Introduction
Helicobacter pylori (H. pylori)
infection first induces chronic superficial gastritis, which can
progress to chronic atrophic gastritis, intestinal metaplasia, and
dysplasia that leads toward gastric carcinoma (1). Lipopolysaccharide (LPS), which is a
component of the outer membrane of Gram-negative bacteria including
H. pylori, is a signaling molecule for the innate immune
system and is one of the main sources of inflammation (2). LPS binding to TLR4 activates signal
transduction through MyD88, IRAK and TRAF6 to activate NF-κB
(3). Activation of NF-κB by H.
pylori induces nuclear translocation, which causes an increase
in IL-8 messenger RNA and protein levels (4). Other NF-κB responsive genes
including pro-inflammatory cytokines have also been found in
elevated levels in H. pylori-infected gastric mucosa. In
addition, the NF-κB pathway is responsible for the generation of
several cell adhesion molecules including ICAM-1 whose expression
is significantly correlated with an increase in H.
pylori-induced gastritis (5).
Thus, H. pylori is a potent activator of NF-κB in gastric
epithelial cells and NF-κB is a major molecule in H.
pylori-induced inflammation (4,6).
On the other hand, NF-κB activation is known to regulate cellular
growth responses, including apoptosis, and is required for the
induction of inflammatory and tissue-repair genes (7). These facts suggest that NF-κB plays
an important role in inflammation-associated carcinogenesis. In
fact, H. pylori infection, activating NF-κB, is now accepted
as a crucial event in the development of peptic ulcer disease and
atrophic gastritis, and it is implicated in the development of
gastric carcinoma, especially not located in the cardia (8–10).
Several cancers, including gastric tumors, show
methylations of multiple genes (11,12). Some genes are methylated in
non-neoplastic tissues with aging (13,14) and these methylations are also
under the influence of chronic inflammation (15,16). In non-cancerous gastric mucosa,
methylation of CpG islands was induced by H. pylori
infection (17,18) and considered as the precancerous
conditions in gastric carcinogenesis (19). Among several genes, E-cadherin
(CDH1), death-associated protein kinase (DAPK) and
cyclin-dependent kinase inhibitor 2A (CDKN2A) are frequently
methylated in non-neoplastic gastric mucosa in relation to age,
H. pylori infection, histological degree of gastritis, and
gastric carcinogenesis (17,20). Therefore, there is a possibility
that NF-κB activation may affect the gene methylations in H.
pylori-induced chronic inflammation. Recently, many studies
have reported the association between the polymorphism, −94 ins/del
ATTG (rs28362491) of NFKB1 encoding NF-κB, and various
inflammatory diseases (21), as
well as malignant neoplasm (22).
However, these results do not always lead to the same conclusions.
Furthermore, the genetic variation −449 C>G in the 5′-UTR of
NFKB1 (rs72696119) has been identified. There are no reports
for the association of this polymorphism and human disorders.
Then, we attempted to clarify the association
between the −94 ins/del ATTG polymorphism (rs28362491) of
NFKB1 and gene methylations in H. pylori-infected
Japanese subjects. In addition, the −449 C>G polymorphism
(rs72696119) was also investigated.
Materials and methods
Clinical samples
The 330 H. pylori-infected subjects without
peptic ulcers and gastric malignancies, who were enrolled at the
Endoscopy Center of Fujita Health University Hospital or Kanazawa
Medical University Hospital from January in 2006 to December in
2009, were selected. As a control, 205 H. pylori-uninfected
subjects were randomly selected from our stocked DNA collected
during the same period. Thus, the overall studied population
comprised 535 subjects.
All subjects underwent upper endoscopy with biopsy
from non-cancerous mucosa in the antrum. Parts of each specimen was
fixed in 10% buffered-formalin and embedded in paraffin, while the
other part was immediately frozen and stored at −85°C. Later,
genomic DNA was isolated from frozen specimens using proteinase K.
The patients with severe systemic diseases, malignancies in other
organs, and who had received nonsteroidal anti-inflammatory drugs,
antibiotics, and H. pylori eradication treatment were
excluded. H. pylori infection status was assessed by
serology, histological examination, or the urea breath test.
Patients were diagnosed as having infection when at least one of
the diagnostic tests was positive.
The subjects with 2 or more methylations of 4 genes
(p14ARF, p16INK4a, DAPK
and CDH1) were classified into the CpG island high
methylation (CIHM) group, whereas the others except the CIHM group
were classified into the non-CIHM group.
The Ethics Committee of the Fujita Health University
and the Kanazawa Medical University approved the protocol, and
prior, written informed consent was obtained from all participating
subjects.
Bisulfate modification and
methylation-specific PCR (MSP)
In 402 of 535 subjects (243 H.
pylori-infected and 159 uninfected), the methylation status of
4 candidate promoter CpG islands (p14, p16,
CDH1, and DAPK), which have been thought to be most
susceptible for methylation in the stomach (12,19,22,23), were assessed. For the examination
of DNA methylation, genomic DNA was treated with sodium bisulfite
using the BislFast DNA Modification kit for methylated DNA
detection (Toyobo, Co., Ltd., Osaka, Japan). Methylation status of
four candidate promoter CpG islands were examined by MSP as
previously described (24). The
primer pairs and experimental conditions for MSP are the same as in
our previous study (13,23,24). The MSP was carried out in a volume
of 20 μl containing 0.1 μg of bislufite-modified DNA.
The bands of MSP were detected by electrophoresis in 3.0% agarose
gels stained with ethidium bromide. Hypermethylation was defined as
the presence of positive methylation band, separated by
electrophoresis on 2.5% agarose gels under UV illumination using an
ethidium bromide staining, showing signals approximately equivalent
to or greater than that of size marker (10 ng/μl: 100 bp DNA
ladder; Takara Bio, Inc., Shiga, Japan), irrespective of the
presence of unmethylated bands. We used DNA from the peripheral
blood of a young individual without H. pylori infection, as
the negative control (unmethylated DNA), and also used DNA being
treated with SssI methylase (New England Biolabs, Inc.,
Beverly, MA, USA), as the positive control (methylated DNA).
