Introduction
With the change of life style and diet structure,
obesity has become increasingly prevalent all over the world. The
incidence of obesity is attributable to many interrelated factors,
of which high-calorie intake, high-fat diet and lack of physical
activity are important risk factors. Excess energy is stored as fat
and leads to obesity. Moreover, obesity is closely associated with
the development of many diseases, including hypertension, type 2
diabetes, metabolic syndrome, dyslipidemia. The excessive
accumulation of visceral adipose tissue, especially the
accumulation of abdominal adipose tissue is an important risk
factor of cardiovascular disease.
Endothelial progenitor cells (EPCs) originate from
bone marrow and are progenitor cells which have the capacity to
migrate to the peripheral circulation and to differentiate into
mature endothelial cells. Under the circumstances of vessel
impairment and tissue ischemia, the EPCs in the bone marrow can be
mobilized into the blood circulation and settle in impaired and
ischemic locations. EPCs are then differentiated into mature
endothelial cells and participate in angiogenesis and
re-endothelialization. Therefore, EPCs play an important role in
maintaining the integrity of endothelial structures and the
function of vascular endothelium. EPCs are influenced by many
factors, such as physical activity and smoking (1,2).
Many diseases can cause the decrease in the quantity of EPCs. EPCs
in diabetic patients decrease significantly in quantity with an
impaired restoration capacity of vessels (3–5).
Hyperglycemia, hyperinsulinemia and hyperlipidemia can all lead to
the impairment of EPCs.
Overweight and obesity are closely related to the
incidence and mortality of cardiovascular diseases. According to
the studies, dysfunction of vascular endothelium exists in
overweight and obese populations. Because endothelial damage and
dysfunction are considered to be a major underlying mechanism for
cardiovascular disease, the prompt endothelial repair/regeneration
is very meaningful in maintaining a normal endothelium function and
preventing cardiovascular events (6–9).
Recent studies indicate that the function of circulating EPCs is
impaired in obese individuals. However, the underlying mechanism
remains unclear.
Adipose tissue can secrete many different
adipokines. Studies indicate that abdominal obesity can change the
levels of many adipokines (10–15). Visfatin is an adiponectin
discovered in 2005. The effect of visfatin on the vascular
inflammation in obesity and type 2 diabetes draws more and more
attention. Visfatin possesses many biological functions. The
function of visfatin is intimately correlated with glucose and
lipid metabolism and can be considered a new proinflammatory factor
which modulates the inflammatory processes of atherosclerosis
(16–21). Research shows that there is an
increased visfatin level in diabetic and obese patients (22,23), which is related to vascular
dysfunction (24).
Transcriptional factor nuclear factor-κB (NF-κB) is
a key inflammatory mediator, which can modulate the expression of a
series of factors in the inflammatory processes. It is found that
visfatin can upregulate the expression of NF-κB in human
endothelium in umbilical veins, and lead to endothelial
inflammation (17,25). Based on these findings, we
hypothesized that visfatin is an upstream influential factor
leading to the decrease of EPCs in obese individuals by
upregulating NF-κB in EPCs. Upregulated NF-κB induce inflammation
and apoptosis of EPCs and results in decreased quantities of EPCs.
At present, the relation of visfatin to EPCs is less
investigated.
In the current study, we measured the quantity of
EPCs, serum visfatin and expression of visfatin in visceral adipose
tissue in high-fat-fed obese rats. The correlations of the above
indices were analyzed. To observe the effect of visfatin on EPCs
and to study the possible underlying mechanism, cultured primary
EPCs were incubated with different concentrations of visfatin. The
migration and adhesion ability and the protein expression of NF-κB
in nuclei of EPCs were detected. We hope the present study can
provide a potential new target for intervening with and preventing
the development of vascular diseases in obese individuals.
Materials and methods
Animals
Male Wistar rats, 200–250 g of weight, were obtained
from Hebei Medical University Animal Laboratory. Rats were randomly
divided into two groups: normal control group (NC, n=11) and
high-fat-fed group (HF, n=11). The animals were kept in a
temperature-controlled room (22±1°C) on a 12-h light/dark cycle
with free access to food and water. The NC group animals were fed a
standard lab diet (65.5% calories from carbohydrate, 10.3% calories
from fat, and 22.4% calories from protein; 384 kcal/100 g). The HF
group rats were fed a high-fat diet (20% calories from
carbohydrate, 60% calories from fat, and 20% calories from protein;
502 kcal/100 g; Research Diets, Inc., USA). After a 16-week
feeding, the Lee’s index and body weight were measured. Lee’s index
= body weight(g)1/3 × 1,000/body length(cm). Animal
studies and relative protocols were approved by the Animal Care and
Use Committee at the Hebei Medical University.
Measurement of serum insulin and
visfatin
Serum insulin and visfatin were detected by ELISA
(sensitivity, 0.01 ng/ml and 1 ng/ml, respectively) with an ALISEI
microplate reader (Seac Srl, Italy).
Immunoblotting
Adipose tissue samples were homogenized in ice-cold
lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 10
mM NaP, 100 mM NaF, 2 mM Na orthovanadate, 1 mM EDTA, 1 mM EGTA,
10% glycerol), supplemented with protease inhibitor cocktail
tablets (Roche) and DL-dithiothreitol and solubilized for 30 min at
4°C. Protein samples were then denatured in SDS sample buffer (125
mmol/l Tris-HCl, pH 6.8, 50% glycerol, 2% SDS, 5%
β-mercaptoethanol, and 0.01% bromophenol blue). Equal amounts of
tissue lysates (60 μg protein) were resolved by SDS-PAGE and
immunoblotted with appropriate antibodies against visfatin
(Biovision). Immunolabeled bands were quantitated by densitometry.
To determine the protein contents of NF-κB in nuclei in EPCs,
nuclear protein was extracted from cultured EPCs using a nuclear
protein extraction kit (Applygen Technologies). Tissue lysates (60
μg protein) were resolved by SDS-PAGE and immunoblotted with
appropriate antibodies against NF-κB (Santa Cruz Biotechnology,
Inc.). Immunolabeled bands were quantitated by densitometry.
β-tubulin (Abcam) was used as an internal standard.
Culture of EPCs
Hollow bones of rat legs were prepared by standard
surgical procedures, and whole bone marrow was harvested by
flushing marrow with 500 μl PBS using a syringe with a 20-gauge
needle. Briefly, rat bone marrow mononuclear cells (MNCs) were
isolated from flushing liquid by Ficoll density centrifugation.
