Introduction
Diabetic cardiomyopathy (DCM), which occurs in type
I and II diabetes, is a substantial risk factor for the subsequent
development of heart failure and increased mortality (1). Its pathological substrate is
characterized by early-onset diastolic dysfunction and late-onset
systolic dysfunction in the absence of coronary artery disease,
hypertension or significant valvular heart disease (2,3).
The pathological mechanism of DCM may be due to metabolic
disturbances, reactive hypertrophy, small vessel disease, autonomic
dysfunction, insulin resistance and myocardial fibrosis. At
present, conventional therapies for DCM include glycemic control
and early administration of neurohormonal antagonists. DCM,
however, has yet to be completely prevented (4,5).
Therefore, the development of more effective therapeutic strategies
for DCM is necessary.
Endothelial progenitor cells (EPCs) are a subset of
bone marrow-derived stem cells present in peripheral circulation
that have the potential to differentiate into functional and mature
endothelial cells. Since their revolutionary discovery by Asahara
et al (6) in 1997, EPCs
have been demonstrated to be of importance in postnatal
vasculogenesis through pivotal bioactivities, mobilization, homing,
migration, differentiation and proliferation in angiovasculogenic
tissues (7). However, decreased
levels of circulating EPCs have been reported in a wide range of
pathological conditions, such as chronic kidney disease (8), hypercholesterolemia (9), rheumatoid arthritis (10), obesity (11) and coronary artery disease
(12). Furthermore, an altered
status of circulating EPCs represents a diagnostic biomarker for
endothelial dysfunction and individual diseases. Due to their role
in enhacing angiogenesis, there has been a growing interest in
using EPCs as therapeutic agents to supply the potent origin of
neovascularization under pathological conditions (13–16). Endothelial dysfunction is a
hallmark of diabetic vascular complications and reduction in
circulating EPCs and functional impairment of cultured EPCs have
been reported in type I and II diabetic patients (17,18). Recently, the ex vivo
therapy of culture-expanded EPC transplantation has been proposed
as a novel therapeutic strategy for diabetes mellitus and its
associated vascular complications (19–21). However, the possibility of
cellular therapy with EPCs for the treatment of DCM has yet to be
investigated.
Based on recent advances in studies of EPCs in
diabetes and the lack of treatment strategies that improve cardiac
repair in diabetics, we hypothesized that EPCs might improve the
cardiac function of diabetic rats. To test this hypothesis, a
streptozotocin (STZ)-induced diabetic rat model was used.
Subsequent to EPC isolation and proliferation in vitro, a
series of experiments was conducted to determine the changes in
morphology and other biological characteristics, and to assess
whether transplantation of EPCs is to be considered a therapeutic
candidate for the treatment of DCM.
Materials and methods
Animals and ethics statement
Male Sprague-Dawley rats at a postnatal age of 7–8
weeks (body weight, 200–220 g) were obtained from the Experimental
Animal Center of the Mudanjiang Medical College. The rats were kept
on straw bedding in cages under light/dark cycle conditions and
were acclimatized to the laboratory conditions for 7 days prior to
commencing the experiments. The animals were fed standard rodent
diet and water ad libitum, unless otherwise indicated. The
experiment protocol was approved by the Institutional Animal Care
and Use Committee and was performed in accordance with the Guide
for the Care and Use of Laboratory Animals (NIH Publication no.
85-23, National Academy Press, Washington, DC, revised 1996).
Isolation and culture of putative
EPCs
EPCs were isolated from the femurs and tibias of
donor rats, according to the method reported in previous studies
(22,23). In brief, the animals were
anesthetized with chloral hydrate (300 mg/kg) and sacrificed using
cervical dislocation. Femur and tibias were excised from each rat
under sterile conditions, while their bone marrow cavities were
rinsed with phosphate-buffered saline (PBS). The rinsing solution
was then subjected to density gradient centrifugation with
Histopaque-1083 (Sigma, St. Louis, MO, USA). The obtained
mononuclear cells were grown in M199 medium supplemented with 20%
fetal bovine serum (FBS), 0.05 mg/ml bovine pituitary extract
(Invitrogen Life Technologies, Carlsbad, CA, USA), antibiotics and
10 U/ml heparin on fibronectin-coated dishes. Seeded cells were
cultured in a humidified incubator at 37°C in 5% CO2.
After 10 days of culture, the cells were detached with trypsin-EDTA
and passed through a cell strainer. To detect transplanted EPCs,
cells were pre-labelled with a red fluorescent marker, CM-Dil
(Invitrogen Life Technologies) following the manufacturer’s
instructions.
Flow cytometry
To confirm that the isolated EPCs maintain their
phenotypic characteristics after culture, flow cytometry was
performed on freshly isolated cells (at day 0), as well as after 10
days of culture. Briefly, after being washed with PBS, cells were
incubated for 30 min at 4°C with mouse anti-rat antibodies for CD31
and CD34 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
rabbit anti-rat antibodies for CD133 and vascular endothelial
growth factor receptor 2 (VEGFR2) (Abnova, Taipei, Taiwan),
followed by anti-mouse or anti-rabbit fluorescein isothiocyanate
(FITC)-labeled secondary antibodies. Finally, flow cytometry was
performed with a FACSCalibur cytometer (BD Biosciences, San Jose,
CA, USA) and data were analyzed using CellQuest software.
Induction of diabetes
A rat DCM model was induced as described previously
(24). In brief, 30 rats were
injected intraperitoneally with 60 mg/kg body weight of STZ (Sigma)
prepared in 0.1 ml citrate buffer (0.1 mmol/l, pH 4.5) to induce
diabetic conditions, while 8 rats received an equal volume of
vehicle and served as a non-diabetic group. Seven days subsequent
to STZ injection, blood samples were collected from the tail vein
after 12 h of fasting. Nineteen rats reached the diabetic rat
standard of a fasting blood glucose level >300 mg/dl, while the
normal value in the control rats injected with vehicle ranged from
80 to 120 mg/dl. Eight weeks subsequent to vehicle and STZ
injection, 16 diabetic rats remained, while the control group
survived. The remaining animals were used for additional
experiments.
