Introduction
Cancer cells frequently exhibit alterations in
protein glycosylation when compared to their normal counterparts
and this is assumed to result from disruptions in expression levels
or activity of the enzymes of glycosylation, the
glycosyltransferases and glycosidases (1). Aberrant glycosylation of membrane
components due to specific alterations of glycosyltransferase
activity is a common feature of carcinoma cells and is usually
associated with invasion and metastasis of cancer. For example,
GCNT2, which is a gene-encoding glucosaminyl (N-acetyl) transferase
2, contributes to breast cancer metastasis with preferential
expression in basal-like breast cancer (2). ST6Gal-I expression in ovarian cancer
cells promotes an invasive phenotype by altering integrin
glycosylation and function (3).
Since aberrant glycoproteins as a result of these enzymes may be
involved in promoting tumor invasion and metastasis, these enzymes
could also be used as cancer biomarkers (4). Therefore, cancer-specific changes in
the expression of glycosyltransferases exhibit the most marked and
consistent change of activity in tumorigenesis.
Gastric cancer is the fourth most common malignancy
and the second leading cause of cancer-related mortality in the
world. Several reports indicate a complexity in glycosyltransferase
activities which lead to several tumor associated carbohydrate
structures in gastric carcinoma. Glycosyltransferase mRNA
expression has been found to be significantly altered in gastric
carcinomas isolated from surgical specimens (5). Upregulation of
glycan:sulfotransferase activities and downregulation of
α,2-fucosyltransferase activity appear to be associated with human
gastric tumori-genesis (6).
Shimizu et al(7) confirmed
that α4GnT, which forms a unique glycan,
GlcNAcα1-->4Galβ-->R, is detectable in 80% of 5 patients with
an early stage of gastric cancer and the expression level of α4GnT
mRNA is increased in association with tumor progression. Recently,
β3Gn-T8, which can extend polylactosamine on N-glycan, was also
reported to be involved in malignancy in gastric cancer cells
(8). However, our previous study
demonstrated that polypeptide N-acetylgal actosaminyltransferase 2
(ppGalNAc-T2) was also involved in gastric cancer migration and
invasion.
ppGalNAc-T2 is a member of the ppGalNAc-T family
which catalyzes the attachment of the first N-acetylgalactosamine
(GalNAc) monosaccharide to the polypeptide at the initiation of
O-linked glycosylation of proteins. All ppGalNAc-Ts in mammals are
type II transmembrane proteins that have a Golgi lumenal region
that contains a catalytic domain with glycosyltransferase activity
and a C-terminal R-type lectin domain (9). The human ppGalNAc-T family contains
more than 18 members, each of which has unique transferase
activity, different peptide substrate specificities, and dissimilar
patterns of expression (10,11). Among all the ppGalNAc-Ts
identified in mammals thus far, the ppGalNAc-T2 gene was highly
expressed in cancer and may play an important role in the
occurrence and development of tumor. Brooks et al(1) reported that levels of ppGalNAc-T2
expression may change with the differentiation of breast carcinoma.
Mandel et al(12) showed
that ppGalNAc-T2 expression was strong in poorly differentiated
tumors. It has also been confirmed that ppGalNac-T2 is involved in
vanadium-induced HL-60 cell differentiation (13). Moreover, both acute T cell
leukemia Jurkat cell lines and human heptocarcinoma HepG-2 cell
lines clearly express ppGalNAc-T2 (14). The invasion and metastasis of
human glioma cells can also be regulated by ppGalNAc-T2 (15). Our previous studies revealed that
ppGalNAc-T2 expression appears to be higher in gastric cancer
SGC7901 cells than in other poorly differentiated human cancer
cells, indicating that ppGalNAc-T2 may play a vital role in the
process of gastric cancer emergence and development. However, a
comprehensive understanding of how ppGalNAc-T2 correlates with the
invasive potential of human gastric cancer is not currently
available.
Numerous studies have shown that overexpression of
Matrix metalloproteinases (MMPs) is correlated with the progression
of gastric cancer, which contributes to tumor invasion, metastasis
and angiogenesis. Thus, this study was undertaken to evaluate the
role of ppGalNAc-T2 in gastric cancer invasion and metastasis by
creating stable transfectants and evaluating them for invasive and
metastatic potential in vitro. In order to elucidate the
role of ppGalNAc-T2 in the gastric cancer metastasis process,
SGC7901 cells were treated with ppGalNAc-T2 sense or antisense
vectors and examined for the following: i) the relationship between
the ppGalNAc-T2 expression and the cell proliferation, adhesion,
and invasion ability, in order to clarify whether ppGalNAc-T2 is
correlated with SGC7901 cell metastasis-associated behavior; ii)
the impact of ppGalNAc-T2 on MMP-2, MMP-14 and transforming growth
factor (TGF)-β1 regulation including mRNA and protein levels, to
investigate the molecular mechanisms of the anti-metastasic
activities in human gastric carcinoma. These data suggested that
high expression of the ppGalNAc-T2 gene in gastric cancer SGC7901
cells might exert anti-growth and anti-metastasic activity through
the decrease of MMP-2 and TGF-β1. Our findings indicate that
ppGalNAc-T2 is useful in regulating gastric carcinoma invasion and
metastasis, and this may be used as a novel approach for cancer
therapy.
Materials and methods
Cell culture
The SGC7901 human gastric cancer, SHG44 glioma,
SHI-1 leukemia, A549 lung adenocarcinoma, and HO8910 ovarian cancer
cell lines were obtained from Shanghai Cell Bank (Shanghai, China).
They were cultured in RPMI-1640 (Gibco, USA) containing 10% fetal
bovine serum (FBS) in a humidified atmosphere with 5%
CO2 at 37°C. They were selected as all these poorly
differentiated cells often have aberrant terminal sugar structures
of O-glycan chains.
Generation and selection of cells stably
transfected with pEGFP-C1-ppGalNAc-T2 sense vectors and
pEGFP-C1-ppGalNAc-T2 antisense vectors
Transfection was carried out using Lipofectamine™
2000 (Invitrogen, Carlsbad, USA), according to the manufacturer’s
instructions. SGC7901 cells (2x105) were plated onto
6-well plates until they reached 70–90% confluency before
transfection. Cells were transfected with 4 μg of
pEGFP-C1-ppGalNAc-T2 sense vectors (SGC7901-T2s group) and
pEGFP-C1-ppGalNAc-T2 antisense vectors (SGC7901-T2as group),
followed by selection with G418 (500 μg/ml). Individual clones were
isolated and expanded for further characterization. The empty
vector pEGFP-C1 was also transfected into SGC7901 cells and served
as the control group. Transfection efficiency was detected by
fluorescence microscopy (Zeiss; Gottingen, Germany). All plasmids
were constructed and conserved in our laboratory (13,15).
Cell proliferation assay
Cell proliferation was measured with MTT assay.
Briefly, SGC7901 cells and the stably transfected clones were
plated in 96-well plates at a density of cells (5x103)
and 180 μl culture medium was added to each well. The cells were
incubated at 37°C for 24, 48, 72, 96 or 120 h, at which time the
cells were incubated with 100 μl of MTT solution (5 g/l; Sigma, St.
Louis, MO, USA) for 4 h. The reaction was stopped by the addition
of l50 μl DMSO (Sigma) and the absorbance of samples at 570 nm was
then measured. A growth curve was plotted for each sample as the
log cell number vs. time, and the growth rates were derived from
the slope of each growth curve. Three independent experiments were
performed and the results were used for plotting the relative
growth rate with SD.