Cells were centrifuged for 30 min at room temperature (1,000 rpm)
for 10 min. MNCs were isolated and washed with PBS. MNCs were
resuspended in EGM-2MV medium (Lonza). Six-well or 24-well tissue
culture plates precoated with fibronectin (Solarbio, China) were
seeded at a density of 2x106/ml and cultured in a
humidified incubator. After 48 h of culture, adherent cells were
washed with EGM-2MV, and EGM-2MV medium was added to each well. The
medium was changed daily for 7 days and then every other day until
the first passage. Cells were observed daily under inverted
microscopy.
Determination of EPCs numbers and
cellular characterization
Immunocytochemistry was performed in cultured cells
to detect the expression of CD34 and kinase insert domain receptor
(KDR). CD34 and KDR also termed Flk-1 are surface markers of EPCs.
Briefly, EPCs were fixed in 4% paraformaldehyde in PBS for 20 min,
washed 3 times with PBS, and respectively stained with various EPCs
specific markers: rabbit anti-rat CD34, mouse anti-rat KDR (Boster,
China). The cells were incubated with secondary antibodies (either
anti-mouse or anti-rabbit) and then in third antibody. Cells were
colored with DAB and stained with hematoxylin.
Moreover, EPCs were characterized by cellular uptake
of DiI-labeled acetylated LDL (DiI-acLDL; Molecular Probes, Eugene,
OR, USA) and binding of fluorescein isothiocyanate-conjugated
lectin from Ulex europaeus agglutinin (FITC-Lectin-UEA-1;
Sigma). Briefly, after 10-day culture, Dil-acLDL (2.5 μg/ml) were
added on cells for 4 h and washed for 3 times. After fixing in 2%
paraformaldehyde for 20 min, FITC-Lectin-UEA-1 (10 μg/ml) were
added and cultured for 1 h. The cells were then observed under
laser scanning confocal microscopy. Orange double-stained cells
positive for both DiI-acLDL and FITC-Lectin-UEA-1 were identified
as EPCs. EPCs were counted under laser scanning confocal
microscopy.
Treatment of EPCs by visfatin
incubation
Bone marrow MNCs from male Wistar rats fed a
standard diet were isolated and cultured as described. EPCs were
determined from MNCs and treated with visfatin at different
concentrations (0, 50, 100, 150, 200 ng/ml) for 48 h.
Evaluation of the migration capacity of
EPCs
Single cell suspension were planted in 24-well plate
as a density of 5×104/ml. After 24-h culture, cells were
digested with 0.25% trypsin solution and counted. Culture medium
were added into the lower chamber of a modified Boyden chamber;
2×104/ml of EPCs were suspended in 150 μl of medium and
added into the upper chamber. After 24 h of culture, the unmoved
cells were scratched from the filtration membrane. The migrated
cells were fixed with formalin and stained with hematoxylin. Ten
fields were randomly chosen to count the migrated cells under
inverted microscopy (x400).
Evaluation of adhesion capacity of
EPCs
Single cell suspensions were plated in 24-well
plates at a density of 5×104/ml. After 24-h culture,
cells were digested with 0.25% trypsin solution. Equal numbers of
cells were plated into 96-well plate and incubated for 30 min at
37°C. Unattached cells were washed out. Ten fields were randomly
chosen to count the attached cells under inverted microscopy
(x400).
Statistical analyses
Data are presented as means ± SE. The data were
tested by homogeneity test for variance. The t-test was used for
comparison of normally distributed data with homogenous variance.
The rank sum test was used for comparison of normally distributed
data with heterogenous variance. One-way analysis of variance was
used for comparison of normally distributed data with homogeneous
variance relevant groups. Linear regression was used to detect a
correlation. Differences at P<0.05 were considered to be
statistically significant.
Results
Comparison of baseline characteristics
between NC and HF groups
Lee’s index, body weight (BW), visceral adipose
tissue (VAT), fasting blood glucose (FBG), fasting insulin in serum
(FINS), homeostasis model assessment-insulin resistance (HOMA-IR),
plasma triglyceride (TG), plasma total cholesterol (TC) were all
significantly higher in the HF group than in the NC group (Table I).
 | Table I.Basic characteristic data in rats of
the two groups (mean ± SD). |
Table I.
Basic characteristic data in rats of
the two groups (mean ± SD).
| Index | Lee’s index | BW (g) | VAT (g) | FBG (mmol/l) | FINS (ng/l) | HOMA-IR | TG (mmol/l) | TC (mmol/l) |
|---|
| NC | 296.66±9.01 | 405±18 | 15.45±1.13 | 4.06±0.40 | 2.03±0.56 | 0.37±0.11 | 0.61±0.12 | 0.91±0.13 |
| HF | 310.57±9.52a | 453±36b | 19.45±3.05b | 5.77±0.84b | 3.17±0.87b | 0.83±0.29b | 0.97±0.24b | 1.29±0.20b |
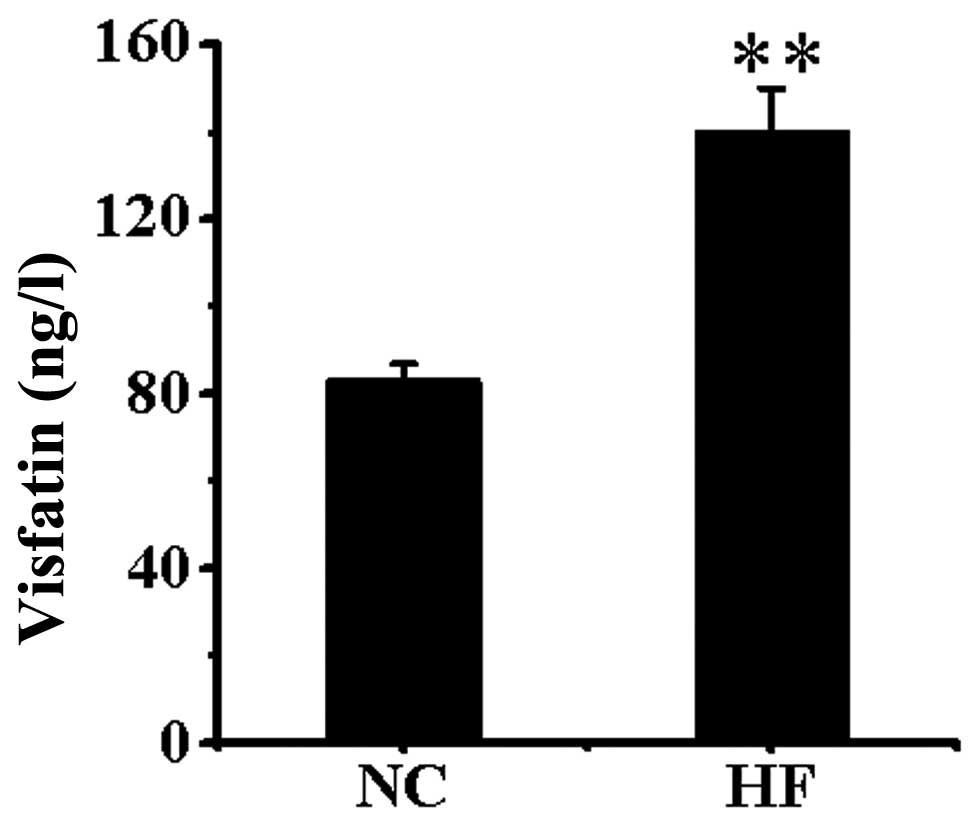
Visfatin level in serum
Serum visfatin was significantly higher in the HF
group than in the NC group (P<0.01) (Fig. 1).
