Introduction
The vascular endothelial growth factor (VEGF) is
considered a major factor mediating endothelial cell survival,
migration, and proliferation during angiogenesis, and has been
shown to be upregulated in various types of tumor cells and also
plays a major role in prostate carcinoma development (1,2).
Therefore, the inhibition of VEGF expression has been considered as
a potential approach for cancer therapy (3–5).
RNA interference (RNAi), initiated by small interfering RNA
(siRNA), induces the sequence-specific degradation of complementary
mRNA and leads to the loss of target gene expression (6,7).
Human VEGF (hVEGF)-siRNA has been used to silence VEGF expression
in PC-3 cells. However, inherent instability along with poor or
non-specific cellular uptake has limited its usefulness.
The application of non-viral systems for gene
delivery is being increasingly advocated due to low immunogenicity,
unlimited payload capacity, absence of endogenous viral
recombination, as wel as low production costs. Nanoparticles (NPs)
that are non-toxic, biocompatible and biodegradable have been
widely used as efficient carrier materials for gene delivery. In
our previous study, mPEG-PLGA-PLL triblock copolymers were
constructed for siRNA delivery (8). The NPs could successfully transfered
siRNA into the tumor cells, and demonstrated higher gene inhibition
efficiency than the control groups, while showing no cytotoxicity
(8). On the basis of previous
findings, this study focused on the surface-modification of
mPEG-PLGA-PLL triblock copolymers with cyclic arginine
(Arg)-glycine (Gly)-aspartic acid (Asp) (cRGD) ligands to recognize
the target site, integrin αvβ3, expressed in high quantities in
activated endothelial cells and certain tumor cells (9,10),
including PC-3 prostate cancer cells (11). The ligand binding of the cRGD
peptide is expected to significantly enhance the ability of
mPEG-PLGA-PLL-cRGD to bind to PC-3 cells and targeted NPs (TNPs)
are expected to be a powerful vector for effective gene therapy
against cancer. In our study, human-VEGF-siRNA was encapsulated in
TNPs to inhibit VEGF expression in PC-3 cells. Non-targeted NPs
(NNPs) were also encapsulated as the control.
Ultrasound-targeted microbubble destruction (UTMD)
has been proven to enhance gene transfer and may serve as a
potential site-specific gene transfer modality. Sonoporation
induced by UTMD leads to the transient and reversible increase in
the permeability of cell membranes when exposed to ultrasound. In
our previous as well as other studies, UTMD was utilized to
facilitate the transfer of siRNA-loaded NPs across the cell
membrane (12,13). Although the process of
sonoporation is not yet well understood and the bioeffects of
sonoporation are similar to the side-effects (14–16), the transient and long-term
viability of PC-3 cells did not decrease significantly under
optimized experimental conditions. This study aimed to explore the
efficient and safe delivery of siRNA for cancer therapy.
Materials and methods
Cell culture
Human prostate carcinoma cells (PC-3, Chinese
Academy of Sciences) were maintained in RPMI-1640 medium containing
10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C
in a humidified incubator with 5% CO2. Cell culture
reagents were all purchased from Gibco (Grand Island, NY, USA).
Cells were plated on a 12-well plate at a density of
1.5×105 cells/well.
Ultrasound exposure protocol
A therapeutic US machine (Physiomed, Erlangen,
Germany) was used and the area of the probe (1 MHz) was ~6.15
cm2. The groups were exposed to optimized ultrasound
conditions (power, 1.2 W cm−2; 20% duty cycle; exposure
time, 20 sec). The SonoVue powder (Bracco, Milan, Italy) was mixed
with 5 ml saline. After agitation for 30 sec, white galactoid
microbubble suspension was prepared.
Preparation of mPEG-PLGA-PLL NPs loading
siRNA
The preparation methods of mPEG-PLGA-PLL
siRNA-loaded NPs and their physicochemical characterization have
been described in our previous study (8). The particle size of the siRNA-loaded
NPs increased as the hVEGF-siRNA molecules were encapsulated in the
inner water phase of mPEG-PLGA-PLL NPs and cyclic Arg-Gly-Asp
(cRGD) peptides were conjugated to the surface of the NPs. NPs
loaded with siRNA but without cRGD were also constructed as the
control. The NPs were termed TNPs and NNPs. The siRNA-loaded NPs
were observed under an atomic force microscope (MultiMode 8;
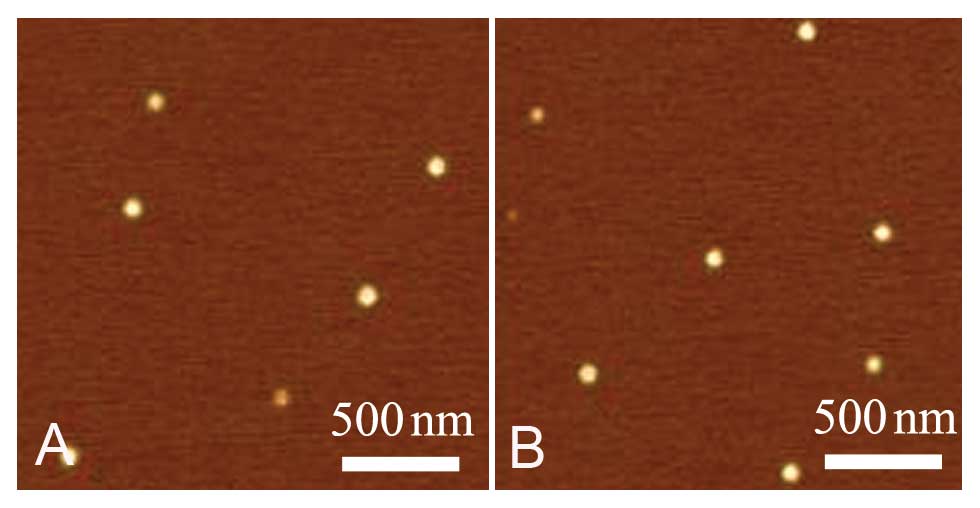
Veeco-Bruker, USA) and were shown to be spherical in shape. The
mean diameter of the NPs was ~112.0±4.0 nm (Fig. 1). All reagents for NPs were
purchased from Shanghai Yuanju Biotechnology Co. (Shanghai,
China).
SiRNA targeting human VEGF labeled with or without
Cy3 and cRGD were purchased from RayBiotech, Inc., Guangzhou,
China. Sequences were as follows: sense, 5′-GGAGUACCCUG AU
GAGAUCdTdT-3′ and antisense, 5′-GAUCUCAUCAGGGUA CUCCdTdT-3′.
Experimental grouping
In this study, the cells were divided into the
following 4 groups: i) the control group P, PC-3 cells with 60 μl
PBS; ii) the control group S, PC-3 cells with 60 μl siRNA; iii) the
control group N, PC-3 cells with 60 μl NNPs loaded with siRNA; and
iv) the test group T, PC-3 cells with 60 μl TNPs loaded with siRNA.
