Introduction
The Greek feta cheese is a traditional semi-soft
white pickled cheese produced from 0-30% goat milk and 70-100%
sheep milk or exclusively from sheep milk (1). Since 2002, it has been recognised as
a protected designation of origin (PDO) product, in accordance with
Commission Regulation (EC) No 1829/2002 (2,3). PDO
cheeses ensure the authenticity of tte product linked to its
geographical indication where it is produced, processed and
developed with recognised expertise characterizing traditional and
craft production. Greek feta PDO is a regional product of
particular areas of Greece (Macedonia, Epirus, Thessaly and
mainland Greece) and is matured in tins or wooden barrels for at
least 60 days (4). Although there
is a variety of >600 different oak species globally (5), only four have been reported to be
suitable for feta PDO cheese production (6). These are Quercus frainetto,
Quercus pubescens, Quercus petraea and Quercus robur and
in Greece, these are found in Western Macedonia, Thrace, Eurytania
and Pindus National Park (7-10).
These species are endemic to specific Greek regions and have
traditionally been used in the construction of wooden barrels for
the maturation of feta PDO cheese. Alongside oak, beech wood is
also considered appropriate.
Wood in contact with raw or pasteurised milk and
cheese is rapidly covered by a microbial biofilm (11,12).
In previous studies, researchers have shown a high biodiversity of
microorganisms that are present in biofilms, not only between the
different types of cheeses studied, but also within the same cheese
variety, between different wooden vats (tina or gerle) used in
different cheese plants (13,14).
The biodiversity confirmed and demonstrated the strong
characterisation of the territorial origin of the products
(15-17).
In cheese, the correct maintenance of wooden vats promotes the
selection of microbial flora, able to play an active role in the
achievement of food safety through the biocompetitive activity of
lactic acid bacteria (LAB) and their inhibitory activity against
pathogenic bacteria, particularly Listeria monocytogenes
(13,18-20).
Greek dairies have traditionally produced feta PDO cheese, using
equipment consisting of wooden utensils and wooden barrels of a
capacity of up to 60 kg (6) (as
illustrated in Fig. 1).
According to the guidelines of the Hellenic Food
Safety Authority (EFET) (21), and
Hazard Analysis and Critical Control Point (HACCP) principles for
the traditional small dairy production units in Greece (22), the wooden barrel can be used among
other wooden utensils, to mature traditional feta PDO and white
cheese, such as barrel-aged goat cheese. Their porous structure
supports beneficial microflora, while their use enhances the
texture and flavour through the natural maturation process
(23-25).
On the other hand, the use of traditional and reusable equipment,
particularly in small and medium-sized enterprises producing
barrel-aged feta PDO (2), requires
special attention concerning the implementation of critical control
points (CCPs), that are designed to reduce microbiological (M),
chemical (C) and physical (P) risks in line with HACCP guidelines.
These CCPs and the related Operational Prerequisite Programs
(OPRPs) are outlined throughout the production routine, as
illustrated in the flowchart of the feta PDO cheese production
(Fig. 2) (26). Some studies have demonstrated that
the wooden vats used for raw milk cheese production allow for the
development of a biofilm on the inner surface, which actively
participates in the production process by adding unique sensory
characteristics to the traditional products, such as aroma,
appearance, taste and texture, which is associated with the total
viable count of microorganisms, mesophilic LAB, lactic
cocci/streptococci and yeast/moulds (11,12,27,28).
The present study aimed to monitor the
microbiological parameters during all the stages of the ripening
period of the Fagus sylvatica (beech) barrel-aged feta PDO
cheese (2), to enhance the
established control according to the methodology that complies with
food safety in the final product.
Materials and methods
Wood type selection for the use of the
barrel
A hand-crafted Greek beech barrel constructed by
artisans of the Metsovo area was used to produce and mature feta
PDO cheese. The evaluation of the suitability of the beech wood for
its use in the dairy sector is approved by the State General
Chemistry of Greece and the competent Forest Service, which
verifies the origin of the timber, lawful harvesting and intended
use through official forest marking (29,30).
Beech barrel cleaning
The barrel was inspected for leak tightness and was
then vigorously and thoroughly brushed with pressurised water to
remove any gross solids, such as salts, brine residues, fat,
bacteria and any crumbled feta PDO due to previous usage, which
could impede any further natural sanitation treatments reaching
into the barrel wood. It was then filled with heated whey of 95˚C
[from which the majority of the whey proteins had been removed
through myzithra cheese production (31)] and left at room temperature for 24
h. The barrel was then emptied of the whey, washed with
high-pressure water, and allowed to dry at ambient temperature for
a further 24 h.
Flow diagram of feta PDO cheese
production
Following milking (step 1), raw milk is cooled to
<4˚C and maintained at this temperature during transportation to
the dairy facility (steps 2 and 3). Upon arrival, a milk sample is
collected and analysed for antibiotic residues, and the pH is
measured (step 4) (32,33). The milk is then filtered (step 5)
and stored in large silos (step 6).
The milk is processed within 48 h of milking.
Pasteurization is performed at 63˚C for 30 min (step 7), followed
by cooling to 32˚C (step 8) with intermittent stirring. At this
point, before the stainless steel vat is filled, a starter culture
and calcium chloride are added to its base (step 9). Once filled,
rennet is added under constant stirring, and coagulation occurs
within 45-50 min (step 10).
Following coagulation, the curd is inspected, cut
(step 11), and allowed to rest for 10 min until whey appears on the
cheese surface (step 12). It is then transferred into perforated
moulds (step 13), where it drains and is subsequently reversed
(step 14). The mould is removed, the cheese is cut (step 15) and
dry salt is applied to the surface (step 16). After two hours, the
blocks are flipped and salted again (step16).
Depending on production needs, the salted cheese
blocks are placed into wooden barrels or metal tins on pallets
(step 17), where they remain in the facility area for an additional
24 h. The following day, they are transferred to the ripening
chamber and held at 18˚C for ~15 days, until the pH decreases to
<4.6 (step 18). Finally, the barrels or tins are moved to cold
storage at 2-4˚C for maturation (step 19). The cheese becomes
suitable for consumption 2 months following production (step 19)
(32). Following that period, feta
PDO cheese is being packed into retail packages and is transported
at the point of sale (step 20) (Fig.
2).
Cheese sampling
Feta PDO cheese was produced according to the
requirements described at EL/PDO/0017/0427(34). Moreover, the sale of the cheese as
feta PDO requires certification by the competent controlling
authority ELGO-DEMETER (2,35). In brief, fresh cheese was left to
mature in beech-aged wooden barrels. All samples, namely fresh
milk, wood, curd and brine, were collected and analysed
microbiologically at different time intervals following the
maturation schedule of the 1st day of cheese-making, the 3rd and
45th day of cheese-making, and on the 150th day of the ripening
period.
