Introduction
Periodontitis is one of the most common chronic
infectious diseases in adults. It often coincides with increased
alveolar bone resorption, causing tooth loss and eventually
exfoliation. Smoking is recognized as the primary behavioral risk
factor for periodontitis (1–4).
However, the mechanisms underlying the effects of smoking on
periodontal tissue destruction remain unclear (5). Compared with non-smoking
periodontitis, the height and density of alveolar bone in the
periodontal tissue of smoking-associated periodontitis is lower,
and closely related to smoking dose and time (6). Nicotine, the main toxic component in
tobacco, was confirmed as the main effect of smoking on periodontal
tissue destruction. Previous studies have demonstrated that daily
quantitative injection of nicotine causes significantly heavier
alveolar bone resorption in rats than injection of saline (7,8).
As the specific agonist of α7 nicotinic acetylcholine receptor (α7
nAChR), nicotine also upregulated the expression of α7 nAChR in rat
periodontal tissue and human periodontal ligament (PDL) cells, and
these effects could be partially suppressed by pretreatment with
α-BTX (8,9). A number of studies suggested that
CD4+ T cell-mediated immune responses are critical to
the process of periodontitis; large amounts of CD4+ T
cells infiltrated periodontal tissue and the proportion of
CD4+ T cells in peripheral blood was significantly
changed in periodontitis (10–12). Other studies showed
CD4+ T cell-mediated immune responses regulated alveolar
bone resorption in periodontitis (13–16).
The receptor activator of NF-κB ligand
(RANKL)/RANK/osteoprotegerin (OPG) axis is the key factor for
regulating differentiation of osteoclast and bone resorption
(17,18). Nicotine affected the balance of
the RANKL/RANK/OPG axis (19) and
also upregulated the level of interleukin-1β (IL-1β) in human PDL
cells (9) and gingival crevicular
fluid (GCF) (20,21). Upregulated expression of IL-1β can
further affect the balance of the RANKL/RANK/OPG axis, causing
alveolar bone resorption in periodontitis (22).
We hypothesized that nicotine could alter the
secretion of IL-1β in serum of human PDL cell-CD4+ T
cell co-culture, further affecting the expression of RANKL in human
PDL cells. Therefore, in this study, we investigated the stimulated
human PDL cell-CD4+ T cell co-culture with nicotine for
72 h and analyzed the effects of this stimulation on the
expressions of RANKL and OPG and the secretion of IL-1β, examining
whether the effects of nicotine were affected by the presence of
CD4+ T cells.
Materials and methods
Cell culture
Cultures of human PDL cells from three healthy young
patients undergoing first premolar tooth extractions at the
Department of Maxillofacial Surgery, were explanted from the
middle-third of the root surface and grown in RPMI-1640 medium
supplemented with 15% fetal calf serum (FCS) and antibiotics
(penicillin, 50 U/ml and streptomycin, 50 mg/ml). Experiments were
performed in 6-well plates that were then placed in a 37°C humid
atmosphere of 5% CO2 and 95% O2. Passage-5
cells were used for all the experiments. This study was
independently reviewed and approved by the Ethics Committee of the
School of Stomatology, The Fourth Military Medical University.
CD4+ T cell sort and
culture
Peripheral venous blood was derived from healthy
non-smoking volunteers. Peripheral blood mononuclear cells (PBMCs)
were separated by density gradient centrifugation (Ficoll-Hypaque
solution; Sigma, USA), stained with CD4 fluorescein isothiocyanate
antibody (BD Biosciences, USA) and incubated at 4°C for 30 min.
CD4+ T cell sorting was performed by flow cytometry.
Only samples with >90% CD4+ T cells were grown in
RPMI-1640 medium supplemented with 15% FCS and antibiotics
(penicillin, 50 U/ml and streptomycin, 50 mg/ml) for further
experiments.
To determine the effects of nicotine and/or α-BTX,
human PDL cells and CD4+ T cells were divided into two
systems: the mono-culture and the co-culture system (ratio 1:4
human PDL cells:CD4+ T cells), then incubated for 72 h.
