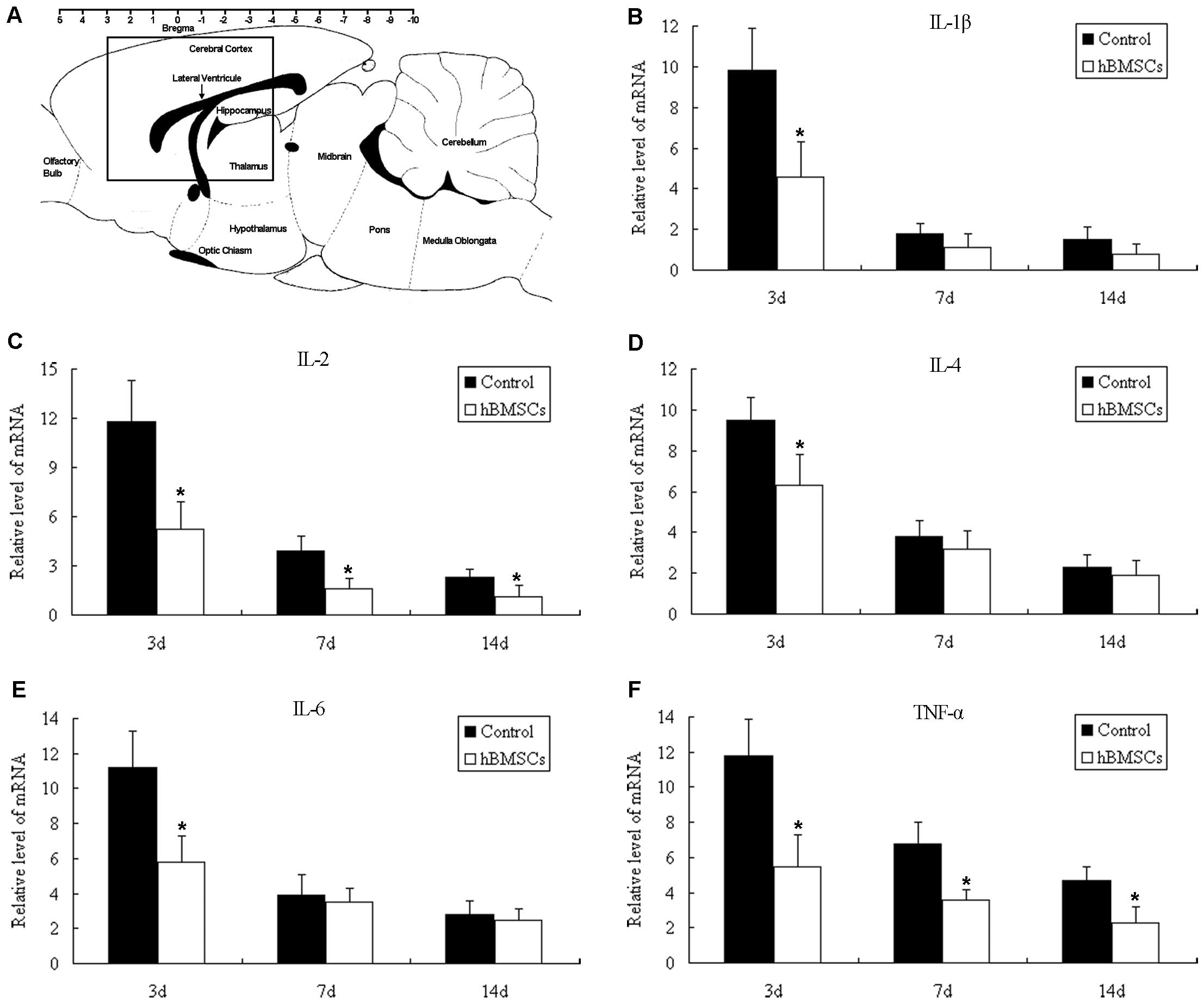
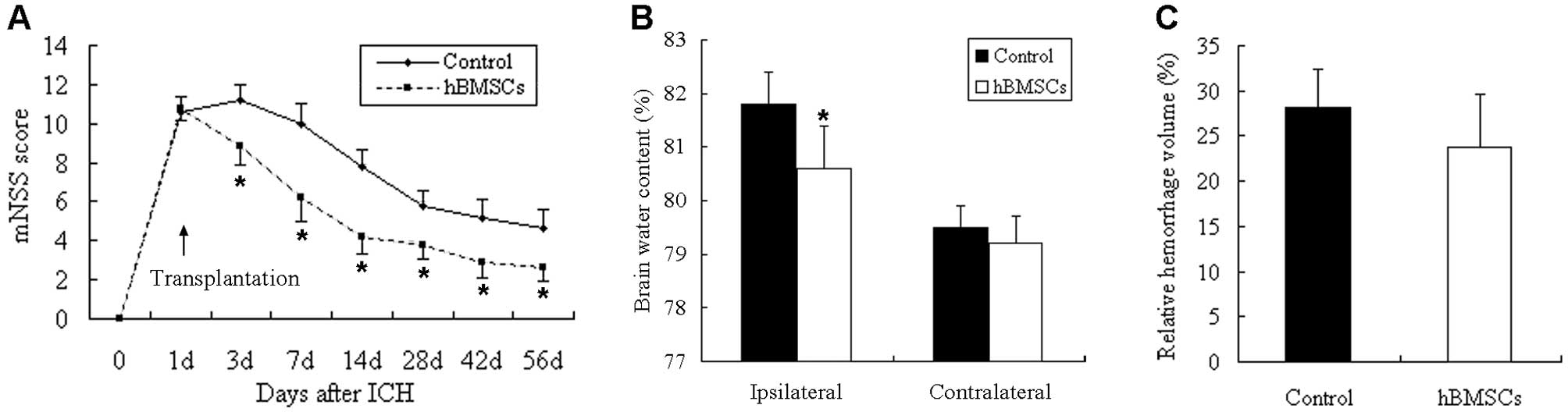
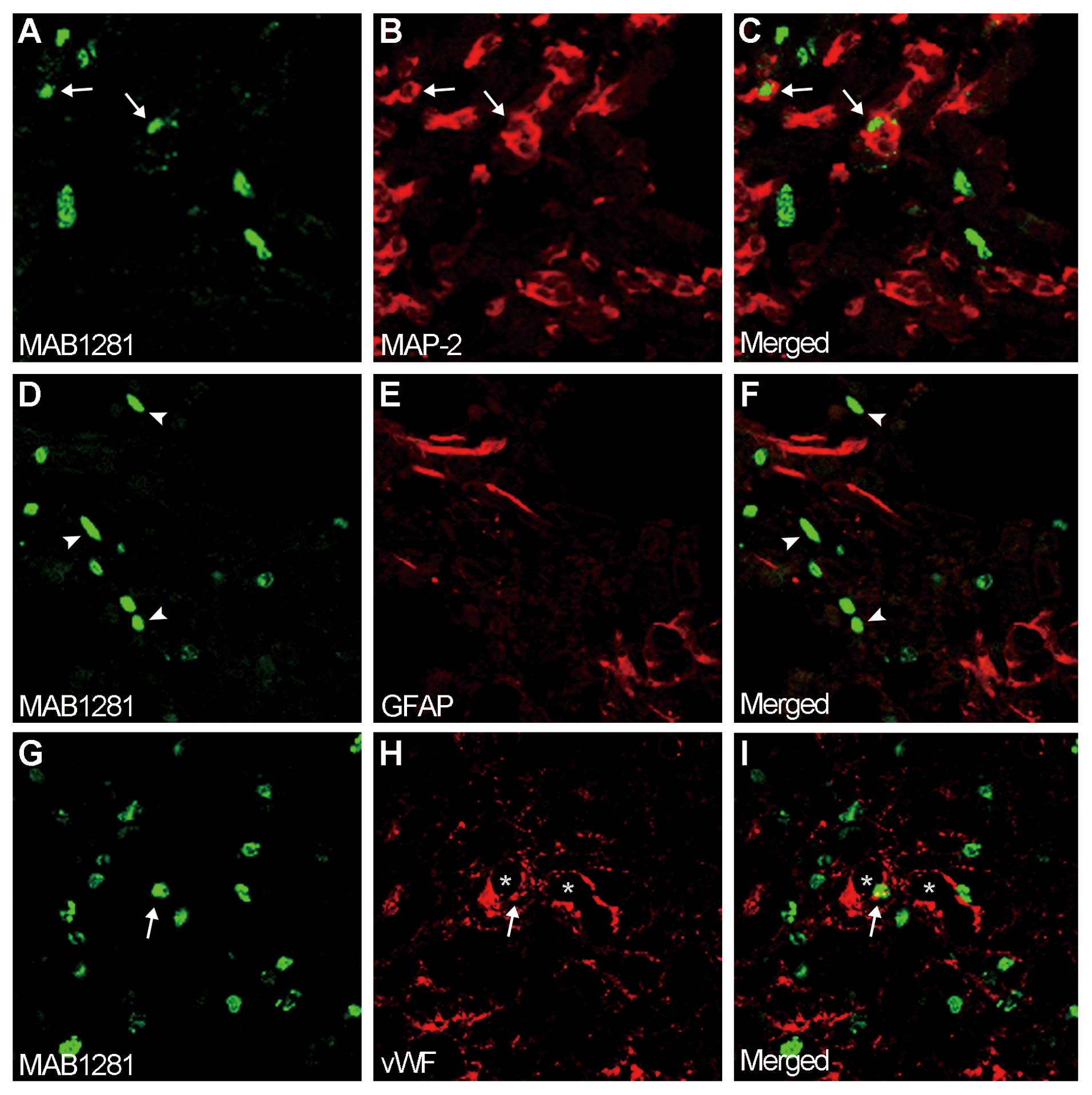
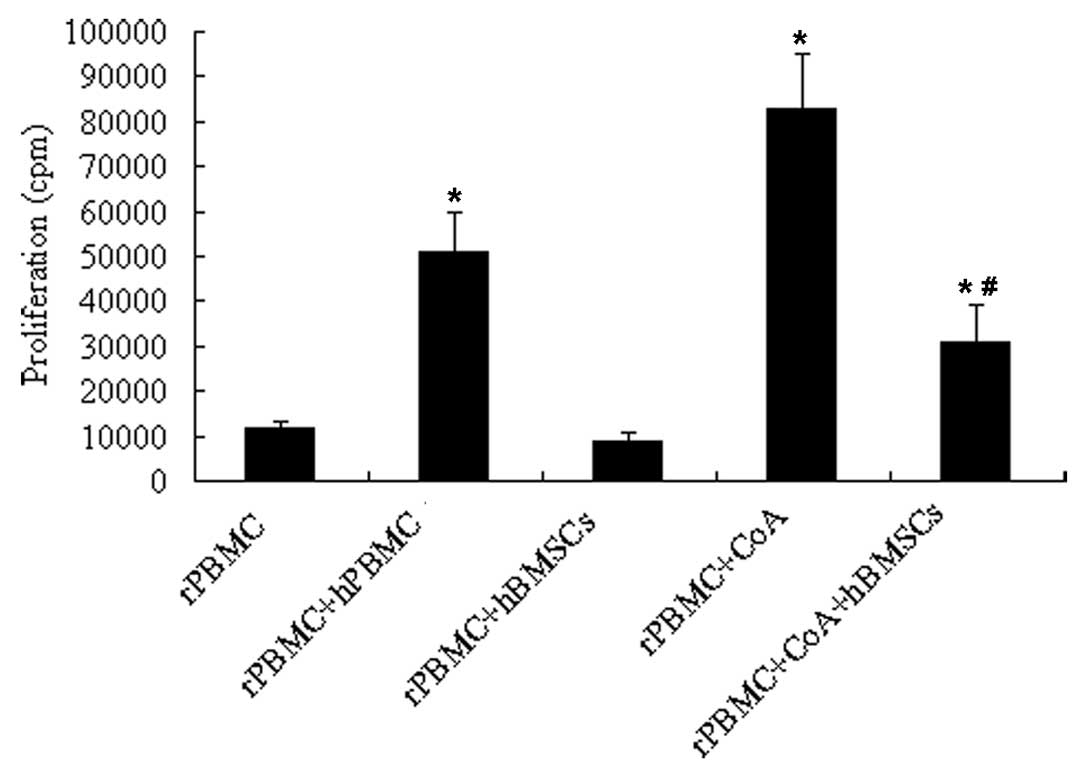
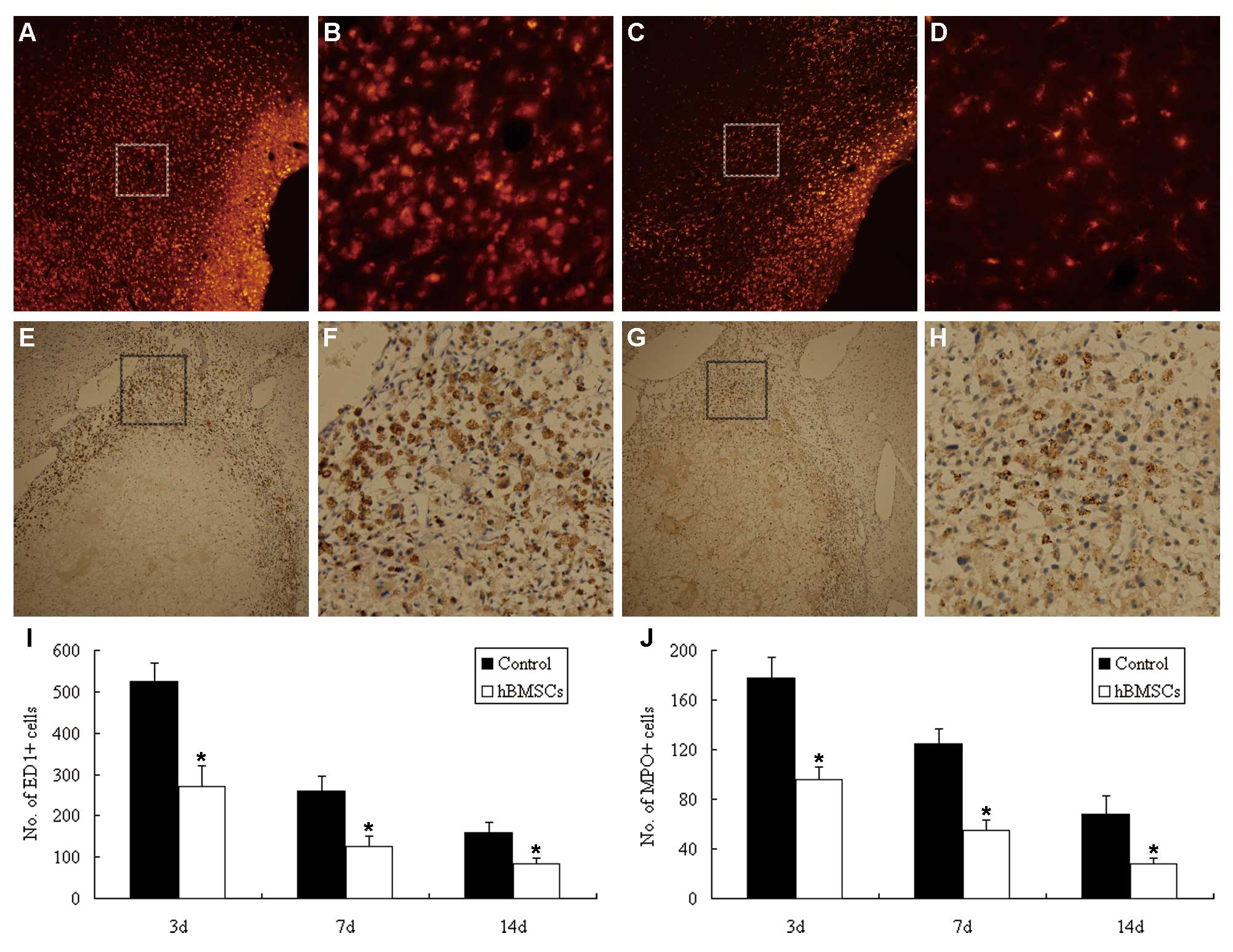
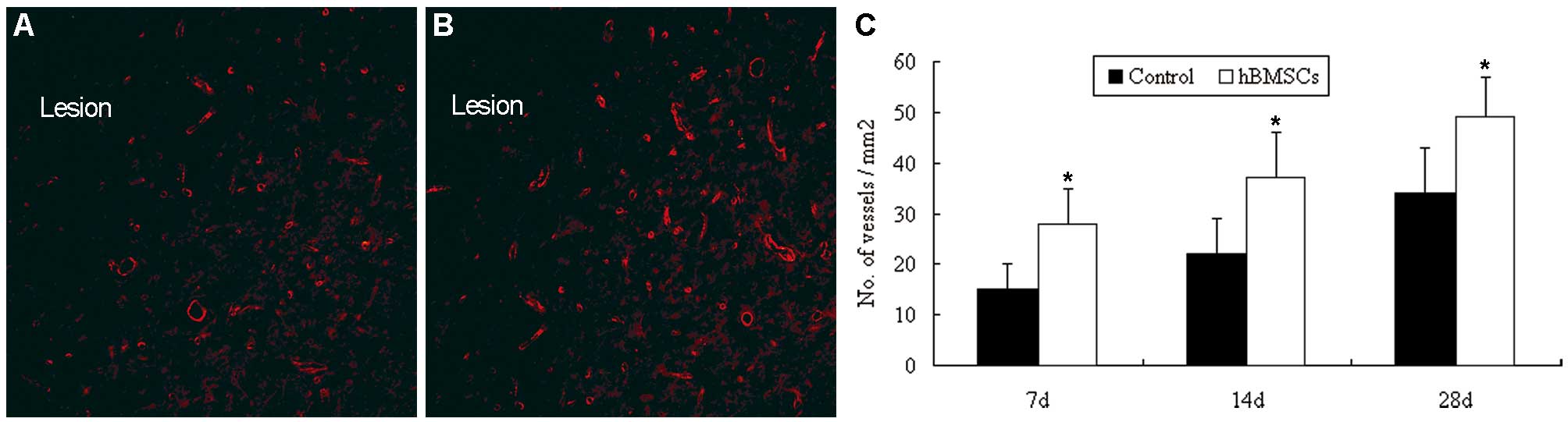
