1. Introduction
Medicinal herbs and spices have been used throughout
human history for their healing properties and their
quality-of-life benefits. They have constituted the major
therapeutics in ancient medicinal systems, such as traditional
Chinese medicine (TCM) (1),
Ayurveda (2), Kampo (Japan) and
numerous others (3). The interest
in medicinal plants and their extracts and multi-component drugs
that include active substances of natural origin has increased
exponentially over the past few decades. The rationale and main
goal is the development of not only potent, but also safe
therapeutic compounds that would not elicit the plethora of
unwanted side-effects otherwise caused by synthetic allopathic
medications. Therefore, the immunomodulating activity of numerous
traditionally used herbs and spices has gained increasing attention
in recent years.
The immune system is relatively complex; thus, any
factors influencing the functions of the immune system may also
influence other systems in the human body, such as the nervous
system, endocrine system, metabolism etc. Consequently, research in
this field is quite diverse, and the modulation of the immune
system aims to prevent disease, as well as to identify novel
targets that may serve as the basis for novel and more effective
therapeutics. One of the main approaches in all traditional
medicine systems for preserving health and wellbeing and at the
same time preventing disease, is a healthy wholesome diet that
includes a number of plant-based foods. This diet has a specific
focus on herbs and spices, as these can support the healthy and
balanced functioning of the immune system. In this respect, studies
have indicated that diet influences the various intrinsic and
extrinsic factors of the immune system (4,5).
Herbs and spices have been a staple in the natural
diets of all cultures globally. They have been highly valued for
their anti-inflammatory properties, particularly considering that
one of the major causes for the development of disease is low-grade
chronic inflammation. Taken together, there are countless
inflammation-lowering plants in different parts of the globe; some
of these are widely known and can be found relatively easily around
the globe, including thyme, oregano, rosemary, sage, basil, mint,
dill, parsley, fenugreek, clove, nutmeg, cinnamon, turmeric, tulsi,
lemon grass, ginger, chili pepper, pepper and numerous others
(6). A vast variety of their
constituents, such as flavonoids, polysaccharides, lactones,
alkaloids, diterpenoids, glycosides etc. have been reported to be
responsible for the immunomodulatory and anti-inflammatory
properties of these plants. Notably, some of these compounds, such
as curcumin, gingerol and capsaicin, appear to inhibit one or more
of the steps linking pro-inflammatory stimuli to cyclooxygenase
(COX) activation (7). In addition,
the activation of NF-κB, a key regulator of the production of
COX-2, is associated with a variety of inflammatory diseases,
including cancer, atherosclerosis, myocardial infarction, diabetes,
allergies, asthma, arthritis, Crohn's disease, multiple sclerosis,
Alzheimer's disease, osteoporosis, psoriasis and septic shock
(8,9); in this regard, the inhibition of COX
should prevent the activation of the alternative NF-κB pathway and
in this manner, may reduce inflammation and related symptoms
(10).
Summarizing the nutraceutical properties of such an
immense variety of herbs would be an extremely difficult, if not
impossible, task, for any review article. Hence, the present review
concentrated on a selection of promising, yet at the same time,
quite popular and widely accessible plants, such as
Andrographis (Andrographis paniculata),
Astragalus (Astragalus propinquus/membranaceus),
black cumin (Nigella sativa), cardamom (Elettaria
cardamomum), purple coneflower (Echinacea), ginger
(Zingiber officinale), licorice (Glycyrrhiza glabra),
shiitake (Lentinula edodes) and turmeric (Curcuma
longa). These plants are often promoted as natural immune
boosters in the form of nutritional supplements. The key signal
transduction pathways and activation methods for each herb are
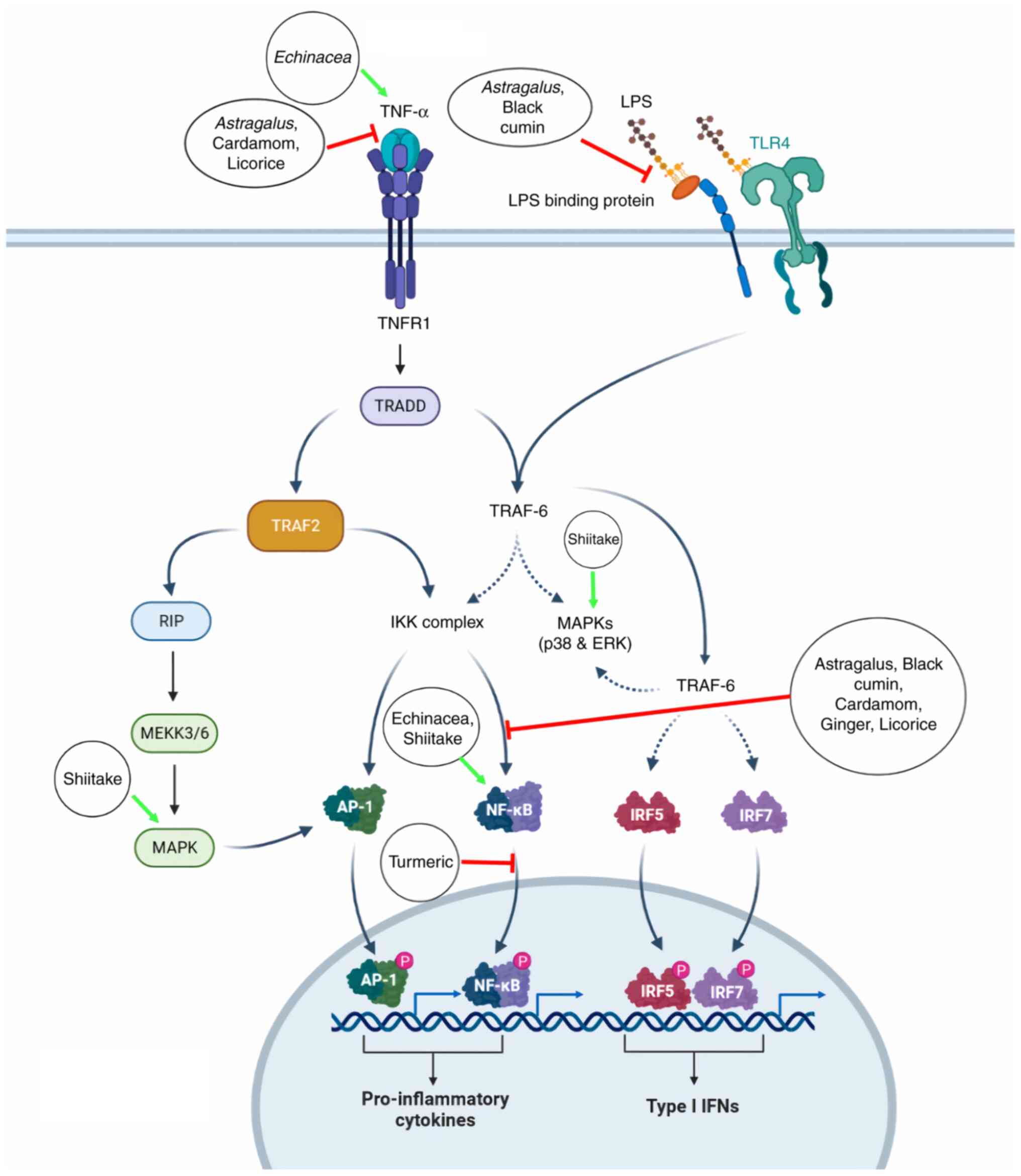
illustrated in Fig. 1.
2. Andrographis (Andrographis
paniculata)
Andrographis is a herbaceous plant of the
Acanthaceae family, known in Europe as the ‘King of Bitters’.