Samples giving faint positive signals were analyzed a further two
times and only those samples with consistent positive methylation
band were considered as hypermethylation status.
Genotyping of polymorphisms
The DNA isolated from biopsy specimens or peripheral
blood was used. The polymorphisms were genotyped by the PCR-SSCP
method as previously described (25,26). To detect NFKB1 −94 ins/del
ATTG using the primer pairs (94-F, 5′-gctatggaccgcatgactctatcag-3′
and 94-R, 5′-ggggctctggcttcctagcag-3′), PCR was carried out in a
volume of 20 μl containing 0.1 μg of genomic DNA. The
DNA was denatured at 95°C for 3 min, followed by 35 cycles at 96°C
for 15 sec, 58°C for 40 sec, and 72°C for 30 sec, with a final
extension at 72°C for 5 min. Thereafter, 2 μl of the PCR
product was denatured with 10 μl of formamide (Sigma-Aldrich
Co., St. Louis, MO, USA) at 90°C for 5 min. SSCP was carried out at
6°C using a GenePhor DNA separation system with GeneGel Excel
12.5/24 (Amersham Biosciences Corp., USA), after which the
denatured single strand DNA bands were detected using a DNA Silver
Staining kit (Amersham Biosciences Corp.).
To detect the NFKB1 −449 C>G, using the
primer pairs (449-F, 5′-cgtgtgtccgtctgtctgtatgctc-3′ and 449-R,
5′-cgctggtgcacttctctctctttct-3′), PCR was carried out in a volume
of 20 μl containing 0.1 μg of genomic DNA. The DNA
was denatured at 95°C for 3 min, followed by 35 cycles at 95°C for
30 sec, 57°C for 40 sec, and 72°C for 45 sec, with a final
extension at 72°C for 5 min. Thereafter, SSCP was carried out as
described above.
Histological evaluation
In 400 of 535 subjects (256 H.
pylori-infected and 144 uninfected subjects), the severity of
chronic gastritis was classified according to the updated Sydney
system (27) by a pathologist who
had no access to any clinical information.
Statistical analysis
The data were expressed as mean ± SD. The mean age
among the two groups was compared by the Student’s t-test. The
ratios of gender and gene methylation were compared by the Fisher’s
extract test. The strength of association between allele
frequencies and the methylation status was assessed by calculating
the odds ratio (OR) and 95% confidence intervals (CI) by logistic
regression analysis. Adjusted ORs were calculated after adjustment
for age and gender. Each updated Sydney system score between the 2
groups were compared by the Mann-Whitney U-test. The methylation
status was compared among the 2 groups by ANOVA. The relationship
between age and the number of methylated genes was also assessed by
ANOVA. Concerning the power of study, the β-value was calculated
when setting α=0.05. For all analyses, the level of significance
was set at p<0.05.
Results
Subjects and genotype
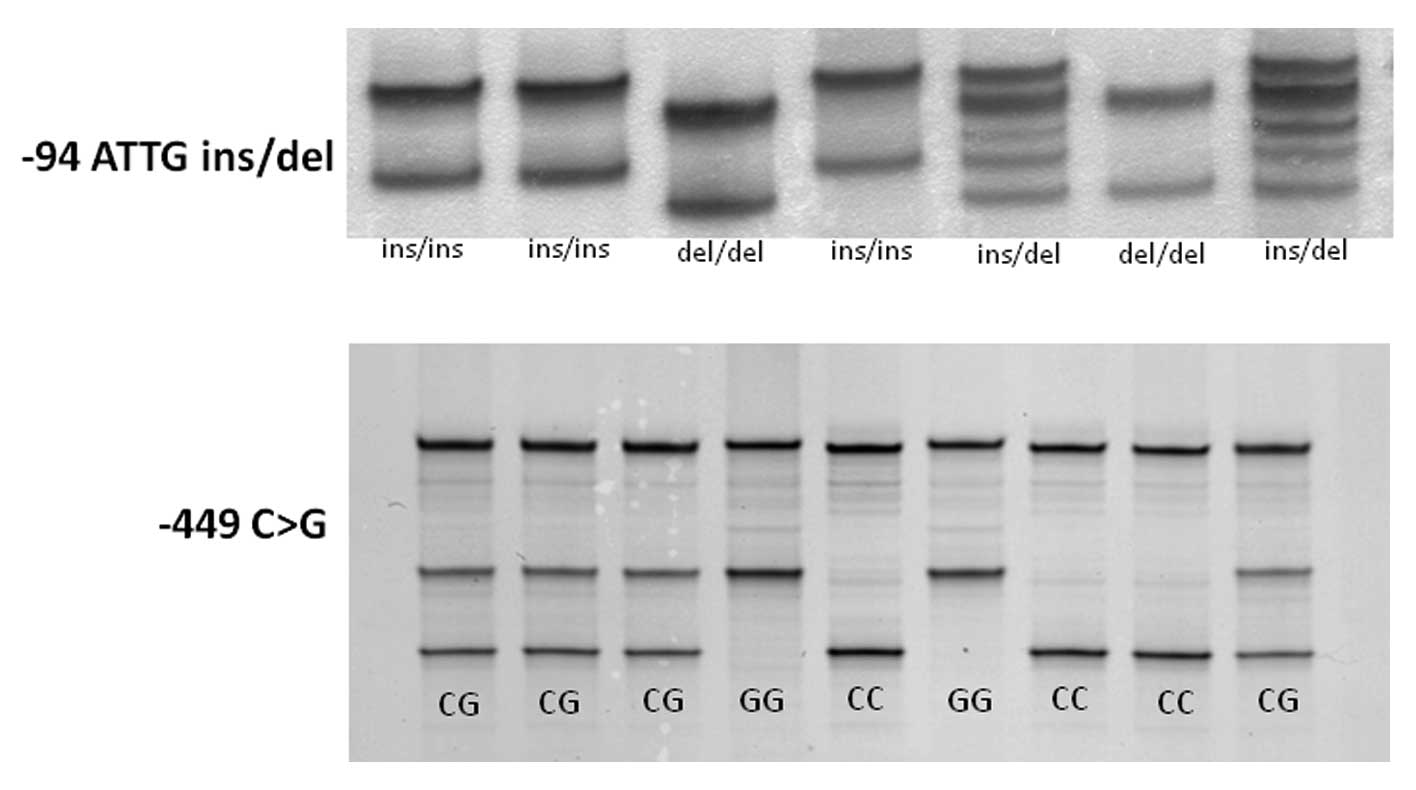
As shown in Fig.
