Introduction
The human body has both an innate and adaptive
immune system that operates in tandem, with phagocytes and natural
killer (NK) cells driving innate immune responses and T and B
lymphocytes driving adaptive immune responses (1,2). The
overstimulation of the immune system is harmful, while a
well-regulated immune system protects the body from infections and
cancer growth (3). Innate immune
cell activities, such as phagocytosis and antibody-dependent
cell-mediated cytotoxicity (ADCC) are major strategies to suppress
or eliminate microorganisms and tumor cells (4). In addition, activated macrophages
release cytokines, such as tumor necrosis factor-α (TNF-α),
interleukin (IL)-6 and IL-12, and chemical mediators, such as
nitric oxide (NO), prostaglandins and reactive oxygen species (ROS)
that stimulate the proliferation of nearby innate and adaptive
immune system cells (5). The RAW
264.7 murine macrophage cell line is a broadly applied model for
the in vitro testing of new potential medicines on
macrophage activity (6).
The main goal of Traditional medicine is to develop
immunostimulators that are safer, entirely biodegradable and
cost-effective in order to prevent diseases (7). Notably, several foods and dietary
herbs have been consumed as formulations to improve the quality of
life, and thus these can be employed as a valuable source of agents
that can boost host immune responses by stimulating or suppressing
both specific and non-specific immunity (8). In fact, the immunostimulatory
qualities of natural herbs are due to their abundance in
antioxidants and bioactive compounds, such as vitamins, phenolic
acid and flavonoids (9).
Therefore, the rigorous search for natural products or herbs and
their immunoregulatory processes has markedly increased in recent
years.
Garlic (Allium sativum) is a culinary and
medicinal herb known for its antioxidant, anticancer,
antibacterial, cholesterol-lowering, anti-inflammatory and other
properties (10,11). The presence of sulfur-containing
substances, such as allicin, alliin, ajoene and their metabolites,
allyl mercaptan and allyl methyl sulfide, has been attributed to
the diverse biological effects reported in garlic preparations and
extracts (12,13). As previously demonstrated, at low
doses, garlic oil stimulates a Th1 immune response, whereas, at
high levels, it promotes a type 2 T helper (Th2)-type response
(14). Alliin, a garlic component,
protects against lipopolysaccharide (LPS)-induced pro-inflammatory
responses, as evidenced by the upregulation of anti-inflammatory
genes and the downregulation of pro-inflammatory genes, such as
IL-6 and monocyte chemoattractant protein-1(15). In addition, allicin has been shown
to improve the immunological response of peripheral blood cells, as
well as macrophage phagocytic activity (16). Recently, fermented garlic extract
(FGE) has been discovered to be a more effective immune booster and
anti-tumor agent than fresh garlic extract (GE). This is due to the
fact that the fermentation procedure and type of microbial strain
change organosulfur compounds in raw garlic to S-allyl cysteine and
S-allyl mercaptocysteine (17),
and enhance antioxidant activity and immune-stimulating potency by
increasing the levels of total polyphenols, tetrahydro-carboline
derivatives, vitamins and flavonoids. In addition, proteolytic
enzymes from microorganisms hydrolyze polyphenol complexes into
free and soluble phenols, which are more active and effectively
absorbed during fermentation (18-20).
Taking these tendencies into account, garlic and
aged GEs have been widely examined for their immune-enhancing
effects in vitro and ex vivo (15,21),
although there are no available studies to date on the
immunological modifying effects of FGE, at least to the best of our
knowledge. As a result, in the present study, it was hypothesized
that supplementation with FGE could exert immune-modulating
effects. To determine this hypothesis, the present study focused on
immunological responses in RAW264.7 cells, primary mouse
splenocytes and NK cell cytotoxicity in BALB/c mice. In addition,
the present study aimed to investigate whether FGE may contribute
to the intestinal immune response of C3H/HeN mice.
Materials and methods
Preparation of samples
A total of 5 g of air-dried garlic (obtained from
Dongsung farm of Dongsun Biopharmaceutical) was extracted with 100%
water (100 ml) for 8 h at room temperature. The aqueous extract was
filtered through filter paper (1 µm) and lyophilized to produce
white GE powder. The GE was then stored at 4˚C.
Solid-state fermentation using Bacillus subtilis
(B. subtilis) KCTC 1028 which was procured from KCTC (The
Korean Collection for Type Cultures) for 3-15 days at 20-40˚C was
performed to yield FG extract in the aqueous phase. The amounts of
water, garlic and B subtilis were in the proportions of
85:5:1. The saccharides and B. subtilis were added to the
water in the first fermentation step to activate the bacteria, and
the garlic was then fermented for 3 days. The second fermentation
step was preferably performed for 7 days at 20-40˚C. The
fermented extract was then concentrated to 500 ml (4.2 Brix) in a
concentrator before being dried in a freeze drier. Dried materials
were suspended in sterile distilled water to make a stock solution
of 10 mg/ml, which was then filtered through a 0.45-µm pore
membrane and sterilized. Sub-stock solutions were then generated
for tests by diluting (broth medium, Difco; BD Biosciences) to the
required concentration, and the samples were further stored at
4˚C.
Cells and cell culture
The RAW 264.7, a murine macrophage cell line, was
obtained from the Korea Cell Line Bank (KCLB 40071) and was
routinely cultured in DMEM (HyClone; Cytiva SH30243.01) medium
supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin (P/S) (GenDEPOT, Inc.) at 37˚C in a
humidified chamber of 95% air and 5% CO2 atmosphere.
Cell viability assay
RAW264.7 cells (2x106 cells/well) were
seeded on 96-well plates and cultured for 24 h to evaluate the cell
viability of the sample. Stock solutions of 10 mg/ml FGE were
employed and were then diluted with medium to the necessary
concentrations. The cells were then pre-treated for 24 h with
0.016-10 mg/ml of FGE, with untreated cells serving as a control.
Following 24 h of incubation at 37˚C, the cells were examined using
an EZ-cytox cell viability assay kit (Daeil Lab Service, Co., Ltd.)
for 30 min before the absorbance was measured at 450 nm (Multiscan
GO, Thermo Fisher Scientific, Inc.) to test cell viability.
Viability was expressed as relative viability (%) with respect to
the group treated with distilled water (control group).
Determination of NO levels
The Griess Reagent System (G2930; Promega
Corporation) was used to measure nitric oxide levels in the cell
culture supernatants. Briefly, the RAW264.7 cells were treated for
24 h with various concentrations of FGE (0.016, 0.08, 0.4, 2 and 10
mg/ml) and 1 µg/ml LPS (MilliporeSigma) as a control. The obtained
supernatants were then incubated (1:1) with Griess reagent [1%
sulfanilamide and 0.1% N-(1-naphthyl) ethylenediamine
dihydrochloride in 5% phosphoric acid]. The absorbance was then
measured at 540 nm (Multiscan GO, Thermo Fisher Scientific, Inc.),
and the NO concentration was calculated using a standard curve.
Determination of cytokine levels using
enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-α, IL-6 and IL-12 in the cell
culture supernatants were determined using a commercial sandwich
ELISA kit: Mouse TNF-α ELISA set, cat. no. 555268, BD Biosciences;
mouse IL-6 ELISA set, cat. no. 555240 BD Biosciences; and mouse
IL-12 ELISA set, cat. no. 555165, R&D Systems, Inc.). For 24 h,
the RAW264.7 cells and peritoneal macrophages were pre-treated with
FGE (0.016, 0.08, 0.4, 2 and 10 mg/ml) and LPS (1 µg/ml)
respectively. The culture medium was collected, and the TNF-α, IL-6
and IL-12 levels were measured in the supernatant using ELISA kits
according to the manufacturer's instructions.
Supernatants were obtained from splenocytes that had
been incubated in the presence or absence of various concentrations
(0.016, 0.08, 0.4, 2 and 10 mg/ml) of FG and LPS (1 µg/ml) for 24 h
to quantify the levels of IL-6, interferon (IFN)-γ and
granulocyte-macrophage colony-stimulating factor (GM-CSF). In
addition, the absorbance was determined using a spectrophotometer
(Multiscan GO, Thermo Fisher Scientific, Inc.) at 450 nm, as per
the manufacturer's instructions (mouse IL-6 ELISA set, cat. no.
555240, BD Biosciences; mouse INF-γ ELISA set, cat. no. DY485
R&D Systems, Inc.).
