Cerebral ischemia occurs when the blood flow to the
brain is restricted, and it claims the lives of millions worldwide
(1,2). In total, 16% of humans will have a
stroke during their lifetime, with >15 million cases noted
annually (1,3). Stroke is a complex disease with a
narrow time window for therapeutic intervention to restore the
blood supply and prevent permanent brain tissue damage (2). As a result, currently available
strategies are considered inadequate (2). Therefore, there is a need for further
research in order to understand the pathophysiology of the disease
and to identify techniques that can reduce its severe complications
(2).
Clinically relevant models are essential for
cerebral ischemia research. These models should be clinically
relevant and reproducible to aid in the understanding of the
pathophysiology of ischemic stroke, as well as to function as a
platform for the development of novel therapeutic approaches for
stroke treatment.
For a number of years, plants and natural remedies
have been the primary tool for folk medicine. Medicinal plants
provide a cost-effective source of drugs with significant
therapeutic benefits and few side-effects in comparison to
commercial synthetic drugs. Herbal remedies may provide a source of
novel compounds that may present novel therapeutic tools for
cerebral ischemia and stroke. However, with the variants of models
of cerebral ischemia, the literature lacks the link between the
efficacy of the plant and the model used, which may represent a
possible strategy with which to understand the mechanisms of these
natural remedies.
The present review aimed to summarize and discuss
the literature for data related to animal models utilized in the
research of cerebral ischemia, along with the herbal-based
treatment approaches used in cerebral ischemia, in order to draw a
full picture of the model used for treatment. The present review
also aimed to illustrate the possible association between the
natural ingredient in question that proved effective and the models
of cerebral ischemia.
Following the guidelines of Preferred Reporting
Items For Systematic Reviews And Meta-Analyses (PRISMA) (4), the present review was conducted. The
present review aimed to illustrate the data from peer-reviewed
original articles on cerebral ischemia phytotherapy or from studies
using in vivo models of cerebral ischemia. A search was
conducted to obtain targeted articles through PubMed (https://pubmed.ncbi.nlm.nih.gov/) using the
following keywords: ‘Cerebral ischemia and herbal medicine’. The
search span was between 1990 and 2020. In addition, the results
were restricted to studies in the English language.
The inclusion criteria were as follows: i) Only
original studies between 1990 and 2020 were included in the present
review; ii) any original article that assessed or used herbal
extracts as a treatment approach for animal models of cerebral
ischemia. On the other hand, the exclusion criteria were expanded
to the following: i) Articles that reported the combination of
plants as a formula/recipe; ii) research that was not published in
the English language and were between 1990 and 2020; iii) case
reports, review articles, or any secondary publications.
Search results were imported into Endnote X8
(Thompson Reuter) for the deletion of duplicates. The references
were then screened by 3 reviewers, independently, using the
eligibility criteria. The included articles were then reviewed, and
data extraction was performed by 3 independent reviewers. Any
disagreement in the extraction steps was raised to the supervisor
to reach a consensus.
The included articles were investigated through the
‘The Cochrane Collaboration's tool for assessing the risk of bias’
(5). Any disagreements were
discussed between authors to reach a consensus.
The search revealed 830 records; following
title/abstract screening, 370 articles were selected. Following the
screening of the full text of the articles, 52 studies were
included in the present review.
Using the Cochrane risk of bias tool, we were
uncertain of the bias regarding domains 4, 5 and 6(5). However, most of the included studies
showed a low risk of bias in the other domains.
Ischemic stroke accounts for approximately 90% of
all stroke cases in humans, followed by intracerebral hemorrhage
(9%) and subarachnoid hemorrhage (3%). Ischemic stroke occurs due
to the blockage of the middle cerebral artery (MCA). Cerebral
tissue hypoxia and ischemia follow within minutes, leading to
neuronal cell death and permanent damage to the brain (6). Thrombolytics and the rapid
restoration of the blood supply remain the only treatment options
with which to prevent further neuronal damage and decrease
disability (6).
Ischemic damage to both white and grey matter causes
permanent damage to brain tissue (7-9).
Chronic cerebral hypoperfusion causes microglia/astrocyte
activation, matrix metalloproteinase stimulation, blood-brain
barrier disruption and endothelial abnormalities (10-12).
Chronic cerebral hypoperfusion generates neuroinflammation,
oxidative stress and apoptosis of the oligodendroglia (10-12).
Aging, diabetes, atherosclerosis and hypertension are the most
common risk factors that lead to chronic cerebral hypoperfusion
(10-12).