We used a semi-permeable membrane (0.4 μm; Millipore, Germany) to
establish the human PDL cell-CD4+ T cell co-culture
system. Each system was divided into four groups, and received one
of the following treatments randomly: i) no treatment; ii) nicotine
(10−5 M); iii) α-BTX (10−8 M); iv) α-BTX
(10−8 M) followed by nicotine (10−5 M) after
30 min.
ELISA analysis
Supernatants of mono-culture human PDL cells,
mono-culture CD4+ T cells and human PDL
cell-CD4+ T cell co-culture challenged as described
above were collected and centrifuged. Concentrations of IL-1β were
quantified using highly sensitive enzyme-linked immunoassay from
R&D Systems (Abingdon, UK) according to the manufacturer’s
instructions, and normalized to the number of cells. Culture
supernatants were thawed only once and assayed in the same run.
RNA analysis and real-time quantitative
PCR
We then determined the constancy of the
transcriptional alterations following stimulation with nicotine for
72 h. Total mRNA of human PDL cells was isolated with RNeasy kit
(Omega, USA). mRNA concentration was measured by using NanoDrop
Spectrophotometer (Thermo-Fischer Scientific, USA), and
reverse-transcribed to cDNA by using iScript Select cDNA Synthesis
kit (Bio-Rad, Hercules, CA, USA). Real-time PCR was performed with
iQ SYBR-Green Supermix (Bio-Rad) and primers for RANKL and OPG
(Takara, Ohtsu, Japan), and were then analyzed with the
2−ΔΔCt method. β-actin served as the housekeeping gene.
Real-time PCR was performed on an ABI PRISM 7500 (Applied
Biosystems, USA). Primer sequences are listed in Table I, all PCR efficiencies were
comparable.
 | Table IPrimers used for real-time
quantitative PCR. |
Table I
Primers used for real-time
quantitative PCR.
| β-actin | F:
5′-TGGCACAGCACAATGAA-3′ |
| R:
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
| RANKL | F:
5′-TGATGTGCTGTGATCCAACGA-3′ |
| R:
5′-AAGATGGCACTCACTGCATTTATAG-5′ |
| OPG | F:
5′-GAAGGTGAGGTTAGCATGTCC-3′ |
| R:
5′-CAAAGTAAACGCAGAGAGTGTAGA-3′ |
Western blot analysis
Whole-cell protein lysates of mono-culture human PDL
cells and human PDL cell-CD4+ T cell co-culture as
described above were collected on ice and resuspended in SDS-sample
buffer. Protein concentration was determined by the BCA protein
assay kit (Pierce, Rockford, IL, USA). Samples containing equal
amounts of protein were separated on 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in a minigel
apparatus. The proteins in the gel were transferred to a
polyvinylidene difluoride (PVDF) membrane. The membrane was blocked
with 5% fat-free dry milk in TBST (TBS containing 0.1% Tween-20),
washed with TBST (3×5 min), and incubated with rabbit anti-human
RANKL and OPG (Santa Cruz Biotechnology, Inc., USA), dilution
1:500, and with mouse anti-human β-actin (Abcam, Cambridge, MA,
USA), dilution 1:500, respectively. Then, the membranes were
incubated overnight with primary antibodies, followed by incubation
with horseradish peroxidase (HRP)-conjugated secondary antibodies,
dilution 1:10,000. Immunoreactive bands were visualized with the
Western-Light chemiluminescent detection system (Peiqing, Shanghai,
China).
Statistical analysis
All data are from experiments performed in
triplicate and recorded as the means ± standard error (SE). The
significant differences in mRNA and protein expressions of
differences between groups were analyzed through one way-ANOVA and
Tukey’s test using SPSS 18.0 software. A P-value <0.05 was
considered to indicate statistically significant differences.