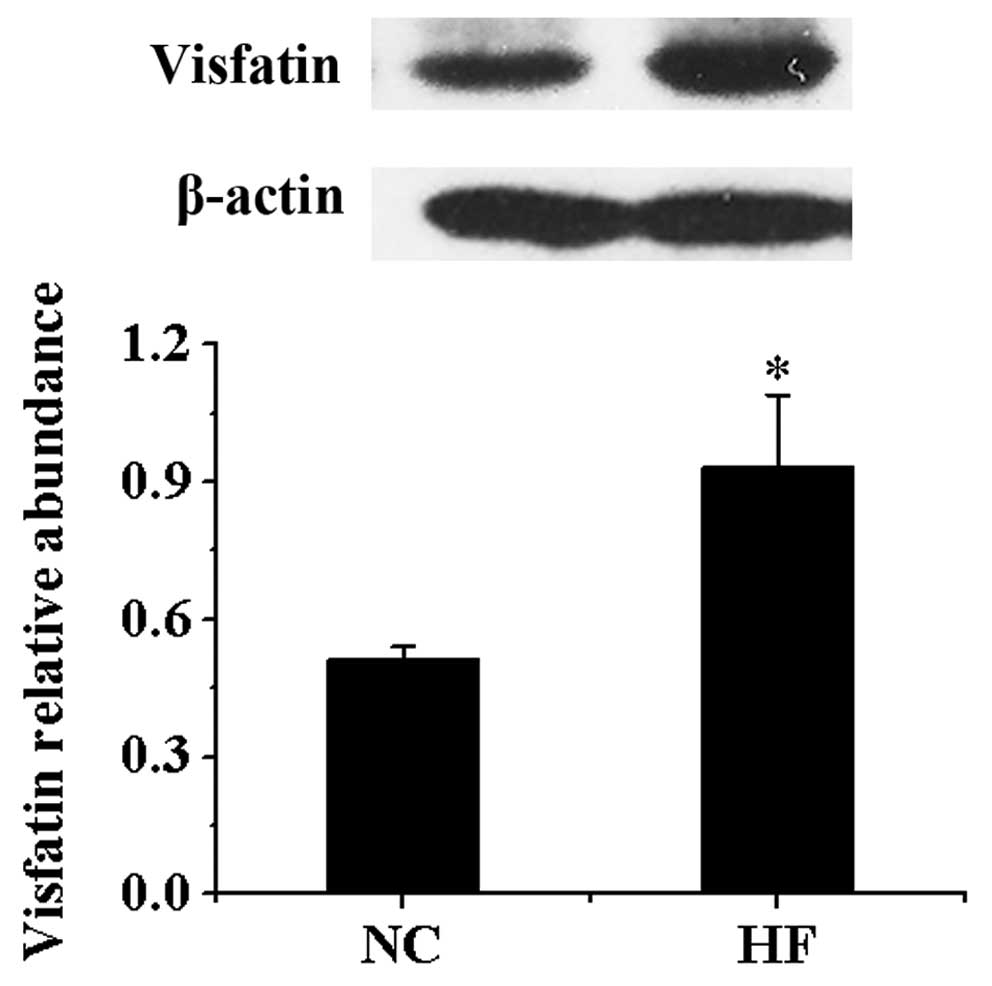
Protein expression of visfatin in
VAT
Compared with NC group, the protein contents of
visfatin in VAT in HF group were significantly higher compared with
the NC group (P<0.05) (Fig.
2).
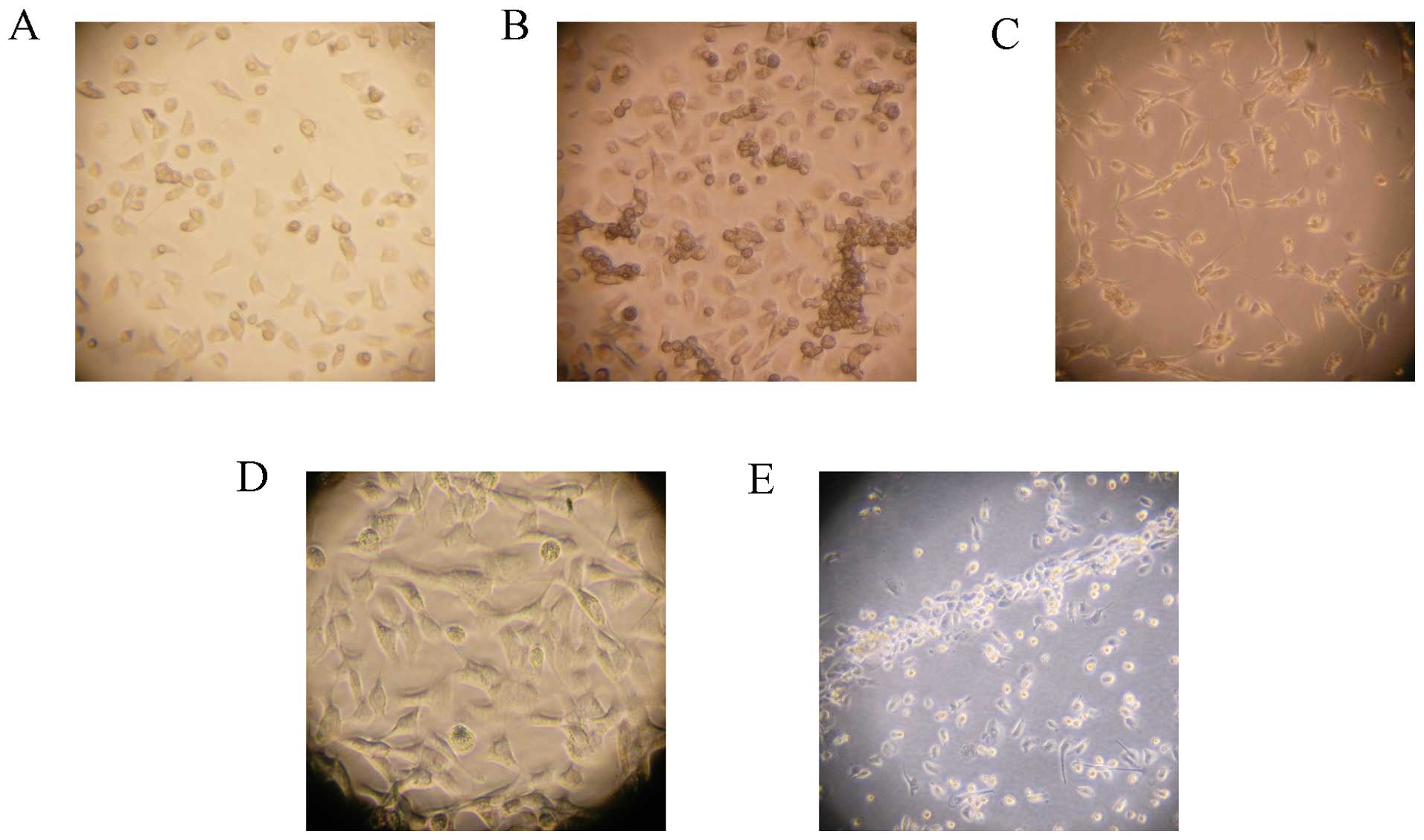
Culture of EPCs
Newly isolated bone marrow mononuclear cells were
round, transparent and suspended in medium. After 48 h of plating,