In vitro cell adhesion assay
The adhesion of SGC7901 cells stably transfected
with sense or antisense ppGalNAc-T2 vectors was performed using
standard methods. A flat-bottomed 96-well plate was coated
overnight at 4°C with 0.2 ml Matrigel (200 μg/ml). Some wells were
left uncoated as negative control. The plate was washed twice with
phosphate-buffered saline (PBS), blocked with 1 mg/ml bovine serum
albumin (BSA) for 2 h at 37°C and then 0.5 ml suspension of tumor
cells (5x103) were added. After the plate was incubated
at 0.5, 1 and 1.5 h intervals at 37°C, unattached cells were
removed by washing with PBS. MTT was added to each well and the
absorbance value obtained by seeding uncoated wells represented
100% adhesion and all other values were divided by this to
calculate percentage adhesion.
Furthermore, to investigate the adhesion of cells to
various extra-cellular matrix (ECM) components, 96-well plates were
precoated with either 1 mg/ml hyaluronic acid (HA) or 50 1 g/ml
fibronectin (FN). The adhesive ability of gastric cancer cells was
also detected as described above.
In vitro cell invasion assay
The invasiveness of different SGC7901 stable cells
was evaluated in 24-well Transwell chambers (Costar Corporation,
Cambridge, MA, USA), according to the manufacturer’s instructions.
Briefly, Transwell chambers equipped with polycarbonate membrane
(12 mm pore size) were precoated with 6.25 mg/l Matrigel on the
upper chamber. The cells were cultured in serum-free medium for
12–24 h. Cells (1x105) were seeded in each transwell
insert containing 200 μl of serum-free medium with BSA. Then 500 μl
of culture medium with 10% FBS was added into each well of a
24-well plate. The cells and Matrigel on the upper chamber were
removed using a cotton stick after 12 h. Cell penetration through
the membrane was quantified by counting the number of cells that
penetrated the membrane in ten microscopic fields (at x200
magnification) per filter. The experiment was repeated 3 times.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total-RNA was isolated from equal cell numbers using
Tri Reagent (Sigma) following the manufacturer’s instructions.
Reverse transcription was as previously described and 5 μl of the
resultant cDNA was used as template for PCR (16). The sequences for primers with
annealing temperatures indicated in brackets were as follows:
ppGalNAc-T2, 5′-AAGAAAGACCTTCATCACAGCAATGGAGAA-3′ (forward) and
5′-ATCAAAACCGCCCTTCAAGTCAGCA-3′ (reverse) (60°C); MMP-2,
5′-AGATCTGCAAACAGGACA TTGTATT-3′ (forward) and
5′-TTCTTCTTCACCTCATTG TATCTCC-3′ (reverse) (56°C); MMP-14,
5′-TGGCGGGTGA GGAATAAC-3′ (forward) and 5′-GGGAACGCTGGCAGT AGAG-3′
(reverse) (56°C); TGF-β1, 5′-TGTGGCTACTGGT GCTGAC-3′ (forward) and
5′-ATAGATTTCGTTGTGGG TTTC-3′ (reverse) (56°C); β-actin,
5′-CATGTACGTTGCTA TCCAGGC-3′ (forward) and 5′-CTCCTTAATGTCACGCA
CGAT-3′ (reverse) (52°C). The number of PCR cycles used was 30 and
the expected product size after primer amplification was as
follows: ppGalNAc-T2, 669 bp; MMP-2, 332 bp; MMP-14, 690 bp;
TGF-β1, 317 bp; and β-actin, 330 bp. The PCR products were
separated by electrophoresis on 10 g/l agarose gels and visualized
by ethidium bromide staining.
Western blot analysis
To detect ppGalNAc-T2, MMP-2, MMP-14 and TGF-β1
protein expression in gastric cancer cells after the indicated
treatment, cells were harvested and extracted using the standard
methods. Equal amounts of protein (50 μg) were separated by
SDS-PAGE and transferred to a polyvinylidene difluoride membrane.
The membrane was blocked with 5% skim milk in Tris-buffered saline
and then incubated with primary antibodies for 1 h at room
temperature. The proteins were analyzed using specific antibodies
as indicated. Horseradish peroxidase (HRP)-conjugated secondary
antibodies and an enhanced chemiluminescence (ECL) kit were used
for detection. Anti-human ppGalNAc-T2 monoclonal antibody was
produced from rabbits in our laboratory (14). Anti-β-actin rabbit mAb, anti-MMP-2
rabbit mAb, anti-MMP-14 rabbit mAb and anti-TGF-β1 rabbit mAb as
well as the anti-rabbit second antibody were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Statistical analysis
The results shown are the mean ± SD. A P-value
<0.05 was considered to indicate statistically significant
differences. Statistical analyses were calculated using SPSS 11.5.
Each experiment was repeated 3 times.
Results
Expression of ppGalNAC-T2 mRNA in human
poorly differentiated malignant tumor cells
To investigate the potential role of ppGalNAc-T2 in
human malignant tumors, we first detected the expression panel of
ppGalNAc-T2 in 5 types of poorly differentiated malignant tumor
cell lines. The mRNA level of ppGalNAc-T2 in these cells was
determined by RT-PCR. All poorly differentiated tumor cells which
have aberrant terminal sugar structures of O-glycan chains,
including SGC7901 gastric cancer, SHG44 glioma, SHI-1 leukemia,
A549 lung adenocarcinoma, and HO8910 ovarian cancer cells expressed
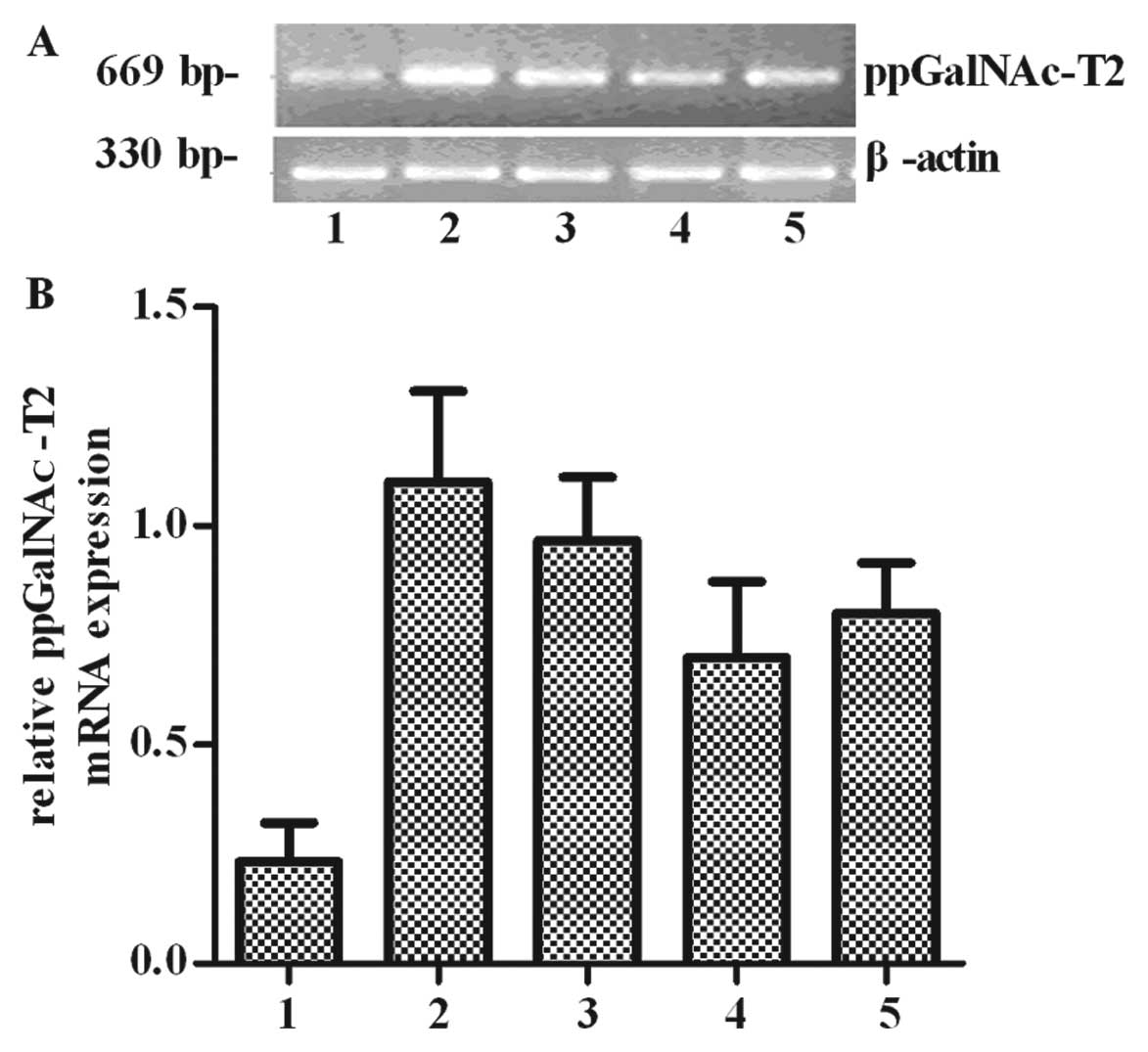
ppGalNAc-T2 (Fig. 1). Thus
ppGalNAc-T2 may be markers for poorly differentiated carcinomas. In
addition, we found that ppGalNAc-T2 mRNA was differentially
expressed, as shown in Fig. 1A.
