Introduction
Oral bacteria have been associated not only with
dental and periodontal diseases, but also with serious systemic
diseases (1,2). Oral hygiene is essential,
particularly for hospitalized patients who are undernourished. This
is due to the fact that in immunosuppressed patients, poor oral
hygiene is more likely to result in aspiration pneumonia (AP),
which is caused by the aspiration of foreign materials into the
bronchial airway (3-8).
AP is among the most common causes of mortality among elderly
Japanese individuals and nursing homes residents, and is most often
caused by the pathogenic bacterium, Streptococcus pneumoniae
(9). Previous studies have found
that the quantity of aspirated bacteria is a major factor in the
development of pneumonia (4,10).
Moreover, in high-risk patients, such as those who are bedridden,
have dysphagia or other eating disorders, and/or are
immunosuppressed, the oral cavity may comprise a reservoir of
pathogens that potentially cause AP.
Oral hygiene, including professional oral care,
reduces bacterial levels in the oral cavity (9,11).
In the elderly, daily oral care has been shown to decrease the
frequency of fever and pneumonia-related mortality (8). Oral health assessment, management and
care are of particular importance for groups of patients that
require nutritional support team (NST) intervention, including
those with head and neck cancer, and those who are malnourished,
are older and are frail (12,13).
Manabe et al reported that sputum suctioning, the
deterioration of swallowing function, dehydration and dementia were
risk factors for AP in older adults; however, they did not evaluate
the effects of oral assessment on the risk of AP (14).
Despite strong literature-based evidence
highlighting the role of oral health in the prevention of AP, no
published study to date, to the best of our knowledge, has
investigated the association between the point-rating systems used
during oral health assessments and the detection of
pneumonia-causing bacteria. Therefore, the present study aimed to
investigate a novel oral assessment system that is simpler than
existing versions. To this end, a cross-sectional study was
conducted to identify the association between oral health
examination based on an original point-rating system and the
detection of pneumonia-causing bacteria.
Patients and methods
Study population
A total of 61 patients requiring NST intervention
who were hospitalized at Kanazawa University Hospital between
January 1, 2013, and June 30, 2013, were possible candidates for
the present study. Data on sex, body mass index (BMI), and
hemoglobin, albumin and transthyretin levels were obtained from
each patient. In total, 6 patients were excluded if they were too
ill to allow the collection of the required data. The present study
was performed in accordance with the Declaration of Helsinki with
the approval of the both of the Institutional Review Board of
Kanazawa University Hospital (approval no. 2441-2) and Yamanashi
University Hospital (approval no. 1746). A trial registration
number was also obtained (UMIN000030913). Informed consent was
obtained from all study participants. Patients were free to
withdraw from the study at any time.
The NST intervened in the nutritional management of
each patient in response to a request by the attending physician
and in the event that albumin levels decreased to ≤3.0 g/dl. The
NST held weekly clinical conferences aimed at providing better
nutritional supplies and care to patients with nutrition deficiency
and at ensuring the administration of necessary clinical
examinations and treatments to improve patients' overall
nutritional conditions.
Oral status evaluation, and blood and
sputum test data collection
The body heights and weights, BMIs and blood test
results of the enrolled patients were recorded at the onset of the
NST intervention. The participants underwent routine blood and
sputum culture testing together with a bedside oral assessment
conducted by a dentist. Sample and oral assessment findings were
collected within the first or second week following the onset of
NST intervention. Additionally, blood cultures (two sets, aerobic
and anaerobic) and sputum cultures were drawn from patients each
time pneumonia was suspected, based on clinical symptoms such as
fever, cough, sputum production, dyspnea, chest pain, or the
appearance of new infiltrates on chest radiography images during
hospitalization. The causative bacteria were estimated from the
results of sputum cultures and blood cultures. Data used to detect
pneumonia-causing bacteria were tracked and updated for all
patients.
Oral health assessments were performed by a single
dentist who was blinded to the results of the laboratory tests,
including the pneumonia-causing bacterial culture tests, and who
evaluated and recorded the prompt non-invasive oral assessment
scores using our original oral health assessment panel (Fig. S1). Eilers' Oral Assessment Guide
is among the most well-known oral health assessment methods and is
used frequently at other institutions (15,16).
However, this system requires the assessment of multiple
components, rendering it more time-consuming, and as such, it was
found to be too complicated and intolerable for patients in poor
general conditions. Moreover, ward rounds often did not provide
sufficient time for the NST bedside evaluations of several
components. Therefore, the present study devised a simple oral
health assessment method that was developed from other available
systems including Eilers' Oral Assessment Guide and the revised
oral assessment guide (17). In
the Eilers' Oral Assessment, 4 evaluation items were considered to
be important: Hygiene, xerostomia, mucositis and occlusion
(18-23).
The 4 evaluation items were each scored as follows: 0, excellent;
1, slight dysfunction; 2, moderate dysfunction; and 3, severe
dysfunction. For example, a score of 0 points for ‘hygiene’
indicated a clean oral cavity without plaque, whereas a score of 3
points indicated a highly insanitary oral cavity with large amounts
of plaque and food residues. The classifications of hygiene,
xerostomia and mucositis were according to the Oral Health
Assessment Tool and the Eilers' Oral Assessment Guide (15,24,25).