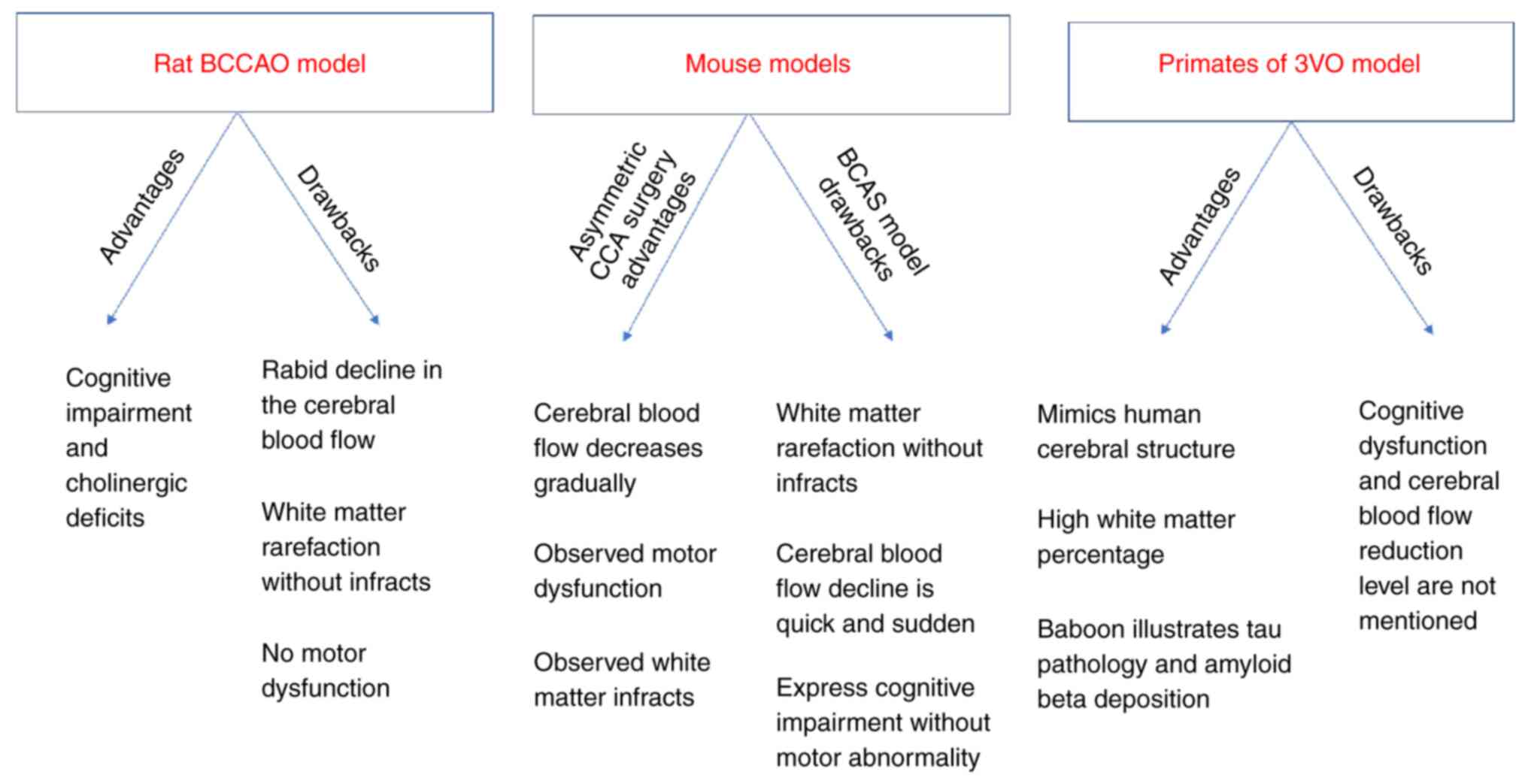
Although there are apparent differences between the
human brain and the brains of other species, animal studies are
critical in translational research. The debate continues as to
whether these differences render the use of animal models in stroke
studies irrelevant to the clinical application (16). These differences are evident in
infarct localization (16).
Another significant difference is the amount of white matter in the
brain. The white matter accounts for 60% of brain tissue in humans,
compared to 35% in dogs, 20% in rabbits, 15% in rats and only 10%
in mice (17). This white matter
difference poses a problem, as the ischemic damage of the white
matter is a key player in the pathophysiology of stroke in humans
(18).
The majority of the stroke preclinical studies are
conducted using small animals, particularly rodents. These studies
have assisted researchers in understanding the molecular and
biochemical processes within the ischemic tissue (23), as well as in understanding the
different aspects of the injury mechanisms (24). However, despite the ease of
handling rodents and the cognitive impairment produced as a
consequence of chronic cerebral hypoperfusion, no motor
abnormalities or white matter infarcts have been generated
(10,25,26).
The guidelines of the Stroke Therapy Academic
Industry Roundtable (STAIR), dictate that clinical studies cannot
be performed on humans before testing the new proposed
pharmaceutical drugs on higher animal species (27-29).
Nonetheless, the use large animal models in research is associated
with various issues. One of these is the need for invasive surgery
to generate and monitor ischemia and this causes a high mortality
rate (24). Furthermore, large
animal models are costly to maintain and are labor-intensive
(24). Moreover, animal rights
organizations have raised several concerns regarding the use of
large animals (16).
One of the apparent advantages of using large
animals, such as dogs, cats, pigs, sheep and primates (30) is that imaging is easier than with
the use of small animals (31). It
is also more suitable to monitor the physiology, e.g., blood
pressure, and blood gases in large animals compared to small ones
(24).
Another advantage is that the brain of large animals
is similar to the human brain in terms of functionality and
structure, i.e. gyrencephalic, while rodents have lissencephalic
brains (24,32). In addition, the ratio of the
neocortex to the basal ganglia and the volume of white matter
indicate that large animals are closer to the human neuroanatomy
(17,33,34).
Furthermore, large animals are considerably similar to humans in
several behavioral aspects, as well as sensorimotor integration
(35). Primates exhibit
similarities to humans regarding the cerebral structure and high
white matter percentage (29,36).
Furthermore, the baboon 3-vessel occlusion (3VO) model has
exhibited a tau pathology and amyloid-β deposition similar to
humans (37).
On the other hand, small animals, particularly
rodents, are less costly to maintain compared to large animals
(17,38). Moreover, the rat physiology and
cerebrovascular anatomy are similar to those of humans (38,39),
and mice have homogeneous genes, and as a result, genetic mutations
are easily achievable to generate transgenic mice, commonly used to
investigate the molecular pathophysiology of stroke (40,41).
The small brain size in rats and mice may be seen as an advantage,
as various fixation procedures can be performed for neurochemical
and biochemical investigations (21).
On the other hand, this technique can lead to the
injury of the underlying cortex or rupture of vessels by drilling
or electrocoagulation (46).
Additionally, the intracranial pressure and blood-brain barrier
(BBB) function are greatly affected, and, as a result, it requires
superior surgical skills (46).
All in all, this method is highly invasive, with several
complications. Therefore, other models have been introduced to
avoid such complications.
This model is reproducible in terms of primary
ischemic injury and, consequently, cell death, blood-brain barrier
(BBB) damage and glial activation (33,52).
It is also appropriate for neuroprotection studies, due to the
considerable existence of ischemic penumbra at the early stages
post-occlusion (33). The infarct
size in this model can be affected by the rat or mouse strain and
the coating material for sutures. Strokes in spontaneously
hypertensive rats (SHRs) are comparatively large and consistent in
size. By contrast, in Sprague-Dawley (SD) rats, the infarcts are
small and vary in size (53). An
inadequate suture type may result in insufficient MCAO, leading to
vessel rupture and subsequent subarachnoid hemorrhage (SAH).
Silicone or poly-l-lysine coating suture is more adherent to
neighboring vascular endothelium, compared to the uncoated suture,
which leads to larger infarcts and minimizes inter-animal
variability (54,55).
