|
1
|
American Psychiatric A, American
Psychiatric Association DSMTF (eds): Diagnostic and statistical
manual of mental disorders: DSM-5. American Psychiatric
Association, Arlington, VA, 2013.
|
|
2
|
Chand SP and Arif H: Depression.
StatPearls (Internet): StatPearls Publishing, Treasure Island, FL,
2022.
|
|
3
|
Mekonen T, Chan GCK, Connor JP, Hides L
and Leung J: Estimating the global treatment rates for depression:
A systematic review and meta-analysis. J Affect Disord.
295:1234–1242. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Global prevalence and burden of depressive
and anxiety disorders in 204 countries and territories in 2020 due
to the COVID-19 pandemic. Lancet. 398:1700–1712. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Proudman D, Greenberg P and Nellesen D:
The growing burden of major depressive disorders (MDD):
Implications for researchers and policy makers. Pharmacoeconomics.
39:619–625. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Marasine NR, Sankhi S, Lamichhane R,
Marasini NR and Dangi NB: Use of antidepressants among patients
diagnosed with depression: A scoping review. Biomed Res Int.
2021(6699028)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karrouri R, Hammani Z, Benjelloun R and
Otheman Y: Major depressive disorder: Validated treatments and
future challenges. World J Clin Cases. 9:9350–9367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Roden DM, McLeod HL, Relling MV, Williams
MS, Mensah GA, Peterson JF and Van Driest SL: Pharmacogenomics.
Lancet. 394:521–532. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou J, Li M, Wang X, He Y, Xia Y, Sweeney
JA, Kopp RF, Liu C and Chen C: Drug response-related DNA
methylation changes in schizophrenia, bipolar disorder, and major
depressive disorder. Front Neurosci. 15(674273)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Boku S, Nakagawa S, Toda H and Hishimoto
A: Neural basis of major depressive disorder: Beyond monoamine
hypothesis. Psychiatry Clin Neurosci. 72:3–12. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shadrina M, Bondarenko EA and Slominsky
PA: Genetics factors in major depression disease. Front Psychiatry.
9(334)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Boas GR, de Lacerda RB, Paes MM, Gubert P,
da Cruz AWL, Rescia VC, de Carvalho PMG, de Carvalho AAV and
Oesterreich SA: Molecular aspects of depression: A review from
neurobiology to treatment. Eur J Pharmacol. 851:99–121.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marathe SV, D'Almeida PL, Virmani G,
Bathini P and Alberi L: Effects of monoamines and antidepressants
on astrocyte physiology: Implications for monoamine hypothesis of
depression. J Exp Neurosci. 12(1179069518789149)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tian H, Hu Z, Xu J and Wang C: The
molecular pathophysiology of depression and the new therapeutics.
MedComm (2020). 3(e156)2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Chávez-Castillo M, Núñez V, Nava M, Ortega
Á, Rojas M, Bermúdez V and Rojas-Quintero J: Depression as a
neuroendocrine disorder: Emerging neuropsychopharmacological
approaches beyond monoamines. Adv Pharmacol Sci.