EPC transplantation
Sixteen diabetic rats were randomized into the EPC
and DCM groups (n=8 per group). Rats in the EPC group were
transplanted intravenously through the tail vein ∼1×106
EPCs (in 0.5 ml culture medium). Rats in the control and DCM groups
received an equal volume of culture medium at the same time point.
The rats were sacrificed at 4 weeks subsequent to EPC
transplantation. Prior to being sacrificed, blood was drawn to
detect the level of fasting blood glucose.
Echocardiographic evaluation
Prior to being sacrificed, each rat was anesthetized
with chloral hydrate (300 mg/kg). A catheter (PE-50;
Becton-Dickinson, Parsippany, NJ, USA) was inserted into the right
carotid artery and then advanced into the left ventricular (LV)
chamber to measure the following indices: heart rate (HR), LV
systolic blood pressure (LVSP), LV end diastolic blood pressure
(LVEDP), maximum rising rate of LV pressure (LV+dp/dtmax) and
minimum declining rate of LV pressure (LV-dp/dtmin), as delineated
previously (25). The rectal
temperature was maintained at 36–38°C using a heating pad
throughout the procedure. Rats were sacrificed immediately after
echocardiographic evaluation. The hearts were harvested and cut
into 2 sections, 1 was immersed in 10% formalin solution for
Masson’s trichrome staining, while the other in liquid nitrogen for
western blot analysis.
Masson’s trichrome staining
The middle of the LV was fixed in 10%
neutral-buffered formalin overnight, embedded in paraffin and
sectioned to a thickness of 5 μm. The sections were stained
with Masson’s trichrome staining to measure interstitial fibrosis.
Interstitial collagen was quantified at a final magnification of
×200 with a microscope (BA400 Binocular Microscope; Motic, Xiamen,
China) connected to a video camera. The resulting image was
processed on a computer image-analysis system. The content of
interstitial collagen (expressed as the fractional area of the
entire cross section) was averaged on 9 fields selected across the
wall thickness in the septum and the free wall.
Protein extraction and western blot
analysis
Western blot analysis was performed as previously
described (24). The hearts were
lysed in radioimmunoprecipitation (RIPA) assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). The heart tissue
homogenates were clarified by centrifugation and protein
concentrations of the lysates were determined using a bovine serum
albumin standard line. Equal amounts of protein were boiled at
100°C for 10 min and chilled on ice, subjected to sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis, and
then electrotransferred to polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). The membranes were blocked with 5%
non-fat dry milk (w/v) and then incubated overnight at 4°C with
rabbit anti-caspase-3, rabbit anti-Bcl-2, mouse anti-Bax, rabbit
anti-collagen I, rabbit anti-manganese superoxide dismutase (MnSOD)
and rabbit anti-p67phox (dilution 1:1000; Abcam, Cambridge, MA,
USA), followed by their respective horseradish
peroxidase-conjugated secondary antibodies. After extensive
washing, the band detection was revealed by an ECL plus
chemiluminescence kit (Millipore).
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Statistical differences in the groups were evaluated by
one-way analysis of variance, while the least-significant
difference (LSD) test was used for multiple comparisons. Graphs
were plotted and statistical calculations were performed using
SigmaPlot 12.0 (Systat Software, Inc., San Jose, CA, USA).
P<0.05 was considered to indicate statistically significant
differences.
Results
Characterization of bone marrow-derived
EPCs
After a 10-day culture, the isolated cells were
observed as bone marrow-derived EPC colony-forming units,
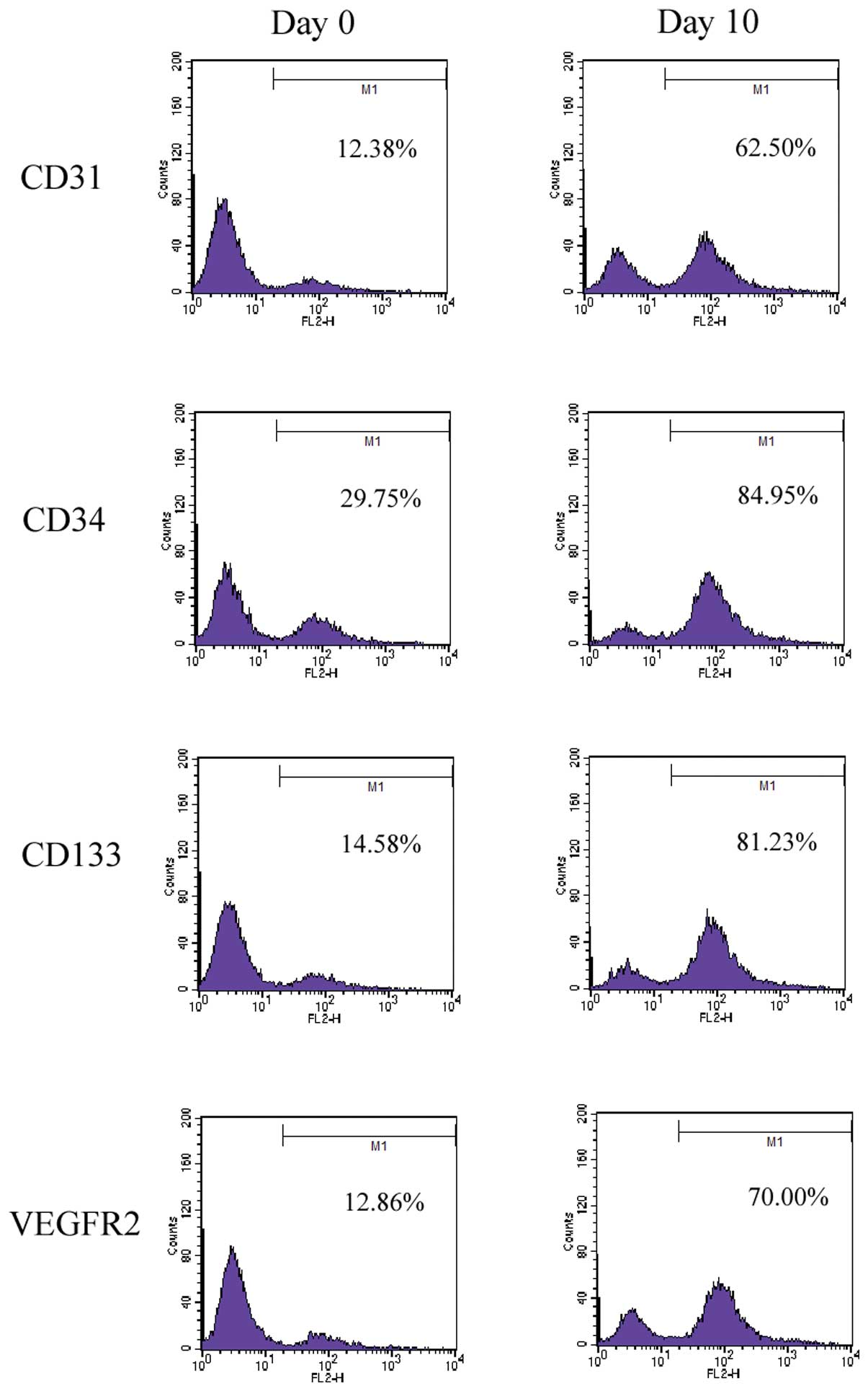
exhibiting endothelium-specific cord-like structures. Flow
cytometry showed that endothelial lineage-specific markers such as
CD31, CD34, CD133 and VEGFR2 significantly increased in 10 days
subsequent to plating (Fig. 1).