Biofilm sampling
The biofilms were collected for the enumeration of
total mesophilic count (TMC), and the estimation of the hygiene
indicator bacteria from the inside of the barrel. The biofilm was
scraped off from the beech wood surface using a sterilised razor
blade within a random interior surface of 100 cm2 area,
delimited with a 10x10 cm sterile plastic mask (80 cm2
from the bottom and 20 cm2 from the side surface) at a
depth of ~1.5 mm within the beech barrel. The detection of
Listeria monocytogenes, Staphylococcus aureus and
Salmonella spp. was performed on a larger surface, using a
20x20 cm mask and it was then cleaned with a sterile gauze.
Microbiological analysis
All samples were obtained from the dairy company,
Pagonis Sisters and Co., and were microbiologically analysed. More
specifically, samples derived from the pasteurised sheep and goat
milk, biofilm samples from the empty barrel, samples of feta cheese
during its maturation stages, biofilm samples in the opened barrel
and samples of brine were analysed. The enumeration of the TMC was
conducted in plate count agar (PCA; Biokar diagnostics) incubated
at 30˚C for 72 h (ISO 4833-1:2013) (36), Coliforms on Violet Red Bile Agar
(VRBA; Oxoid; Thermo Fisher Scientific, Inc.), incubated at 30˚C
for 24 h (ISO 4832:2006) (37),
Enterobacteria on Violet Red Bile Glucose Agar (VRBG,
Biolife) incubated at 30˚C for 24 h (ISO 21528-2:2017) (38), Escherichia coli in Tryptone
Bile X-Gluc Agar-TBX at 44˚C for 24 h (ISO16649-2/2001) (39,40),
and in chromIDTM Coli Agar (COLI ID-F) NF VALID (AFNOR BIO
12/19-12/06) for further confirmation.
Listeria monocytogenes, Staphylococcus
aureus and Salmonella spp. were detected in compliance
with the microbiological criteria for foodstuffs determined in
Commission Regulation (EC) No 2073/2005 (41,42).
Listeria monocytogenes and Salmonella spp. testing
was performed using a mini VIDAS Compact automated immunoassay
system based on the enzyme linked fluorescent assay (ELFA)
principles by Biomerieux using methods according to the protocols,
VIDAS Listeria monocytogenes II LMO2 BIO-12/11-03/04 and
VIDAS Easy Salmonella SLM BIO-12/16-09/05, respectively.
Both methods were accredited under ISO/IEC 17025:2005. The
enumeration of coagulase-positive staphylococci (CPS,
Staphylococcus aureus) was performed with ISO
6888-2:1999/AMD 1:2003. All methods used for the detection of
foodborne pathogens were accredited according to ISO/IEC 17025 by
the Hellenic Accreditation System E.SY.D. with certification no.
1111(43).
pH value measurement
The pH value of cheese was measured using a RL150 pH
meter (Russel) throughout the maturation process. The pH was
measured by immersing the glass electrode into the cheese sample at
independent time points, without interfering with the
microbiological assessment.
Results
The data obtained from the microbiological analysis
carried out on samples of feta PDO, brine, and biofilm are reported
in Table I, Table II and Table III. The levels of total coliform
bacteria, Enterobacteriaceae and Escherichia coli, in the
pasteurised goat and sheep's milk were zero. Similarly, the 7.6%
(w/w) NaCl microbial loads of brine were null. The TMC ranged from
5.67x105 cfu/cm2 in the biofilm of the empty
barrel to 5.91x105 cfu/cm2 in the biofilm of
the opened barrel, which according to the literature (12,44),
confirms the slight quantitative variation over the ripening period
of feta PDO. Coagulase-positive staphylococci, Listeria
monocytogenes and Salmonella spp. were absent in all
cheese samples. The absence of pathogens was observed in the
biofilm samples taken from the inner surface of the wooden barrel
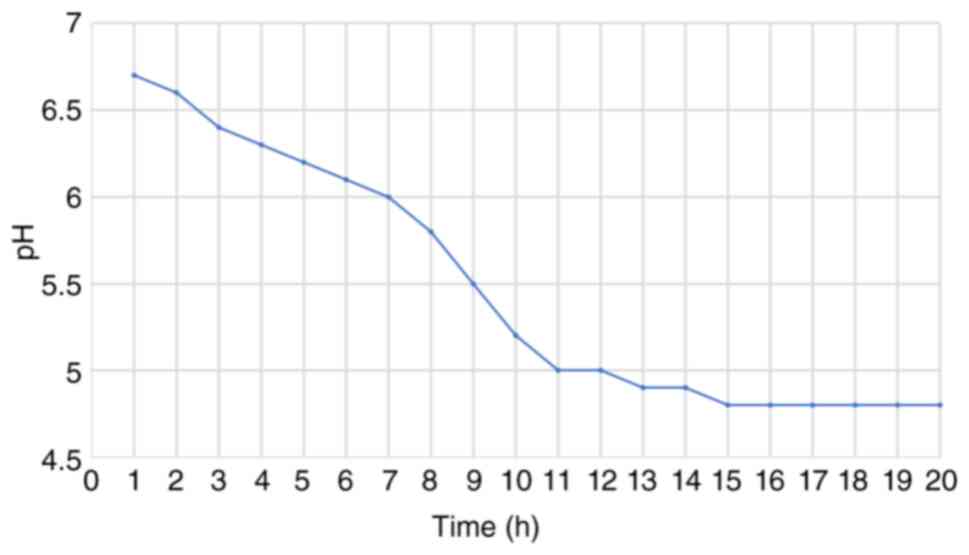
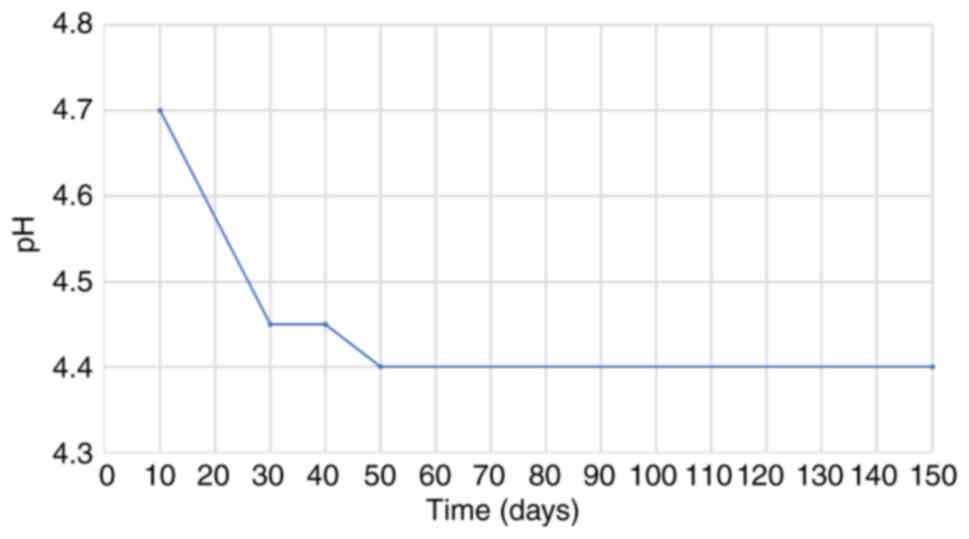
(45). The follow-up of pH
measurements in feta PDO revealed a gradual decline from 6.7 to 4.5
within the period of monitoring lasted from the first hours of
repining to the fifth month, as shown in Figs. 3 and 4, verifying that acidification proceeded
as expected and that pH levels fell within the target range for the
ripening of feta PDO cheese.
 | Table IMicrobiological analysis of
pasteurised milk and feta PDO cheese. |
Table I
Microbiological analysis of
pasteurised milk and feta PDO cheese.
| |
Enterobacteriaceae | E. coli | L.