2019(7943481)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Richter-Levin G and Xu L: How could stress
lead to major depressive disorder? IBRO Rep. 4:38–43.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
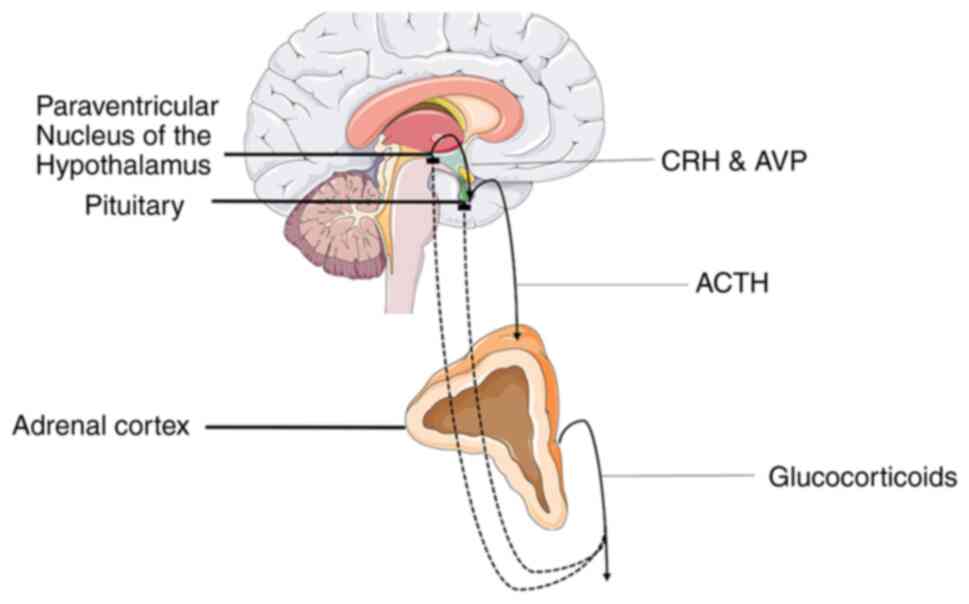
Tsigos C, Kyrou I, Kassi E and Chrousos
GP: Stress: Endocrine physiology and pathophysiology. In: Feingold
KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et
al., (eds). Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright© 2000-2023, MDText.com, Inc.; 2020.
|
|
18
|
Menke A: Is the HPA axis as target for
depression outdated, or is there a new hope? Front Psychiatry.
10(101)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nicolaides NC, Pavlaki AN, Maria Alexandra
MA, Chrousos GP, Feingold KR, Anawalt B, Blackman MR, Boyce A,
Chrousos G, Corpas E, et al: Glucocorticoid therapy and adrenal
suppression. Copyright © 2000-2023, MDText.com, Inc.;
2018.
|
|
20
|
Chen H, Amazit L, Lombès M and Le Menuet
D: Crosstalk between glucocorticoid receptor and early-growth
response protein 1 accounts for repression of brain-derived
neurotrophic factor transcript 4 expression. Neuroscience.
399:12–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Budziñski ML, Sokn C, Gobbini R, Ugo B,
Antunica-Noguerol M, Senin S, Bajaj T, Gassen NC, Rein T, Schmidt
MV, et al: Tricyclic antidepressants target FKBP51 SUMOylation to
restore glucocorticoid receptor activity. Mol Psychiatry.
27:2533–2545. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ronaldson A, Carvalho LA, Kostich K,
Lazzarino AI, Urbanova L and Steptoe A: The effects of six-day SSRI
administration on diurnal cortisol secretion in healthy volunteers.
Psychopharmacology (Berl). 235:3415–3422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roohi E, Jaafari N and Hashemian F: On
inflammatory hypothesis of depression: What is the role of IL-6 in
the middle of the chaos? J Neuroinflammation. 18(45)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Miller AH and Raison CL: The role of
inflammation in depression: From evolutionary imperative to modern
treatment target. Nat Rev Immunol. 16:22–34. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Innes S, Pariante CM and Borsini A:
Microglial-driven changes in synaptic plasticity: A possible role
in major depressive disorder. Psychoneuroendocrinology.
102:236–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schramm E and Waisman A: Microglia as
central protagonists in the chronic stress response. Neurol
Neuroimmunol Neuroinflamm. 9(e200023)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arčan IS, Kouter K and Paska AV:
Depressive disorder and antidepressants from an epigenetic point of
view. World J Psychiatry. 12:1150–1168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grygiel-Górniak B, Limphaibool N and
Puszczewicz M: Cytokine secretion and the risk of depression
development in patients with connective tissue diseases. Psychiatry
Clin Neurosci. 73:302–316. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chockalingam R, Gott BM and Conway CR:
Tricyclic antidepressants and monoamine oxidase inhibitors: Are
they too old for a new look? Handb Exp Pharmacol. 250:37–48.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moraczewski J and Aedma KK: Tricyclic
Antidepressants. StatPearls. Treasure Island (FL): StatPearls
Publishing Copyright©. 2022, StatPearls Publishing LLC.; 2022.