part of the cells attached. The adherent cells gradually enlarged
and stretched. After 4–7 days of plating adherent cells grew as
colonies. Cells were round, triangle, oval or irregular. After 10
days, cell were linearly arranged (Fig. 3).
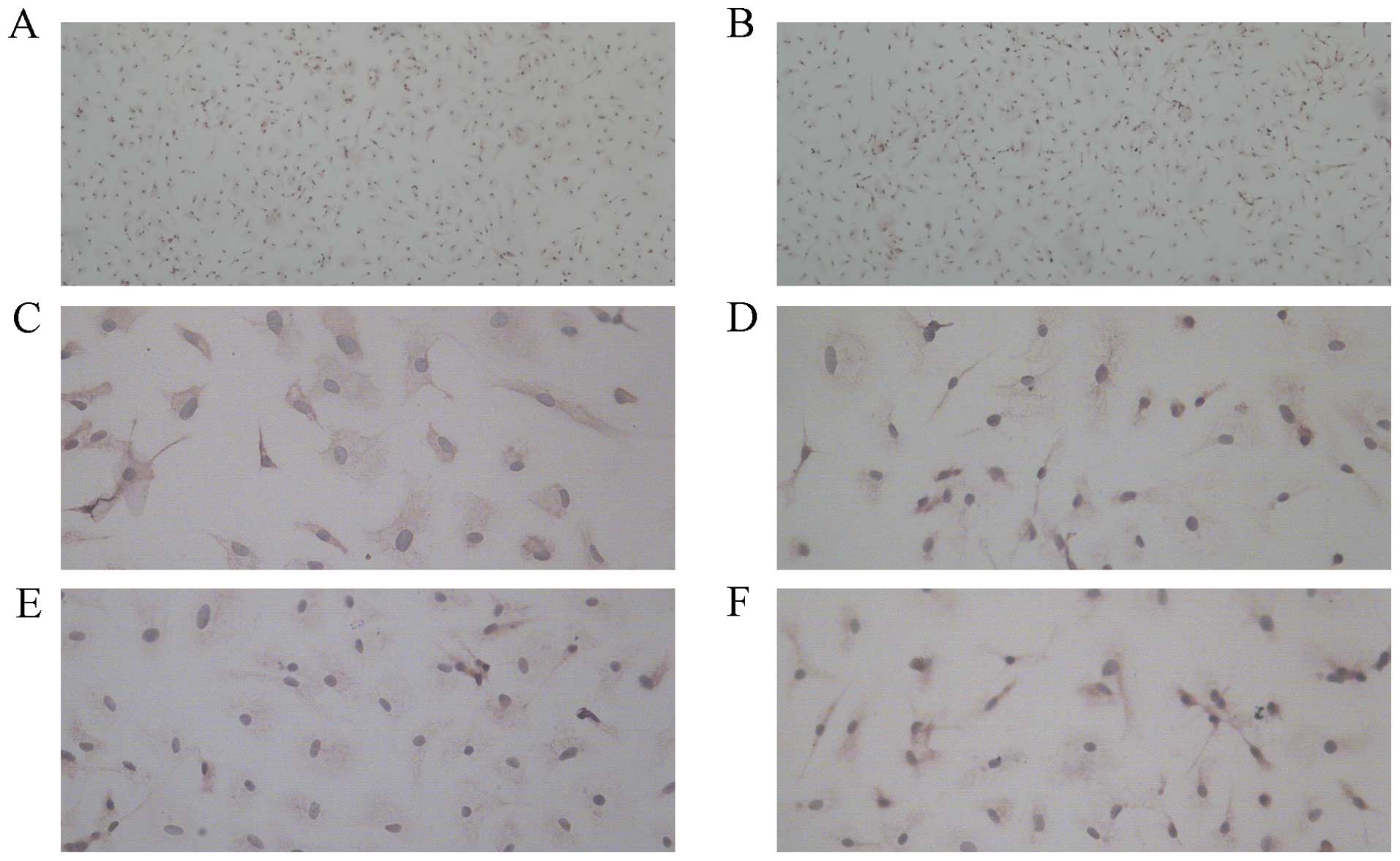
Determination of EPCs numbers and
cellular characterization
EPCs were analyzed by immunohistochemical staining
under a microscope. CD34 and KDR are surface markers of EPCs
(Fig. 4).