Occlusions were defined usingEichner's classification (26,27).
The total scores were defined as the sum of individual item scores,
and ranged from 0 (best oral health) to 12 points (worst oral
health). The scores were then used by the NST for patient
assessment.
The following 8 species that have been strongly
associated with the incidence of pneumonia were targeted (4,28-31):
Methicillin-sensitive Staphylococcus aureus,
methicillin-resistant Staphylococcus aureus, Klebsiella
pneumonia, Pseudomonas aeruginosa, Enterobacter
cloacae, Escherichia coli, Haemophilus influenzae
and Streptococcus pneumoniae. For sputum culture, the
patient was initially instructed to gargle with water several
times, after which sputum was collected into a sterile Petri dish
to avoid contaminating the saliva and dental plaque. Moreover, to
avoid collecting sputum samples contaminated with saliva and upper
respiratory secretions, the Geckler's classification of macroscopic
sputum findings and the Miller & Jones' classification of
microscopic findings were employed to exclude unsuitable specimens
(32,33). Per the Miller classification, M1
class sputum that is viscous and consists mostly of saliva was
excluded (33). Furthermore, good
quality sputum specimens were selected as per the Geckler
classification (classes 3, 4 and 5) (32,33).
The sample was delivered to the laboratory immediately after
collection and subjected to Gram staining and isolation as per
standard techniques. Three types of agar plates were used:
Bromothymol blue lactose, sheep blood, and chocolate. The globally
adopted BacT/ALERT® microbial detection system
(bioMérieux) for blood culturing was used (34). Blood culture bottles were placed in
a BacT/ALERT® instrument upon arrival at the laboratory
and incubated at 37˚C for 7 days (34). Upon the detection of growth, Gram
staining and sub-culturing were performed on the 3 aforementioned
types of agar plates using standard techniques. Bacterial
identification was conducted 24-48 h following sub-culture
(34). Positive bacterial culture
findings were confirmed by the presence of colonies on the agar
plates. A neutral third party at the Clinical Microbiology
Laboratory at Kanazawa University Hospital performed the bacterial
analyses while blinded to the oral health assessment data.
Statistical analyses were performed by professionals blinded to
both the oral assessment and laboratory data. These procedures
ensured a blinded, cross-sectional study.
Statistical analyses
Welch's t-test was used to determine significant
differences in clinical parameters between the detection and
non-detection groups. The Kolmogorov-Smirnov test was used to
confirm that the distribution of each clinical parameter in the
detection and non-detection groups was parametric. Fisher's exact
test was used to determine significant differences in prompt
non-invasive oral assessment scores between the groups in terms of
each of the 4 components. Differences in categorical variables were
assessed using the Chi-squared test. The associations were
expressed as P-values and odds ratios with 95% confidence intervals
(CIs). In the multivariable analysis, logistic regression was
applied to adjust for potential confounding factors. Receiver
operating characteristic (ROC) analysis was used to determine the
cut-off point for separating the detection and non-detection
groups. All statistical analyses were performed using the SPSS 23
software (SPSS, Inc.). Differences were considered statistically
significant at a P-value <0.05.
Results
In total, 61 patients were candidates for the
present study. Among these, 6 patients were excluded; specifically,
3 of these 6 patients refused bedside oral assessments due to
mental diseases, such as major depression and severe anorexia, 1
patient was unable to complete a sputum test due to a severe
breathing disorder, and the remaining 2 patients had undergone
total gastrectomy and hepatic transplantation; however, they had
not recovered sufficiently in order to be assessed for a dentist's
examination. Therefore, the prompt non-invasive oral assessment was
applied to 55 patients who were hospitalized for the following
reasons: Hematological disease (mainly leukemia), 17 patients who
received oral care and management in the department of oral and
maxillofacial surgery at the time of admission; pneumonic disease
(mainly lung cancer), 14 patients; psychiatric diseases, 8
patients; cardiovascular disease, 5 patients; gastroenterological
disease, 3 patients (2 with hepatitis C and 1 with Crohn's
disease); skin disease, 3 patients; laryngeal cancer: 2 patients;
uterine cancer: 1 patient; spinal tumors, 1 patient; and upper arm
sarcoma, 1 patient.
Patients from whom at least 1 of the aforementioned
8 bacterial species were cultured comprised the ‘detection group’
(n=13), while the remainder comprised the ‘non-detection group’
(n=42). No significant inter-group differences were observed with
respect to the blood test results or demographic data (e.g., age,
sex and BMI) (Table I). The
sputum-cultured pneumonia-causing bacteria in the detection group
are shown in Table SI. Notably,
E. coli and H. influenzae were not detected in any of
the patients. The number of patients with only Gram-negative
bacilli (patient nos. 20, 26, 27, 36, 39 and 41) was higher than
that of patients with only Gram-positive cocci (patient nos. 44, 45
and 50). The group in which only Gram-negative bacilli (n=6) were
detected had lower albumin levels than the group in which only
Gram-positive cocci (n=3) were detected (mean serum albumin levels
were 2.37 and 3.58 g/dl, respectively) (Table SI).
