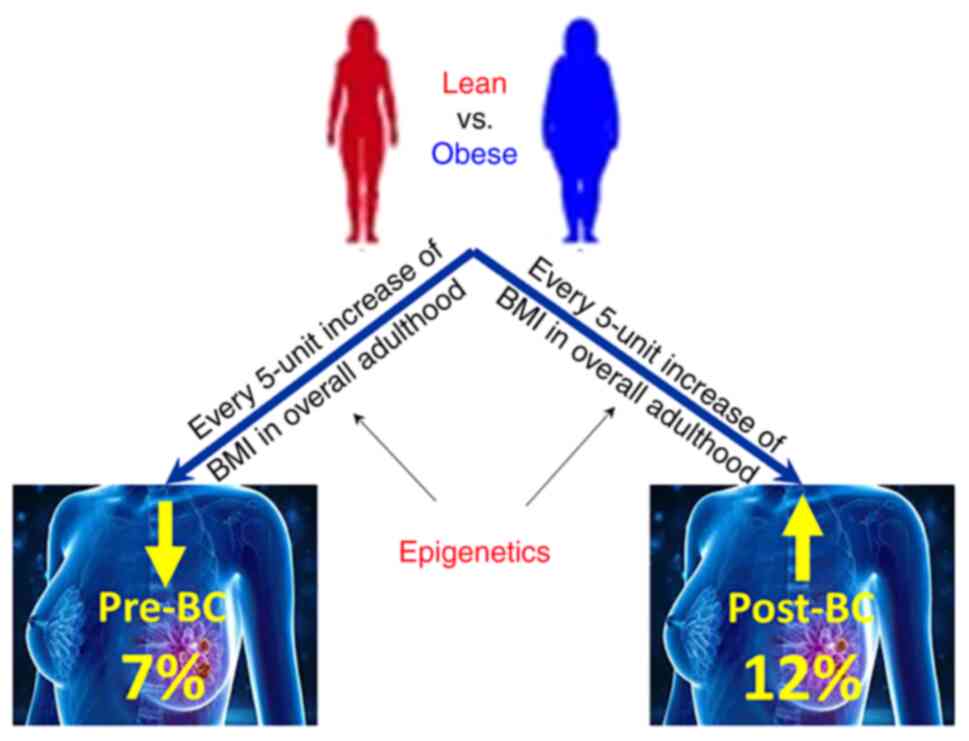
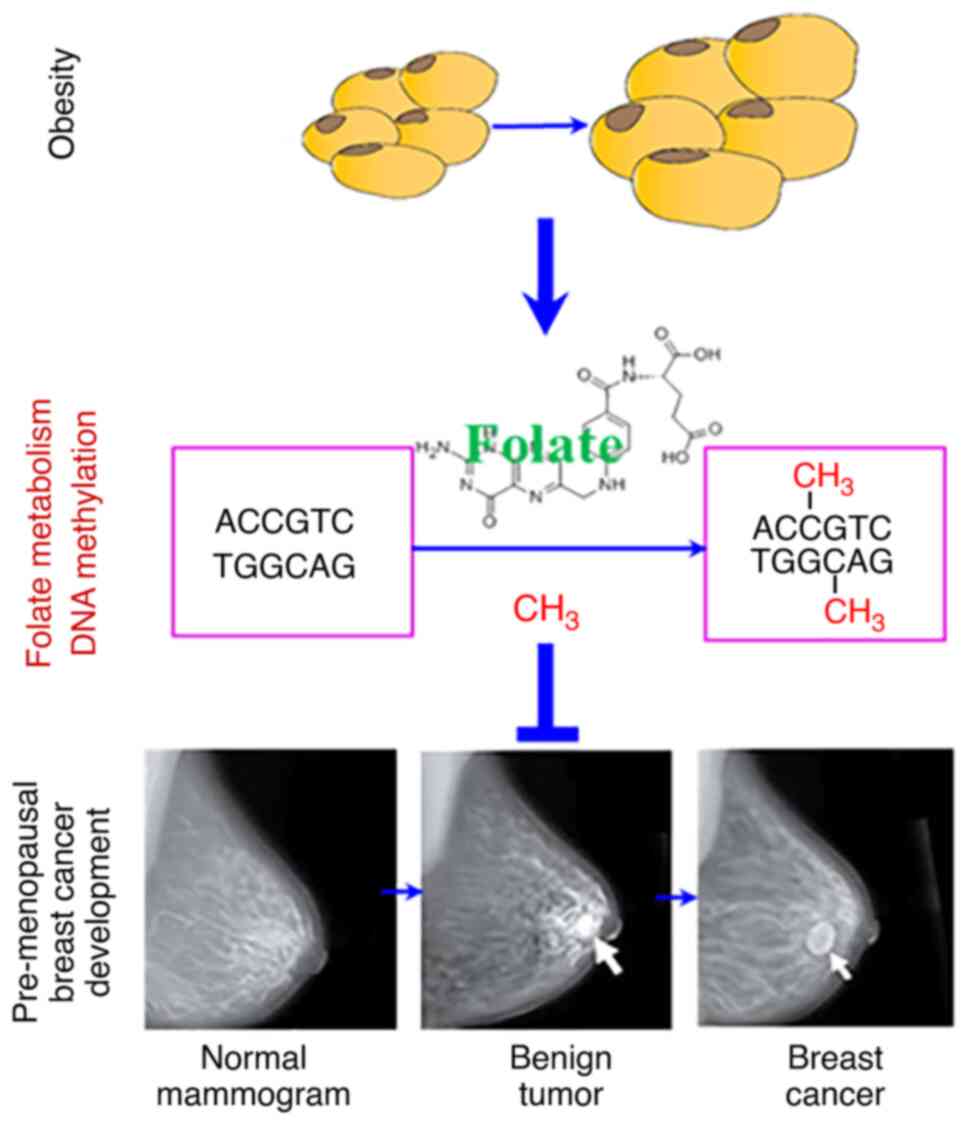
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Torre LA, Islami F, Siegel RL, Ward EM and Jemal A: Global cancer in women: Burden and trends. Cancer Epidemiol Biomarkers Prev. 26:444–457. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al: Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 288:321–333. 2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Breen N, Cronin KA, Tiro JA, Meissner HI, McNeel TS, Sabatino SA, Tangka FK and Taplin SH: Was the drop in mammography rates in 2005 associated with the drop in hormone therapy use? Cancer. 117:5450–5460. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Breen N, Gentleman JF and Schiller JS: Update on mammography trends: Comparisons of rates in 2000, 2005, and 2008. Cancer. 117:2209–2218. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer statistics, 2019. CA Cancer J Clin. 69:438–4351. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pfeiffer RM, Webb-Vargas Y, Wheeler W and Gail MH: Proportion of U.S. Trends in breast cancer incidence attributable to long-term changes in risk factor distributions. Cancer Epidemiol Biomarkers Prev. 27:1214–1222. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
World Health Organization (WHO): Obesity and Overweight. WHO, Geneva, 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed June 9, 2021.
|
|
10
|
Hales CM, Carroll MD, Fryar CD and Ogden CL: Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 360:1–8. 2020.PubMed/NCBI
|
|
11
|
Centers for Disease Control and Prevention (CDC): Overweight & Obesity. CDC, Atlanta, GA, 2021. https://www.cdc.gov/obesity/data/adult.html. Last reviewed June 7, 2021.
|
|
12
|
Calle EE and Kaaks R: Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 4:579–591. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B and Dietz W: Obesity and severe obesity forecasts through 2030. Am J Prev Med. 42:563–570. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Heart: Lung and Blood Institute (NIH): Managing overweight and obesity in adults: Systematic evidence review from the Obesity Expert Panel. NIH, Bethesda, MD, 2013.