Monocytogenes | Salmonella
spp. | S. aureus
(coagulase+) |
|---|
| Sample | TMC (log cfu/ml or
cfu/g) | Coliforms (log
cfu/ml or log cfu/g) | log cfu/ml | Limita,b | log cfu/ml or log
cfu/g | Limita | cfu/25 g | Limita,b | cfu/25 g | Limit | cfu/g | Limita,b |
|---|
| D1 milk | 3.00 | 0.00 | 0.00 | n=5, c=2, m<1
cfu/ml, M=5 cfu/ml | 0.00 | | | | | | | |
| D1 cheese | | 3.08 | | | 0.00 | | Absent | | Absent | | <10 | |
| D2 cheese | | 0.00 | | | 0.00 | | Absent | | Absent | | <10 | |
| D4 cheese | | 0.00 | | | 0.00 | | Absent | | Absent | | <10 | |
| D15 cheese | | 0.00 | | | 0.00 | | Absent | | Absent | | <10 | |
| D150 cheese | | 0.00 | | | 0.00 | n=5, c=2, m=100
cfu/g M=1.000 cfu/g | <10 | n=5, c=2, m=100
cfu/g, M=1,000 cfu/g | Absent | n=5, c=0, Absent in
25 g | Absent | n=5, c=0, Absent in
25 g |
 | Table IIEvaluation of microbial load (log
CFU/ml) of brine following a period of 150 days of feta PDO cheese
maturation. |
Table II
Evaluation of microbial load (log
CFU/ml) of brine following a period of 150 days of feta PDO cheese
maturation.
| Sample-brine | TMC (log
cfu/ml) | Coliforms (log
cfu/ml) | Enterobacteria (log
cfu/ml) | E. coli (log
cfu/ml) | L.
monocytogenes (presence/absence) | Salmonella
spp. (presence/absence) | S. aureus
(coagulase+) (cfu/g) | Regulatory limit
for natural brine (reference) |
|---|
| D150 | 5.77 | 0.00 | 0.00 | 0.00 | Absent | Absent | <10 | No regulatory
limits exist for brine |
 | Table IIIBiofilm microbiological analysis. |
Table III
Biofilm microbiological analysis.
| Biofilm sample/time
point | TMC (log
cfu/cm2) | E. coli (log
cfu/cm2) | L.
monocytogenes (cfu/cm2) | Salmonella
spp. (cfu/cm2) | S. aureus
coagulase+ (cfu/cm2) | Regulatory limit
for biofilm (reference) |
|---|
| Biofilm sample of
D1 before feta PDO was transferred into the barrel | 5.75 | 0.00 | Absent | Absent | <10 | No regulatory
limits exist for biofilm |
| Biofilm sample of
feta PDO on D150 of ripening | 5.77 | 0.00 | Absent | Absent | <10 | Same as above |
Discussion
Greek feta PDO cheese is a national treasure,
representing 90% of the total cheese production of the country
(44). The essential ingredients
for its production include milk, salt, rennet and microorganisms
(46). The matrix of dairy
products facilitates microorganism proliferation, such as
Staphylococcus aureus, Listeria monocytogenes,
Escherichia coli, Brucella abortus, Campylobacter jejuni,
Salmonella, Yersinia enterocolitica, and several others,
compromising the public health status (26,47).
Traceability plays a key role in food chain
management, particularly in the case of rapidly deteriorating
products, such as milk and cheese (48). In this context, traceability has
become a requirement for a better understanding of the life cycle
of products and conscientious delight. Thus, the major concern of
the dairy companies is the respect of the traditional practices
through a certified quality control system according to ISO
22000:2005 and implementing a HACCP daily workflow, illustrated by
Casino et al (49), and in
the workflow of Fig. 2, to
guarantee excellent quality.
Food safety is an increasingly critical public
health concern, and the proposed traceability procedure enhances
the improved process control for managing any future product
recalls and/or product quality. Microbial food contamination in
dairy plants is of utmost significance for the monitoring of food
quality and safety (6,50). For instance, it is known that
Listeria monocytogenes has the potential to survive at low
temperatures (0˚C), low pH values (pH 4.4) and in salt media with
10-20% (w/v) of NaCl (28),
conditions that are close to the physicochemical characteristics of
feta cheese. For the purpose of the present study, further
investigated the food safety of beech barrel-aged Feta PDO, 2.5%
(w/w) NaCl, following the monitoring of pH and through the in-depth
microbiological analysis across the time-points of the production
workflow (Fig. 2).
Previous studies describe that the microbial ecology
found in cheese-making establishments exhibits a great
differentiation in composition, regarding taxonomy classification,
due to the processing procedures (51-53).
The conditions that favour microbiome settlement are several and
are associated with the age, conservation, quality and nature of
the wooden vats. Additionally, the presence of microbial ecosystems
and environmental conditioning (54), such as temperature, humidity, or
pH, markedly affects the selective survival of the microbiome
(55). Humidity, cleaning
protocols, nutrient availability and composition also have a
regulatory impact on the ability of microbes to develop biofilms.
The availability of nutrients in wooden barrels can promote biofilm
development. However, this effect is modulated by key environmental
factors, such as salinity, moisture and pH, which suppress the the
proliferation of pathogenic microorganisms even in nutrient-rich
conditions. This specific microenvironment favours the development
of stable, functional biofilms that enhance the microbial stability
and hygiene of the final product (56). In particular, the limited moisture,
regulated by the high salinity of the brine, combined with the
acidic pH (~4.4), creates a selective ecosystem. Within this
environment, the growth of pathogens, such as Listeria
monocytogenes, Staphylococcus aureus and
Salmonella spp. is inhibited, while microorganisms naturally
adapted to high-salt and low-pH conditions are favoured. These
salt- and acid-tolerant microbiota include lactic acid bacteria
such as Lactobacillus spp., as well as probiotic strains
such as Lactobacillus paracasei and Bifidobacterium
spp., which form resilient, protective biofilms (17). In the present study, the
aggregation of microorganisms referred to as ‘biofilm’ formed in
the wooden barrels used in the production of feta PDO cheese is
characterised by minimal variation in TMC, with a quantitative
change of <5% following 150 days of ripening. Additionally, the
structural and chemical properties of beech wood exert further
selective pressure. Cleaning protocols effectively eliminate
pathogens while preserving these non-pathogenic, functionally
advantageous biofilms. Nutrient limitation during advanced ripening
likely restricts microbial overgrowth, supporting a balanced
beneficial microbiota and ensuring the microbiological safety and
quality of the final product (57-59).