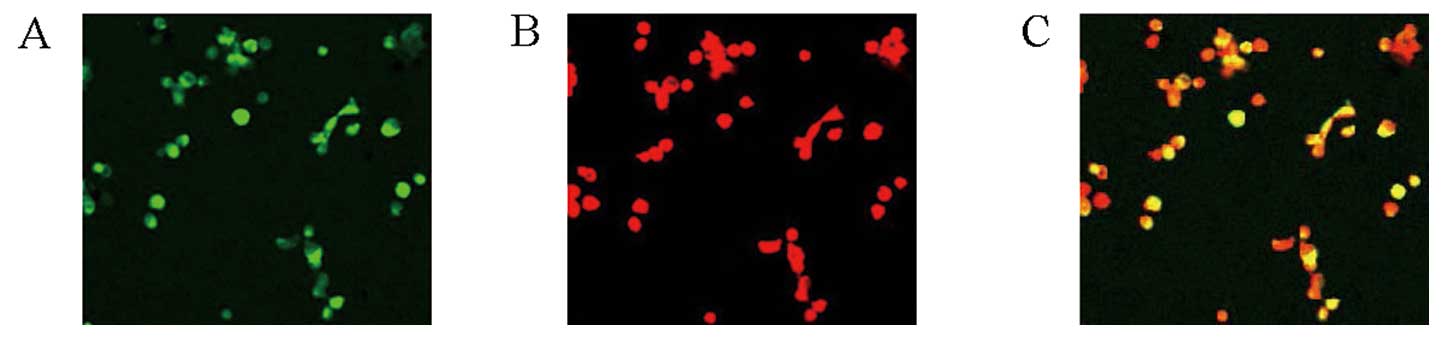
After a 10-day culture, by uptake of DIL-Ac-LDL and
binding of FITC-Lectin-UEA-1, EPCs which were double-stained cells
as yellow were observed and counted under laser scanning confocal
microscopy. The numbers of EPCs were significantly lower in the HF
group compared with those in the NC group (P<0.01) (Table II) (Fig. 5).
 | Table II.Number of EPCs in the normal control
(NC) and high-fat-fed (HF) groups (mean ± SD). |
Table II.
Number of EPCs in the normal control
(NC) and high-fat-fed (HF) groups (mean ± SD).
| NC | HF |
|---|
| EPCs | 72.59±4.22 | 63.23±5.33a |
Correlation analysis of different
index
The Pearson correlation analysis indicated that the
numbers of EPCs were negatively correlated with serum visfatin
(r=−0.886, P<0.01), CRP (r=−0.849, P<0.01), FBG (r=−0.753,
P<0.01), HOMA-IR (r=−0.775, P<0.01), TC (r=−0.744,
P<0.01), TG (r=−0.821, P<0.01), VAT (r=−0.631, P<0.01) and
BW (r=−0.656, P<0.01).
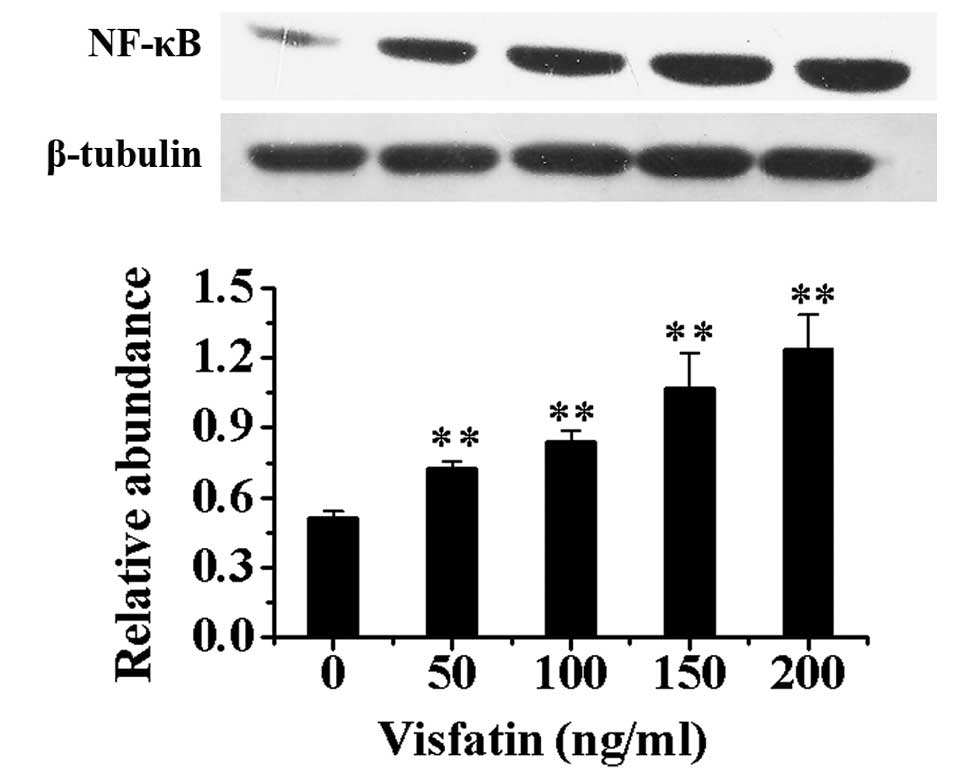
Effect of visfatin treatment on EPCs
Compared with EPCs without incubation of visfatin,
the protein expression of NF-κB in nuclei in EPCs after treatment
with different concentrations of visfatin increased in a
dose-dependent manner (P<0.01) (Fig. 6).