Traditional applications in Ayurvedic medicine and TCM, among
others, vary widely and include supporting liver and gallbladder
function, detoxification, digestion, immune response (through the
modulation of the levels of immune cells), maintaining normal body
temperature, as well as use for its antiviral, cardio protective
and hepatoprotective properties (11). According to previous phytochemical
studies, the aerial parts of the plant possess most of the
medicinal properties and are the source for the isolation of
diterpenoid lactones, which are the major phytochemical and
flavonoid constituents; the roots are the source of several
different compounds, such as xanthones, some noriridoids and
trace/macro elements (12-14).
Other researchers, using a broad variety of formulations, extracts
and pure compounds, have demonstrated anti-inflammatory,
immunomodulating, antioxidant, cytotoxic, anti-microbial and
anti-malarial properties (15).
Various extraction methods of Andrographis
paniculata have been tested, based on determining free radical
scavenging of extracts using the high-performance liquid
chromatography-ultraviolet mass spectrometry method and the DPPH
test (16). The main antioxidant
compounds in plants are known as flavonoids and phenols (17). A previous study concluded that an
aqueous extract has a higher concentration of total flavonoids
compared to an ethanol extract, and a radical scavenging activity
of 66.8 vs. 57.8% in ethanol; however, the ethanol extract appears
to have more phenols compared to the aqueous extract, even though
the potency of the aqueous extract is higher in antioxidant
activities (16). Several
phytochemicals, extracted from the leaves of Andrographis
(namely andrographolide, neoandrographolide, isoandrographolide,
andrograpanin, 7-O-methyl-wogonin,
14-deoxy-11,12-didehydroandro-grapholide and skullcapflavone) have
been examined for their anti-inflammatory and anti-allergic effects
in vitro (18,19). The majority of these compounds have
been shown to induce a concentration-dependent inhibition of the
release of inflammatory mediators from cultured macrophages,
stimulated by lipopolysaccharides (LPS), such as nitric oxide (NO)
and prostaglandin E2, interleukin (IL)-1β and IL-6. The compound
with the broadest effect appeared to be andrographolide, whereas
7-O-methylwogonin was unique in its potent dose-dependent
ability to inhibit A23187-induced (calcimycin-stimulated) histamine
release in RBL-2H3 rat basophil leukemic cells (18). In a previous study,
andrographolide, dehydroandrographolide and neo-andrographolide
were shown to exhibit anti-inflammatory effects by interfering with
both COX-1 and COX-2 enzyme activity (20). Dehydroandrographolide demonstrated
the highest efficacy in modulating the level of LPS-induced TNF-α,
IL-6, IL-1β and IL-10 secretion in human blood, in a
concentration-dependent manner; its mechanism of action may be
related to the downregulation of the expression of genes involved
in the inflammatory cascade (20),
as research using mice has demonstrated that dehydroandrographolide
can inhibit NF-κB activation in mice (21). The diterpenes, andrographolide and
neoandrographolide, have been shown to induce significant antibody
responses in mice, delayed hypersensitivity response against sheep
red blood cells, as well as non-specific immune responses (22). When ethanol extract was applied
instead of purified diterpenes, an immune response was still
observed, albeit considerably milder, suggesting that other
constituents in the alcohol extract may provide an entourage
immunostimulating effect (22).
Further studies investigating the mechanisms of
action are expected to contribute towards an improved assessment
and understanding of the complex pharmacological effects of this
plant. In addition, safety should be evaluated through laboratory
and clinical studies on the toxicity of the different extracts and
certain pure phytochemical isolates.
3. Astragalus (Astragalus
propinquus/membranaseus)
Astragalus is a leguminous plant and its main
chemical composition includes astragaloside, Astragalus
polysaccharide (APS), astragalus flavonoids, selenium and other
trace elements (23). In TCM,
where it is known as Huangqi, it is used both as a medicine and
food; it is believed to stimulate the spleen and to replenish the
vital energy that is considered to circulate the body in currents,
also known as the qi (23).
Astragalus is used to treat weakness, wounds, anemia, fever,
multiple allergies, chronic fatigue, loss of appetite, uterine
bleeding and prolapse (24). The
main pharmacologically active ingredient is APS, a type of
water-soluble heteropolysaccharide extracted from the stems or
dried roots (25). APS exerts
multiple bioactive effects, several of which have been thoroughly
investigated, particularly for their activity in immune regulation
and anti-aging. APS has further gained attention due to its ability
to reduce blood sugar levels and lower blood lipid levels, as well
as due to its antitumor, anti-fibrotic, antibacterial and antiviral
effects, and its radiation shielding properties (23). As regards immunomodulation, APS has
been shown to activate mouse macrophages and B-cells, rather than
T-cells, leading to proliferation and cytokine production via the
activation of Toll-like receptor (TLR)4(1). It has also been reported to inhibit
the growth of the breast cancer cell line, MDA-MB-468(26). Xu et al (27) demonstrated that APS strongly
promoted the phagocytosis of Mycobacterium tuberculosis and
the secretion of IL-1α, IL-6 and TNF-α by activated macrophages.
Experimental data have also indicated that this particular
polysaccharide can initiate splenocytes to secrete IL-2, IL-3, IL-4
and IL-6, and also induce interferon (IFN) production (23), with substantial evidence also
pointing towards a concentration-dependent initiation of IL-10,
IL-12 and IL-2 secretion (28).
Furthermore, in carp models, APS has been shown to increase the
levels of IL1-β, lysozyme C and TNF-α in the kidneys, gills and
spleen, in a dose-dependent manner (29). Various experiments using H22
tumor-bearing mice have also demonstrated that the administration
of APS increases the production of IL2, IL6 and TNF-α, which
further highlights the effects of APS on the immune system and
subsequently, its antitumor activity (30,31).
Several preclinical studies and clinical trials have
demonstrated that Astragalus has more potent anticancer
effects than immunomodulatory properties (32-34)
and for this reason, it is recommended to cancer patients as a
complementary and alternative therapy (35). Nonetheless, it has also exhibited
immense potential in balancing Treg/Th17 cells, and is thus being
investigated as a potential complementary treatment for patients
with asthma (36) and perhaps for
patients with diabetic nephropathy (37).
4. Black cumin (Nigella sativa)
Black cumin, also known as Nigella sativa, is
a widely used medicinal plant of the Ranunculaceae family. Its
popularity encompasses almost all traditional medicinal systems,
such as Indian, Ayurveda and Siddha, Islamic Tibb-e-Nabawi and
Western herbalism. In Bulgarian folklore, it is referred to as ‘the
herb that cures everything but death’. Black cumin has been broadly
used as a (skin) tonic, a digestive, an anti-diarrheal, an appetite
stimulant, an anti-bacterial, an antihypertensive, a diuretic and
an analgesic. The majority of its therapeutic effects are
considered to be due to thymoquinone (TQ), a major bioactive
component of black cumin essential oil (38).
In vitro studies have demonstrated that the
aqueous extract of black cumin exerts an anti-inflammatory and
immunomodulatory effect, as it significantly enhances splenocyte
proliferation in BLAB/c mice and C57/BL6 primary cells in a
concentration-dependent manner (39). Specifically, the aqueous extract
appears to favor the secretion of Th2, as opposed to Th1 cytokines
by splenocytes; the secretion of key pro-inflammatory mediators,
such as IL-6, TNF-α and NO by macrophages is significantly
suppressed and the cytotoxic activity of natural killer (NK) cells
against YAC-1 tumor cells is significantly enhanced (40). This suggests that black cumin
bioactive compounds may be used as therapeutic agents for the
regulation of several immune reactions in variety of conditions and
diseases, such as cancer (40). In
addition, the immunomodulatory activity of black cumin seed can be
used for the prophylaxys of opportunistic infections and as
adjuvant treatment in certain oncological patients (39). Other observations indicate that the
seeds of Nigella sativa may represent a potential
immunosuppressive cytotoxic agent (41). Preventative oral administration of
N. sativa oil to rats daily has been shown to significantly reverse
the reduction of hemolysin antibody titers related to the applied
whole body gamma irradiation (42). This suggests that the oil may
constitute a promising radioprotective agent against ionizing
radiation, particularly against the immunosuppressive and oxidative
effects of the latter (42).