Each group was then devided into 3 subgroups: 1, no ultrasound (P1,
S1, N1, T1); 2, ultrasound alone (P2, S2, N2 and T2); and 3,
ultrasound + 80 μl SonoVue microbubbles (P3, S3, N3 and T3).
The mixture volume per well was added to 1 ml with
culture medium and the final concentration of siRNA was adjusted to
0.03 pmol/μl in the different groups. All experiments were carried
out in triplicate.
Transfection
We compared the cellular uptake efficiencies of the
different groups of cells as mentioned above. Cells were plated on
a 12-well plate at a density of 1.5×105 cells/well.
After 48 h of incubation, the medium was replaced by fresh medium
containing 10% FBS and the desired siRNA formulations were added to
the cells. siRNA labeled with fluorescent Cy3 dyes was used to
examine the uptake of PC-3 cells. After 48 h of incubation, a
fluorescent microscope was used for observation and the
quantitative cellular uptake of Cy3-siRNA was estimated using a
FACSCalibur flow cytometer. NNPs (without cRGD) loaded with siRNA
were observed in the perinuclear cytoplasmic region in our previous
studies. The intracellular localization of TNPs but not absorption
by the surface of cells was also confirmed by a confocal
microscope.
Real-time PCR
We used siRNA without Cy3 for relative molecular
experiments. To establish the hVEGF-siRNA silencing efficiency at
the mRNA level, a real-time PCR analysis of PC-3 cells was carried
out. Total RNA was extracted from the different groups at 2
time-points (after 24 and 48 h of transfection). Subsequently,
reverse transcription to synthesize the cDNA was carried out using
the First-Strand cDNA Synthesis kit (Takara, Tokyo, Japan).
Real-time PCR was then performed with cDNA using the
SYBR® Premix Ex Taq™ kit (Takara). The final results
were evaluated by the 2-ΔΔCT analytical method.
Programmed cell death 5 (PDCD5) as the sensitive
index of apoptosis was dynamicly monitored with real-time PCR at 6
time-points (after 2, 6, 12, 24, 48 and 72 h of transfection). The
expression level of PDCD5 was significantly higher during apoptosis
than in the normal cells. Therefore, it can be used to further
investigate the apoptosis-promoting effects of the VEGF-siRNA
interruption in PC-3 cells. PCR primers were designed and producted
by the Invitrogen Co. The PCR primer sequences were as follows:
VEGF forward, 5′-AAGATCCGC AGACGTGTAAATGTT-3′ and reverse,
5′-CGGCTTGTC ACATGCAAGTA-3′; PDCD5 forward, 5′-CTGAGGAGA
CAGAGGCTGGC-3′ and reverse, 5′-TTTCTGCTTCCCT GTGCTTTG-3′; and the
internal standard, GAPDH forward, 5′-CTTAGCACCCCTGGCCAAG-3′ and
reverse, 5′-GATGTT CTGGAGAGCCCCG-3′.
Detection of apoptosis by flow
cytometry
Phosphatidylserine (PS) externalization is one of
the main events occurring during the early stages of apoptosis. To
detect PS externalization, the transfected cells were harvested by
trypsinization and washed twice with PBS. The washed cells were
resuspended in 200 μl binding buffer (PBS containing 1 mM calcium
chloride). FITC-conjugated Annexin V and propidium iodide were
added according to the manufacturer’s instructions (Biosea
Biotechnology Co., Ltd., Beijing, China). After incubation for 20
min at room temperature, 400 μl binding buffer was added, and
samples were immediately analyzed on a FACSCalibur flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA) with excitation using a
488 nm argon ion laser. The samples were stained with propidium
iodide (PI) to distinguish necrotic and late apoptotic events from
early apoptotic events. The experiment was also monitored at 6
time-points by analyzing PDCD5 expression (after 2, 6, 12, 24, 48
and 72 h of transfection). Similar to real-time PCR, flow cytometry
is still able to cover the wide dynamic range required for
quantification.
VEGF protein assay with ELISA
The detection of VEGF protein expression following
transfection was carried out by ELISA quantitative assay. Cells
were plated on a 12-well plate at a density of 1.5×105
cells/well. After 48 h of incubation, the medium was replaced by
fresh medium without 10% FBS and the desired siRNA formulations
were added to the cells. PC-3 cell culture supernatant was
collected after 12, 24 and 48 h of transfection. The
human-VEGF-ELISA kit (4iBIO, Beijing, China) was used to determine
VEGF protein expression according to the manufacturer’s
instructions.
Cell viability assay
We performed trypan blue assay immediately after the
cells were treated to measure the transient cytotoxicity of UTMD
and NPs. The cells were then analyzed under a microscope to
determine the proportion of positive blue-stained cells.
Clonogenic assay
To investigate the effect of the siRNA interruption
on the proliferative ability of cells, and to evaluate the
side-effects induced by UTMD or NPs, clonogenic cell survival assay
was carried out. Cell viability depends not only on the intact cell
membrane but also on many other factors. PI commonly used in
sonoporation studies or drug toxicity, may not be a reliable
measure of cell long-term viability.
After incubation for 48 h at 37°C, the 12 groups of
cells were trypsinized and seeded into 12-well plates (100
cells/well). Ordinary culture medium was added. Two weeks later,
colonies were fixed and stained with Giemsa, and clones containing
>10 cells were counted. Each group was assayed in triplicate
wells.
Statistical analysis
Data are expressed as the means and standard
deviation (means ± SD). An independent samples t-test was used to
determine the significance of the difference between 2 groups. The
Kruskal-Wallis test was used to examine the significance by
multiple comparisons. Differences were considered significant at
P<0.05. Statistical analysis was performed with a software
package (SPSS, version 13.0; SPSS, Chicago, IL, USA).