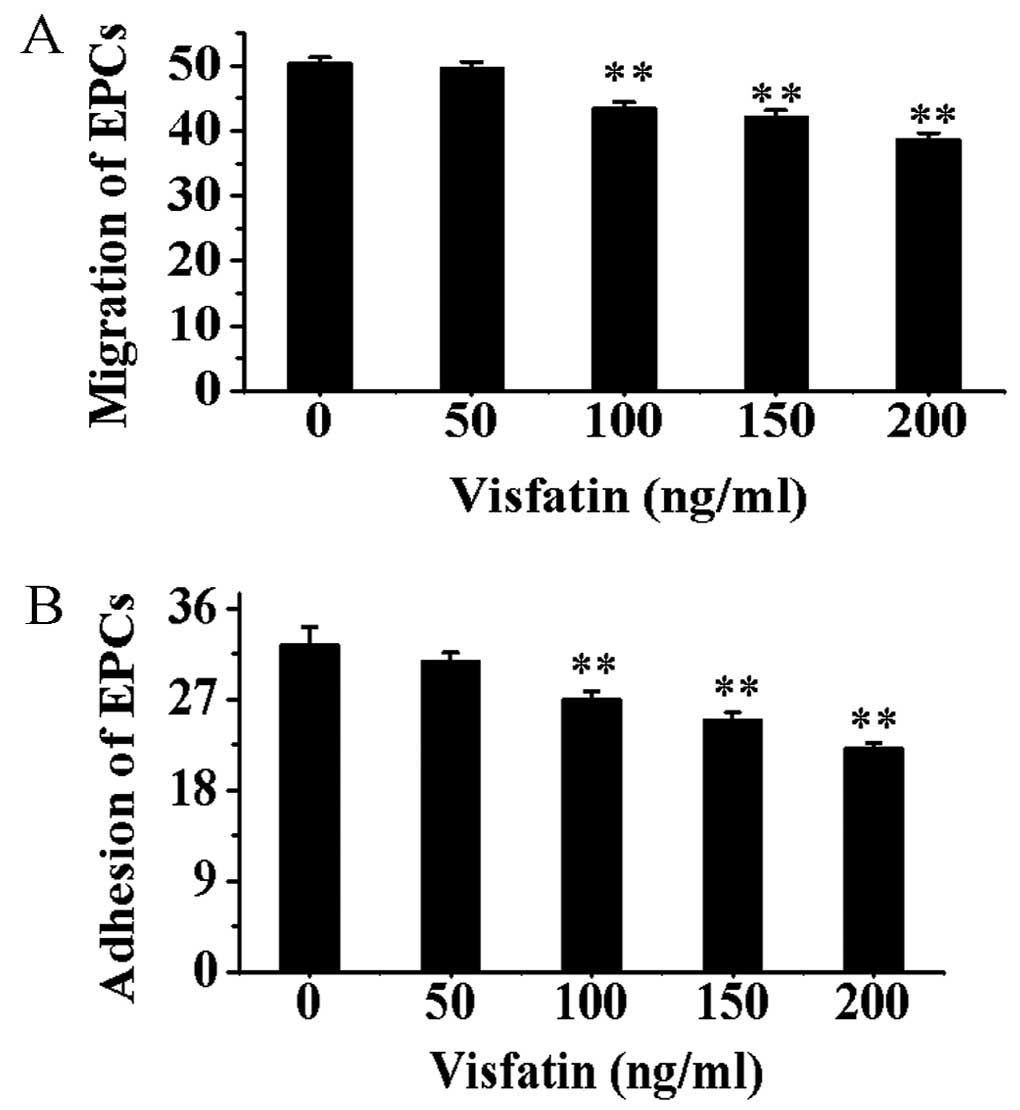
Compared with EPCs without incubation of visfatin,
the migration and adhesion capacities of EPCs treated with visfatin
were gradually decreased in a dose-depent manner (P<0.01)
(Fig. 7).
Discussion
Obesity has become increasingly prevalent and is an
important risk factor of cardiovascular disease (26–29). Studies indicate that obesity,
similarly to other risk factors including diabetes mellitus,
hypertension and smoking, can impair the function of the vascular
endothelium (30–33) and lead to arteriosclerosis and
other cardiovascular diseases (6–9).
Endothelial progenitor cells (EPCs) play an important role in
maintaining the complement of endothelial structure and normal
function of the vascular endothelium. EPCs originate from the bone
marrow. EPCs released into the blood circulation after stimulation
can differentiate into mature endothelial cells and especially
settle in ischemic locations and participate in the
re-endothelialization of injured blood vessels. According to
previous studies, the alterations in EPCs exist in overweight and
obese individuals. Several recent studies show that the numbers of
EPCs decreased in overweight and obese populations with an impaired
proliferation capacity (34–39). Heida et al (34) found that the numbers of EPCs in
the blood circulation decreased in obese individuals and obese
mice; meanwhile, the migration and adhesion capacity of EPCs were
impaired. MacEneaney et al (35) also found an decrease in EPCs
quantity in overweight and obese individuals with an weakened
proliferation capacity. However, the underlying mechanism by which
the quantity and function of EPCs are impaired remain unclear.
Visfatin is an adipokine which is secreted by
adipose tissue. The effects of visfatin include promoting the
differentiation of adipose cells and the synthesis and storage of
adipose tissue, promoting inflammation of vascular endothelial
cells and leading to the development of arteriosclerosis (16,40–43). By far, the possible relationship
of visfatin to EPCs is less investigated. We hypothesized that
visfatin might be involved in the impairment of EPCs function in
the situation of obesity. In the present study, we fed the rats
with a high-fat diet for 16 weeks and induced obesity in rats along
with the development of whole body insulin resistance (as shown by
increased FBG, FINS and HOMA-IR). EPCs were significantly decreased
in rats in HF group, which is consistent with previous studies.
Meanwhile, serum visfatin and the protein contents of visfatin were
significantly increased by high fat feeding. Correlation analysis
indicated that the quantity of EPCs are negatively correlated with
visfatin levels, indicating a possible relationship of visfatin to
EPCs and that visfatin is possibly an influential factor of the
quantity of EPCs.
A possible underlying mechanism of the influence of
visfatin on EPCs quantity might be the inflammatory effect of
visfatin. Previous studies found that visfatin induces the
upregulation of inflammatory factors and adhesion molecules in
human umbilical vein endothelial cells (HUVECs) through the NF-κB
pathway (17,20,21,25). Lee et al (17) found that the activity of NF-κB was
increased in HUVECs after incubation with visfatin, with the
upregulation of IL-6, IL-8, ICAM-1, VCAM-1 and E-selectin genes.
The activity of NF-κB increased in both HUVECs and epithelial tumor
cells when incubated with visfatin for 24 h; the activity increased
with the treatment of visfatin in a dose-dependent manner (25). Furthermore, visfatin can increase
the expression of NF-κB, tumor necrosis factor-α (TNF-α), matrix
metalloproteinase-9 (MMP-9), interleukin-8 (IL-8), IL-6 in
monocytes by activating insulin receptor (IR)-Ras-MAPK signaling
pathway and the insulin-independent p38 pathway (16). Based on these findings, we presume
that visfatin might influence the function of EPCs through the
NF-κB pathway.
In our study, we incubated the EPCs with different
concentrations of visfatin to observe the effect of visfatin on the
expression of NF-κB in nuclei of EPCs. The concentration of
visfatin was based on serum visfatin levels measured in the animal
study. The results show that the expression of NF-κB in nuclear
EPCs were significantly increased by visfatin treatment in a
dose-dependent manner, which is consistent with the previous study
that visfatin possesses inflammatory effects. The effect of
visfatin on NF-κB in EPCs support our hypothesis that visfatin may
have an effect on EPCs through the NF-κB pathway. Meanwhile, the
capacity of EPCs to migrate and adhere was impaired by visfatin
treatment. The possible mechanism is that inflammation induced by
visfatin causes the upregulation of a series of inflammatory
factors including NF-κB and TNF-α, leading to the aging and
apoptosis of EPCs and resulting in decreased quantities and
impaired functions of EPCs (44–46). Further investigation is
warranted.