Mice
A total of 40 BALB/c mice 6 weeks old, weighing
18-20 g, female and 15 C3H/HeN mice (6 weeks old, weighing 16-18 g,
female) were purchased from Core Tech Co., Ltd. The mice were
allowed to acclimatize to their environment for 1 week prior to the
experiment on a 12-h light/dark cycle at 23˚C and 55% humidity. The
mice were provided with access to water and a standard pellet diet
ad libitum. The animal health and behavior was monitored
daily and the animals were cared for according to the Guide for the
Care and Use of Laboratory Animals of the Kyung Hee University. The
experiment was carried out for 1.5 months, for which 40 BALB/c mice
and 15 C3H/HeN mice were used. All animal health and behaviors were
monitored daily. The 55 mice were euthanized by cervical
dislocation at the end of the experiment. However, no mortality
occurred during the experiment. The human endpoints used to
determine when animals ought to be euthanized in the experiment
included the inability to rest in a sternal position, being unable
to eat or drink, respiratory issues, paralysis, uncontrollable
bleeding and irreversible weight loss. The assessment criteria for
the confirmation of death in the experiment included no heart rate,
no signs of breathing, and dilated and unreactive pupils with
light. All animal welfare considerations taken, including efforts
to minimize suffering and distress, the use of analgesics or
anesthetics (including the dose), or special housing conditions
according to the Institutional Animal Care and Use Committee of
Dongsung Cancer Center and Kyung Hee University guidelines. The
experimental protocol was approved by the Institutional Animal Care
and Use Committee of Kyung Hee University (KHUSBC-R-SPA 2018-0601)
and the Institutional Animal Care and Use Committee of the Dongsung
Cancer Center under protocol IACUC #ds002205112-EUTO3. The
experiments were carried out in compliance with the ARRIVE
guidelines.
Isolation of peritoneal macrophages
from BALB/c mice
Peritoneal fluid was collected from the BALB/c mice
on the 4th day following the administration of 1 ml 5%
thioglycollate media (MilliporeSigma) intraperitoneally to extract
murine peritoneal macrophages (MPMs). Subsequently, the cell
suspension was dispensed and cultured for 4 h in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS) (cat. no. CM002-050,
GenDEPOT) in an incubator (5% CO2, 95% air).
Furthermore, to remove non-adherent cells, the dishes were gently
rinsed with the medium.
Peritoneal macrophage cell viability
assay
The MPMs (1x106 cells/ml) were seeded in
96-well plates (SPL Life Sciences Co., Ltd.). To determine the
effects of FGE and LPS on the viability of peritoneal macrophages,
the cells were pre-treated with 0.016, 0.08, 0.4, 2 and 10 mg/ml
FGE and LPS (1 µg/ml) for 24 h. A 100 µl solution of
Ez-cytox/phosphate-buffered saline (PBS) was added, and the
absorbance at 450 nm was measured using a microplate reader (Tecan
Austria GmbH). Cell viability was expressed as the relative
activity (%) with respect to the group treated with distilled water
(control group).
Isolation of splenocytes
The splenocytes were harvested from the spleen
following the cervical dislocation of BALB/c mice. A single-cell
suspension was obtained by grinding the spleen under aseptic
conditions and passing through a 100-µm cell strainer. Red blood
cells were lysed with 0.2% NaCl lysis solution (cat. no. 00-4300,
Thermo Fisher Scientific, Inc.) prior to being washed in PBS and
kept in RPMI-1640 complete medium.
Determination of splenocyte
proliferation
The effects of FGE or LPS on the cell proliferation
of splenocytes were detected using the EZ-cytox cell viability
assay kit (Daeil Lab Service, Co., Ltd.). Splenocytes were seeded
into 96-well culture plates at a density of 2.5x106
cells/ml and incubated at 37˚C for 24 h. The cells were then
treated with various concentrations of FGE (0.016, 0.08, 0.4, 2 and
10 mg/ml) and LPS (1 µg/ml) at 37˚C in a 5% CO2
incubator. A total of 20 µl EZ-cytox solution was added to each
well and incubated for 3 h to assess splenocyte proliferation. The
absorbance was measured at 450 nm using a microplate reader (Tecan
Austria GmbH).
Isolation of Peyer's patch (PP) cells
and bone marrow cells
PPs were removed aseptically from the exposed small
intestine of the C3H/HeN mice. The tissue was sorted into
single-cell suspensions by grinding and filtering through a
200-gauze metal mesh. Finally, harvested cells were washed twice
with Hank's balanced saline solution (HBSS; cat. no. 14175095,
Gibco; Thermo Fisher Scientific, Inc.), and suspended in a complete
medium (RPMI-1640 with 10% FBS).
On the other hand, bone marrow cells were recovered
from the femurs of the same mice. Single-cell suspensions were
obtained by drawing femoral bone tissue into 1 ml of a syringe, and
further, the collected tissue was treated several times with
sterile 0.2% NaCl to remove all red blood cells. The obtained cells
were then passed through a 100-µM cell strainer, centrifuged at 200
x g, 5 min at 4˚C and suspended in RPMI-1640 medium.
Bone marrow cell proliferation
activity via PP
The cells from PPs were washed in 10% FBS containing
RPMI-1640 and seeded at a density of 2x106 cells/ml. For
5 days, aliquots of cell suspension (180 µl) derived from PPs were
dispensed into a 96-well plate and cultured with 20 µl of diluted
concentrations of FGE. The cultured supernatant (conditioned
medium) was then tested for bone marrow cell proliferation and the
presence of GM-CSF, an immunoreactive cytokine produced by PP
cells.
Furthermore, the isolated bone marrow cells were
adjusted to 2.5x105 cells/ml and 100 µl were dispensed
into each well of the PP cell culture. To examine the ability to
grow on bone marrow cells, 50 µl of the resultant supernatant were
mixed with 50 µl bone marrow cell suspension in DMEM full medium
(DMEM; cat. no. SH30243.01, HyClone; Cytiva) and grown for 6 days.
Bone marrow cell proliferation via PP cells stimulated with FGE was
detected using the EZ-cytox cell viability assay kit (Daeil Lab
Service, Co., Ltd.), and the absorbance (Multiscan GO, Thermo
Fisher Scientific, Inc.) was measured at 450 nm to determine the
relative activity (%) for myeloid cell proliferation.
Cytotoxic activity assay of NK cells
from splenocytes against cancer cells
Female BALB/c mice (6-8 weeks old) were
intravenously injected with 0.1, 1 and 10 mg/kg of FGE in the
drug-treated groups and PBS in the vehicle-treated groups, for 4
days and 1 day before sacrifice. The mice were sacrificed by
cervical dislocation and the spleens were aseptically extracted.
Splenocytes that had destroyed all red blood cells using 0.2% NaCl
were recovered in serum-free medium (SFM) containing 1%
penicillin/streptomycin (cat. no. CA005-010, GenDepot) and were
adjusted to a density of 5x107 cells/ml.
Briefly, YAC-1 lymphoma cells (CVCL_2244; American
Type Culture Collection) were used as target cells to detect NK
cell cytotoxicity. The cytotoxic capacity of harvested mouse
splenocytes as effector cells was investigated. Splenocytes and
YAC-1 cells were co-cultured at 50:1, 25:1 and 12.5:1 ratios of
effector:target cells (1x105 cells/ml) in 96-well
plates. After 24 h of mixed culture of the target cell and the
effector cell, the effector cell toxicity was determined. The
amount of lactate dehydrogenase (LDH) generated in the target cell
was measured using the LDH assay kit, and the tumor cell killing
ability of NK cells was assessed using the following formula:
Statistical analysis
The experimental results are expressed as the mean ±
SD of the results of experiments performed in triplicate samples.
Statistical analysis was performed using one-way ANOVA (with Graph
Pad Prism 5.0 software, GraphPad Software, Inc.) followed by post
hoc Tukey's multi comparison test. P<0.05 was considered to
indicate a statistically significant difference. In addition,
Duncan's multiple range tests were used to confirm the significance
of each measurement value.