 | Table IDemographic and clinical data of the
patients. |
Table I
Demographic and clinical data of the
patients.
| Variable | Detection group
(n=13) (mean ± SD, range) | Non-detection group
(n=42) (mean ± SD, range) | P-value |
|---|
| Sex [male, n
(%)] | 7 (54%) | 23 (55%) | 0.95 |
| Age (years) | 63.5±17.1
(29,92) | 60.9±17.4
(17,90) | 0.63 |
| Body mass index
(kg/m2) | 18.9±4.2
(13.5,25.0) | 19.7±4.4
(10.4,28.1) | 0.48 |
| Hemoglobin
(g/dl) | 10.1±2.4
(7.2,14.1) | 10.4±2.0
(6.9,13.7) | 0.59 |
| C-reactive protein
(mg/dl) | 1.8±2.4
(0.2,7.8) | 3.6±4.9
(0,17.9) | 0.79 |
| Albumin (g/dl) | 2.7±0.7
(1.7,3.8) | 3.2±1.1
(1.5,7.4) | 0.14 |
| Transthyretin
(g/dl) | 16.8±8.3
(3.0,31.0) | 18.6±9.9
(2.0,37.0) | 0.61 |
A blood culture test was performed on 33 (60%)
patients. Among these, pneumonia-causing bacteria were detected in
4 (12%) patients, and the bacteria were the same as those found in
the sputum (data not shown).
The total prompt non-invasive oral assessment scores
were significantly higher in the detection group than in the
non-detection group [median (25th, 75th percentile), total score, 6
(4, 7) vs. 3 (1, 5), P=0.02; Table
II]. Similarly, the hygiene item scores were significantly
higher in the detection group [median (25th, 75th percentile),
hygiene score, 2 (1, 3) vs. 1 (0, 2), P=0.02; Table II]. By contrast, the xerostomia,
mucositis and occlusion scores did not significantly differ between
the detection and non-detection groups [median (25th, 75th
percentile), xerostomia score, 1 (0, 2) vs. 1 (0, 2), P=0.49;
Table II]; mucositis [0 (0, 1)
vs. 0 (0, 0), P=0.18] and occlusion [2 (1, 3) vs. 1 (0, 2), P=0.06]
(Table II). Mucositis
consistently received the lowest scores among the 4 components.
 | Table IIScore of each category between the
detection and non-detection group. |
Table II
Score of each category between the
detection and non-detection group.
| Score of each
category (range) | Detection group
(n=13) Median (25th, 75th percentile) | Non-detection group
(n=42) Median (25th, 75th percentile) | P-value |
|---|
| Total point
(0-12) | 6 (4,7) | 3 (1,5) | 0.02a |
| Hygiene (0-3) | 2 (1,3) | 1 (0,2) | 0.02a |
| Xerostomia
(0-3) | 1 (0,2) | 1 (0,2) | 0.49 |
| Mucositis
(0-3) | 0 (0,1) | 0 (0,0) | 0.18 |
| Occlusion
(0-3) | 2 (1,3) | 1 (0,2) | 0.06 |
When the participants were divided into a good
hygiene group (category 0, clean; and 1, slight local debris; n=36)
and the poor hygiene group (category 2, moderate local debris; and
3, general debris; n=19), according to the departments in which
they had been hospitalized, hematology inpatients had a
significantly higher ratio of good cases than the other
departments, as demonstrated by the following data. Of the 17
hematology department inpatients, 15 (88%) were good hygiene cases.
By contrast, of the 38 inpatients of other departments, 21 (55%)
were good hygiene cases (P=0.018). The pneumonia-causing bacteria
detection rate was significantly higher among patients hospitalized
in departments other than hematology (P=0.038) and among those with
poor hygiene (P=0.048) (Table
III). Additionally, a multivariable analysis revealed a high
odds ratio for the presence of pneumonia-causing bacteria only in
patients with poor hygiene (odds ratio, 2.09; 95% CI, 1.04 to 4.22)
(Table IV).
 | Table IIIAssociation between prevalence of
pneumonia-causing bacteria and clinical variables. |
Table III
Association between prevalence of
pneumonia-causing bacteria and clinical variables.
| | Pneumonia-causing
bacteria | | |
|---|
| Variables | Positive (n=13)
(%) | Negative (n=42)
(%) | Total | P-value |
|---|
| Age (years) | | | | |
|
65≥ | 6 (26.1) | 17 (73.9) | 23 | 0.72 |
|
<65 | 7 (21.9) | 25 (78.1) | 32 | |
| Sex | | | | |
|
Male | 7 (23.3) | 23 (76.7) | 30 | 0.95 |
|
Female | 6(24) | 19(76) | 25 | |
| Hospital
department | | | | |
|
Hematology | 1 (5.9) | 16 (94.1) | 17 | 0.038a |
|
Other | 12 (31.6) | 26 (68.4) | 38 | |
| Hygiene | | | | |
|
Clean
(category 0) | 2(10) | 20(90) | 22 | 0.048a |
|
Slight local
debris (category1) | 4(29) | 10(71) | 14 | |
|
Moderate
local debris (category2) | 2(20) | 8(80) | 10 | |
|
General
debris (category 3) | 5(56) | 4(44) | 9 | |
 | Table IVRisk of detection of
pneumonia-causing bacteria by hospital departments or the status of
oral hygiene. |
Table IV
Risk of detection of
pneumonia-causing bacteria by hospital departments or the status of
oral hygiene.