Results
Analysis of transfection efficiency
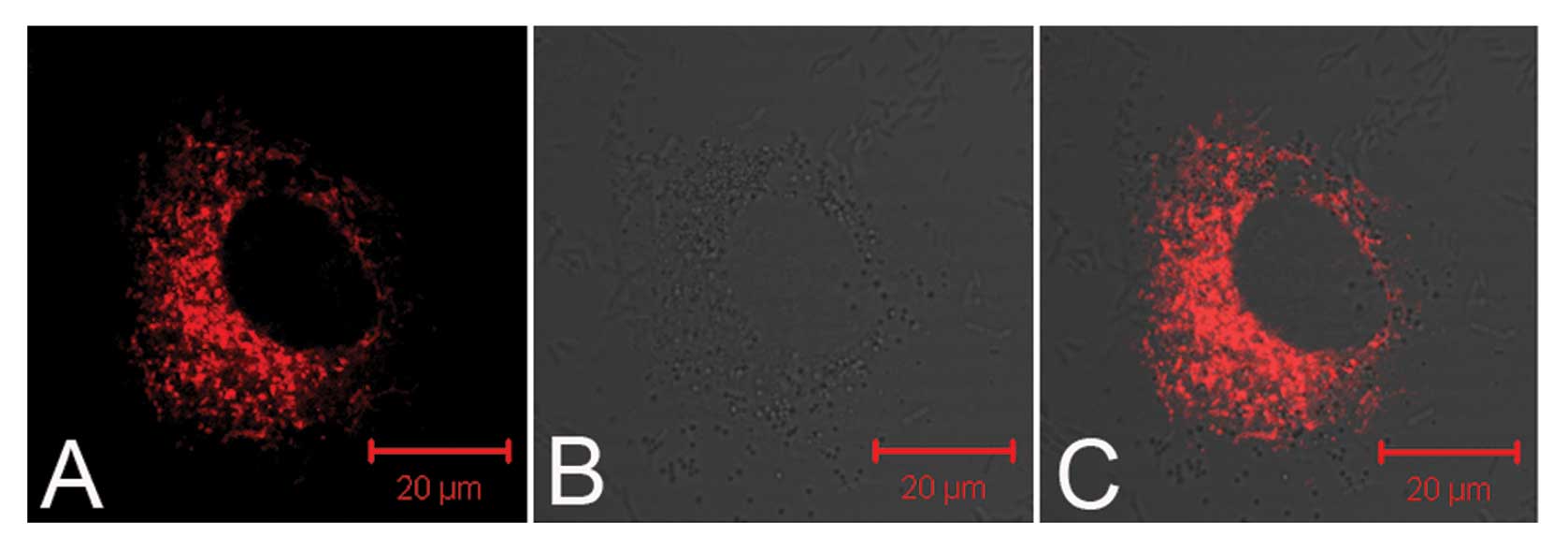
TNPs loaded with siRNA delivered by UTMD were
observed in the perinuclear cytoplasmic region by a confocal
microscope (Fig. 2), which was
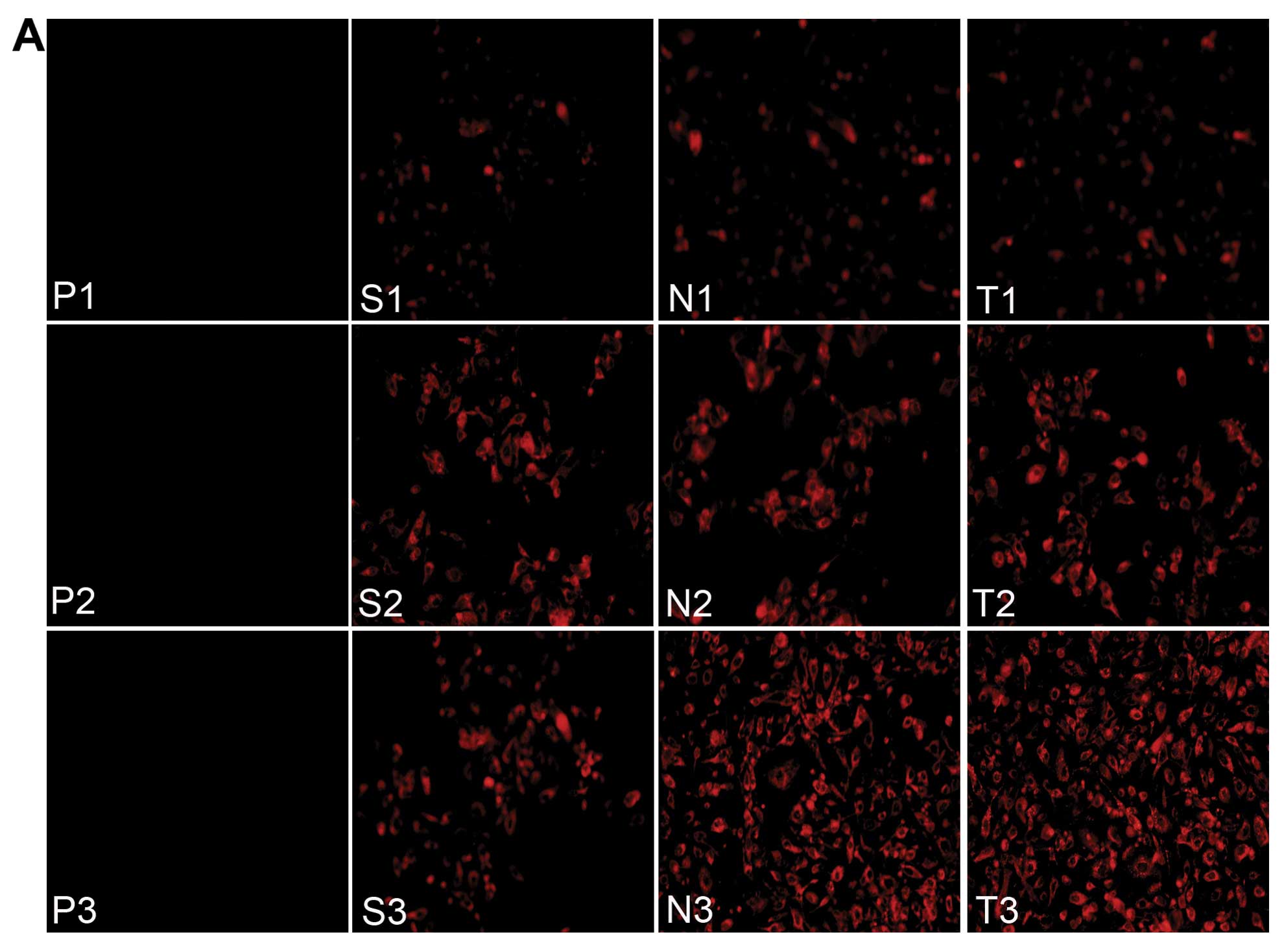
the first step towards successful gene transfection. Fig. 3A shows the fluorescence images of
the cellular uptake of siRNA in the different groups. Examination
of the cultured PC-3 cells with a flow cytometer showed that the T3
group (TNPs + UTMD) had a maximal fluorescence intensity index
(27.18±0.91) among all the groups, slightly higher than the N3
group (NNPs + UTMD) (18.39±0.90) (P<0.05), while the T3 group
demonstrated a significant difference compared to the other control
groups (P<0.001) (Fig.
3B).