Results
Effect of FGE on the viability of
RAW264.5 cells
The cytotoxic effects of FGE and GE on murine
RAW264.7 macrophage cells were assessed before the immunomodulatory
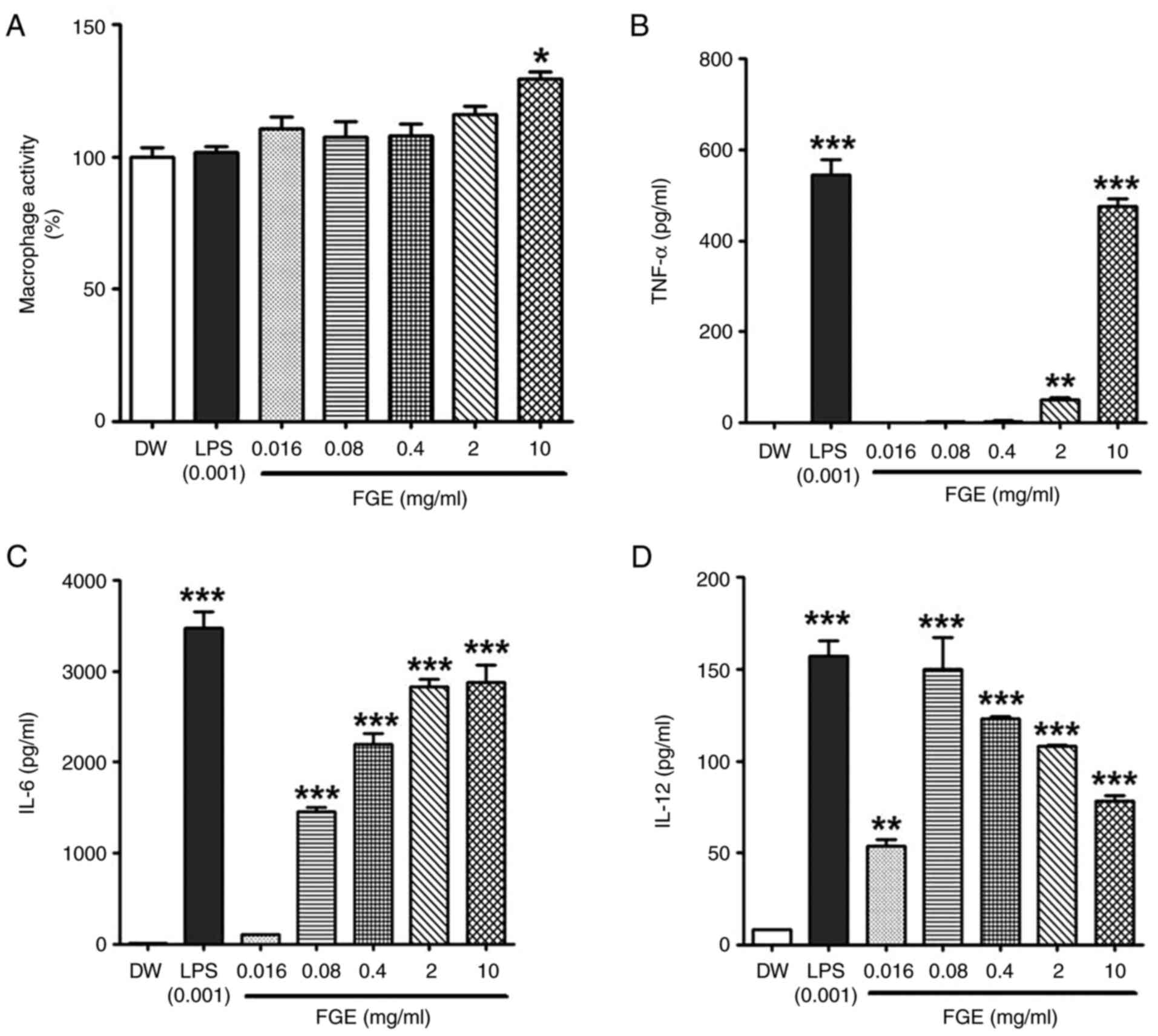
impact was established, as shown in Fig. 1A. The RAW264.7 cells treated with
FGE at concentrations of 0.016, 0.08, 0.4, 2 and 10 mg/ml exhibited
a viability of 161.7±14.46, 179.62±11.35, 169.79±11.30,
147.71±10.36 and 154±14.16%, respectively. On the other hand, the
cells treated with GE at concentrations of 0.016, 0.08, 0.4, 2 and
10 mg/ml exhibited a viability of 123.83±8.08, 130.64±8.71,
143.27±11.34, 89.79±10.25 and 65.77±6.82%, respectively. Indeed,
these findings revealed that FGE had no negative effect on
macrophage viability at any of the concentrations examined.
However, at the concentrations of 2 and 10 mg/ml, GE was cytotoxic
to the RAW264.7 cells.
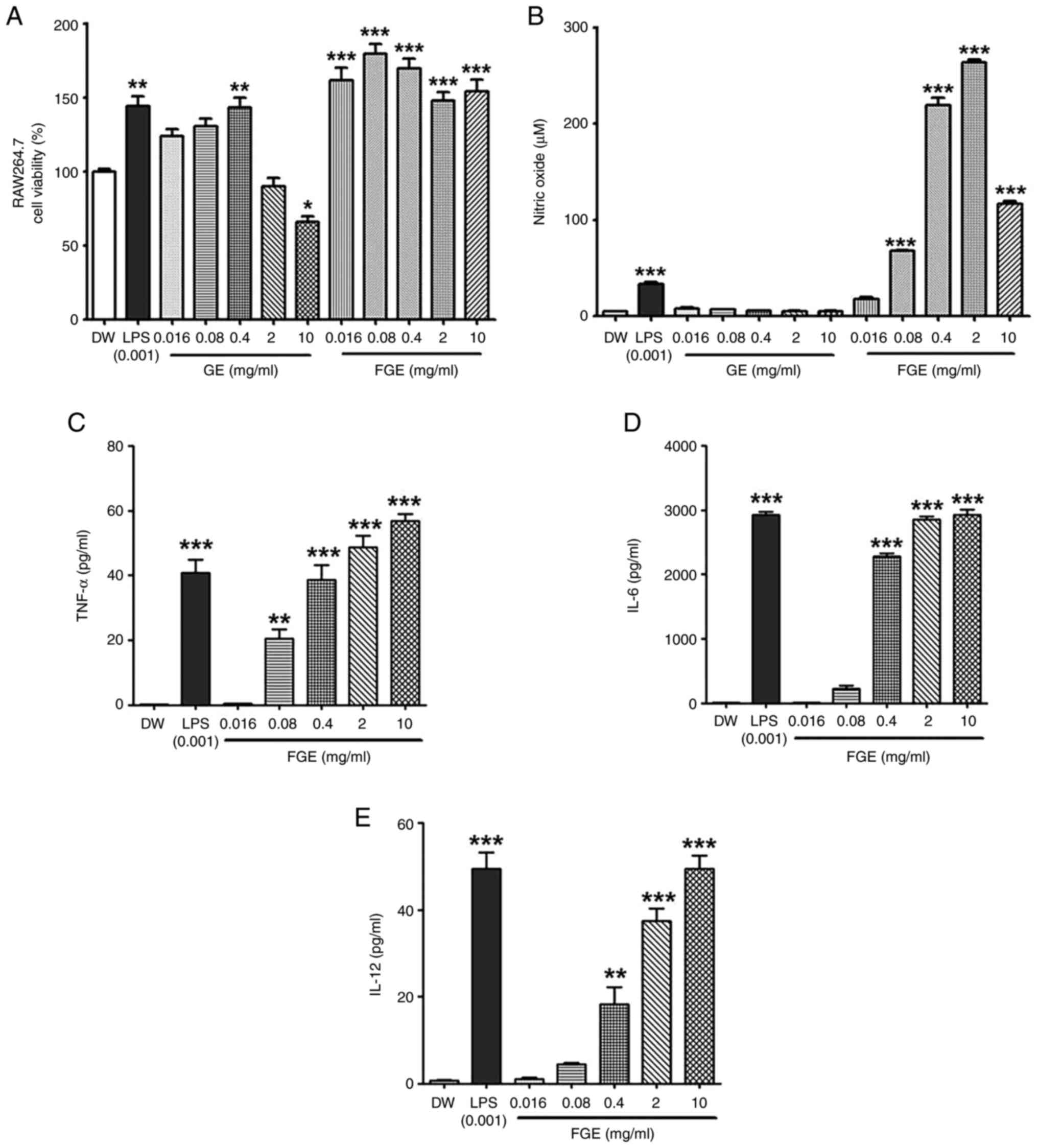 | Figure 1In vitro effect of FGE on
cytotoxicity, and NO and cytokine production in RAW264.5 cells. (A)
Effects of GE and FGE on cell viability during the first 24 h in
RAW264.7 cells. FG exhibited no toxicity at any concentrations,
while GE exhibited cytotoxicity at the concentrations of 2 and 10
mg/ml. MTT assays of RAW264.7 cells were used to assess the
cytotoxicity of GE, FGE, LPS (0.001 mg/ml or 1 µg/ml) and DW
treatments for 24 h. The effects of FGE and GE on (B) NO and only
FGE on (C) TNF-α, (D) IL-6, and (E) IL-12 production were confirmed
using ELISA. The data are presented as the mean ± standard
deviation (n=3). *P<0.05, **P<0.01 and
***P<0.001, compared to the vehicle (DW)-treated
control. DW, distilled water; FGE, fermented garlic extract; GE,
garlic extract; LPS, lipopolysaccharide; NO, nitric oxide. |
Effect of FGE on NO and cytokine
production in RAW264.7 cells
To confirm the immunostimulatory activity of FGE, NO
production was evaluated after the RAW264.7 macrophages were
treated with FGE and GE. FGE treatment at 0.016, 0.08, 0.4, 2 and
10 mg/ml upregulated NO production in a concentration-dependent
manner compared to the GE-treated and untreated cells (Fig. 1B). NO generation was markedly
promoted by FGE at the concentration of up to 2 mg/ml, followed by
a reduction at the concentration of 10 mg/ml. Nonetheless, GE
equivalent to the concentrations of FGE did not increase NO levels
in RAW264.7 cells. The production of cytokines in RAW264.7
macrophages was likewise assessed to assess the effects of FGE on
immunological activation. The levels of all three immunostimulatory
cytokines, TNF-α, IL-6 and IL-12, were enhanced by FGE treatment
compared to the untreated cells, as shown in Fig. 1C-E. In comparison to the
LPS-treated control, TNF-α production was observed to be greater at
2 and 10 mg/ml.
