Introduction
At the time of the East Japan earthquake disaster of
March 2011, a large quantity of radioactive material was scattered
by the accident at the Fukushima Daiichi nuclear power plant, and
workers and inhabitants were exposed to radiation (1–3).
Acute radiation syndrome (ARS) is caused by exposure to a high dose
of ionizing radiation within several months (4,5).
Generally, the effects of ARS such as gastrointestinal disturbances
and blood and bone marrow disorders are known to occur within
several hours to several weeks after 1–6 Gy of radiation exposure
(4,6).
Hair loss is also an effect of ARS, but little is
known about the mechanism underlying radiation-induced hair loss.
In humans, hair loss is caused by radiation of more than 3 Gy, and
almost complete hair loss occurs within weeks of exposure to 6 Gy
(4,6). Since blood stem cells are sensitive
to radiation (7), hair loss is
thought to be caused by irradiation-induced stem cell damage, yet
no studies have investigated this hypothesis. Additionally, as the
skin and hair are the parts of the body most affected by radiation,
skin and hair proteins may be biological markers for ARS.
Hair stem cells and many types of keratin protein
are present in hair follicles (8–10).
Keratin is an intermediate filament expressed region-specifically
in epidermal cells, and many researchers have focused on
cytokeratin as an epidermal cell differentiation marker. The large
keratin multigene family comprises cytokeratins, which are
differentially expressed in various types of epithelia, and hair
keratins expressed in hard keratinized structures such as hairs,
nails, and claws. These keratins can be divided into acidic type I
and basic-to-neutral type II members, which form the 10-nm
intermediate filament network through the obligatory association of
equimolar amounts of type I and type II keratins. Cytokeratins are
known to assemble into strong networks that help attach
keratinocytes together and anchor the epidermis to underlying
layers of skin (11–13). Cytokeratins are also very useful
as differentiation markers of cornified cells in the epidermis.
Keratin (Krt)15 is a type I keratin, expressed in mouse bulge cells
in the basal layer of the outer root sheath, and is known as a hair
follicle stem cell marker (14–17). CD34 is expressed in a variety of
stromal cells such as vascular endothelial, dermal dendritic and
epithelial stem cells (14,18–20).
A basal layer of cells undergoes proliferation in
the epithelial tissue of the skin, differentiates to form spinous
cells and granule cells, and then cornifies and falls off. Stem
cells remain throughout life in the basal layer as the ongoing
source of epidermal renewal. Basal cells and spinous cells are well
known to be highly susceptible to the effects of radiation, and
cell division stops in cells affected by radiation (21).
Krt1 is specifically expressed in the spinous layer
of the epidermis along with Krt10, and Krt5 and Krt14 are expressed
in undifferentiated keratinocytes and are downregulated during
differentiation of the basal layer (22–24).
In this study, we investigated the mechanism of
radiation-induced alopecia by focusing on alterations of these
proteins in the hair and skin of irradiated mice, and we
hypothesized that proteins such as keratins may be novel biological
markers for ARS.
Materials and methods
Animals
Male C57/BL6 mice (Clea, Tokyo, Japan) 4 weeks of
age, were housed in plastic cages in air-conditioned rooms with a
12 h light/dark cycle, and had free access to water and food. This
study was carried out in accordance with the Guidelines for Animal
Experimentation, Hirosaki University.
Exposure conditions
Five mice were irradiated with X-rays (150 kV, 20
mA) using 0.5-mm aluminum and 0.3-mm copper filters at a distance
of 45 cm from the focus at a dose of 6 Gy administered at a rate of
1.1 Gy/min (MBR-1520R-3; X-ray generator; Hitachi Medical
Corporation, Tokyo, Japan).
Histological analysis and
immunohistochemistry
For whole-mount examination, skin and hair were
observed under a microscope (Biomedical Science, Tokyo, Japan). For
histological examination, skin tissues were fixed in 10%
formaldehyde and embedded in paraffin. Tissue sections (4 μm) were
passed through xylene and a graded alcohol series and stained with
hematoxylin and eosin (H&E). Immunohistochemical staining was
performed with the Vectastain ABC-AP kit (Vector Laboratories,
Inc., Burlingame, CA). The following primary antibodies were used:
rabbit anti-Krt1 (1:500, v/v; Applied Biological Materials, Inc.),
rabbit anti-Krt5 (1:500, v/v; Epitomics), rabbit anti-Krt10 (1:500,
v/v; Assay Biotech), mouse anti-Krt15 (1:500, v/v; Santa Cruz
Biotechnology, Inc.), and rat anti-CD34 (1:100, v/v; Abcam).