|
|
15
|
Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E and Colditz GA: Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 161:1581–1586. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sturm R and An R: Obesity and economic environments. CA Cancer J Clin. 64:337–350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA and Speizer FE: Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 132:501–513. 1990.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brown JC, Carson TL, Thompson HJ and Agurs-Collins T: The triple health threat of diabetes, obesity, and cancer-epidemiology, disparities, mechanisms, and interventions. Obesity (Silver Spring). 29:954–959. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Scully T, Ettela A, LeRoith D and Gallagher EJ: Obesity, type 2 diabetes, and cancer risk. Front Oncol. 10(615375)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rahman I, Athar MT and Islam M: Type 2 diabetes, obesity, and cancer share some common and critical pathways. Front Oncol. 10(600824)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC and Hu FB: Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 73:61–67. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wilding JPH and Jacob S: Cardiovascular outcome trials in obesity: A review. Obes Rev. 22(e13112)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Swanson SM, Harper J and Zisman TL: Obesity and inflammatory bowel disease: Diagnostic and therapeutic implications. Curr Opin Gastroenterol. 34:112–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dai ZH, Xu XT and Ran ZH: Associations between obesity and the effectiveness of anti-tumor necrosis factor-α agents in inflammatory bowel disease patients: A literature review and meta-analysis. Ann Pharmacother. 54:729–741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Horesh A, Tsur AM, Bardugo A and Twig G: Adolescent and childhood obesity and excess morbidity and mortality in young adulthood-a systematic review. Curr Obes Rep: May 5, 2021 (Epub ahead of print).
|
|
26
|
Yates T, Razieh C, Zaccardi F, Rowlands AV, Seidu S, Davies MJ and Khunti K: Obesity, walking pace and risk of severe COVID-19 and mortality: Analysis of UK Biobank. Int J Obes (Lond). 45:1155–1159. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Masters RK, Reither EN, Powers DA, Yang YC, Burger AE and Link BG: The impact of obesity on US mortality levels: The importance of age and cohort factors in population estimates. Am J Public Health. 103:1895–1901. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brown KA: Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 17:350–363. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Centers for Disease Control and Prevention (CDC): Cancers Associated with Overweight and Obesity Make up 40 percent of Cancers Diagnosed in the United States. CDC, Atlanta, GA, 2017. Accessed October 3, 2017.
|
|
30
|
World Cancer Research Fund (WCRF): Continuous Update Project Report: Diet, Nutrition, Physical Activity and Breast Cancer. WCRF, London, 2017. https://www.wcrf.org/sites/default/files/Breast-Cancer-2017-Report.pdf. Accessed July 20, 2020.
|
|
31
|
Calle EE, Rodriguez C, Walker-Thurmond K and Thun MJ: Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 348:1625–1638. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tamimi RM, Spiegelman D, Smith-Warner SA, Wang M, Pazaris M, Willett WC, Eliassen AH and Hunter DJ: Population attributable risk of modifiable and nonmodifiable breast cancer risk factors in postmenopausal breast cancer. Am J Epidemiol. 184:884–893. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Silvera SA, Jain M, Howe GR, Miller AB and Rohan TE: Energy balance and breast cancer risk: A prospective cohort study. Breast Cancer Res Treat. 97:97–106. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Anderson GL and Neuhouser ML: Obesity and the risk for premenopausal and postmenopausal breast cancer. Cancer Prev Res (Phila). 5:515–521. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nelson HD, Zakher B, Cantor A, Fu R, Griffin J, O'Meara ES, Buist DS, Kerlikowske K, van Ravesteyn NT, Trentham-Dietz A, et al: Risk factors for breast cancer for women aged 40 to 49 years: A systematic review and meta-analysis. Ann Intern Med. 156:635–648. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Munsell MF, Sprague BL, Berry DA, Chisholm G and Trentham-Dietz A: Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 36:114–1136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Renehan AG, Tyson M, Egger M, Heller RF and Zwahlen M: Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 371:569–578. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ursin G, Longnecker MP, Haile RW and Greenland S: A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 6:137–1341. 1995.PubMed/NCBI View Article : Google Scholar
|
|
39
|
van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, et al: Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 152:514–527. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
De Pergola G and Silvestris F: Obesity as a major risk factor for cancer. J Obes. 2013(291546)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Frederick AM, Guo C, Meyer A, Yan L, Schneider SS and Liu Z: The influence of obesity on folate status, DNA methylation and cancer-related gene expression in normal breast tissues from premenopausal women. Epigenetics. 16:458–467. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
NIH Roadmap Epigenomics Project. Overview. http://www.roadmapepigenomics.org/overview. Access June 1, 2021.