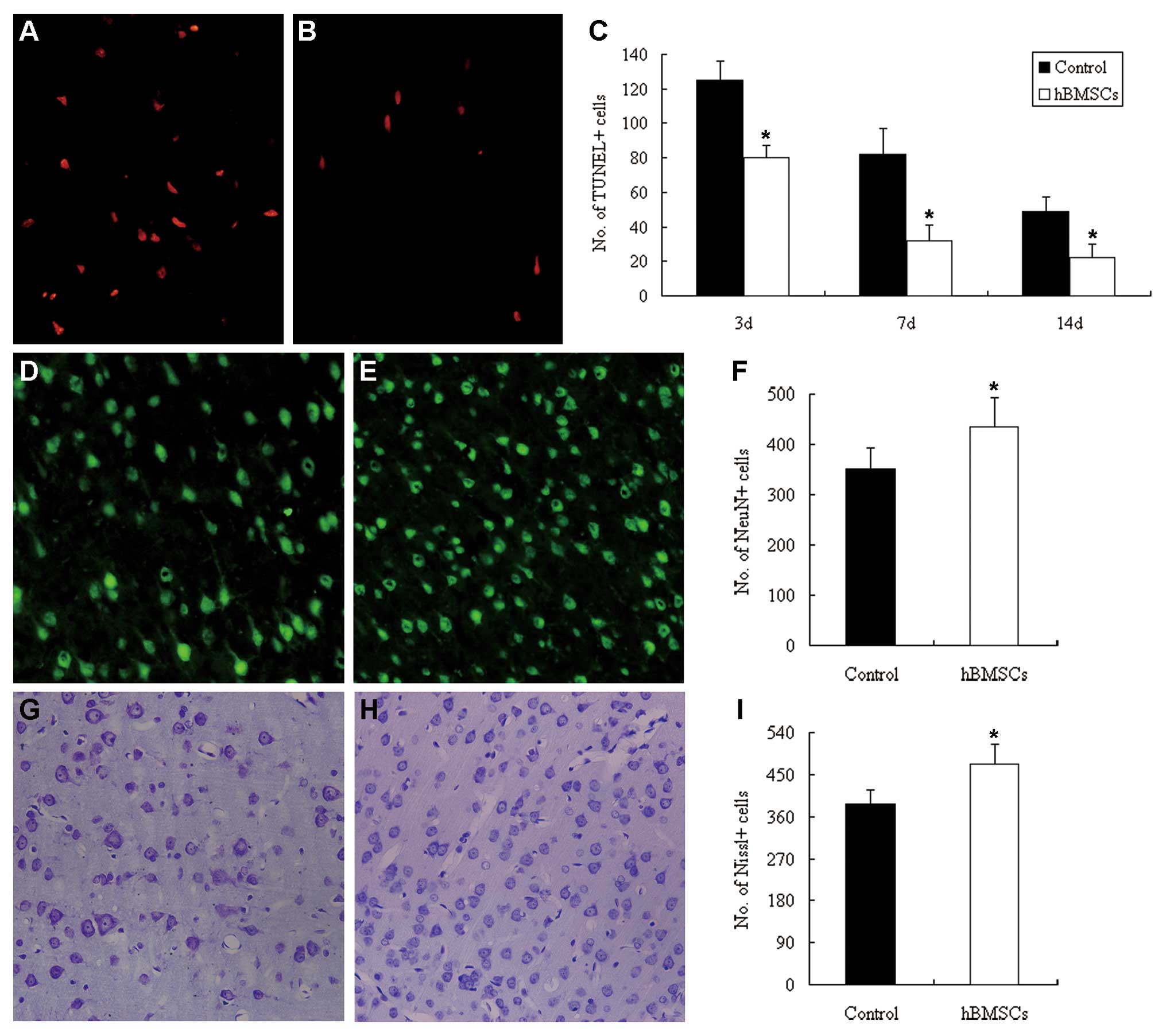
|
1
|
Heiskanen O: Treatment of spontaneous
intracerebral and intracerebellar hemorrhages. Stroke. 24:I94–I95.
1993.PubMed/NCBI
|
|
2
|
Qureshi AI, Tuhrim S, Broderick JP, Batjer
HH, Hondo H and Hanley DF: Spontaneous intracerebral hemorrhage. N
Engl J Med. 344:1450–1460. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lyden PD and Zivin JA: Hemorrhagic
transformation after cerebral ischemia: mechanisms and incidence.
Cerebrovasc Brain Metab Rev. 5:1–16. 1993.PubMed/NCBI
|
|
4
|
Hacke W, Kaste M, Bluhmki E, et al:
Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic
stroke. N Engl J Med. 359:1317–1329. 2008. View Article : Google Scholar
|
|
5
|
Aronowski J and Hall CE: New horizons for
primary intracerebral hemorrhage treatment: experience from
preclinical studies. Neurol Res. 27:268–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qureshi AI, Mendelow AD and Hanley DF:
Intracerebral haemorrhage. Lancet. 373:1632–1644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J and Doré S: Inflammation after
intracerebral hemorrhage. J Cereb Blood Flow Metab. 27:894–908.