Both the aqueous and alcoholic extracts of black
cumin exhibit anti-inflammatory and analgesic properties, which are
considered to be exerted by their TQ content. The concentration of
the latter varies greatly throughout the different parts of the
plant; it is 12-fold higher in the leaf callus than the seed
extract. The anti-inflammatory effects of TQ on inflamed rat mix
glial cells is evidenced by a significant reduction in NO
production (43). In addition,
oxidative stress and inflammation related to osteoporosis appear to
be beneficially influenced by TQ via the inhibition of inflammatory
cytokine production, such as IL-1 and IL-6, and the element of the
NF-κB consensus site (44). It
also appears to reduce the synthesis of monocyte chemoattractant
protein (MCP)-1, TNF-α, IL-1β and COX-2 by pancreatic ductal
adenocarcinoma cells in a concentration- and time-dependent manner.
These results suggest that TQ may be a promising agent that
combines the inhibition of proinflammatory pathways with a
pro-apoptotic mode of action (45). Other researchers have also
demonstrated the anti-inflammatory effects of TQ during an allergic
response in the lungs, through the inhibition of prostaglandin D2
synthesis and Th2-driven immune responses (46).
Last but not least, a clinical trial was conducted
as a prospective and double-blind study with descriptive analysis
to investigate the anti-inflammatory effects of Nigella
sativa in patients with allergic rhinitis (47). The results revealed that Nigella
sativa reduced the congestion of the nasal mucosa, itching,
mucosal pallor, runny nose and sneezing attacks, thereby suggesting
that it may be beneficial as a treatment option when other
anti-allergic drugs must be avoided for various reasons (47).
5. Cardamom (Elettaria
cardamomum)
Cardamom is a traditional aromatic plant from the
family of Zingiberaceae. The chemical composition of cardamom
varies, depending on its origin and maturity stage. The seed
contains 2 to 5% of the volatile compounds that contribute to the
sweet and spicy flavor. The essential oil contains a considerable
number of bioactive compounds, such as α-pinene, α-phellandrene,
β-pinene, myrcene, limonene, sabinene, 1,8-cineole, linalool,
terpinolene, γ-terpinene, linalyl acetate, α-terpineol, terpinen
4-ol, α-terpinyl acetate, geraniol, citronellol, trans-nerolidol
and methyl eugenol (48). The
particular flavor of cardamom is mainly owned by α-terpinyl acetate
and 1,8-cineole (19). Over the
years, cardamom has been used in the treatment of disorders, such
as asthma, indigestion and congestive jaundice (49).
It has been demonstrated that cardamom possesses
various pharmacological properties, such as antioxidant,
anti-inflammatory, anticancer and antimicrobial properties
(48). The aqueous extract exerts
immunomodulatory effects as well, which have been validated in
vivo. In particular, it has been shown to significantly enhance
splenocyte proliferation in a synergistic and
concentration-dependent manner; based on an enzyme-linked
immunosorbent assay, cardamom was shown to significantly inhibit
the release of Th1-cytokines from splenocytes and to enhance
Th2-cytokine release (50).
Previous research has indicated that cardamom extract exerts
anti-inflammatory effects in BALB/c mice, as it significantly
enhances the cytotoxic activity of NK cells, thereby indicating an
anticancer potential (50).
Additional research has demonstrated that cardamom modulates the
status of proliferation, and the modification of COX-2 and
inducible nitric oxide synthase (iNOS) expression in apoptotic
processes, suggesting that it may confer a protective effect in
experimentally-induced colon carcinogenesis (51).
6. Echinacea
Echinacea belongs to a genus of herbaceous
flowering plants, commonly known as coneflowers, with Echinacea
purpurea being the herb most commonly used either to treat or
prevent the common cold, despite the existence of contradictory
data regarding its efficacy. For example, a 2014 systematic review
claimed that Echinacea was ineffective against the common
cold (51), whereas a subsequent
meta-analysis found some evidence that it reduces the risk of
repetitive respiratory infections (52,53).
Three species of Echinacea are widely used
medicinally: E. purpurea, E. angustifolia, and E.
pallida (54). Preparations of
the root and aerial parts of the three species are all currently
being promoted as immune stimulants (55). The immune-boosting effects of
Echinacea are considered to result primarily through the
targeting of non-specific immune mechanisms, including phagocytic
activity, macrophage activation and NK cell activity. These effects
have been demonstrated in vitro, where both the juice from
the aerial part of E. purpurea and alcohol extracts from
roots of E. purpurea, E. angustifolia and E.
pallida have been used (56,57).
In macrophages, phagocytosis and cytokine production (evidenced as
increased levels of TNF-α, IL-1 and IFN-β) appear to be enhanced
following treatment with Echinacea extracts (58). Increased leukocyte mobility and the
activation of NK cells has also been reasonably demonstrated in
animals and humans (58-61).
Notably, E. purpurea polysaccharide-enriched extracts can
promote the phenotypic and functional maturation of dendritic cells
via the modulation of the JNK, p38 MAPK and NF-κB pathways
(62). In addition, Fu et
al (63) demonstrated that
Echinacea extract was a potent activator of murine bone
marrow-derived macrophages by increasing the expression of CD80,
CD86 and MHCII molecules, and by upregulating the levels of markers
of classically activated macrophages (M1), including CCR7, and the
production of IL-1β, IL-6, IL-12p70, TNF-α and NO.
There are still open questions related to the
long-term use of Echinacea. Although primarily considered
for therapeutic purposes, some authors suggest the use of
Echinacea as a prophylactic during winter (64). The long-term effects of
Echinacea (in years) are unknown.
7. Ginger (Zingiber officinale)
Ginger (Zingiber officinale Rosc.) belongs to
the family of Zingiberaceae. It has long been cultivated as a
flavoring spice to Indian food (65) and is generally widely used in South
East Asia. The constituents of ginger are numerous and vary,
depending on the place of origin and form of rhizomes, e.g., fresh
or dry. The ginger rhizome contains multiple compounds of interest,
such as carbohydrates, minerals, phytochemicals, phenolic
compounds, terpenes, polysaccharides, lipids, organic acids, and
raw fibers (66). It also contains
appreciable amounts of vitamins and minerals and some enzymes,
among which the potent proteolytic enzyme, zingibain, appears to be
of particular interest (67).
Ginger contains >40 antioxidant compounds, which can be used to
treat various inflammatory conditions (68).
Ginger, being both a traditional spice and medicine
in TCM and Ayurveda, is also consumed in various forms in order to
boost immunity, reduce nausea and to ease digestion, as evidenced
by several clinical trials, while the dietary supplementation of
the powdered rhizome (vertical root) daily may increase
testosterone levels (69,70). As regards immune function, ginger
has been shown to successfully lower TNF-α and IL-1β levels
compared to placebo in patients knee osteoarthritis, where pain is
caused by joint inflammation due to the overproduction of synovial
cytokines; this demonstrates its ability to modulate inflammatory
cytokine production in chronic disease (71). The structurally related substances,
gingerol and shogaol in ginger inhibit prostaglandins by preventing
the biosynthesis of prostaglandin synthase, and may also be
effective against arachidonate 5-lipoxygenase, which is part of the
leukotriene biosynthesis (2).