However, in the case of feta PDO production, which has always been
an artisanal process, the improvements through empirical trials
have emerged long before the current scientific knowledge of these
fermentations.
The establishment of the LAB protects against
spoilage bacteria through differential colonisation capability
(60), which creates food
bio-protection conditions, providing antifungal and antimicrobial
activity. This could be explained through mechanisms of competition
for nutrients (61). In the case
of the traditional art that characterises feta PDO aged in a wooden
barrel, surface spoilage is subject to the irregularity of the
surface material, which represents a cardinal issue for compliance
with standard hygiene regulations in food processing plants
(50). In the present study, it
was demonstrated that the traditional practice for the production
of feta PDO cheese using wooden-beech barrels for cheese ageing met
the standards of Good Manufacturing Practice (GΜP) (62), including the appropriate practices
of sanitation to ensure food safety.
Wood, due to its organic properties, particularly
its porosity and hygroscopic nature, is a renewable natural
resource that allows a progressive inlet of oxygen into the barrel
and attributes a sharp flavour to the cheese, but also a slightly
harder texture (23,54,60).
Previous studies have demonstrated that some commonly used wood
species have antimicrobial activities and are suitable for food
contact surfaces (63,64). Wooden barrels remain an
indispensable requirement characterising the traditional practice
of barrel-aged feta PDO. This peculiar maturing process could
significantly influence the future and the perspective of this
iconic Greek product.
Specifically, the use of beech barrels (Fagus
sylvatica) allows for controlled micro-oxygenation, as wood
enables oxygen to reach the inside of feta cheese as it matures.
This oxygen interaction enhances the flavour, contributes to the
development of a sharp, piquant and distinct sensory profile, but
also defines the texture of the cheese. These organoleptic
characteristics are associated to the presence of wood-derived
compounds, such as phenolics, for example, syringyl derivatives,
and mild, insignificant tree lactones (65-67),
as well as tannins, that exert antimicrobial efficacy and
structural resilience in brine (68). This maturation technique highlights
the artisanal and terroir-specific identity of feta, affirming its
value as a PDO product.
Additionally, wooden barrels used in the production
of feta PDO support sustainability goals, as they are biodegradable
and eco-friendly. Their local sourcing supports circular production
and promotes the cultural legacy of cheese, which in turn fosters
the rural economy and helps prevent land abandonment. Furthermore,
the implementation of forest certification protocols could help
prevent and mitigate the decline of tree species commonly used for
timber of beech (Fagus spp.) populations in Mediterranean
regions, while contributing to improved stand structure by
protecting mature trees and promoting natural regeneration
(69).
Consequently, the long-term commitment to using
beech wooden barrels in the production of feta PDO cheese (8,70,71)
ensures not only the preservation of the cultural and nutritional
heritage, but also highlights the use of sustainable forest
management (72,73). This contributes to establishing the
continuity and the viability of agricultural livelihoods in
Greece.
Despite its advantages, wooden barrel sanitisation
poses challenges for product safety, as it carries the risk of
microbial contamination due to the porous nature of the wood
(74). Nevertheless,
forward-looking strategies could further develop Greek feta PDO
cheese, ensuring product safety and enhancing its nutritional
profile, with the potential to qualify as a functional food through
targeted fortification and the integration of adjunct co-culture
strains exhibiting probiotic and technological potential (28,75-76).
These strategies may not only satisfy consumer expectations for
authenticity and safety, but may also boost the market prospects of
traditional cheeses, particularly feta PDO cheese.
Although the specifications for Greek feta PDO
clearly define the origin of the milk, they do not impose specific
requirements on the type of wood used for barrels, since the
regulation does not include this parameter (2). For example, chestnut (Castanea
sativa) has been recommended for fresh cheeses due to its
antioxidant properties and its capacity to enhance product
stability as well as the shelf life in other agro-industrial
by-products (56,77). Likewise, sweet cherry (Prunus
avium L.) is known for its high phenolic content, contributing
to organoleptic, antioxidant, and anti-hyperglycemic benefits
(78,79). At the same time, walnut (Juglans
regia L.) has been incorporated into functional cheese
fortification, as seen in the case of Beyaz cheese (78,79).
Despite these favourable attributes, none of these woods aligns
with the cultural and historical framework that governs the
production of feta PDO.
Although oak is beneficial for cheese ageing, the
porosity of the wood, creates an organic wicking effect that
favours the conditions of drying and ageing of cheese, because it
sustains bacterial growth and the development of biofilms. This is
beneficial, as the prevalence of desirable organisms that are
immobilised within the thick layer of polysaccharides, promotes the
contribution of flavour and positive organoleptic characteristics
to the Feta PDO cheese. Specifically, lactococci and lactobacilli
are critical as they contribute to the cheese acidification and
flavour formation of the feta cheese (23).
To better illustrate how exogenous or endogenous
environmental factors impact microbial populations during feta PDO
cheese ripening, Table IV
summarises the selective pressure acting on several bacterial
species, deduced from both our observations and the literature
evidence. The perspective is that characterisation and mapping of
the microbiome of feta PDO cheese may become a valuable tool for
the monitoring of environmental contamination, to improve the
quality control in the dairy plants, particularly under routine and
experimental conditions such as salinity, moisture, temperature and
pH adjustments. Therefore, understanding the interactions between
feta cheese and selected bacterial flora could be meaningful for
feta PDO cheese manufacturing of a standard quality (50) and variability of organoleptic
features. Moreover, the growth of pathogens and the risk of their
survival is a fundamental issue and needs to be addressed by
validated and frequently monitored good sanitation practices.
 | Table IVSummary of endogenous and exogenous
conditions affecting bacterial species during feta PDO cheese
production. |
Table IV
Summary of endogenous and exogenous
conditions affecting bacterial species during feta PDO cheese
production.
| Environmental
factor | Effect on pathogens
(Refs.) | Effect on
beneficial bacteria (LAB) (Refs.) | Remarks |
|---|
| pH
<4.6-(endogenous) | Inhibits pathogen
growth such L. monocytogenes, Salmonella spp., E.