Results
IL-1β secretion in serum of co-culture
and mono-culture systems
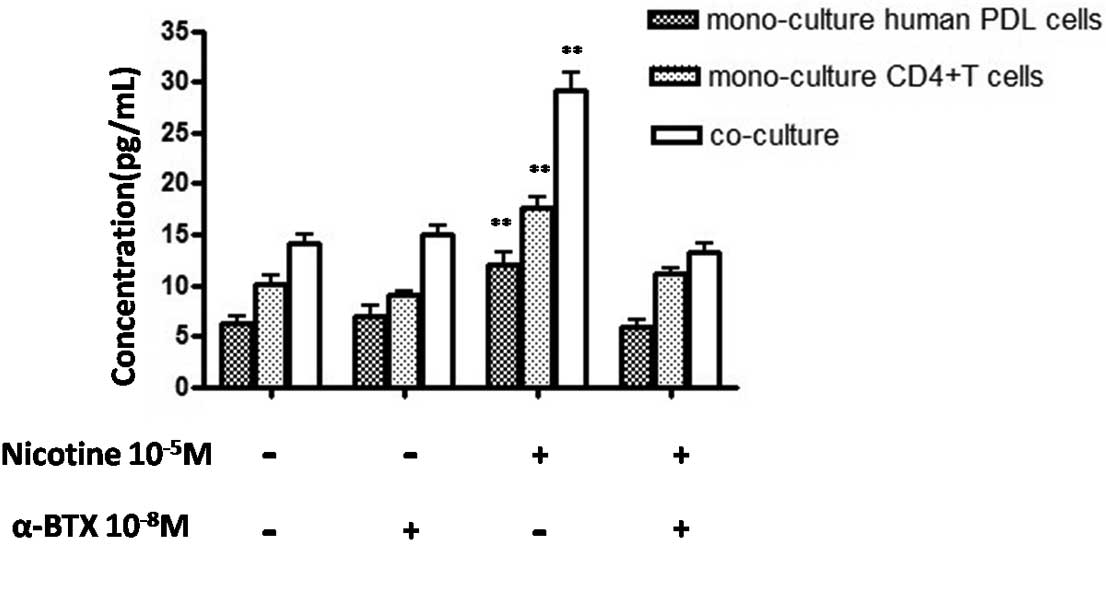
The secretion of IL-1β was subsequently quantified
in co-culture and mono-culture systems by ELISA. The secretion of
IL-1β was significantly upregulated following stimulation with
nicotine (P<0.01) in mono-culture human PDL cells, and the
secretion of IL-1β in mono-culture CD4+ T cells showed a
similar tendency to mono-culture human PDL cells (P<0.01).
Furthermore, the secretion of IL-1β in human PDL
cell-CD4+ T cell co-culture was significantly higher
than both mono-culture systems (P<0.01) (Fig. 1).
Gene expressions of RANKL and OPG in
human PDL cells
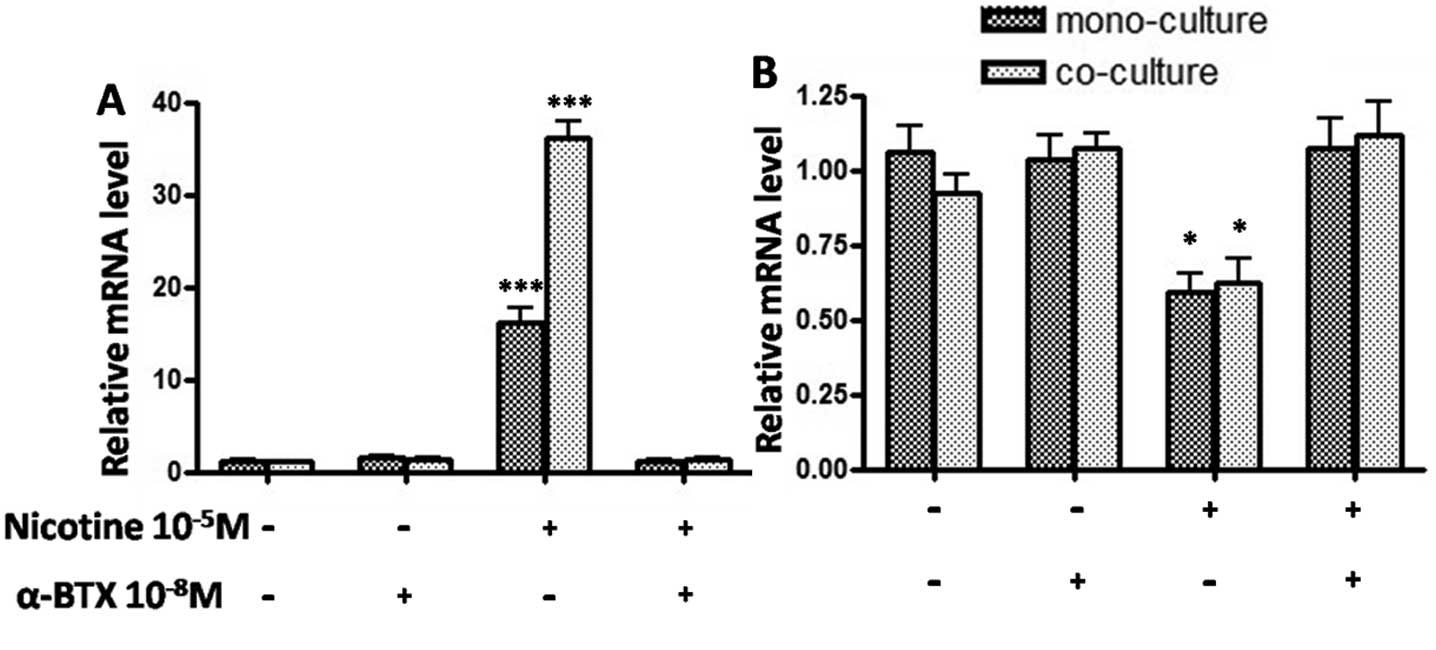
Subsequently, we examined whether nicotine regulated
expressions of RANKL and OPG, thus potentially modulating alveolar
bone metabolism. We used real-time PCR to amplify the mRNAs of
RANKL and OPG in mono-culture human PDL cells and human PDL
cell-CD4+ T cell co-culture. In the mono-culture system,
gene expression of RANKL was significantly upregulated after
stimulation with nicotine (P<0.001) (Fig. 2A), while OPG was significantly
downregulated (P<0.05) (Fig.
2B). In the co-culture system, gene expression of RANKL was
significantly higher than the mono-culture system after stimulation
with nicotine (P<0.01) (Fig.
2A), while no differences were observed in OPG mRNA expression
between the co-culture and mono-culture systems (P>0.05)
(Fig. 2B). β-actin used as the
housekeeping gene was unaffected by the different stimulations.
RANKL and OPG protein expression in human