|
|
43
|
Lo PK and Sukumar S: Epigenomics and breast cancer. Pharmacogenomics. 9:1879–1902. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang Y, Nayak S, Jankowitz R, Davidson NE and Oesterreich S: Epigenetics in breast cancer: What's new? Breast Cancer Res. 13(225)2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kasiappan R and Rajarajan D: Role of MicroRNA regulation in obesity-associated breast cancer: Nutritional perspectives. Adv Nutr. 8:868–888. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gyorffy B, Bottai G, Fleischer T, Munkácsy G, Budczies J, Paladini L, Børresen-Dale AL, Kristensen VN and Santarpia L: Aberrant DNA methylation impacts gene expression and prognosis in breast cancer subtypes. Int J Cancer. 138:87–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mahdavi M, Nassiri M, Kooshyar MM, Vakili-Azghandi M, Avan A, Sandry R, Pillai S, Lam AK and Gopalan V: Hereditary breast cancer; Genetic penetrance and current status with BRCA. J Cell Physiol. 234:5741–5750. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Luque M, Arranz F, Cueva JF, de Juan A, García-Teijido P, Calvo L, Peláez I, García-Palomo A, García-Mata J, Antolín S, et al: Breast cancer management in the elderly. Clin Transl Oncol. 16:351–361. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Daly AA, Rolph R, Cutress RI and Copson ER: A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer (Dove Med Press). 13:241–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al: The consensus coding sequences of human breast and colorectal cancers. Science. 314:268–274. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Esteller M: Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 8:286–298. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Esteller M: Epigenetics in cancer. N Engl J Med. 358:1148–1159. 2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al: The genomic landscapes of human breast and colorectal cancers. Science. 318:1108–1113. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Holliday R: A new theory of carcinogenesis. Br J Cancer. 40:513–522. 1979.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Laird PW: The power and the promise of DNA methylation markers. Nat Rev Cancer. 3:253–266. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tang Q, Cheng J, Cao X, Surowy H and Burwinkel B: Blood-based DNA methylation as biomarker for breast cancer: A systematic review. Clin Epigenetics. 8(115)2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Meeks KAC, Henneman P, Venema A, Burr T, Galbete C, Danquah I, Schulze MB, Mockenhaupt FP, Owusu-Dabo E, Rotimi CN, et al: An epigenome-wide association study in whole blood of measures of adiposity among Ghanaians: The RODAM study. Clin Epigenetics. 9(103)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Crujeiras AB, Diaz-Lagares A, Stefansson OA, Macias-Gonzalez M, Sandoval J, Cueva J, Lopez-Lopez R, Moran S, Jonasson JG, Tryggvadottir L, et al: Obesity and menopause modify the epigenomic profile of breast cancer. Endocr Relat Cancer. 24:351–363. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Szyf M, Pakneshan P and Rabbani SA: DNA methylation and breast cancer. Biochem Pharmacol. 68:1187–1197. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H and Jaenisch R: Induction of tumors in mice by genomic hypomethylation. Science. 300:489–492. 2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Van Tongelen A, Loriot A and De Smet C: Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Lett. 396:130–137. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hon GC, Hawkins RD, Caballero OL, Lo C, Lister R, Pelizzola M, Valsesia A, Ye Z, Kuan S, Edsall LE, et al: Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome Res. 22:246–258. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kuchiba A, Iwasaki M, Ono H, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Tsugane S and Yoshida T: Global methylation levels in peripheral blood leukocyte DNA by LUMA and breast cancer: A case-control study in Japanese women. Br J Cancer. 110:2765–2771. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ennour-Idrissi K, Dragic D, Durocher F and Diorio C: Epigenome-wide DNA methylation and risk of breast cancer: A systematic review. BMC Cancer. 20(1048)2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Huang TH, Perry MR and Laux DE: Methylation profiling of CpG islands in human breast cancer cells. Hum Mol Genet. 8:459–470. 1999.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jovanovic J, Ronneberg JA, Tost J and Kristensen V: The epigenetics of breast cancer. Mol Oncol. 4:242–254. 