The expression changes in these factors are characteristic of those
of EPCs (26).
In vivo tracking of EPCs in myocardial
tissues
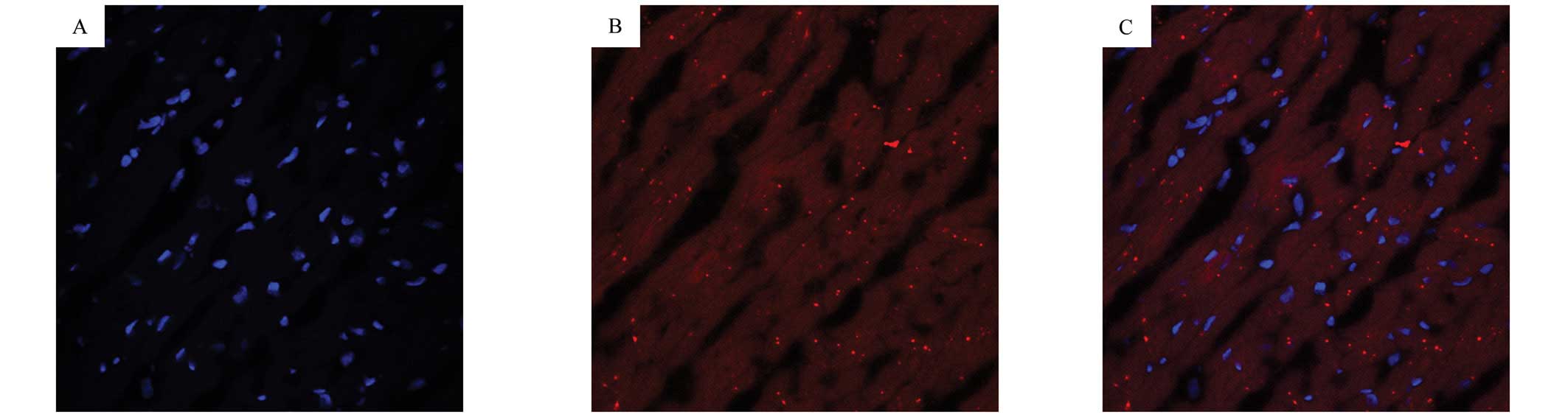
The trafficking of EPCs into the myocardial tissues
of STZ-induced diabetic rats was monitored using double staining of
CM-Dil and 4′,6-diamidino-2-phenylindole (DAPI). As shown in
Fig. 2, numerous CM-Dil-labeled
EPCs showing red fluorescence adoptively transferred into the
myocardial tissues of STZ-induced diabetic rats, indicating that
EPCs had the potential to migrate into the myocardium in the
STZ-induced diabetic model at 4 weeks after transplantation.
EPC transplantation improved myocardial
function in diabetic rats
Twelve weeks after the induction of diabetes, rat
cardiac function was evaluated by invasive hemodynamic
measurements. The metabolic and hemodynamic parameters of the
animals are presented in Table I.
A lower LVSP and higher LVEDP were observed in the DCM compared to
the control group. STZ-diabetic animals showed significant
impairments in the contraction (+dp/dt) and relaxation rate
(−dp/dt), compared to the control animals, indicating that the
systolic and diastolic functions in the diabetic rat heart were
significantly impaired. Four weeks subsequent to EPC
transplantation, the above-mentioned hemodynamic abnormalities were
notably attenuated. However, there were no significant differences
in the heart rate in the 3 groups. In addition, plasma glucose
concentrations in diabetic rats exceeded 16.7 mmol/l, however,
these concentrations decreased significantly subsequent to EPC
transplantation.
 | Table IHemodynamic parameters evaluated by
invasive measurements. |
Table I
Hemodynamic parameters evaluated by
invasive measurements.