Sections were then lightly counterstained with hematoxylin for
microscopic examination. The specimens were examined and
photographed using a fluorescence microscope (FSX100; Olympus,
Tokyo, Japan).
SDS-polyacrylamide gel electrophoresis
(PAGE) and western blotting
Hair and skin proteins were extracted from the hairs
of mice, as described by Winter et al (25). Western blotting was performed
according to the method of Towbin et al (26). In brief, extracted hair proteins
were separated by SDS-PAGE (27)
on 7.5% (w/v) polyacrylamide gels and electroblotted to Hybond
nitrocellulose membranes (GE Healthcare). Blots were probed with
the primary antibody as described in ‘Histological analysis and
immunohistochemistry’ followed by horseradish peroxidase-conjugated
goat anti-rabbit IgG (1:2,000, v/v; GE Healthcare). Signals were
detected with an ECL kit (GE Healthcare) according to the
manufacturer’s protocol.
Two-dimensional electrophoresis
(2-DE)
The hair proteins from alopecic mice were separated
on a series of 7-cm pH 5.0–8.0 immobilized pH gradient strips. The
electrophoretic separation of protein was performed as described by
O’Farrell (28). The 2-DE was
carried out using Protean® IEF gels (Bio-Rad), according
to the manufacturer’s protocol. The gels were stained with
Coomassie Brilliant Blue R-250.
To purify hair proteins, the gel portions containing
each mouse hair protein resolved by 2-DE were cut with a razor,
homogenized in 1% SDS, 20 mM Tris-HCl (pH 8.0), and then rotated
overnight. After centrifugation at 15,000 x g for 10 min, the
supernatant fractions were dialyzed against distilled water and
then lyophilized.
Liquid
chromatography/electrospray-ionization mass spectrometry
(LC/ESI-MS)
After being reduced with dithiothreitol and
alkylated with iodoacetamide, proteins were dissolved in 100 mM
NH4HCO3 containing 50 ng/μl trypsin and
incubated at 37°C for 16 h. The samples were applied to a Nano
Frontier LC column, C18 (75 μm id x 150 mm; Hitachi High-Tech,
Tokyo, Japan) and eluted by a gradient flow of acetonitrile and
distilled water containing 0.1% formic acid (flow rate 200 nl/min).
The eluted peptide fragments were analyzed using online coupled
linear trap electrospray-ionization mass spectrometry (Nano
Frontier L; Hitachi High-Tech) at a heated capillary temperature of
140°C, and voltage of 1.0 kV. Peptide sequence analysis was
performed using BioLynx software (Micromass). The sequence
information was submitted to the Mascot programs (http://www.matrixscience.com/), and Mascot scores
>34 were considered to indicate the corresponding proteins.
TUNEL assay
Cell death was localized in tissue sections by TUNEL
analysis (29). Paraffin tissue
sections (4-μm) were dewaxed at 60°C for 30 min and washed with
toluene twice for 5 min each. Sections were hydrated through a
graded series of ethanol and PBS and then incubated with proteinase
K (20 μg/ml in PBS) for 30 min at 37°C. Thereafter, the ApopTag
Plus Peroxidase in situ apoptosis detection kit (Intergen
Discovery Products, Purchase, NY) was used for nick-end labeling
according to the manufacturer’s instructions. Fluorescein-linked
nucleotides incorporated into DNA breaks were observed with a
fluorescence microscope (FSX100; Olympus). Postweaning mammary
tissue was included as a positive control.