Immunostimulatory effects of FGE on
peritoneal macrophage activity and cytokine production
The effects of FGE on peritoneal macrophage cell
viability were examined by treating the cells with the respective
concentrations of FGE (0.016, 0.08, 0.4, 2 and 10 mg/ml) or LPS (1
µg/ml) for 24 h. As regards the peritoneal macrophages, FGE had no
effect on cell survival, while it significantly increased
macrophage activity at 10 mg/ml (Fig.
2A). As shown in Fig. 2B and
C, FGE at concentrations of 10
mg/ml also markedly increased the production of TNF-α and IL-6 by
peritoneal macrophages in a concentration-dependent manner. In
peritoneal macrophages, TNF-α production increased with 0.4 to 10
mg/ml of FGE, whereas IL-6 production increased with 0.016 to 10
mg/ml of FGE. In the evaluation of IL-12 production ability,
however, a different aspect was observed compared with TNF-α or
IL-6. IL-12 exhibited a 3-fold greater capacity for production at a
low concentration level of 0.08 mg/ml of FGE than at the 10 mg/ml
concentration. On the other hand, IL-12 production increased with
FGE at the concentration of 0.08 mg/ml and then decreased with
concentrations from 0.4 to 10 mg/ml. Nuclear factor κ-B (NF-κB) and
interferon regulatory factor-1 (IRF-1) are involved in the
production of IL-12, which is then activated by IFN-γ (22). T-cells and NK cells, which release
a substantial amount of IFN-γ, are found in insignificant numbers
in adherent peritoneal exudate cells (23). As a result, FGE treatment may have
induced peritoneal macrophages to create more IL-12 in response to
IFN-γ. Thus, FGE has the ability to stimulate peritoneal
macrophages, prompting them to generate effector molecules, such as
TNF-α, IL-6 and IL-12.
FGE enhances the splenocyte
proliferative capacity (mitogen activity)
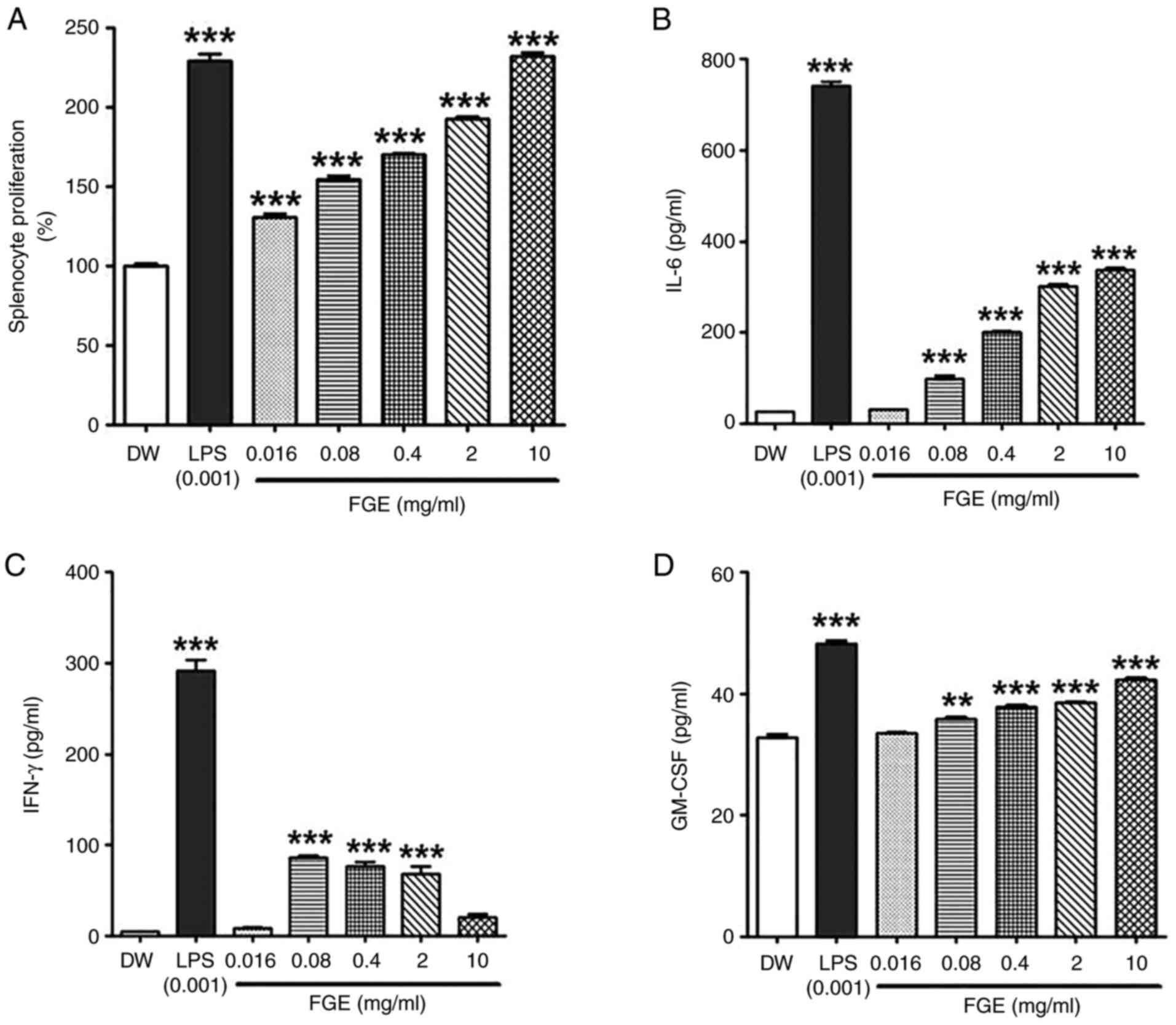
Mitogen activity employing splenocytes revealed that
the FGE-treated groups were able to proliferate splenocytes. FGE
treatment led to a statistically significant increase in
proliferative activity at the lowest concentration of 0.016 mg/ml
in comparison to the distilled water-treated control. The
proliferative activity induced by FGE at concentrations of 0.016-10
mg/ml was 1.30-2.32-fold higher than that of the distilled
water-treated control. In particular, mitogen activity was similar
to that of the LPS-treated positive control group at the maximum
concentration of 10 mg/ml (Fig.
3A).
FGE modulates the IL-6, IFN-γ and
GM-CSF cytokine levels
Splenocytes have a complex immune response mechanism
where one cytokine is involved and other cytokines follow.
Therefore, depending on the type of cytokine identified when the
splenocytes are stimulated, this has been used as evidence of other
immune responses (24). In the
present study, the ability of FGE to affect hemopoietic and T
helper 1 cell (Th1) cytokines was assessed by examining its
immunomodulatory effects on the splenocyte secretion of IL-6,
GM-CSF and IFN-γ. The secretion of IL-6, IFN-γ, and GM-CSF was
measured in BALB/c splenocytes cultured in medium supplemented with
the vehicle, LPS and FGE (0.016, 0.08, 0.4, 2, and 10 mg/ml). As
demonstrated in Fig. 3B, at the
lower concentration of 0.016 mg/ml, FGE had a minimal effect on
IL-6 (Th17) cytokine production. However, the production of IL-6
was considerably increased following treatment with FGE from the
concentration of 0.08 mg/ml in a concentration-dependent manner, as
compared to the distilled water-treated negative control group. The
secretion of GM-CSF was likewise increased and exhibited a similar
trend as that of IL-6 (Fig. 3D).