| Groups
|
|---|
| Parameters | Control (n=8) | DCM (n=8) | EPC (n=8) |
|---|
| Plasma glucose
(mmol/l) | 4.7±0.8 | 28.4±2.0a | 13.7±2.2b |
| HR (beats/min) | 327±20 | 338±32 | 307±22 |
| LVSP (mmHg) | 123.4±7.9 | 95.6±7.1a | 109.5±5.6b |
| LVEDP (mmHg) | 4.7±0.8 | 15.4±2.3a | 9.2±2.2b |
| +dp/dt (mmHg/sec) | 5697±304 | 2761±458a | 3762±473b |
| −dp/dt (mmHg/sec) | 4788±337 | 2614±281a | 3524±315b |
EPC transplantation attenuated myocardial
interstitial fibrosis in diabetic rats
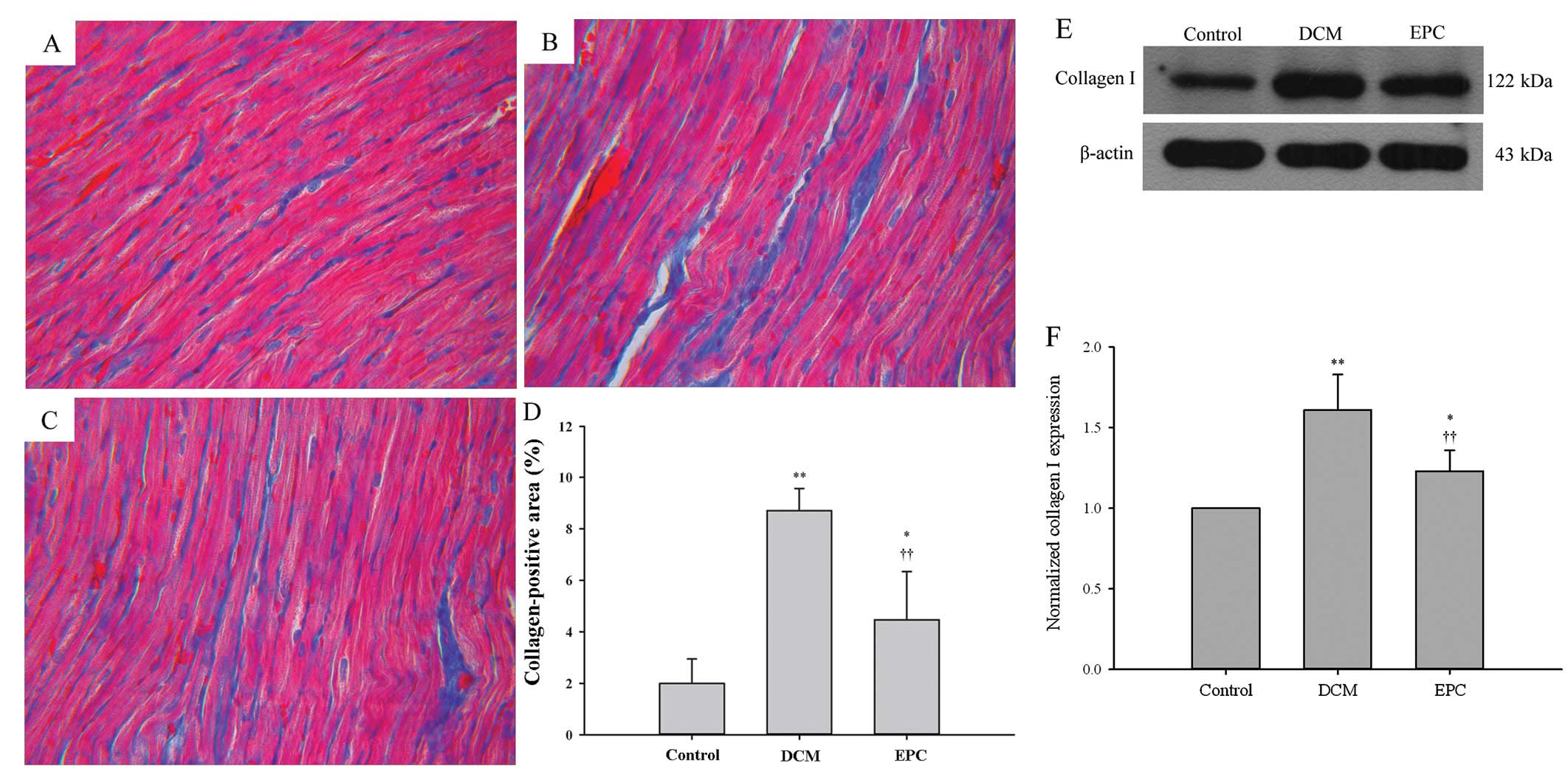
Myocardial fibrosis is a pathological hallmark in
the development of DCM. Masson’s trichrome staining was used to
examine whether EPC transplantation affected the myocardial
fibrosis in a STZ-induced diabetic rat model. Obvious interstitial
and perivascular fibrosis were detected in the myocardium of the
DCM group compared with the control group (Fig. 3A–C). Quantification of the
fibrosis area showed a statistically significant increase of
fibrosis in the DCM group, whereas EPC transplantation markedly
attenuated fibrosis progression in the diabetic hearts (P<0.01)
(Fig. 3D). Moreover, western blot
analysis showed that the type I collagen expression was markedly
upregulated in the DCM group, but inhibited by the EPC
transplantation (P<0.01) (Fig.
3E–F). These results suggest that EPC transplantation has the
potential to attenuate diabetes-induced myocardial interstitial
fibrosis.