|
|
31
|
Andersen J, Stuhr-Hansen N, Zachariassen
L, Toubro S, Hansen SM, Eildal JN, Bond AD, Bøgesø KP,
Bang-Andersen B, Kristensen AS and Strømgaard K: Molecular
determinants for selective recognition of antidepressants in the
human serotonin and norepinephrine transporters. Proc Natl Acad Sci
USA. 108:12137–12142. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cottingham C, Percival S, Birky T and Wang
Q: Tricyclic antidepressants exhibit variable pharmacological
profiles at the α(2A) adrenergic receptor. Biochem Biophys Res
Commun. 451:461–466. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Laban TS and Saadabadi A: Monoamine
oxidase inhibitors (MAOI). StatPearls. Treasure Island (FL):
StatPearls Publishing Copyright ©. 2022, StatPearls Publishing
LLC.; 2022.
|
|
34
|
Edinoff AN, Akuly HA, Hanna TA, Ochoa CO,
Patti SJ, Ghaffar YA, Kaye AD, Viswanath O, Urits I, Boyer AG, et
al: Selective serotonin reuptake inhibitors and adverse effects: A
narrative review. Neurol Int. 13:387–401. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fuentes AV, Pineda MD and Venkata KCN:
Comprehension of top 200 prescribed drugs in the US as a resource
for pharmacy teaching, training and practice. Pharmacy (Basel).
6(43)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chu A and Wadhwa R: Selective serotonin
reuptake inhibitors. Statpearls. treasure island (FL): StatPearls
publishing copyright©. 2022, StatPearls Publishing LLC.; 2022.
|
|
37
|
Takano A, Halldin C and Farde L: SERT and
NET occupancy by venlafaxine and milnacipran in nonhuman primates:
A PET study. Psychopharmacology (Berl). 226:147–153.
2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fanelli D, Weller G and Liu H: New
serotonin-norepinephrine reuptake inhibitors and their anesthetic
and analgesic considerations. Neurol Int. 13:497–509.
2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li J, Lu C, Gao Z, Feng Y, Luo H, Lu T,
Sun X, Hu J and Luo Y: SNRIs achieve faster antidepressant effects
than SSRIs by elevating the concentrations of dopamine in the
forebrain. Neuropharmacology. 177(108237)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Haller E, Geier M and Finley P:
Antidepressants, pharmacology of. In: Aminoff MJ, Daroff RB,
editors. Encyclopedia of the Neurological Sciences (Second
Edition). Oxford: Academic Press; 2014. p. 219-23.
|
|
41
|
Onaolapo AY and Onaolapo OJ: Glutamate and
depression: Reflecting a deepening knowledge of the gut and brain
effects of a ubiquitous molecule. World J Psychiatry. 11:297–315.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pal MM: Glutamate: The master
neurotransmitter and its implications in chronic stress and mood
disorders. Front Hum Neurosci. 15(722323)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pochwat B, Nowak G and Szewczyk B: An
update on NMDA antagonists in depression. Expert Rev Neurother.
19:1055–1067. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li Y: Modern epigenetics methods in
biological research. Methods. 187:104–113. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sun L, Zhang H and Gao P: Metabolic
reprogramming and epigenetic modifications on the path to cancer.
Protein Cell. 13:877–919. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gougousis S, Petanidis S, Poutoglidis A,
Tsetsos N, Vrochidis P, Skoumpas I, Argyriou N, Katopodi T and
Domvri K: Epigenetic editing and tumor-dependent immunosuppressive
signaling in head and neck malignancies. Oncol Lett.
23(196)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dawson MA and Kouzarides T: Cancer
epigenetics: From mechanism to therapy. Cell. 150:12–27.