Overall, ginger is believed to inhibit the synthesis of IL-1, TNF-α
and IL-8, acting in a pro-inflammatory cytokine manner, through a
mechanism that includes influencing of coding genes (72,73).
In another study, [6]-shogaol was shown to downregulate
inflammatory iNOS levels by inhibiting the expression of the COX-2
gene in macrophages, and the activation of NF-κB by disrupting the
activation PI3K/Akt/IκB-kinases IKK and MAPK (74).
8. Liquorice (Glycyrrhiza
glabra)
Liquorice (or licorice) is a plant of the family
Fabaceae (Glycyrrhiza glabra L.). The use of licorice is
well described and already has a history of several thousands of
years. The plant has been implemented in TCM and medieval medicine
as a tonic, to promote vitality, and to treat ulcers, gastritis, a
variety of infections and other inflammatory conditions. The most
significant compounds in terms of pharmacological activity include
flavonoids, triterpenes and saponins, where the main bioactive
compound is glycyrrhizin (5-24%) (75). The composition varies greatly
depending on the species, geographical location and environmental
conditions, with the species growing in Iran, China and Russia
having the highest glycyrrhizin contents (76).
Licorice polysaccharides have demonstrated a
potential immunomodulatory and anticancer effect in mice, by
inducing and upregulating the expression of IL-7, an antitumor
cytokine, as well as by affecting T-lymphocytes (77). Glycyrrhizin, glabridin and
isoliquiritigenin have exhibited apoptotic and anti-proliferative
activity on cancer cells in vitro (78,79).
Similarly, 18β-glycyrrhetinic acid, has exhibited immunomodulatory
activity by enhancing T-cell proliferation, thereby increasing
leukocyte concentration in murine models (80). The polyphenols of licorice induce
apoptosis by upregulating the expression of Bax and Bid proteins
and via the downregulation of Bcl-2, thus affecting the caspase
pathway (81).
In 4T1 murine mammary cancer cells, licorice has
been observed to inhibit and attenuate the progression of
angiogenesis and metastasis of cancer cells, by reducing
inflammation and tumor growth (82,83).
The introduction of licorice compounds as adjuvant treatment in
chemotherapy has demonstrated increased anticancer activity and
hepatoprotection in murine models (84,85).
Furthermore, licorice appears to downregulate the levels of the
inflammatory cytokines, IL-6, IL-1 and TNFα in vitro
(86). Such observations highlight
the promising activity of licorice compounds in vitro, and
undoubtedly warrant their testing in clinical trials to determine
their safety and efficacy in various clinical settings.
9. Shiitake (Lentinula edodes)
Shiitake is a type of mushroom that grows on the
Shii tree [Castanopsis cuspidata (Thunb.) Schottky]. It is
well known as a dense, nutrient-rich food, and as a medicine for
thousands of years (3,87). The mushroom has been proven to
exert anticarcinogenic, antitumor, hepatoprotective, cardiovascular
and immunomodulating effects. It also has antibacterial and
antiviral properties and has been found to be a potent antioxidant
(88). The chemical composition of
the Shiitake mushroom consists of ~58-60% carbohydrates, 20-23%
protein (with 80-87% digestibility), 9-10% fiber and 3-4% lipids
(89). It is also an excellent
source of vitamins, including D2 and B 1, 2, 5 and 12. In addition,
Shiitake contains a plethora of minerals, namely zinc, copper,
phosphorus, manganese, iron, potassium, calcium, magnesium and
cadmium (87).
The antitumor polysaccharide, lentinan (LNT), a
β-d-glucan, is regarded to be the most significant of the medicinal
properties of the Shiitake mushroom; LNT forms a worm-like triple
helix, that is heat-stable, water-soluble and alkali labile
(90). However, due to limitations
in 3D structure identification technology and crystal structure
absence, there is still no detailed structure information of
lentinan (91). Studies have
suggested that LNT can cause the regression of solid type tumors of
sarcoma 180 almost completely, as well as several other tumors,
including methylcholanthrene-induced fibrosarcoma (92,93).
It was later shown that the antitumor activity also affects
synergic and autochthonous tumors and prevents viral and chemical
oncogenesis (94). Overall, it has
been demonstrated that β-glucans can serve as pathogen-associated
molecular patterns, initiating immune responses through the binding
of pattern recognition receptors, such as dectin-1, TLR2/4/6 and
CR3(95). Among, these, dectin-1
appears to be of the highest significance, as it constitutes a
cell-like-receptor type II membrane protein, expressed in variety
of cells, but mainly in neutrophils, dendritic cells and
macrophages (96). The binding of
LNT to these receptors leads to the activation of MAPK/NF-κB and
spleen tyrosine kinase (Syk)/protein kinase C signaling (97). Following this, T-lymphocytes
upregulate the expression of TNF-α, TLR4 and TLR9, whereas
B-lymphocytes secrete IgG and enhance macrophage activity in mice
(98). Additional activities of
LNT include NK cell activation and an increased production of IFN-γ
and IL-12(99). These findings
strongly suggest that lentinan and similar polysaccharides have key
immunomodulatory properties and can be used to supplement ongoing
treatment in a variety of cancerous diseases and tumors.
10. Turmeric (Curcuma longa)
Curcumin is a yellow polyphenolic pigment from the
Curcuma longa L. (turmeric) rhizome, a flowering plant of
the ginger family. It has been used throughout history for culinary
and medicinal purposes, specifically in Ayurvedic and Chinese
medicine. The use of the spice dates ~5,000 years back in history
(100). Apart from its use as a
culinary seasoning, turmeric has been historically used as an
antimicrobial agent, an insect repellent and a natural coloring
agent (101). Curcumin,
demethoxycurcumin and bisdemethoxycurcumin are bioactive
polyphenolic compounds, identified in turmeric, which have been
collectively referred to as curcuminoids (CCMs) (4). It can be safely ingested in very high
doses, as confirmed by several clinical studies (102).
The chemical composition of turmeric consists of
~70% carbohydrates, 6% proteins, 6% essential oils (phellandrene,
sabinene, cineol, borneol, zingiberene and sesquiterpenes), 5% fat,
3% minerals (potassium, calcium, phosphorus, iron and sodium), 3-5%
curcuminoids, and trace amounts of vitamins (B1, B2, C and niacin)
(103,104). One of extensively researched
effects of curcumin on inflammation is the inhibition of the
TNF-α-induced activation and nuclear translocation of NF-κB
(105). It also appears to exert
inhibitory effects on several inflammatory cytokines, such as
IL-1β, IL-2, IL-5, IL-6, IL-8, IL-12 and IL18(106). Other activities include the
downregulation of monocytes via MCP-1, and macrophage recruitment
via macrophage inflammatory protein-1 α (107). Curcumin can also suppress the
activity of protein kinases, including protein kinase A,
phosphorylase kinase, mTOR and MAPKs, which play essential roles in
various cellular responses, including the regulation of cell
growth, proliferation, division, survival and death (103). Although curcumin lacks analgesic
and antipyretic properties (106), the suppression of inflammation is
a vital therapeutic effect.
Overall, curcumin is well-tolerated and has multiple
beneficial effects; however, it has a low bioavailability which is
a main obstacle to its application as a therapeutic agent (109). Nonetheless, adding piperine, an
alkaloid present in black pepper (Piper nigrum), to the
curcumin compound appears to increase its bioavailability by
2,000%, which allows for a highly improved therapeutic effect
(110).