2007.
|
|
8
|
Kim J, Lee S, Kon C, et al: Systemic
transplantation of human adipose stem cells attenuated cerebral
inflammation and degeneration in a hemorrhagic stroke model. Brain
Res. 1183:43–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee S, Chu K, Jung K, et al:
Anti-inflammatory mechanism of intravascular neural stem cell
transplantation in haemorrhagic stroke. Brain. 131:616–629. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seyfried D, Ding J, Han Y, Li Y, Chen J
and Chopp M: Effects of intravenous administration of human bone
marrow stromal cells after intracerebral hemorrhage in rats. J
Neurosurg. 104:313–318. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Huang Z, Xu Y and Zhang S:
Differentiation and neurological benefit of the mesenchymal stem
cells transplanted into the rat brain following intracerebral
hemorrhage. Neurol Res. 28:104–112. 2006. View Article : Google Scholar
|
|
12
|
Feng M, Zhu H, Zhu Z, et al: Serial
18F-FDG PET demonstrates benefit of human mesenchymal
stem cells in treatment of intracerebral hematoma: a translational
study in a primate model. J Nucl Med. 52:90–97. 2011.
|
|
13
|
Deng W, Han Q, Liao L, et al: Allogeneic
bone marrow-derived Flk-1+Sca-1− mesenchymal
stem cells leads to stable mixed chimerism and donor-specific
tolerance. Exp Hematol. 32:861–867. 2004.PubMed/NCBI
|
|
14
|
Xu G, Zhang L, Ren G, Yuan Z, Zhang Y,
Zhao RC and Shi Y: Immunosuppressive properties of cloned bone
marrow mesenchymal stem cells. Cell Res. 17:240–248.
2007.PubMed/NCBI
|
|
15
|
Zhang B, Liu R, Shi D, et al: Mesenchymal
stem cells induce mature dendritic cells into a novel
Jagged-2-dependent regulatory dendritic cell population. Blood.
113:46–57. 2009. View Article : Google Scholar
|
|
16
|
Tan J, Wu W, Xu X, et al: Induction
therapy with autologous mesenchymal stem cells in living-related
kidney transplants: a randomized controlled trial. JAMA.
307:1169–1177. 2012. View Article : Google Scholar
|
|
17
|
Saito S, Nakayama T, Hashimoto N, et al:
Mesenchymal stem cells stably transduced with a dominant-negative
inhibitor of CCL2 greatly attenuate bleomycin-induced
lung damage. Am J Pathol. 179:1088–1094. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams AR and Hare JM: Mesenchymal stem
cells: biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar
|
|
19
|
Morando S, Vigo T, Esposito M, et al: The
therapeutic effect of mesenchymal stem cell transplantation in
experimental autoimmune encephalomyelitis is mediated by peripheral
and central mechanisms. Stem Cell Res Ther. 3:32012. View Article : Google Scholar
|
|
20
|
Fang B, Liao L, Shi M, Yang S and Zhao RC:
Multipotency of Flk1+CD34− progenitors
derived from human fetal bone marrow. J Lab Clin Med. 143:230–240.
2004.PubMed/NCBI
|
|
21
|
Fang B, Shi M, Liao L, Yang S, Liu Y and
Zhao RC: Multiorgan engraftment and multilineage differentiation by
human fetal bone marrow
Flk1+/CD31−/CD34− progenitors. J
Hematother Stem Cell Res. 12:603–613. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Sun Z, Chen B, et al: Ex vivo
expansion and in vivo infusion of bone marrow-derived
Flk-1+CD31−CD34− mesenchymal stem
cells: feasibility and safety from monkey to human. Stem Cells Dev.