Results
Whole mount and H&E staining
analysis
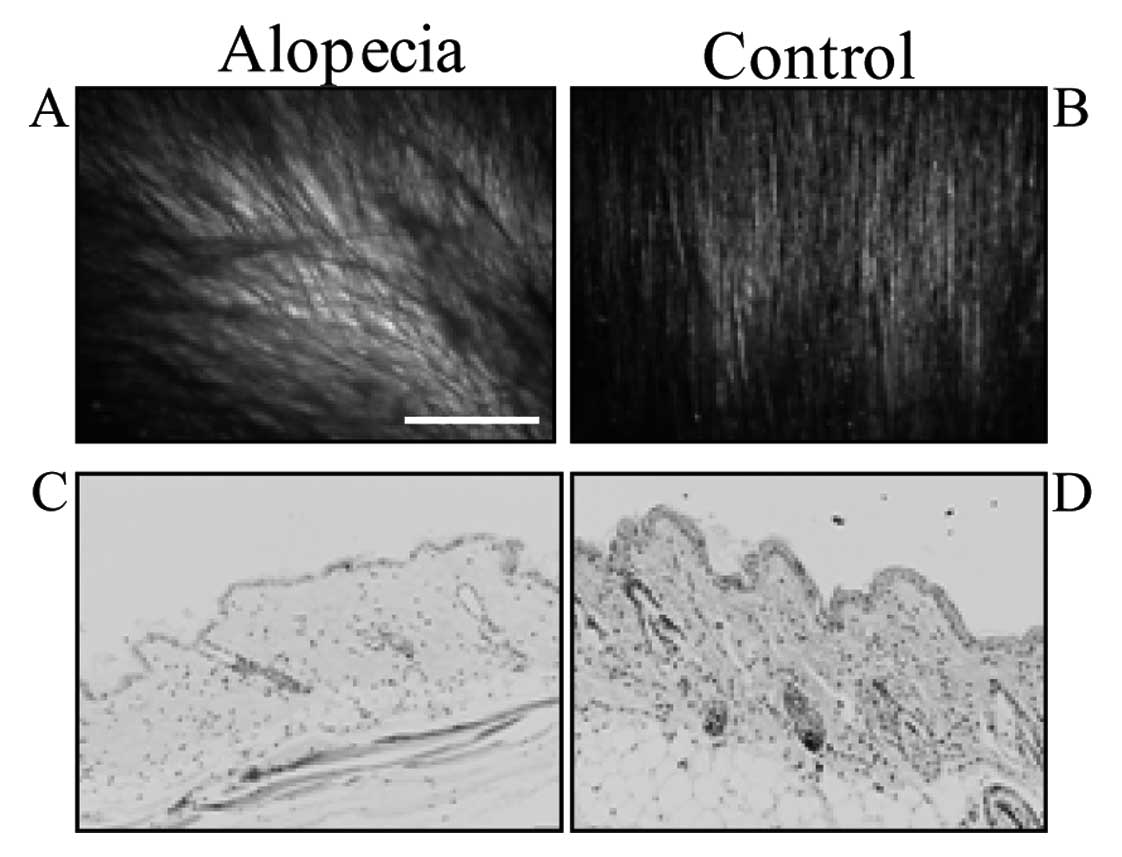
In X-ray irradiated mice, hair density decreased,
and alopecia was induced after 5 weeks (Fig. 1A and B). Some hair became white,
but inflammation was not observed in the skin (Fig. 1C and D).
SDS-PAGE and 2-DE analysis
To examine whether hair protein profiles differed
between control mice and mice with radiation-induced alopecia,
proteins extracted from hairs were subjected to SDS-PAGE and 2-DE.
Protein staining with Coomassie Brilliant Blue revealed 2 major
bands at 51 kDa (basic hair keratin, Hb) and 40 kDa (acidic hair
keratin, Ha) in each mouse (Fig.
2A). The protein band pattern did not differ between control
and alopecia hair. The hair protein spots from alopecic mice were
shifted toward acidic pH (Fig.
2B) on 2-DE. This result suggested that irradiation changed the
isoelectric point (PI) of proteins. To identify the proteins, gels
were cut at 3 spots (X1-3) for the alopecia samples and 6 spots
(C1-6) in the control mice, and subjected to LC/ESI-MS analysis
(Fig. 2B).
Protein profiling by LC/ESI-MS
analysis
The samples recovered from SDS gels were digested
with trypsin, and their fragments were subjected to LC/ESI-MS
analysis. This analysis revealed that most hair proteins were hair
keratins (Table I) such as Krt86,
Krt81, and Krt83 in the spots C1–3 and X1–2 from both groups
(Fig. 2B). The composition of
major hair keratins demonstrated no change, but the expression of
Krt15, a marker of hair follicle stem cells, was detected in the
control mice (Fig. 2B, spot C1)
but not in the mice with alopecia. The absence of Krt15 expression
in the alopecic mice, suggests that their hair stem cells were
damaged. Krt10 was detected in X1, and Krt1 and Krt5b were detected
in the X2 spot (Fig. 2B) from the
hair of alopecic mice (Table I).
Since Krt1, Krt5 and Krt10 are known as epidermal markers, we
focused on these cytokeratins in our immunohistochemical analysis.
Furthermore, many additional proteins such as vimentin and tubulin
were decreased in the hair of alopecic mice (Table I).
 | Table I.Identification of hair-derived
proteins by LC/ESI-MS. |
Table I.
