Introduction
Endometrial cancer is the most common malignant
tumor of the female genital tract, and its incidence has increased
in recent years (1,2). Furthermore, the search for agents
effective in the treatment of either advanced or recurrent
endometrial cancer has proved to be disappointing (2,3).
Therefore, innovative approaches are required for the treatment of
endometrial cancer.
Peroxisome proliferator-activated receptor (PPAR)γ
is a nuclear hormone receptor and its ligands, troglitazone and
pioglitazone, have been shown to induce apoptosis in several types
of cancer cells, including endometrial cancer cells (4–6).
15-Deoxy-Δ12,14-prostaglandin J2
(15d-PGJ2) is a PPARγ ligand that activates PPARγ at
micromolar concentrations in humans in vivo (7–9).
Recently, 15d-PGJ2 was reported to have
antiproliferative activity in certain types of cancer (4,10–12). However, the effect of
15d-PGJ2 on endometrial cancer cells has not yet been
investigated.
The present study aimed to investigate the
biological and therapeutic effects of 15d-PGJ2 on
endometrial cancer. We examined whether this compound can mediate
cell growth inhibition, cell cycle arrest and apoptosis in
endometrial cancer cell lines (HHUA, Ishikawa and HEC-59).
Furthermore, to identify potential and novel target genes
responsive to the anticancer effect in 15d-PGJ2-treated
endometrial cancer cells, we analyzed the global changes in gene
expression in HHUA cells following treatment with
15d-PGJ2 using cDNA microarrays. The expression of
candidate proteins was confirmed by western blot analysis in the 3
endometrial cancer cell lines.
Materials and methods
Cell lines
The HHUA human endometrial cancer cell line was
obtained from Riken (Ibaraki, Japan). The Ishikawa human
endometrial cancer cell line was kindly provided by Dr Masato
Nishida (Tsukuba University, Ibaraki, Japan). The HEC-59 human
endometrial cancer cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA). The cells were maintained
as monolayers at 37°C in 5% CO2/air in Dulbecco’s
modified Eagle’s medium (DMEM; Gibco, Rockville, MD, USA)
containing 10% heat-inactivated fetal bovine serum (FBS; Omega,
Tarzana, CA, USA).
Chemicals
15d-PGJ2 was obtained from Enzo Life
Sciences (Plymouth Meeting, Montgomery County, PA, USA), and
prepared as a 20 mg/ml stock solution in dimethyl sulfoxide (DMSO).
The stock solution was stored in aliquots at −20°C.
Assessment of cell proliferation and cell
viability
The cell proliferation and cell viability were
determined in 96-well plates by a modified methylthiazol
tetrazolium (MTT) assay using WST-1 (Roche Diagnostics, Penzberg,
Germany) following the manufacturer’s instructions. We distributed
5×103 cells in DMEM supplemented with 10% FBS into each
well of a 96-well flat-bottomed microplate (Corning, Inc., New
York, NY, USA) and incubated them overnight. The medium was then
removed, and the cells were incubated for 48 h with 100 μl of
experimental medium containing various concentrations of
15d-PGJ2. Thereafter, 10 μl of WST-1 dye was added to
each well, and the cells were further incubated for 4 h. All
experiments were performed in the presence of 10% FBS. Cell
proliferation was evaluated by measuring the absorbance at 540 nm.
Data were calculated as the ratio of the values obtained for the
15d-PGJ2-treated cells to those for the untreated
controls.
Cell cycle analysis by flow
cytometry
The cell cycle was analyzed by flow cytometry after
2 days of culturing. Cells (5×104) were exposed to
15d-PGJ2 in 6-well flat-bottomed plates for 48 h.
Analysis was performed immediately after staining using the CellFIT
program (Becton-Dickinson, San Jose, CA, USA), whereby the S phase
was calculated using an RFit model.
Measurement of apoptosis [flow-cytometric
analysis with the Annexin V/propidium iodide (PI) assay]
Cells were plated and grown overnight until they
reached 80% confluence and then treated with 15d-PGJ2.
After 48 h, detached cells in the medium were collected, and the
remaining adherent cells were harvested by trypsinization. The
cells (1×105) were washed with PBS and resuspended in
250 μl of binding buffer (Annexin V-FITC kit; Becton-Dickinson)
containing 10 μl of 20 μg/ml PI and 5 μl of Annexin V-FITC, which
binds to phosphatidylserine translocated to the exterior of the
cell membrane early in the apoptotic pathway as well as during
necrosis. After incubation for 10 min at room temperature in a
light-protected area, the samples were analyzed on a FACSCalibur
flow cytometer (Becton-Dickinson). FITC and PI emissions were
detected in the FL-1 and FL-2 channels, respectively. For each
sample, data from 30,000 cells were recorded in list mode on
logarithmic scales. Subsequent analysis was performed with
CellQuest software (Becton-Dickinson).
Mitochondrial transmembrane potential
(MTP)
Cells were prepared for FACS analysis as described
above and stained using a Mitocapture Apoptosis Detection kit
obtained from BioVision (Palo Alto, CA, USA) with a fluorescent
lipophilic cationic reagent that assesses mitochondrial membrane
permeability, according to the manufacturer’s recommendations.
Microarray analysis
Total RNA was extracted from the
15d-PGJ2-treated and untreated HHUA cells using an
RNeasy mini kit (Qiagen, Valencia, CA, USA) in accordance with the
manufacturer’s instructions. Prior to hybridization, the quantity
and quality of the total RNA were evaluated using a
spectrophotometer and a 2100 Bioanalyzer (Agilent Technologies,
Santa Clara, CA, USA), respectively. Cy3-labeled cRNA targets were
generated using a Low RNA Input Fluorescent Linear Amplification
kit (Agilent Technologies). A human 44 K oligoarray was used for
hybridization, in accordance with the manufacturer’s
recommendations (Agilent Technologies). A laser confocal scanner
(Agilent Technologies) was used to measure signal intensities in
the expression microarray analysis. Feature Extraction software
(Version 9.1; Agilent Technologies) with the manufacturer’s
recommended settings was applied for the microarray image analysis.
Analysis of the microarray images was performed with GeneSpring
7.3.1 software (Agilent Technologies). For comparison among
multiple arrays, probe set data were median-normalized/chip. The
data were then centered across the genes in 6 normal controls,
followed by filtering based on a signal intensity of ≥100, and
contained no flagged values. Among these differentially expressed
genes, those designated as ‘upregulated’ were overexpressed
>2-fold in comparison with the controls (P<0.05), whereas
those designated as ‘downregulated’ were underexpressed
<0.75-fold compared with the controls (P<0.05). Annotations
including chromosomal loci were provided by Agilent
Technologies.