| Parameter | Unadjusted odds
ratio (95% CI) | P-value | Adjusted odds ratio
(95% CI)a | P-value |
|---|
| Hospital
department | | | | |
|
Hematology | 1.70
(0.085-33.91) | 0.73 | 2.01
(0.09-45.24) | 0.66 |
|
Other | 1.0
(reference) | | 1.0
(reference) | |
| Oral hygiene
status | | | | |
|
Poor
(category 2 or 3) | 2.09
(1.04-4.22) | 0.038b | 2.90
(1.23-6.83) | 0.015b |
|
Good
(category 0 or 1) | 1.0
(reference) | | 1.0
(reference) | |
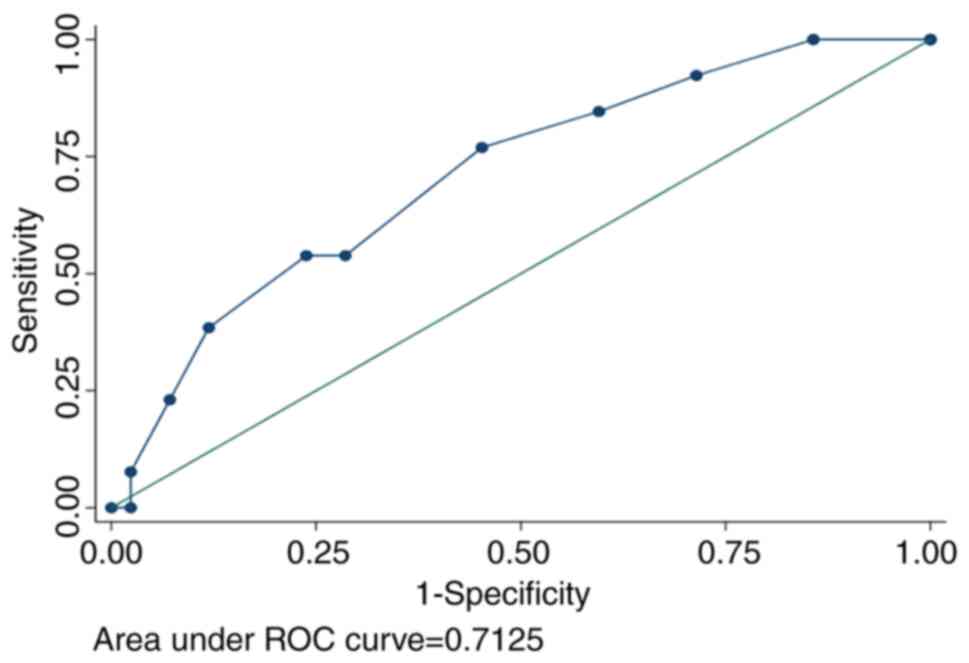
As shown in Fig. 1,
a cut-off point of >3.5 corresponded to the highest numerical
Youden index value (130.7). As scores were calculated as whole
numbers (0-12), the clinical cut-off point was set at 4. This value
exhibited a high sensitivity and high negative predictive value (77
and 89%, respectively), with a relatively low specificity and low
positive predictive value (55 and 35%, respectively). Finally, ROC
analysis validated the values of ≥4 and <4 as discriminatory of
patients with and without pneumonia-causing bacteria,
respectively.
Discussion
A previous systematic review demonstrated that good
oral health care could reduce the incidence of pneumonia by 40% and
the associated mortality rate by 10% (35). Additionally, a previous study found
that oral health care reduced the incidence of
ventilator-associated pneumonia in patients on ventilatory support
(36). Moreover, according to the
study by Senpuku et al, attention to oral hygiene and
professional care that include elimination of pneumonia-causing
bacteria and fungi (such as Pseudomonas spp. and Candida
albicans) could diminish the risk of developing systemic
diseases (37). Recently, it was
reported that in mechanically ventilated patients, bacterial
species may migrate rapidly from the mouth and upper airways, and
this can contribute to the pathogenesis of pneumonia (4). Taken together, oral cleaning is
considered an effective tool for the suppression and prevention of
AP (38,39). The findings of the present study
are consistent with those of these above-mentioned studies.
El-Solh et al (40) reported that among 67 patients with
AP, bronchial sampling indicated that Gram-negative bacilli were
the most predominant organisms (49%), followed by anaerobes (16%)
and S. aureus (12%). In the present study, Gram-negative
bacilli were predominantly detected over Gram-positive cocci, as in
previous studies. Pathogenic bacteria, including Gram-negative
species, which are not observed in the oral cavity of the normal
host, may emerge in hospitalized patients (41). The cleavage of cell-surface
fibronectin exposes receptors for Gram-negative rods on underlying
airway epithelial cells and is closely associated with host
factors, such as acute illness (42). Furthermore, a number of studies
have already demonstrated that undernourishment increases the risk
of developing AP (43-45).