Identification of hair-derived
proteins by LC/ESI-MS.
| Protein | Gene | Score |
|---|
| Alopecia | | |
| aKeratin, type II cuticular
Hb6 | Krt86 | 3580a |
| aKeratin, type II cuticular
Hb3 | Krt83 | 3316 |
| aKeratin, type II cuticular
Hb1 | Krt81 | 2770 |
| aKeratin, type II cuticular
Hb5 | Krt85 | 2481 |
| aKeratin, type I cuticular
Ha3-I | Krt33a | 1892 |
| aKeratin, type I cuticular
Ha4 | Krt34 | 1687 |
| aKeratin, type I cuticular
Ha1 | Krt31 | 1631 |
| aKeratin, type I cuticular
Ha3-II | Krt33b | 1625 |
| Keratin, type I
cuticular Ha5 | Krt35 | 236 |
| Vimentin | Vim | 196 |
| Keratin, type II
cytoskeletal 5 | Krt5b | 141 |
| Keratin, type II
cytoskeletal 1 |
Krt1 | 116 |
| Keratin, type I
cytoskeletal 10 |
Krt10 | 108 |
| Keratin, type II
cytoskeletal 1b | Krt77 | 65 |
| Keratin, type I
cytoskeletal 14 | Krt14 | 65 |
| Tubulin α-1B
chain | Tuba1b | 63 |
| Keratin, type II
cytoskeletal 6B | Krt6b | 54 |
| Keratin, type II
cytoskeletal 5 | Krt5a | 48 |
| Keratin, type II
cytoskeletal 79 | Krt79 | 40 |
|
| Control | | |
| aKeratin, type II cuticular
Hb6 | Krt86 | 4894 |
| aKeratin, type II cuticular
Hb1 | Krt81 | 4709 |
| aKeratin, type II cuticular
Hb3 | Krt83 | 4620 |
| aKeratin, type I cuticular
Ha3-I | Krt33a | 3449 |
| aKeratin, type I cuticular
Ha4 | Krt34 | 3404 |
| aKeratin, type I cuticular
Ha1 | Krt31 | 3360 |
| aKeratin, type I cuticular
Ha3-II | Krt33b | 3144 |
| aKeratin, type II cuticular
Hb5 | Krt85 | 2964 |
| Actin, cytoplasmic
1 | Actb | 979 |
| Tubulin α-1B
chain | Tuba1b | 502 |
| Tubulin β-5
chain | Tubb5 | 400 |
| Keratin, type I
cuticular Ha5 | Krt35 | 384 |
| Vimentin | Vim | 374 |
| Keratin, type II
cytoskeletal 75 | Krt75 | 309 |
| Elongation factor
1-α 1 | Eefla1 | 205 |
| α-enolase | Eno1 | 193 |
| Keratin, type I
cytoskeletal 15 |
Krt15 | 129 |
| Keratin, type II
cytoskeletal 79 | Krt79 | 91 |
| Keratin, type I
cytoskeletal 17 | Krt17 | 86 |
|
Fructose-bisphosphate aldolase A | Aldoa | 83 |
| Keratin, type II
cytoskeletal 72 | Krt72 | 78 |
| Phosphoglycerate
kinase 1 | Pgk1 | 76 |
| Heterogeneous
nuclear ribonucleoprotein K | Hnrnpk | 67 |
| Keratin, type II
cytoskeletal 8 | Krt8 | 57 |
| Calpain-12 | Capn12 | 57 |
| Pyruvate kinase
isozymes M1/M2 | Pkm2 | 54 |
| Aldehyde
dehydrogenase, cytosolic 1 | Aldh1a7 | 51 |
| Glycine
tyrosine-rich hair keratin protein |
Krtap6-1 | 50 |
| Keratin, type II
cytoskeletal 71 | Krt71 | 43 |
| Protein
disulfide-isomerase | P4hb | 41 |
Immunohistochemistry
We next examined whether hair stem cells were
damaged after irradiation using immunohistochemistry to detect
CD34, a marker of hair follicle stem cells, as well as Krt15. With
the anti-Krt15 antibody, the bulge and outer root sheath in the
hair follicle of control mice showed a positive reaction, while the
staining of hair follicles from irradiated mice was negative
(Fig. 3A and B). Staining with
the anti-CD34 antibody revealed a positive reaction in the
peripheral layer of the outer root sheath (Fig. 3C and D). These positive cells were
putative hair follicle stem cells. Krt1 and Krt10 were expressed in
the spinous layer of epidermis, and levels did not differ between
alopecic and control mice (Fig.
3E–H). Krt5 expression was clearly increased in the basal layer
of alopecic mice (Fig. 3I and J).
Krt5 consists of subtypes Krt5a and b, and the anti-Krt5 antibody
used in this study detected both subtypes.