EPC transplantation protected
cardiomyocytes against apoptosis in diabetic rats
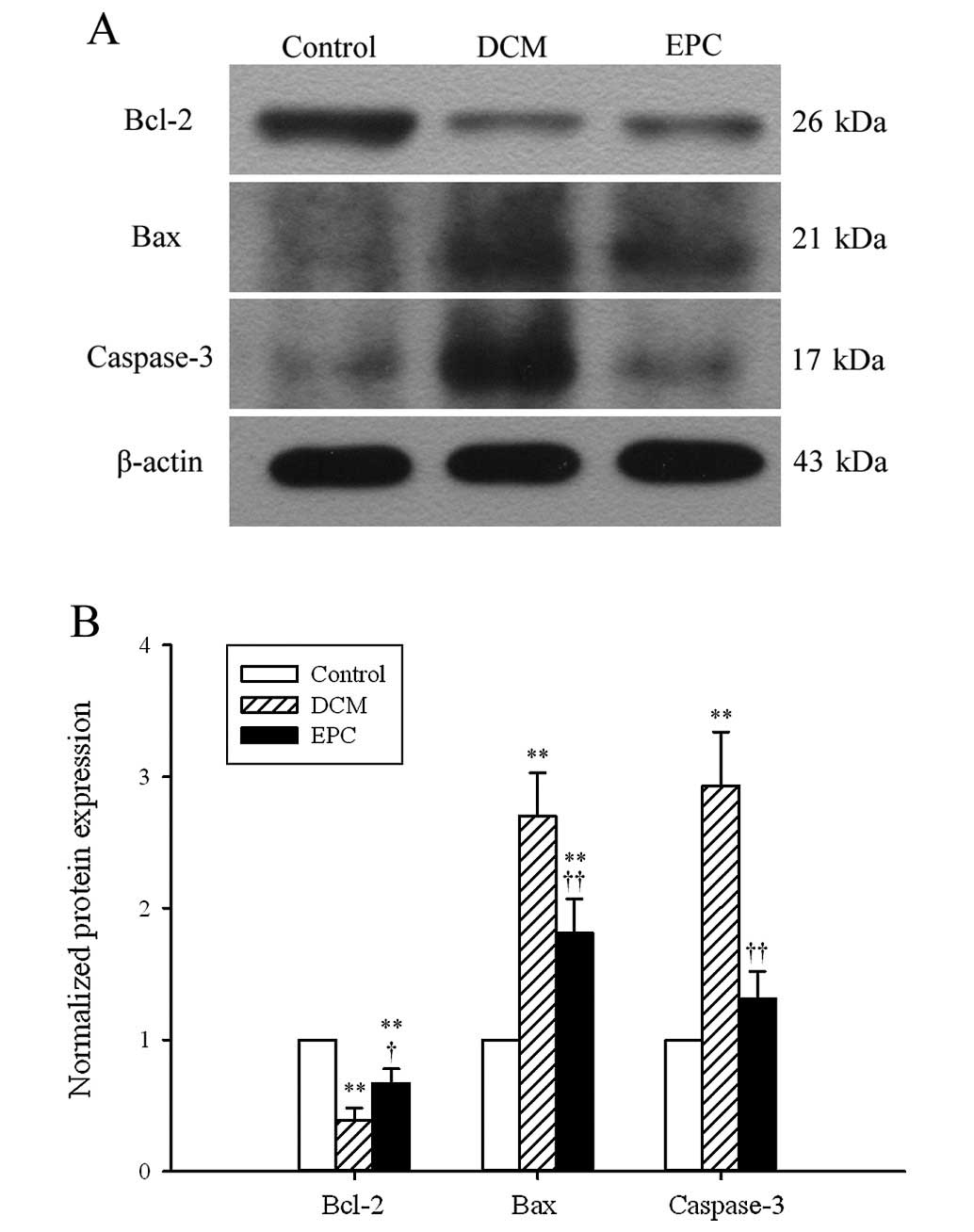
Cardiomyocyte apoptosis is a critical process in the
pathogenesis of DCM. The expression of apoptotic markers, such as
Bcl-2, Bax and caspase-3, was examined by western blot analysis in
order to investigate whether or not transplantation of EPCs
improved myocardial function by protecting cardiomyocytes against
apoptosis. Bcl-2 was found to be downregulated, whereas Bax and
caspase-3 were upregulated in the DCM group (Fig. 4). These changes, however, were
markedly attenuated by the administration of EPCs. Since Bcl-2 is
characterized by anti-apoptosis, while Bax and caspase-3 by
pro-apoptosis, our results suggest that transplantation of EPCs has
the potential to protect cardiomyocytes against apoptosis.
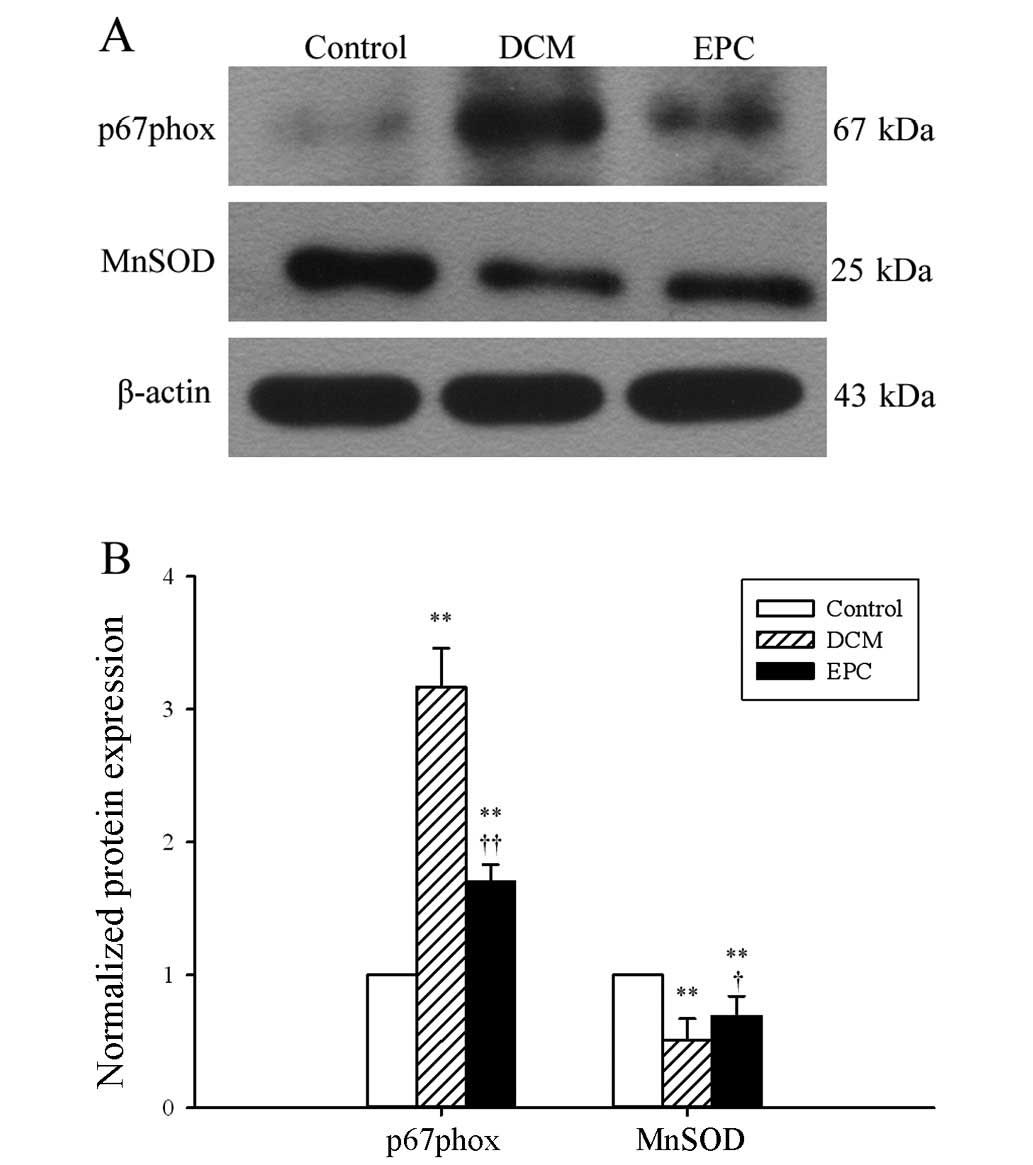
EPC transplantation prevented oxidative
stress in diabetic rats
The effects of EPC transplantation on the endogenous
pro-oxidants and antioxidants, including p67phox and MnSOD, were
determined by western blot analysis. The results showed an elevated
expression of p67phox in dietetic hearts, suggesting that an
endogenous pro-oxidant system was triggered under diabetic
conditions, which may further aggravate oxidative stress. EPC
transplantation markedly attenuated the p67phox expression
(Fig. 5). However, a
statistically significant decrease in the MnSOD expression in
STZ-induced diabetic hearts was observed, compared to control
hearts. Transplantation of EPCs resulted in a statistically
significant increase in the MnSOD expression in the DCM group.
These findings demonstrate that transplantation of EPC exerts its
protective effects by targeting the nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase and mitochondrial redox
enzymes.
Discussion
In this study, we have shown that transplantation of
EPCs significantly improved cardiac function and attenuated cardiac
fibrosis in a rat model of STZ-induced diabetes, as evidenced by
echocardiography, Masson’s trichrome staining and the analysis of
type I collagen expression. Moreover, EPC implantation effectively
prevented STZ-induced cardiac apoptosis and oxidative stress,
indicating the possible mechanisms whereby EPC transplantation
exerts its cardioprotective effects against myocardial fibrosis and
improves diastolic dysfunction in diabetic rats. The present study
is known to be the first to report the potential therapeutic
effects of EPC transplantation in an animal model of DCM.
Although in recent years an excessive number of
studies have been available on EPCs, the definition of EPCs has yet
to be determined due to the lack of unique cell surface molecules
that specifically recognize EPCs (27,28). Currently, the most widely accepted
definition is the co-expression of the surface markers CD31, CD133,
CD34 and VEGFR2 (13). The
characteristics of EPCs delineated in the present study are
consistent with the common features of EPCs reported in previous
