Introduction
Antibiotic resistance has become a serious and
increasing threat to human health. For example, it is estremely
difficult to treat infected wounds caused by antibiotic-resistant
pathogens using common antibiotics (1). There is an urgent need for the
development of new antibacterial agents. Natural product
anti-infectious agents are attractive options.
Maggot (fly larva) therapy with the larvae of
Lucilia sericata is an effective and simple method for
cleaning infected and necrotic wounds (2–8).
Its use dates back to the beginning of civilization, and it became
popular and prevalent for the treatment of chronic or infected
wounds worldwide during the 1930s (2). With the introduction and production
of antibiotics in the 1940s, however, the academic and clinical
interest in this biosurgery was unfortunately lost. In the 1990s
the rising incidence of antibiotic resistance resulted in the
renaissance of maggot therapy. Despite the repeated falling out of
favor and the persistent public disdain which hamper its
acceptance, the practice of maggot therapy is on the rise,
attributed to its efficacy, safety and simplicity. Our group has
performed successful maggot bio-debridement on diabetic foot
ulcers, pressure ulcers after spinal cord injury and infected
wounds after forearm replantation (7,8).
Sherman (3) and Mumcuoglu
(4) have confirmed that maggot
therapy benefits patients through rapid wound debridement and
elimination of infection. Recently, there is an increasing interest
in investigating antimicrobial protein from maggots (MAMP) that
belongs to the family of antimicrobial peptides (AMPs). Several
reports have described that the maggot contains a diversity of
bioactive proteins with antimicrobial function, either in the whole
body or in the excretion/secretion (9–11).
AMPs have broad-spectrum activity against a wide
range of micro-organisms including viruses, Gram-positive and
Gram-negative bacteria, protozoa, yeasts and fungi (12,13). However, the mechanisms of action
for its effects on infected wounds remain unclear.
Staphylococci are common commensal bacteria of the skin
(14) and are also an important
pathogen in foreign-pathogen infections (15). The Gram-positive Staphylococcus
(S.) aureus is a major human pathogen of many nosocomial
infections, including life-threatening diseases such as toxic shock
syndrome, endocarditis and chronic infected wounds (16). The emergence of
multidrug-resistant strains of S. aureus, such as
methicillin-resistant S. aureus (MRSA), has intensified the
need for the development of new treatment modalities.
In the present study, we purified antimicrobial
proteins from the body of maggots with ultrafiltration, and then
investigated their in vitro and in vivo biological
activities and the mechanisms of action on infected wounds caused
by S. aureus. The results provide a basis for the further
development of AMP-based therapy.
Materials and methods
Ethical considerations concerning the
human subjects and animal use and care
The present study involving human participants was
reviewed and approved by the Clinical Research Ethics Committee of
Dalian Medical University, Dalian, China. Written consent was
obtained from each of the participants who provided blood samples
voluntarily for the study. All animal experiments were in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals, and the protocols were reviewed and approved by the
Committee on Research and Animal Care of Dalian Medical
University.
Bacterial strains
S. aureus (ATCC 25923, 29213 and AB94004) was
purchased from the China Center of Type Culture Collection.
Clinical isolates were obtained from the Department of Clinical
Laboratory, The First Affiliated Hospital of Dalian Medical
University, including penicillin-resistant S. aureus
12SAU130, penicillin-resistant S. aureus 12SAU133,
methicillin-resistant S. aureus 12SAU124 and
methicillin-resistant S. aureus 12SAU145.
Crude protein extract from maggots
Eggs of L. sericata were collected from the
eyes of Scomberomorus niphonius and were disinfected in 1%
sodium sulfite solution for 3 min, followed by 3% Lysol
disinfectant for 5 min. The disinfected eggs were transferred to
sterile vials for cloning. The third stage L. sericata
larvae were placed in 3.5% methanol/normal saline solution for 5
min, 2% hydrogen peroxide solution for 3 min, and 5% dilute
hydrochloric acid solution for 5 min. Following a two-step
disinfection, larvae were homogenized by a mortar in precooling TAE
buffer solution (1:3 v/v). The homogenate was then centrifuged
thrice at 12,000 × g at 4°C, for 30 min. The supernatant was
collected and used as a crude protein extract.
Purification of the crude protein
extract
The crude extract was primarily passed through a
30-kDa cut-off ultrafiltration membrane on a Labscale™ TFF System
(Millipore, USA). The filtrate was then ultrafiltrated by a 10-kDa
cut-off ultrafiltration membrane, and the filtrate (<10 kDa) and
the cut-off fluid (10–30 kDa) were obtained for further
testing.
Determination of protein
concentration
The protein concentration was determined by the
Bradford method (17) and bovine
serum albumin (BSA) was used as the standard. The standard curve
was established as follows: y = 0.0082× − 0.0035
(R2=0.9995) [where the x-axis represents the protein
concentration and the y-axis represents the optical density (OD)].
The final concentration of protein in subsequent assays was 50
μg/ml.
Antimicrobial assays in vitro
Identification of the antimicrobial
peptide from maggots (MAMP) using a turbidometric (TB) assay
The TB assay was modified from that of Thomas et
al (18). S. aureus
(ATCC 25923) was grown, washed in PBS and adjusted to a
concentration of 105 CFUs in fresh tryptic soy broth
(TSB). The crude protein extracts, cut-off fluid or filtrate (200
μl) were mixed, respectively, with 22 μl of 10% peptone water and
the pH was measured. One hundred and fifty milliliters of the
samples was incubated with 30 μl of the bacterial suspension in
triplicate in a 96-well microtiter plate (Nunc; Fisher Scientific
UK, Leicestershire, UK). Samples were incubated at 37°C for 24 h,
and the optical density at 589 nm (OD589) was measured every 3 h.