2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ling C and Rönn T: Epigenetics in human
obesity and type 2 diabetes. Cell Metab. 29:1028–1044.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Surace AEA and Hedrich CM: The role of
epigenetics in autoimmune/inflammatory disease. Front Immunol.
10(1525)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Menke A, Klengel T and Binder EB:
Epigenetics, depression and antidepressant treatment. Curr Pharm
Des. 18:5879–5889. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fardi M, Solali S and Hagh MF: Epigenetic
mechanisms as a new approach in cancer treatment: An updated
review. Genes Dis. 5:304–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Singh KP, Miaskowski C, Dhruva AA, Flowers
E and Kober KM: Mechanisms and measurement of changes in gene
expression. Biol Res Nurs. 20:369–382. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Corbett AH: Post-transcriptional
regulation of gene expression and human disease. Curr Opin Cell
Biol. 52:96–104. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Landini A, Trbojević-Akmačić I, Navarro P,
Tsepilov YA, Sharapov SZ, Vučković F, Polašek O, Hayward C,
Petrović T, Vilaj M, et al: Genetic regulation of
post-translational modification of two distinct proteins. Nat
Commun. 13(1586)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li X, Zhao Q, Wei W, Lin Q, Magnan C,
Emami MR, Wearick-Silva LE, Viola TW, Marshall PR, Yin J, et al:
The DNA modification N6-methyl-2'-deoxyadenosine (m6dA) drives
activity-induced gene expression and is required for fear
extinction. Nat Neurosci. 22:534–544. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kiselev IS, Kulakova OG, Boyko AN and
Favorova OO: DNA methylation as an epigenetic mechanism in the
development of multiple sclerosis. Acta Naturae. 13:45–57.
2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Dhar GA, Saha S, Mitra P and Chaudhuri RN:
DNA methylation and regulation of gene expression: Guardian of our
health. Nucleus (Calcutta). 64:259–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lee YS: Are we studying non-coding RNAs
correctly? Lessons from nc886. Int J Mol Sci.
23(4251)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Diamantopoulos MA, Tsiakanikas P and
Scorilas A: Non-coding RNAs: The riddle of the transcriptome and
their perspectives in cancer. Ann Transl Med. 6(241)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kumar S, Gonzalez EA, Rameshwar P and
Etchegaray JP: Non-Coding RNAs as mediators of epigenetic changes
in malignancies. Cancers (Basel). 12(3657)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Padda IS, Mahtani AU and Parmar M: Small
interfering RNA (siRNA) based therapy. StatPearls. Treasure island
(FL): StatPearls Publishing Copyright ©. 2022, StatPearls
Publishing LLC.; 2022.
|
|
62
|
Zhang X, Wang W, Zhu W, Dong J, Cheng Y,
Yin Z and Shen F: Mechanisms and functions of long non-coding RNAs
at multiple regulatory levels. Int J Mol Sci.
20(5573)2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chen JJ, Stermer D and Tanny JC: Decoding
histone ubiquitylation. Front Cell Devel Biol.