Real-time PCR
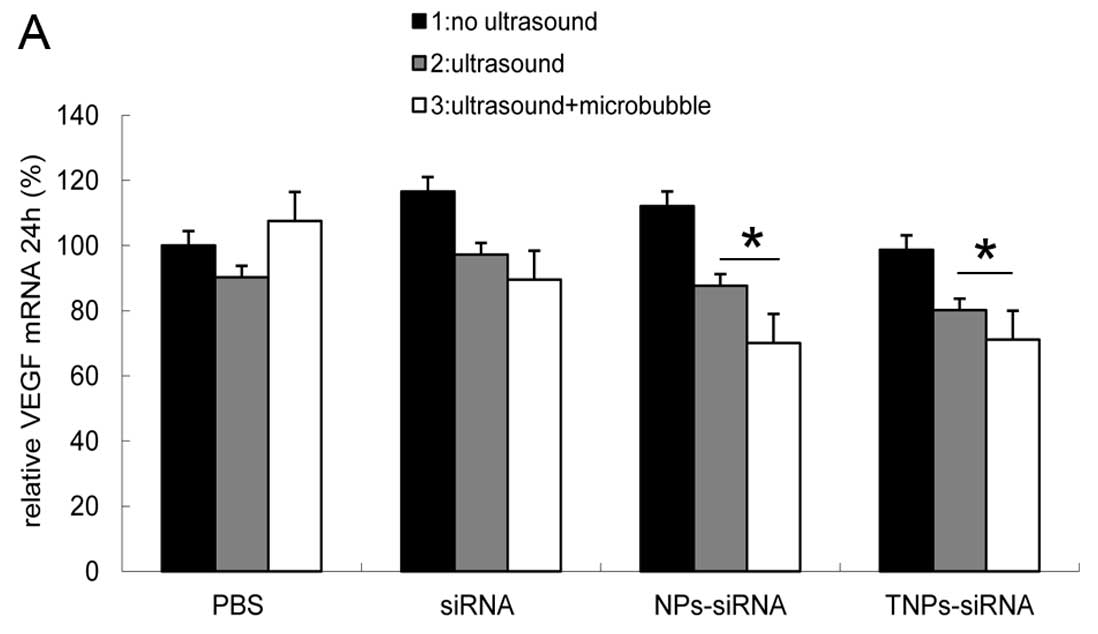
To establish the inhibitory effect of hVEGF-siRNA at
the mRNA level, relative VEGF mRNA expression levels was evaluated
after 24 and 48 h of transfection. The results showed that the T3
group ranked last at 24 h (71%) (Fig.
4A)and 48 h (53%) (Fig. 4B),
while the N3 group showed similar results to the T3 group at 24 h
(70%) and 48 h (70%). The 2 groups showed inhibitory effects as
compared to the other samples (P<0.05). No difference between
these groups was observed at 24 h, but a significant difference was
observed between these groups at 48 h (P<0.05).
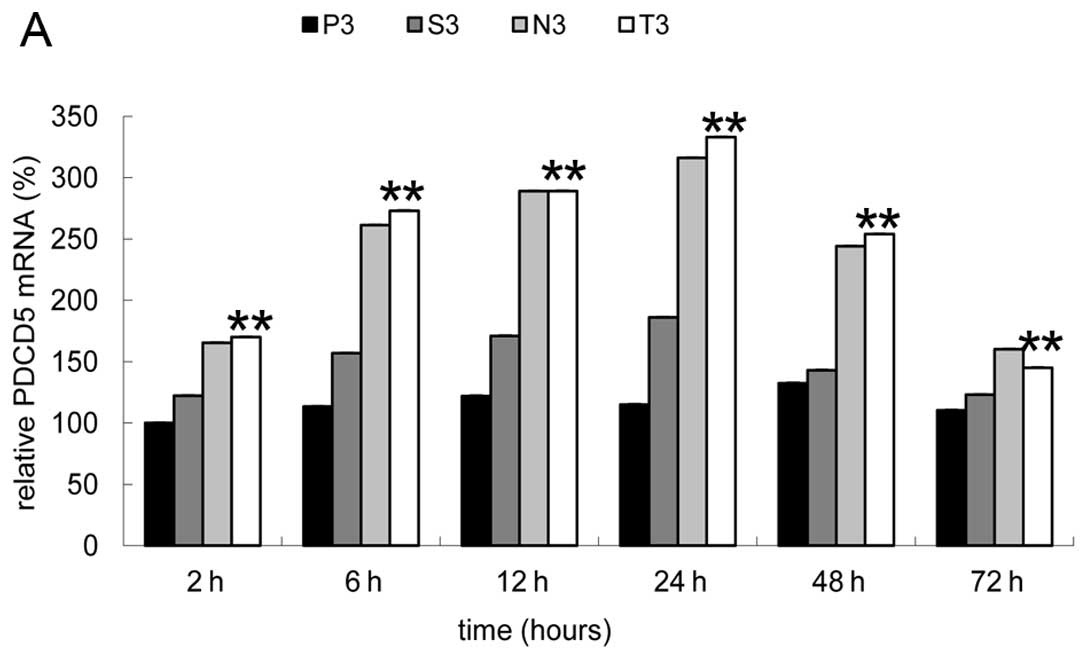
Given that cell apoptosis can be induced by
hVEGF-siRNA, as a sensitive index of cell apoptosis, PDCD5 mRNA
expression can reflect the gene silencing effect of siRNA
indirectly and was therefore examined by real-time PCR analysis.
Cell apoptosis is a complex process involving many stages;
therefore, we selected 6 time-points to depict the expression
trend. The results of real-time PCR analysis demonstrated that all
the control groups without microbubbles added and the P3 group had
very similar performance characteristics. No difference was
observed in PDCD5 mRNA expression beween these groups at different
time-points (data not shown). However, there was no significant
difference between the N3 and T3 groups (P<0.20), showing that
the targeted group failed to exceed the non-targeted group
concerning inhibitory effects, while differences were noted when
compared to the S3 and P3 groups, (P<0.001) (Fig. 5A). Fig. 5B shows that PDCD5 mRNA levels in
the N3 and T3 groups remarkably increased with time after 2 h of
transfection, and reached the peak (3-fold the expression of P3) at
24 h, then decreased significantly, while the S3 group (naked
siRNA-transfected cells) displayed a weak increase at all
time-points.