11. Conclusions and future perspectives
The present general overview of the few selected,
very broadly promoted and highly popular immunomodulatory herbs
(Fig. 2 and Table I), clearly demonstrates that all
plants have very promising profiles as preventative tools in a
general naturopathic setting, and most likely, in the therapy
setting as well. It is evident however, that there is still a long
way to go before consistent data can be collected, that is easily
applicable and reliable. At present, the available information in
the scientific literature is relatively scattered and inconsistent.
A broad variety of approaches have been applied to study each one
of the different herbs and for this reason, the data cannot be
easily summarized and processed. For some herbs, the main focus is
on phytochemical constituent research, combined with some in
vitro data regarding the application of various extracts on a
broad spectrum of models, such as cell cultures, research animals,
etc. (111). For other herbs,
there is a comparison between the effects of single-isolated and
purified compounds and some water-based or alcohol-based extracts
(112). It is not very clear,
however, what will occur when whole extracts are applied, or which
of the pharmacologically active constituents play the major role in
the observed effect. Presumably, the entourage effect is much more
significant in terms of conferring a therapeutic benefit rather
than the effect of purified single compounds. Furthermore, whole
extracts may have a much safer profile. As such, a considerable
amount of research is required to provide ample evidence.
 | Table ISummary of the names, active
ingredients and chemical formulas of the included herbs and
plants. |
Table I
Summary of the names, active
ingredients and chemical formulas of the included herbs and
plants.
| Plant name | Main active
ingredient | Chemical
formula | (Refs.) |
|---|
| Andrographis
(Andrographis paniculata) |
Andrographolide |
C20H30O5 | (15,16) |
| Astragalus
(Astragalus propinquus/membranaseus) | Astragaloside,
Astragalus polysaccharide |
C28H32O17 | (22) |
| Black cumin
(Nigella sativa) | Thymoquinone |
C10H12O2 | (38) |
| Cardamom
(Elettaria cardamomum) | Terpinyl acetate,
1,8-cineole |
C12H20O2,
C10H18O | (48) |
|
Echinacea | Alkamides, caffeic
acid derivatives |
C9H8O4 | (54) |
| Ginger (Zingiber
officinale) | Gingerol,
shogaol |
C17H26O4,
C17H24O3 | (67) |
| Liquorice
(Glycyrrhiza glabra) | Glycyrrhizin, other
polysaccharides |
C42H62O16 | (76) |
| Shiitake
(Lentinula edodes) | Lentinan, other
β-glucans |
C42H72O36 | (87) |
| Turmeric
(Curcuma longa) | Turmeric |
C21H20O6 | (104) |
In addition, the available data on safety (the
majority of data have been obtained via hepatocyte culture models)
are not sufficient (5,113-115).
Human clinical data are even more limited. There is partial
information from some prospective observational trials; however,
double-blinded, randomized, placebo-controlled trials (which are
the golden standard) are lacking. Adequate research on all of these
promising plants, even the few that were selected for discussion in
the present review, would require a very consistent and structured
approach, numerous resources invested in terms of time and funding,
and more importantly, a good collaboration between research teams.
However, despite all these visible hurdles, it can be considered
that plant-based therapeutics represent a very promising area of
pharmacological research and development, which may prove to be
effective, both in terms of patient well-being and therapeutics,
and in terms of industry profits.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
IT, KD and AZ performed the initial collection and
curation of the data to be included in the review. AZ, NS, RH and
TK reviewed the collected data and extracted relevant information.
IT, KD, TK, AZ and NS summarized the data for each presented
example. DAS, RH, MA and VZ reviewed and edited the manuscript. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Honorary Editor of the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have competing interests.
References
|
1
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem
Biophys Res Commun. 320:1103–1111. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Azeez TB and Lunghar J: 6-Antiinflammatory
effects of turmeric (Curcuma longa) and ginger (Zingiber
officinale). In: Gopi S, Amalraj A, Kunnumakkara A and Thomas S
(eds). Inflammation and Natural Products. Academic Press,
pp127-146, 2021.
|
|
3
|
Rahman T and Choudhury MBK: Shiitake
mushroom: A tool of medicine. Bangladesh J Med Biochem. 5:24–32.
2012.
|
|
4
|
Isbill J, Kandiah J and Kružliaková N:
Opportunities for health promotion: Highlighting herbs and spices
to improve immune support and well-being. Integr Med (Encinitas).
19:30–42. 2020.PubMed/NCBI
|
|
5
|
Pelvan E, Karaoğlu Ö, Önder Fırat E, Betül
Kalyon K, Ros E and Alasalvar C: Immunomodulatory effects of
selected medicinal herbs and their essential oils: A comprehensive
review. J Funct Foods. 94(105108)2022.
|
|
6
|
Rubió L, Motilva MJ and Romero MP: Recent
advances in biologically active compounds in herbs and spices: A
review of the most effective antioxidant and anti-inflammatory
active principles. Crit Rev Food Sci Nutr. 53:943–953.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tapsell LC, Hemphill I, Cobiac L, Patch
CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM,
Williams PG, et al: Health benefits of herbs and spices: The past,
The present, the future. Med J Aust. 185 (S4):S1–S24.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lim JW, Kim H and Kim KH: Nuclear
factor-kappaB regulates cyclooxygenase-2 expression and cell
proliferation in human gastric cancer cells. Lab Invest.
81:349–360. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi G and Li D, Fu J, Sun Y, Li Y, Qu R,
Jin X and Li D: Upregulation of cyclooxygenase-2 is associated with
activation of the alternative nuclear factor kappa B signaling
pathway in colonic adenocarcinoma. Am J Transl Res. 7:1612–1620.
2015.PubMed/NCBI
|
|
11
|
Kishore V, Yarla NS, Bishayee A, Putta S,
Malla R, Neelapu NRR, Challa S, Das S, Shiralgi Y, Hegde G and
Dhananjaya BL: Multi-targeting andrographolide and its natural
analogs as potential therapeutic agents. Curr Top Med Chem.
17:845–857. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu C, Chou GX, Wang CH and Wang ZT: Rare
noriridoids from the roots of Andrographis paniculata.
Phytochemistry. 77:275–279. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Behera PR, Nayak P, Barik DP, Rautray TR,
Thirunavoukkarasu M and Chand PK: ED-XRF spectrometric analysis of
comparative elemental composition of in vivo and in vitro roots of
Andrographis paniculata (Burm.f.) Wall. ex Nees-a
multi-medicinal herb. Appl Radiat Isot. 68:2229–2236.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Okhuarobo A, Falodun JE, Erharuyi O,
Imieje V, Falodun A and Langer P: Harnessing the medicinal
properties of Andrographis paniculata for diseases and
beyond: A review of its phytochemistry and pharmacology. Asian Pac
J Trop Dis. 4:213–222. 2014.
|
|
15
|
Li X, Yuan W, Wu J, Zhen J, Sun Q and Yu
M: Andrographolide, a natural anti-inflammatory agent: An update.
Front Pharmacol. 13(920435)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhao Y, Kao CP, Wu KC, Liao CR, Ho YL and
Chang YS: Chemical compositions, chromatographic fingerprints and
antioxidant activities of andrographis herba. Molecules.
19:18332–18350. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mussard E, Cesaro A, Lespessailles E,
Legrain B, Berteina-Raboin S and Toumi H: Andrographolide, a
natural antioxidant: An update. Antioxidants (Basel).
8(571)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chandrasekaran CV, Thiyagarajan P, Deepak
HB and Agarwal A: In vitro modulation of LPS/calcimycin induced
inflammatory and allergic mediators by pure compounds of
Andrographis paniculata (King of bitters) extract. Int
Immunopharmacol. 11:79–84. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Choi MJ and Kim YR: Anti-allergic effect
of fermented extracts of medicinal plants andrographis paniculate,
salvia plebeia R. Br., canavalia gladiate, eleuthorococcus
senticosus, ulmus davidiana var. japonica, and clerodendrum
trichotomum thunb. ex murray. Microbiol Biotechnol Lett.