In summary, serum visfatin and protein contents of
visfatin in VAT increased in obese rats fed a high-fat diet,
accompanied with decreased quantities of bone-marrow originating
EPCs. Visfatin may be involved in the development of decreased EPC
numbers and impaired functions through the NF-κB pathway. The
present study provides a new target for prevention of the
development of cardiovascular disease in obese populations.
Acknowledgements
We thank Mr. Chao Wang for continuous
advice, support and technical assistance.
Abbreviations:
|
EPCs
|
endothelial progenitor cells;
|
|
VAT
|
visceral adipose tissue;
|
|
NF-κB
|
nuclear factor-κB;
|
|
MNCs
|
marrow mononuclear cells;
|
|
EGM-2MV
|
endothelial growth media-2MV;
|
|
KDR
|
kinase insert domain receptor;
|
|
Flk-1
|
fetal liver kinase-1;
|
|
DAB
|
diaminobenzidine;
|
|
DiI-acLDL
|
DiI-labeled acetylated LDL;
|
|
FITCLectin-UEA-1
|
fluorescein isothiocyanate-conjugated
lectin from Ulex europaeus agglutinin;
|
|
BW
|
body weight;
|
|
FBG
|
fasting blood glucose;
|
|
FINS
|
fasting insulin in serum;
|
|
TG
|
triglyceride;
|
|
TC
|
total cholesterol;
|
|
ICAM-1
|
intercellular adhesion molecule 1;
|
|
VCAM-1
|
vascular cell adhesion molecule 1;
|
|
TNF-α
|
tumor necrosis factor-α;
|
|
MMP-9
|
matrix metalloproteinase-9;
|
|
IL-8
|
interleukin-8;
|
|
IR
|
insulin receptor;
|
|
Ras-MAPK
|
Ras-mitogen-activated protein
kinases
|
References
|
1.
|
U LaufsA UrhausenN WernerRunning exercise
of different duration and intensity: effect on endothelial
progenitor cells in healthy subjectsEur J Cardiovasc Prev
Rehabil12407414200510.1097/01.hjr.0000174823.87269.2e16079651
|
|
2.
|
JM HillG ZalosJP HalcoxCirculating
endothelial progenitor cells, vascular function, and cardiovascular
riskN Engl J Med348593600200310.1056/NEJMoa02228712584367
|
|
3.
|
GP FadiniM MiorinM FaccoCirculating
endothelial progenitor cells are reduced in peripheral vascular
complications of type 2 diabetes mellitusJ Am Coll
Cardiol4514491457200510.1016/j.jacc.2004.11.06715862417
|
|
4.
|
GP FadiniS SartoreM AlbieroNumber and
function of endothelial progenitor cells as a marker of severity
for diabetic vasculopathyArterioscler Thromb Vasc
Biol2621402146200610.1161/01.ATV.0000237750.44469.8816857948
|
|
5.
|
GP FadiniS SartoreC AgostiniA
AvogaroSignificance of endothelial progenitor cells in subjects
with diabetesDiabetes
Care3013051313200710.2337/dc06-230517277037
|
|
6.
|
MR MeyersN GokceEndothelial dyfuntion in
obesity: etiological role in atherosclerosisCurr Opin Endocrinol
Diabetes Obes14365369200710.1097/MED.0b013e3282be90a817940464
|
|
7.
|
MK RerianiLO LermanA LermanEndothelial
function as a functional expression of cardiovascular risk
factorsBiomark Med4351360201010.2217/bmm.10.6120550469
|
|
8.
|
IM ChungYM KimMH YooImmobilization stress
induces endothelial dysfunction by oxidative stress via the
activation of the angiotensin II/its type I receptor
pathwayAtherosclerosis213109114201010.1016/j.atherosclerosis.2010.08.052
|
|
9.
|
S SitiaL TomasoniF AtzeniFrom endothelial
dysfunction to atherosclerosisAutoimmun
Rev9830834201010.1016/j.autrev.2010.07.01620678595
|
|
10.
|
S KouidhiS JarbouiR MarrakchiAdiponectin
expression and metabolic markers in obesity and type 2 diabetesJ
Endocrinol Invest34e16e23201110.1007/BF0334705620651470
|
|
11.
|
H ManggeG AlmerM
Truschnig-WildersInflammation, adiponectin, obesity and
cardiovascular riskCurr Med
Chem1745114520201010.2174/09298671079418300621062254
|
|
12.
|
JE YunH KimmJ JoSH JeeSerum leptin is
associated with metabolic syndrome in obese and nonobese Korean
populationsMetabolism59424429201010.1016/j.metabol.2009.08.01219846168
|
|
13.
|
PA HeckerKM O’SheaTF GalvaoRole of
adiponectin in the development of high fat diet-induced metabolic
abnormalities in miceHorm Metab
Res43100105201110.1055/s-0030-126989821165812
|
|
14.
|
J ChangY LiY HuangAdiponectin prevents
diabetic premature senescence of endothelial progenitor cells and
promotes endothelial repair by suppressing the p38 MAP
kinase/p16INK4A signaling
pathwayDiabetes5929492959201010.2337/db10-0582
|
|
15.
|
V LavoieAE KernaleguenG CharronFunctional
effects of adiponectin on endothelial progenitor
cellsObesity19722728201110.1038/oby.2010.18720814418
|
|
16.
|
AR MoschenA KaserB EnrichVisfatin, an
adipocytokine with proinflammatory and immunomodulating propertiesJ
Immunol17817481758200710.4049/jimmunol.178.3.174817237424
|
|
17.
|
WJ LeeCS WuH LinVisfatin-induced
expression of inflammatory mediators in human endothelial cells
through the NF-kappaB pathwayInt J
Obes33465472200910.1038/ijo.2009.2419223849
|
|
18.
|
F HuangXF XiongS YouVisfatin upregulates
MMP-2 and MMP-9 expressions in human monocytes through activating
NF-kappaBZhonghua Xin Xue Guan Bing Za Zhi384554592010(In
Chinese)
|
|
19.
|
SR KimYH BaeSK BaeVisfatin enhances ICAM-1
and VCAM-1 expression through ROS-dependent NF-kappaB activation in
endothelial cellsBiochim Biophys
Acta1783886895200810.1016/j.bbamcr.2008.01.00418241674
|
|
20.