1, single strand DNAs of each polymorphism were clearly
identified by SSCP. The characteristics of the subjects are
summarized in Table I. The
overall distribution of −94 ins/del ATTG genotype was 198 ins/ins,
269 ins/del and 68 del/del. The distribution of −449 C>G was 207
CC, 252 CG and 76 GG. There was a strong allelic association
between −94 ins/del ATTG and −449 C>G. The frequencies of
distributions of both genotypes, in the Hardy-Weinberg equilibrium
(p=0.12 and 1.00, respectively), were not significant difference
among H. pylori-infected a d uninfected subjects. The
male/female ratio was lower and each gene methylation ratio, except
p14ARF, was higher in H. pylori-infected
subjects than uninfected subjects. The CIHM/non-CIHM ratio was also
significantly higher in H. pylori-infected subjects.
 | Table ICharacteristics and prevalence
polymorphisms and methylation status. |
Table I
Characteristics and prevalence
polymorphisms and methylation status.
|
Characteristics | H.
pylori-infected | H.
pylori-uninfected | p-valuea |
|---|
| Number of
subjects | 330 | 205 | |
| Mean age ± SD | 61.0±12.4 | 59.2±15.0 | NS |
| Male:female | 211:119 | 98:107 | 0.0003 |
| NFKB1−94
ins/del ATTG | | | |
| ins/ins | 119 | 79 | |
| ins/del | 172 | 97 | |
| del/del | 39 | 29 | |
| del allele
frequency | 37.9% | 37.8% | NS |
| NFKB1−449
G>C | | | |
| CC | 124 | 83 | |
| CG | 161 | 91 | |
| GG | 45 | 31 | |
| G allele
frequency | 38.0% | 37.3% | NS |
|
Methylated:unmethylated | | | |
|
p14ARF | 90:153 | 44:115 | 0.053 |
|
p16INK4a | 91:152 | 21:138 | <0.0001 |
| CDH1 | 109:134 | 38:121 | <0.0001 |
| DAPK | 139:104 | 58:101 | <0.0001 |
| CIHM/non-CIHM | 136/107 | 47/112 | <0.0001 |
Association between NFKB1 polymorphisms
and CIHM
We defined the subjects with 2 or more gene
methylations as the CIHM group, because the overall average number
of gene methylation was 1.58. NFKB1 −94 del/del homozygotes
had an increased risk for the development of CIHM in H.
pylori-infected over 60-year-old subjects (OR, 4.16; 95% CI,
1.14–15.3; p=0.031 and β=0.674) (Table II), although no significant risk
was seen in overall infected subjects. In H.
pylori-uninfected subjects, there was no association between
−94 ins/del ATTG polymorphism and CIHM.
 | Table IIAssociation between NFKB1−94
ins/del ATTG polymorphism and CIHM. |
Table II
Association between NFKB1−94
ins/del ATTG polymorphism and CIHM.
| Genotype (n)
| del/del vs. ins
carrier
| |
|---|
| ins/ins | ins/del | del/del | OR (95% CI) | p-value |
|---|
| H.
pylori-infected | | | | | |
| Overall | | | | | |
| Non-CIHM
(n=107) | 40 | 57 | 10 | Reference
value | - |
| CIHM
(n=136) | 50 | 65 | 21 | 1.58
(0.698–3.56) | 0.27 |
| 60≤ | | | | | |
| Non-CIHM
(n=61) | 23 | 35 | 3 | Reference
value | - |
| CIHM
(n=81) | 27 | 39 | 15 | 4.16
(1.14–15.3) | 0.031 |
| H.
pylori-uninfected | | | | | |
| Overall | | | | | |
| Non-CIHM
(n=112) | 45 | 50 | 17 | Reference
value | - |
| CIHM
(n=47) | 19 | 20 | 8 | 1.15
(0.456–2.88) | 0.77 |
| 60≤ | | | | | |
| Non-CIHM
(n=56) | 21 | 25 | 10 | Reference
value | - |
| CIHM
(n=27) | 13 | 10 | 4 | 0.814
(0.228–2.91) | 0.75 |
The association of −449 G>C with CIHM was similar
to that of −94 ins/del ATTG with CIHM (Table III), because both polymorphisms
were in strong linkage disequilibrium. So, in H.
pylori-infected subjects over 60-years-old, −449 GG homozygote
had an increased risk for CIHM (OR, 3.31; 95%CI, 1.04–10.6; p=
0.044).
 | Table IIIAssociation between NFKB1−449
C>G polymorphism and CIHM. |
Table III
Association between NFKB1−449
C>G polymorphism and CIHM.
| Genotype (n)
| GG vs. C carrier
| |
|---|
| CC | CG | GG | OR (95% CI) | p-value |
|---|
| H.
pylori-infected | | | | | |
| Overall | | | | | |
| Non-CIHM
(n=107) | 42 | 52 | 13 | Reference
value | - |
| CIHM
(n=136) | 48 | 65 | 23 | 1.30
(0.612–2.74) | 0.50 |
| 60≤ | | | | | |
| Non-CIHM
(n=61) | 25 | 32 | 4 | Reference
value | - |
| CIHM
(n=81) | 27 | 38 | 16 | 3.31
(1.04–10.6) | 0.044 |
| H.