studies. In addition, the co-expression of these markers also
characterizes the isolated EPCs at a specific maturation stage
(14). EPCs have been reported to
ameliorate acute myocardial infarction and diabetic erectile
dysfunction by promoting angiogenesis (29–30). However, few data are available
regarding the role of EPCs in other diabetic complications,
particularly in DCM. In the present study, EPC administration
markedly improved cardiac function, while fluorescent-labeled EPCs
were identified in cardiac tissues by immunofluorescence analysis,
suggesting that EPCs act via a direct transdifferentiation into
mature cardiomyocytes. These results corresponded to those drawn by
Badorff et al (31),
showing that EPCs differentiate into cardiomyocytes when
co-cultured with neonatal rat cardiomyocytes. Notably, the
discovery rate of EPCs was very low in the cardiac tissues of
STZ-induced diabetic rats, which further supports the generally
accepted concept that apart from direct cell fusion, EPCs may exert
a cardioprotective benefit via their paracrine effects (32).
Increased myocardial interstitial fibrosis is a
hallmark pathologic feature of DCM and has been considered to be
responsible for the diastolic dysfunction occurring in this
condition (33). In the present
study, 12-week diabetic condition was found to induce interstitial
fibrosis in the left ventricle of STZ rats, as demonstrated by
morphologic data and the analysis of type I collagen expression.
However, EPC administration significantly reduced left ventricular
interstitial fibrosis in diabetic rats. These results are
consistent with recent findings showing that EPC administration
prevents renal interstitial fibrosis in a murine chronic renal
failure model (34), as well as
in a mouse model of unilateral ureteral obstruction (35). In addition, Liu et al
(36) have demonstrated that
transplanted EPCs ameliorate carbon tetrachloride-induced liver
fibrosis in rats. These results, along with results drawn from
previous studies, suggest an anti-fibrotic effect of EPCs under
different pathologic conditions. Nonetheless, the detailed
mechanism involved in the anti-fibrotic effects of EPC
transplantation needs to be further elucidated.
Hyperglycemia-induced apoptosis is an early event in
the pathophysiology of DCM (37).
Caspase-3 is considered a primary executioner of apoptosis. In the
present study, caspase-3 was observed to have been activated in
STZ-induced diabetic rat hearts. The Bcl-2 family is thought to be
the critical factor in the apoptotic signaling pathways, and the
relative ratio of anti- and pro-apoptotic proteins is crucial for
the determination of cell survival or death (38). In this study, the hearts in the
DCM group were found to have exhibited an increased Bax expression
and a decreased Bcl-2 expression at the protein level. However,
these changes were markedly reversed by EPC administration,
suggesting that transplantation of EPCs might have the potential to
protect cardiomyocytes from apoptotic death in diabetic conditions.