PDL cells
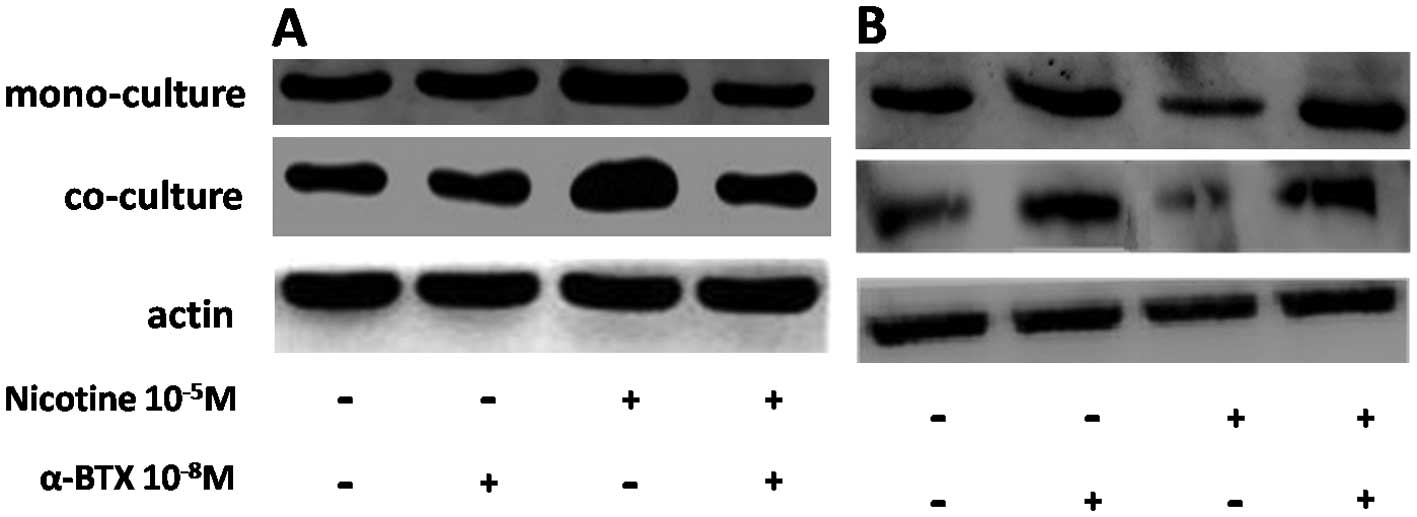
Since human PDL cells constitutively expressed RANKL
and OPG on mRNA levels, protein levels of RANKL and OPG were
assessed by western blot analysis. Protein levels showed a similar
tendency to mRNA levels (Fig. 3).
Consequently, the observed minor changes at the protein and mRNA
levels do not evidently affect OPG, identifying this cytokine as a
stable protein synthesized by human PDL cells.
Discussion
In the present study, we found that nicotine
significantly upregulated RANKL expression and downregulated OPG
expression in mono-culture human PDL cells; it also upregulated the
secretion of IL-1β in serum of mono-culture human PDL cells and
mono-culture CD4+ T cells. The secretion of IL-1β in
human PDL cell-CD4+ T cell co-culture was significantly
higher than in mono-culture systems, and the expression of RANKL
was further upregulated in human PDL cells co-cultured with
CD4+ T cells.
Tobacco smoking aggravated periodontal tissue
destruction, pocket formation, alveolar bone resorption in
periodontitis (2–4,23,24). It also reduced alveolar bone
height and bone mineral density and led to alveolar bone resorption
in rats (25). Meanwhile, its
main component, nicotine, also aggravated the alveolar bone
resorption in rat experimental periodontitis, and it was positively
related to the expression of α7 nAChR (8,9).
In addition, local accumulation of excessive CD4+ T
cells may be associated with periodontal tissue destruction in
periodontitis (26).
CD4+ T cells in peripheral blood of smoking-associated