pylori-uninfected | | | | | |
| Overall | | | | | |
| Non-CIHM
(n=112) | 48 | 45 | 19 | Reference
value | - |
| CIHM
(n=47) | 18 | 20 | 9 | 1.14
(0.473–2.75) | 0.77 |
| 60≤ | | | | | |
| Non-CIHM
(n=56) | 23 | 22 | 11 | Reference
value | - |
| CIHM
(n=27) | 12 | 10 | 5 | 0.939
(0.287–3.07) | 0.92 |
In H. pylori-infected over 60-year-old
subjects, both −94 del/del ATTG and −449 GG homozygote had an
increased risk for the development of DAPK methylation (OR,
5.35; 95% CI, 1.17–24.5; p=0.031 and β=0.726; and OR, 3.75; 95% CI,
1.04–13.6; p=0.044, respectively) (Table IV). In addition, −94 del/del
homozygote had an increased risk for CDH1, as well as
DAPK, methylation (OR, 2.91; 95% CI, 1.02–8.30; p=0.046). On
the other hand, both polymorphisms were not associated with
CDKN2A (p14ARF and
p16INK4a) methylation.
 | Table IVAssociations between NFKB1
polymorphisms and each gene methylation in the H.
pylori-infected subjects older than 60-year-old. |
Table IV
Associations between NFKB1
polymorphisms and each gene methylation in the H.
pylori-infected subjects older than 60-year-old.
| −94 ATTG
ins/del | ins/ins | ins/del | del/del | del/del vs. ins
carrier; OR (95% CI) | p-value |
|
|
p14ARF-unmethylated
(n=86) | 35 | 39 | 12 | Reference
value | - |
|
p14ARF-methylated
(n=56) | 15 | 35 | 6 | 0.661
(0.228–1.92) | 0.45 |
|
p16INK4a-unmethylated
(n=90) | 29 | 52 | 9 | Reference
value | - |
|
p16INK4a-methylated
(n=52) | 21 | 22 | 9 | 1.83
(0.671–4.97) | 0.24 |
|
CDH1-unmethylated (n=79) | 29 | 44 | 6 | Reference
value | - |
|
CDH1-methylated (n=63) | 21 | 30 | 12 | 2.91
(1.02–8.30) | 0.046 |
|
DAPK-unmethylated (n=53) | 19 | 32 | 2 | Reference
value | - |
|
DAPK-methylated (n=89) | 31 | 42 | 16 | 5.35
(1.17–24.5) | 0.031 |
|