For Gene Ontology (GO) analysis, differentially
expressed genes were defined as those with a >2-fold increase or
decrease in expression relative to the controls. GO term enrichment
in the upregulated or downregulated gene sets was assessed using
the GOstat web tool (13).
Western blot analysis
Cells were washed twice in PBS, suspended in lysis
buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS, 0.5% sodium
deoxycholate, 1% NP-40, phenylmethylsulfonyl fluoride at 100 μg/ml,
aprotinin at 2 μg/ml, pepstatin at 1 μg/ml and leupeptin at 10
μg/ml], and placed on ice for 30 min. After centrifugation at
15,000 × g for 15 min at 4°C, the suspension was collected. Protein
concentrations were quantified using the Bio-Rad protein Assay Dye
Reagent Concentrate (Bio-Rad Laboratories, Hercules, CA, USA)
according to the manufacturer’s recommendations. Whole-cell lysates
(40 μg) were resolved by SDS-polyacrylamide gel electrophoresis on
a 4–15% gel, transferred onto a polyvinylidene difluoride membrane
(Immobilon; Amersham, Arlington Heights, IL, USA), and probed
sequentially with antibodies against anterior gradient homolog 3
(AGR3; 1:1,000; GeneTex, Irvine, CA, USA), aldo-keto reductase
family 1 member C1 (AKR1C1; 1:1,000; GeneTex), aldo-keto reductase
family 1 member C3 (AKR1C3; 1:1,000; ProteinTech, Chicago, IL,
USA), α-1-microglobulin/bikunin precursor (AMBP; 1:1,000; Abnova,
Taipei, Taiwan), complement component 3a receptor 1 (C3AR1;
1:1,000; Abnova), chondroadherin (CHAD; 1:1,000; Avia Systems
Biology, San Diego, CA, USA), Fer3-like (Drosophila)
(FERDL3; 1:1,000; Avia Systems Biology), ferritin, light
polypeptide (FTL; 1:1,000; GeneTex),
galactose-3-O-sulfotransferase 3 (GAL3ST3; 1:1,000; Avia
Systems Biology), glutamate-cysteine ligase, modifier subunit
(GCLM; 1:1,000; Abnova), heme oxygenase (decycling) 1 (HMOX1;
1:1,000; Abnova), intercellular adhesion molecule 4 (ICAM4;
1:1,000; Abnova), potassium voltage-gated channel, shaker-related
subfamily, β member 1 (KCNAB1; 1:1,000; Osenses, Keswick,
Australia), mitochondrial ribosomal protein L37 (MRPL37; 1:1,000;
ProteinTech), nitric oxide synthase 2A (NOS2A; 1:1,000; Applied
Biological Materials, Kampenhout, Belgium), phosphorylated
eukaryotic translation initiation factor 4E (p-eIF4E; 1:1,000;
Bioworld Technology, Minneapolis, MN, USA), pirin (PIR; 1:1,000;
Avia Systems Biology), tripartite motif-containing 16 (TRIM16;
1:1,000; Avia Systems Biology), thioredoxin reductase 1 (TXNRD1;
1:1,000; ProteinTech), UDP glucuronosyltransferase 1 family,
polypeptide A6 (UGT1A6; 1:1,000; LifeSpan Biosciences, Seattle, WA,
USA) and GAPDH monoclonal antibody (mAb) (1:10,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The blots were developed
using an enhanced chemiluminescent (ECL) kit (Amersham). Band
intensity was measured using the public domain Image program ImageJ
version 1.44, and fold increase in expression as compared with
control, untreated cells was calculated.
Statistical analysis
Data are presented as the means ± SD of
representative experiments and were analyzed by the Bonferroni-Dunn
test using StatView 4.5 software (Abacus Concepts, Berkeley, CA,
USA). A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
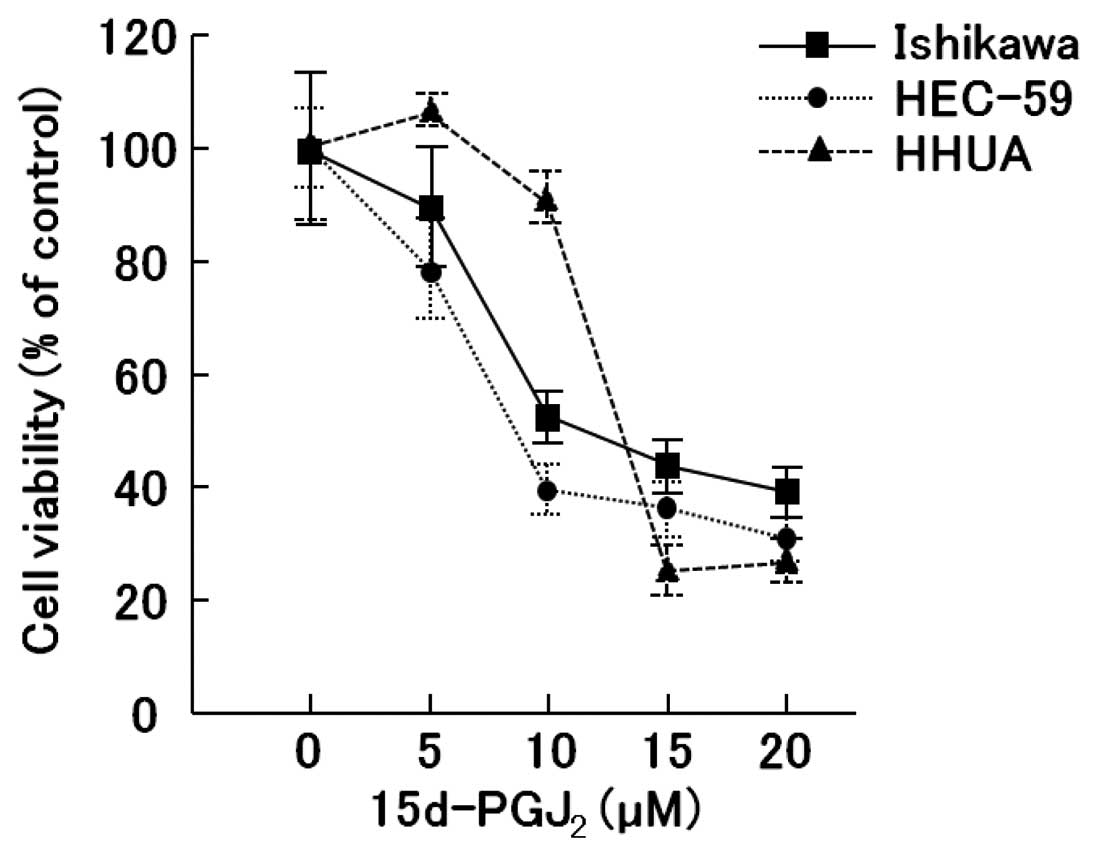
Effects of 15d-PGJ2 on the
proliferation and viability of endometrial cancer cell lines in
vitro
The antitumor effects of 15d-PGJ2 on 3
endometrial cancer cell lines in vitro were examined using a
WST-1 assay of the 2-day exposure to 15d-PGJ2.