periodontitis was significantly higher than that of non-smokers
(27). These findings suggested
that CD4+ T cell-mediated immune responses play a
critical role in the process of periodontitis.
The RANKL/RANK/OPG axis is significantly involved in
osteoclast differentiation and bone remodeling (17,18). Any deregulation of this axis can
alter bone metabolism, resulting in loss or gain of bone mass.
Compared with periodontal health, RANKL is upregulated, whereas OPG
is downregulated, in periodontitis, resulting in an enhanced
RANKL/OPG ratio (28–30). Compared with health or gingivitis,
GCF RANKL level is also significantly upregulated and OPG level is
significantly downregulated in chronic and generalized aggressive
periodontitis, and positively related to probing pocket depth and
clinical attachment level (31).
In smoking-associated periodontitis, the GCF levels of RANKL and
OPG showed a similar tendency to chronic and generalized aggressive
periodontitis (32). The RANKL
level was higher in gingival biopsies of smokers with periodontitis
than in controls, whereas the OPG level was lower (33). Smoking also affected the status of
periodontal treatment; the untreated as well as the treated smokers
exhibited higher RANKL and lower OPG concentrations than
non-smokers in saliva (34).
Nicotine downregulated the expression of OPG and upregulated the
expression of RANKL in human PDL cells (19). High RANKL/OPG ratio could promote
differentiation of osteoclasts. These results suggested that
nicotine may affect the RANKL/RANK/OPG axis involved in alveolar
bone resorption of smoking-associated periodontitis.