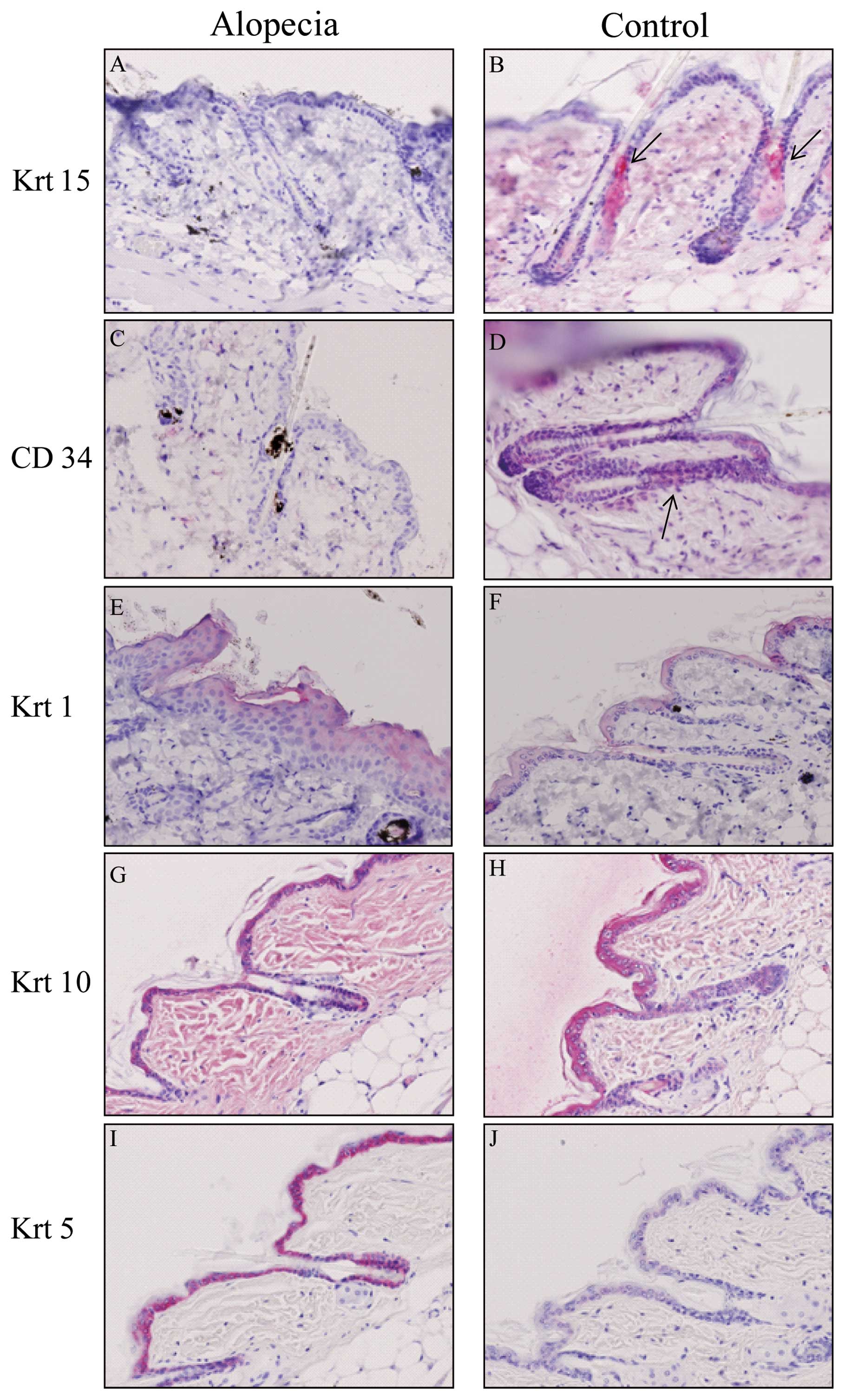 | Figure 3.Immunohistochemical staining of skin.