The mRNA expression ratios (ppGalNAc-T2/β-actin) were 0.38±0.016,
1.23±0.017, 0.82±0.035, 0.69±0.014 and 0.78±0.023, respectively.
The results showed that ppGalNAc-T2 expression in SGC7901 cells was
higher than in other cells (P<0.05) (Fig. 1B), suggesting that this gene may
play a key role in gastric tumorigenesis. We therefore used gastric
cancer as our research model to determine whether ppGalNAc-T2 is
correlated with cell invasion and metastasis.
Establishment of ppGalNAc-T2
overexpression or downregulation of cells
To further explore the role of ppGalNAc-T2 in
gastric cancer, SGC7901 cells were used to reconstitute the
expression of ppGalNAc-T2 by stable overexpression (SGC7901-T2s) or
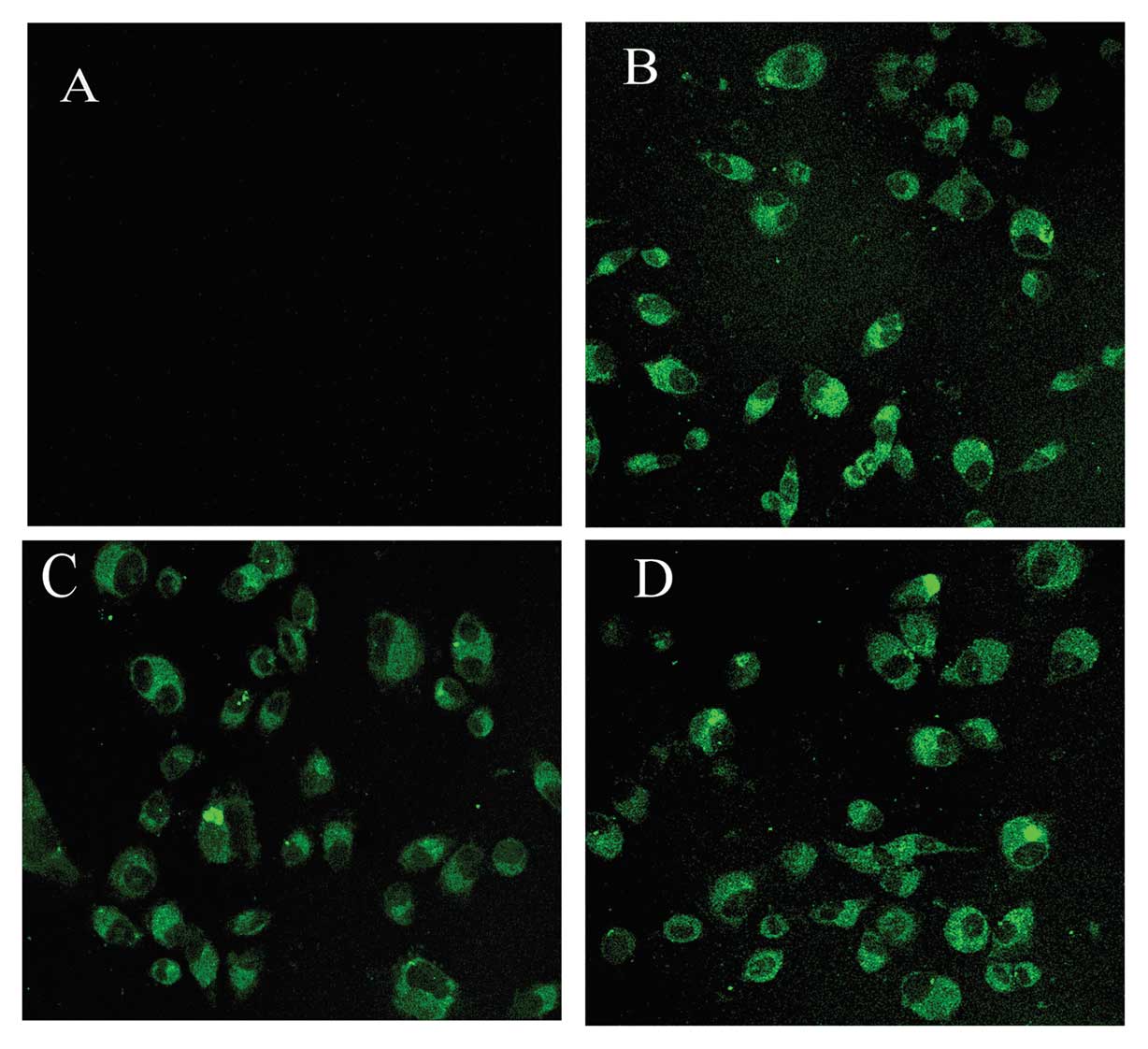
downregulation (SGC7901-T2as) of ppGalNAc-T2. Transfection
efficiency was measured using fluorescence microscopy to detect
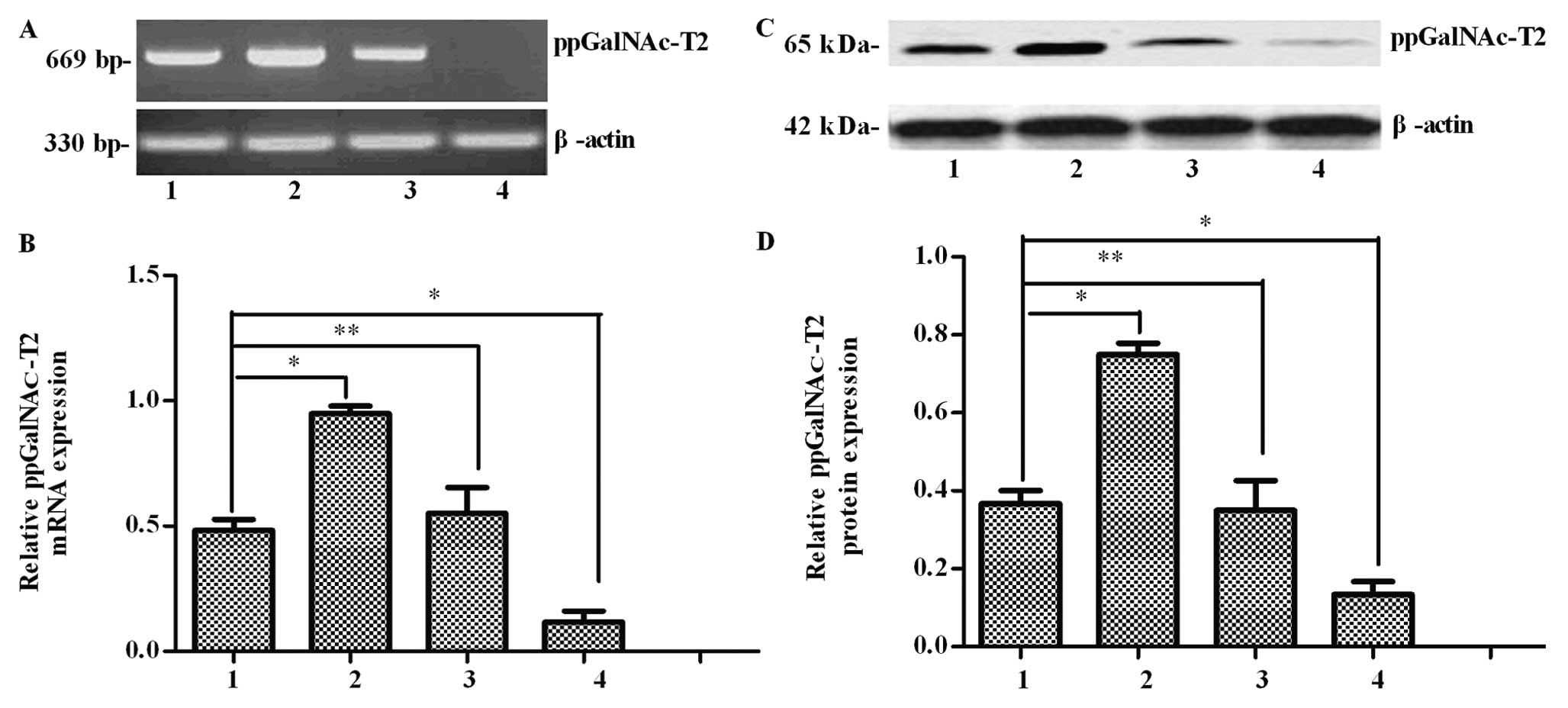
expression of the plasmid-encoded eGFP gene (Fig. 2). Then, the ppGalNAc-T2 mRNA and
protein levels in the SGC7901 cells were measured by RT-PCR and
western blot analysis, respectively. When compared with untreated
cells, ppGalNAc-T2 transcripts were increased in the
pEGFP-C1-ppGalNAc-T2 sense vector transfected cells (P<0.05)
(Fig. 3A and B). Consistent with
the RT-PCR results, the expression of the ppGalNAc-T2 protein was
clearly increased in this group (P<0.05) (Fig. 3C and D). Furthermore, ppGalNAc-T2