coli (80,81) | Favours
Lactobacillus spp., L. paracasei, Lactococcus
spp. (82,83) | Acid-tolerant LAB
dominate in low pH environments |
| NaCl curd 2.5-2.8%
(w/w) (endogenous) | Osmotic stress
inhibits pathogens, such as S. aureus, Bacillus cereus,
Yersinia spp. (84) | Enhances LAB
survival; stimulates biofilm formation Lactobaccilus
bulgaricus, Lactococcus lactis (85) | LAB possess
salt-tolerance mechanisms |
| Low temperature
(4-10˚C) (exogenous) | Suppresses
mesophilic pathogens (85)’ i.e.,
L. monocytogenes, Salmonella spp., E.
coli | Psychrotolerant
LAB, such as Lactococcus lactis survive and metabolise
slowly (85) | Cold ripening
favours LAB over pathogens |
| Environmental
humidity (exogenous) and brine (endogenous) | Exogenous factor:
Reduces desiccation (86), but
limits aerobic pathogen survival (endogenous factors) (87) | Both exogenous and
endogenous factors support LAB surface colonisation and
stability | Moist environment
promotes biofilm integrity on the wood surface |
| Wooden barrel
(beech or oak) | Surface
irregularities complicate pathogen removal (56) | Promotes biofilm of
LAB (e.g., Lactobacillus, Bifidobacterium) (56) | Wood porosity
allows oxygen gradients; enhances flavour development |
| Cleaning (hot
whey) | Effectively removes
planktonic pathogens | Preserves
biofilm-associated beneficial flora | Traditional
cleaning helps balance hygiene with microbial stability |
In conclusion, the present study provides a
comprehensive assessment of the microbiological safety of
handcrafted feta PDO cheese aged in wooden barrels, in accordance
with current food safety regulations in Greece. The results confirm
that the traditional maturation process using beech barrels, when
accompanied by proper cleaning protocols inhibit the presence of
key foodborne pathogens, including Listeria monocytogenes,
Salmonella spp. and Staphylococcus aureus. In
particular, to the best of our knowledge, the present study is the
first to formally document and evaluate under microbiological
criteria the traditional cleaning method of hot whey rinsing during
the ripening of Greek artisanal feta PDO cheesemaking in Fagus
sylvatica barrels, demonstrating that it contributes to the
effective hygienic maintenance of wooden barrels used in the
maturation process.
The observed TMC in biofilm samples revealed minimal
fluctuations (≤5%) over a 150-day ripening period, suggesting a
stable microbial ecosystem. The dominance of beneficial LAB,
fostered by selective environmental pressures such as low pH and
high salinity, supports the formation of functional, protective
biofilms that contribute to both microbial safety and flavour
development. While the complexity and variability of the cheese
microbiota pose challenges for standardisation, they also highlight
the unique sensory identity of feta PDO. In particular, the
presence of beneficial microbial communities within the beech
wood-associated biofilm contributes to both microbial stability and
the organoleptic profile of the cheese during ripening. Future
studies utilizing advanced molecular approaches, such as next
generation sequencing (NGS) and metagenomic analysis, will be
essential for elucidating the role of the microbiome in ripening
dynamics, biofilm formation, and sensory development.
Overall, the present study reinforces the
compatibility of traditional wooden-barrel aging practices with
modern food safety requirements and highlights the potential of
microbial mapping as a future tool for quality control and process
optimisation in artisanal dairy production. Furthermore, the
presence of beneficial microbial populations, particularly
probiotic strains, provides a promising basis for the development
of functional feta cheese with potential health-promoting
properties.
Acknowledgements
The authors gratefully acknowledge Mr. Konstantinos
Pagonis, founder of Pagonis Sis & Co., for supporting this
research by sharing his expertise and practical experience in feta
PDO production and Mrs. Eirini Christodoulou, Quality Control
manager of Pagonis Sis & Co., for her insightful suggestions.
The authors would also thank the cooperage company of Barsoukis
Sons and Metsovo Forest Service for specifying the tree
species.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MP, VZ, GTT and ND conceptualised and designed the
study. MP, AP, IM and AT were involved in the study methodology.
MP, DP and IM provided software (Excel for graph design, GIMP for
image editing and LAB Fit for curve fitting). MP and AP were
involved in the investigative aspects of the study and in the
provision of resources (milk, cheese, brine and biofilm samples).
MP and AP confirm the authenticity of all the raw data. AP, IM, DP
and NPR were involved in data analysis and curation. MP, VZ and AP
were involved in the writing and preparation of the original draft
of the manuscript. MP, DP, AP, IM, NPR and GTT were involved in the
writing, reviewing and editing of the manuscript. AP, ND and GTT
supervised the study. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MP and AP are co-owners of the company Pagonis Sis
& Co. from which the feta was procured. DP, VZ, IM, AT, NPR,
GTT and ND declare that they have no competing interests.
References
|
1
|
Manolopoulou E, Sarantinopoulos P, Zoidou
E, Aktypis A, Moschopoulou E, Kandarakis IG and Anifantakis EM:
Evolution of microbial populations during traditional feta cheese
manufacture and ripening. Int J Food Microbiol. 82:153–161.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Commission: Commission Regulation
(EC) No 1829/2002 of 14 October 2002 amending the Annex to
Regulation (EC) No 1107/96 with regard to the name ‘Feta’, 2002.
https://eur-lex.europa.eu/eli/reg/2002/1829/oj.
Accessed on December 6, 2024.
|
|
3
|
Bozoudi D, Kotzamanidis C, Hatzikamari M,
Tzanetakis N, Menexes G and Litopoulou-Tzanetaki E: A comparison
for acid production, proteolysis, autolysis and inhibitory
properties of lactic acid bacteria from fresh and mature feta PDO
Greek cheese, made at three different mountainous areas. Int J Food
Microbiol. 200:87–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kritikou AS, Aalizadeh R, Damalas DE,
Barla IV, Baessmann C and Thomaidis NS: MALDI-TOF-MS integrated
workflow for food authenticity investigations: An untargeted
protein-based approach for rapid detection of PDO feta cheese
adulteration. Food Chem. 370(131057)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chalyan T, Magnus I, Konstantaki M,
Pissadakis S, Diamantakis Z, Thienpont H and Ottevaere H:
Benchmarking spectroscopic techniques combined with machine
learning to study oak barrels for wine ageing. Biosensors (Basel).
12(227)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Katsouri E, Magriplis E, Zampelas A,
Nychas GJ and Drosinos EH: Nutritional characteristics of prepacked
feta PDO cheese products in greece: Assessment of dietary intakes
and nutritional profiles. Foods. 9(253)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Biagi P, Starnini E, Efstratiou N, Nisbet
R, Hughes PD and Woodward JC: Mountain landscape and human
settlement in the Pindus Range: The Samarina Highland Zones of
Western Macedonia, Greece. Land. 12(96)2023.
|
|
8
|
Fyllas NM, Koufaki T, Sazeides CI,
Spyroglou G and Theodorou K: Potential impacts of climate change on
the habitat suitability of the dominant tree species in Greece.
Plants (Basel). 11(1616)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Opgenoorth L, Dauphin B, Benavides R, Heer
K, Alizoti P, Martínez-Sancho E, Alía R, Ambrosio O, Audrey A,
Auñón F, et al: The GenTree platform: Growth traits and tree-level
environmental data in 12 European forest tree species. Gigascience.