| −449 C>G | CC | CG | GG | GG vs. C carrier;
OR (95% CI) | p-value |
|
|
p14ARF-unmethylated
(n=86) | 37 | 37 | 12 | Reference
value | - |
|
p14ARF-methylated
(n=56) | 15 | 33 | 8 | 0.925
(0.346–2.48) | 0.88 |
|
p16INK4a-unmethylated
(n= 90) | 31 | 48 | 11 | Reference
value | - |
|
p16INK4a-methylated
(n=52) | 21 | 22 | 9 | 1.45
(0.555–3.81) | 0.45 |
|
CDH1-unmethylated (n=79) | 31 | 40 | 8 | Reference
value | - |
|
CDH1-methylated (n=63) | 21 | 30 | 12 | 2.12
(0.802–5.59) | 0.13 |
|
DAPK-unmethylated (n=53) | 20 | 30 | 3 | Reference
value | - |
|
DAPK-methylated (n=89) | 32 | 40 | 17 | 3.75
(1.04–13.6) | 0.044 |
Relationship between NFKB1 polymorphisms
and methylated gene number
We found strong allelic association between −94
ins/del ATTG and −449 C>G. That is, 66 of 68 del/del homozygote
had −449 GG genotype. In H. pylori-infected subjects, all of
39 del/del homozygote had −449 GG genotype. Therefore, we
investigated the association of only −94 ins/del ATTG polymorphism
with gastric inflammation and gene methylation. In H.
pylori-infected subjects over 60-years-old or more, methylated
gene number was significantly higher in del/del homozygotes than in
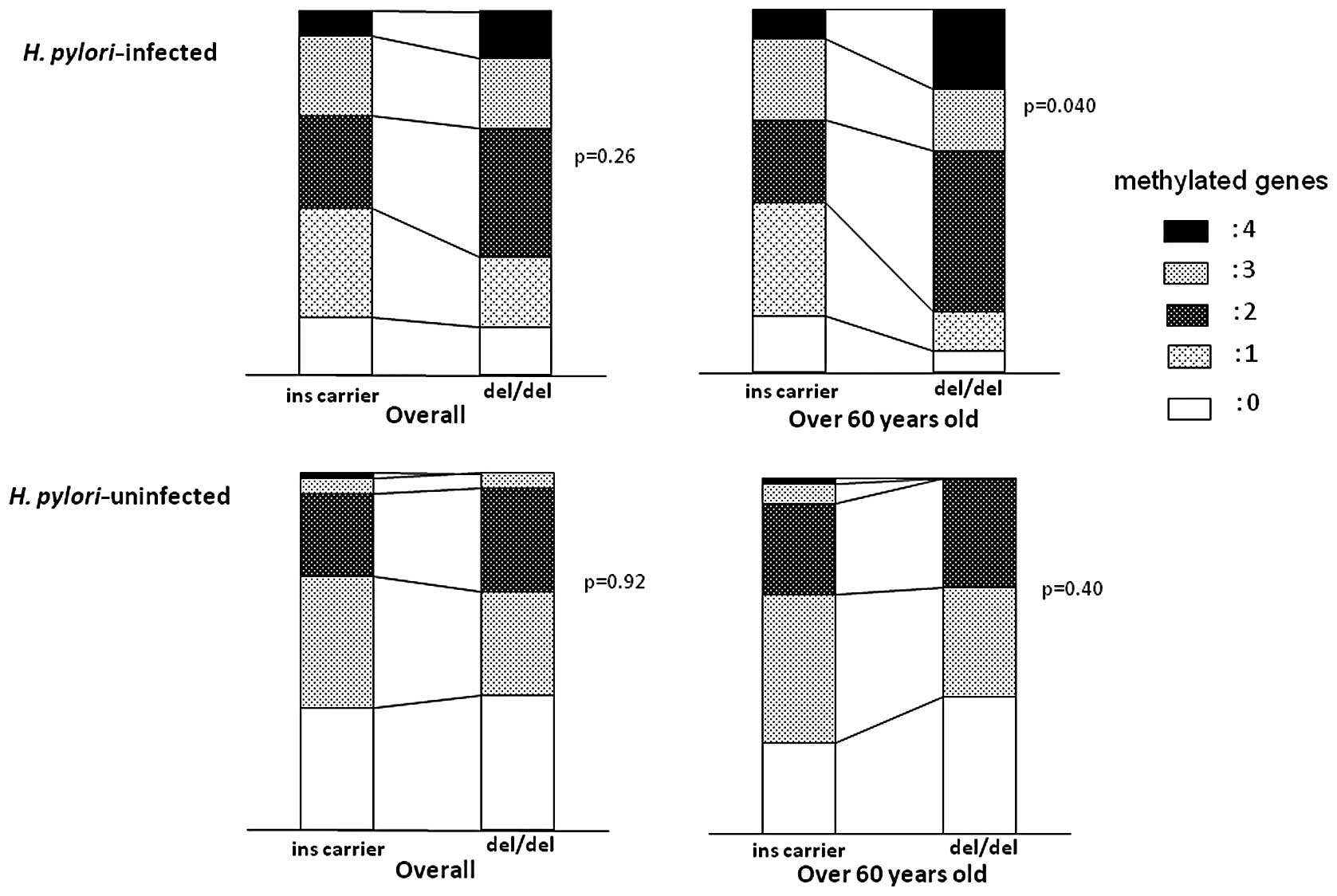
ins carriers (p=0.040 by ANOVA) (Fig.
2), although no significant difference was seen in overall
H. pylori-infected subjects. In H. pylori-uninfected
subjects, there was no significant difference in the methylated
gene number among two genotypes.
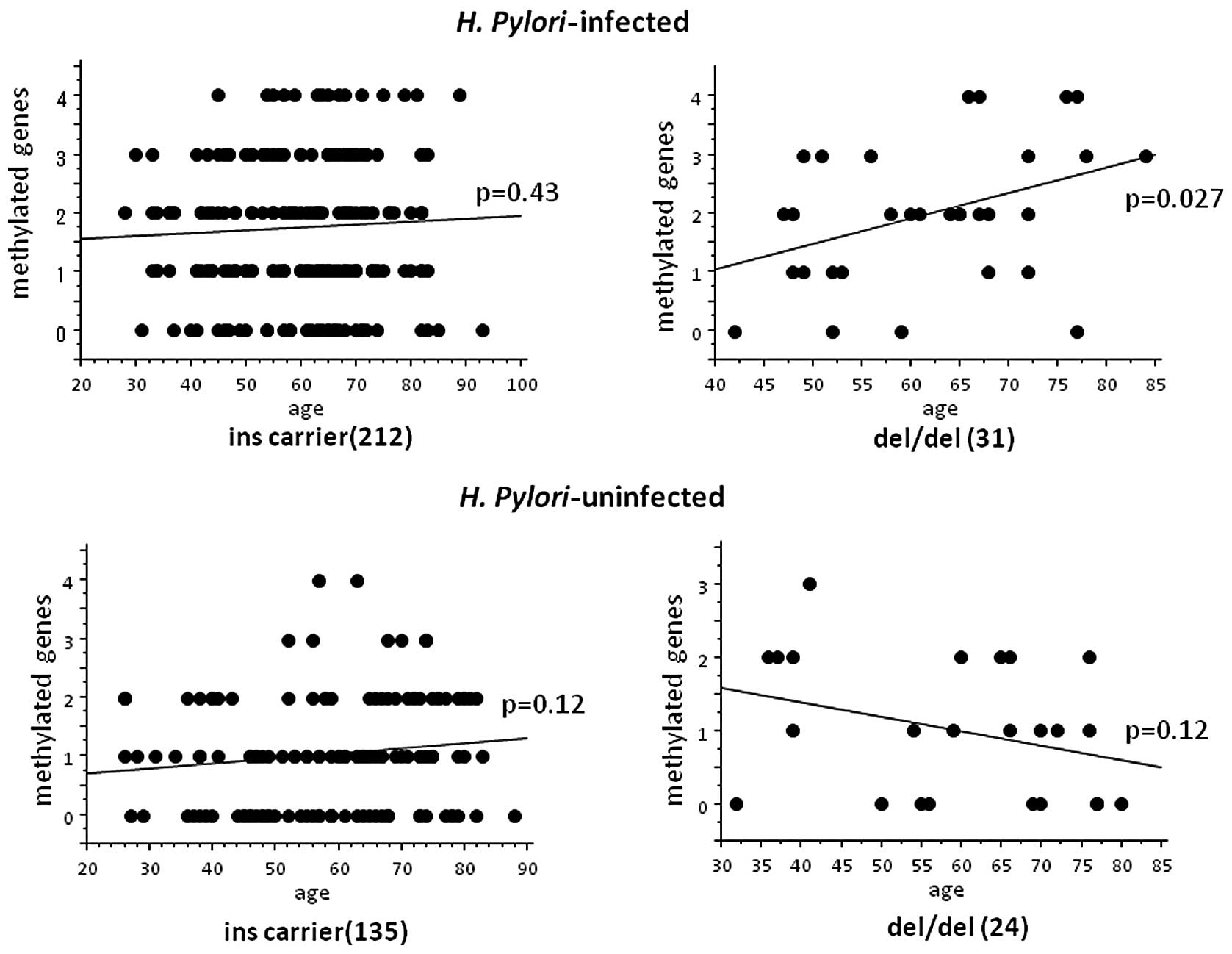
In H. pylori-infected del/del homozygote,
methylated gene number was significantly correlated to age (p=
0.027 by ANOVA) (Fig. 3), whereas
no significant correlation was seen in the H.
pylori-infected ins carrier and the uninfected groups.