At the concentration of 0.08 mg/ml, FGE had a significant effect on
IFN-γ (Th1) cytokine secretion, which then exhibited a
concentration-dependent suppression at 0.4 mg/ml (Fig. 3C). These results demonstrated that
FGE stimulated cytokine secretion (IL-6) at higher concentrations,
while stimulating IFN-γ secretion at lower concentrations,
suggesting that FGE can interfere with the Th1 and hematopoietic
cytokine profiles.
FGE influences the activity of the gut
immune-system through PP stimulation
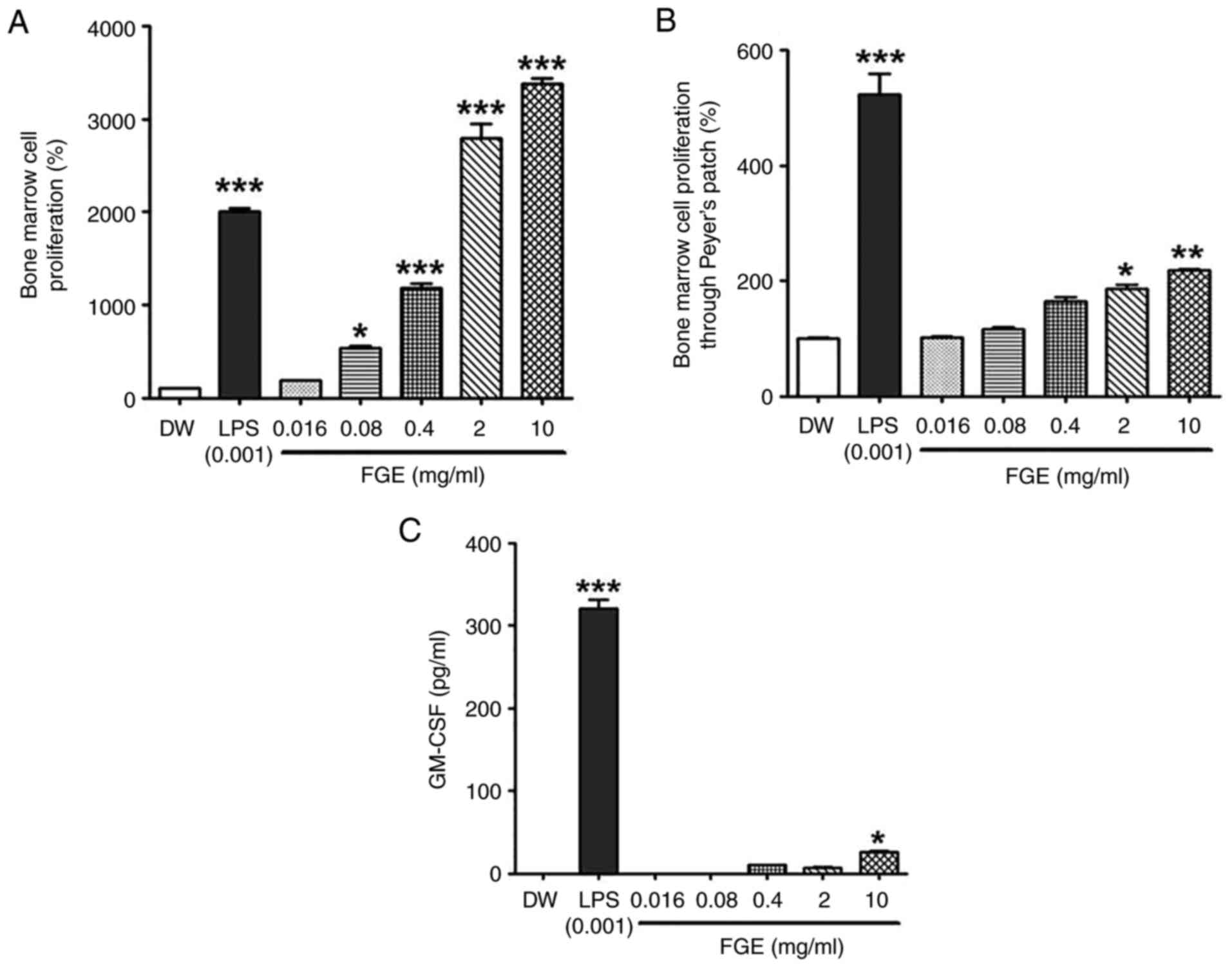
First, the effect of garlic fermentation broth on
bone marrow cells was evaluated. As shown in Fig. 4A, the garlic fermented broth
exhibited a concentration-dependent trend to enhance the
proliferation of bone marrow cells. FGE at all concentration ranges
(0.016-10 mg/ml) significantly enhanced the proliferation of bone
marrow cells compared to the distilled water-treated control group.
It exhibited cell proliferative activity (2.8-3.4-fold) compared to
the LPS-treated positive control group.
On the other hand, by measuring the bone marrow cell
proliferative activity mediated by PP cells, the effects of various
concentrations of FGE on the intestinal immune system were also
determined. In the present study, PP cells were stimulated directly
with FGE at 0.016-10 mg/ml, and the supernatant was subsequently
administered to a bone marrow cell culture to mimic hematopoietic
growth factors (HGFs). As shown in Fig. 4B, FGE at the highest concentration
of 10 mg/ml exerted the most prominent promoting effect on bone
marrow cell proliferative activity through PPs. T-cells and B-cells
comprise PP cells, where T-cells are the primary source of CSF and
cytokines (25,26). In the present study, the level of
the hematopoietic cytokine, GM-CSF, increased following treatment
with 0.4 to 10 mg/ml FGE, with the maximum production observed at
10 mg/ml (Fig. 4C). As a
consequence, the 10 mg/ml concentration of FG exceedingly
stimulated HGFs from specialized immune cells, such as T-cells and
intestinal PP cells, culminating in bone marrow cell
differentiation. FGE, however, did not promote bone marrow cell
proliferation and cytokine production as much as when compared to
the LPS-treated positive control. Therefore, these findings
indicate that FGE is helpful in increasing intestinal
immunostimulatory activity.
Effects of FGE on the cytotoxic
activity of NK cells from BALB/c mice
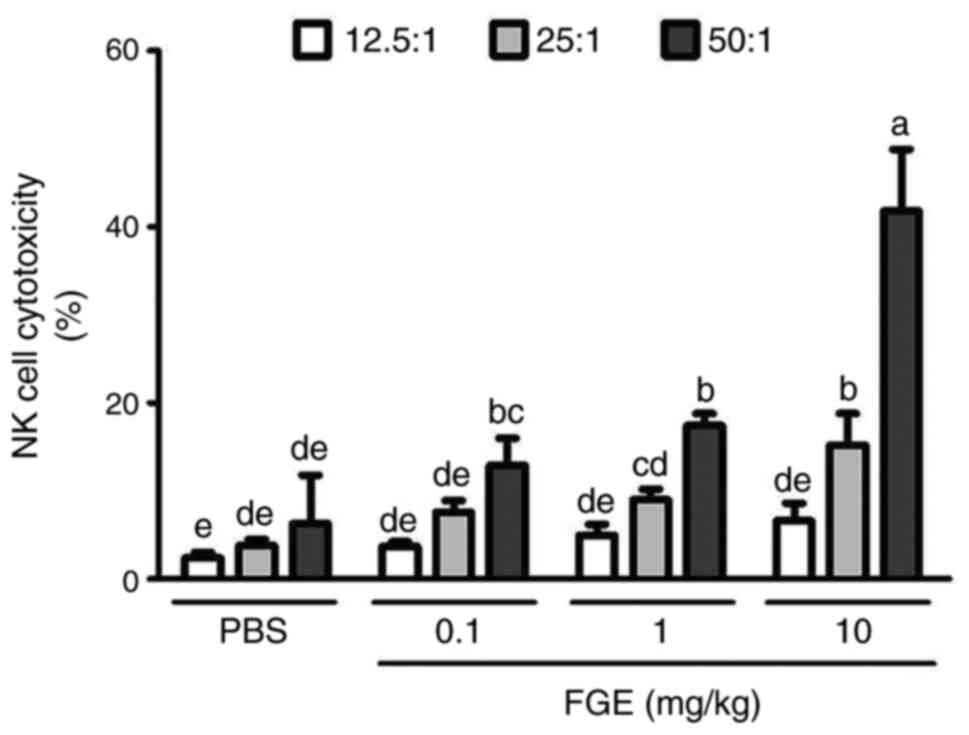
In the present study, 6-week-old BALB/c mice were
treated with PBS and, following the intravenous administration of
FGE (0.1, 1 and 10 mg/kg), mouse splenocytes were employed to
assess NK cell activity using YAC-1 cells as target cells. As a
result, the effector (NK cells) and target (cancer cells) were
compared to the saline-treated control in a ratio-dependent manner.