10(giab010)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Crous PW, Wingfield MJ, Jurjevic Z,
Balashov S, Osieck ER, Marin-Felix Y, Luangsa-Ard JJ, Mejía LC,
Cappelli A, Parra LA, et al: Fungal Planet description sheets:
1697-1780. Fungal Syst Evol. 14:325–577. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lortal S, Di Blasi A, Madec MN,
Pediliggieri C, Tuminello L, Tanguy G, Fauquant J, Lecuona Y, Campo
P, Carpino S and Licitra G: Tina wooden vat biofilm: A safe and
highly efficient lactic acid bacteria delivering system in PDO
Ragusano cheese making. Int J Food Microbiol. 132:1–8.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Didienne R, Defargues C, Callon C,
Meylheuc T, Hulin S and Montel MC: Characteristics of microbial
biofilm on wooden vats (‘gerles’) in PDO Salers cheese. Int J Food
Microbiol. 156:91–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bonanno A, Tornambe G, Bellina V, De
Pasquale C, Mazza F, Maniaci G and Di Grigoli A: Effect of farming
system and cheesemaking technology on the physicochemical
characteristics, fatty acid profile, and sensory properties of
Caciocavallo Palermitano cheese. J Dairy Sci. 96:710–724.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scatassa ML, Cardamone C, Miraglia V,
Lazzara F, Fiorenza G, Macaluso G, Arcuri L, Settanni L and Mancuso
I: Characterisation of the microflora contaminating the wooden vats
used for traditional sicilian cheese production. Ital J Food Saf.
4(4509)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lortal S, Licitra G and Valence F: Wooden
tools: Reservoirs of microbial biodiversity in traditional
cheesemaking. Microbiol Spectr. 2(CM-0008-2012)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gaglio R, Cruciata M, Di Gerlando R,
Scatassa ML, Cardamone C, Mancuso I, Sardina MT, Moschetti G,
Portolano B and Settanni L: Microbial activation of wooden vats
used for traditional cheese production and evolution of neoformed
biofilms. Appl Environ Microbiol. 82:585–595. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cruciata M, Gaglio R, Scatassa ML, Sala G,
Cardamone C, Palmeri M, Moschetti G, La Mantia T and Settanni L:
Formation and characterization of early bacterial biofilms on
different wood typologies applied in dairy production. Appl Environ
Microbiol. 84:e02107–e02117. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Quigley L, O'Sullivan O, Beresford TP,
Ross RP, Fitzgerald GF and Cotter PD: Molecular approaches to
analysing the microbial composition of raw milk and raw milk
cheese. Int J Food Microbiol. 150:81–94. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Licitra G, Caccamo M, Valence F and Lortal
S: Traditional wooden equipment used for cheesemaking and their
effect on quality. In: Global Cheesemaking Technology, pp 157-172,
2017.
|
|
20
|
Papadimitriou K, Anastasiou R, Georgalaki
M, Bounenni R, Paximadaki A, Charmpi C, Alexandraki V, Kazou M and
Tsakalidou E: Comparison of the microbiome of artisanal homemade
and industrial feta cheese through amplicon sequencing and shotgun
metagenomics. Microorganisms. 10(1073)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Varzakas TH, Tsigarida E, Apostolopoulos
C, Kalogridou-Vassiliadou D and Jukes D: The role of the Hellenic
food safety authority in Greece-Implementation strategies. Food
Control. 17:957–965. 2006.
|
|
22
|
Koutou A: The framework of hygiene
inspections in the food sector in greece-implementation of HACCP
principles and penalties in case of non-compliance. Health Sci J.
12(573)2018.
|
|
23
|
Thodis P, Kosma IS, Nesseris K, Badeka AV
and Kontominas MG: Evaluation of a new bulk packaging container for
the ripening of feta cheese. Foods. 12(2176)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Carrascosa C, Millan R, Saavedra P, Jaber
JR, Raposo A and Sanjuan E: Identification of the risk factors
associated with cheese production to implement the hazard analysis
and critical control points (HACCP) system on cheese farms. J Dairy
Sci. 99:2606–2616. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ruta S, Belvedere G, Licitra G, Nero LA,
Caggia C, Randazzo CL and Caccamo M: Recent insights on the
multifaceted roles of wooden tools in cheese-making: A review of
their impacts on safety and final traits of traditional cheeses.
Int J Food Microbiol. 435(111179)2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Geronikou A, Srimahaeak T, Rantsiou K,
Triantafillidis G, Larsen N and Jespersen L: Occurrence of yeasts
in white-brined cheeses: Methodologies for identification, spoilage
potential and good manufacturing practices. Front Microbiol.
11(582778)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Settanni L, Di Grigoli A, Tornambe G,
Bellina V, Francesca N, Moschetti G and Bonanno A: Persistence of
wild Streptococcus thermophilus strains on wooden vat and during
the manufacture of a traditional Caciocavallo type cheese. Int J
Food Microbiol. 155:73–81. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Papadopoulou OS, Argyri AA, Varzakis EE,
Tassou CC and Chorianopoulos NG: Greek functional Feta cheese:
Enhancing quality and safety using a Lactobacillus plantarum strain
with probiotic potential. Food Microbiol. 74:21–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Independent Authority for Public Revenue:
Food and Drinks Code, 2024. https://www.aade.gr/en/chemical-laboratories/food-materials-contact-food/chemical-laboratory/food-and-drinks-code.
Accessed on December 15, 2024.
|
|
30
|
Koulelis PP, Proutsos N, Solomou AD,
Avramidou EV, Malliarou E, Athanasiou M, Xanthopoulos G and
Petrakis PV: Effects of climate change on greek forests: A review.
Atmosphere. 14(1155)2023.
|
|
31
|
Andritsos ND and Mataragas M:
Characterization and antibiotic resistance of listeria
monocytogenes strains isolated from greek myzithra soft whey cheese
and related food processing surfaces over two-and-a-half years of
safety monitoring in a cheese processing facility. Foods.
12(1200)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lappa IK, Natsia A, Alimpoumpa D,
Stylianopoulou E, Prapa I, Tegopoulos K, Pavlatou C, Skavdis G,
Papadaki A and Kopsahelis N: Novel probiotic candidates in
artisanal feta-type kefalonian cheese: Unveiling a
still-undisclosed biodiversity. Probiotics Antimicrob Proteins: Mar
13, 2024 (Epub ahead of print). doi:
10.1007/s12602-024-10239-x.
|
|
33
|
EFSA Panel on Biological Hazards (BIOHAZ).
Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid
S, Chemaly M, Davies R, De Cesare A, Hilbert F, et al: Scientific
opinion on the update of the list of QPS-recommended biological
agents intentionally added to food or feed as notified to EFSA
(2017-2019). EFSA J. 18(e05966)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
European Commission: eAmbrosia: Union
register of geographical indications, 2002. https://ec.europa.eu/agriculture/eambrosia/geographical-indications-register/details/EUGI00000013179.