2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ordway JM, Budiman MA, Korshunova Y, Maloney RK, Bedell JA, Citek RW, Bacher B, Peterson S, Rohlfing T, Hall J, et al: Identification of novel high-frequency DNA methylation changes in breast cancer. PLoS One. 2(e1314)2007.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Mukherjee N, Bhattacharya N, Alam N, Roy A, Roychoudhury S and Panda CK: Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Sci. 103:210–220. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Li Z, Guo X, Wu Y, Li S, Yan J, Peng L, Xiao Z, Wang S, Deng Z, Dai L, et al: Methylation profiling of 48 candidate genes in tumor and matched normal tissues from breast cancer patients. Breast Cancer Res Treat. 149:767–779. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
O'Donovan PJ and Livingston DM: BRCA1 and BRCA2: Breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis. 31:961–967. 2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Zhang L and Long X: Association of BRCA1 promoter methylation with sporadic breast cancers: Evidence from 40 studies. Sci Rep. 5(17869)2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hedenfalk I, Duggan D, Chen Y, Radmacher M, Bittner M, Simon R, Meltzer P, Gusterson B, Esteller M, Kallioniemi OP, et al: Gene-expression profiles in hereditary breast cancer. N Engl J Med. 344:539–548. 2001.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Duffy MJ, Napieralski R, Martens JW, Span PN, Spyratos F, Sweep FC, Brunner N, Foekens JA and Schmitt M: EORTC PathoBiology Group. Methylated genes as new cancer biomarkers. Eur J Cancer. 45:335–346. 2009.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Brooks J, Cairns P and Zeleniuch-Jacquotte A: Promoter methylation and the detection of breast cancer. Cancer Causes Control. 20:1539–1550. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
García-Giménez JL, Sanchis-Gomar F, Lippi G, Mena S, Ivars D, Gomez-Cabrera MC, Viña J and Pallardó FV: Epigenetic biomarkers: A new perspective in laboratory diagnostics. Clin Chim Acta. 413:1576–1582. 2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Parashar S, Cheishvili D, Mahmood N, Arakelian A, Tanvir I, Khan HA, Kremer R, Mihalcioiu C, Szyf M and Rabbani SA: DNA methylation signatures of breast cancer in peripheral T-cells. BMC Cancer. 18(574)2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Szyf M: DNA methylation signatures for breast cancer classification and prognosis. Genome Med. 4(26)2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Li Y, Melnikov AA, Levenson V, Guerra E, Simeone P, Alberti S and Deng Y: A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer. 15(417)2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Stirzaker C, Zotenko E, Song JZ, Qu W, Nair SS, Locke WJ, Stone A, Armstong NJ, Robinson MD, Dobrovic A, et al: Methylome sequencing in triple-negative breast cancer reveals distinct methylation clusters with prognostic value. Nat Commun. 6(5899)2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Samblas M, Milagro FI and Martinez A: DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 14:421–444. 2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ouni M and Schurmann A: Epigenetic contribution to obesity. Mamm Genome. 31:134–145. 2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Donovan MG, Wren SN, Cenker M, Selmin OI and Romagnolo DF: Dietary fat and obesity as modulators of breast cancer risk: Focus on DNA methylation. Br J Pharmacol. 177:1331–1350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Perfilyev A, Dahlman I, Gillberg L, Rosqvist F, Iggman D, Volkov P, Nilsson E, Risérus U and Ling C: Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: A randomized controlled trial. Am J Clin Nutr. 105:991–1000. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Jacobsen SC, Brøns C, Bork-Jensen J, Ribel-Madsen R, Yang B, Lara E, Hall E, Calvanese V, Nilsson E, Jørgensen SW, et al: Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia. 55:3341–3349. 2012.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Milagro FI, Campion J, Cordero P, Goyenechea E, Gómez-Uriz AM, Abete I, Zulet MA and Martínez JA: A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 25:1378–1389. 2011.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Bouchard L, Rabasa-Lhoret R, Faraj M, Lavoie ME, Mill J, Pérusse L and Vohl MC: Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am J Clin Nutr. 91:309–320. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Voisin S, Eynon N, Yan X and Bishop DJ: Exercise training and DNA methylation in humans. Acta Physiol (Oxf). 213:39–59. 2015.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ and Zierath JR: Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15:405–411. 2012.