At the ratio of 12.5:1, NK cells isolated from mice administered
intravenously with physiological saline killed 2.4% of YAC-1 cells,
whereas splenocytes from 0.1, 1 and 10 mg/kg FGE-treated mice
killed 3.7 to 6.6% of YAC-1 cells. PBS-NK cells eliminated YAC-1
cells at a rate of 3.8% when the ratio was 25:1; however, FGE-NK
cells killed cancer cells at a rate of 7.6-15.2%. When the ratio of
NK cells to cancer cells was set to 50:1, PBS-NK cells cleared 6.3%
of cancer cells, whereas FGE-NK cells eliminated 41.8% of YAC-1
cells. Treatment with FGE increased cytotoxic NK cell activity in
the spleen and demonstrated that they can enhance the innate immune
response to tumor cells (Fig.
5).
Discussion
In the present study, FG was prepared by
fermentation with B. subtilis. It has been previously
demonstrated that extracts from garlic fermented with B.
subtilis are promising for the treatment of cardiovascular
disease and blood cholesterol levels; the elevated level of stable
nitrite present in the garlic contributes to these positive effects
(27). However, for the first
time, to the best of our knowledge, the present study established
that FG prepared by using B. subtilis exerted
immune-stimulating and non-toxic effects in vitro and ex
vivo. The present study demonstrated the immunomodulatory
properties of FG in vitro in a model of RAW264.7 cells.
Macrophages, which are phagocytizing immune cells, are known to aid
the body's innate and adaptive immunological responses (28). Following treatment with FGE, the
present study determined that FGE activated macrophages by
releasing immunostimulatory cytokines, such as TNF-α, IL-6, and
IL-12 from RAW264.7 cells. In macrophages, FG promoted
immunostimulatory cytokine production in a concentration-dependent
manner. It is known that the excessive secretion of
immunomodulatory factors by macrophages is known to serve as a
chronic inflammatory factor or to be lethal to macrophages
(29). As a result, the present
study evaluated whether FGE cytotoxicity was observed in RAW264.7
cells. It was confirmed that FGE at concentrations ranging from
0.016 to 10 mg/ml was not cytotoxic to RAW264.7 macrophages,
although FGE considerably promoted cell multiplication by 50 to 80%
in comparison to the control. At the concentration of 10 mg/ml, FGE
also increased peritoneal macrophage proliferation to the greatest
extent. GE in comparison to FGE exerted a less prominent promoting
effect on cell proliferation at low concentrations, while
cytotoxicity was observed at higher concentrations. These findings
suggest that FGE is not toxic to cells, and the immunomodulators
generated in macrophages by FGE strengthen the human immune system
in comparison to GE.
NO is an inflammatory mediator generated by
macrophages and neutrophils after L-arginine activates nitric oxide
synthase (30). Previous research
has demonstrated that arginine in aged garlic and nitrites
contained in fermented garlic contribute to the promotion of NO
generation (18,31-33).
The present study also found that FGE markedly increased NO
generation in RAW264.7 cells in a concentration-dependent manner in
comparison to the distilled water control and GE-treated groups.
Although GE promoted RAW264.7 cell proliferation at lower
concentrations, it was unable to increase NO production at all
concentrations. On the contrary, FGE increased cell proliferation
and NO production (which reached maximum levels at the
concentration of 2 mg/ml FGE). However, a concentration of FG (0.08
mg/ml) that stimulated cell proliferation, but did not produce NO
suggests that the increased NO production by FGE may not result in
increased macrophage proliferation. Such NO generated through
garlic extracts has been proven to induce expansion and circulation
by relaxing the muscles of blood vessels (34-36).
Other research also suggests that FGEs, due to their greater NO
generation capabilities, may have a vasorelaxing effect (33). Therefore, further research is
required to dissect the role of FG in vascular endothelial
function. Increased NO production, on the other hand, contributes
to macrophage phagocytic activity as macrophages are innate immune
cells that proliferate host defense against infections (37). In addition, FGE increased the
production of pro-inflammatory cytokines, such as IL-6 and TNF-α in
mouse peritoneal macrophages, which was consistent with the results
obtained in vitro. However, a higher level and different
pattern of IL-12 production were detected at a lower concentration
of FGE in comparison to that in RAW264.7 cells. Kim et al
(23) discovered that the
activation of NF-κB and IRF-1 proteins by IFN-γ plays a role in the
production of IL-12. Furthermore, T-cells, NK cells that release a
substantial amount of IFN-γ, are found in insignificant numbers in
adherent fractions of peritoneal exudate cells (23). Thus, FG therapy may have caused
peritoneal macrophages to create more IL-12 in response to released
IFN-γ; however, RAW264.7 cells were unable to develop such a
response. They exhibited a higher INF-γ level at lower FGE
concentrations and a lower INF-γ level with increasing FGE
concentrations, which was consistent with the findings on
splenocytes. These results are in accordance with those of previous
research, in that in comparison to unfermented garlic, heat-dried
garlic fermented with Lactobacillus plantarum boosted PBMC
proliferation and NO production, and suggested that these
biological activities were linked to greater phenolic and flavonoid
content released by microorganisms during the fermentation process
(19). Moreover, the present study
found that fermenting garlic with B. subtilis increased
macrophage proliferation, cytokine production and NO
generation.
Splenocyte proliferation, together with an increase
in cytokine production, leads to early humoral and cell-mediated
immune enhancement (38). In the
present study, for the first time, to the best of our knowledge,
the immunomodulatory effects of FGE were assessed by determining
mitogen activity in splenocytes. It was discovered that FGE
promoted splenocyte proliferation in a concentration-dependent
manner and led to a higher mitogen activity with a higher
concentration than the LPS-treated group. As a major
macrophage-activating cytokine, INF-γ plays a critical role in
cell-mediated adaptive immunity against intracellular parasitic
bacteria (39). In response to
antigen recognition, a cluster of differentiation 4+
(CD4+) Th1 cells produce INF-γ, which is amplified by
the production of IL-12 and IL-18(40). As a result, in the present study,
INF-γ followed a nearly identical trend to macrophage-derived IL-12
production. Splenocyte-produced IL-6 stimulates B-cell development
and functions as a co-stimulator for T-cells and hepatocytes. It
also promotes T-cell proliferation and stimulates the production of
antibodies that trigger B-cell differentiation (41). In the present study, FGE at a low
concentration level of 0.08 mg/ml led to a notable increase in IL-6
production (21.3-fold that of the distilled water control group).
Following that, as the concentration increased, the production
exhibited a tendency to gradually decrease. On the other hand, FGE
also exhibited a concentration-dependent tendency to increase the
production of splenocyte-derived GM-CSF, a powerful hematopoietic
cell stimulator. By acting on the bone marrow precursor, GM-CSF
facilitates the development of diverse circulating leukocytes of
the innate immune system (42). As
a result, FGE increased splenocyte secretion of the hemopoietic
cytokines IL-6 and GM-CSF.
In the present study, the activity of ex vivo
splenic cytotoxic NK cells was assessed to determine whether there
were any links between FGE and tumor-related immunity. The effect
of FGE on NK cell activation and cytotoxicity on YAC-1 cells, which
lack class I major histocompatibility complex (MHC) molecules, was
investigated in BALB/c mice by co-cultivation of isolated
splenocytes with the NK cell sensitive YAC-1 target cell. The
results revealed a concentration-related significant effect on NK
cytotoxic activity in relation to the control in all ratios of
effector cell to the target cell. These associations indicate that
FG exerts antitumorigenic activities through the promotion of NK
cell responses to YAC-1 tumor cell lysis. In vitro cytotoxic
studies have revealed that aged garlic extract significantly
improves the cytotoxic capability of human peripheral blood NK
cells against K562 tumor cells (43,44),
which is consistent with the findings of the present study. Thus,
the ability of FGE to stimulate immune cell IL-12 release must have
aided the generation of INF-γ-producing NK cells. As demonstrated
herein and in previous research, the inhibition of tumor cell
lines, such as YAC-1 may be further aided by extract-induced NK
cells (43).