50:512–521. 2022.
|
|
20
|
Parichatikanond W, Suthisisang C,
Dhepakson P and Herunsalee A: Study of anti-inflammatory activities
of the pure compounds from Andrographis paniculata (burm.f.)
Nees and their effects on gene expression. Int Immunopharmacol.
10:1361–1373. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Weng Z, Chi Y, Xie J, Liu X, Hu J, Yang F
and Li L: Anti-inflammatory activity of dehydroandrographolide by
TLR4/NF-κB signaling pathway inhibition in bile duct-ligated mice.
Cell Physiol Biochem. 49:1124–1137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Puri A, Saxena R, Saxena RP, Saxena KC,
Srivastava V and Tandon JS: Immunostimulant agents from
Andrographis paniculata. J Nat Prod. 56:995–999.
1993.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zheng Y, Ren W, Zhang L, Zhang Y, Liu D
and Liu Y: A review of the pharmacological action of Astragalus
polysaccharide. Front Pharmacol. 11(349)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim C, Ha H, Kim JS, Kim YT, Kwon SC and
Park SW: Induction of growth hormone by the roots of Astragalus
membranaceus in pituitary cell culture. Arch Pharm Res. 26:34–39.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han J, Guo D, Sun XY, Wang JM, Ouyang JM
and Gui BS: Repair effects of Astragalus polysaccharides with
different molecular weights on oxidatively damaged HK-2 cells. Sci
Rep. 9(9871)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ye MN, Chen HF, Zhou RJ and Liao MJ:
Effects of Astragalus polysaccharide on proliferation and Akt
phosphorylation of the basal-like breast cancer cell line. Zhong Xi
Yi Jie He Xue Bao. 9:1339–1346. 2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
27
|
Xu HD, You CG, Zhang RL, Gao P and Wang
ZR: Effects of Astragalus polysaccharides and astragalosides on the
phagocytosis of Mycobacterium tuberculosis by macrophages. J
Int Med Res. 35:84–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yin JY, Chan BCL, Yu H, Lau IYK, Han XQ,
Cheng SW, Wong CK, Lau CB, Xie MY, Fung KP, et al: Separation,
structure characterization, conformation and immunomodulating
effect of a hyperbranched heteroglycan from radix astragali.
Carbohydr Polym. 87:667–675. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yuan C, Pan X, Gong Y, Xia A, Wu G, Tang J
and Han X: Effects of Astragalus polysaccharides (APS) on the
expression of immune response genes in head kidney, gill and spleen
of the common carp, Cyprinus carpio L. Int Immunopharmacol.
8:51–58. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tian QE, Li HD, Yan M, Cai HL, Tan QY and
Zhang WY: Astragalus polysaccharides can regulate cytokine and
P-glycoprotein expression in H22 tumor-bearing mice. World J
Gastroenterol. 18:7079–7086. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang B, Xiao B and Sun T: Antitumor and
immunomodulatory activity of Astragalus membranaceus
polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol.
62:287–290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Balakrishnan B, Liang Q, Fenix K, Tamang
B, Hauben E, Ma L and Zhang W: Combining the anticancer and
immunomodulatory effects of Astragalus and shiitake as an
integrated therapeutic approach. Nutrients. 13(2564)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu ZG, Xiong ZM and Yu XY: Effect of
astragalus injection on immune function in patients with congestive
heart failure. Zhongguo Zhong Xi Yi Jie He Za Zhi. 23:351–353.
2003.PubMed/NCBI(In Chinese).
|
|
34
|
Li S, Sun Y, Huang J, Wang B, Gong Y, Fang
Y, Liu Y, Wang S, Guo Y, Wang H, et al: Anti-tumor effects and
mechanisms of Astragalus membranaceus (AM) and its specific
immunopotentiation: Status and prospect. J Ethnopharmacol.
258(112797)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhou L, Liu Z, Wang Z, Yu S, Long T, Zhou
X and Bao Y: Astragalus polysaccharides exerts immunomodulatory
effects via TLR4-mediated MyD88-dependent signaling pathway in
vitro and in vivo. Sci Rep. 7(44822)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang W, Liu QB and Jing W: Astragalus
membranaceus improves therapeutic efficacy of asthmatic children by
regulating the balance of Treg/Th17 cells. Chin J Nat Med.
17:252–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li M, Wang W, Xue J, Gu Y and Lin S:
Meta-analysis of the clinical value of Astragalus membranaceus in
diabetic nephropathy. J Ethnopharmacol. 133:412–419.
2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fatima Shad K, Soubra W and Cordato DJ:
The role of thymoquinone, a major constituent of Nigella
sativa, in the treatment of inflammatory and infectious
diseases. Clin Exp Pharmacol Physiol. 48:1445–1453. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ghonime M, Eldomany R, Abdelaziz A and
Soliman H: Evaluation of immunomodulatory effect of three herbal
plants growing in Egypt. Immunopharmacol Immunotoxicol. 33:141–145.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Majdalawieh AF, Hmaidan R and Carr RI:
Nigella sativa modulates splenocyte proliferation, Th1/Th2
cytokine profile, macrophage function and NK anti-tumor activity. J
Ethnopharmacol. 131:268–275. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Torres MP, Ponnusamy MP, Chakraborty S,
Smith LM, Das S, Arafat HA and Batra SK: Effects of thymoquinone in
the expression of mucin 4 in pancreatic cancer cells: Implications
for the development of novel cancer therapies. Mol Cancer Ther.
9:1419–1431. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Assayed ME: Radioprotective effects of
black seed (Nigella sativa) oil against hemopoietic damage
and immunosuppression in gamma-irradiated rats. Immunopharmacol
Immunotoxicol. 32:284–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alemi M, Sabouni F, Sanjarian F, Haghbeen
K and Ansari S: Anti-inflammatory effect of seeds and callus of
Nigella sativa L. extracts on mix glial cells with regard to
their thymoquinone content. AAPS PharmSciTech. 14:160–167.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shuid AN, Mohamed N, Mohamed IN, Othman F,
Suhaimi F, Mohd Ramli ES, Muhammad N and Soelaiman IN: Nigella
sativa: A potential antiosteoporotic agent. Evid Based
Complement Alternat Med. 2012(696230)2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chehl N, Chipitsyna G, Gong Q, Yeo CJ and
Arafat HA: Anti-inflammatory effects of the Nigella sativa
seed extract, thymoquinone, in pancreatic cancer cells. HPB
(Oxford). 11:373–381. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
El Mezayen R, El Gazzar M, Nicolls MR,
Marecki JC, Dreskin SC and Nomiyama H: Effect of thymoquinone on
cyclooxygenase expression and prostaglandin production in a mouse
model of allergic airway inflammation. Immunol Lett. 106:72–81.
2006.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nikakhlagh S, Rahim F, Aryani FHN,
Syahpoush A, Brougerdnya MG and Saki N: Herbal treatment of
allergic rhinitis: The use of Nigella sativa. Am J
Otolaryngol. 32:402–407. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Souissi M, Azelmat J, Chaieb K and Grenier
D: Antibacterial and anti-inflammatory activities of cardamom
(Elettaria cardamomum) extracts: Potential therapeutic
benefits for periodontal infections. Anaerobe.
61(102089)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jamal A, Siddiqui A, Aslam M, Javed K and
Jafri MA: Antiulcerogenic activity of Elettaria cardamomum
maton. and amomum subulatum Roxb. Seeds. Indian J Tradit Knowl.