Accessed on July 12, 2024.
|
|
35
|
Hellenic Agricultural
Organization-Demeter: Control and certification of PDO and PGI
products, 2025. https://www.elgo.gr/index.php?option=com_content&view=category&id=188&Itemid=1259.
Accessed on June 24, 2024.
|
|
36
|
International Organization for
Standardization: ISO 4833-1: Microbiology of the Food
Chain-Horizontal Method for the Enumeration of Microorganisms-Part
1: Colony Count at 30˚C by the Pour Plate Technique, 2013.
https://www.iso.org/standard/53728.html. Accessed on
August 11, 2020.
|
|
37
|
International Organization for
Standardization: ISO 4832:2006: Microbiology of food and animal
feeding stuffs-Horizontal method for the enumeration of
coliforms-Colony-count technique, 2006. https://www.iso.org/standard/38282.html. Accessed on
July 17, 2024.
|
|
38
|
Ogura A, Iwasaki M, Ogihara H and Teramura
H: Evaluation of the novel dry sheet culture method for the
enumeration of enterobacteriaceae. Biocontrol Sci. 23:235–240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Teramura H, Ogura A, Everis L and Betts G:
MC-Media pad EC for enumeration of escherichia coli and coliforms
in a variety of foods. J AOAC Int. 102:1502–1515. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
International Organization for
Standardization: ISO 16649-2:2001: Microbiology of food and animal
feeding stuffs-Horizontal method for the enumeration of
beta-glucuronidase-positive Escherichia coli: Part 2:
Colony-count technique at 44 degrees C using
5-bromo-4-chloro-3-indolyl beta-D-glucuronide, 2001. https://www.iso.org/standard/29824.html. Accessed on
August 24, 2024.
|
|
41
|
European Commission: Commission Regulation
(EC) No 2073/2005 of 15 November 2005 on microbiological criteria
for foodstuffs, 2005. https://eur-lex.europa.eu/eli/reg/2005/2073/oj.
Accessed on August 5, 2024.
|
|
42
|
European Commission: Commission Regulation
(EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No
2073/2005 on microbiological criteria for foodstuffs, 2007.
https://eur-lex.europa.eu/eli/reg/2007/1441/oj.
Accessed on August 5, 2024.
|
|
43
|
(ESYD) HAS: Certificate Number: 1111-3,
2025. http://esydops.gr/portal/p/esyd/en/showOrgInfo.jsp?id=224143.
Accessed on September 14, 2023.
|
|
44
|
Papadakis P, Konteles S, Batrinou A,
Ouzounis S, Tsironi T, Halvatsiotis P, Tsakali E, Van Impe JFM,
Vougiouklaki D, Strati IF and Houhoula D: Characterization of
bacterial microbiota of P.D.O. Feta cheese by 16S metagenomic
analysis. Microorganisms. 9(2377)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bintsis T, Litopoulou-Tzanetaki E and
Robinson RK: Microbiology of brines used to mature Feta cheese. Int
J Food Microbiol. 53:106–112. 2000.
|
|
46
|
Anagnostopoulos AK and Tsangaris GT: Feta
cheese proteins: Manifesting the identity of Greece's National
treasure. Data Brief. 19:2037–2040. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rantsiou K, Urso R, Dolci P, Comi G and
Cocolin L: Microflora of Feta cheese from four Greek manufacturers.
Int J Food Microbiol. 126:36–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mania I, Delgado AM, Barone C and Parisi
S: Traceability in the dairy industry in Europe. In: Traceability
in the Dairy Industry in Europe, pp129-139, 2018.
|
|
49
|
Casino F, Kanakaris V, Dasaklis TK,
Moschuris S, Stachtiaris S, Pagoni M and Rachaniotis NP:
Blockchain-based food supply chain traceability: A case study in
the dairy sector. Int J Prod Res. 59:5758–5770. 2021.
|
|
50
|
Stellato G, De Filippis F, La Storia A and
Ercolini D: Coexistence of lactic acid bacteria and potential
spoilage microbiota in a dairy processing environment. Appl Environ
Microbiol. 81:7893–7904. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Afshari R, Pillidge CJ, Dias DA, Osborn AM
and Gill H: Cheesomics: The future pathway to understanding cheese
flavour and quality. Crit Rev Food Sci Nutr. 60:33–47.
2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mahony J, McDonnell B, Casey E and van
Sinderen D: Phage-Host interactions of cheese-making lactic acid
bacteria. Annu Rev Food Sci Technol. 7:267–285. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Montel MC, Buchin S, Mallet A, Delbes-Paus
C, Vuitton DA, Desmasures N and Berthier F: Traditional cheeses:
Rich and diverse microbiota with associated benefits. Int J Food
Microbiol. 177:136–154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bokulich NA and Mills DA:
Facility-specific ‘house’ microbiome drives microbial landscapes of
artisan cheesemaking plants. Appl Environ Microbiol. 79:5214–5223.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Funari R and Shen AQ: Detection and
characterization of bacterial biofilms and biofilm-based sensors.
ACS Sens. 7:347–357. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gaglio R, Cruciata M, Scatassa ML, Tolone
M, Mancuso I, Cardamone C, Corona O, Todaro M and Settanni L:
Influence of the early bacterial biofilms developed on vats made
with seven wood types on PDO Vastedda della valle del Belìce cheese
characteristics. Int J Food Microbiol. 291:91–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rather MA, Gupta K, Bardhan P, Borah M,
Sarkar A, Eldiehy KSH, Bhuyan S and Mandal M: Microbial biofilm: A
matter of grave concern for human health and food industry. J Basic
Microbiol. 61:380–395. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Licitra G, Ogier JC, Parayre S,
Pediliggieri C, Carnemolla TM, Falentin H, Madec MN, Carpino S and
Lortal S: Variability of bacterial biofilms of the ‘tina’ wood vats
used in the ragusano cheese-making process. Appl Environ Microbiol.
73:6980–6987. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Skowron K, Wiktorczyk N, Grudlewska K,
Kwiecińska-Piróg J, Wałecka-Zacharska E, Paluszak Z and
Gospodarek-Komkowska E: Drug-susceptibility, biofilm-forming
ability and biofilm survival on stainless steel of Listeria spp.
Strains isolated from cheese. Int J Food Microbiol. 296:75–82.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Munir MT, Pailhories H, Eveillard M, Irle
M, Aviat F, Dubreil L, Federighi M and Belloncle C: Testing the
antimicrobial characteristics of wood materials: A review of
methods. Antibiotics (Basel). 9(225)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Siedler S, Balti R and Neves AR:
Bioprotective mechanisms of lactic acid bacteria against fungal
spoilage of food. Curr Opin Biotechnol. 56:138–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
de Oliveira CAF, da Cruz AG, Tavolaro P
and Corassin CH: Chapter 10-food safety: Good manufacturing
practices (GMP), Sanitation standard operating procedures (SSOP),
hazard analysis and critical control point (HACCP). In:
Antimicrobial food packaging. Barros-Velázquez J (ed.) Academic
Press, San Diego, pp 129-139, 2016.
|
|
63
|
Aviat F, Gerhards C, Rodriguez-Jerez JJ,
Michel V, Bayon IL, Ismail R and Federighi M: Microbial safety of
wood in contact with food: A review. Compr Rev Food Sci Food Saf.