These results are consistent with previous ones demonstrating that
several cytokines, released by peripheral blood or bone
marrow-derived EPC, are potent inhibitors of apoptosis (39–40). Furthermore, a recent study by Sen
et al (41) showed that
autologous transplantation of the genetic modification of EPCs also
inhibited cardiac apoptosis in a rat model of myocardial
infarction. Collectively, results in the current study confirmed
previous findings demonstrating that cardiomyocytes apoptosis is
associated with the progression of DCM, and that EPC implantation
has the potential to prevent cardiomyocytes apoptosis in diabetic
conditions.
STZ treatment induces systemic generation of
reactive oxygen species (ROS) by activating NADPH oxidase, thus
leading to cardiac dysfunction (42). To further investigate the
mechanisms by which EPCs exert their cardioprotective effects, the
expression of NADPH oxidase subunit p67phox and mitochondrial
ROS-eliminating enzyme MnSOD was examined. The results showed that
EPC transplantation attenuated the increased p67phox expression and
the decreased MnSOD expression that were induced by STZ injection.
These findings indicate that EPC transplantation ameliorated
diabetic cardiac damage by targeting the redox signaling
pathways.
In summary, transplantation of bone-marrow EPCs
improved cardiac function, protected cardiomyocytes against
apoptosis and reduced the extent of myocardial interstitial
fibrosis in a STZ-induced diabetic rat model. Additional
experiments showed that this cardioprotective effect may be
associated with the antioxidative properties of EPCs. Thus, EPC
transplantation may be a novel therapeutic approach for the
prevention of diabetic myocardial complications.
Acknowledgements
This study was supported by a grant
from the Heilongjiang Province Science Foundation for Youths (Grant
no. QC2010040).
References
|
1.
|
PK BattiproluTG GilletteZV WangS
LavanderoJA HillDiabetic cardiomyopathy: mechanisms and therapeutic
targetsDrug Discov Today Dis
Mech7e135e143201010.1016/j.ddmec.2010.08.00121274425
|
|
2.
|
SA HayatB PatelRS KhattarRA MalikDiabetic
cardiomyopathy: mechanisms, diagnosis and treatmentClin Sci
(Lond)107539557200410.1042/CS2004005715341511
|
|
3.
|
S BoudinaED AbelDiabetic cardiomyopathy
revisitedCirculation11532133223200710.1161/CIRCULATIONAHA.106.67959717592090
|
|
4.
|
E AdeghateMolecular and cellular basis of
the aetiology and management of diabetic cardiomyopathy: a short
reviewMol Cell
Biochem261187191200410.1023/B:MCBI.0000028755.86521.1115362503
|
|
5.
|
A AnejaWH TangS BansilalMJ GarciaME
FarkouhDiabetic cardiomyopathy: insights into pathogenesis,
diagnostic challenges, and therapeutic optionsAm J
Med121748757200810.1016/j.amjmed.2008.03.04618724960
|
|
6.
|
T AsaharaT MuroharaA SullivanIsolation of
putative progenitor endothelial cells for
angiogenesisScience275964967199710.1126/science.275.5302.9649020076
|
|
7.
|
M HristovW ErlPC WeberEndothelial
progenitor cells: mobilization, differentiation, and
homingArterioscler Thromb Vasc
Biol2311851189200310.1161/01.ATV.0000073832.49290.B512714439
|
|
8.
|
G KrenningPY DankersJW DrouvenEndothelial
progenitor cell dysfunction in patients with progressive chronic
kidney diseaseAm J Physiol Renal
Physiol296F1314F1322200910.1152/ajprenal.90755.200819339628
|
|
9.
|
JZ ChenFR ZhangQM TaoXX WangJH ZhuNumber
and activity of endothelial progenitor cells from peripheral blood
in patients with hypercholesterolaemiaClin Sci
(Lond)107273280200410.1042/CS2003038915099190
|
|
10.
|
J AvouacG UzanA KahanC BoileauY
AllanoreEndothelial progenitor cells and rheumatic disordersJoint
Bone Spine75131137200810.1016/j.jbspin.2007.09.00618314371
|
|
11.
|
J Muller-EhmsenD BraunT SchneiderDecreased
number of circulating progenitor cells in obesity: beneficial
effects of weight reductionEur Heart
J2915601568200810.1093/eurheartj/ehn21318515295
|
|
12.
|
M VasaS FichtlschererA AicherNumber and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery
diseaseCirc Res89E1E7200110.1161/hh1301.09395311440984
|
|
13.
|
A AllegraG CoppolinoD BolignanoEndothelial
progenitor cells: pathogenetic role and therapeutic perspectivesJ
Nephrol22463475200919662601
|
|
14.
|
S SenSP McDonaldPT CoatesCS
BonderEndothelial progenitor cells: novel biomarker and promising
cell therapy for cardiovascular diseaseClin Sci
(Lond)120263283201121143202
|
|
15.
|
C AlevM IiT AsaharaEndothelial progenitor
cells: a novel tool for the therapy of ischemic diseasesAntioxid
Redox Signal15949965201110.1089/ars.2010.387221254837
|
|
16.
|
JC GrisarF HaddadFA GomariJC WuEndothelial
progenitor cells in cardiovascular disease and chronic
inflammation: from biomarker to therapeutic agentBiomark
Med5731744201110.2217/bmm.11.9222103609
|
|
17.
|
A GeorgescuVascular dysfunction in
diabetes: The endothelial progenitor cells as new therapeutic
strategyWorld J Diabetes29297201110.4239/wjd.v2.i6.9221860692
|
|
18.
|
KA KimYJ ShinJH KimDysfunction of
endothelial progenitor cells under diabetic conditions and its
underlying mechanismsArch Pharm
Res35223234201210.1007/s12272-012-0203-y22370777
|
|
19.