The small intestine's lumen is exposed to a range of
bacteria and antigens, and gut-associated lymphoreticular tissues,
such as PPs play a role in immune monitoring and intramucosal
immune response (45). M-cells,
which are specialized cells, transport external antigens directly
from the lumen to antigen-presenting cells. After lymphocytes
triggered by antigens travel to the mesenteric lymph nodes,
activated T- and B-cells flow into the circulation via the thoracic
duct, augmenting the immune response. Therefore, if the food item
stimulates the PP in the colon, it could be produced as a
functional food that helps the body's systemic immune response
(46). To the best of our
knowledge, there are no available studies to date on the impact of
fermented garlic on the gut immune system via PP cells. Hence, the
present study experiment is the first to demonstrate that FG
increased bone marrow cell proliferation via PP cells. As PP cells
are comprised of T- and B-cells, and T-cells produce GM-CSF, it can
be hypothesized that FGE increased T-cell activation and the
production of the hematopoietic growth factor, GM-CSF. As a result,
it was found that FGE strongly promoted splenocytes to create the
hematopoietic cytokines, IL-6 and GM-CSF, which were sufficient to
cause mouse bone marrow cells to proliferate and differentiate into
various hematopoietic cells. Therefore, FG has the potential to
significantly proliferate both murine peritoneal macrophages and
splenocytes in a concentration-dependent manner. Hence, to confirm
the link with systemic immunity, GM-CSF generation in the culture
supernatant of PP cells with FGE and bone marrow cell proliferation
by treatment with PPs were assessed in the present study. As a
result, FGE may act as an inducer for strengthening the weakened
intestinal immune system and promoting intestinal immunity through
a functional diet.
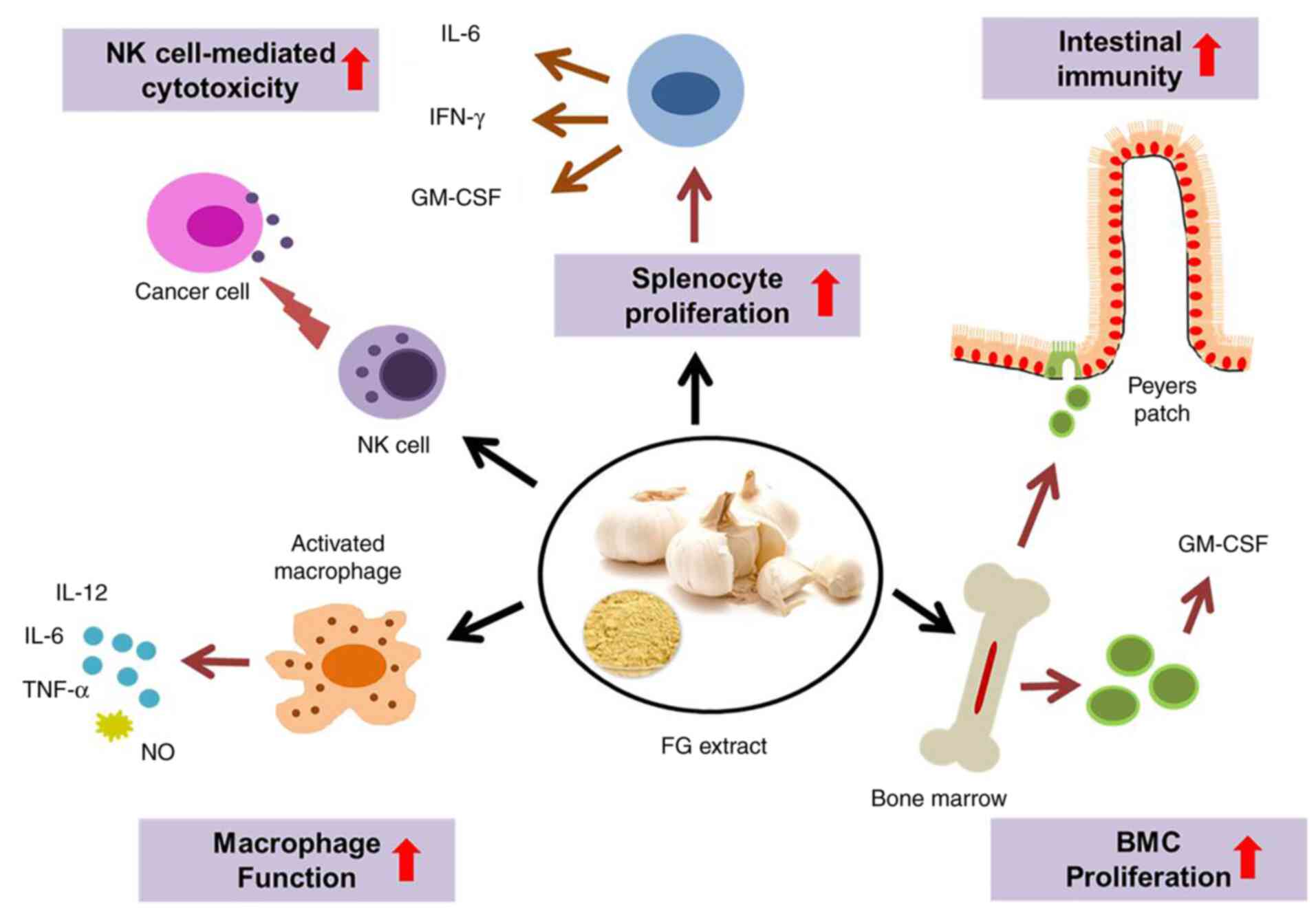
Taken together, the present study strongly suggests
that FG stimulates NK cell activity, the proliferation of
macrophages, and splenic T- and B-cells, suggesting that it can
improve cell-mediated immunity. FGE can enhance the cellular immune
response by upregulating Th1 cytokines. It has intestinal
immunostimulatory activity and positive effects on hematopoiesis
and the production of immune cells. A conceptual diagram of FG and
its proposed targets for immunomodulation is presented in Fig. 6.
In conclusion, the present study demonstrates that
FG has immunomodulatory properties in both ex vivo and in
vitro settings. Its action is mediated by the stimulation of
macrophage functions, the production of NO and the lytic activity
of NK cells-induced by INF-γ. The production of IL-6 and GM-CSF by
FG plays a crucial role in bone marrow cell proliferation, which
involves hematopoietic regulation. Furthermore, FG also has a
favorable effect on intestinal health by increasing gut immunity
through immune cell stimulation and cytokine production. Several
hypotheses can be advanced to explain the mechanism underlying the
reported modulation of local and systemic immune responses through
intestinal immune responses by FG, which may be related to the
substantial number of transformable organosulfur components formed
by B. subtilis. However, further studies are warranted to
concentrate on isolating the active components found in FGE and
assess the impact of these compounds on a variety of immune system
parameters. On the whole, FG may have an advantage in the
development of nutraceutical products and functional agents that
can help strengthen and sustain a strong immune system.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
All the experiments were devised by YWK and PG.
Experiments were carried out by PG at the Kyung Hee University Skin
Biotechnology Center and Dong-Sung Cancer Center. PG wrote the
manuscript, prepared all the figures and revised the manuscript.
YWK supervised the study and provided advice on the design, as well
as manuscript preparation. JL assisted with the experiments and in
manuscript preparation. TBTM and RS assisted with the study design
and manuscript preparation, and revised the manuscript. YWK and PG
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Institutional Animal Care and Use Committee of Kyung Hee University
(KHUSBC-R-SPA 2018-0601) and the Institutional Animal Care and Use
Committee of the Dongsung Cancer Center under protocol IACUC
#ds002205112-EUTO3. The experiments were carried out in compliance
with the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
Note that the authors are affiliated with the
company (Dongsung Biopharmaceutical) that provided the fermented
garlic whose effects have been investigated in this study.
References
|
1
|
Müller U, Vogel P, Alber G and Schaub GA:
The innate immune system of mammals and insects. Contrib Microbiol.
15:21–44. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Warrington R, Watson W, Kim HL and
Antonetti FR: An introduction to immunology and immunopathology.
Allergy Asthma Clin Immunol. 7: (Suppl 1)(S1)2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Blach-Olszewska Z and Leszek J: Mechanisms
of over-activated innate immune system regulation in autoimmune and
neurodegenerative disorders. Neuropsychiatr Dis Treat. 3:365–372.