The bilateral common carotid artery stenosis (BCAS)
model is a modification that causes carotid stenosis; the severity
of cerebral hypoperfusion can be easily controlled by changing the
diameter of micro-coils inserted in the carotid artery. This model
is used worldwide and can be regarded as one of the most promising
models of chronic cerebral hypoperfusion (10,20,62,63).
This model mimics the white matter lesions induced by chronic
cerebral hypoperfusion in humans (64).
An alternative method is the direct injection of
thrombin into the MCA or internal carotid artery (ICA) to cause
vascular occlusion (66). In this
model, polymerized fibrin with a low number of cells and platelets
are used to produce clots, while most of the human clots contain an
accumulation of both platelets and fibrin, a deposition of
neutrophils and monocytes and a high aggregation percentage of
erythrocyte (67). In addition,
the intravascular introduction of clots causes, in most cases,
multifocal infarcts with noticeable variance in size and the
localization of the lesion (68).
Another method with which to induce embolic stroke
is by using microsphere/macrospheres to block blood flow. The major
discrepancy with this model is that the fabricated spheres lead to
permanent ischemia as they do not dissolve (65). An advantage associated with this
model is the fact that the occlusion in rats may be postponed,
while the animal is under monitor by PET or a magnetic resonance
imaging (MRI) device (69).
In conclusion, as demonstrated through various
sudies, there are various animal models, and associated techniques
to produce cerebral ischemia/reperfusion, and each model has its
advantages and weaknesses. The selection of the model should be
based on the objectives and goal of the research study, bearing in
mind that none of these models is identical to the human stroke
pathophysiology.
Since stroke is associated with problems in the
sensory and motor pathways, researchers have focused on studying
the behavioral and cognitive aspects post-stroke (82). Various functional tests (Table I) are available for use in animals,
including the Rotarod, the grip and string test, the wire hanging
test, the adhesive removal test, the open field maze and the water
maze test (83-95).
The MCAO model has been extensively studied with
numerous natural herbal extracts. Cerebral ischemic injury results
in the production of ROS, which cause lipid, DNA and protein
oxidation, therefore causing cell damage and death (96). A number of these herbs possess
antioxidant, anti-inflammatory and neuroprotective activity, such
as Artemisia absinthium L. (97), Lavandula angustifolia
(98), Scutellaria
baicalensis (99) and several
others. Their active ingredients exert significant neuroprotective
effects and improvement in behavioral function when used before or
after ischemic injury (98-100).
Chinese plants provide a rich source of herbal
extracts for medicinal purposes. Some of these have been widely
investigated in cerebral ischemia. Plants, such as Erigeron
breviscapus (101,102) contain breviscapine, which is
considered to target autophagy mechanisms, leading to a reduction
in infarct size and functional improvements in rats. Scutellarin is
another component of Erigeron breviscapus, which exerts a
decrease in infarct size and functional improvement when injected
into rats (102). Other Chinese
plants include Fructus Chebulae (103) and Fructus Schisandrae
(Chinese magnolia vine fruit) (104), which protect against
metalloproteinase degradation. In rats, these fruits lead to a
reduction in the expression of TNF-a and IL-1b, as well as
reduction in the degradation of the metalloproteinases, MMP-2 and
MMP-9, in ischemic hemispheres, which leads to a reduction in
infarct size (104). Panax
ginseng (105-107)
reduces infarcts size in rats and mice and improves function
through the downregulation of calpain I and caspase 3(107), and the induction of Nrf 2
downstream targets (106).
Panax ginseng has also been used in global and focal
ischemic models (108); it causes
a decrease in lipid peroxidation and mitochondrial swelling
(108).
Other herbal extracts and their active ingredients
used in rodent models of MCAO are summarized in Table II (97-107,113-129).
In Table III, the commonly used
herbal extracts and their active ingredients used in other rodent
models of cerebral ischemia are also listed (98,108-112,130-147).
The possible mechanisms of action of each are highlighted.
Not applicable.
The present study was partially funded by a research
support grant (SSE-BIOL-A.A-FY20) from the American University in
Cairo.
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
NME, NS, EK, AA were involved in the conception and
design of the study, and in the writing and revision of the
manuscript. NE and NS were involved in the production of the figure
and tables.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ,
Howard VJ, et al: Heart disease and stroke statistics-2015 update:
A report from the American Heart Association. Circulation.
131:e29–e322. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dong B, Yang Y, Zhang Z, Xie K, Su L and
Yu Y: Hemopexin alleviates cognitive dysfunction after focal
cerebral ischemia-reperfusion injury in rats. BMC Anesthesiol.
19(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Di Carlo A: Human and economic burden of
stroke. Age Ageing. 38:4–5. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. Ann Intern Med. 151:264–269.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Woodruff TM, Thundyil J, Tang SC, Sobey
CG, Taylor SM and Arumugam TV: Pathophysiology, treatment, and
animal and cellular models of human ischemic stroke. Mol
Neurodegener. 6(11)2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–1706. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kalaria RN: Neuropathological diagnosis of
vascular cognitive impairment and vascular dementia with
implications for Alzheimer's disease. Acta Neuropathol.
131:659–685. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hainsworth AH and Markus HS: Do in vivo
experimental models reflect human cerebral small vessel disease? A
systematic review. J Cereb Blood Flow Metab. 28:1877–1891.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bink DI, Ritz K, Aronica E, Van Der Weerd
L and Daemen MJ: Mouse models to study the effect of cardiovascular
risk factors on brain structure and cognition. J Cereb Blood Flow
Metab. 33:1666–1684. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gorelick PB, Counts SE and Nyenhuis D:
Vascular cognitive impairment and dementia. Biochim Biophys Acta.
1862:860–868. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Venkat P, Chopp M and Chen J: Models and
mechanisms of vascular dementia. Exp Neurol. 272:97–108.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hanke T: Lessons from TGN1412. Lancet.
368:1569–1570; author reply 1570. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Römer PS, Berr S, Avota E, Na SY,
Battaglia M, ten Berge I, Einsele H and Hünig T: Preculture of
PBMCs at high cell density increases sensitivity of T-cell
responses, revealing cytokine release by CD28 superagonist TGN1412.
Blood. 118:6772–6782. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Holloway PM and Gavins FN: Modeling
ischemic stroke in vitro: status quo and future perspectives.
Stroke. 47:561–569. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cook DJ and Tymianski M: Nonhuman primate
models of stroke for translational neuroprotection research.