Significant inhibitory effects of 15d-PGJ2 on the cell
growth were observed in all 3 endometrial cancer cell lines
(Ishikawa, HHUA and HEC-59) (Fig.
1).
Cell cycle analysis of endometrial cancer
cells following exposure to 15d-PGJ2
We then investigated whether 15d-PGJ2
would lead to the induction of apoptosis and/or cell cycle arrest
in the endometrial cancer cells (Table I). 15d-PGJ2 led to an
increase in the sub G0/G1 apoptotic cell population and the cell
population in the G2/M phase of the cell cycle compared to
treatment with the vehicle alone, with a concomitant decrease in
the proportion of cells in the S phase.
 | Table ICell cycle changes in endometrial
cancer cell lines. |
Table I
Cell cycle changes in endometrial
cancer cell lines.
| Cell line | Vehicle | 15d-PGJ2
(10 μM) |
|---|
| Ishikawa |
| Sub G0/G1 (%) | 3.1±0.1 | 6.2±0.3a |
| G0/G1 (%) | 53.4±12.4 | 35.0±17.7a |
| S (%) | 36.1±5.6 | 38.4±7.5 |
| G2/M (%) | 10.5±7.7 | 26.6±11.8a |
| HEC-59 |
| Sub G0/G1 (%) | 2.7±0.1 | 0.5±0.1a |
| G0/G1 (%) | 51.9±0.8 | 54.7±0.9 |
| S (%) | 35.8±0.6 | 31.6±1.2 |
| G2/M (%) | 12.3±0.3 | 13.6±0.7a |
| HHUA |
| Sub G0/G1 (%) | 3.9±0.8 | 5.2±2.0a |
| G0/G1 (%) | 53.5±3.6 | 44.0±8.1 |
| S (%) | 35.3±1.7 | 44.0±4.0 |
| G2/M (%) | 11.3±2.0 | 12.1±4.0 |
Apoptotic changes in endometrial cancer
cells treated with 15d-PGJ2
To assess the ability of the endometrial cancer
cells to undergo apoptosis in response to 15d-PGJ2
exposure and to distinguish between the different types of cell
death, we double-stained the 15d-PGJ2-treated cells with
Annexin V and PI and analyzed the results using flow cytometry.
Annexin V binding combined with PI labeling was performed for the
distinction of early apoptotic (Annexin
V+/PI−) and necrotic (Annexin
V+/PI+) cells. At increasing doses of
15d-PGJ2, a simultaneous increase in both the Annexin
V+/PI− fraction (early apoptotic) and Annexin
V+/PI+ (regarded as necrotic) subpopulations
was detected (Table II).
 | Table IICell death measured by Annexin V and
mitochondrial transmembrane potential assay in endometrial cancer
cell lines. |
Table II
Cell death measured by Annexin V and
mitochondrial transmembrane potential assay in endometrial cancer
cell lines.
| Assay/cell line | Vehicle | 15d-PGJ2
(10 μM) |
|---|
| Annexin V assay |
| Ishikawa |
| Viable (LL)
(%) | 92.5±0.1 | 48.4±1.6a |
| Apoptosis (LR)
(%) | 4.9±0.1 | 35.8±0.8a |
| Necrosis (UR)
(%) | 2.5±0.2 | 15.3±0.8a |
| HEC-59 |
| Viable (LL)
(%) | 86.7±0.3 | 56.3±1.0a |
| Apoptosis (LR)
(%) | 4.7±0.3 | 6.5±0.3 |
| Necrosis (UR)
(%) | 5.4±0.1 | 16.7±0.4a |
| HHUA |
| Viable (LL)
(%) | 79.2±8.5 | 61.9±5.9a |
| Apoptosis (LR)
(%) | 6.7±1.1 | 12.9±3.3a |
| Necrosis (UR)
(%) | 4.6±1.1 | 17.1±4.3a |
| MTP assay |
| Ishikawa |
| Viable (%) | 76 | 44 |
| Apoptosis
(%) | 25 | 59 |
| HEC-59 |
| Viable (%) | 77 | 58 |
| Apoptosis
(%) | 23 | 44 |
| HHUA |
| Viable (%) | 68 | 14 |
| Apoptosis
(%) | 34 | 87 |
Loss of MTP in response to treatment with
15d-PGJ2
It has been shown that the loss of MTP occurs prior
to nuclear condensation and caspase activation and is linked to
cytochrome c release in many, but not all, apoptotic cells
(14,15). It was found that the treatment of
endometrial cancer cells with 15d-PGJ2 resulted in the
loss of MTP (Table II).