An animal study showed that CD4+ T cells,
but not CD8+ T cells or B cells, were identified as
essential mediators of alveolar bone destruction (35). Extract of smokeless tobacco at low
concentrations enhanced the production of IL-1β in lymphocytes and
also induced lymphocyte proliferation (36). IL-1β could be part of host
responses involved in the process of local periodontitis and
promote differentiation of osteoclasts, causing periodontal tissue
destruction and alveolar bone absorption (22). Gursoy et al (37) suggested that salivary IL-1β could
be referred to as the marker of periodontitis. Clinical studies
showed that the GCF IL-1β level is significantly associated with
pocket depth and bleeding on probing in periodontitis (38). Smoking status affected the GCF
IL-1β level; GCF IL-1β from deep bleeding sites of heavy smokers
was significantly higher than that of non-smokers (20). Moreover, smoking could also affect
the GCF IL-1β level following periodontal therapy. IL-1β
concentration was significantly greater in smokers following
therapy than in non-smokers (39). Any factors affecting the balance
of the RANKL/RANK/OPG axis could lead to the imbalance of bone
metabolism, both in physiological and pathological conditions.
Nakao et al (40)
suggested IL-1β as an autocrine factor regulating compressive
force-induced RANKL expression in human PDL cells. IL-1β induced
RANKL expression at the mRNA and protein levels, as well as RANKL
activity, through partial suppression of prostaglandin E2 synthesis
in human PDL cells directly or indirectly (41). Cell-cell interactions between
PBMCs and periodontal ligament fibroblasts significantly favor the
expression of osteoclastogenesis-related genes (RANKL, RANK, TNF-α
and IL-1β) and the ultimate formation of osteoclasts (42). CD4+ T cells also derive
from PBMCs, indicating human PDL cell-CD4+ T cell
co-culture could show similar effects as human PDL cell-PBMC
co-culture. Stimulated human PDL cell-PBMC co-culture with IL-1β
has a long-lasting effect, leading to a significantly increased
osteoclastogenesis, upregulating mRNA expression of intercellular
adhesion molecule-1 (ICAM-1), macrophage colony stimulating factor
(M-CSF) and IL-1β, augmenting formation of TRACP+
multinucleated cells (43). These
findings indicate that IL-1β could affect the balance of the
RANKL/RANK/OPG axis, particularly RANKL, causing bone resorption
and inflammation in periodontitis.
In conclusion, our data indicate that nicotine could
favor osteoclastogenesis in human PDL cells co-cultured with
CD4+ T cells by upregulating IL-1β. This may have
implications for the better understanding of affected bone
remodeling in smoking-associated periodontitis. Although we gained
significant insights, these results are based on the responses of
cell lines; further investigations are required to ascertain
whether or not these results reflect processes in the intact
body.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (NSFC grants 30973315 and
81170964). The authors are grateful to Professor Kun Yang for her
guidance in this study.
References
|
1
|
de Heens GL, Kikkert R, Aarden LA, van der
Velden U and Loos BG: Effects of smoking on the ex vivo cytokine
production in periodontitis. J Periodontal Res. 44:28–34.
2009.PubMed/NCBI
|
|
2
|
Tomar SL and Asma S: Smoking-attributable
periodontitis in the United States: findings from NHANES III.
National Health and Nutrition Examination Survey. J Periodontol.
71:743–751. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haber J, Wattles J, Crowley M, Mandell R,
Joshipura K and Kent RL: Evidence for cigarette smoking as a major
risk factor for periodontitis. J Periodontol. 64:16–23. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomson WM, Broadbent JM, Welch D, Beck JD
and Poulton R: Cigarette smoking and periodontal disease among
32-year-olds: a prospective study of a representative birth cohort.
J Clin Periodontol. 34:828–834. 2007.PubMed/NCBI
|
|
5
|
Bergström J: Tobacco smoking and chronic
destructive periodontal disease. Odontology. 92:1–8. 2004.
|
|
6
|
Levin L and Levine J: Cigarette smoking
and radiographic alveolar bone height and density. NY State Dent J.