Comparison of each updated Sydney system
score among del-G and non-del-G groups
In H. pylori-infected subjects, the
inflammation score was significantly higher in del/del homo-zygote
than ins carrier (p=0.0091 by Mann-Whitney U-test), whereas the
other scores were not different among two genotypes (Table V). In H. pylori-uninfected
subjects, there were no significant differences of all scores among
two genotypes.
 | Table VComparison of each updated Sydney
system score among del/del and ins carrier. |
Table V
Comparison of each updated Sydney
system score among del/del and ins carrier.
| H.
pylori-infected | del/del (n=28) | ins carrier
(n=228) | p-value |
|
| Activity | 0.929±0.813 | 0.851±0.858 | NS |
| Inflammation | 2.179±0.476 | 1.860±0.628 | 0.0091 |
| Atrophy | 1.607±0.685 | 1.575±0.773 | NS |
| Metaplasia | 0.786±1.067 | 0.982±1.015 | NS |
|
| H.
pylori-uninfected | del/del (n=15) | ins carrier
(n=129) | p-value |
|
| Activity | 0.067±0.258 | 0.155±0.441 | NS |
| Inflammation | 0.533±0.516 | 0.659±0.667 | NS |
| Atrophy | 0.133±0352 | 0.326±0.614 | NS |
| Metaplasia | 0 | 0.109±0.437 | NS |
Discussion
Accumulation of DNA damage and aberrant methylation
of various genes in gastric mucosa were induced by H. pylori
infection and confer risk for developing gastric cancer. However,
all the H. pylori-infected patients do not show the same
hypermethylation status of genes. This suggests that some host
genetic factor, such as genetic variations related to the immune
response or inflammation, may be relevant to the hypermethylation
of genes during gastric carcinogenesis.
Here, we evaluated the association between
NFKB1, encoding NF-κB which plays an important role in
inflammation and carcinogenesis, polymorphisms and aberrant
methylation of genes in non-neoplastic gastric mucosa. NFKB1
−94 ins/del ATTG and −449 C>G was in linkage disequilibrium and
all of 39 H. pylori-infected −94 del/del homozygote had the
−449 GG genotype. Therefore, the effects of the −94 and −449 mutant
haplotype was equal to that of the −94 mutant variation. In the
present study, we demonstrated that the −94 del/del ATTG
homozygotes had increased risk of aberrant methylation of
DAPK and CDH1 in comparatively older H.
pylori-infected subjects. We also found that in H.
pylori-infected del/del homozygotes, the number of methylated
genes was higher in subjects over 60-years-old and was correlated
to age. In addition, gastric mucosal inflammation was more severe
in infected del/del homozygotes. These findings suggest that, in
the NFKB1 −94 del/del ATTG homozygote, H. pylori
infection may accelerate severe mucosal inflammation, resulting in
high age-related gene methylation. The methylation of genes is
frequently observed in H. pylori-infected non-neoplastic
mucosa (17–19), and is closely correlated with
gastric cancer occurrence (12,18,20). Therefore, this epigenetic effect
seems to be an early step in carcinogenesis in the stomach. Our
data suggest that NFKB1 polymorphisms may have a role in
gastric carcinogenesis in the early phase via gene
methylation-related pathway. In the present study, sample selection
may affect the outcome, because our subjects came to hospital in
order to have endoscopic examination for the compliant of abdominal
discomfort, or for complete check up of gastric cancer following to
barium X-ray examination in the health check, not complete healthy
subjects. Moreover, the effect of type II error cannot be excluded
in relatively small sample sizes. Another limitation of this study
was that the male/female ratio was different among H.
pylori-infected and uninfected subjects. However, adjustment of
age and gender was performed in genotype analysis using logistic
regression.
It has been reported that the NFKB1 −94 ATTG
deletion variant in the promoter region destroys a transcription
factor binding site, resulting in lower expression of NF-κB
(28). Due to their important
role in inflammation, the lower expression of NF-κB protein seems
to suppress inflammation. Furthermore, the NFKB1 −94
deletion mutant has been associated with reduced risk for the
auto-immune disorders in China (29). In stomach, Lo et al
(30) showed that −94 deletion
variant had a significantly reduced risk for the gastric
carcinogenesis in China. For hepatocarcinogenesis, He et al
(31) also showed that −94
deletion mutant had a reduced risk under the influence of hepatitis
B virus infection in China. Contrary to these results, several
studies have showed that −94 deletion variant is associated with
increased risk for the development of inflammatory or auto-immune
diseases in Caucasian (28,29,32). In colorectal carcinogenesis,
Andersen et al (33) have
showed that carriers of NFKB1 −94 deletion were at 1.45-fold
higher risk than homozygous carriers of the insertion allele. On
the other hand, the lack of an association between the NFKB1
−94 ins/del polymorphism and the inflammatory or autoimmune
diseases has also been reported (34–37). These contrasting observations may
be explained by differences in the genotypic composition of
populations in different countries with different racial groups. In
fact, the frequency of −94 deletion allele seems to be rather
higher in Chinese healthy subjects (45–55%). However, in our study
of Japanese subjects, the frequency was ∼38%, similar to the value
in Caucasians. Our study as well as the Caucasian study indicate
that the −94 deletion variant may be an inflammation promoting
allele.
NF-κB encompasses a number of different
transcription factors that are homo- or heterodimers of p65, p50,
p105, c-Rel and RelB (38). NF-κB
is involved in both inflammatory and anti-inflammatory process
(39). The role of NF-κB in
inflammation is determined by the subunit type. NFKB1
encodes both the subunits p105 and p50 of the transcription factor
NF-κB by alternative splicing (40). As part of the p65/p50 NF-κB
transcription factor complex, it is pro-inflammatory, controlling
transcription of pro-inflammatory cytokines (41). Conversely, since p50 lacks this
COOH-terminal transactivation domain which is necessary for the
positive regulation of gene expression, p50 has anti-inflammatory
properties in the p50 homodimer by repressing transcription
(42). The relative abundance of
p65/p50 heterodimers and p50 homodimers may determine the magnitude
of inflammation by balancing the pro-inflammatory and
anti-inflammatory response (38).