Neurotherapeutics. 9:371–379. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krafft PR, Bailey EL, Lekic T, Rolland WB,
Altay O, Tang J, Wardlaw JM, Zhang JH and Sudlow CL: Etiology of
stroke and choice of models. Int J Stroke. 7:398–406.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ahmad AS, Satriotomo I, Fazal J, Nadeau SE
and Doré S: Considerations for the optimization of induced white
matter injury preclinical models. Front Neurol.
6(172)2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Edrissi H, Schock SC, Cadonic R, Hakim AM
and Thompson CS: Cilostazol reduces blood brain barrier
dysfunction, white matter lesion formation and motor deficits
following chronic cerebral hypoperfusion. Brain Res. 1646:494–503.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shibata M, Ohtani R, Ihara M and Tomimoto
H: White matter lesions and glial activation in a novel mouse model
of chronic cerebral hypoperfusion. Stroke. 35:2598–2603.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Enmi J, Kitamura A, Yamamoto Y,
Saito S, Takahashi Y, Iguchi S, Tsuji M, Yamahara K, Nagatsuka K,
et al: A novel mouse model of subcortical infarcts with dementia. J
Neurosci. 35:3915–3928. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen A, Akinyemi RO, Hase Y, Firbank MJ,
Ndung'u MN, Foster V, Craggs LJ, Washida K, Okamoto Y, Thomas AJ,
et al: Frontal white matter hyperintensities, clasmatodendrosis and
gliovascular abnormalities in ageing and post-stroke dementia.
Brain. 139:242–258. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McCabe C, Arroja MM, Reid E and Macrae IM:
Animal models of ischaemic stroke and characterisation of the
ischaemic penumbra. Neuropharmacology. 134:169–177. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Traystman RJ: Animal models of focal and
global cerebral ischemia. ILAR J. 44:85–95. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Farkas E, Luiten PG and Bari F: Permanent,
bilateral common carotid artery occlusion in the rat: A model for
chronic cerebral hypoperfusion-related neurodegenerative diseases.
Brain Res Rev. 54:162–180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nishio K, Ihara M, Yamasaki N, Kalaria RN,
Maki T, Fujita Y, Ito H, Oishi N, Fukuyama H, Miyakawa T, et al: A
mouse model characterizing features of vascular dementia with
hippocampal atrophy. Stroke. 41:1278–1284. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stem Cell Therapies as an Emerging
Paradigm in Stroke Participants: Stem cell therapies as an emerging
paradigm in stroke (STEPS): Bridging basic and clinical science for
cellular and neurogenic factor therapy in treating stroke. Stroke
40: 510-515, 2009.
|
|
28
|
Savitz SI, Chopp M, Deans R, Carmichael S,
Phinney D and Wechsler L: STEPS Participants: Stem cell therapy as
an emerging paradigm for stroke (STEPS) II. Stroke. 42:825–829.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stroke Therapy Academic Industry
Roundtable (STAIR): Recommendations for standards regarding
preclinical neuroprotective and restorative drug development.
Stroke 30: 2752-2758, 1999.
|
|
30
|
Bacigaluppi M, Comi G and Hermann DM:
Animal models of ischemic stroke. Part two: Modeling cerebral
ischemia. Open Neurol J. 4:34–38. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Marshall J, Ridley R, Baker H, Hall L,
Carpenter T and Wood N: Serial MRI, functional recovery, and
long-term infarct maturation in a non-human primate model of
stroke. Brain Res Bull. 61:577–585. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cook DJ, Teves L and Tymianski M:
Treatment of stroke with a PSD-95 inhibitor in the gyrencephalic
primate brain. Nature. 483:213–217. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Howells DW, Porritt MJ, Rewell SS,
O'collins V, Sena ES, Van Der Worp HB, Traystman RJ and Macleod MR:
Different strokes for different folks: The rich diversity of animal
models of focal cerebral ischemia. J Cereb Blood Flow Metab.
30:1412–1431. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Macrae I: Preclinical stroke
research-advantages and disadvantages of the most common rodent
models of focal ischaemia. Br J Pharmacol. 164:1062–1078.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Canazza A, Minati L, Boffano C, Parati E
and Binks S: Experimental models of brain ischemia: A review of
techniques, magnetic resonance imaging, and investigational
cell-based therapies. Front Neurol. 5(19)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Madigan JB, Wilcock DM and Hainsworth AH:
Vascular contributions to cognitive impairment and dementia:
Topical review of animal models. Stroke. 47:1953–1959.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ndung'u M, Härtig W, Wegner F, Mwenda J,
Low R, Akinyemi R and Kalaria RN: Cerebral amyloid β(42) deposits
and microvascular pathology in ageing baboons. Neuropathol Appl
Neurobiol. 38:487–499. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: Overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu F and McCullough LD: Middle cerebral
artery occlusion model in rodents: Methods and potential pitfalls.
J Biomed Biotechnol. 2011(464701)2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kraft P, Göb E, Schuhmann MK, Göbel K,
Deppermann C, Thielmann I, Herrmann AM, Lorenz K, Brede M, Stoll G,
et al: FTY720 ameliorates acute ischemic stroke in mice by reducing
thrombo-inflammation but not by direct neuroprotection. Stroke.
44:3202–3210. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Göb E, Reymann S, Langhauser F, Schuhmann
MK, Kraft P, Thielmann I, Göbel K, Brede M, Homola G, Solymosi L,
et al: Blocking of plasma kallikrein ameliorates stroke by reducing
thromboinflammation. Ann Neurol. 77:784–803. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dirnagl U and Macleod MR: Stroke research
at a road block: The streets from adversity should be paved with
meta-analysis and good laboratory practice. Br J Pharmacol.
157:1154–1156. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yanamoto H, Nagata I, Niitsu Y, Xue JH,
Zhang Z and Kikuchi H: Evaluation of MCAO stroke models in
normotensive rats: Standardized neocortical infarction by the 3VO
technique. Exp Neurol. 182:261–274. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dirnagl U: Rodent models of stroke:
Springer, 2010.
|
|
45
|
Buchan AM, Xue D and Slivka A: A new model
of temporary focal neocortical ischemia in the rat. Stroke.