mRNA and protein expression was suppressed in the SGC7901-T2as
group when compared to the untreated group (P<0.05), while no
difference was found between the control group and the untreated
cells (P>0.05). The above results indicate the successful
construction of the ppGalNAc-T2 overexpression or downregulation
cell lines. These stable cell lines can be effectively used to
further examine the role of ppGalNAc-T2.
Effect of ppGalNAc-T2 on the viability of
SGC7901 cells
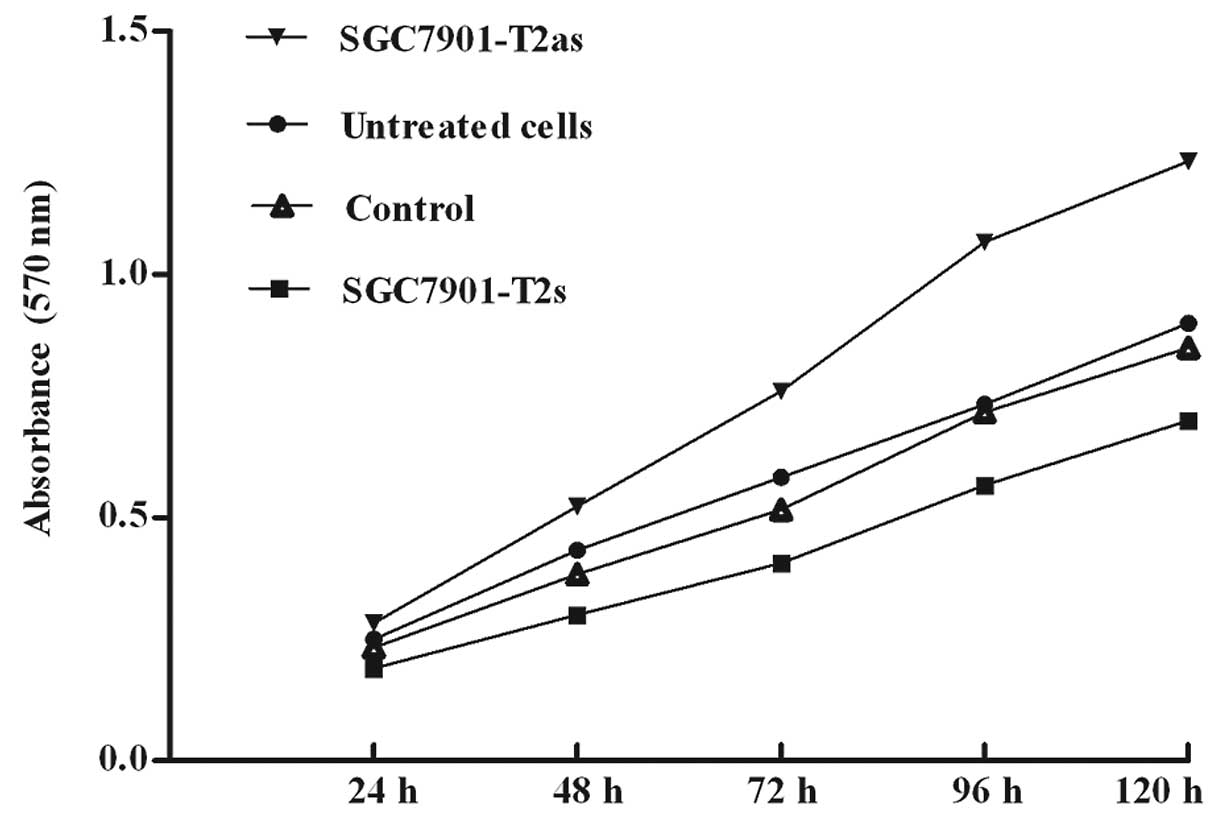
To examine whether modulation of ppGalNAc-T2
expression affects the tumorigenic properties of the gastric cancer
cells, we measured the abilities of in vitro cell
proliferation by MTT assay. The untreated SGC7901, control, as well
as the SGC7901-T2s and SGC7901-T2as cells were grown in culture for
5 days. The ability of cell proliferation in the SGC7901-T2s cells
was decreased compared with the control or untreated cells but
increased in the SGC7901-T2as cells (P>0.05) (Fig. 4). Treatment of SGC7901 cells with
ppGalNAc-T2 sense vectors was associated with a time-dependent
inhibition of cell growth, whereas no significant inhibitory effect
was observed in the untreated and control cells. These results
indicate that multi-step molecular events are necessary for the
function of ppGalNAc-T2 to switch the SGC7901 cells from a
proliferative state to an inhibited state of cell growth.