In fact, p50-deficient mice have an increased sensitivity to
lipopolysaccharide (LPS) and have increased LPS-induced
inflammation (43,44). In subjects with the del/del
genotype, decreased p50 synthesis may lead to decreased repressive
homodimers and increased active heterodimers of the NF-κB complex.
This balance may promote the H. pylori-induced inflammation,
resulting in hypermethylation of genes.
In current study, 66 of 68 del/del homozygotes had
the −449 GG genotype and, all of 39 del/del homozygotes had the
−449 GG genotype in H. pylori-infected subjects. Therefore,
we suspect that −94 ins/del ATTG polymorphism may mainly regulate
the expression and function of NF-κB. From our results in this
study, we could not show the role of −449 C>G polymorphism. Our
results showed that, in −94 del/del homozygote, gastric
inflammation was more severe and gene methylation was promoted over
60-year-old under influence of H. pylori infection. Because
NF-κB is activated by some stimulation such as infection and
stress, it is reasonable that NFKB1 polymorphism is
associated with the gastric inflammation process under H.
pylori infection. In addition, it is also reasonable that
increased gene methylation is revealed in elder subjects, because
gene methylation gradually progresses with age and accumulates for
a long time. Interestingly, although gastric inflammation was more
severe in −94 del/del homozygote, atrophy and metaplasia scores
were not different among del/del homozygote and ins carrier. This
reason is unclear. The decreased p50 production may affect the
action of not only the p50 homodimer but also of the p65/p50
heterodimer.
In conclusion, the NFKB1 −94 ins/del ATTG
polymorphism (rs28362491) was significantly associated with an
increased risk for the development of age related-gene methylations
in non-cancerous gastric mucosa under H. pylori-induced
inflammation. The −94 del/del homozygote may have an increased risk
for the development of age-related and inflammation-induced gene
methylation, as a precancerous condition, in gastric mucosa.
References
|
1.
|
P CorreaHuman gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and PreventionCancer
Res52673567401992
|
|
2.
|
S KiechlE LorenzM ReindlToll-like receptor
4 polymorphisms and atherogenesisN Engl J
Med347185192200210.1056/NEJMoa01267312124407
|
|
3.
|
K HoshinoO TakeuchiT KawaiCutting edge:
Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to
lipopolysaccharide: evidence for TLR4 as the Lps gene productJ
Immunol16237493752199910201887
|
|
4.
|
S KeatesYS HittiM UptonCP
KellyHelicobacter pylori infection activates NF-kappa B in
gastric epithelial
cellsGastroenterology11310991109199710.1053/gast.1997.v113.pm9322504
|
|
5.
|
RA HatzG RiederM StoltePattern of adhesion
molecule expression on vascular endothelium in Helicobacter
pylori associated antral
gastritisGastroenterology11219081919199710.1053/gast.1997.v112.pm91786839178683
|
|
6.
|
S MaedaH YoshidaK OguraH. pylori
activates NF-kappaB through a signaling pathway involving IkappaB
kinases, NF-kappaB-inducing kinase, TRAF2, and TRAF6 in gastric
cancer cellsGastroenterology11997108200010.1053/gast.2000.8540
|
|
7.
|
MG SmithGL HoldE TaharaEM El-OmarCellular
and molecular aspects of gastric cancerWorld J
Gastroenterol12297929902006
|
|
8.
|
J ParsonnetGD FriedmanDP
VandersteenHelicobacter pylori infection and the risk of
gastric carcinomaN Engl J
Med32511271131199110.1056/NEJM199110173251603
|
|
9.
|
MJ BlaserJ ParsonnetParasitism by the
‘slow’ bacterium Helicobacter pylori leads to altered
gastric homeostasis and neoplasiaJ Clin Invest94481994
|
|
10.
|
JQ HuangS SridharY ChenRH
HuntMeta-analysis of the relationship between Helicobacter
pylori seropositivity and gastric
cancerGastroenterology11411691179199810.1016/S0016-5085(98)70422-69609753
|
|
11.
|
M EstellerPG CornSB BaylinJG HermanA gene
hypermethylation profile of human cancerCancer
Res6132253229200111309270
|
|
12.
|
T TaharaT ShibataM NakamuraIncreased
number of CpG island hypermethylation in tumor suppressor genes of
nonneoplastic gastric mucosa correlates with higher risk of gastric
cancerDigestion822736201010.1159/00025276620150736
|
|
13.
|
JP IssaYL OttavianoP CelanoSR HamiltonNE
DavidsonSB BaylinMethylation of the oestrogen receptor CpG island
links ageing and neoplasia in human colonNat
Genet4536540199410.1038/ng0894-5367951326
|
|
14.
|
N AhujaQ LiAL MohanSB BaylinJP IssaAging
and DNA methylation in colorectal mucosa and cancerCancer
Res235489549419989850084
|
|
15.
|
JP IssaN AhujaM ToyotaMP BronnerTA
BrentnallAccelerated age-related CpG island methylation in
ulcerative colitisCancer Res6135733577200111325821
|
|
16.
|
YS BianMC OsterheldC FontollietFT BosmanJ
Benhattarp16 inactivation by methylation of the CDKN2A promoter
occurs early during neoplastic progression in Barrett’s
esophagusGastroenterology122111311212001
|
|
17.
|
T MaekitaK NakazawaM MiharaHigh levels of
aberrant DNA methylation in Helicobacter pylori-infected
gastric mucosae and its possible association with gastric cancer
riskClin Cancer Res12989995200616467114
|
|
18.