76:31–35. 2010.PubMed/NCBI
|
|
7
|
Bosco AF, Bonfante S, de Almeida JM, Luize
DS, Nagata MJ and Garcia VG: A histologic and histometric
assessment of the influence of nicotine on alveolar bone loss in
rats. J Periodontol. 78:527–532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu YF, Wu LA, Wang J, Wen LY and Wang XJ:
Micro-computerized tomography analysis of alveolar bone loss in
ligature- and nicotine-induced experimental periodontitis in rats.
J Periodontal Res. 45:714–719. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XJ, Liu YF, Wang QY, et al:
Functional expression of alpha 7 nicotinic acetylcholine receptors
in human periodontal ligament fibroblasts and rat periodontal
tissues. Cell Tissue Res. 340:347–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ito H, Honda T, Domon H, et al: Gene
expression analysis of the CD4+ T-cell clones derived
from gingival tissues of periodontitis patients. Oral Microbiol
Immunol. 20:382–386. 2005.
|
|
11
|
Takeichi O, Haber J, Kawai T, Smith DJ,
Moro I and Taubman MA: Cytokine profiles of T-lymphocytes from
gingival tissues with pathological pocketing. J Dent Res.
79:1548–1555. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sigusch B, Klinger G, Glockmann E and
Simon HU: Early-onset and adult periodontitis associated with
abnormal cytokine production by activated T lymphocytes. J
Periodontol. 69:1098–1104. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alayan J, Ivanovski S and Farah CS:
Alveolar bone loss in T helper 1/T helper 2 cytokine-deficient
mice. J Periodontal Res. 42:97–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baker PJ, Dixon M, Evans RT, Dufour L,
Johnson E and Roopenian DC: CD4(+) T cells and the proinflammatory
cytokines gamma interferon and interleukin-6 contribute to alveolar
bone loss in mice. Infect Immun. 67:2804–2809. 1999.
|
|
15
|
Baker PJ, Garneau J, Howe L and Roopenian
DC: T-cell contributions to alveolar bone loss in response to oral
infection with Porphyromonas gingivalis. Acta Odontol Scand.
59:222–225. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taubman MA, Valverde P, Han X and Kawai T:
Immune response: the key to bone resorption in periodontal disease.
J Periodontol. 76:2033–2041. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HJ, Pi SH, Kim Y, et al: Effects of
nicotine on antioxidant defense enzymes and RANKL expression in
human periodontal ligament cells. J Periodontol. 80:1281–1288.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rawlinson A, Grummitt JM, Walsh TF and Ian
Douglas CW: Interleukin 1 and receptor antagonist levels in
gingival crevicular fluid in heavy smokers versus non-smokers. J
Clin Periodontol. 30:42–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toker H, Akpinar A, Aydin H and Poyraz O:
Influence of smoking on interleukin-1beta level, oxidant status and
antioxidant status in gingival crevicular fluid from chronic
periodontitis patients before and after periodontal treatment. J
Periodontal Res. 47:572–577. 2012. View Article : Google Scholar
|
|
22
|
Liu YC, Lerner UH and Teng YT: Cytokine
responses against periodontal infection: protective and destructive
roles. Periodontol 2000. 52:163–206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson GK and Hill M: Cigarette smoking
and the periodontal patient. J Periodontol. 75:196–209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergström J: Periodontitis and smoking: an
evidence-based appraisal. J Evid Based Dent Pract. 6:33–41.
2006.
|
|
25
|
Benatti BB, Cesar-Neto JB, Goncalves PF,
Sallum EA and Nociti FH Jr: Smoking affects the self-healing
capacity of periodontal tissues. A histological study in the rat.