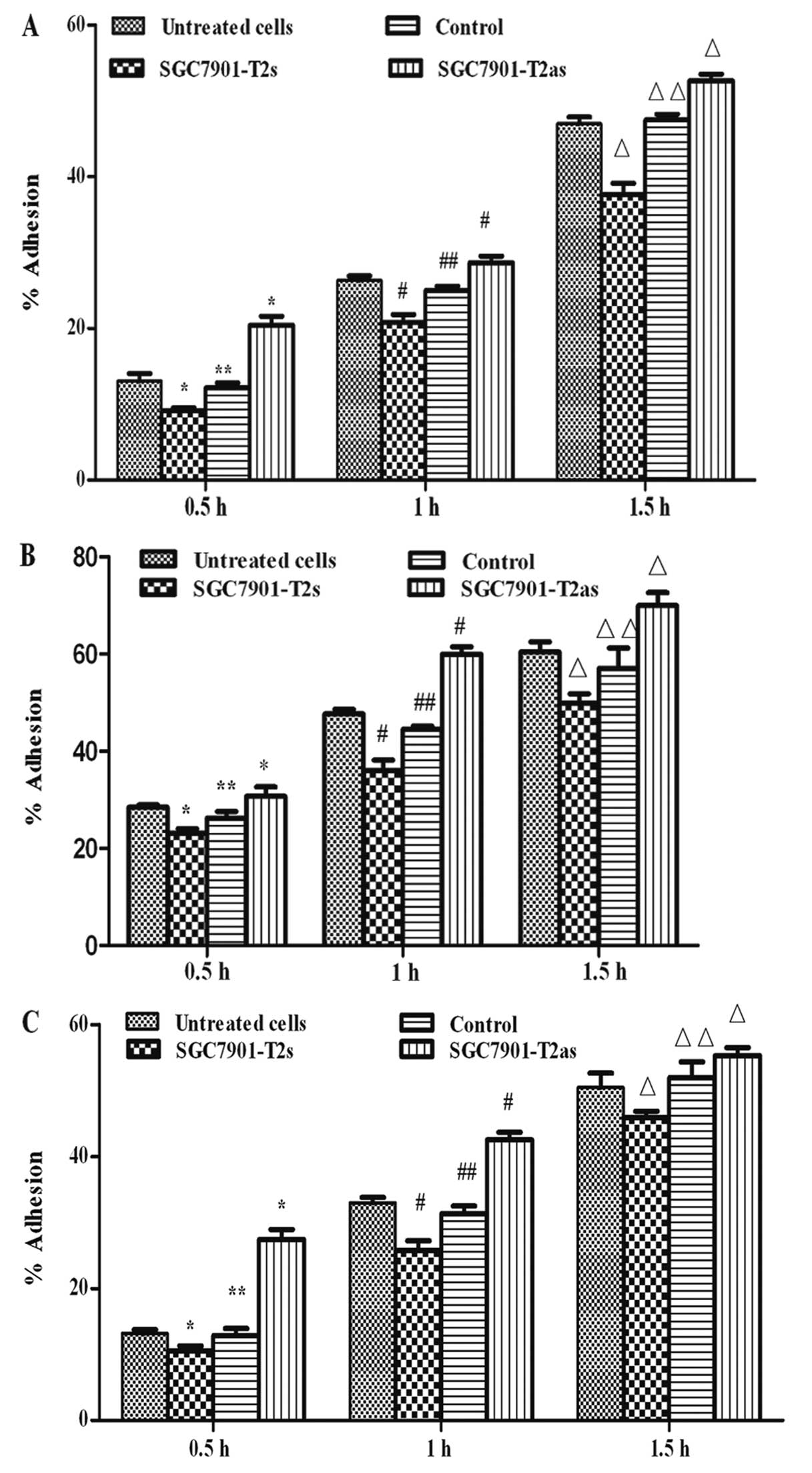
Effect of ppGalNAc-T2 on cell
adhesion
Adhesion is a key event in the metastasic process
where cells must first adhere to the ECM prior to its degradation.
To examine whether ppGalNAc-T2 expression is associated with
adhesion of gastric cancer, in vitro adhesion assay was
carried out to evaluate the adhesive ability of the untreated
SGC7901, control, SGC7901-T2s and SGC7901-T2as cells. The ability
of cell adhesion in the SGC7901-T2s group cells was decreased
compared with untreated or control SGC7901 cells (P<0.05), but
increased in the SGC7901-T2as group cells at different time points
(P<0.05) (Figs. 5A, 5B and
5C).
To further investigate the behavior of cells in the
presence of ECM components, adhesion assays were carried out in the
presence of HA and FN. Increased cell-cell signaling and contact is
also mediated by increased expression of cell adhesion molecules.
The control, untreated, as well as the SGC7901-T2s and SGC7901-T2as
group cells were cultured in the presence of HA and FN.
Overexpression of ppGalNAc-T2 led to an average of 32.5% decreased
adhesive ability compared with untreated clones at different time
points. Conversely, downregulated ppGalNAc-T2 expression caused an
average of 58.2% increase in the adhesive ability in the
SGC7901-T2as group at different time points (Fig. 5B and C), while no difference was
found between the control group and the untreated SGC7901 cells
(P>0.05). These results suggest that ppGalNAc-T2 expression is
associated with the adhesion of SGC7901 cells in vitro;
therefore, overexpression of ppGalNAc-T2 has a significant
anti-adhesion effect at all intervals.