10(968398)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
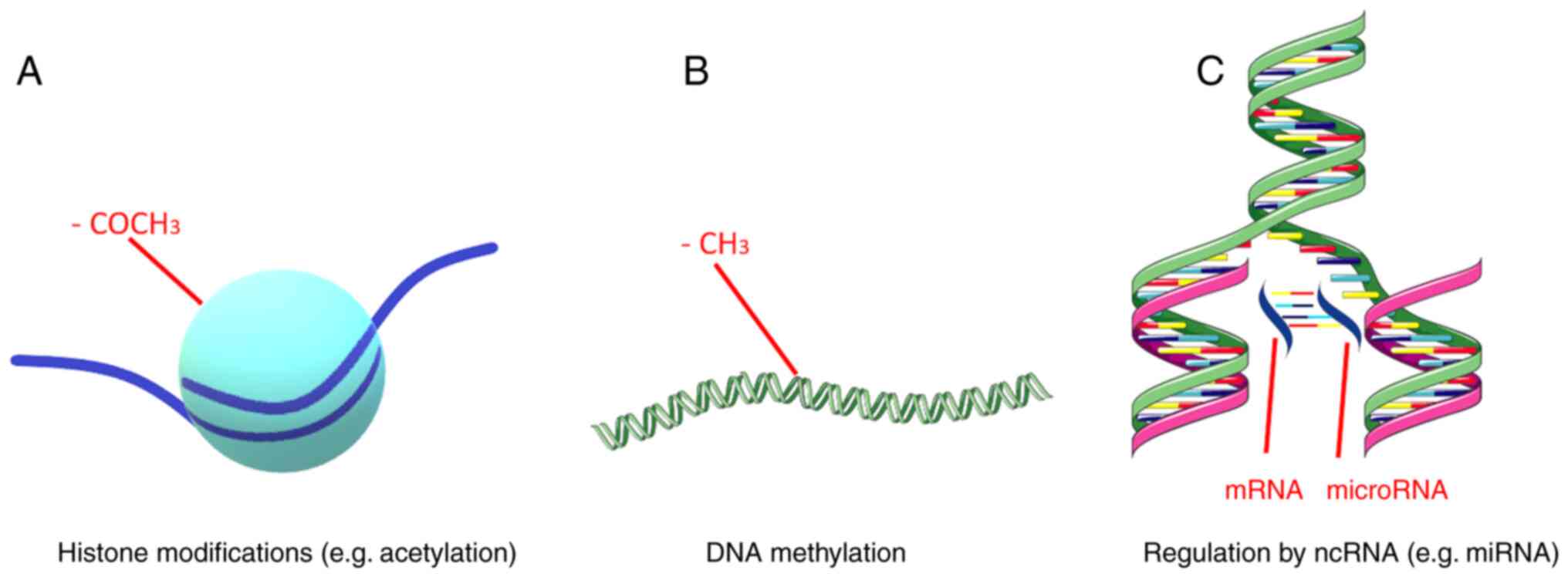
Miller JL and Grant PA: The role of DNA
methylation and histone modifications in transcriptional regulation
in humans. Subcell Biochem. 61:289–317. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhang Y, Sun Z, Jia J, Du T, Zhang N, Tang
Y, Fang Y and Fang D: Overview of histone modification. Adv Exp Med
Biol. 1283:1–16. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Alhamwe BA, Khalaila R, Wolf J, von Bülow
V, Harb H, Alhamdan F, Hii CS, Prescott SL, Ferrante A, Renz H, et
al: Histone modifications and their role in epigenetics of atopy
and allergic diseases. Allergy Asthma Clin Immunol.
14(39)2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Barnes CE, English DM and Cowley SM:
Acetylation & Co: An expanding repertoire of histone acylations
regulates chromatin and transcription. Essays Biochem. 63:97–107.
2019.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sekiguchi M and Matsushita N: DNA damage
response regulation by histone ubiquitination. Int J Mol Sci.
23(8187)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wang J, Qiu Z and Wu Y: Ubiquitin
regulation: The histone modifying Enzyme's story. Cells.
7(118)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Penner-Goeke S and Binder EB: Epigenetics
and depression. Dialogues Clin Neurosci. 21:397–405.
2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Menke A and Binder EB: Epigenetic
alterations in depression and antidepressant treatment. Dialogues
Clin Neurosci. 16:395–404. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wankerl M, Miller R, Kirschbaum C, Hennig
J, Stalder T and Alexander N: Effects of genetic and early
environmental risk factors for depression on serotonin transporter
expression and methylation profiles. Transl Psychiatry.
4(e402)2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Lee JS, Jaini PA and Papa F: An epigenetic
perspective on lifestyle medicine for depression: Implications for
primary care practice. Am J Lifestyle Med. 16:76–88.
2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Meng L, Bai X and Zheng Y, Chen D and
Zheng Y: Altered expression of norepinephrine transporter
participate in hypertension and depression through regulated TNF-α
and IL-6. Clin Exp Hypertens. 42:181–189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Xu Q, Jiang M, Gu S, Wang F and Yuan B:
Early life stress induced DNA methylation of monoamine oxidases
leads to depressive-like behavior. Front Cell Dev Biol.