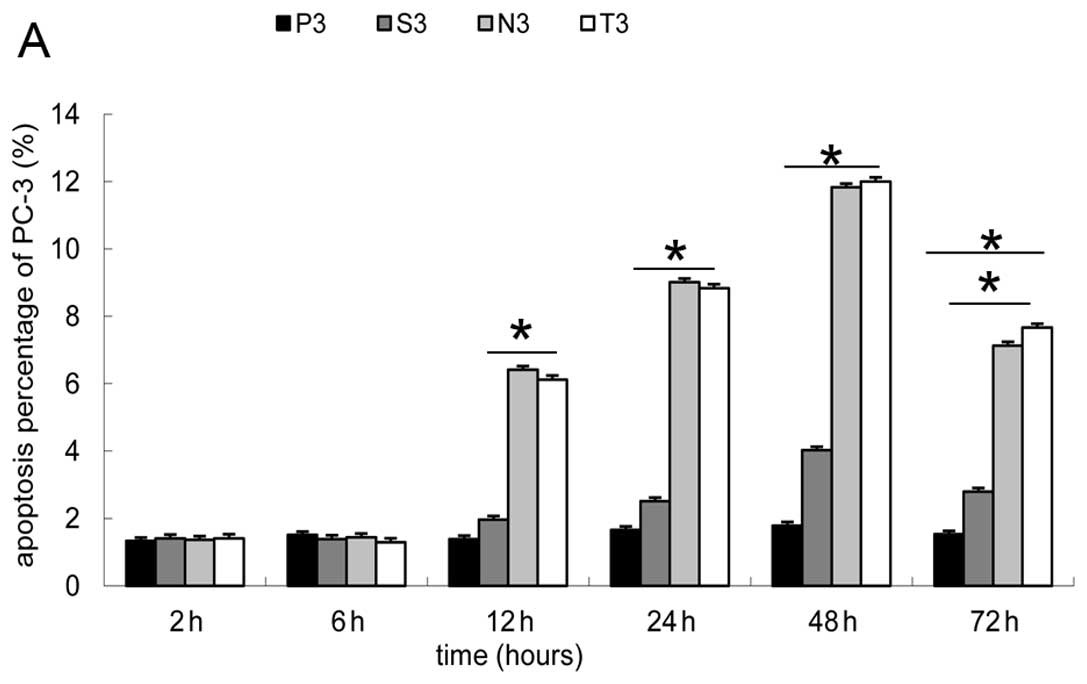
Detection of apoptosis by flow
cytometry
Similar to the results of PDCD5 analysis, the 8
groups without microbubbles showed similar results to the P3 group,
while no difference was observed between the N3 and T3 group
(P<0.15) (Fig. 6A). The
difference was that the cell apoptotic percentage in the N3 and T3
groups notably increased after 12 h of transfection (P<0.05),
and reached the peak (6.7-fold the percentage of P3) at 48 h, then
decreased slightly (Fig. 6B).
This shows that PS externalization began later than PDCD5 mRNA
alteration during early apoptosis.
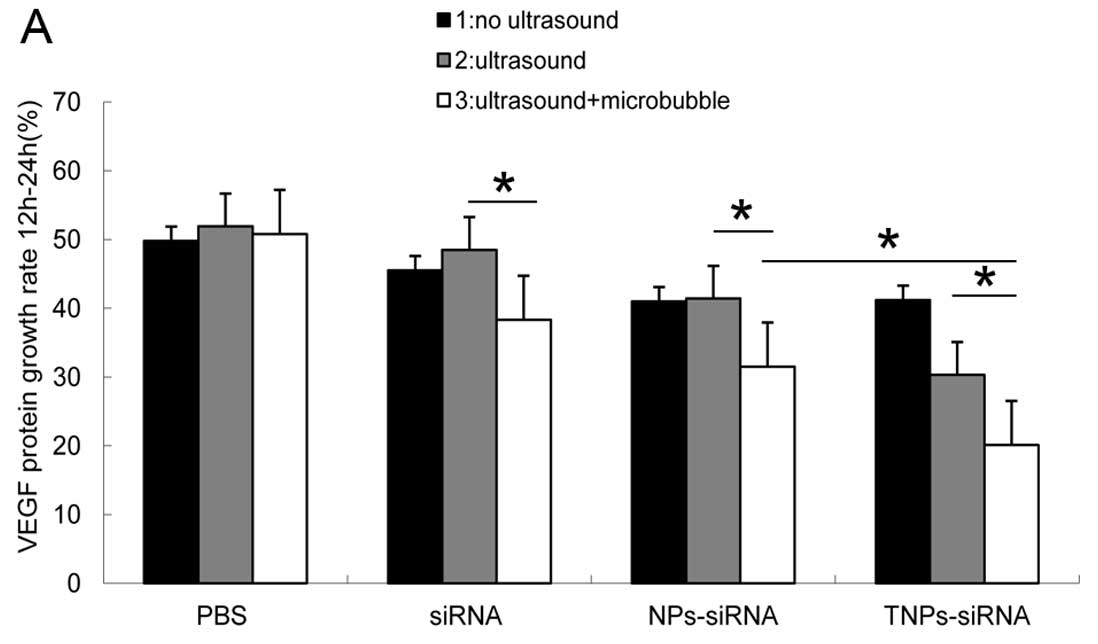
ELISA
In order to maximally reduce protein secretion
induced by the number of cells, the 12–24 h growth rate (GR) and
24–48 h GR were compared among the groups. The T3 group showed the
lowest protein GR (20% at both time-points), while the N3 group had
the lowest GR (31 and 33%) (Fig.
7). Compared with the other control groups, the protein
expression of the T3 group was significantly suppressed,
demonstraring a statistical significance compared with the N3 group
(P<0.05). In this experiment, the S3, N3 and T3 groups showed a
significant decline in GR (P<0.05), indicating that the UTMD
method can be used for efficient gene delivery. The GR was
determined using the following equation: GR = [protein quatity of
24 h (48 h) - protein quatity of 12 h (24 h)/protein quatity of 12
h (24 h)] ×100%.
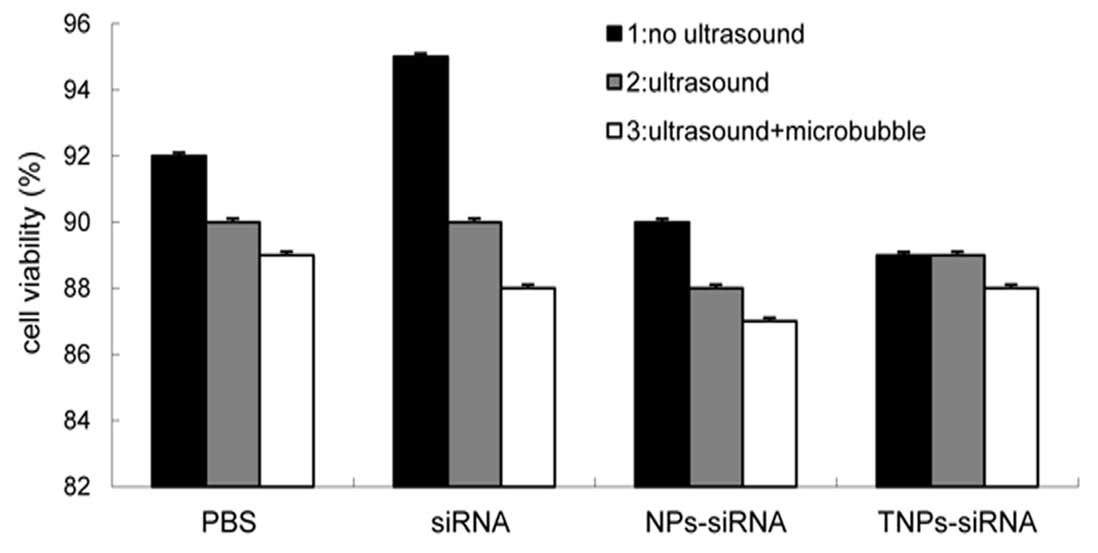
Cell viability assay
Under optimized conditions, no significant
difference in cell viability was observed between the groups
(P<0.5) (Fig. 8). The results
demonstrated that cell membrane integrity remained intact during
the different treatments and was not be affected by UTMD and NPs.