It is reasonable to hypothesize that undernutrition and poor oral
hygiene cause inflammation and damage to the airway mucosa,
resulting in a high detection rate of Gram-negative bacilli. In
fact, the group in which only Gram-negative bacilli were detected
in the present study had a poor oral hygiene condition and had
significantly lower albumin levels than the group in which only
gram-positive cocci were detected (Table SI).
The present study demonstrated that the oral
cleaning state differed, depending on the department of
hospitalization and that hematology inpatients had a high cleaning
ability. This finding may be attributed to the fact that hematology
inpatients are routinely referred to the department of oral and
maxillofacial surgery at the time of hospitalization for the
purpose of preventing severe odontogenic infections that may have
occurred when the immune system was weakened by high-dose cancer
chemotherapy. Moreover, patients who are hospitalized for pulmonary
diseases are likely to have unclean oral cavities due to the
long-term use of endotracheal intubation and ventilation through
the oral cavity. In addition, oral hygiene management may be
difficult for otolaryngology inpatients as the oral cavity is close
to or included in the surgical wound.
Adequate salivary flow may prevent the accumulation
of pneumonia-causing bacteria, and can thus maintain oral health
while regulating the oral microbiome. However, the xerostomia
scores did not significantly differ between the detection and
non-detection groups in the present study. The standardization of
non-stimulated saliva collection and the time of its collection
should be performed in future research so that the evaluation of
xerostomia detection is not based solely on visual findings, which
are likely to be inaccurate (46,47).
The detection group in the present study tended to receive worse
occlusion scores than the non-detection group, although this
difference was not significant. To evaluate chewing ability,
laborious tests, such as checking the rate of disintegration of
items, such as gummy jelly are required. However, the aim of the
present study was to promote a non-invasive and relatively prompt
examination. Therefore, the present study evaluated chewing ability
based on the Eichner index of occlusion, which indicates a
significant association with masticatory functions, such as maximum
biting force and occlusal contact area in elderly Japanese
individuals (26,27). Flores-Orozco et al reported
that being underweight and having a low body fat percentage were
associated with a long chewing cycle duration and masticatory
lateral asymmetry even in young adults with natural dentition
(48), suggesting that chewing and
swallowing may be decreased in malnourished patients. Similarly, it
has been reported that tooth loss is associated with dysfunctional
mastication, a risk of aspiration and increased malnutrition,
including diabetes (49-51).
Furthermore, Furuta et al reported that having a low number
of teeth was associated with dysphagia, whereas wearing dentures
contributed to a recovery of swallowing functions (22). Therefore, an unfavorable occlusal
status may eventually lead to AP due to the aspiration of
bacterially contaminated saliva and food under unsanitary
conditions.
In the present study, ROC analysis revealed that a
total oral assessment cut-off score of 4 was useful for
distinguishing between the detection and non-detection groups. As
this cut-off score exhibited both a moderate sensitivity and high
negative predictive value (77 and 89%, respectively), the prompt
non-invasive oral assessment may be clinically useful for screening
patients at risk of developing AP. By contrast, the low specificity
and positive predictive value of this cut-off (55 and 35%,
respectively) may lead to an increase in the number of
false-positive cases. The reason for the large number of
false-positive cases may be that some patients did not brush after
eating before the oral evaluation. Therefore, oral hygiene may have
been rated worse than normal oral hygiene at the time of the oral
assessment.
Further research, including a time-series database,
is required to determine the associations of blood and sputum
culture results with oral status. Although the present study
carefully conducted sputum sampling to reduce the risk of oral
bacterial contamination, the possibility that oral bacterial
contamination could lead to inaccurate sputum culture results
should be considered. Moreover, molecular biology techniques,
including the metabolomic profiling of saliva should be adopted to
detect and quantify pathogens, as it was recently reported that
such methods can detect a higher proportion of streptococcal
infections in the oral cavity than bronchoalveolar lavage fluid
culturing (52,53). Importantly, the evaluation method
used herein can be easily implemented and evaluated by nurses, who
should be trained in these evaluation methods, and help improve
their reliability. Involving nurses would further enhance the
utility of the prompt non-invasive oral assessment used herein as a
universal clinical and research tool for patients in a poor
condition, particularly as this tool may be more practical than
traditional oral assessment systems (54-56).
One strength of the present study was that the data
management and analysis were performed by an independent
biostatistician to exclude evaluator subjectivity. A limitation of
the present study is that it was conducted at a single facility,
and thereby the findings were subject to possible selection bias as
a specific group of patients with malnutrition who had been
admitted to a tertiary medical care center were targeted. To ensure
inter-observer reliability, future studies should consider the
validity of multiple examiners. Furthermore, the small sample size
may have resulted in bias, and therefore may have reduced
generalizability. A large-scale, prospective cohort study is
required to confirm the present findings and validate the prompt
non-invasive oral assessment. This may enable the prompt detection
of pneumonia-causing bacteria using the system used herein on a
broader scale, and facilitate better and more timely treatment.