4:298–302. 2005.
|
|
50
|
Majdalawieh AF and Carr RI: In Vitro
Investigation of the potential immunomodulatory and anti-cancer
activities of black pepper (Piper nigrum) and cardamom
(Elettaria cardamomum). J Med Food. 13:371–381.
2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sengupta A, Ghosh S and Bhattacharjee S:
Dietary cardamom inhibits the formation of azoxymethane-induced
aberrant crypt foci in mice and reduces COX-2 and iNOS expression
in the colon. Asian Pac J Cancer Prev. 6:118–122. 2005.PubMed/NCBI
|
|
52
|
Karsch-Völk M, Barrett B, Kiefer D, Bauer
R, Ardjomand-Woelkart K and Linde K: Echinacea for
preventing and treating the common cold. Cochrane Database Syst
Rev. 2014(CD000530)2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schapowal A, Klein P and Johnston SL:
Echinacea reduces the risk of recurrent respiratory tract
infections and complications: A meta-analysis of randomized
controlled trials. Adv Ther. 32:187–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Manayi A, Vazirian M and Saeidnia S:
Echinacea purpurea: Pharmacology, phytochemistry and
analysis methods. Pharmacogn Rev. 9:63–72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Block KI and Mead MN: Immune system
effects of Echinacea, ginseng, and astragalus: A review.
Integr Cancer Ther. 2:247–267. 2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Bauer R: Echinacea drugs-effects
and active ingredients. Z Arztl Fortbild (Jena). 90:111–115.
1996.PubMed/NCBI(In German).
|
|
57
|
Coeugniet EG and Elek E: Immunomodulation
with Viscum album and Echinacea purpurea extracts.
Onkologie. 10 (3 Suppl):S27–S33. 1987.PubMed/NCBI View Article : Google Scholar
|
|
58
|
McCann DA, Solco A, Liu Y, Macaluso F,
Murphy PA, Kohut ML and Senchina DS: Cytokine- and
interferon-modulating properties of Echinacea spp. root
tinctures stored at -20 degrees C for 2 years. J Interferon
Cytokine Res. 27:425–436. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Percival SS: Use of Echinacea in
medicine. Biochem Pharmacol. 60:155–158. 2000.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Luettig B, Steinmüller C, Gifford GE,
Wagner H and Lohmann-Matthes ML: Macrophage activation by the
polysaccharide arabinogalactan isolated from plant cell cultures of
Echinacea purpurea. J Natl Cancer Inst. 81:669–675.
1989.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Vetvicka V and Vetvickova J: Natural
immunomodulators and their stimulation of immune reaction: True or
false? Anticancer Res. 34:2275–2282. 2014.PubMed/NCBI
|
|
62
|
Li Y, Wang Y, Wu Y, Wang B, Chen X and Xu
X, Chen H, Li W and Xu X: Echinacea pupurea extracts promote
murine dendritic cell maturation by activation of JNK, p38 MAPK and
NF-κB pathways. Dev Comp Immunol. 73:21–26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fu A, Wang Y, Wu Y, Chen H, Zheng S, Li Y,
Xu X and Li W: Echinacea purpurea extract polarizes M1
macrophages in murine bone marrow-derived macrophages through the
activation of JNK. J Cell Biochem. 118:2664–2671. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Rondanelli M, Miccono A, Lamburghini S,
Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M
and Perna S: Self-care for common colds: The pivotal role of
vitamin D, vitamin C, zinc, and Echinacea in three main
immune interactive clusters (physical barriers, innate and adaptive
immunity) involved during an episode of common colds-practical
advice on dosages and on the time to take these
nutrients/botanicals in order to prevent or treat common colds.
Evid Based Complement Alternat Med. 2018(5813095)2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Park EJ and Pezzuto JM: Botanicals in
cancer chemoprevention. Cancer Metastasis Rev. 21:231–255.
2002.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H,
Beta T and Li HB: Bioactive compounds and bioactivities of ginger
(Zingiber officinale Roscoe). Foods. 8(185)2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Butt MS and Sultan MT: Ginger and its
health claims: Molecular aspects. Crit Rev Food Sci Nutr.
51:383–393. 2011.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Poles J, Karhu E, McGill M, McDaniel HR
and Lewis JE: The effects of twenty-four nutrients and
phytonutrients on immune system function and inflammation: A
narrative review. J Clin Transl Res. 7:333–376. 2021.PubMed/NCBI
|
|
69
|
Nikkhah Bodagh M, Maleki I and Hekmatdoost
A: Ginger in gastrointestinal disorders: A systematic review of
clinical trials. Food Sci Nutr. 7:96–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Banihani SA: Ginger and testosterone.
Biomolecules. 8(119)2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Mozaffari-Khosravi H, Naderi Z, Dehghan A,
Nadjarzadeh A and Fallah Huseini H: Effect of ginger
supplementation on proinflammatory cytokines in older patients with
osteoarthritis: outcomes of a randomized controlled clinical trial.
J Nutr Gerontol Geriatr. 35:209–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tjendraputra E, Tran VH, Liu-Brennan D,
Roufogalis BD and Duke CC: Effect of ginger constituents and
synthetic analogues on cyclooxygenase-2 enzyme in intact cells.
Bioorg Chem. 29:156–163. 2001.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Verma SK, Singh M, Jain P and Bordia A:
Protective effect of ginger, Zingiber officinale Rosc on
experimental atherosclerosis in rabbits. Indian J Exp Biol.
42:736–738. 2004.PubMed/NCBI
|
|
74
|
Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H,
Sang S and Ho CT: 6-Shogaol induces apoptosis in human colorectal
carcinoma cells via ROS production, caspase activation, and GADD
153 expression. Mol Nutr Food Res. 52:527–537. 2008.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Bahmani M, Rafieian-Kopaei M, Jeloudari M,
Eftekhari Z, Delfan B, Zargaran A and Forouzan S: A review of the
health effects and uses of drugs of plant licorice (Glycyrrhiza
glabra L.) in Iran. Asian Pac J Trop Dis. 4 (Suppl
2):S847–S849. 2014.
|
|
76
|
Wahab S, Annadurai S, Abullais SS, Das G,
Ahmad W, Ahmad MF, Kandasamy G, Vasudevan R, Ali MS and Amir M:
Glycyrrhiza glabra (licorice): A comprehensive review on its
phytochemistry, biological activities, clinical evidence and
toxicology. Plants (Basel). 10(2751)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang
Y, Uzayisenga R and Otachi EO: Immunomodulatory and anticancer
potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides
by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7
upregulation in vitro. BMC Complement Altern Med.
16(206)2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung
JW, Yang SR, Park JS, Hwang JW, Aruoma OI, et al: Chemopreventive
properties of the ethanol extract of chinese licorice (Glycyrrhiza
uralensis) root: Induction of apoptosis and G1 cell cycle arrest in
MCF-7 human breast cancer cells. Cancer Lett. 230:239–247.
2005.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Bode AM and Dong Z: Chemopreventive
effects of licorice and its components. Curr Pharmacol Rep.
1:60–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sasaki H, Suzuki N, Alshwaimi E, Xu Y,
Battaglino R, Morse L and Stashenko P: 18β-glycyrrhetinic acid
inhibits periodontitis via glucocorticoid-independent nuclear
factor-κB inactivation in interleukin-10-deficient mice. J
Periodontal Res. 45:757–763. 2010.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Zhou Y and Ho WS: Combination of
liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell
death through upregulating p53 and p21 in the A549 non-small cell
lung cancer cells. Oncol Rep. 31:298–304. 2014.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Park SY, Kwon SJ, Lim SS, Kim JK, Lee KW
and Park JHY: Licoricidin, an active compound in the hexane/ethanol
extract of Glycyrrhiza uralensis, inhibits lung metastasis of 4T1
murine mammary carcinoma cells. Int J Mol Sci.