Although UTMD is thought to assist the delivery of molecules into a
cell by transiently increasing the membrane permeability, cell
membrane integrity would be restored in a short while.
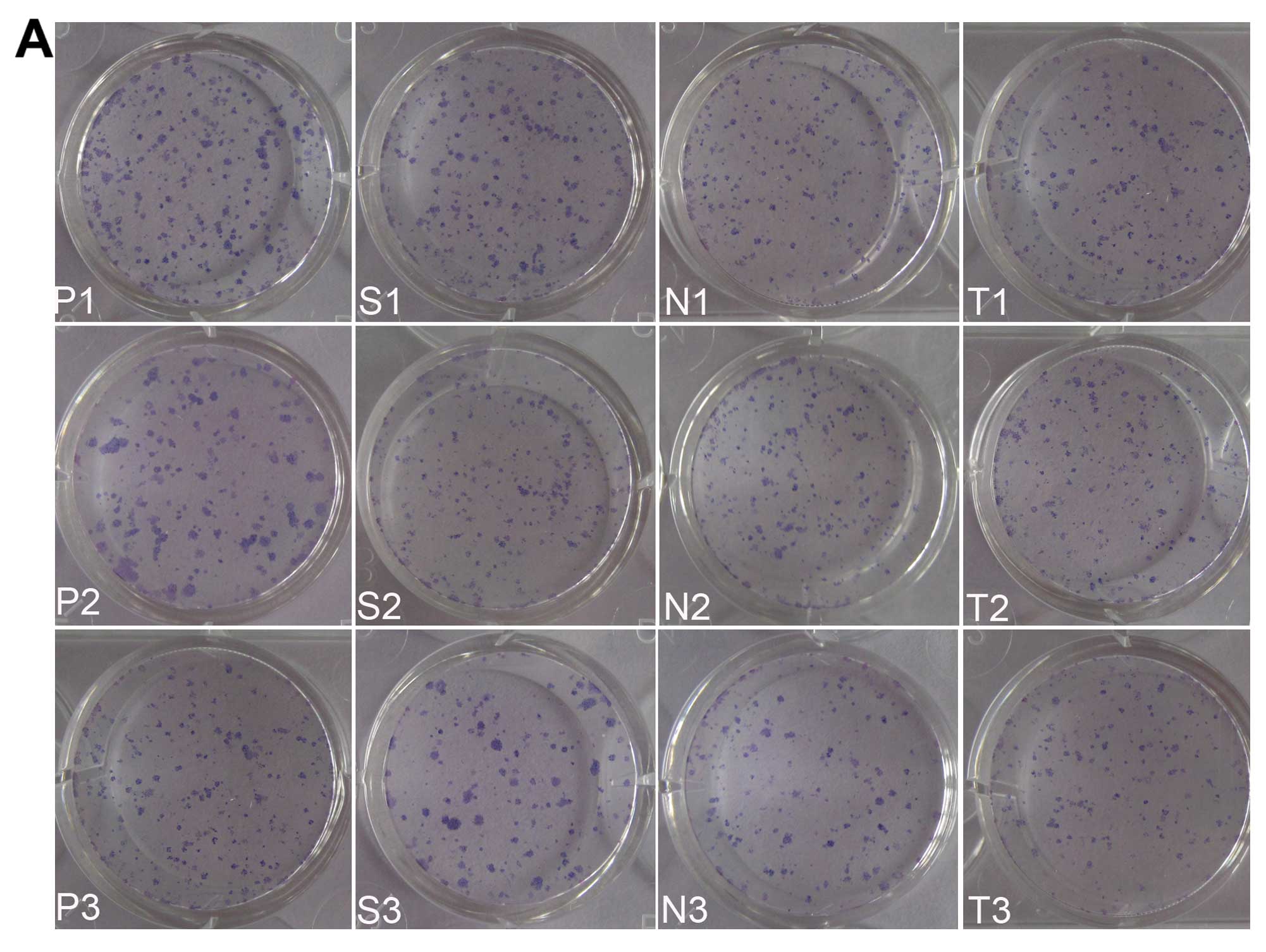
Clonogenic assay
The cell colonies formed by the 12 groups are shown
in Fig. 9A. There was no
significant difference between these groups (P1, P2, P3, S1, N1 and
T1) (Fig. 9B), which demonstrated
that UTMD technology and NPs loaded with siRNA had no potential to
inhibit cells to proliferate. The number of cell colonies formed by
the S2, N2 and T2 groups was less than the groups mentioned above;
however, there was no statistical significance. The N3 and T3
groups formed the least number of cell colonies, significantly less
than the other groups (P<0.05), which demonstrates that the
proliferative ability of the cells had been badly impaired by
hVEGF-siRNA delivered with NPs combined with UTMD. There was no
difference observed between these 2 groups (P<0.1) and the S3
group ranked in the middle position.
Discussion
Prostate cancer is one of the most common types of
cancer and the main leading cause of cancer-related mortality in
males. Most late stage prostate cancer patients may become
non-responsive to hormone therapy, resulting in deterioration and
even death. To date, no single or combination therapy has shown
efficacy in inhibiting tumor progression. Genetic therapies
represent promising approaches for the treatment of this neoplastic
disease. Hormone refractory PC-3 prostate cancer cellss and siRNA
targeting the hVEGF gene were selected in our study. It is known
that the instability and poor cellular uptake of siRNA has limited
its usefulness. To minimize the loss of siRNA and increase cellular
uptake efficiencies, hVEGF-siRNA were encapsulated in biodegradable
NPs and were delivered into the cell plasma by sonoporation induced
by UTMD, which was expected to achieve the downregulation of VEGF
gene expression, and to inhibit tumor cell proliferation and induce
tumor cell apoptosis.
In our previous study, we successfully constructed
NPs made of mPEG-PLGA-PLL triblock copolymers (8). As a non-viral siRNA delivery vector,
its stability and biocompatibility, lower cytotoxicity and higher
gene transfection efficiency was verified. On the basis of these
studies, cRGD ligands were conjugated to the surface of
mPEG-PLGA-PLL triblock copolymers to recognize the target site,
integrin αvβ3, highly expressed in PC-3 cells. cRGD is a
commonly-used ligand in many targeting studies for specific
recognition (17,18). Targeted NPs loaded with siRNA
combined with UTMD techonology were used to further improve the
gene delivery efficiencies.
We used siRNA labeled with Cy3 for morphological
experiments. After the intracellular localization of TNPs was
confirmed, the fluorescence intensity of the T3 group was the
highest among the 12 groups, which indicated that much more TNPs
were aggregated and adhered on the surface of PC-3 cells with the
help of the targeting ligand, cRGD.