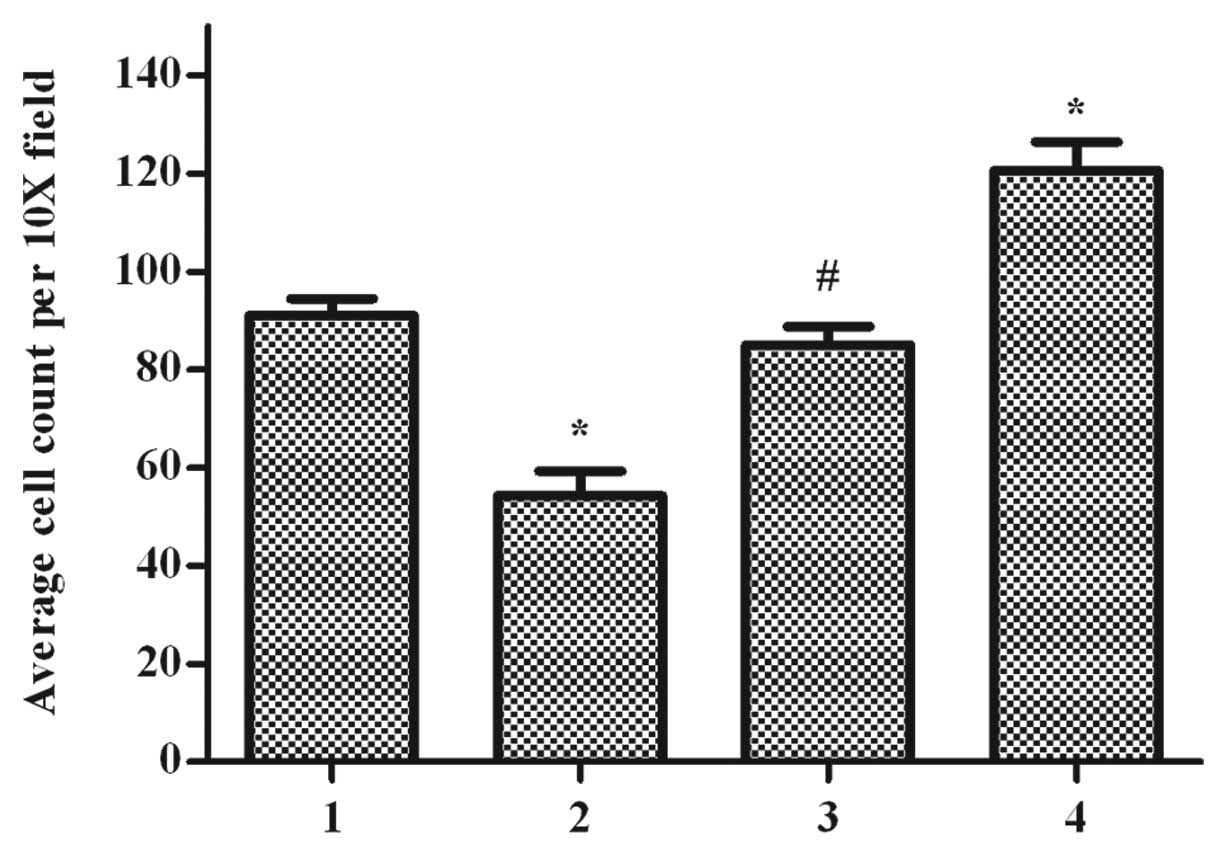
Effect of ppGalNAc-T2 on the invasive
capability of cells
Since ECM degradation is key to tumor cell invasion,
the in vitro invasiveness of these cell lines through
Matrigel coated membranes was compared. Different invasiveness was
observed in the control, untreated, as well as the SGC7901-T2s and
SGC7901-T2as group cells, respectively (Fig. 6). The control group cells showed
little invasion in comparison to the untreated cells (P>0.05),
whereas overexpression of ppGalNAc-T2 in SGC7901-T2s cells
decreased their migratory capacity (P<0.05). By contrast,
downregulation of ppGalNAc-T2 increased the invasive ability in the
SGC7901-T2as group (P<0.05). These results suggested that
ppGalNAc-T2 expression was inversely associated with the
invasiveness of cells in vitro. The inverse correlation
tendency between ppGalNAc-T2 expression in SGC7901 cells and their
in vitro invasive ability indicates that ppGalNAc-T2 is
likely to be a metastasis suppressor gene in SGC7901.
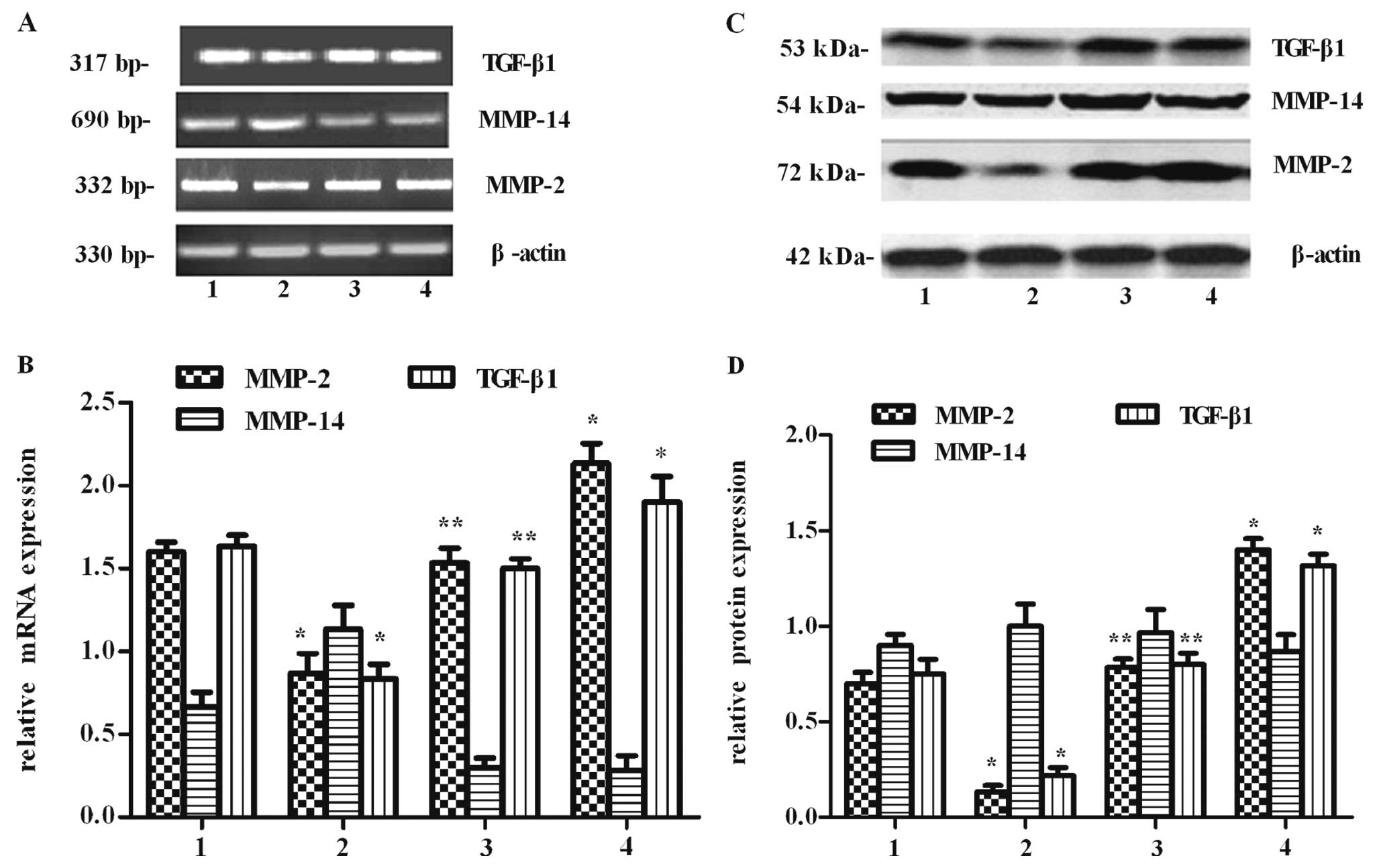
Effect of ppGalNAc-T2 on MMP-2 and MMP-14
expression
Among the MMP family that has been identified, MMP-2
is considered a key enzyme since it is responsible for degradation
of the ECM. Meanwhile, MMP-2 activity can be activated by MMP-14,
and this activity may be involved in tumor invasion and metastasis.
Therefore, to investigate whether the metastasic inhibitory effect
of ppGalNAc-T2 resulted from the suppression of MMP-2 and MMP-14
expression, MMP-2 and MMP-14 mRNA and protein levels were measured.
Using RT-PCR, we found that the expression of MMP-2 at the mRNA
level was lower in the SGC7901-T2s group than in the SGC7901-T2as
group (P<0.05), and there was no difference between untreated
and control group cells (P>0.05) (Fig. 7A and B). However, there was no
evident change on the mRNA transcriptional expression of
MMP-14.
The protein levels from whole-cell lysates of MMP-2
and MMP-14 were further assessed by western blot analysis (Fig. 7C and D), respectively. We found
that the expression of MMP-2 at the protein level was increased in
the ppGalNAc-T2-downregulation cells, but decreased in the
ppGalNAc-T2-overexpressing cells (P<0.05). Similar to the RT-PCR
results, the expression of the MMP-14 protein presented no
noticeable difference in all groups (P>0.05). The changes in the
protein levels of MMP-2 and MMP-14 coincided with their mRNA
levels, indicating that ppGalNAc-T2 might regulate MMP-2 but not
MMP-14 expressions at the transcriptional level.