23:273–279. 1992.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sugimori H, Yao H, Ooboshi H, Ibayashi S
and Iida M: Krypton laser-induced photothrombotic distal middle
cerebral artery occlusion without craniectomy in mice. Brain Res
Brain Res Protoc. 13:189–196. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bogousslavsky J, Van Melle G and Regli F:
The Lausanne Stroke Registry: Analysis of 1,000 consecutive
patients with first stroke. Stroke. 19:1083–1092. 1988.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemic brain edema. 1. A new
experimental model of cerebral embolism in rats in which
recirculation can be introduced in the ischemic area. Jpn J Stroke.
8:1–8. 1986.
|
|
49
|
Smith HK, Russell JM, Granger DN and
Gavins FN: Critical differences between two classical surgical
approaches for middle cerebral artery occlusion-induced stroke in
mice. J Neurosci Methods. 249:99–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chiang T, Messing RO and Chou WH: Mouse
model of middle cerebral artery occlusion. J Vis Exp.
(e2761)2011.PubMed/NCBI View
Article : Google Scholar
|
|
51
|
Garcia JH, Liu KF and Ho KL: Neuronal
necrosis after middle cerebral artery occlusion in Wistar rats
progresses at different time intervals in the caudoputamen and the
cortex. Stroke. 26:636–643, Discussion 643. 1995.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kuraoka M, Furuta T, Matsuwaki T, Omatsu
T, Ishii Y, Kyuwa S and Yoshikawa Y: Direct experimental occlusion
of the distal middle cerebral artery induces high reproducibility
of brain ischemia in mice. Exp Anim. 58:19–29. 2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Duverger D and MacKenzie ET: The
quantification of cerebral infarction following focal ischemia in
the rat: Influence of strain, arterial pressure, blood glucose
concentration, and age. J Cereb Blood Flow Metab. 8:449–461.
1988.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Belayev L, Alonso OF, Busto R, Zhao W and
Ginsberg MD: Middle cerebral artery occlusion in the rat by
intraluminal suture. Neurological and pathological evaluation of an
improved model. Stroke. 27:1616–1623. 1996.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schmid-Elsaesser R, Zausinger S,
Hungerhuber E, Baethmann A and Reulen HJ: A critical reevaluation
of the intraluminal thread model of focal cerebral ischemia.
Stroke. 29:2162–2170. 1998.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li F, Omae T and Fisher M: Spontaneous
hyperthermia and its mechanism in the intraluminal suture middle
cerebral artery occlusion model of rats. Stroke. 30:2464–2470,
Discussion 2470-2471. 1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Barber PA, Hoyte L, Colbourne F and Buchan
AM: Temperature-regulated model of focal ischemia in the mouse: A
study with histopathological and behavioral outcomes. Stroke.
35:1720–1725. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hossmann KA: The two pathophysiologies of
focal brain ischemia: Implications for translational stroke
research. J Cereb Blood Flow Metab. 32:1310–1316. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Demarin V, Zavoreo I and Kes VB: Carotid
artery disease and cognitive impairment. J Neurol Sci. 322:107–111.
2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
de Bruijn RF, Heeringa J, Wolters FJ,
Franco OH, Stricker BH, Hofman A, Koudstaal PJ and Ikram MA:
Association between atrial fibrillation and dementia in the general
population. JAMA Neurol. 72:1288–1294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Adelborg K, Szépligeti S, Sundbøll J,
Horváth-Puhó E, Henderson VW, Ording A, Pedersen L and Sørensen HT:
Risk of stroke in patients with heart failure: A population-based
30-year cohort study. Stroke. 48:1161–1168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shibata M, Yamasaki N, Miyakawa T, Kalaria
RN, Fujita Y, Ohtani R, Ihara M, Takahashi R and Tomimoto H:
Selective impairment of working memory in a mouse model of chronic
cerebral hypoperfusion. Stroke. 38:2826–2832. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ihara M, Taguchi A, Maki T, Washida K and
Tomimoto H: A mouse model of chronic cerebral hypoperfusion
characterizing features of vascular cognitive impairment. Methods
Mol Biol. 1135:95–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Washida K, Hattori Y and Ihara M: Animal
models of chronic cerebral hypoperfusion: From mouse to primate.
Int J Mol Sci. 20(6176)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sommer CJ: Ischemic stroke: Experimental
models and reality. Acta Neuropathol. 133:245–261. 2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Orset C, Macrez R, Young AR, Panthou D,
Angles-Cano E, Maubert E, Agin V and Vivien D: Mouse model of in
situ thromboembolic stroke and reperfusion. Stroke. 38:2771–2778.
2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Smith WS, Sung G, Starkman S, Saver JL,
Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM,
et al: Safety and efficacy of mechanical embolectomy in acute
ischemic stroke: Results of the MERCI trial. Stroke. 36:1432–1438.
2005.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Niessen F, Hilger T, Hoehn M and Hossmann
KA: Differences in clot preparation determine outcome of
recombinant tissue plasminogen activator treatment in experimental
thromboembolic stroke. Stroke. 34:2019–2024. 2003.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Walberer M and Rueger MA: The macrosphere
model-an embolic stroke model for studying the pathophysiology of
focal cerebral ischemia in a translational approach. Ann Transl
Med. 3(123)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Macrae IM, Robinson MJ, Graham DI, Reid JL
and McCulloch J: Endothelin-1-induced reductions in cerebral blood
flow: Dose dependency, time course, and neuropathological
consequences. J Cereb Blood Flow Metab. 13:276–284. 1993.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Bogaert L, Scheller D, Moonen J, Sarre S,
Smolders I, Ebinger G and Michotte Y: Neurochemical changes and
laser Doppler flowmetry in the endothelin-1 rat model for focal
cerebral ischemia. Brain Res. 887:266–275. 2000.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Biernaskie J, Corbett D, Peeling J, Wells
J and Lei H: A serial MR study of cerebral blood flow changes and
lesion development following endothelin-1-induced ischemia in rats.
Magn Reson Med. 46:827–830. 2001.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hughes PM, Anthony DC, Ruddin M, Botham
MS, Rankine EL, Sablone M, Baumann D, Mir AK and Perry VH: Focal
lesions in the rat central nervous system induced by endothelin-1.
J Neuropathol Exp Neurol. 62:1276–1286. 2003.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Horie N, Maag AL, Hamilton SA, Shichinohe
H, Bliss TM and Steinberg GK: Mouse model of focal cerebral
ischemia using endothelin-1. J Neurosci Methods. 173:286–290.