|
T TaharaT ArisawaT SibataRisk prediction
of gastric cancer by analysis of aberrant DNA methylation in
non-neoplastic gastric
epitheliumDigestion755461200710.1159/00010177517438355
|
|
19.
|
GH KangHJ LeeKS HwangS LeeJH KimJS
KimAberrant CpG island hypermethylation of chronic gastritis, in
relation to aging, gender, intestinal metaplasia, and chronic
inflammationAm J
Pathol16315511556200310.1016/S0002-9440(10)63511-014507661
|
|
20.
|
GH KangS LeeJS LimHY JungProfile of
aberrant CpG island methylation along the multistep pathway of
gastric carcinogenesisLab
Invest83635641200310.1097/01.LAB.0000067481.08984.3F12746473
|
|
21.
|
YF ZouF WangXL FengAssociation of
NFKB1 −94ins/delATTG promoter polymorphism with
susceptibility to auto-immune and inflammatory diseases: a
meta-analysisTissue Antigens779172010
|
|
22.
|
YF ZouFL YuanXL FengAssociation between
NFKB1 −94ins/delATTG promoter polymorphism and cancer risk:
a meta-analysisCancer Invest2978852010
|
|
23.
|
T TaharaT ArisawaT ShibataIncreased number
of methylated CpG islands correlates with Helicobacter
pylori infection, histological and serological severity of
chronic gastritisEur J Gastroenterol
Hepatol21613619200910.1097/MEG.0b013e32830e28b219307977
|
|
24.
|
T TaharaT ShibataT ArisawaImpact of
catechol-O-methyltransferase (COMT) gene polymorphism on promoter
methylation status in gastric mucosaAnticancer
Res2928572861200919596974
|
|
25.
|
T ArisawaT TaharaT ShibataThe relationship
between Helicobacter pylori infection and promoter
polymorphism of the Nrf2 gene in chronic gastritisInt J Mol
Med191431482007
|
|
26.
|
H ShiroedaT TaharaT ShibataFunctional
promoter polymorphisms of macrophage migration inhibitory factor
(MIF) in peptic ulcer diseasesInt J Mol Med26707711201020878093
|
|
27.
|
MF DixonRM GentaJH YardleyP
CorreaClassification and grading of gastritis: the updated Sydney
systemAm J Surg
Pathol2011611181199610.1097/00000478-199610000-000018827022
|
|
28.
|
AS KarbanT OkazakiCI PanhuysenFunctional
annotation of a novel NFKB1 promoter polymorphism that
increases risk for ulcerative colitisHum Mol
Genet133545200414613970
|
|
29.
|
H LiL GaoZ ShenAssociation study of
NFKB1 and SUMO4 polymorphisms in Chinese patients with
psoriasis vulgarisArch Dermatol Res3004254332008
|
|
30.
|
SS LoJH ChenCW WuWY LuiFunctional
polymorphism of NFKB1 promoter may correlate to the
susceptibility of gastric cancer in aged
patientsSurgery1452802852009
|
|
31.
|
Y HeH ZhangJ YinIκBα gene promoter
polymorphisms are associated with hepatocarcinogenesis in patients
infected with hepatitis B virus genotype
CCarcinogenesis30191619222009
|
|
32.
|
A KurylowiczY HiromatsuB
Jurecka-LubienieckaAssociation of NFKB1 −94ins/del ATTG
promoter polymorphism with susceptibility to and phenotype of
Graves’ diseaseGenes Immun85325382007
|
|
33.
|
V AndersenJ ChristensenK OvervadA
TjønnelandU VogelPolymorphisms in NFκB, PXR, LXR and risk of
colorectal cancer in a prospective study of DanesBMC
Cancer104842010
|
|
34.
|
A MartínezE SánchezA ValdiviaEpistatic
interaction between FCRL3 and NFkappaB1 genes in Spanish patients
with rheumatoid arthritisAnn Rheum Dis6511881191200616476711
|
|
35.
|
MM MirzaSA FisherC OnnieNo association of
the NFKB1 promoter polymorphism with ulcerative colitis in a
British case control cohortGut5412051206200516009698
|
|
36.
|
J GlasHP TörökL TonenchiRole of the
NFKB1 −94ins/delATTG promoter polymorphism in IBD and
potential interactions with polymorphisms in the CARD15/NOD2, IKBL,
and IL-1RN genesInflamm Bowel Dis126066112006
|
|
37.
|
EK BajwaPC CremerMN GongAn NFKB1
promoter insertion/deletion polymorphism influences risk and
outcome in acute respiratory distress syndrome among CaucasiansPLoS
One6e19469201121573030
|
|
38.
|
SG PereiraF OakleyNuclear factor-kappaB1:
regulation and functionInt J Biochem Cell
Biol4014251430200810.1016/j.biocel.2007.05.00417693123
|
|
39.
|
MP de WintherE KantersG KraalMH
HofkerNuclear factor kappaB signaling in atherogenesisArterioscler
Thromb Vasc Biol25904914200515731497
|
|
40.
|
L LinGN DeMartinoWC GreeneCotranslational
biogenesis of NF-kappaB p50 by the 26S
proteasomeCell92819828199810.1016/S0092-8674(00)81409-99529257
|
|
41.
|
ND PerkinsIntegrating cell-signalling
pathways with NF-kappaB and IKK functionNat Rev Mol Cell
Biol84962200710.1038/nrm208317183360
|
|
42.
|
MS HaydenS GhoshShared principles in
NF-kappaB
signalingCell132344362200810.1016/j.cell.2008.01.02018267068
|
|
43.
|
M GadjevaMF TomczakM ZhangA role for
NF-kappaB subunits p50 and p65 in the inhibition of
lipopolysaccharide-induced shockJ
Immunol17357865793200410.4049/jimmunol.173.9.578615494531
|
|
44.
|
W HanM JooMB EverhartMyeloid cells control
termination of lung inflammation through the NF-kappaB pathwayAm J
Physiol Lung Cell Mol
Physiol296L320L327200910.1152/ajplung.90485.200819098124
|