In conclusion, the present study demonstrated an
association between the original point-rating-based oral
examination system and the detection of pneumonia-causing bacteria
in patients with poor nutrition. Notably, hygiene was the only
assessment component associated with the detection of
pneumonia-causing bacteria.
Supplementary Material
Clinical images of patients
participating in the oral health assessment. Each of the four items
evaluated (‘hygiene’, ‘xerostomia’, ‘mucositis’ and ‘occlusion’)
received a score from 0 (excellent assessment) to 3 points
(extremely poor assessment). The total score ranged from 0 points
(excellent oral health) to 12 points (worst oral health).
Pneumonia-causing bacteria detected
among the 13 patients in the detection group in the present
study.
Acknowledgements
The authors are grateful to Dr Takashi Hase
(Department of Oral and Maxillofacial Surgery, Noto General
Hospital) and Professor Yasuaki Kakinoki (Department of Special
Needs and Geriatric Dentistry, Kyushu Dental University) for
providing the clinical images in presented in Fig. S1.
Funding
No funding was received.
Availability of data and materials
The data used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
KY designed the study, and drafted and wrote the
manuscript. TF participated in the design of the study and in the
collection of the data. SK participated in the design of the study
and its conception. TaT and ToT contributed to interpretation of
the findings of the study. HY participated in the design of the
study and performed the statistical analyses. AM, ToT and KY
corrected the manuscript. AM and KU supervised the interpretation
of the data and the drafting of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki with the approval of the both of the
Institutional Review Board of Kanazawa University Hospital
(approval no. 2441-2) and Yamanashi University Hospital (approval
no. 1746). A trial registration number was also obtained
(UMIN000030913). Informed consent was obtained from all study
participants. Patients were free to withdraw from the study at any
time.
Patient consent for publication
The images presented in Fig. S1 were reprinted with each
patient's informed consent. The images were submitted without any
identifying information to ensure patient anonymity. Some of the
clinical images of ‘Mucositis’ illustrated in Fig. 1 were reprinted with permission from
the ‘Manual for the management of individual serious adverse drug
reactions’ (https://www.mhlw.go.jp/topics/2006/11/dl/tp1122-1l09.pdf)
of the Japanese Ministry of Health, Labour and Welfare, and
permission from Department of Dentistry, National Cancer Center
Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zimmermann H, Hagenfeld D, Diercke K,
El-Sayed N, Fricke J, Greiser KH, Kühnisch J, Linseisen J,
Meisinger C, Pischon N, et al: Pocket depth and bleeding on probing
and their associations with dental, lifestyle, socioeconomic and
blood variables: A cross-sectional, multicenter feasibility study
of the German National Cohort. BMC Oral Health.
15(7)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bágyi K, Haczku A, Márton I, Szabó J,
Gáspár A, Andrási M, Varga I, Tóth J and Klekner A: Role of
pathogenic oral flora in postoperative pneumonia following brain
surgery. BMC Infect Dis. 9(104)2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van der Maarel-Wierink CD, Vanobbergen JN,
Bronkhorst EM, Schols JM and de Baat C: Oral health care and
aspiration pneumonia in frail older people: A systematic literature
review. Gerodontology. 30:3–9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
de Carvalho Baptista IM, Martinho FC,
Nascimento GG, da Rocha Santos CE, Prado RFD and Valera MC:
Colonization of oropharynx and lower respiratory tract in critical
patients: Risk of ventilator-associated pneumonia. Arch Oral Biol.
85:64–69. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoneyama T, Hashimoto K, Fukuda H, Ishida
M, Arai H, Sekizawa K, Yamaya M and Sasaki H: Oral hygiene reduces
respiratory infections in elderly bed-bound nursing home patients.
Arch Gerontol Geriatr. 22:11–19. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kikuchi R, Watabe N, Konno T, Mishina N,
Sekizawa K and Sasaki H: High incidence of silent aspiration in
elderly patients with community-acquired pneumonia. Am J Respir
Crit Care Med. 150:251–253. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoshino A, Ebihara T, Ebihara S, Fuji H
and Sasaki H: Daily oral care and risk factors for pneumonia among
elderly nursing home patients. JAMA. 286:2235–2236. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoneyama T, Yoshida M, Matsui T and Sasaki
H: Oral care and pneumonia. Oral Care Working Group. Lancet.
354(515)1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abe S, Ishihara K and Okuda K: Prevalence
of potential respiratory pathogens in the mouths of elderly
patients and effects of professional oral care. Arch Gerontol
Geriatr. 32:45–55. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Inglis TJ, Sherratt MJ, Sproat LJ, Gibson
JS and Hawkey PM: Gastroduodenal dysfunction and bacterial
colonisation of the ventilated lung. Lancet. 341:911–913.
1993.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hayashida S, Funahara M, Sekino M,
Yamaguchi N, Kosai K, Yanamoto S, Yanagihara K and Umeda M: The
effect of tooth brushing, irrigation, and topical tetracycline
administration on the reduction of oral bacteria in mechanically
ventilated patients: A preliminary study. BMC Oral Health.