2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ansari S, Azari H, Caldwell KJ, Regenhardt
RW, Hedna VS, Waters MF, Hoh BL and Mecca AP: Endothelin-1 induced
middle cerebral artery occlusion model for ischemic stroke with
laser Doppler flowmetry guidance in rat. J Vis Exp.
(50014)2013.PubMed/NCBI View
Article : Google Scholar
|
|
76
|
Kim GW, Sugawara T and Chan PH:
Involvement of oxidative stress and caspase-3 in cortical
infarction after photothrombotic ischemia in mice. J Cereb Blood
Flow Metab. 20:1690–1701. 2000.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kleinschnitz C, Braeuninger S, Pham M,
Austinat M, Nölte I, Renné T, Nieswandt B, Bendszus M and Stoll G:
Blocking of platelets or intrinsic coagulation pathway-driven
thrombosis does not prevent cerebral infarctions induced by
photothrombosis. Stroke. 39:1262–1268. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Watson BD, Dietrich WD, Busto R, Wachtel
MS and Ginsberg MD: Induction of reproducible brain infarction by
photochemically initiated thrombosis. Ann Neurol. 17:497–504.
1985.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Dietrich WD, Ginsberg MD, Busto R and
Watson BD: Photochemically induced cortical infarction in the rat.
1. Time course of hemodynamic consequences. J Cereb Blood Flow
Metab. 6:184–194. 1986.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Lee VM, Burdett NG, Carpenter A, Hall LD,
Pambakian PS, Patel S, Wood NI and James MF: Evolution of
photochemically induced focal cerebral ischemia in the rat.
Magnetic resonance imaging and histology. Stroke. 27:2110–2119.
1996.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Provenzale JM, Jahan R, Naidich TP and Fox
AJ: Assessment of the patient with hyperacute stroke: Imaging and
therapy. Radiology. 229:347–359. 2003.PubMed/NCBI View Article : Google Scholar
|
|
82
|
DeVries AC, Nelson RJ, Traystman RJ and
Hurn PD: Cognitive and behavioral assessment in experimental stroke
research: Will it prove useful? Neurosci Biobehav Rev. 25:325–342.
2001.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Shiotsuki H, Yoshimi K, Shimo Y, Funayama
M, Takamatsu Y, Ikeda K, Takahashi R, Kitazawa S and Hattori N: A
rotarod test for evaluation of motor skill learning. J Neurosci
Methods. 189:180–185. 2010.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Balkaya M, Kröber JM, Rex A and Endres M:
Assessing post-stroke behavior in mouse models of focal ischemia. J
Cereb Blood Flow Metab. 33:330–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Lee JK, Park MS, Kim YS, Moon KS, Joo SP,
Kim TS and Kim SH: Photochemically induced cerebral ischemia in a
mouse model. Surg Neurol. 67:620–625. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
De Luca A, Tinsley J, Aartsma-Rus A, van
Putten M, Nagaraju K, de La Porte S, Dubach-Powell J and Carlson G:
Use of grip strength meter to assess the limb strength of mdx mice.
SOP DMD_M.2. 2008.
|
|
87
|
Ishrat T, Sayeed I, Atif F and Stein DG:
Effects of progesterone administration on infarct volume and
functional deficits following permanent focal cerebral ischemia in
rats. Brain Res. 1257:94–101. 2009.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Hoffman E and Winder SJ: A modified wire
hanging apparatus for small animal muscle function testing. PLoS
Curr 8.
(ecurrents.md.1e2bec4e78697b7b0ff80ea25a1d38be)2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Gerlai R, Thibodeaux H, Palmer JT, van
Lookeren Campagne M and Van Bruggen N: Transient focal cerebral
ischemia induces sensorimotor deficits in mice. Behav Brain Res.
108:63–71. 2000.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Bouet V, Boulouard M, Toutain J, Divoux D,
Bernaudin M, Schumann-Bard P and Freret T: The adhesive removal
test: A sensitive method to assess sensorimotor deficits in mice.
Nat Protoc. 4:1560–1564. 2009.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Seibenhener ML and Wooten MC: Use of the
Open Field Maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. (e52434)2015.PubMed/NCBI View
Article : Google Scholar
|
|
92
|
Gould TD, Dao DT and Kovacsics CE: The
open field test. Mood and anxiety related phenotypes in mice.
Springer, pp1-20, 2009.
|
|
93
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Chen J, Sanberg PR, Li Y, Wang L, Lu M,
Willing AE, Sanchez-Ramos J and Chopp M: Intravenous administration
of human umbilical cord blood reduces behavioral deficits after
stroke in rats. Stroke. 32:2682–2688. 2001.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Clark WM, Lessov NS, Dixon MP and
Eckenstein F: Monofilament intraluminal middle cerebral artery
occlusion in the mouse. Neurol Res. 19:641–648. 1997.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Niizuma K, Endo H and Chan PH: Oxidative
stress and mitochondrial dysfunction as determinants of ischemic
neuronal death and survival. J Neurochem. 109:133–138.
2009.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Bora KS and Sharma A: Neuroprotective
effect of Artemisia absinthium L. on focal ischemia and
reperfusion-induced cerebral injury. J Ethnopharmacol. 129:403–409.
2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang D, Yuan X, Liu T, Liu L, Hu Y, Wang Z
and Zheng Q: Neuroprotective activity of lavender oil on transient
focal cerebral ischemia in mice. Molecules. 17:9803–9817.
2012.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Dai J, Qiu YM, Ma ZW, Yan GF, Zhou J, Li
SQ, Wu H, Jin YC and Zhang XH: Neuroprotective effect of baicalin
on focal cerebral ischemia in rats. Neural Regen Res. 13:2129–2133.
2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Cao ZQ, Quan W, Hou SX, Guo C, Ma SB,
Zhang W and Li X: The natural therapeutic magnesium lithospermate B
potently provides neuroprotective effects on cerebral
ischemia/reperfusion injury in rats. J Ethnopharmacol. 162:191–198.
2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Pengyue Z, Tao G, Hongyun H, Liqiang Y and
Yihao D: Breviscapine confers a neuroprotective efficacy against
transient focal cerebral ischemia by attenuating neuronal and
astrocytic autophagy in the penumbra. Biomed Pharmacother.