|
YP JarajapuMB GrantThe promise of
cell-based therapies for diabetic complications: challenges and
solutionsCirc
Res106854869201010.1161/CIRCRESAHA.109.21314020299675
|
|
20.
|
A GeorgescuN AlexandruA ConstantinescuI
TitorencuD PopovThe promise of EPC-based therapies on vascular
dysfunction in diabetesEur J
Pharmacol66916201110.1016/j.ejphar.2011.07.03521839073
|
|
21.
|
L GrapensparrJ OlerudS VasylovskaPO
CarlssonThe therapeutic role of endothelial progenitor cells in
Type 1 diabetes mellitusRegen
Med6599605201110.2217/rme.11.4521916595
|
|
22.
|
SJ ZhangH ZhangM HouIs it possible to
obtain ‘true endothelial progenitor cells’ by in vitro culture of
bone marrow mononuclear cells?Stem Cells Dev166836902007
|
|
23.
|
F TimmermansJ PlumMC YoderDA IngramB
VandekerckhoveJ CaseEndothelial progenitor cells: identity
defined?J Cell Mol
Med1387102200910.1111/j.1582-4934.2008.00598.x19067770
|
|
24.
|
Y ChengG LiuQ PanS GuoX YangElevated
expression of liver X receptor alpha (LXRalpha) in myocardium of
streptozotocin-induced diabetic
ratsInflammation34698706201110.1007/s10753-010-9281-521136146
|
|
25.
|
R WichiC MalfitanoK RosaNoninvasive and
invasive evaluation of cardiac dysfunction in experimental diabetes
in rodentsCardiovasc
Diabetol614200710.1186/1475-2840-6-1417462095
|
|
26.
|
AJ DevanesanKA LaughlanHR GirnS
Homer-VanniasinkamEndothelial progenitor cells as a therapeutic
option in peripheral arterial diseaseEur J Vasc Endovasc
Surg38475481200910.1016/j.ejvs.2009.05.01919560945
|
|
27.
|
DN PraterJ CaseDA IngramMC YoderWorking
hypothesis to redefine endothelial progenitor
cellsLeukemia2111411149200710.1038/sj.leu.240467617392816
|
|
28.
|
KK HirschiDA IngramMC YoderAssessing
identity, phenotype, and fate of endothelial progenitor
cellsArterioscler Thromb Vasc
Biol2815841595200810.1161/ATVBAHA.107.15596018669889
|
|
29.
|
X GouWY HeMZ XiaoTransplantation of
endothelial progenitor cells transfected with VEGF165 to restore
erectile function in diabetic ratsAsian J
Androl13332338201110.1038/aja.2010.11621113173
|
|
30.
|
JH ParkJY YoonSM KoEndothelial progenitor
cell transplantation decreases lymphangiogenesis and adverse
myocardial remodeling in a mouse model of acute myocardial
infarctionExp Mol Med43479485201110.3858/emm.2011.43.8.054
|
|
31.
|
C BadorffRP BrandesR
PoppTransdifferentiation of blood-derived human adult endothelial
progenitor cells into functionally active
cardiomyocytesCirculation10710241032200310.1161/01.CIR.0000051460.85800.BB12600917
|
|
32.
|
A ZampetakiJP KirtonQ XuVascular repair by
endothelial progenitor cellsCardiovasc
Res78413421200810.1093/cvr/cvn08118349136
|
|
33.
|
J AsbunFJ VillarrealThe pathogenesis of
myocardial fibrosis in the setting of diabetic cardiomyopathyJ Am
Coll Cardiol47693700200610.1016/j.jacc.2005.09.05016487830
|
|
34.
|
O SangidorjSH YangHR JangBone
marrow-derived endothelial progenitor cells confer renal protection
in a murine chronic renal failure modelAm J Physiol Renal
Physiol299F325F335201010.1152/ajprenal.00019.2010
|
|
35.
|
YY MaD SunJ LiZC YinTransplantation of
endothelial progenitor cells alleviates renal interstitial fibrosis
in a mouse model of unilateral ureteral obstructionLife
Sci86798807201010.1016/j.lfs.2010.03.013
|
|
36.
|
F LiuZD LiuN WuTransplanted endothelial
progenitor cells ameliorate carbon tetrachloride-induced liver
cirrhosis in ratsLiver
Transpl1510921100200910.1002/lt.2184519718641
|
|
37.
|
L CaiW LiG WangL GuoY JiangYJ
KangHyperglycemia-induced apoptosis in mouse myocardium:
mitochondrial cytochrome C-mediated caspase-3 activation
pathwayDiabetes5119381948200210.2337/diabetes.51.6.193812031984
|
|
38.
|
A BurlacuRegulation of apoptosis by Bcl-2
family proteinsJ Cell Mol
Med7249257200310.1111/j.1582-4934.2003.tb00225.x14594549
|
|
39.
|
T KinnairdE StabileMS
BurnettMarrow-derived stromal cells express genes encoding a broad
spectrum of arteriogenic cytokines and promote in vitro and in vivo
arteriogenesis through paracrine mechanismsCirc
Res94678685200410.1161/01.RES.0000118601.37875.AC
|
|
40.
|
C UrbichA AicherC HeeschenSoluble factors
released by endothelial progenitor cells promote migration of
endothelial cells and cardiac resident progenitor cellsJ Mol Cell
Cardiol39733742200510.1016/j.yjmcc.2005.07.00316199052
|
|
41.
|
S SenJ MerchanJ DeanAutologous
transplantation of endothelial progenitor cells genetically
modified by adeno-associated viral vector delivering insulin-like
growth factor-1 gene after myocardial infarctionHum Gene
Ther2113271334201010.1089/hum.2010.006
|
|
42.
|
M OelzeM KnorrS SchuhmacherVascular
dysfunction in streptozotocin-induced experimental diabetes
strictly depends on insulin deficiencyJ Vasc
Res48275284201110.1159/00032062721273782
|