Effect of ppGalNAc-T2 on TGF-β1
expression
TGF-β1 promotes tumor progression through the
upregulation of MMP-2. To investigate whether the MMP-2 inhibitory
effect of ppGalNAc-T2 resulted from the suppression of TGF-β1
expression, TGF-β1 mRNA and protein levels were measured. The
SGC7901 cells transfected with ppGalNAc-T2 sense vectors exhibited
a direct reduction at the levels of TGF-β1 mRNA and protein
(P<0.05) (Fig. 7). Meanwhile,
the expression of TGF-β1 in the SGC7901-T2as group was contrary to
that in the SGC7901-T2s group (P<0.05). The change of TGF-β1 at
the mRNA and protein level displayed a similar trend with that of
MMP-2. These results suggest that the MMP-2 inhibitory effect by
high expression of ppGalNAc-T2 is probably through regulation of
TGF-β1 expression.
Discussion
Although multiple factors contribute to aberrant
glycosylation in cancer, such as the availability and localization
of nucleotide sugar donors and substrates, one of the primary
mechanisms seems to be the differential expression of
glycosyltransferases involved in the synthesis of glycans.
Mucin-type linkages (GalNAcα1-O-Ser/Thr) of proteins begin with the
addition of a single GalNAc monosaccharide to a serine or threonine
residue on the polypeptide. Attachment is catalyzed by a family of
glycosyltransferases called the UDP-N-acetylgalactosamine:
polypeptide N-acetylgalactosaminyltransferases (ppGalNAc-Ts, EC
2.4.1.41), which is a crucial regulatory step (17,18). These structural changes can alter
the function of the cell, and its antigenic and adhesive
properties, as well as its potential to invade and metastasize.
PpGalNAc-T2, an important member of ppGalNAc-Ts, is often variable
in cells of different types and differentiation, and the expression
may change in cancer cells (12).
Herein, we investigated the mRNA expression of ppGalNAc-T2 in
several human poorly differentiated cancer cells, including SGC7901
gastric cancer cells (19), SHG44
glioma cells (20), SHI-1
leukemia cells (21), A549 lung
adenocarcinoma cells (22), and
HO8910 ovarian cancer cells (23). We found that all of these cells
could express ppGalNAc-T2, however, mRNA expression in SGC7901
cells appeared to be higher than in other cells. Furthermore,
ppGalNAc-T2 was identified as a metastasis-associated gene, which
was highly expressed in human acute T cells, heptocarcinoma HepG-2
cells, and glioma U251 cells (14,15). However, the expression of
ppGalNAc-T2 in gastric cancer has not been previously reported. In
this regard, we first demonstrated that SGC7901 cells show
significantly increased expression of ppGalNAc-T2 compared with
other poorly differentiated tumor, suggesting that expression
levels of ppGalNAc-T2 in gastric cancer are associated with a high
risk of metastasis.
Tumor metastasis occurs by a series of steps
including cell attachment, invasion, and proliferation, and is
regulated by extremely complicated mechanisms (24,25). For example, patients with gastric
cancer generally have metastasis when clinically examined. There is
a low 5-year survival rate and poor quality of life even after
tumor resection. The SGC-7901 human gastric cell line was first
established from the metastatic lymph node of a 56-year-old female
patient suffering from gastric adenocarcinoma (26). To examine the potential
anti-metastasic effects of ppGalNAc-T2, proliferation, adhesion and
invasion assays were performed on SGC7901 cells. Regardless of the
exact mechanism of ppGalNAc-T2 in the cell metastatic process,
pEGFP-C1-ppGalNAc-T2 sense vectors or pEGFP-C1-ppGalNAc-T2
antisense vectors were transfected into SGC7901 cells to
reconstitute the expression of ppGalNAc-T2, focusing on the changes
in the characteristics of cell metastasis-associated behavior. In
ppGalNAc-T2 overexpressed cells (SGC7901-T2s group), proliferation,
adhesion, and invasion were decreased compared with untreated
SGC7901 cells, whereas the values for the same assays were
increased in ppGalNAc-T2 downregulated cells (SGC7901-T2as
group).
Since adhesion is considered a key in regulating
cell growth at the metastatic secondary site (27,28), in this study we first proved
ppGalNAc-T2 expression affected the adhesive ability of SGC7901
cells by in vitro cell adhesion assay. In addition, the
degradation of basal membrane and ECM of primary tumor are crucial
steps for tumor invasion and metastasis. We also looked at adhesion
of the cells on various ECM components including FN and HA. The
ppGalNAc-T2 antisense vectors transfected cells attached better to
FN and HA than any other cell line. Thus, upregulated expression of
ppGalNAc-T2 is likely to inhibit the growth of the cancer cells in
the metastatic sites due to a decrease in cell adhesive
ability.
Proteins of the MMP family are involved in the
breakdown of ECM in normal physiological processes, such as
embryonic development, reproduction, and tissue remodeling, as well
as in disease processes, such as arthritis and metastasis (29). Among the many MMPs that have been
identified, MMP-2 encodes an enzyme which degrades type IV
collagen, the major structural component of basement membranes.
MMP-14 activates MMP-2 protein, and this activity may be involved
in tumor invasion (30). Thus, we
examined the effect of ppGalNAc-T2 on the expression of MMP-2 and
MMP-14 in SGC7901 cells. Our study revealed that expression of
ppGalNAc-T2 had an inverse correlation with the expression of MMP-2
at the mRNA and protein levels. It is highly likely that
ppGalNAc-T2 regulates the proliferation, adhesion, and
invasiveness, all of which are essential steps for the
establishment of metastasis of SGC7901 cells, due to the regulation
of the MMP-2 signaling pathway. However, it did not exhibit any
apparent correlation with MMP-14 expression. MMP-14 has been shown
to interact with tissue inhibitor of metalloproteinase-2 (TIMP-2)
(31). Further experiments are
required to determine if ppGalNAc-T2 functions in SGC7901 cells, in
part, by TIMP-2 signaling pathways.
The multifunctional cytokine transforming growth
factor-β1 (TGF-β1) plays a dual role in the process of
carcinogenesis by promoting tumor progression by enhancing
migration, invasion and survival of tumor cells. TGF-β1 has been
proven to play a key role in activating MMP-2 (32). We also investigated the effect of
ppGalNAc-T2 on the expression of TGF-β1 in SGC7901 cells.
Consistent with this concept, we confirmed that in the SGC7901-T2s
group, TGF-β1 was decreased compared with control or untreated
cells, whereas downregulation of ppGalNAc-T2 mRNA and protein
levels induced activation of TGF-β1. These results suggest that the
MMP-2 inhibitory effect by high expression of ppGalNAc-T2 is
probably through regulation of TGF-β1 expression.
In conclusion, the present study demonstrated that
high expression of ppGalNAc-T2 significantly inhibits the
metastasic ability of SGC7901 human gastric cancer cells. The
proposed anti-growth and anti-metastasic mechanisms might be
mediated through the inhibition of MMP-2 and TGF-β1. These results
indicate that ppGalNAc-T2 is also a metastasis regulation gene of
human gastric cancer. We suggest that ppGalNAc-T2 can be used as a
novel therapeutic target for human gastric treatment. However, to
provide a potential valuable therapeutic strategy for gastric
cancer metastasis, it is necessary to further investigate the
underlying molecular mechanism of ppGalNAc-T2 in suppressing
SGC7901 cell metastasis.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant nos. 30670462
and 31170772).