Differential gene expression in
15d-PGJ2-treated cells
In order to identify potential and novel target
genes responsive to the anticancer effects in
15d-PGJ2-treated endometrial cancer cells, we examined
the global changes in gene expression in the HHUA cells following
treatment with 10 μM of 15d-PGJ2 for 48 h (Tables III and IV). Of the 44,000 genes, GO analysis
was carried out on the genes upregulated and downregulated by the
treatment (Tables V and VI).
 | Table IIIUpregulated genes following treatment
with 15d-PGJ2 in HHUA cells. |
Table III
Upregulated genes following treatment
with 15d-PGJ2 in HHUA cells.
| Fold changes | Gene symbol | Description | GenBank | UniGene | Map |
|---|
| 17.50674 | AKR1C1 | Aldo-keto reductase
family 1, member C1 (dihydrodiol dehydrogenase 1; 20-α
(3-α)-hydroxysteroid dehydrogenase) | NM_001353 | Hs.460260 | 10p15-p14 |
| 15.647071 | AKR1C3 | Aldo-keto reductase
family 1, member C3 (3-α hydroxysteroid dehydrogenase, type
II) | NM_003739 | Hs.78183 | 10p15-p14 |
| 6.0476165 | AMBP |
α-1-microglobulin/bikunin precursor | NM_001633 | Hs.436911 | 9q32-q33 |
| 5.751939 | HMOX1 | Heme oxygenase
(decycling) 1 | NM_002133 | Hs.517581 | 22q12 |
| 4.723847 | A_32_P157671 | | | | 17p11.2 |
| 4.683617 | TRIM16 | Tripartite
motif-containing 16 | NM_006470 | Hs.123534 | 17p11.2 |
| 4.5855446 | PIR | Pirin (iron-binding
nuclear protein) | NM_003662 | Hs.495728 | Xp22.2 |
| 4.380505 | UGT1A6 | UDP
glucuronosyltransferase 1 family, polypeptide A6 | NM_001072 | Hs.654499 | 2q37 |
| 4.2928677 | TXNRD1 | Thioredoxin
reductase 1 | NM_003330 | Hs.654922 | 12q23-q24.1 |
| 3.9529867 | GCLM | Glutamate-cysteine
ligase, modifier subunit | NM_002061 | Hs.315562 | 1p22.1 |
| 3.817845 |
ENST00000313481 | | | | 19p13.3 |
| 3.6058688 | FTL | Ferritin, light
polypeptide | NM_000146 | Hs.433670 | 19q13.3-q13.4 |
| 3.4976888 | CR598364 | Full-length cDNA
clone CS0CAP007YJ17 of Thymus of Homo sapiens (human) | CR598364 | Hs.596052 | |
| 3.4703205 | G6PD | Glucose-6-phosphate
dehydrogenase | NM_000402 | Hs.461047 | Xq28 |
| 3.410493 | SRXN1 | Sulfiredoxin 1
homolog (S. cerevisiae) | NM_080725 | Hs.516830 | 20p13 |
| 3.343625 | SPP1 | Secreted
phosphoprotein 1 (osteopontin, bone sialoprotein I, early
T-lymphocyte activation 1) | NM_000582 | Hs.313 | 4q21-q25 |
| 3.3256302 | A_24_P281683 | | | | 11q23.3 |
| 3.108579 | TXNRD1 | Thioredoxin
reductase 1 | BG001037 | Hs.654922 | 12q23-q24.1 |
| 3.0549212 | PFKFB3 |
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase
3 | NM_004566 | Hs.195471 | 10p14-p15 |
| 3.0234468 | FLJ35767 | FLJ35767
protein | NM_207459 | Hs.231897 | 17q25.3 |
| 2.9421628 | EPHX1 | Epoxide hydrolase
1, microsomal (xenobiotic) | NM_000120 | Hs.89649 | 1q42.1 |
| 2.833596 | GCNT3 | Glucosaminyl
(N-acetyl) transferase 3, mucin type | NM_004751 | Hs.194710 | 15q21.3 |
| 2.699825 | OSGIN1 | Oxidative stress
induced growth inhibitor 1 | NM_013370 | Hs.128055 | 16q23.3 |
| 2.6840672 | GSR | Glutathione
reductase | BC035691 | Hs.271510 | 8p21.1 |
| 2.6485877 | IKBKG | Inhibitor of κ
light polypeptide gene enhancer in B-cells, kinase γ | NM_003639 | Hs.43505 | Xq28 |
| 2.6185443 |
ENST00000313774 | Homo sapiens
glucosaminyl (N-acetyl) transferase 3, mucin type, mRNA (cDNA clone
MGC:9086 IMAGE:3851937), complete cds. [BC017032] | | Hs.194710 | 15q22.2 |
| 2.5760007 | DDC | Dopa decarboxylase
(aromatic L-amino acid decarboxylase) | NM_000790 | Hs.359698 | 7p11 |
| 2.5224981 | LMNB1 | Lamin B1 | NM_005573 | Hs.89497 | 5q23.3-q31.1 |
| 2.5161438 | A_32_P7974 | | | | 1q21.3 |
| 2.505143 | NADSYN1 | NAD synthetase
1 | AL512694 | Hs.556986 | 11q13.4 |
| 2.4961286 | HSPA1A | Heat shock 70 kDa
protein 1A | NM_005345 | Hs.520028 | 6p21.3 |
| 2.47789 | GCLC | Glutamate-cysteine
ligase, catalytic subunit | NM_001498 | Hs.654465 | 6p12 |
| 2.361285 | CN272797 | 17000600009278
GRN_PREHEP Homo sapiens cDNA 5′, mRNA sequence. | CN272797 | | 9p23 |
| 2.3579373 | HSPA8 | Heat shock 70 kDa
protein 8 | BU731317 | Hs.180414 | 11q24.1 |
| 2.3155344 | C16orf28 | Chromosome 16 open
reading frame 28 | NM_023076 | Hs.643536 | 16p13.3 |
| 2.2990816 | ABCB6 | ATP-binding
cassette, sub-family B (MDR/TAP), member 6 | NM_005689 | Hs.107911 | 2q36 |
| 2.2683835 | ALDH3A2 | Aldehyde
dehydrogenase 3 family, member A2 | NM_000382 | Hs.499886 | 17p11.2 |
| 2.2379045 |
ENST00000238571 | Homo sapiens
(clone zap3) mRNA, 3′ end of cds. [L40403] | | Hs.531111 | 14q24.3 |
| 2.2241304 | GLA | Galactosidase,
α | NM_000169 | Hs.69089 | Xq22 |
| 2.2137868 | PRDX1 | Peroxiredoxin
1 | NM_002574 | Hs.180909 | 1p34.1 |
| 2.2122195 | ANGPTL4 | Homo sapiens
angiopoietin-like 4 (ANGPTL4), transcript variant 2, mRNA
[NM_016109] | NM_016109 | Hs.9613 | 19p13.2 |
| 2.165274 | GCLC | Hlutamate-cysteine
ligase, catalytic subunit | M90656 | Hs.654465 | 6p12 |
| 2.1589625 | THC2309960 | Q7ZX66 (Q7ZX66)
RNPC7 protein (Fragment), partial (9%) [THC2309960] | | Hs.527551 | Xq23 |
| 2.150731 | THC2269657 | Q6QI74 (Q6QI74)
LRRG00134, partial (10%) [THC2269657] | | | chr10 |
| 2.1422243 | PLXND1 | Plexin D1 | NM_015103 | Hs.301685 | 3q21.3 |
| 2.141769 | ABCB6 | ATP-binding
cassette, sub-family B (MDR/TAP), member 6 | NM_005689 | Hs.107911 | 2q36 |
| 2.1359584 | GSR | Glutathione
reductase | NM_000637 | Hs.271510 | 8p21.1 |
| 2.119439 | A_24_P178167 | | | | Xp11.23 |
| 2.1153674 | AIFM2 | Apoptosis-inducing
factor, mitochondrion-associated, 2 | NM_032797 | Hs.655377 | 10q22.1 |
| 2.0789819 | KCNMB4 | Potassium large
conductance calcium-activated channel, subfamily M, β member 4 | NM_014505 | Hs.525529 | 12q |
 | Table IVDownregulated genes following
treatment with 15d-PGJ2 in HHUA cells. |
Table IV
Downregulated genes following
treatment with 15d-PGJ2 in HHUA cells.