17(934)2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Seon MR, Park SY, Kwon SJ, Lim SS, Choi
HJ, Park H, Lim DY, Kim JS, Lee CH, Kim J and Park JH:
Hexane/ethanol extract of Glycyrrhiza uralensis and its active
compound isoangustone A induce G1 cycle arrest in DU145 human
prostate and 4T1 murine mammary cancer cells. J Nutr Biochem.
23:85–92. 2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lee CK, Park KK, Lim SS, Park JHY and
Chung WY: Effects of the licorice extract against tumor growth and
cisplatin-induced toxicity in a mouse xenograft model of colon
cancer. Biol Pharm Bull. 30:2191–2195. 2007.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Huo HZ, Wang B, Liang YK, Bao YY and Gu Y:
Hepatoprotective and antioxidant effects of licorice extract
against CCl4-induced oxidative damage in rats. Int J Mol Sci.
12:6529–6543. 2011.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kim KR, Jeong CK, Park KK, Choi JH, Park
JHY, Lim SS and Chung WY: Anti-inflammatory effects of licorice and
roasted licorice extracts on TPA-induced acute inflammation and
collagen-induced arthritis in mice. J Biomed Biotechnol.
2010(709378)2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hobbs C: Medicinal value of lentinus
edodes (Berk.) sing. (agaricomycetideae). A literature review. Int
J Med Mushrooms. 2:2000.
|
|
88
|
Zhang Y, Zhang M, Jiang Y, Li X, He Y,
Zeng P, Guo Z, Chang Y, Luo H, Liu Y, et al: Lentinan as an
immunotherapeutic for treating lung cancer: A review of 12 years
clinical studies in China. J Cancer Res Clin Oncol. 144:2177–2186.
2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Mizuno T: Shiitake, lentinus edodes:
Functional properties for medicinal and food purposes. Food Rev
Int. 11:109–128. 1995.
|
|
90
|
Wang X, Xu X and Zhang L: Thermally
induced conformation transition of triple-helical lentinan in NaCl
aqueous solution. J Phys Chem B. 112:10343–10351. 2008.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wu X, Zheng Z, Guo T, Wang K and Zhang Y:
Molecular dynamics simulation of lentinan and its interaction with
the innate receptor dectin-1. Int J Biol Macromol. 171:527–538.
2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Chihara G, Hamuro J, Maeda Y, Arai Y and
Fukuoka F: Fractionation and purification of the polysaccharides
with marked antitumor activity, especially lentinan, from lentinus
edodes (Berk.) Sing. (an edible mushroom). Cancer Res.
30:2776–2781. 1970.PubMed/NCBI
|
|
93
|
Chihara G, Hamuro J, Maeda Y, Arai Y and
Fukuoka F: Antitumor polysaccharide derived chemically from natural
glucan (pachyman). Nature. 225:943–944. 1970.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Mayer S, Raulf MK and Lepenies B: C-type
lectins: Their network and roles in pathogen recognition and
immunity. Histochem Cell Biol. 147:223–237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Brown J, O'Callaghan CA, Marshall ASJ,
Gilbert RJC, Siebold C, Gordon S, Brown GD and Jones EY: Structure
of the fungal beta-glucan-binding immune receptor dectin-1:
Implications for function. Protein Sci. 16:1042–1052.
2007.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zhang M, Zhang Y, Zhang L and Tian Q:
Mushroom polysaccharide lentinan for treating different types of
cancers: A review of 12 years clinical studies in China. Prog Mol
Biol Transl Sci. 163:297–328. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang G, Lin W, Zhao R and Lin N: Effects
of six polysaccharides extracted from plants on the immunological
cells of mice. Wei Sheng Yan Jiu. 37:577–580. 2008.PubMed/NCBI(In Chinese).
|
|
99
|
Kupfahl C, Geginat G and Hof H: Lentinan
has a stimulatory effect on innate and adaptive immunity against
murine Listeria monocytogenes infection. Int Immunopharmacol.
6:686–696. 2006.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Petrovska BB: Historical review of
medicinal plants' usage. Pharmacogn Rev. 6:1–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Moghadamtousi SZ, Kadir HA, Hassandarvish
P, Tajik H, Abubakar S and Zandi K: A review on antibacterial,
antiviral, and antifungal activity of curcumin. Biomed Res Int.
2014(186864)2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Kotha RR and Luthria DL: Curcumin:
Biological, pharmaceutical, nutraceutical, and analytical aspects.
Molecules. 24(2930)2019.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Prasad S, Gupta SC, Tyagi AK and Aggarwal
BB: Curcumin, a component of golden spice: From bedside to bench
and back. Biotechnol Adv. 32:1053–1064. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Priyadarsini KI: The chemistry of
curcumin: From extraction to therapeutic agent. Molecules.
19:20091–20112. 2014.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Olivera A, Moore TW, Hu F, Brown AP, Sun
A, Liotta DC, Snyder JP, Yoon Y, Shim H, Marcus AI, et al:
Inhibition of the NF-κB signaling pathway by the curcumin analog,
3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31):
anti-inflammatory and anti-cancer properties. Int Immunopharmacol.
12:368–377. 2012.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Fahey AJ, Adrian Robins R and
Constantinescu CS: Curcumin modulation of IFN-beta and IL-12
signalling and cytokine induction in human T cells. J Cell Mol Med.
11:1129–1137. 2007.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Zhou H, Beevers CS and Huang S: Targets of
curcumin. Curr Drug Targets. 12:332–347. 2011.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Srimal RC and Dhawan BN: Pharmacology of
diferuloyl methane (curcumin), a non-steroidal anti-inflammatory
agent. J Pharm Pharmacol. 25:447–452. 1973.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ireson CR, Jones DJL, Orr S, Coughtrie
MWH, Boocock DJ, Williams ML, Farmer PB, Steward WP and Gescher AJ:
Metabolism of the cancer chemopreventive agent curcumin in human
and rat intestine. Cancer Epidemiol Biomarkers Prev. 11:105–111.
2002.PubMed/NCBI
|
|
110
|
Shoba G, Joy D, Joseph T, Majeed M,
Rajendran R and Srinivas PS: Influence of piperine on the
pharmacokinetics of curcumin in animals and human volunteers.
Planta Med. 64:353–356. 1998.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Ali M, Khan T, Fatima K, Ali QUA, Ovais M,
Khalil AT, Ullah I, Raza A, Shinwari ZK and Idrees M: Selected
hepatoprotective herbal medicines: Evidence from ethnomedicinal
applications, animal models, and possible mechanism of actions.
Phytother Res. 32:199–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Sasidharan S, Chen Y, Saravanan D, Sundram
KM and Yoga Latha L: Extraction, isolation and characterization of
bioactive compounds from plants' extracts. Afr J Tradit Complement
Altern Med. 8:1–10. 2011.PubMed/NCBI
|
|
113
|
Kaur P, Robin Mehta RG, Arora S and Singh
B: Progression of conventional hepatic cell culture models to
bioengineered HepG2 cells for evaluation of herbal bioactivities.
Biotechnol Lett. 40:881–893. 2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Zhang Y, Wu J, Guo S, Lin W, Zhang B, Chen
X, Mo H and Zhan T: The clinical efficacy and safety of the Chinese
herbal medicine Astragalus (Huangqi) preparation for the treatment
of acute myocardial infarction: A systematic review of randomized
controlled trials. Medicine (Baltimore). 98(e15256)2019.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ardjomand-Woelkart K and Bauer R: Review
and assessment of medicinal safety data of orally used echinacea
preparations. Planta Med. 82:17–31. 2016.PubMed/NCBI View Article : Google Scholar
|