8(582247)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Humphreys KL, Moore SR, Davis EG, MacIsaac
JL, Lin DTS, Kobor MS and Gotlib IH: DNA methylation of HPA-axis
genes and the onset of major depressive disorder in adolescent
girls: A prospective analysis. Transl Psychiatry.
9(245)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Murgatroyd C, Patchev AV, Wu Y, Micale V,
Bockmühl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OFX and
Spengler D: Dynamic DNA methylation programs persistent adverse
effects of early-life stress. Nat Neurosci. 12:1559–1566.
2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Duan Z and Lu J: DNA methyltransferases in
depression: An update. Front Psychiatry. 11(538683)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Crawford B, Craig Z, Mansell G, White I,
Smith A, Spaull S, Imm J, Hannon E, Wood A, Yaghootkar H, et al:
DNA methylation and inflammation marker profiles associated with a
history of depression. Hum Mol Genet. 27:2840–2850. 2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ryan J, Pilkington L, Neuhaus K, Ritchie
K, Ancelin ML and Saffery R: Investigating the epigenetic profile
of the inflammatory gene IL-6 in late-life depression. BMC
Psychiatry. 17(354)2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Peña CJ and Nestler EJ: Progress in
epigenetics of depression. Prog Mol Biol Transl Sci. 157:41–66.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Park HS, Kim J, Ahn SH and Ryu HY:
Epigenetic targeting of histone deacetylases in diagnostics and
treatment of depression. Int J Mol Sci. 22(5398)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wu MS, Li XJ, Liu CY, Xu Q, Huang JQ, Gu S
and Chen JX: Effects of histone modification in major depressive
disorder. Curr Neuropharmacol. 20:1261–1277. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Rey R, Chauvet-Gelinier JC, Suaud-Chagny
MF, Ragot S, Bonin B, d'Amato T and Teyssier JR: Distinct
expression pattern of epigenetic machinery genes in blood
leucocytes and brain cortex of depressive patients. Mol Neurobiol.
56:4697–4707. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Policarpo R, Sierksma A, De Strooper B and
d'Ydewalle C: From junk to function: LncRNAs in CNS health and
disease. Front Mol Neurosci. 14(714768)2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Lin R and Turecki G: Noncoding RNAs in
depression. Adv Exp Med Biol. 978:197–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Shi Y, Wang Q, Song R, Kong Y and Zhang Z:
Non-coding RNAs in depression: Promising diagnostic and therapeutic
biomarkers. EBioMedicine. 71(103569)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Yoshino Y and Dwivedi Y: Non-coding RNAs
in psychiatric disorders and suicidal behavior. Front Psychiatry.
11(543893)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Wu Y, Rong W, Jiang Q, Wang R and Huang H:
Downregulation of lncRNA GAS5 alleviates hippocampal neuronal
damage in mice with depression-like behaviors via modulation of
MicroRNA-26a/EGR1 axis. J Stroke Cerebrovasc Dis.
30(105550)2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhou Y and Chen B: GAS5-mediated
regulation of cell signaling (Review). Mol Med Rep. 22:3049–3056.
2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Webb LM, Phillips KE, Ho MC, Veldic M and
Blacker CJ: The relationship between DNA methylation and
antidepressant medications: A systematic review. Int J Mol Sci.
21(826)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Czarny P, Białek K, Ziółkowska S,
Strycharz J, Barszczewska G and Sliwinski T: The importance of
epigenetics in diagnostics and treatment of major depressive
disorder. J Person Med. 11(167)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kanherkar RR, Getachew B, Ben-Sheetrit J,
Varma S, Heinbockel T, Tizabi Y and Csoka AB: The effect of
citalopram on genome-wide DNA methylation of human cells. Int J
Genomics. 2018(8929057)2018.PubMed/NCBI View Article : Google Scholar
|