90:69–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Guo H, Hu LM, Wang SX, Wang YL, Shi F, Li
H, Liu Y, Kang LY and Gao XM: Neuroprotective effects of
scutellarin against hypoxic-ischemic-induced cerebral injury via
augmentation of antioxidant defense capacity. Chin J Physiol.
54:399–405. 2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gaire BP and Kim HJ: Neuroprotective
effects of Fructus Chebulae extracts on experimental models of
cerebral ischemia. J Tradit Chin Med. 34:69–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Lee TH, Jung CH and Lee DH:
Neuroprotective effects of Schisandrin B against transient focal
cerebral ischemia in Sprague-Dawley rats. Food Chem Toxicol.
50:4239–4245. 2012.PubMed/NCBI View Article : Google Scholar
|
|
105
|
He B, Chen P, Yang J, Yun Y, Zhang X, Yang
R and Shen Z: Neuroprotective effect of 20(R)-ginsenoside Rg(3)
against transient focal cerebral ischemia in rats. Neurosci Lett.
526:106–111. 2012.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Liu L, Vollmer MK, Fernandez VM, Dweik Y,
Kim H and Doré SJ: Korean red ginseng pretreatment protects against
long-term sensorimotor deficits after ischemic stroke likely
through Nrf2. Front Cell Neurosci. 12(74)2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Dong X, Zheng L, Lu S and Yang YJ:
Neuroprotective effects of pretreatment of ginsenoside Rb1 on
severe cerebral ischemia-induced injuries in aged mice: Involvement
of anti-oxidant signaling. Geriatr Gerontol Int. 17:338–345.
2017.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Chen LM, Zhou XM, Cao YL and Hu WX:
Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion
injury in rats. J Asian Nat Prod Res. 10:439–445. 2008.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Duan W, Wang L, Lv J, Gao K, Lu Y, Qin S,
Ma X, Li J and Ge X: Metabolomics study on the effects of
salvianolic acid B and borneol for treating cerebral ischemia in
rats by ultra-performance liquid chromatography quadrupole
time-of-flight mass spectrometry. Rejuvenation Res. 22:313–324.
2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Hong JT, Ryu SR, Kim HJ, Lee JK, Lee SH,
Kim DB, Yun YP, Ryu JH, Lee BM and Kim PY: Neuroprotective effect
of green tea extract in experimental ischemia-reperfusion brain
injury. Brain Res Bull. 53:743–749. 2000.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Graham HN: Green tea composition,
consumption, and polyphenol chemistry. Prev Med. 21:334–350.
1992.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Mukherjee PK, Ahamed KN, Kumar V,
Mukherjee K and Houghton PJ: Protective effect of biflavones from
Araucaria bidwillii Hook in rat cerebral ischemia/reperfusion
induced oxidative stress. Behav Brain Res. 178:221–228.
2007.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Nazam Ansari M, Bhandari U, Islam F and
Tripathi CD: Evaluation of antioxidant and neuroprotective effect
of ethanolic extract of Embelia ribes Burm in focal cerebral
ischemia/reperfusion-induced oxidative stress in rats. Fundam Clin
Pharmacol. 22:305–314. 2008.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ferreira Ede O, Fernandes MY, Lima NM,
Neves KR, Carmo MR, Lima FA, Fonteles AA, Menezes AP and Andrade
GM: Neuroinflammatory response to experimental stroke is inhibited
by eriodictyol. Behav Brain Res. 312:321–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Lee D, Park J, Yoon J, Kim MY, Choi HY and
Kim HJ: Neuroprotective effects of Eleutherococcus senticosus bark
on transient global cerebral ischemia in rats. J Ethnopharmacol.
139:6–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Luo L, Kim SW, Lee HK, Kim ID, Lee H and
Lee JK: Anti-Zn2+-toxicity of 4-hydroxybenzyl alcohol in
astrocytes and neurons contribute to a robust neuroprotective
effects in the postischemic brain. Cell Mol Neurobiol. 38:615–626.
2018.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Akhtar M, Maikiyo AM, Najmi AK, Khanam R,
Mujeeb M and Aqil M: Neuroprotective effects of chloroform and
petroleum ether extracts of Nigella sativa seeds in stroke model of
rat. J Pharm Bioallied Sci. 5(119)2013.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Wang C, Zhang D, Ma H and Liu JJ:
Neuroprotective effects of emodin-8-O-beta-d-glucoside in vivo and
in vitro. Eur J Pharmacol. 577:58–63. 2007.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Guo C, Tong L, Xi M, Yang H, Dong H and
Wen AJ: Neuroprotective effect of calycosin on cerebral ischemia
and reperfusion injury in rats. Cell Physiol Biochem. 144:768–774.
2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Chang Y, Hsieh CY, Peng ZA, Yen TL, Hsiao
G, Chou DS, Chen CM and Sheu JR: Neuroprotective mechanisms of
puerarin in middle cerebral artery occlusion-induced brain
infarction in rats. J Biomed Sci. 16(9)2009.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Meng X, Xie W, Xu Q, Liang T, Xu X, Sun G
and Sun X: Neuroprotective effects of radix scrophulariae on
cerebral ischemia and reperfusion injury via MAPK pathways.
Molecules. 23(2401)2018.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Kaneko Y, Eve DJ, Yu S, Shojo H, Bae EC,
Park DH, Roschek B Jr, Alberte RS, Sanberg PR, Sanberg CD, et al:
Acute treatment with herbal extracts provides neuroprotective
benefits in in vitro and in vivo stroke models, characterized by
reduced ischemic cell death and maintenance of motor and
neurological functions. Cell Med. 1:137–142. 2010.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Guo C, Yin Y, Duan J, Zhu Y, Yan J, Wei G,
Guan Y, Wu X, Wang Y, Xi M and Wen A: Neuroprotective effect and
underlying mechanism of sodium danshensu [3-(3,4-dihydroxyphenyl)
lactic acid from Radix and Rhizoma Salviae miltiorrhizae=Danshen]
against cerebral ischemia and reperfusion injury in rats.
Phytomedicine. 22:283–289. 2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Lam BY, Lo AC, Sun X, Luo HW, Chung SK and
Sucher NJ: Neuroprotective effects of tanshinones in transient
focal cerebral ischemia in mice. Phytomedicine. 10:286–291.
