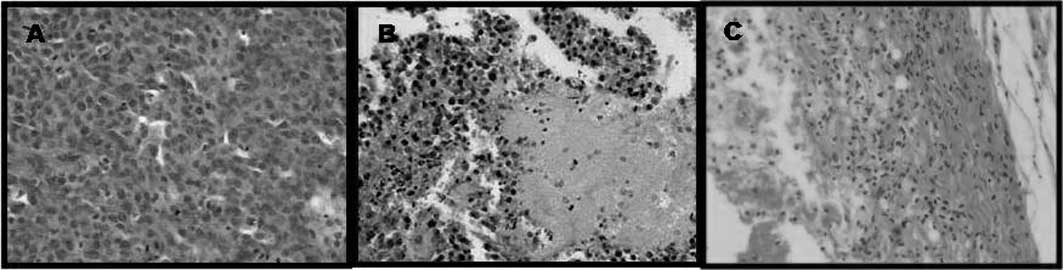
Tumor histological examination. The
histological evaluation of the group of mice (n=5) treated at 210
J/cm2 after injection with
ZnPc(CF3)4 or
ZnPc(OCH3)4 (0.2 mg/kg bw) showed tumors with
few focal necrotic areas evident following PDT treatment in most
mice (Fig. 7B and C,
respectively). This tumor cell death was probably the direct result
of PDT cell killing mechanisms. In contrast, tumor regression in
the mouse groups treated with ZnPc(OCH3)4 or
ZnPc(CF3)4 after treatment of PDT was
observed by a decrease in tumor volume (Fig. 5A and B, respectively).
In contrast, in tumors treated with 210
J/cm2 alone, control and
ZnPc(CF3)4 or
ZnPc(OCH3)4 in dark condition, no significant
effect on tissue damage and tumor growth was noted (Fig. 7A).
Tumor to skin ratio
Tumor uptake of a PS depends on
hydrophobicity/hydrophilicity of the sensitizer.
Previous investigation has revealed that most of the
liposome-released PSs are associated with high density lipoproteins
(HDL), low density lipoproteins (LDL) and very low density
lipoproteins (VLDL), whereas free PSs are distributed evenly
between albumin and HDL (12).
LDL-bound lipophilic PSs can be selectively incorporated into tumor
cells, whereas hydrophilic PSs bind preferentially to serum albumin
and often accumulate in the vascular stroma of tumors. It has been
suggested that when liposomal PSs are administered to animals, the
PS is more efficiently transferred to LDL than an aqueous
formulation (13,14).
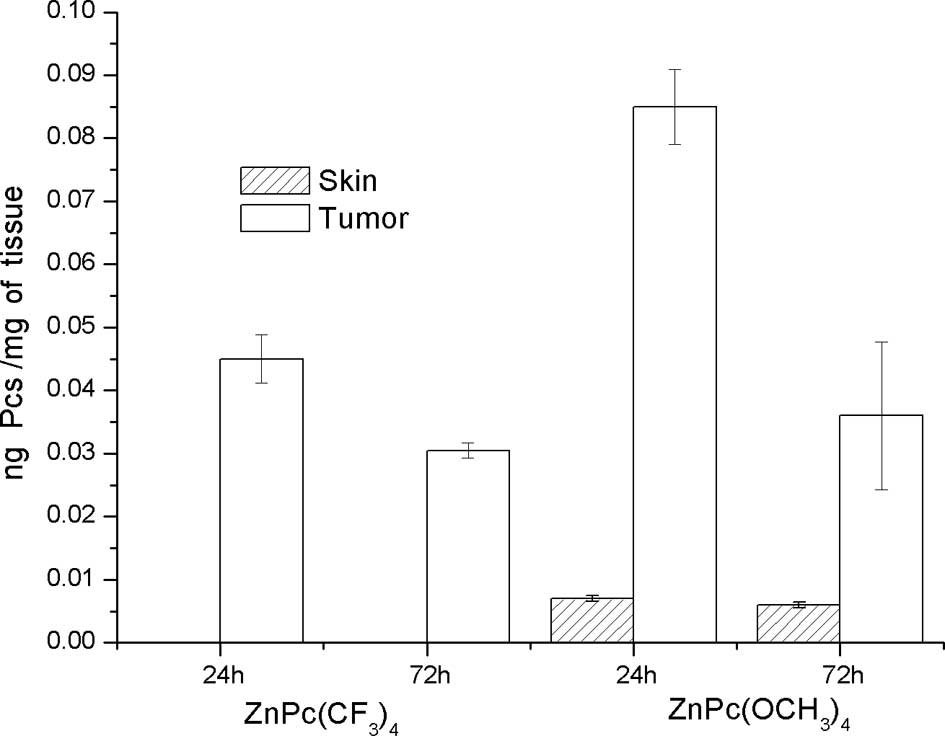
In the present study, the
ZnPc(OCH3)4 and
ZnPc(CF3)4 concentrations in the tumors were
at a maximum level 24 h after injection of 0.085 and 0.045 ng/mg
tissue, respectively, and then the levels of both drugs decreased.