15:491–505. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Goncalves JM, Rodrigues KL, Almeida ATS,
Pereira GM and Buchweitz MRD: Assessment of good practices in
hospital food service by comparing evaluation tools. Nutr Hosp.
32:1796–1801. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Issa-Issa H, Lipan L, Cano-Lamadrid M,
Nems A, Corell M, Calatayud-García P, Carbonell-Barrachina AA and
López-Lluch D: Effect of aging vessel (Clay-Tinaja versus Oak
Barrel) on the Volatile composition, descriptive sensory profile,
and consumer acceptance of red wine. Beverages. 7(35)2021.
|
|
66
|
Formato M, Scharenberg F, Pacifico S and
Zidorn C: Seasonal variations in phenolic natural products in Fagus
sylvatica (European beech) leaves. Phytochemistry.
203(113385)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wang J, Minami E, Asmadi M and Kawamoto H:
Effect of delignification on thermal degradation reactivities of
hemicellulose and cellulose in wood cell walls. J Wood Sci.
67(19)2021.
|
|
68
|
Das AK, Islam MN, Faruk MO, Ashaduzzaman M
and Dungani R: Review on tannins: Extraction processes,
applications and possibilities. South African J Botany. 135:58–70.
2020.
|
|
69
|
Morales-Molino C, Steffen M, Samartin S,
van Leeuwen JFN, Hürlimann D, Vescovi E and Tinner W: Long-Term
responses of mediterranean mountain forests to climate change, fire
and human activities in the Northern Apennines (Italy). Ecosystems.
24:1361–1377. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Stagakis S, Markos N, Vanikiotis T,
Levizou E and Kyparissis A: Multi-year monitoring of deciduous
forests ecophysiology and the role of temperature and precipitation
as controlling factors. Plants (Basel). 11(2257)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Varsamis G, Papageorgiou AC, Merou T,
Takos I, Malesios C, Manolis A, Tsiripidis I and Gailing O:
Adaptive diversity of beech seedlings under climate change
scenarios. Front Plant Sci. 9(1918)2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Winkel G, Lovrić M, Muys B, Katila P,
Lundhede T, Pecurul M, Pettenella D, Pipart N, Plieninger T,
Prokofieva I, et al: Governing Europe's forests for multiple
ecosystem services: Opportunities, challenges, and policy options.
For Policy Econ. 145:2022.
|
|
73
|
Food and Agriculture Organization of the
United Nations: Sustainable Forest Management Toolbox, 2025.
https://www.fao.org/sustainable-forest-management/toolbox.
Accessed on August 15, 2025.
|
|
74
|
Sainz-García A, González-Marcos A,
Múgica-Vidal R, Muro-Fraguas I, Escribano-Viana R,
González-Arenzana L, López-Alfaro I, Alba-Elías F and Sainz-García
E: Application of atmospheric pressure cold plasma to sanitize oak
wine barrels. LWT. 139(110509)2021.
|
|
75
|
Vettorazzi A, de Cerain AL, Sanz-Serrano
J, Gil AG and Azqueta A: European regulatory framework and safety
assessment of food-related bioactive compounds. Nutrients.
12(613)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
The European Food Information Council:
Bioactive compounds, 2021. https://www.eufic.org/en/whats-in-food/category/bioactive-compounds.
Accessed on May 1, 2023.
|
|
77
|
Ferreira SM, Matos LC and Santos L:
Harnessing the potential of chestnut shell extract to enhance fresh
cheese: A sustainable approach for nutritional enrichment and
shelf-life extension. Journal of Food Measurement and
Characterization. 18:1559–1573. 2024.
|
|
78
|
Nunes AR, Gonçalves AC, Pinto E, Amaro F,
Flores-Félix JD, Almeida A, de Pinho PG, Falcão A, Alves G and
Silva LR: Mineral content and volatile profiling of prunus avium L.
(Sweet Cherry) By-products from fundão region (Portugal). Foods.
11(751)2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Salık MA and Çakmakçı S: Development and
techno-functional characterization of beyaz cheese fortified with
walnut (Juglans regia L.) leaf powder and lactobacillus acidophilus
LA-5. Probiotics Antimicrob Proteins: Jun 14, 2025 (Epub ahead of
print). doi: 10.1007/s12602-025-10629-9, 2025.
|
|
80
|
Suehr QJ, Chen F, Anderson NM and Keller
SE: Effect of Ph on survival of escherichia coli O157, escherichia
coli O121, and salmonella enterica during desiccation and
short-term storage. J Food Prot. 83:211–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Chacón-Flores NA, Olivas-Orozco GI,
Acosta-Muñiz CH, Gutiérrez-Méndez N and Sepúlveda-Ahumada DR:
Effect of water activity, pH, and lactic acid bacteria to inhibit
escherichia coli during chihuahua cheese manufacture. Foods.
12(3751)2023.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Samelis J and Kakouri A: Microbiological
characterization of greek galotyri cheese PDO products relative to
whether they are marketed fresh or ripened. Fermentation.
8(492)2022.
|
|
83
|
Li Y, Yu H, Tang Z, Wang J, Zeng T and Lu
S: Effect of Coreopsis tinctoria microcapsules on tyramine
production by Enterococcus faecium in smoked horsemeat sausage.
LWT. 170(113974)2022.
|
|
84
|
Akbulut S, Dasdemir E, Ozkan H and
Adiguzel A: Determination of bacteriocin genes and antimicrobial
activity of Lactiplantibacillus plantarum isolated from feta cheese
samples. FEMS Microbiol Lett. 372(fnaf002)2025.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tzora A, Nelli A, Voidarou C, Fthenakis G,
Rozos G, Theodorides G, Bonos E and Skoufos I: Microbiota
‘Fingerprint’ of greek feta cheese through ripening. Appl Sci.
11(5631)2021.
|
|
86
|
Georgalaki M, Anastasiou R, Zoumpopoulou
G, Charmpi C, Lazaropoulos G, Bounenni R, Papadimitriou K and
Tsakalidou E: Feta cheese: A pool of Non-Starter Lactic Acid
Bacteria (NSLAB) with anti-hypertensive potential. Int Dairy J.
162(106144)2025.
|
|
87
|
Doukaki A, Papadopoulou OS, Baraki A,
Siapka M, Ntalakas I, Tzoumkas I, Papadimitriou K, Tassou C,
Skandamis P, Nychas GJ and Chorianopoulos N: Effect of the
bioprotective properties of lactic acid bacteria strains on quality
and safety of feta cheese stored under different conditions.
Microorganisms. 12(1870)2024.PubMed/NCBI View Article : Google Scholar
|