|
YC ChangTJ ChangWJ LeeLM ChuangThe
relationship of visfatin/pre-B-cell colony-enhancing
factor/nicotinamide phosphoribosyltransferase in adipose tissue
with inflammation, insulin resistance, and plasma
lipidsMetabolism599399201010.1016/j.metabol.2009.07.011
|
|
21.
|
YS KangHK SongMH LeePlasma concentration
of visfatin is a new surrogate marker of systemic inflammation in
type 2 diabetic patientsDiabetes Res Clin
Pract89141149201010.1016/j.diabres.2010.03.02020409603
|
|
22.
|
T DogruA SonmezI TasciPlasma visfatin
levels in patients with newly diagnosed and untreated type2
diabetes mellitus and impaired glucose toleranceDiabetes Res Clin
Pract762429200710.1016/j.diabres.2006.07.03116956691
|
|
23.
|
A FukuharaM MatsudaM NishizawaVisfatin: a
protein secreted by visceral fat that mimics the effects of
insulinScience307426430200510.1126/science.109724315604363
|
|
24.
|
K TakebayashiM SuetsuguS
WakabyashiAssociation between plasma visfatin and vascular
endothelial function in patients with type 2 diabetes
mellitusMetabolism56451458200710.1016/j.metabol.2006.12.00117378999
|
|
25.
|
R AdyaBK TanJ ChenHS RandevaNuclear
factor-kappaB induction by visfatin in human vascular endothelial
cells: role in MMP-2/9 production and activationDiabetes
Care31758760200810.2337/dc07-154418184904
|
|
26.
|
J DoupisS RahangdaleC GnardellisEffects of
diabetes and obesity on vascular reactivity, inflammatory
cytokines, and growth
factorsObesity19729735201110.1038/oby.2010.19320829804
|
|
27.
|
LL YanML DaviglusK LiuMidlife body mass
index and hospitalization and mortality in older
ageJAMA295190198200610.1001/jama.295.2.19016403931
|
|
28.
|
L AkilHA AhmadRelationships between
obesity and cardiovascular diseases in four southern states and
ColoradoJ Health Care Poor Underserved22Suppl
46172201110.1353/hpu.2011.016622102306
|
|
29.
|
V DeClercqC TaylorP ZahradkaAdipose
tissue: the link between obesity and cardiovascular
diseaseCardiovasc Hematol Disord Drug
Targets8228237200810.2174/18715290878584908018781935
|
|
30.
|
M BartonObesity and aging: determinants of
endothelial cell dysfunction and atherosclerosisPflugers
Arch460825837201010.1007/s00424-010-0860-y20635093
|
|
31.
|
R KobayasiEH AkamineAP DavelOxidative
stress and inflammatory mediators contribute to endothelial
dysfunction in high-fat diet-induced obesity in miceJ
Hypertens2821112119201010.1097/HJH.0b013e32833ca68c20616756
|
|
32.
|
J KetonenJ ShiE MartonenE
MervaalaPeriadventitial adipose tissue promotes endothelial
dysfunction via oxidative stress in diet-induced obese C57Bl/6
miceCirc J7414791487201010.1253/circj.CJ-09-066120526041
|
|
33.
|
KR ShortPR BlackettAW GardnerKC
CopelandVascular health in children and adolescents: effects of
obesity and diabetesVasc Health Risk Manag5973990200919997578
|
|
34.
|
NM HeidaJP MüllerIF ChengEffects of
obesity and weight loss on the functional properties of early
outgrowth endothelial progenitor cellsJ Am Coll
Cardiol55357367201010.1016/j.jacc.2009.09.03120117442
|
|
35.
|
OJ MacEneaneyEJ KushnerGP Van
GuilderEndothelial progenitor cell number and colony-forming
capacity in overweight and obese adultsInt J Obes
(Lond)33219225200910.1038/ijo.2008.26219079361
|
|
36.
|
OJ MacEneaneyEJ KushnerCM
WestbyEndothelial progenitor cell function, apoptosis, and telomere
length in overweight/obese
humansObesity1816771682201010.1038/oby.2009.49420057362
|
|
37.
|
K ToblerA FreudenthalerSM
Baumgartner-ParzerReduction of both number and proliferative
activity of human endothelial progenitor cells in obesityInt J Obes
(Lond)34687700201010.1038/ijo.2009.28020065973
|
|
38.
|
PE WesterweelFL VisserenGR
HajerEndothelial progenitor cell levels in obese men with the
metabolic syndrome and the effect of simvastatin monotherapy vs.
simvastatin/ezetimibe combination therapyEur Heart
J2928082817200810.1093/eurheartj/ehn43118824462
|
|
39.
|
K EspositoM CiotolaMI MaiorinoCirculating
CD34+ KDR+ endothelial progenitor cells
correlate with erectile function and endothelial function in
overweight menJ Sex Med61071142009
|
|
40.
|
J MalyszkoJS MalyszkoM MysliwiecVisfatin
and endothelial function in dialyzed patientsNephrology
(Carlton)15190196201010.1111/j.1440-1797.2009.01180.x20470278
|
|
41.
|
AE SchutteHW HuismanR SchutteAdipokines
and cardiometabolic function: How are they interlinked?Regul
Pept164133138201010.1016/j.regpep.2010.06.00820615436
|
|
42.
|
AR MoschenRR GernerH TilgPre-B cell colony
enhancing factor/NAMPT/visfatin in inflammation and obesity-related
disordersCurr Pharm
Des1619131920201010.2174/13816121079120894720370672
|
|
43.
|
AR MoschenS GeigerR GernerH TilgPre-B cell
colony enhancing factor/NAMPT/visfatin and its role in
inflammation-related bone diseaseMutat
Res69095101201010.1016/j.mrfmmm.2009.06.01219583971
|
|
44.
|
S VermaMA KuliszewskiSH LiC-reactive
protein attenuates endothelial progenitor cell survival,
differentiation, and function: further evidence of a mechanistic
link between C-reactive protein and cardiovascular
diseaseCirculation10920582067200410.1161/01.CIR.0000127577.63323.24
|
|
45.
|
W SuhKL KimJH ChoiC-reactive protein
impairs angiogenic functions and decreases the secretion of
arteriogenic chemo-cytokines in human endothelial progenitor
cellsBiochem Biophys Res
Commun3216571200410.1016/j.bbrc.2004.06.107
|
|
46.
|
JL NanJJ LiJG HeC-reactive protein
decreases interleukin-8 production in human endothelial progenitor
cells by inhibition of p38 MAPK pathwayChin Med J
(Engl)12219221928200919781372
|