The absolute amounts of ZnPc(OCH3)4 in the
tumors were higher than that in the skin 24 or 72 h after i.p.
injection. The tumor to skin accumulation ratio was 12.14 at 24 h,
and 6.0 at 72 h after the injection.
After 24 and 72 h post-injection of
ZnPc(CF3)4 there was no accumulation in the
skin (Fig. 8). The lower level of
the drug in the skin is appropriated in tumor treatments since only
a low cutaneous phototoxicity is found in experimental carcinoma
models in vivo. Delivery of
ZnPc(OCH3)4 or
ZnPc(CF3)4 in liposomal solution gains access
to cells in the tumor mass in a relatively short period of time and
localizes with a high degree of specificity.
Discussion
The uptake, distribution and retention of a
sensitizer in a tumor depend on the route and mode of delivery, as
well as on the physicochemical properties (e.g., lipophilicity) of
the drug. For instance, lipophilic PSs need to be incorporated into
delivery vehicles, for example liposomes in in vivo
administration. Liposomes are known to enhance the clinical effects
of PSs, facilitate uptake by tumor cells due to direct contact of
the liposomal drug with the tumor, reduce their toxicity and
protect them from immune responses. The tumor cells express higher
levels of receptors to the lipoprotein LDL than normal cells,
facilitating entry of the drug into neoplastic cells. Both
phthalocyanine derivatives [ZnPc(CF3)4 and
ZnPc(OCH3)4] exhibit lipophilic properties.
The phthalocyanine derivative ZnPc(OCH3)4
exhibited a high lipophilic character which allowed it to remain in
the tumor, improving the phototherapeutic action. Therefore, one
factor which enhances the specificity of the PS for neoplastic
tissues is its highly hydrophobic character. However, this
characteristic leads to poor solubility of the molecules in
physiologically compatible solvent media, and thus they must be
administered in vivo by means of a delivery system (15). Various drug delivery systems, such
as liposomes, polymeric micelles, Cremophor emulsion, microspheres
and nanoparticles, have been developed to deliver PSs. Both
phthalocyanine derivatives [ZnPc(CF3)4 and
ZnPc(OCH3)4] incorporated in liposomes
containing cholesterol would probably favor interaction with the
lipoprotein LDL and the accumulation in macrophages of RES and
tumor tissue, which are known in the literature to have high
expression of LDL receptors (16).
This property confers enhanced efficiency to the phototherapeutic
action, due to the fact that PSs can cross membranes and organelles
interacting with intracellular proteins or structures that contains
hydrophobic elements.
Both PSs did not affect the hepatic and renal
function. The histological section of the treated tumors showed the
presence of areas of necrosis and, in addition, demonstrated signs
of inflammatory response; in addition, 10 days post-TFD the treated
tumors showed signs of tumor death.
Tumor necrosis was achieved 10 days post-PDT at 24 h
after i.p. injection of ZnPc(CF3)4 or
ZnPc(OCH3)4. These results in vivo
were confirmed by the use of Evan’s blue dye. This dye is a direct
and easy method for evaluating the mechanism of tumor destruction
and measuring the depth of necrosis after PDT treatment. This
method is suitable for assessing tumor death after PDT.
One limiting factor for treatment success is the
penetration depth of ZnPc(CF3)4.
While ZnPc(OCH3)4 was shown
to penetrate more deeply, no highly significant difference in the
therapeutic effect has been demonstrated so far. This result showed
that ZnPc(OCH3)4 has more rapid clearance
from the body tissue, particularly from the skin. In conclusion,
our results demonstrate that ZnPc(CF3)4 and
ZnPc(OCH3)4 accumulate in the tumor and that
these sensitizers lead to tumor destruction upon photodynamic
treatment.
Abbreviations:
|
bw
|
body weight;
|
|
DMEM
|
Dulbecco’s modified Eagle’s
medium;
|
|
ERS
|
endothelial-reticulum system;
|
|
GPT
|
glutamic-pyruvic transaminase;
|
|
H&E
|
hematoxylin and eosin;
|
|
HDL
|
high-density lipoproteins;
|
|
i.p.
|
intraperitoneally;
|
|
LDL
|
low-density lipoproteins;
|
|
PCs
|
phthalocyanines;
|
|
PDT
|
photodynamic therapy;
|
|
PS
|
photosensitizer;
|
|
VLDL
|
very low-density lipoproteins
|
Acknowledgements
The authors are grateful to
Secretaría de Ciencia y Técnica (SECYT) of Universidad Nacional de
Río Cuarto and Consejo Nacional de Investigaciones Científicas y
Técnicas (CONICET) for financial support. E.I.Y., E.N.D. and V.A.R.
are scientific members of CONICET.