2007.PubMed/NCBI
|
|
4
|
Patel DK, Dutta SD, Ganguly K, Cho SJ and
Lim KT: Mushroom-derived bioactive molecules as immunotherapeutic
agents: A review. Molecules. 26(1359)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Camille N and Dealtry G: Regulation of
M1/M2 macrophage polarization by sutherlandia frutescens via NFkB
and MAPK signaling pathways. S Afr J Bot. 116:42–51. 2018.
|
|
7
|
Yeap SK, Omar AR, Ho WY, Beh BK, Ali AM
and Alitheen NB: Rhaphidophora korthalsii modulates peripheral
blood natural killer cell proliferation, cytokine secretion and
cytotoxicity. BMC Complement Altern. 13(145)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu D, Lewis ED, Pae M and Meydani SN:
Nutritional modulation of immune function: Analysis of evidence,
mechanisms, and clinical relevance. Front Immunol.
9(3160)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yanishlieva NV, Marinova E and Pokorný J:
Natural antioxidants from herbs and spices. Eur J Lipid Sci
Technol. 108:776–793. 2006.
|
|
10
|
Londhe V, Gavasane AT, Nipate SS,
Bandawane DD and Chaudhari PD: Role of garlic (Allium
sativum) in various diseases: An overview. Angiogenesis.
12(13)2011.
|
|
11
|
Borrelli F, Capasso R and Izzo AA: Garlic
(Allium sativum L.): Adverse effects and drug interactions
in humans. Mol Nutr Food Res. 51:1386–1397. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
El-Saber Batiha G, Magdy Beshbishy A, G
Wasef L, Elewa YHA, A Al-Sagan A, Abd El-Hack ME, Taha AE, M
Abd-Elhakim Y and Prasad Devkota H: Chemical constituents and
pharmacological activities of garlic (Allium sativum L.): A
review. Nutrients. 12(872)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mikaili P, Maadirad S, Moloudizargari M,
Aghajanshakeri S and Sarahroodi S: Therapeutic uses and
pharmacological properties of garlic, shallot, and their
biologically active compounds. Iran J Basic Med Sci. 16:1031–1048.
2013.PubMed/NCBI
|
|
14
|
Sung J, Harfouche Y, De La Cruz M, Zamora
MP, Liu Y, Rego JA and Buckley NE: Garlic (Allium sativum)
stimulates lipopolysaccharide-induced tumor necrosis factor-alpha
production from J774A.1 murine macrophages. Phytother Res.
29:288–294. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Arreola R, Quintero-Fabián S, López-Roa
RI, Flores-Gutiérrez EO, Reyes-Grajeda JP, Carrera-Quintanar L and
Ortuño-Sahagún D: Immunomodulation and anti-inflammatory effects of
garlic compounds. J Immunol. 2015(401630)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Salman H, Bergman M, Bessler H, Punsky I
and Djaldetti M: Effect of a garlic derivative (alliin) on
peripheral blood cell immune responses. Int J Immunopharmacol.
21:589–597. 1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ichikawa M, Ide N and Ono K: Changes in
organosulfur compounds in garlic cloves during storage. J Agric
Food Chem. 54:4849–4854. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shang A, Cao SY, Xu XY, Gan RY, Tang GY,
Corke H, Mavumengwana V and Li HB: Bioactive compounds and
biological functions of garlic (Allium sativum L.). Foods.
8(246)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daliri EBM, Kim SH, Park BJ, Kim HS, Kim
JM, Kim HS and Oh DH: Effects of different processing methods on
the antioxidant and immune stimulating abilities of garlic. Food
Sci Nutr. 7:1222–1229. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Najman K, Sadowska A and Hallmann E:
Influence of thermal processing on the bioactive, antioxidant, and
physicochemical properties of conventional and organic agriculture
black garlic (Allium sativum L.). Appl Sci.
10(8638)2020.
|
|
21
|
Venkatesh YP: Immunomodulatory attributes
of aged garlic extract and its components. Immunology, Elsevie,
pp203-224, 2018.
|
|
22
|
Ogawa K, Funaba M and Tsujimoto M:
Suppression of NF-kappaB and IRF-1-induced transcription of the
murine IL-12 p40 by transforming growth factor-beta Smad pathway in
macrophages. Mol Cell Biochem. 308:9–15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim BG, Song Y, Lee MG, Ku JM, Jin SJ,
Hong JW, Lee S and Kang H: Macrophages from mice administered rhus
verniciflua stokes extract show selective anti-inflammatory
activity. Nutrients. 10(1926)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Albert MJ, Raghupathy R, Khan I and
Azizieh FY: In vitro spleen cell cytokine responses of adult mice
immunized with a recombinant PorA [major outer membrane protein
(MOMP)] from campylobacter jejuni. Sci Rep. 9(12024)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jung C, Hugot JP and Barreau F: Peyer's
patches: The immune sensors of the intestine. Int J Inflam.
2010(823710)2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ingelfinger F, De Feo D and Becher B:
GM-CSF: Master regulator of the T cell-phagocyte interface during
inflammation. Semin Immunol. 54(101518)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee HJ, Yoon DK, Lee NY and Lee CH: Effect
of aged and fermented garlic extracts as natural antioxidants on
lipid oxidation in pork patties. Food Sci Anim Resour. 39:610–622.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Diskin C and Pålsson-McDermott EM:
Metabolic modulation in macrophage effector function. Front
Immunol. 9(270)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu W, Zhao M, Fu X, Hou J, Wang Y, Shi F
and Hu S: Molecular mechanisms underlying macrophage
immunomodulatory activity of Rubus chingii Hu polysaccharides. Int
J Biol Macromol. 185:907–916. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Palmieri EM, McGinity C, Wink DA and
McVicar DW: Nitric oxide in macrophage immunometabolism: Hiding in
plain sight. Metabolites. 10(429)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Das I, Khan NS and Sooranna SR: Potent
activation of nitric oxide synthase by garlic: A basis for its
therapeutic applications. Curr Med Res Opin. 13:257–263.
1995.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park BM, Cha SA, Kim HY, Kang DK, Yun K,
Chun H, Chae SW and Kim SH: Fermented garlic extract decreases
blood pressure through nitrite and sGC-cGMP-PKG pathway in
spontaneously hypertensive rats. J Funct Foods. 22:156–165.
2016.
|
|
34
|
Cruz C, Correa-Rotter R, Sánchez-González
DJ, Hernández-Pando R, Maldonado PD, Martínez-Martínez CM,
Medina-Campos ON, Tapia E, Aguilar D, Chirino YI and
Pedraza-Chaverri J: Renoprotective and antihypertensive effects of
S-allylcysteine in 5/6 nephrectomized rats. Am J Physiol Renal
Physiol. 293:F1691–F1698. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Asdaq S and Inamdar M: Potential of garlic
and its active constituent, S-allyl cysteine, as antihypertensive
and cardioprotective in presence of captopril. Phytomedicine.
17:1016–1026. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takashima M, Kanamori Y, Kodera Y,
Morihara N and Tamura K: Aged garlic extract exerts
endothelium-dependent vasorelaxant effect on rat aorta by
increasing nitric oxide production. Phytomedicine. 24:56–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tümer C, Bilgin HM, Obay BD, Diken H,
Atmaca M and Kelle M: Effect of nitric oxide on phagocytic activity
of lipopolysaccharide-induced macrophages: Possible role of
exogenous L-arginine. Cell Biol Int. 31:565–569. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bronte V and Pittet MJ: The spleen in
local and systemic regulation of immunity. Immunity. 39:806–818.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5(491)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Griffin G, Shenoi S and Hughes GC:
Hemophagocytic lymphohistiocytosis: An update on pathogenesis,
diagnosis, and therapy. Best Pract Res Clin Rheumatol.
34(101515)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6(a016295)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Johnson CB, Zhang J and Lucas D: The role
of the bone marrow microenvironment in the response to infection.
Front Immunol. 11(585402)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hassan ZM, Yaraee R, Zare N, Ghazanfari T,
Sarraf Nejad AH and Nazori B: Immunomodulatory affect of R10
fraction of garlic extract on natural killer activity. Int
Immunopharmacol. 3:1483–1489. 2003.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kyo E, Uda N, Suzuki A, Kakimoto M,
Ushijima M, Kasuga S and Itakura Y: Immunomodulation and antitumor
activities of aged garlic extract. Phytomedicine. 5:259–267.
1998.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hashizume T, Togawa A, Nochi T, Igarashi
O, Kweon MN, Kiyono H and Yamamoto M: Peyer's patches are required
for intestinal immunoglobulin A responses to Salmonella spp. Infect
Immun. 76:927–934. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim H, Kim HW, Yu KW and Suh HJ:
Polysaccharides fractionated from enzyme digests of Korean red
ginseng water extracts enhance the immunostimulatory activity. Int
J Biol Macromol. 121:913–920. 2019.PubMed/NCBI View Article : Google Scholar
|