Skin sections of (A, C, E, G and I) alopecic and (B, D, F, H and J)
control mice were stained with (A and B) anti-Krt15, (C and D)
anti-CD34, (E and F) anti-Krt1, (G and H) anti-Krt5 and (I and J)
anti-Krt10. The arrows in B and D indicate Krt15- and CD34-positive
cells, respectively. Original magnification, x200. |
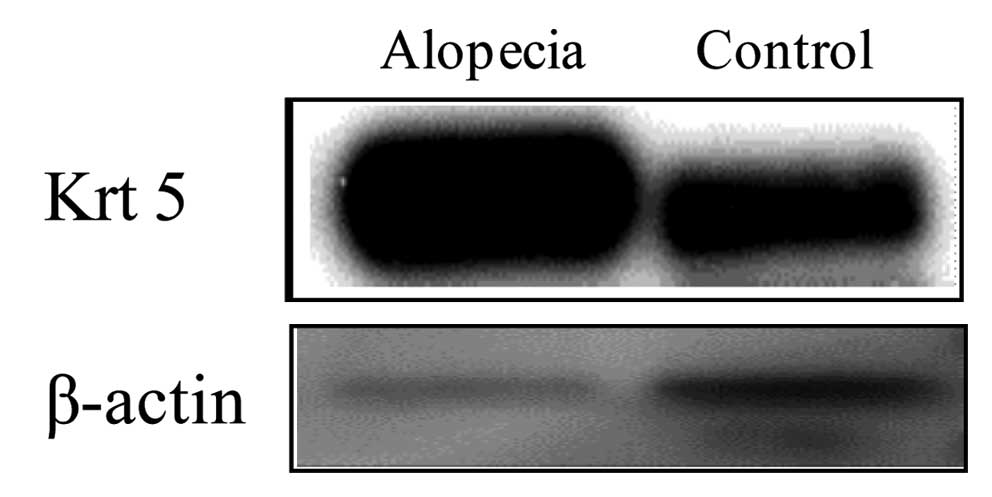
Western blot analysis
As expression of Kr5 was increased in alopecic mice
using immunohistochemistry, to examine whether it could be used as
a novel biomarker, we performed western blotting to analyze its
expression. Krt5 was increased in alopecic mice, suggesting that it
may be a useful novel biological marker for acute radiation
symptoms (Fig. 4).
Discussion
Acute alopecia is a symptom of ARS, and although
hair loss is thought to occur due to damage of hair follicle stem
cells no previous study has investigated this hypothesis. In
addition, no previous study has focused on the alteration of hair
proteins such as keratins in radiation-induced alopecia. Therefore,
we examined the mechanism of radiation-induced hair loss and
profiling of hair proteins. When mice were irradiated with 6 Gy,
hair loss began after 5 weeks. As hair loss usually depends on
apoptosis (30–32), we examined whether apoptotic cells
were increased in alopecic mice by TUNEL assay. However,
TUNEL-positive cells were not detected in alopecic mice, suggesting
that alopecia was caused by necrosis of hair follicle stem cells in
this study (data not shown).
To examine whether hair protein profiles differed
between control and radiation-induced alopecic mice, hair proteins
were extracted and subjected to SDS-PAGE and 2-DE for analysis of
molecular mass and PI. Although the molecular mass did not differ,
the hair protein spots from the alopecic mice were shifted toward
an acidic pH (Fig. 2B). This
result suggested that irradiation altered the PI of the proteins.
Protein modifications such as phosphorylation or glycosylation are
known to alter PI (33). To
examine whether phosphorylation of these protein was modified by
radiation, western blotting was performed with anti-phosphotyrosine
and anti-phosphoserine antibodies. However, phosphorylation was not
detected in hair proteins from the control or irradiated mice,
suggesting that these proteins undergo another modification (data
not shown).
With regard to LC/ESI-MS analysis and
immunohistochemical staining, Krt15 and CD34 were decreased in the
hair follicles of irradiated mice but not in the control mice
(Table I and Fig. 3). These results suggest that
damaged hair follicle stem cells could not differentiate normally,
leading to hair loss in mice. Since vimentin is a mesenchymal cell
marker, similar to CD34, our findings suggested damage to
mesenchymal cells in alopecic mice (Table I). The hair keratin score did not
change. As hair keratin is differentiated dead material, it may be
less affected by radiation. Cytokeratins such as Krt5 were
increased in hair protein from irradiated mice (Table I and Fig. 4). Since expression of Krt5
increases in the basal layer of the epidermis, keratinocytes may
lose differentiation potential as a result of X-ray irradiation in
alopecic mice.
Furthermore, after irradiation with a radioactivity
dose of 6 Gy, hair loss occurred, but expression of cytokeratins
such as Krt1 and Krt10 increased as determined by LC/ESI-MS
analysis. However, we could not detect differences between alopecic
mice and control mice by immunohistochemistry. LC/ESI-MS analysis
is a much more sensitive method compared to immunohistochemistry or
western blotting, and therefore LC/ESI-MS analysis may have been
able to reveal the increase in Krt1 and Krt10 in alopecic mice.
This result suggested that Krt1 and Krt10 are not suitable for use
as biological markers for acute radiation symptoms.
Tubulin is known to form microtubules and the
centrosome and is an important protein in the differentiation of
cells including keratinocytes (34). Both α and β-tubulin decreased in
alopecic mice (Table I),
suggesting that cell proliferation of keratinocyte was decreased in
alopecic mice. These proteins may be associated with alopecia, but
the details of this possible association remain to be
clarified.