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kanzleiter T, Jahnert M, Schulze G, Selbig J, Hallahan N, Schwenk RW and Schürmann A: Exercise training alters DNA methylation patterns in genes related to muscle growth and differentiation in mice. Am J Physiol Endocrinol Metab. 308:E912–E920. 2015.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Sailani MR, Halling JF, Møller HD, Lee H, Plomgaard P, Pilegaard H, Snyder MP and Regenberg B: Lifelong physical activity is associated with promoter hypomethylation of genes involved in metabolism, myogenesis, contractile properties and oxidative stress resistance in aged human skeletal muscle. Sci Rep. 9(3272)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Maugeri A: The effects of dietary interventions on DNA methylation: Implications for obesity management. Int J Mol Sci. 21(8670)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ling C and Ronn T: Epigenetics in Human Obesity and type 2 diabetes. Cell Metab. 29:1028–1044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Bombonati A and Sgroi DC: The molecular pathology of breast cancer progression. J Pathol. 223:307–317. 2011.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Robertson KD: DNA methylation, methyltransferases, and cancer. Oncogene. 20:3139–3155. 2001.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Fleischer T, Tekpli X, Mathelier A, Wang S, Nebdal D, Dhakal HP, Sahlberg KK and Schlichting E: Oslo Breast Cancer Research Consortium (OSBREAC). Børresen-Dale AL, et al: DNA methylation at enhancers identifies distinct breast cancer lineages. Nat Commun. 8(1379)2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Jin W, Li QZ, Zuo YC, Cao YN, Zhang LQ, Hou R and Su WX: Relationship Between DNA methylation in key region and the differential expressions of genes in human breast tumor tissue. DNA Cell Biol. 38:49–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Hair BY, Xu Z, Kirk EL, Harlid S, Sandhu R, Robinson WR, Wu MC, Olshan AF, Conway K, Taylor JA and Troester MA: Body mass index associated with genome-wide methylation in breast tissue. Breast Cancer Res Treat. 151:453–463. 2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hair BY, Troester MA, Edmiston SN, Parrish EA, Robinson WR, Wu MC, Olshan AF, Swift-Scanlan T and Conway K: Body mass index is associated with gene methylation in estrogen receptor-positive breast tumors. Cancer Epidemiol Biomarkers Prev. 24:580–586. 2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Dragic D, Ennour-Idrissi K, Michaud A, Chang SL, Durocher F and Diorio C: Association Between BMI and DNA methylation in blood or normal adult breast tissue: A systematic review. Anticancer Res. 40:1797–1808. 2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Bosviel R, Garcia S, Lavediaux G, Michard E, Dravers M, Kwiatkowski F, Bignon YJ and Bernard-Gallon DJ: BRCA1 promoter methylation in peripheral blood DNA was identified in sporadic breast cancer and controls. Cancer Epidemiol. 36:e177–e182. 2012.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Sturgeon SR, Balasubramanian R, Schairer C, Muss HB, Ziegler RG and Arcaro KF: Detection of promoter methylation of tumor suppressor genes in serum DNA of breast cancer cases and benign breast disease controls. Epigenetics. 7:1258–1267. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Hasan TN, Leena Grace B, Shafi G and Syed R: Association of BRCA1 promoter methylation with rs11655505 (c.2265C>T) variants and decreased gene expression in sporadic breast cancer. Clin Transl Oncol. 15:555–562. 2013.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Buyru N, Altinisik J, Ozdemir F, Demokan S and Dalay N: Methylation profiles in breast cancer. Cancer Invest. 27:307–312. 2009.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Iwamoto T, Yamamoto N, Taguchi T, Tamaki Y and Noguchi S: BRCA1 promoter methylation in peripheral blood cells is associated with increased risk of breast cancer with BRCA1 promoter methylation. Breast Cancer Res Treat. 129:69–77. 2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Dobrovic A and Simpfendorfer D: Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 57:3347–3350. 1997.PubMed/NCBI
|
|
106
|
Gupta S, Jaworska-Bieniek K, Narod SA, Lubinski J, Wojdacz TK and Jakubowska A: Methylation of the BRCA1 promoter in peripheral blood DNA is associated with triple-negative and medullary breast cancer. Breast Cancer Res Treat. 148:615–622. 2014.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Peplonska B, Bukowska A, Wieczorek E, Przybek M, Zienolddiny S and Reszka E: Rotating night work, lifestyle factors, obesity and promoter methylation in BRCA1 and BRCA2 genes among nurses and midwives. PLoS One. 12(e0178792)2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