References
|
1
|
Brooks SA, Carter TM, Bennett EP, Clausen
H and Mandel U: Immunolocalisation of members of the polypeptide
N-acetylgalactosaminyl transferase (ppGalNAc-T) family is
consistent with biologically relevant altered cell surface
glycosylation in breast cancer. Acta Histochem. 109:273–284. 2007.
View Article : Google Scholar
|
|
2
|
Zhang H, Meng F, Wu S, et al: Engagement
of I-branching {beta}-1, 6-N-acetylglucosaminyltransferase 2 in
breast cancer metastasis and TGF-{beta} signaling. Cancer Res.
71:4846–4856. 2011.PubMed/NCBI
|
|
3
|
Christie DR, Shaikh FM, Lucas JA IV, Lucas
JA III and Bellis SL: ST6Gal-I expression in ovarian cancer cells
promotes an invasive phenotype by altering integrin glycosylation
and function. J Ovarian Res. 1:32008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meany DL and Chan DW: Aberrant
glycosylation associated with enzymes as cancer biomarkers. Clin
Proteomics. 8:72011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petretti T, Schulze B, Schlag PM and
Kemmner W: Altered mRNA expression of glycosyltransferases in human
gastric carcinomas. Biochim Biophys Acta. 1428:209–218. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chandrasekaran EV, Xue J, Piskorz C, et
al: Potential tumor markers for human gastric cancer: an elevation
of glycan:sulfotransferases and a concomitant loss of
alpha1,2-fucosyltransferase activities. J Cancer Res Clin Oncol.
133:599–611. 2007. View Article : Google Scholar
|
|
7
|
Shimizu F, Nakayama J, Ishizone S, et al:
Usefulness of the real-time reverse transcription-polymerase chain
reaction assay targeted to alpha1,4-N-acetylglucosaminyltransferase
for the detection of gastric cancer. Lab Invest. 83:187–197. 2003.
View Article : Google Scholar
|
|
8
|
Liu Z, Shen L, Xu L, Sun X, Zhou J and Wu
S: Downregulation of β-1,3-N-acetylglucosaminyltransferase-8 by
siRNA inhibits the growth of human gastric cancer. Mol Med Rep.
4:497–503. 2011.
|
|
9
|
Zlocowski N, Sendra VG, Lorenz V, et al:
Catalytic and glycan-binding abilities of ppGalNAc-T2 are regulated
by acetylation. Biochem Biophys Res Commun. 410:140–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Wang J, Li W, et al:
Characterization of ppGalNAc-T18, a member of the
vertebrate-specific Y subfamily of
UDP-N-acetyl-alpha-D-galactosamine: polypeptide
N-acetylgalactosaminyltransferases. Glycobiology. 22:602–615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng C, Togayachi A, Kwon YD, et al:
Identification of a novel human UDP-GalNAc transferase with unique
catalytic activity and expression profile. Biochem Biophys Res
Commun. 402:680–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mandel U, Hassan H, Therkildsen MH, et al:
Expression of polypeptide GalNAc-transferases in stratified
epithelia and squamous cell carcinomas: immunohistological
evaluation using monoclonal antibodies to three members of the
GalNAc-transferase family. Glycobiology. 9:43–52. 1999. View Article : Google Scholar
|
|
13
|
Gao Y, Tu YB, Guo Y, et al: PpGalNacT2
participating in vanadium-induced HL-60 cell differentiation. Mol
Biol Rep. 38:1483–1489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Lin D, Xu L, Jiang Z, Zhou Y and Wu
S: An anti-human ppGalNAcT-2 monoclonal antibody. Hybridoma.
30:549–554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Yang L, Jin M, Xu L and Wu S:
regulation of the invasion and metastasis of human glioma cells by
polypeptide N-acetylgalactosaminyltransferase 2. Mol Med Rep.
4:1299–1305. 2011.PubMed/NCBI
|
|
16
|
Shen L, Liu Z, Tu Y, Xu L, Sun X and Wu S:
Regulation of MMP-2 expression and activity by
beta-1,3-N-acetylglucosaminyltransferase-8 in AGS gastric cancer
cells. Mol Biol Rep. 38:1541–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milac AL, Buchete NV, Fritz TA, Hummer G
and Tabak LA: Substrate-induced conformational changes and dynamics
of UDP-N-acetylgalactosamine:polypeptide
N-acetylgalactosaminyltransferase-2. J Mol Biol. 373:439–451. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ten Hagen KG, Fritz TA and Tabak LA: All
in the family: the UDP-GalNAc:polypeptide
N-acetylgalactosaminyltransferases. Glycobiology. 13:1R–16R.
2003.PubMed/NCBI
|
|
19
|
Wei M, Wang Z, Yao H, et al: P27(Kip1),
regulated by glycogen synthase kinase-3beta, results in
HMBA-induced differentiation of human gastric cancer cells. BMC
Cancer. 11:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zeng Y, Yang Z, Xu JG, Yang MS, Zeng ZX
and You C: Differentially expressed genes from the glioblastoma
cell line SHG-44 treated with all-trans retinoic acid in vitro. J
Clin Neurosci. 16:285–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu H, Guo XH, Mo JH, Jin MF, Wu SL and
Chen HL: Expressions of polypeptide:
N-acetylgalactosaminyltransferase in leukemia cell lines during
1,25-dihydroxyvitamin D3 induced differentiation. Glycoconj J.
23:575–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia HP, Look DC, Shi L, et al: ACE2
receptor expression and severe acute respiratory syndrome
coronavirus infection depend on differentiation of human airway
epithelia. J Virol. 79:14614–14621. 2005. View Article : Google Scholar
|
|
23
|
Xu S, Mou H, Lu G, et al: Gene expression
profile differences in high and low metastatic human ovarian cancer
cell lines by gene chip. Chin Med J (Engl). 115:36–41.
2002.PubMed/NCBI
|
|
24
|
Langley RR and Fidler IJ: Tumor cell-organ
microenvironment interactions in the pathogenesis of cancer
metastasis. Endocr Rev. 28:297–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fidler IJ: The organ microenvironment and
cancer metastasis. Differentiation. 70:498–505. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Li B, Xiang CP, Zhang Y, Li YY and
Wu XL: Characterization of gastric cancer models from different
cell lines orthotopically constructed using improved implantation
techniques. World J Gastroenterol. 18:136–143. 2012. View Article : Google Scholar
|
|
27
|
Li HZ, Wang Y, Gao Y, et al: Effects of
raf kinase inhibitor protein expression on metastasis and
progression of human epithelial ovarian cancer. Mol Cancer Res.
6:917–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HZ, Gao Y, Zhao XL, et al: Effects of
raf kinase inhibitor protein expression on metastasis and
progression of human breast cancer. Mol Cancer Res. 7:832–840.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao M, Zhang JH, Zhou FX, et al:
Angelica sinensis suppresses human lung adenocarcinoma A549
cell metastasis by regulating MMPs/TIMPs and TGF-β1. Oncol Rep.
27:585–593. 2012.
|
|
30
|
Sato H, Takino T, Okada Y, et al: A matrix
metalloproteinase expressed on the surface of invasive tumour
cells. Nature. 370:61–65. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zucker S, Drews M, Conner C, et al: Tissue
inhibitor of metalloproteinase-2 (TIMP-2) binds to the catalytic
domain of the cell surface receptor, membrane type 1-matrix
metalloproteinase 1 (MT1-MMP). J Biol Chem. 273:1216–1222. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taipale J, Miyazono K, Heldin CH and
Keski-Oja J: Latent transforming growth factor-beta 1 associates to
fibroblast extra-cellular matrix via latent TGF-beta binding
protein. J Cell Biol. 124:171–181. 1994. View Article : Google Scholar : PubMed/NCBI
|