For the evaluation of radiation exposure,
chromosomal aberration is considered the gold standard, yet this
method requires technical expertise. There is a method to measure
free radical production in biological tissues such as teeth and
hair, in order to estimate the radiation exposure dose (35,36). Electron spin resonance (ESR) is
used to measure free radicals, and this method has been tested in
hair (35). However, while the
ESR method has been reported as an irradiation evaluation method
using hair for biological dosimetry, no previous reports have
analyzed hair proteins by proteomics. The findings of this study
suggest that Krt5 is a novel biological marker of ARS. Further
studies are needed to examine whether expression of Krt5 is altered
by differences in the period after irradiation, by the dose of
radioactivity, and due to differences in skin organization between
the mouse and human.
Acknowledgements
This study was supported in part by
grants-in-aid for scientific research from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (nos.
20770096 and 22770119) and a Hirosaki University Grant for the
Exploratory Research by Young Scientists.
References
|
1.
|
K Hirose2011Fukushima Dai-ichi nuclear
power plant accident: summary of regional radioactive deposition
monitoring resultsJ Environ RadioactNov262011(Epub ahead of
print)
|
|
2.
|
S MonzenM HosodaS TokonamiIndividual
radiation exposure dose due to support activities at safe shelters
in Fukushima prefecturePLoS
One6e27761201110.1371/journal.pone.002776122114685
|
|
3.
|
T TanimotoN UchidaY KodamaT TeshimaS
TaniguchiSafety of workers at the Fukushima Daiichi nuclear power
plantLancet37714891490201110.1016/S0140-6736(11)60519-921497897
|
|
4.
|
EH DonnellyJB NemhauserJM SmithAcute
radiation syndrome: assessment and managementSouth Med
J103541546201010.1097/SMJ.0b013e3181ddd57120710137
|
|
5.
|
M XiaoMH WhitnallPharmacological
countermeasures for the acute radiation syndromeCurr Mol
Pharmacol2122133200910.2174/187446721090201012220021452
|
|
6.
|
DO StramS MizunoAnalysis of the DS86
atomic bomb radiation dosimetry methods using data on severe
epilationRadiat Res11793113198910.2307/35772802913611
|
|
7.
|
P MauchL ConstineJ
GreenbergerHematopoietic stem cell compartment: acute and late
effects of radiation therapy and chemotherapyInt J Radiat Oncol
Biol Phys3113191339199510.1016/0360-3016(94)00430-S7713791
|
|
8.
|
L LangbeinMA RogersH WinterS PraetzelJ
SchweizerThe catalog of human hair keratins. II Expression of the
six type II members in the hair follicle and the combined catalog
of human type I and II keratinsJ Biol
Chem2763512335132200110.1074/jbc.M10330520011445569
|
|
9.
|
L LangbeinJ SchweizerKeratins of the human
hair follicleInt Rev
Cytol243178200510.1016/S0074-7696(05)43001-615797458
|
|
10.
|
J SchweizerL LangbeinMA RogersH WinterHair
follicle-specific keratins and their diseasesExp Cell
Res31320102020200710.1016/j.yexcr.2007.02.03217428470
|
|
11.
|
E FuchsThe cytoskeleton and disease:
genetic disorders of intermediate filamentsAnnu Rev
Genet30197231199610.1146/annurev.genet.30.1.1978982454
|
|
12.
|
M HatzfeldK WeberThe coiled coil of in
vitro assembled keratin filaments is a heterodimer of type I and II
keratins: use of site-specific mutagenesis and recombinant protein
expressionJ Cell
Biol11011991210199010.1083/jcb.110.4.11991691189
|
|
13.
|
PM SteinertLN MarekovRD FraserDA
ParryKeratin intermediate filament structure. Crosslinking studies
yield quantitative information on molecular dimensions and
mechanism of assemblyJ Mol Biol2304364521993
|
|
14.
|
G CotsarelisEpithelial stem cells: a
folliculocentric viewJ Invest
Dermatol12614591468200610.1038/sj.jid.570037616778814
|
|
15.
|
DM JihS LyleR ElenitsasDE ElderG
CotsarelisCytokeratin 15 expression in trichoepitheliomas and a
subset of basal cell carcinomas suggests they originate from hair
follicle stem cellsJ Cutan
Pathol26113118199910.1111/j.1600-0560.1999.tb01814.x10235375
|
|
16.
|
Y LiuS LyleZ YangG CotsarelisKeratin 15
promoter targets putative epithelial stem cells in the hair
follicle bulgeJ Invest
Dermatol121963968200310.1046/j.1523-1747.2003.12600.x14708593
|
|
17.