2003.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Cui L, Zhang X, Yang R, Wang L, Liu L, Li
M and Du W: Neuroprotection and underlying mechanisms of oxymatrine
in cerebral ischemia of rats. Neurol Res. 33:319–324.
2011.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Park S, Nam K, Lee H, Cho EY, Koo U and
Mar W: Neuroprotective effects of an alkaloid-free ethyl acetate
extract from the root of Sophora flavescens Ait. against
focal cerebral ischemia in rats. Phytomedicine. 16:1042–1051.
2009.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Li W, Yang Y, Hu Z, Ling S and Fang M:
Neuroprotective effects of DAHP and Triptolide in focal cerebral
ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway
activation. Front Neuroanat. 9(48)2015.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Lee HF, Lee TS and Kou YR:
Anti-inflammatory and neuroprotective effects of triptolide on
traumatic brain injury in rats. Respir Physiol Neurobiol. 182:1–8.
2012.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Gupta S and Gupta YK: Combination of
Zizyphus jujuba and silymarin showed better neuroprotective
effect as compared to single agent in MCAo-induced focal cerebral
ischemia in rats. J Ethnopharmacol. 197:118–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Chen JH, Kuo HC, Lee KF and Tsai TH:
Magnolol protects neurons against ischemia injury via the
downregulation of p38/MAPK, CHOP and nitrotyrosine. Toxicol Appl
Pharmacol. 279:294–302. 2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Gong J, Sun F, Li Y, Zhou X, Duan Z, Duan
F, Zhao L, Chen H, Qi S and Shen J: Momordica charantia
polysaccharides could protect against cerebral ischemia/reperfusion
injury through inhibiting oxidative stress mediated c-Jun
N-terminal kinase 3 signaling pathway. Neuropharmacology.
91:123–134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Bora KS, Arora S and Shri R: Role of
Ocimum basilicum L. in prevention of ischemia and
reperfusion-induced cerebral damage, and motor dysfunctions in mice
brain. J Ethnopharmacol. 137:1360–1365. 2011.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Dringen R: Metabolism and functions of
glutathione in brain. Prog Neurobiol. 62:649–671. 2000.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Siddiqui BS, Aslam H, Ali ST, Begu S and
Khatoon N: Two new triterpenoids and a steroidal glycoside from the
aerial parts of Ocimum basilicum. Chem Pharm Bull (Tokyo).
55:516–519. 2007.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Yanpallewar S, Rai S, Kumar M and Acharya
SB: Evaluation of antioxidant and neuroprotective effect of Ocimum
sanctum on transient cerebral ischemia and long-term cerebral
hypoperfusion. Pharmacol Biochem Behav. 79:155–164. 2004.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Fki I, Sahnoun Z and Sayadi S:
Hypocholesterolemic effects of phenolic extracts and purified
hydroxytyrosol recovered from olive mill wastewater in rats fed a
cholesterol-rich diet. J Agric Food Chem. 55:624–631.
2007.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Mohagheghi F, Bigdeli MR, Rasoulian B,
Zeinanloo AA and Khoshbaten A: Dietary virgin olive oil reduces
blood brain barrier permeability, brain edema, and brain injury in
rats subjected to ischemia-reperfusion. ScientificWorldJournal.
10:180–191. 2010.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Rabiei Z, Bigdeli MR and Rasoulian B:
Neuroprotection of dietary virgin olive oil on brain lipidomics
during stroke. Curr Neurovasc Res. 10:231–237. 2013.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Bayat M, Azami Tameh A, Hossein Ghahremani
M, Akbari M, Mehr SE, Khanavi M and Hassanzadeh G: Neuroprotective
properties of Melissa officinalis after hypoxic-ischemic injury
both in vitro and in vivo. Daru. 20(42)2012.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Rabiei Z and Rafieian-Kopaei M:
Neuroprotective effect of pretreatment with Lavandula officinalis
ethanolic extract on blood-brain barrier permeability in a rat
stroke model. Asian Pac J Trop Med. 7S1:S421–S426. 2014.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Cao Y, Maoa X, Sun C, Zheng P, Gao J, Wang
X, Min D, Sun H, Xie N and Cai J: Baicalin attenuates global
cerebral ischemia/reperfusion injury in gerbils via anti-oxidative
and anti-apoptotic pathways. Brain Res Bull. 85:396–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Han BH, D'Costa A, Back SA, Parsadanian M,
Patel S, Shah AR, Gidday JM, Srinivasan A, Deshmukh M and Holtzman
DM: BDNF blocks caspase-3 activation in neonatal hypoxia-ischemia.
Neurobiol Dis. 7:38–53. 2000.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Zhang ZJ, Li P, Wang Z, Li PT, Zhang WS,
Sun ZH, Zhang XJ and Wang YY: A comparative study on the individual
and combined effects of baicalin and jasminoidin on focal cerebral
ischemia-reperfusion injury. Brain Res. 1123:188–195.
2006.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Kim HJ, Lee SR and Moon KD: Ether fraction
of methanol extracts of Gastrodia elata, medicinal herb protects
against neuronal cell damage after transient global ischemia in
gerbils. Phytother Res. 17:909–912. 2003.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Yu SJ, Kim JR, Lee CK, Han JE, Lee JH, Kim
HS, Hong JH and Kang SG: Gastrodia elata blume and an active
component, p-hydroxybenzyl alcohol reduce focal ischemic brain
injury through antioxidant related gene expressions. Biol Pharm
Bull. 28:1016–1020. 2005.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Joyeux M, Lobstein A, Anton R and Mortier
F: Comparative antilipoperoxidant, antinecrotic and scavenging
properties of terpenes and biflavones from Ginkgo and some
flavonoids. Planta Med. 61:126–129. 1995.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Calapai G, Crupi A, Firenzuoli F, Marciano
MC, Squadrito F, Inferrera G, Parisi A, Rizzo A, Crisafulli C,
Fiore A and Caputi AP: Neuroprotective effects of Ginkgo
biloba extract in brain ischemia are mediated by inhibition of
nitric oxide synthesis. Life Sci. 67:2673–2683. 2000.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Yan XB, Wang SS, Hou HL, Ji R and Zhou JN:
Lithium improves the behavioral disorder in rats subjected to
transient global cerebral ischemia. Behav Brain Res. 177:282–289.
2007.PubMed/NCBI View Article : Google Scholar
|