References
|
1.
|
Buytaert E, Dewaele M and Agostinis P:
Molecular effectors of multiple cell death pathways initiated by
photodynamic therapy. Review Biochim Biophys Acta. 1776:86–107.
2007.PubMed/NCBI
|
|
2.
|
Henderson BW and Miller AC: Effects of
scavengers of reactive oxygen and radical species on cell survival
following photodynamic treatment in vitro: comparison to ionizing
radiation. Radiat Res. 108:196–205. 1986. View Article : Google Scholar
|
|
3.
|
Castano AP, Demidova TN and Hamblin MR:
Mechanisms in photodynamic therapy: part three photosensitizer,
pharmacokinetics, biodistribution, tumor localization and modes of
tumor destruction. Photodiag Photodyn Therapy. 2:91–106. 2005.
View Article : Google Scholar
|
|
4.
|
Chan WS, Zuk M and Ber-Hur E:
Phthalocyanines. Photodynamic Tumor Therapy, 2nd and 3rd Generation
of Photosensitizers. Moser JG: Harwood Academic; Amsterdam: pp.
63–73. 1998
|
|
5.
|
Allison R, Downie G, Cuenca R, Hu X,
Childs C and Sibata C: Photosensitizers in clinical PDT. Photodiag
Photodyn Therapy. 1:27–42. 2004. View Article : Google Scholar
|
|
6.
|
Noodt BB, Berg K, Stokke T, Peng Q and
Nesland JM: Different apoptotic pathways are induced from various
intracellular sites by tetraphenylporphyrins and light. Br J
Cancer. 79:72–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Yslas I, Rivarola V and Durantini EN:
Synthesis and photodynamic activity of zinc (II) phthalocyanine
derivatives bearing methoxy and trifluoromethylbenzyloxy
substituents in homogeneous and biological media. Bioorg Med Chem.
13:39–46. 2005. View Article : Google Scholar
|
|
8.
|
Whitacre CM, Feyes DK, Satoh T, Grossmann
J, Mulvihill JW, Mukhtar H and Oleinik NL: Photodynamic therapy
with the phthalocyanine photosensitizer Pc 4 of SW480 human colon
cancer xenografts in athymic mice. Clin Cancer Res. 6:2021–2027.
2000.PubMed/NCBI
|
|
9.
|
Whitacre CM, Zborowska E, Willson JKV and
Berger NA: Detection of poly (ADP-ribose) polymerase cleavage in
response to treatment with topoisomerase I inhibitors: a potential
surrogate end point to assess treatment effectiveness. Clin Cancer
Res. 5:665–672. 1999.
|
|
10.
|
Schastak S, Jean B, Handzel R, et al:
Improved pharmacokinetics, biodistribution and necrosis in vivo
using a new near infra-red photosensitizer: tetrahydroporphyrin
tetratosylat. J Photochem Photobiol. 78:203–213. 2005. View Article : Google Scholar
|
|
11.
|
Kostenich GA, Zhuravkin IN, Furmanchuk AV
and Zhavrid EA: Photodynamic therapy with chlorin e6. A morphologic
study of tumor damage efficiency in experiment. J Photochem
Photobiol. 11:307–318. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Richter AM, Waterfield E, Jain AK, Canaan
AJ, Allison BA and Levy JG: Liposomal delivery of a
photosensitizer, benzoporphyrin derivative monoacid ring A (BPD),
to tumor tissue in a mouse tumor model. Photochem Photobiol.
57:1000–1006. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jori G and Reddi E: The role of
lipoproteins in the delivery of tumour-targeting photosensitizers.
Int J Biochem. 25:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Love WG, Havenaar EC, Lowe PJ and Peter
WT: Uptake of zinc(II)-phthalocyanine by HepG2 cells expressing the
low density lipoprotein receptor: studies with the liposomal
formulation CGP55847. SPIE Proc. 2078:381–388. 1994. View Article : Google Scholar
|
|
15.
|
Fadel M, Kassab K and Fadeel DA: Zinc
phthalocyanine-loaded PLGA biodegradable nanoparticles for
photodynamic therapy in tumor-bearing mice. Lasers Med Sci.
25:283–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Polo L, Valduga G, Jori G and Redi E:
Low-density lipoprotein receptors in the uptake of tumour
photosensitizers by human and rat transformed fibroblasts. Int J
Biochem Cell Biol. 34:10–23. 2002. View Article : Google Scholar : PubMed/NCBI
|