16(67)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nguyen NP, Moltz CC, Frank C, Vos P, Smith
HJ, Karlsson U, Nguyen LM, Rose S, Dutta S and Sallah S: Evolution
of chronic dysphagia following treatment for head and neck cancer.
Oral Oncol. 42:374–380. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sumi Y, Ozawa N, Miura H, Michiwaki Y and
Umemura O: Oral care help to maintain nutritional status in frail
older people. Arch Gerontol Geriatr. 51:125–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Manabe T, Teramoto S, Tamiya N, Okochi J
and Hizawa N: Risk factors for aspiration pneumonia in older
adults. PLoS One. 10(e0140060)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eilers J and Epstein JB: Assessment and
measurement of oral mucositis. Semin Oncol Nurs. 20:22–29.
2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glenny AM, Gibson F, Auld E, Coulson S,
Clarkson JE, Craig JV, Eden OB, Khalid T, Worthington HV and Pizer
B: Children's Cancer and Leukaemia Group (CCLG)/Paediatric Oncology
Nurses. The development of evidence-based guidelines on mouth care
for children, teenagers and young adults treated for cancer. Eur J
Cancer. 46:1399–1412. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Prendergast V, Kleiman C and King M: The
bedside oral exam and the barrow oral care protocol: Translating
evidence-based oral care into practice. Intensive Crit Care Nurs.
29:282–290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Astvaldsdottir A, Boström AM, Davidson T,
Gabre P, Gahnberg L, Sandborgh Englund G, Skott P, Ståhlnacke K,
Tranaeus S, Wilhelmsson H, et al: Oral health and dental care of
older persons-A systematic map of systematic reviews.
Gerodontology. 35:290–304. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Almirall J, Bolibar I, Serra-Prat M, Roig
J, Hospital I, Carandell E, Agustí M, Ayuso P, Estela A and Torres
A: Community-Acquired Pneumonia in Catalan Countries (PACAP) Study
Group. New evidence of risk factors for community-acquired
pneumonia: A population-based study. Eur Respir J. 31:1274–1284.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Azarpazhooh A and Leake JL: Systematic
review of the association between respiratory diseases and oral
health. J Periodontol. 77:1465–1482. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dennesen P, van der Ven A, Vlasveld M,
Lokker L, Ramsay G, Kessels A, van den Keijbus P, van Nieuw
Amerongen A and Veerman E: Inadequate salivary flow and poor oral
mucosal status in intubated intensive care unit patients. Crit Care
Med. 31:781–786. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Furuta M, Komiya-Nonaka M, Akifusa S,
Shimazaki Y, Adachi M, Kinoshita T, Kikutani T and Yamashita Y:
Interrelationship of oral health status, swallowing function,
nutritional status, and cognitive ability with activities of daily
living in Japanese elderly people receiving home care services due
to physical disabilities. Community Dent Oral Epidemiol.
41:173–181. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Terezakis E, Needleman I, Kumar N, Moles D
and Agudo E: The impact of hospitalization on oral health: A
systematic review. J Clin Periodontol. 38:628–636. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chalmers JM, King PL, Spencer AJ, Wright
FA and Carter KD: The oral health assessment tool-validity and
reliability. Aust Dent J. 50:191–199. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eilers J, Berger AM and Petersen MC:
Development, testing, and application of the oral assessment guide.
Oncol Nurs Forum. 15:325–330. 1988.PubMed/NCBI
|
|
26
|
Miura H, Araki Y, Hirai T, Isogai E,
Hirose K and Umenai T: Evaluation of chewing activity in the
elderly person. J Oral Rehabil. 25:190–193. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Eichner K: Renewed examination of the
group classification of partially edentulous arches by Eichner and
application advices for studies on morbidity statistics. Stomatol
DDR. 40:321–325. 1990.PubMed/NCBI(In German).
|
|
28
|
Jones RN: Microbial etiologies of
hospital-acquired bacterial pneumonia and ventilator-associated
bacterial pneumonia. Clin Infect Dis. 51 (Suppl 1):S81–S87.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Bousbia S, Papazian L, Saux P, Forel JM,
Auffray JP, Martin C, Raoult D and La Scola B: Repertoire of
intensive care unit pneumonia microbiota. PLoS One.
7(e32486)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Noguchi S, Yatera K, Kawanami T, Yamasaki
K, Naito K, Akata K, Shimabukuro I, Ishimoto H, Yoshii C and Mukae
H: The clinical features of respiratory infections caused by the
Streptococcus anginosus group. BMC Pulm Med. 15(133)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Panghal M, Kaushal V, Kadayan S and Yadav
JP: Incidence and risk factors for infection in oral cancer
patients undergoing different treatments protocols. BMC Oral
Health. 12(22)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miller DL and Jones R: The bacterial flora
of the upper respiratory tract and sputum of working men. J Pathol
Bacteriol. 87:182–186. 1964.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Geckler RW, Gremillion DH, McAllister CK
and Ellenbogen C: Microscopic and bacteriological comparison of
paired sputa and transtracheal aspirates. J Clin Microbiol.