SiRNA without Cy3 was used for relative molecular
experiments. Real-time PCR (for the mRNA level) and ELISA (for the
protein level) were carried out to identify the direct inhibitory
effect of hVEGF-siRNA. The results showed that VEGF expression
decreased significantly at both levels. ELISA demonstrated that the
VEGF protein GR of the T3 group was <40% of the P3 group, while
less than 68% of the N3 group at the 2 time-points. These may be
the most significant results in this study, showind that cRGD had a
targeting effect similar to that shown in previous studies
(19,20). The S3, N3 and T3 groups showed a
significant decline in GR comared with the other groups
(P<0.05), demonstrating that UTMD as driving force was an
effective method to facilitate gene delivery.
To examine the induction of apoptosis by the
siRNA-mediated inhibition of VEGF expression in PC-3 cells
(21,22), the detection of apoptosis by flow
cytometry and real-time PCR was carried out after 2 h of
transfection. A biochemical hallmark of apoptotic cell death is the
translocation of PS from the cytoplasmic surface of the cell
membrane to the external cell surface (23). The levels of PS in apoptotic cells
is easily determined by flow cytometry using fluorescence-labelled
Annexin V, which specifically binds PS. The overexpression of PDCD5
precedes the chromosome DNA fragmentation and PS externalization
during early apoptosis. PDCD5 mRNA and protein levels are
upregulated in response to various apoptotic stimuli (24–26). Therefore, PDCD5 mRNA levels were
aslo determined by real-time PCR in our study. As the other 8
groups showed a similar trend with the P3 group, the 4 groups (P3,
S3, N3 and T3) which demonstrated significant differences are
described in this study. The results of flow cytometry demonstrated
that apopotosis of PC-3 cells occurred after 6 h of transfection
and reached the peak at 48 h, while the results of PDCD5 analysis
showed that apopotosis began after 2 h of transfection and reached
the peak at 24 h. PDCD5 has been consistently regarded as a more
sensitive index during early apoptosis as it has been reported that
the upregulation of PDCD5 mRNA precedes PS externalization during
early apoptosis (27). This was
also determined in our study. Certain studies have shown that PDCD5
is possibly involved in the 2 cell death programs, apoptosis and
paraptosis, and that it may be one of the key molecules connecting
these 2 processes (28,29). However, the targeting effect of
the cRGD ligands was similar to that of the than non-targeted NPs
in the 2 experiments. Combined with the VEGF expression results
presented above, although we obtained some results with statistical
significance, from the perspective of absolute value, the results
were limited. The reasons why the gene silencing effect of the
TNP-siRNA complexes failed to dramatically exceed that of the
NNP-siRNA may be as follows: although the physicochemical
characterization of the NNPs loaded with siRNA was introduced in
our previous study, whether the combination of cRGD has an impact
on the inherent nature of NPs should be verified; therefore,
further experiments for constructing TNPs of perfect quality are
warranted. siRNA which were not connected to a self-replication
vector lacked biological amplification effects; experimental
conditions should be further optimized; and a number of tumor cell
lines should be selected, taking into account the differences
between cell lines.
Trypan blue assay showed that UTMD methods and
different NPs had no significant effect on transient cell
viability. MPEG-PLGA-PLL NPs turned out to be stable, biodegradable
and biocompatible, without hampering the metabolic activity of the
cells as revealed by the results of our previous study (8). A number of studies have indicated
the side-effects of inertial cavitation induced by UTMD, such as
cell apoptosis and cell lysis (30,31), capillary rupture (32), hemolysis (33), etc. In our study, UTMD did not
induce transient cell viability decrease under optimized
conditions. Adverse effects were directly related to microbubble
nature and ultrasound exposure conditions. The side-effects should
be reduced to a minimum by optimization.
We selected clonogenic cell survival assay to
further investigate the long-term effect of siRNA and the cytoxity
of UTMD and NPs. The N3 and T3 groups formed the least number of
cell colonies, demonstrating the long-term effect of NPs loaded
with siRNA, which should be mainly attributed to the longer-lasting
siRNA release from the NPs (34,35). Apart from the S3 group, the number
of colonies of the other 9 groups remained similar. The UTMD and
NPs failed to damage the ability of the PC-3 cells to form colonies
and proliferate. The study by Karshafian et al demonstrated
that approximately half of the cells undergoing reversible
permeabilization retained their long-term viability (36). Perhaps the utilization of NPs
facilitates the delivery of siRNA; therefore, side-effects were
significantly reduced with lower ultrasound exposure parameters and
less quatities of reagents, as shown in our study.
Novel biodegradable targeted NPs combined with UTMD
were expected to significantly facilitate gene transfection.
However, not all experimental results in this study indicated that
the TNPs had a more powerful inhibitory effect than the NNPs. The
results from our study indicate that NPs combined with UTMD can
synergistically serve as a non-viral gene delivery system without
notable cell toxicity. Although some positive results were
obtained, the small number of samples and the fact that the
experiments were only repeated a few times may be the first
limitation of the present study. Secondly, it was impossible to
avoid the disturbance of certain personal factors, instrument
errors and environmental alterations. Thirdly, this study was
limited to in vitro study. In conclusion, repeated
experiments are necessary to verify the feasibility of this novel
gene delivery system and further studies using animal models are
required.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81171352/H1805 and
81000617), the National Natural Science Foundation of China (no.
81272568 and 81101738), Biological Pharmaceutical and Agriculture
Fields from Science and Technology Commission of Shanghai Municipal
(no. 114119a 3300).
References
|
1
|
Namiecińska M, Marciniak K and Nowak JZ:
VEGF as an angiogenic, neurotrophic, and neuroprotective factor.
Postepy Hig Med Dosw (Online). 59:573–583. 2005.(In Polish).
|
|
2
|
Anai S, Sakamoto N, Sakai Y, Tanaka M,
Porvasnik S, Urbanek C, Cao W, Goodison S and Rosser CJ: Dual
targeting of Bcl-2 and VEGF: a potential strategy to improve
therapy for prostate cancer. Urol Oncol. 29:421–429. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao J, Tu YT, Huang CZ, Feng AP, Wu Q,
Lian YJ, Zhang LX, Zhang XP and Shen GX: Inhibiting the growth of
malignant melanoma by blocking the expression of vascular
endothelial growth factor using an RNA interference approach. Br J
Dermatol. 153:715–724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rhee J and Hoff PM: Angiogenesis
inhibitors in the treatment of cancer. Expert Opin Pharmacother.
6:1701–1711. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sitohy B, Nagy JA and Dvorak HF:
Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer
Res. 72:1909–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chakraborty C: Potentiality of small
interfering RNAs (siRNA) as recent therapeutic targets for
gene-silencing. Curr Drug Targets. 8:469–482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McNamara JO II, Andrechek ER, Wang Y,
Viles KD, Rempel RE, Gilboa E, Sullenger BA and Giangrande PH: Cell
type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat
Biotechnol. 24:1005–1015. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du J, Sun Y, Shi QS, Liu PF, Zhu MJ, Wang
CH, Du LF and Duan YR: Biodegradable nanoparticles of mPEG-PLGA-PLL
triblock copolymers as novel non-viral vectors for improving siRNA
delivery and gene silencing. Int J Mol Sci. 13:516–533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vamer JA and Cheresh DA: Tumor
angiogenesis and the role of vascular cell integrin alphavbeta3.