| Fold change | Gene symbol | Description | GenBank | UniGene | Map |
|---|
| 0.01 | FERD3L | Fer3-like
(Drosophila) | NM_152898 | Hs.592168 | 7p21.1 |
| 0.025915636 | A_24_P922893 | | | | 7q11.21 |
| 0.027622959 | AGR3 | Anterior gradient
homolog 3 (Xenopus laevis) | NM_176813 | Hs.100686 | 7p21.1 |
| 0.038513284 | MRPL37 | Mitochondrial
ribosomal protein L37 | NM_016491 | Hs.584908 | 1p32.1 |
| 0.05087885 | NOS2A | Nitric oxide
synthase 2A (inducible, hepatocytes) | NM_000625 | Hs.434386 | 17q11.2-q12 |
| 0.095559224 | C18orf23 | Chromosome 18 open
reading frame 23 | AK091537 | Hs.501114 | 18q21.1 |
| 0.10365046 |
ENST00000329610 | Homo sapiens
prepro-NPW mRNA for prepro-Neuropeptide W polypeptide, partial cds.
[AB084276] | | Hs.233533 | 16p13.3 |
| 0.12478433 | CASC1 | Cancer
susceptibility candidate 1 | NM_018272 | Hs.407771 | 12p12.1 |
| 0.14965945 | THC2368014 | AY320849
immunoglobulin κ chain variable region (Homo sapiens),
complete [THC2368014] | | | 2p11.2 |
| 0.15056583 | WBSCR19 | Williams Beuren
syndrome chromosome region 19 | NM_175064 | Hs.645483 | 7p13 |
| 0.15237725 | KIAA1183 | KIAA1183
protein | AB033009 | Hs.7193 | 19q13.32 |
| 0.16197103 | A_24_P932355 | | | | 19p13.11 |
| 0.1684146 | THC2280638 | RL2A_HUMAN (P46776)
60S ribosomal protein L27a, partial (24%) [THC2280638] | | | 4q13.3 |
| 0.16913189 | AK022268 | CDNA FLJ12206 fis,
clone MAMMA1000941 | AK022268 | Hs.658369 | 3 |
| 0.17584784 | GAL3ST3 |
Galactose-3-O-sulfotransferase
3 | NM_033036 | Hs.208343 | 11q13.1 |
| 0.18523274 | TMEM169 | Transmembrane
protein 169 | NM_138390 | Hs.334916 | 2q35 |
| 0.18725868 | THC2441492 | ALU7_HUMAN (P39194)
Alu subfamily SQ sequence contamination warning entry, partial
(12%) [THC2441492] | | | 19q13.12 |
| 0.19028467 |
ENST00000304181 |
GB|AJ009794.1|CAA08833.1 proline rich
domain [NP101191] | | | 10q24.31 |
| 0.20250778 | PDPR | Pyruvate
dehydrogenase phosphatase regulatory subunit | NM_017990 | Hs.655245 | 16q22.1 |
| 0.21064165 | THC2283809 | | | | 10q24.1 |
| 0.21432775 | ZNF791 | Zinc finger protein
791 | NM_153358 | Hs.522545 | 19p13.2-p13.13 |
| 0.21798867 | C3AR1 | Complement
component 3a receptor 1 | NM_004054 | Hs.591148 | 12p13.31 |
| 0.2198143 | CCDC110 | Coiled-coil domain
containing 110 | NM_152775 | Hs.41101 | 4q35.1 |
| 0.2209758 | KCNAB1 | Potassium
voltage-gated channel, shaker-related subfamily, β member 1 | BC043166 | Hs.654519 | 3q26.1 |
| 0.22387888 | CHAD | Chondroadherin | NM_001267 | Hs.97220 | 17q21.33 |
| 0.22510499 | ICAM4 | Intercellular
adhesion molecule 4 (Landsteiner-Wiener blood group) | NM_001544 | Hs.631609 | 19p13.2-cen |
| 0.22763024 | eIF4E | Eukaryotic
translation initiation factor 4E | BM981574 | Hs.249718 | 4q21-q25 |
| 0.2376016 | XPNPEP1 | X-prolyl
aminopeptidase (aminopeptidase P) 1, soluble | NM_020383 | Hs.390623 | 10q25.3 |
| 0.23924729 | CPXM1 | Carboxypeptidase X
(M14 family), member 1 | NM_019609 | Hs.659346 | 20p13-p12.3 |
| 0.24067116 | NDRG2 | NDRG family member
2 | NM_201535 | Hs.525205 | 14q11.2 |
| 0.2438523 | A_24_P916853 | | | 8q24.21 | |
| 0.24945486 | FLJ21272 | Hypothetical
protein FLJ21272 | AK024925 | Hs.612891 | 1q21.2 |
| 0.25685737 | CSNK1G1 | Casein kinase 1, γ
1; Homo sapiens casein kinase 1, γ 1 (CSNK1G1), mRNA | NM_001011664 | Hs.254335 | 15q22.1-q22.31 |
| 0.2718289 | CATSPER1 | Cation channel,
sperm associated 1 | NM_053054 | Hs.189105 | 11q12.1 |
| 0.27470103 | APOA4 | Apolipoprotein
A-IV | NM_000482 | Hs.591940 | 11q23 |