McCullough LE, Chen J, Cho YH, Khankari NK, Bradshaw PT, White AJ, Garbowski G, Teitelbaum SL, Terry MB, Neugut AI, et al: DNA methylation modifies the association between obesity and survival after breast cancer diagnosis. Breast Cancer Res Treat. 156:183–194. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Choi JY, James SR, Link PA, McCann SE, Hong CC, Davis W, Nesline MK, Ambrosone CB and Karpf AR: Association between global DNA hypomethylation in leukocytes and risk of breast cancer. Carcinogenesis. 30:1889–1897. 2009.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Cho YH, Yazici H, Wu HC, Terry MB, Gonzalez K, Qu M, Dalay N and Santella RM: Aberrant promoter hypermethylation and genomic hypomethylation in tumor, adjacent normal tissues and blood from breast cancer patients. Anticancer Res. 30:2489–2496. 2010.PubMed/NCBI
|
|
111
|
Xu X, Gammon MD, Hernandez-Vargas H, Herceg Z, Wetmur JG, Teitelbaum SL, Bradshaw PT, Neugut AI, Santella RM and Chen J: DNA methylation in peripheral blood measured by LUMA is associated with breast cancer in a population-based study. FASEB J. 26:2657–2666. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Friso S, Choi SW, Dolnikowski GG and Selhub J: A method to assess genomic DNA methylation using high-performance liquid chromatography/electrospray ionization mass spectrometry. Anal Chem. 74:4526–4531. 2002.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Beck CR, Garcia-Perez JL, Badge RM and Moran JV: LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 12:187–215. 2011.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Carraro JC, Mansego ML, Milagro FI, Chaves LO, Vidigal FC, Bressan J and Martínez JA: LINE-1 and inflammatory gene methylation levels are early biomarkers of metabolic changes: Association with adiposity. Biomarkers. 21:625–632. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Karimi M, Johansson S and Ekstrom TJ: Using LUMA: A Luminometric-based assay for global DNA-methylation. Epigenetics. 1:45–48. 2006.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Karimi M, Johansson S, Stach D, Corcoran M, Grandér D, Schalling M, Bakalkin G, Lyko F, Larsson C and Ekström TJ: LUMA (LUminometric Methylation Assay)-a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res. 312:1989–1995. 2006.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Kurdyukov S and Bullock M: DNA methylation analysis: Choosing the right method. Biology (Basel). 5(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Marques-Rocha JL, Milagro FI, Mansego ML, Mourao DM, Martinez JA and Bressan J: LINE-1 methylation is positively associated with healthier lifestyle but inversely related to body fat mass in healthy young individuals. Epigenetics. 11:49–60. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Feinberg AP, Irizarry RA, Fradin D, Aryee MJ, Murakami P, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Gudnason V and Fallin MD: Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med. 2(49ra67)2010.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Piyathilake C, Badiga S, Johanning G, Alvarez R and Partridge E: Predictors and health consequences of epigenetic changes associated with excess body weight in women of child-bearing age. In: Proceedings of the 35th Annual Meeting of the American Society of Preventive Oncology. ASPO, Las Vegas, NV, 2011.
|
|
121
|
Zhu ZZ, Hou L, Bollati V, Tarantini L, Marinelli B, Cantone L, Yang AS, Vokonas P, Lissowska J, Fustinoni S, et al: Predictors of global methylation levels in blood DNA of healthy subjects: A combined analysis. Int J Epidemiol. 41:126–139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Boyne DJ, Friedenreich CM, McIntyre JB, Courneya KS and King WD: Associations between adiposity and repetitive element DNA methylation in healthy postmenopausal women. Epigenomics. 9:1267–1277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Xu Z, Sandler DP and Taylor JA: Blood DNA methylation and breast cancer: A prospective case-cohort analysis in the sister study. J Natl Cancer Inst. 112:87–94. 2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Ducker GS and Rabinowitz JD: One-carbon metabolism in health and disease. Cell Metab. 25:27–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Murin R, Vidomanova E, Kowtharapu BS, Hatok J and Dobrota D: Role of S-adenosylmethionine cycle in carcinogenesis. Gen Physiol Biophys. 36:513–520. 2017.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Bird JK, Ronnenberg AG, Choi SW, Du F, Mason JB and Liu Z: Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J Nutr. 145:79–86. 2015.PubMed/NCBI View Article : Google Scholar
|