6:396–399. 1977.PubMed/NCBI
|
|
34
|
Lee DH, Kim SC, Bae IG, Koh EH and Kim S:
Clinical evaluation of BacT/Alert FA plus and FN plus bottles
compared with standard bottles. J Clin Microbiol. 51:4150–4155.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sjogren P, Nilsson E, Forsell M, Johansson
O and Hoogstraate J: A systematic review of the preventive effect
of oral hygiene on pneumonia and respiratory tract infection in
elderly people in hospitals and nursing homes: Effect estimates and
methodological quality of randomized controlled trials. J Am
Geriatr Soc. 56:2124–2130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mori H, Hirasawa H, Oda S, Shiga H,
Matsuda K and Nakamura M: Oral care reduces incidence of
ventilator-associated pneumonia in ICU populations. Intensive Care
Med. 32:230–236. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Senpuku H, Sogame A, Inoshita E, Tsuha Y,
Miyazaki H and Hanada N: Systemic diseases in association with
microbial species in oral biofilm from elderly requiring care.
Gerontology. 49:301–309. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Okuda K, Kimizuka R, Abe S, Kato T and
Ishihara K: Involvement of periodontopathic anaerobes in aspiration
pneumonia. J Periodontol. 76:2154–2160. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Adachi M, Ishihara K, Abe S and Okuda K:
Professional oral health care by dental hygienists reduced
respiratory infections in elderly persons requiring nursing care.
Int J Dent Hyg. 5:69–74. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
El-Solh AA, Pietrantoni C, Bhat A,
Aquilina AT, Okada M, Grover V and Gifford N: Microbiology of
severe aspiration pneumonia in institutionalized elderly. Am J
Respir Crit Care Med. 167:1650–1654. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mandell LA and Niederman MS: Aspiration
pneumonia. N Engl J Med. 380:651–663. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Johanson WG, Pierce AK and Sanford JP:
Changing pharyngeal bacterial flora of hospitalized patients.
Emergence of gram-negative bacilli. N Engl J Med. 281:1137–1140.
1969.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Scrimshaw NS, Taylor CE and Gordon JE:
Interactions of nutrition and infection. Monogr Ser World Health
Organ. 57:3–329. 1968.
|
|
44
|
Higashiguchi T, Ohara H, Kamakura Y,
Kikutani T, Kuzuya M, Enoki H, Sanada H, Matsuzaki M and Maruyama
M: Efficacy of a new post-mouthwash intervention (Wiping Plus Oral
Nutritional Supplements) for preventing aspiration pneumonia in
elderly people: A multicenter, randomized, comparative trial. Ann
Nutr Metab. 71:253–260. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Maeda K, Koga T and Akagi J: Tentative nil
per os leads to poor outcomes in older adults with aspiration
pneumonia. Clin Nutr. 35:1147–1152. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dawes C: The unstimulated salivary flow
rate after prolonged gum chewing. Arch Oral Biol. 50:561–563.
2005.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Proctor GB: The physiology of salivary
secretion. Periodontol. 70:11–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Flores-Orozco EI, Tiznado-Orozco GE,
Osuna-Gonzalez OD, Amaro-Navarrete CL, Rovira-Lastra B and
Martinez-Gomis J: Lack of relationship between masticatory
performance and nutritional status in adults with natural
dentition. Arch Oral Biol. 71:117–121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Holm-Pedersen P, Schultz-Larsen K,
Christiansen N and Avlund K: Tooth loss and subsequent disability
and mortality in old age. J Am Geriatr Soc. 56:429–435.
2008.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mann T, Heuberger R and Wong H: The
association between chewing and swallowing difficulties and
nutritional status in older adults. Aust Dent J. 58:200–206.
2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Azogui-Levy S, Dray-Spira R, Attal S,
Hartemann A, Anagnostou F and Azerad J: Factors associated with
oral health-related quality of life in patients with diabetes. Aust
Dent J. 63:163–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Akata K, Yatera K, Yamasaki K, Kawanami T,
Naito K, Noguchi S, Fukuda K, Ishimoto H, Taniguchi H and Mukae H:
The significance of oral streptococci in patients with pneumonia
with risk factors for aspiration: The bacterial floral analysis of
16S ribosomal RNA gene using bronchoalveolar lavage fluid. BMC Pulm
Med. 16(79)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yatsuoka W, Ueno T, Miyano K, Uezono Y,
Enomoto A, Kaneko M, Ota S, Soga T, Sugimoto M and Ushijima T:
Metabolomic profiling reveals salivary hypotaurine as a potential
early detection marker for medication-related osteonecrosis of the
jaw. PLoS One. 14(e0220712)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Quinn B, Baker DL, Cohen S, Stewart JL,
Lima CA and Parise C: Basic nursing care to prevent nonventilator
hospital-acquired pneumonia. J Nurs Scholarsh. 46:11–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jablonski RA, Kolanowski A, Therrien B,
Mahoney EK, Kassab C and Leslie DL: Reducing care-resistant
behaviors during oral hygiene in persons with dementia. BMC Oral
Health. 11(30)2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Baker D and Niederhauser V: Immunizations:
How do your patients score? Nurse Pract. 37:46–52. 2012.PubMed/NCBI View Article : Google Scholar
|