| 0.2767344 | MMP1 | Matrix
metallopeptidase 1 (interstitial collagenase) | NM_002421 | Hs.83169 | 11q22.3 |
| 0.29412216 | C15orf37 | Chromosome 15 open
reading frame 37 | NM_175898 | Hs.512015 | 15q25.1 |
| 0.3019541 | COPZ2 | Coatomer protein
complex, subunit ζ 2 | NM_016429 | Hs.408434 | 17q21.32 |
| 0.3044736 | RREB1 | Ras responsive
element binding protein 1 | NM_002955 | Hs.298248 | 6p25 |
| 0.31109598 | GMFG | Glia maturation
factor, γ | NM_004877 | Hs.5210 | 19q13.2 |
| 0.3145231 | MGC16121 | Hypothetical
protein MGC16121 | BC007360 | Hs.416379 | Xq26.3 |
| 0.31809595 | MCCD1 | Mitochondrial
coiled-coil domain 1 | NM_001011700 | Hs.558922 | 6p21.33 |
| 0.3269747 | WBSCR27 | Williams Beuren
syndrome chromosome region 27 | NM_152559 | Hs.647042 | 7q11.23 |
| 0.33880442 | DHRS2 |
Dehydrogenase/reductase (SDR family)
member 2 | NM_182908 | Hs.272499 | 14q11.2 |
| 0.342074 | MDFI | MyoD family
inhibitor | NM_005586 | Hs.520119 | 6p21 |
| 0.3541897 | DHRS2 |
Dehydrogenase/reductase (SDR family)
member 2 | NM_182908 | Hs.272499 | 14q11.2 |
| 0.361466 | IFP38 | Homo sapiens
IFP38 (IFP38), mRNA [NM_031943] | NM_031943 | Hs.513128 | chr13 |
| 0.36588448 |
ENST00000329078 | Homo
sapiens, Similar to spinster-like protein, clone IMAGE:4814561,
mRNA, partial cds. [BC041772] | | Hs.556015 | 17p13.2 |
| 0.36866197 | THC2433384 | ALU7_HUMAN (P39194)
Alu subfamily SQ sequence contamination warning entry, partial
(15%) [THC2433384] | | | 17p13.1 |
| 0.37413767 | BG182941 | Transcribed
locus | BG182941 | Hs.635280 | 7 |
 | Table VPermutation analysis of the
correlation between GO terms and upregulated genes following
treatment with 15d-PGJ2. |
Table V
Permutation analysis of the
correlation between GO terms and upregulated genes following
treatment with 15d-PGJ2.
| GO Accession | GO Term | Corrected
P-value | Count in
selection |
|---|
| GO:0055114 | Oxidation
reduction | 5.08E-05 | 10 |
| GO:0016491 | Oxidoreductase
activity | 5.08E-05 | 10 |
| GO:0005829 | Cytosol | 3.99E-04 | 13 |
| GO:0051186 | Co-factor metabolic
process | 0.001967945 | 5 |
| GO:0016209 | Antioxidant
activity | 0.001967945 | 4 |
|
| Genes | GO Term | ID |
Treatment/control |
|
| GO:0055114 | Oxidation
reduction | | |
| AKR1C1 | | A_23_P257971 | 17.29906688 |
| AKR1C3 | | A_23_P138541 | 15.00240358 |
| HMOX1 | | A_23_P120883 | 5.318975127 |
| TXNRD1 | | A_23_P204581 | 4.005567769 |
| GO:0016491 | Oxidoreductase
activity | | |
| AKR1C1 | | A_23_P257971 | 17.29906688 |
| AKR1C3 | | A_23_P138541 | 15.00240358 |
| HMOX1 | | A_23_P120883 | 5.318975127 |
| TXNRD1 | | A_23_P204581 | 4.005567769 |
| GO:0005829 | Cytosol | | |
| AKR1C1 | | A_23_P257971 | 17.29906688 |
| HMOX1 | | A_23_P120883 | 5.318975127 |
| TXNRD1 | | A_23_P204581 | 4.005567769 |
| GCLM | | A_23_P103996 | 3.688437908 |
| GO:0051186 | Co-factor metabolic
process | | |
| AMBP | | A_23_P256504 | 5.657345747 |
| HMOX1 | | A_23_P120883 | 5.318975127 |
| GCLM | | A_23_P103996 | 3.688437908 |
| GCLM | | A_32_P177953 | 3.255236172 |
| GO:0016209 | Antioxidant
activity | | |
| TXNRD1 | | A_23_P204581 | 4.005567769 |
| SRXN1 | | A_23_P320113 | 3.1590489 |
| GSR | | A_32_P31618 | 2.458848635 |
| PRDX1 | | A_23_P11995 | 2.077957762 |
 | Table VIPermutation analysis of the
correlation between GO terms and downregulated genes following
treatment with 15d-PGJ2. |
Table VI
Permutation analysis of the
correlation between GO terms and downregulated genes following
treatment with 15d-PGJ2.