Important Adv Oncol. 1:69–87. 1996.
|
|
10
|
Eliceiri BP and Cheresh DA: The role of
alphav integrins during angiogenesis: insights into potential
mechanisms of action and clinical development. J Clin Invest.
103:1227–1230. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng DQ, Woodard AS, Fornaro M, Tallini G
and Languino LR: Prostatic carcinoma cell migration via
alpha(v)beta3 integrin is modulated by a focal adhesion kinase
pathway. Cancer Res. 59:1655–1664. 1999.PubMed/NCBI
|
|
12
|
Li HL, Zheng XZ, Wang HP, Li F, Wu Y and
Du LF: Ultrasound-targeted microbubble destruction enhances
AAV-mediated gene transfection in human RPE cells in vitro and rat
retina in vivo. Gene Ther. 16:1146–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carson AR, McTiernan CF, Lavery L, Hodnick
A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA and Villanueva
FS: Gene therapy of carcinoma using ultrasound-targeted microbubble
destruction. Ultrasound Med Biol. 37:393–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shohet RV and Grayburn PA: Potential
bioeffects of ultrasonic destruction of microbubble contrast
agents. J Am Coll Cardiol. 47:1469–1470. 2006.PubMed/NCBI
|
|
15
|
Lai CY, Wu CH, Chen CC and Li PC:
Quantitative relations of acoustic inertial cavitation with
sonoporation and cell viability. Ultrasound Med Biol. 32:1931–1941.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ay T, Havaux X, Van Camp G, Campanelli B,
Gisellu G, Pasque A, Denef JF, Melin JA and Vanoverschelde JL:
Destruction of contrast microbubbles by ultrasound: effects on
myocardial function, coronary perfusion pressure and microvascular
integrity. Circulation. 104:461–466. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie J, Shen Z, Li KC and Danthi N: Tumor
angiogenic endothelial cell targeting by a novel integrin-targeted
nanoparticle. Int J Nanomedicine. 2:479–485. 2007.PubMed/NCBI
|
|
18
|
Lin RY, Dayananda K, Chen TJ, Chen CY, Liu
GC, Lin KL and Wang YM: Targeted RGD nanoparticles for highly
sensitive in vivo integrin receptor imaging. Contrast Media Mol
Imaging. 7:7–18. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu Y, Li L and Ayoufu G: Experimental
study of the preparation of targeted microbubble contrast agents
carrying urokinase and RGDS. Ultrasonics. 49:676–681. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dayton PA, Pearson D, Clark J, Simon S,
Schumann PA, Zutshi R, Matsunaga TO and Ferrara KW: Ultrasonic
analysis of peptide- and antibody-targeted microbubble contrast
agents for molecular imaging of alphavbeta3-expressing cells. Mol
Imaging. 3:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rong W, Wang J, Liu X, Jiang L, Wei F, Hu
X, Han X and Liu Z: Naringin treatment improves functional recovery
by increasing BDNF and VEGF expression, inhibiting neuronal
apoptosis after spinal cord injury. Neurochem Res. 37:1615–1623.
2012. View Article : Google Scholar
|
|
22
|
Ferrari G, Pintucci G, Seghezzi G, Hyman
K, Galloway AC and Mignatti P: VEGF, a prosurvival factor, acts in
concert with TGF-beta1 to induce endothelial cell apoptosis. Proc
Natl Acad Sci USA. 103:17260–17265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fadok VA, Bratton DL, Frasch SC, Warner ML
and Henson PM: The role of phosphatidylserine in recognition of
apoptotic cells by phagocytes. Cell Death Differ. 5:551–562. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen
G, Tang J and Ma D: TFAR19, a novel apoptosis-related gene, cloned
from human leukemia cell line TF-1, could enhance apoptosis of some
tumor cells induced by growth factor withdrawal. Biochem Biophys
Res Commun. 254:203–210. 1999. View Article : Google Scholar
|
|
25
|
Xu L, Chen Y, Song Q, Xu D, Wang Y and Ma
D: PDCD5 interacts with Tip60 and functions as a cooperator in
acetyltransferase activity and DNA damage-induced apoptosis.
Neoplasia. 11:345–354. 2009.PubMed/NCBI
|
|
26
|
Zhuge C, Chang Y, Li Y, Chen Y and Lei J:
PDCD5-regulated cell fate decision after
ultraviolet-irradiation-induced DNA damage. Biophys J.
101:2582–2591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Sun R, Han W, Zhang Y, Song Q, Di
C and Ma D: Nuclear translocation of PDCD5 (TFAR19): an early
signal for apoptosis? FEBS Lett. 509:191–196. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang J, Wang N, Guan Z and Houshan LV:
Programmed cell death 5 factor enhances triptolide-induced
fibroblast-like synoviocyte apoptosis of rheumatoid arthritis.
Artif Cells Blood Substit Immobil Biotechnol. 38:38–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Li X, Wang L, Ding P, Zhang Y, Han
W and Ma D: An alternative form of paraptosis-like cell death,
triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell
Sci. 117:1525–1532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feril LB Jr, Kondo T, Zhao QL, Ogawa R,
Tachibana K, Kudo N, Fujimoto S and Nakamura S: Enhancement of
ultrasound-induced apoptosis and cell lysis by echo-contrast
agents. Ultrasound Med Biol. 29:331–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Honda H, Zhao QL and Kondo T: Effects of
dissolved gases and an echo contrast agent on apoptosis induced by
ultrasound and its mechanism via the mitochondria-caspase pathway.
Ultrasound Med Biol. 28:673–682. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Skyba DM, Price RJ, Linka AZ, Skalak TC
and Kaul S: Direct in vivo visualization of intravascular
destruction of microbubbles by ultrasound and its local effects on
tissue. Circulation. 98:290–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miller MW, Everbach EC, Cox C, Knapp RR,
Brayman AA and Sherman TA: A comparison of hemolytic potential of
Optison and Albunex in whole human blood in vitro: acoustic
pressure, ultrasound frequency, donor and passive cavitation
detection considerations. Ultrasound Med Biol. 27:709–721. 2001.
View Article : Google Scholar
|
|
34
|
Danhier F, Pourcelle V, Marchand-Brynaert
J, Jérôme C, Feron O and Préat V: Targeting of tumor endothelium by
RGD-grafted PLGA-nanoparticles. Methods Enzymol. 508:157–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu P, Qi X, Sun Y, Wang H, Li Y and Duan
Y: RGD-conjugated PLA-PLL nanoparticles targeting to Bacp-37 breast
cancer xenografts in vivo. J Nanosci Nanotechnol. 11:10760–10764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karshafian R, Samac S, Bevan PD and Burns
PN: Microbubble mediated sonoporation of cells in suspension:
clonogenic viability and influence of molecular size on uptake.
Ultrasonics. 50:691–697. 2010. View Article : Google Scholar : PubMed/NCBI
|