15:349–357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou H, Guo M, Bian CJ, et al: Efficacy of
bone marrow-derived mesenchymal stem cells in the treatment of
sclerodermatous chronic graft-versus-host disease: clinical report.
Biol Blood Marrow Transplant. 16:403–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao X, Feng M, Wei J, et al:
Transplantation of Flk-1+ human bone marrow-derived
mesenchymal stem cells promotes angiogenesis and neurogenesis after
cerebral ischemia in rats. Eur J Neurosci. 34:87–98. 2011.
|
|
25
|
Gong C, Hoff JT and Keep RF: Acute
inflammatory reaction following experimental intracerebral
hemorrhage in rat. Brain Res. 871:57–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nauta AJ and Fibbe WE: Immunomodulatory
properties of mesenchymal stromal cells. Blood. 110:3499–3506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Zhu H, Liu Y, et al: Human
mesenchymal stem cell transplantation protects against cerebral
ischemic injury and upregulates interleukin-10 expression in
Macaca fascicularis. Brain Res. 1334:65–72. 2010. View Article : Google Scholar
|
|
28
|
Bao X, Wei J, Feng M, et al:
Transplantation of human bone marrow-derived mesenchymal stem cells
promotes behavioral recovery and endogenous neurogenesis after
cerebral ischemia in rats. Brain Res. 1367:103–113. 2011.
View Article : Google Scholar
|
|
29
|
Li Y, Chen J, Chen XG, et al: Human marrow
stromal cell therapy for stroke in rat: neurotrophins and
functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terada N, Hamazaki T, Oka M, et al: Bone
marrow cells adopt the phenotype of other cells by spontaneous cell
fusion. Nature. 416:542–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castro RF, Jackson KA, Goodell MA,
Robertson CS, Liu H and Shine HD: Failure of bone marrow cells to
transdifferentiate into neural cells in vivo. Science.
297:12992002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen J, Zhang ZG, Li Y, et al: Intravenous
administration of human bone marrow stromal cells induces
angiogenesis in the ischemic boundary zone after stroke in rats.
Circ Res. 92:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shen LH, Li Y, Chen J, et al: One-year
follow-up after bone marrow stromal cell treatment in middle-aged
female rats with stroke. Stroke. 38:2150–2156. 2007.PubMed/NCBI
|
|
34
|
Schott RJ and Morrow LA: Growth factors
and angiogenesis. Cardiovasc Res. 27:1155–1161. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zacharek A, Chen J, Cui X, et al:
Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies
angiogenesis and vascular stabilization after stroke. J Cereb Blood
Flow Metab. 27:1684–1691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stem Cell Therapies as an Emerging
Paradigm in Stroke Participants. The STEPS Participants: Stem cell
therapies as an emerging paradigm in stroke (STEPS): bridging basic
and clinical science for cellular and neurogenic factor therapy in
treating stroke. Stroke. 40:510–515. 2009. View Article : Google Scholar
|
|
37
|
Han Q, Sun Z, Liu L, Chen B, Cao Y, Li K
and Zhao RC: Impairment in immuno-modulatory function of
Flk1+ CD31−CD34− MSCs from MDA-RA
patients. Leuk Res. 31:1469–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Parent JM, Valentin VV and Lowenstein DH:
Prolonged seizures increase proliferating neuroblasts in the adult
rat subventricular zone-olfactory bulb pathway. J Neurosci.
22:3174–3188. 2002.PubMed/NCBI
|
|
39
|
Henriksson HB, Svanvik T, Jonsson M,
Hagman M, Horn M, Lindahl A and Brisby H: Transplantation of human
mesenchymal stems cells into intervertebral discs in a xenogeneic
porcine model. Spine. 34:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Swanson RA, Morton MT, Tsao-Wu G, Savalos
RA, Davidson C and Sharp FR: A semiautomated method for measuring
brain infarct volume. J Cereb Blood Flow Metab. 10:290–293.
1990.PubMed/NCBI
|