| GO Accession | GO Term | Corrected
P-value | Count in
selection |
|---|
| GO:0002675 | Positive regulation
of acute inflammatory response | 0.064905845 | 2 |
| GO:0010817 | Regulation of
hormone levels | 0.076163195 | 3 |
| GO:0032101 | Regulation of
response to external stimulus | 0.076163195 | 3 |
| GO:0002673 | Regulation of acute
inflammatory response | 0.076163195 | 2 |
| GO:0002790 | Peptide
secretion | 0.07797773 | 2 |
|
| Genes | GO Term | ID |
Treatment/control |
|
| GO:0002675 | Positive regulation
of acute inflammatory response | | |
| IL6 | | A_23_P71037 | 0.404520132 |
| C3 | | A_23_P101407 | 0.450077889 |
| GO:0010817 | Regulation of
hormone levels | | |
| DHRS2 | | A_23_P321501 | 0.311235885 |
| IL6 | | A_23_P71037 | 0.404520132 |
| EDN1 | | A_23_P214821 | 0.461688691 |
| GO:0032101 | Regulation of
response to external stimulus | | |
| IL6 | | A_23_P71037 | 0.404520132 |
| C3 | | A_23_P101407 | 0.450077889 |
| EDN1 | | A_23_P214821 | 0.461688691 |
| GO:0002673 | Regulation of acute
inflammatory response | | |
| IL6 | | A_23_P71037 | 0.404520132 |
| C3 | | A_23_P101407 | 0.450077889 |
| GO:0002790 | Peptide
secretion | | |
| IL6 | | A_23_P71037 | 0.404520132 |
| EDN1 | | A_23_P214821 | 0.461688691 |
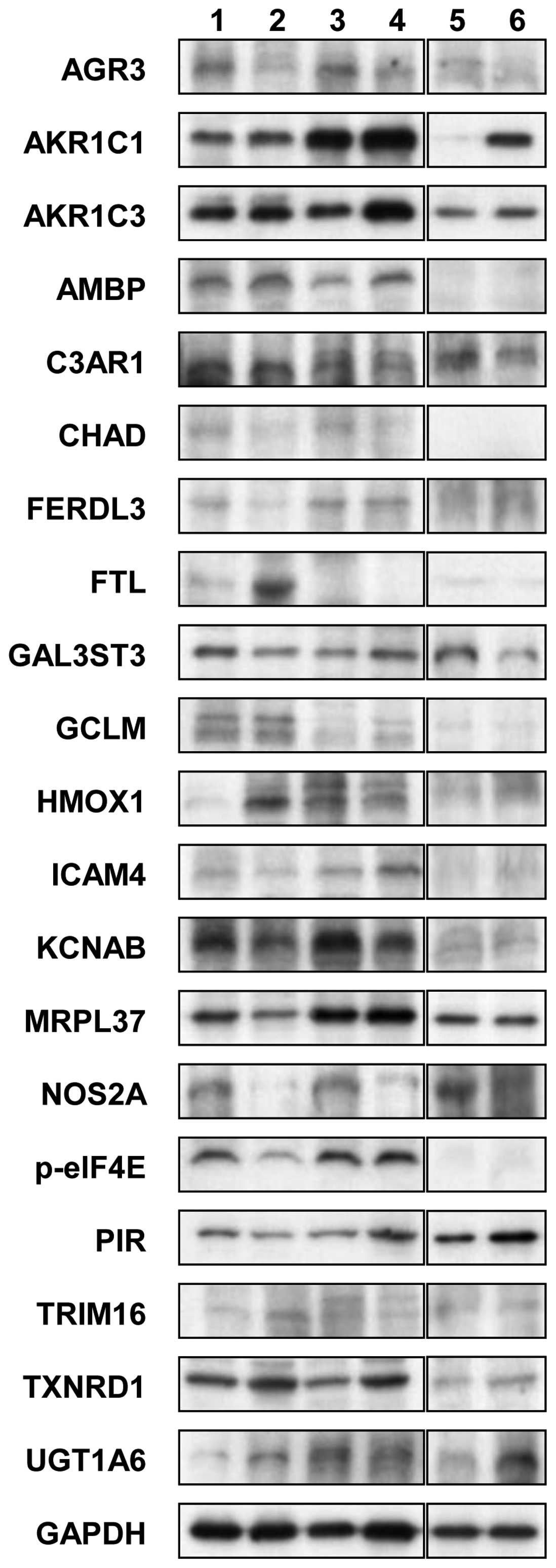
Effects of 15d-PGJ2 on the
expression of novel proteins
To elucidate the common mechanism of action of
15d-PGJ2 in endometrial cancer, we examined the effects
of 15d-PGJ2 on the expression of 20 proteins that were
selected from the cDNA microarray data in 3 endometrial cancer cell
lines using western blot analysis (Fig. 2 and Table VII). 15d-PGJ2
markedly upregulated the levels of AKR1C3 and downregulated the
levels of AGR3 and NOS2A proteins in all 3 endometrial cancer cell
lines.
 | Table VIIResults of western blot analysis in
the 3 cell lines. |
Table VII
Results of western blot analysis in
the 3 cell lines.
| Name | HHUA | Ishikawa | HEC-59 |
|---|
| AGR3 | ↓ | ↓ | ↓ |
| AKR1C1 | ↑ | - | ↑ |
| AKR1C3 | ↑ | ↑ | ↑ |
| AMBP | ↑ | ↑ | NE |
| C3AR1 | - | - | ↓ |
| CHAD | ↓ | ↓ | NE |
| FERDL3 | ↓ | - | - |
| FTL | ↑ | NE | - |
| GAL3ST3 | ↓ | ↑ | ↓ |
| GCLM | - | ↑ | - |
| HMOX1 | ↑ | - | - |
| ICAM4 | - | ↑ | NE |
| KCNAB1 | ↓ | ↓ | - |
| MRPL37 | ↓ | NE | NE |
| NOS2A | ↓ | ↓ | ↓ |
| p-elF4E | ↓ | - | NE |
| PIR | ↓ | ↑ | ↑ |
| TRIM16 | ↑ | - | - |
| TXNRD1 | ↑ | ↑ | - |
| UGT1A6 | ↑ | ↓ | ↑ |
Discussion
In the present study, we demonstrated that
15d-PGJ2 inhibits cell viability in endometrial cancer
cells. The prominent arrest of these cells in the G2/M phase of the
cell cycle and the induction of apoptosis likely account for this
inhibitory effect, suggesting that 15d-PGJ2 has
anticancer activity.
In order to investigate the molecular mechanisms
involved in the effects of 15d-PGJ2 on the cell cycle
arrest and the induction of apoptosis, we investigated the global
gene expression profile changes in HHUA endometrial cancer cells
following treatment with 15d-PGJ2. Surprisingly, the
expression of PPARγ or angiotensin II type 1 receptor (AT1R) was
not altered, although 15d-PGJ2 has been characterized as
a potent PPARγ ligand. To identify novel target genes of
15d-PGJ2, we focused on some GO terms of the numerous
genes upregulated and downregulated by 15d-PGJ2
treatment in the HHUA cells. GO analysis revealed that oxidation
reduction (GO:0055114) and oxidoreductase activity (GO:0016491)
were enriched in genes that were overexpressed in the
15d-PGJ2-treated HHUA cells compared to the untreated
HHUA cells. Both GO terms include AKR1C3.
AKR1C3 is a multifunctional enzyme involved in
androgen, estrogen, progesterone and prostaglandin metabolism.