Eur J Oral Sci. 113:400–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loos BG, Roos MT, Schellekens PT, van der
Velden U and Miedema F: Lymphocyte numbers and function in relation
to periodontitis and smoking. J Periodontol. 75:557–564. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Heens GL, van der Velden U and Loos BG:
Cigarette smoking enhances T cell activation and a Th2 immune
response; an aspect of the pathophysiology in periodontal disease.
Cytokine. 47:157–161. 2009.PubMed/NCBI
|
|
28
|
Belibasakis GN and Bostanci N: The
RANKL-OPG system in clinical periodontology. J Clin Periodontol.
39:239–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Crotti T, Smith MD, Hirsch R, et al:
Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin
(OPG) protein expression in periodontitis. J Periodontal Res.
38:380–387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu D, Xu JK, Figliomeni L, et al:
Expression of RANKL and OPG mRNA in periodontal disease: possible
involvement in bone destruction. Int J Mol Med. 11:17–21.
2003.PubMed/NCBI
|
|
31
|
Bostanci N, Ilgenli T, Emingil G, et al:
Gingival crevicular fluid levels of RANKL and OPG in periodontal
diseases: implications of their relative ratio. J Clin Periodontol.
34:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang TH, Fitzsimmons TR and Bartold PM:
Effect of smoking on concentrations of receptor activator of
nuclear factor kappa B ligand and osteoprotegerin in human gingival
crevicular fluid. J Clin Periodontol. 36:713–718. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cesar-Neto JB, Duarte PM, de Oliveira MC,
Tambeli CH, Sallum EA and Nociti FH Jr: Smoking modulates
interleukin-6:interleukin-10 and RANKL:osteoprotegerin ratios in
the periodontal tissues. J Periodontal Res. 42:184–191. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Buduneli N, Biyikoglu B, Sherrabeh S and
Lappin DF: Saliva concentrations of RANKL and osteoprotegerin in
smoker versus non-smoker chronic periodontitis patients. J Clin
Periodontol. 35:846–852. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teng YT, Nguyen H, Gao X, et al:
Functional human T-cell immunity and osteoprotegerin ligand control
alveolar bone destruction in periodontal infection. J Clin Invest.
106:R59–R67. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seyedroudbari SA and Khan MM: In vitro
effects of smokeless tobacco extract on tumor necrosis factor-alpha
(TNF-alpha) and interleukin-1beta (IL-1beta) production, and on
lymphocyte proliferation. Toxicon. 36:631–637. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gursoy UK, Kononen E, Uitto VJ, et al:
Salivary interleukin-1beta concentration and the presence of
multiple pathogens in periodontitis. J Clin Periodontol.
36:922–927. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhong Y, Slade GD, Beck JD and Offenbacher
S: Gingival crevicular fluid interleukin-1beta, prostaglandin E2
and periodontal status in a community population. J Clin
Periodontol. 34:285–293. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goutoudi P, Diza E and Arvanitidou M:
Effect of periodontal therapy on crevicular fluid interleukin-1beta
and interleukin-10 levels in chronic periodontitis. J Dent.
32:511–520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakao K, Goto T, Gunjigake KK, Konoo T,
Kobayashi S and Yamaguchi K: Intermittent force induces high RANKL
expression in human periodontal ligament cells. J Dent Res.
86:623–628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oikawa A, Kobayashi M, Okamatsu Y, et al:
Mitogen-activated protein kinases mediate interleukin-1beta-induced
receptor activator of nuclear factor-kappaB ligand expression in
human periodontal ligament cells. J Periodontal Res. 42:367–376.
2007. View Article : Google Scholar
|
|
42
|
Bloemen V, Schoenmaker T, de Vries TJ and
Everts V: Direct cell-cell contact between periodontal ligament
fibroblasts and osteoclast precursors synergistically increases the
expression of genes related to osteoclastogenesis. J Cell Physiol.
222:565–573. 2010.
|
|
43
|
Bloemen V, Schoenmaker T, de Vries TJ and
Everts V: IL-1beta favors osteoclastogenesis via supporting human
periodontal ligament fibroblasts. J Cell Biochem. 112:1890–1897.
2011. View Article : Google Scholar : PubMed/NCBI
|