|
S TiedeK BohmN MeierW FunkR PausEndocrine
controls of primary adult human stem cell biology: thyroid hormones
stimulate keratin 15 expression, apoptosis, and differentiation in
human hair follicle epithelial stem cells in situ and in vitroEur J
Cell Biol89769777201010.1016/j.ejcb.2010.06.002
|
|
18.
|
YC HsuHA PasolliE FuchsDynamics between
stem cells, niche, and progeny in the hair
follicleCell14492105201110.1016/j.cell.2010.11.04921215372
|
|
19.
|
BJ NickoloffThe human progenitor cell
antigen (CD34) is localized on endothelial cells, dermal dendritic
cells, and perifollicular cells in formalin-fixed normal skin, and
on proliferating endothelial cells and stromal spindle-shaped cells
in Kaposi’s sarcomaArch Dermatol12752352919912006877
|
|
20.
|
E PobletF JimenezJM GodinezA
Pascual-MartinA IzetaThe immunohistochemical expression of CD34 in
human hair follicles: a comparative study with the bulge marker
CK15Clin Exp
Dermatol31807812200610.1111/j.1365-2230.2006.02255.x16981909
|
|
21.
|
H FukudaRadiation-induced skin
injuriesKaku Igaku402132192003(In Japanese)
|
|
22.
|
H AlamL SehgalST KunduSN DalalMM
VaidyaNovel function of keratins 5 and 14 in proliferation and
differentiation of stratified epithelial cellsMol Biol
Cell2240684078201110.1091/mbc.E10-08-070321900500
|
|
23.
|
E FuchsH GreenChanges in keratin gene
expression during terminal differentiation of the
keratinocyteCell1910331042198010.1016/0092-8674(80)90094-X6155214
|
|
24.
|
R MollM DivoL LangbeinThe human keratins:
biology and pathologyHistochem Cell
Biol129705733200810.1007/s00418-008-0435-618461349
|
|
25.
|
H WinterI HofmannL LangbeinMA RogersJ
SchweizerA splice site mutation in the gene of the human type I
hair keratin hHa1 results in the expression of a tailless keratin
isoformJ Biol
Chem2723234532352199710.1074/jbc.272.51.323459405442
|
|
26.
|
H TowbinT StaehelinJ GordonElectrophoretic
transfer of proteins from polyacrylamide gels to nitrocellulose
sheets: procedure and some applicationsProc Natl Acad Sci
USA7643504354197910.1073/pnas.76.9.4350388439
|
|
27.
|
UK LaemmliCleavage of structural proteins
during the assembly of the head of bacteriophage
T4Nature227680685197010.1038/227680a05432063
|
|
28.
|
PH O’FarrellHigh resolution
two-dimensional electrophoresis of proteinsJ Biol
Chem250400740211975
|
|
29.
|
BJ WaddellS HishehAM DharmarajanPJ
BurtonApoptosis in rat placenta is zone-dependent and stimulated by
glucocorticoidsBiol
Reprod6319131917200010.1095/biolreprod63.6.191311090465
|
|
30.
|
MJ HarriesKC MeyerR PausHair loss as a
result of cutaneous autoimmunity: frontiers in the
immunopathogenesis of primary cicatricial alopeciaAutoimmun
Rev8478483200910.1016/j.autrev.2008.09.003
|
|
31.
|
S HendrixB HandjiskiEM PetersR PausA guide
to assessing damage response pathways of the hair follicle: lessons
from cyclophosphamide-induced alopecia in miceJ Invest
Dermatol1254251200510.1111/j.0022-202X.2005.23787.x15982301
|
|
32.
|
F NakayamaA HagiwaraM KimuraM AkashiT
ImamuraEvaluation of radiation-induced hair follicle apoptosis in
mice and the preventive effects of fibroblast growth factor-1Exp
Dermatol18889892200910.1111/j.1600-0625.2009.00849.x19469896
|
|
33.
|
MR LarsenP RoepstorffMass spectrometric
identification of proteins and characterization of their
post-translational modifications in proteome analysisFresenius J
Anal Chem366677690200010.1007/s00216005156211225779
|
|
34.
|
JY RohSH KeeJW ChoiJH LeeES LeeYS
KimExpression of class II beta-tubulin in non-melanoma cutaneous
tumorsJ Cutan
Pathol34166173200710.1111/j.1600-0560.2006.00583.x17244029
|
|
35.
|
T NakajimaThe use of organic substances as
emergency dosimetersInt J Appl Radiat
Isot3310771084198210.1016/0020-708X(82)90236-86298117
|
|
36.
|
AA RomanyukhaDF RegullaAspects of
retrospective ESR dosimetryAppl Radiat
Isot4712931297199610.1016/S0969-8043(96)00188-19022187
|