AKR1C3-mediated steroid metabolism may play a critical role in the
maintenance of viable normal and abnormal endometrial epithelium
(16). AKR1C3 has been reported
to play important roles in the physiology of endometrial cells and
that suppressed AKR1C3 expression represents a feature that allows
the differentiation of hyperplastic and neoplastic endometrial
epithelium from normal endometrial epithelium (16). In the present study, we
demonstrated that 15d-PGJ2 markedly upregulated the
levels of the AKR1C3 protein in all 3 endometrial cancer cell
lines. Based on these observations, it can be hypothesized that the
15d-PGJ2-induced anticancer activity may be mediated, at
least in part, by the upregulation of AKR1C3 in human endometrial
cancer cells.
We confirmed the downregulation of AGR3 using
western blot analysis in all 3 cell lines examined. AGR genes, a
protein disulfide isomerase (PDI) family, harbour core thioredoxin
folds (CxxS motifs) that have the potential to regulate protein
folding and maturation. AGR3 is overexpressed by a hormone
(estrogen-receptor α)-independent mechanism, identifying a novel
protein-folding associated pathway that can mediate resistance to
DNA-damaging agents in human cancers (17). These findings indicate that the
downregulation of AGR3 by 15d-PGJ2 may cause DNA-damage,
leading to the apoptosis of endometrial cancer cells.
Nitric oxide, a reactive free radical, acts as a
biological mediator in several processes, including
neurotransmission and antimicrobial and antitumor activities. The
NOS2A gene encodes a nitric oxide synthase which is expressed in
the liver and is inducible by a combination of lipopolysaccharide
and certain cytokines. A recent study revealed that NOS2
upregulation contributes primarily to the proliferation and tumor
maintenance in highly tumorigenic human glioma stem cells (18). Therefore, our finding that
15d-PGJ2 downregulated NOS2A expression suggests that
the eicosanoid may inhibit the proliferation and maintenance of
endometrial cancer cells via NOS2A downregulation.
In conclusion, the data from the present study
demonstrate that 15d-PGJ2 exhibits anti-proliferative
activity, potently induces cell cycle arrest, and stimulates
apoptosis in human endometrial cancer cells. These events were
accompanied by the upregulation of AKR1C3 and the downregulation of
AGR3 and NOS2A. It is suggested that 15d-PGJ2 may be a
novel therapeutic option for the treatment of endometrial
cancer.
References
|
1
|
Reinhardt MJ: Gynecologic tumors. Recent
Results Cancer Res. 170:141–150. 2008. View Article : Google Scholar
|
|
2
|
Obel JC, Friberg G and Fleming GF:
Chemotherapy in endometrial cancer. Clin Adv Hematol Oncol.
4:459–468. 2006.
|
|
3
|
Hill EK and Dizon DS: Medical therapy of
endometrial cancer: current status and promising novel treatments.
Drugs. 72:705–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ota K, Ito K, Suzuki T, et al: Peroxisome
proliferator-activated receptor gamma and growth inhibition by its
ligands in uterine endometrial carcinoma. Clin Cancer Res.
12:4200–4208. 2006. View Article : Google Scholar
|
|
5
|
Xin B, Yokoyama Y, Shigeto T, Futagami M
and Mizunuma H: Inhibitory effect of meloxicam, a selective
cyclooxygenase-2 inhibitor, and ciglitazone, a peroxisome
proliferator-activated receptor gamma ligand, on the growth of
human ovarian cancers. Cancer. 110:791–800. 2007. View Article : Google Scholar
|
|
6
|
Yang YC, Tsao YP, Ho TC and Choung IP:
Peroxisome proliferator-activated receptor-gamma agonists cause
growth arrest and apoptosis in human ovarian carcinoma cell lines.
Int J Gynecol Cancer. 17:418–425. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nosjean O and Boutin JA: Natural ligands
of PPARgamma: are prostaglandin J(2) derivatives really playing the
part? Cell Signal. 14:573–583. 2002.PubMed/NCBI
|
|
8
|
Forman BM, Tontonoz P, Chen J, Brun RP,
Spiegeiman BM and Evans RM: 15-Deoxy-delta 12, 14-prostaglandin
J2 is a ligand for the adipocyte determination factor
PPARγ. Cell. 83:803–812. 1995.PubMed/NCBI
|
|
9
|
Kliewer SA, Lenhard JM, Willson TM, Patel
I, Morris DC and Lehmann JM: A prostaglandin J2
metabolite binds peroxisome proliferator-activated receptor g and
promotes adipocyte differentiation. Cell. 83:813–819.
1995.PubMed/NCBI
|
|
10
|
Wang JJ and Mak OT: Induction of apoptosis
in non-small cell lung carcinoma A549 cells by PGD2
metabolite, 15d-PGJ2. Cell Biol Int. 35:1089–1096. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin SW, Seo CY, Han H, et al:
15d-PGJ2 induces apoptosis by reactive oxygen
species-mediated inactivation of Akt in leukemia and colorectal
cancer cells and shows in vivo antitumor activity. Clin Cancer Res.
15:5414–5425. 2009.PubMed/NCBI
|
|
12
|
Mansure JJ, Nassim R and Kassouf W:
Peroxisome proliferator-activated receptor gamma in bladder cancer:
a promising therapeutic target. Cancer Biol Ther. 8:6–15. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beissbarth T and Speed TP: GOstat: find
statistically overrepresented Gene Ontologies within a group of
genes. Bioinformatics. 20:1464–1465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rimon G, Bazenet CE, Philpott KL and Rubin
LL: Increased surface phosphatidylserine is an early marker of
neuronal apoptosis. J Neurosci Res. 48:563–570. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Kramer DL, Diegelman P, Vujcic S
and Porter CW: Apoptotic signaling in polyamine analogue-treated
SK-MEL-28 human melanoma cells. Cancer Res. 61:6437–6444.
2001.PubMed/NCBI
|
|
16
|
Zakharov V, Lin HK, Azzarello J, et al:
Suppressed expression of type 2 3alpha/type 5 17beta-hydroxysteroid
dehydrogenase (AKR1C3) in endometrial hyperplasia and carcinoma.
Int J Clin Exp Pathol. 3:608–617. 2010.PubMed/NCBI
|
|
17
|
Gray TA, MacLaine NJ, Michie CO, et al:
Anterior Gradient-3: a novel biomarker for ovarian cancer that
mediates cisplatin resistance in xenograft models. J Immunol
Methods. 378:20–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eyler CE, Wu Q, Yan K, et al: Glioma stem
cell proliferation and tumor growth are promoted by nitric oxide
synthase-2. Cell. 146:53–66. 2011. View Article : Google Scholar : PubMed/NCBI
|