Controls consisted of 1% peptone water and were adjusted with 1 M
NaOH to equalize the pH of the samples. All data points were
subsequently blanked against time zero to account for the opacity
of the samples. The sample subjected to the lowest OD value was
identified as the antimicrobial peptide from the maggots (MAMP),
and was used in the subsequent experiments.
Minimum inhibitory concentration (MIC)
analysis
The MIC of MAMP was determined by a microdilution
assay in 96-well microtiter plates according to the broth
microdilution guideline of the Clinical and Laboratory Standards
Institute (CLSI) (19).
Overnight-cultured S. aureus (ATCC 25923) was diluted with
Luria-Bertani (LB) medium to 104–106 CFU/ml.
The bacterial suspension and the serially diluted MAMP were added
to 96-well plates at a ratio of 4:1 in a final volume of 100 μl.
The microplates were incubated at 37°C with continuous shaking.
After 16 h, the optical density at 630 nm (OD630) was measured with
a microplate reader. Vancomycin and penicillin were used as
controls. The MIC values were determined at the zero optical
density concentration. All experiments were repeated at least three
times.
Antimicrobial assays in vivo
Experimental animals
Female BALB/c mice weighing 18–20 g were provided by
the Animal Experimental Center of Dalian Medical University and
housed one per cage for one week before study in a room with
controlled environment (12 h/12 h light/dark cycle, 23±2°C and
relative humidity 70%). They were also given free access to
standard laboratory diet and water.
Gel preparation
MAMP was prepared in gel form in 0.5%
hydroxypropylcellulose to a final concentration of 5 mg/ml.
Mouse skin abrasion and infection
model
The activity of MAMP against S. aureus in
vivo was determined according to the method of Cao et al
(20) with minor modification.
Briefly, mice were injected with 150 mg/kg of cyclophosphamide 4
days before infection, and with 100 mg/kg of cyclophosphamide 1 day
before infection. After being anesthetized, the dorsal skin of the
mice was shaved, and abrasions were produced in a 1×1
cm2 area using a blade. These abrasion wounds damaged
only the stratum corneum and the upper layer of the epidermis, but
not the dermis. Five minutes later, the wounds were inoculated with
20 ml of S. aureus (ATCC 25923) (107 CFUs). One
group of mice was sacrificed 4 h after infection to control for the
infectious dose. Four hours after infection, the wounds of the
other mice were smeared with 20 ml of 2.5 mg/ml MAMP gel or a
placebo control gel (0.5% hydroxypropylcellulose). The treatment
lasted for 4 days, with two peptide gel applications being
performed daily (morning and evening at 8 h intervals). At the end
of the study, the mice were sacrificed, and the wound area of the
skin was immediately excised and homogenized. Suitable dilutions of
the homogenates were plated on LB plates to determine the number of
living bacteria (CFUs).
Histological examination
After the animals were euthanized, biopsy specimens
of the excised wound area of the skin were collected and
immediately fixed in phosphate-buffered formalin. The biopsy
specimens were then embedded in paraffin and stained with
hematoxylin and eosin for pathological analysis.
Determination of the antibacterial
mechanism
Membrane permeability
The assay was modified from that of Marri et
al (21). S. aureus
was grown, washed in PBS and adjusted to a concentration of
1×108 CFUs in fresh TSB. Ten microliters of 1× MIC MAMP,
10 μl of ortho-nitrophenylgalactoside (ONPG) and 30 μl of the
bacterial suspension were mixed in triplicate in the wells of a
96-well microtiter plate. Triton X-100 was used as the positive
control. Samples were incubated at 37°C for 3 h, and the optical
density at 405 nm (OD405) was measured every 30 min.
Observation of the effect of MAMP on
S. aureus using scanning electron microscopy (SEM) and transmission
electron microscopy (TEM)
S. aureus was grown overnight to
mid-logarithmic phase and diluted to a final density of
1×108 CFUs. The culture was centrifuged at 8,000 × g for
10 min; the supernatant was removed and 1 ml of 1× MIC MAMP was
added to the bacteria, resuspended and cultured at 37°C for 6 or 12
h. After centrifuged at 8,000 × g for 10 min for the second time,
the supernatant was removed and the bacteria precipitate was fixed
in cold (4°C) 2.5% (v/v) glutaraldehyde in PBS (0.15 M, pH 7.2) for
24 h, and washed 3 times in PBS at 4°C. The bacteria were then
dehydrated through an ascending series of acetone solutions,
critical point dried, placed on aluminium stubs, sputter coated
with gold, and then viewed under a field emission scanning electron
microscope (JSM-6360LV, Japan). After dehydration, some bacteria
were embedded in Epon-Araldite resin mixture, and then cut with an
ultramicrotome. The sections were stained with uranyl/lead and
viewed under a transmission electron microscope (JEM-2000EX,
Japan).
Hemolytic activity
Fresh human blood treated with MAMP was centrifuged
at 1,000 × g for 10 min, and the sediment was washed three times in
HEPES (pH 7.2) by centrifugation at 1,000 × g for 10 min. The red
blood cells were counted and diluted to a concentration of
107–108 cells/ml and then incubated at room
temperature for 1 h with serially diluted MAMP at a ratio of 4:1 in
a final volume of 100 μl. Finally, the OD570 was measured with a
microplate reader. HC50 values were obtained according
to the method of Cao et al (20). Saline solution at a concentration
of 0.85% was used as the negative control, and 0.1% Triton X-100
was used as the positive control.
Statistical analysis
All experimental data are expressed as means ±
standard deviation, and the differences among various groups were
analyzed by one-way ANOVA and then Fisher’s least significant
difference t-test, using the SPSS version 13.0 software (SPSS Inc.,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
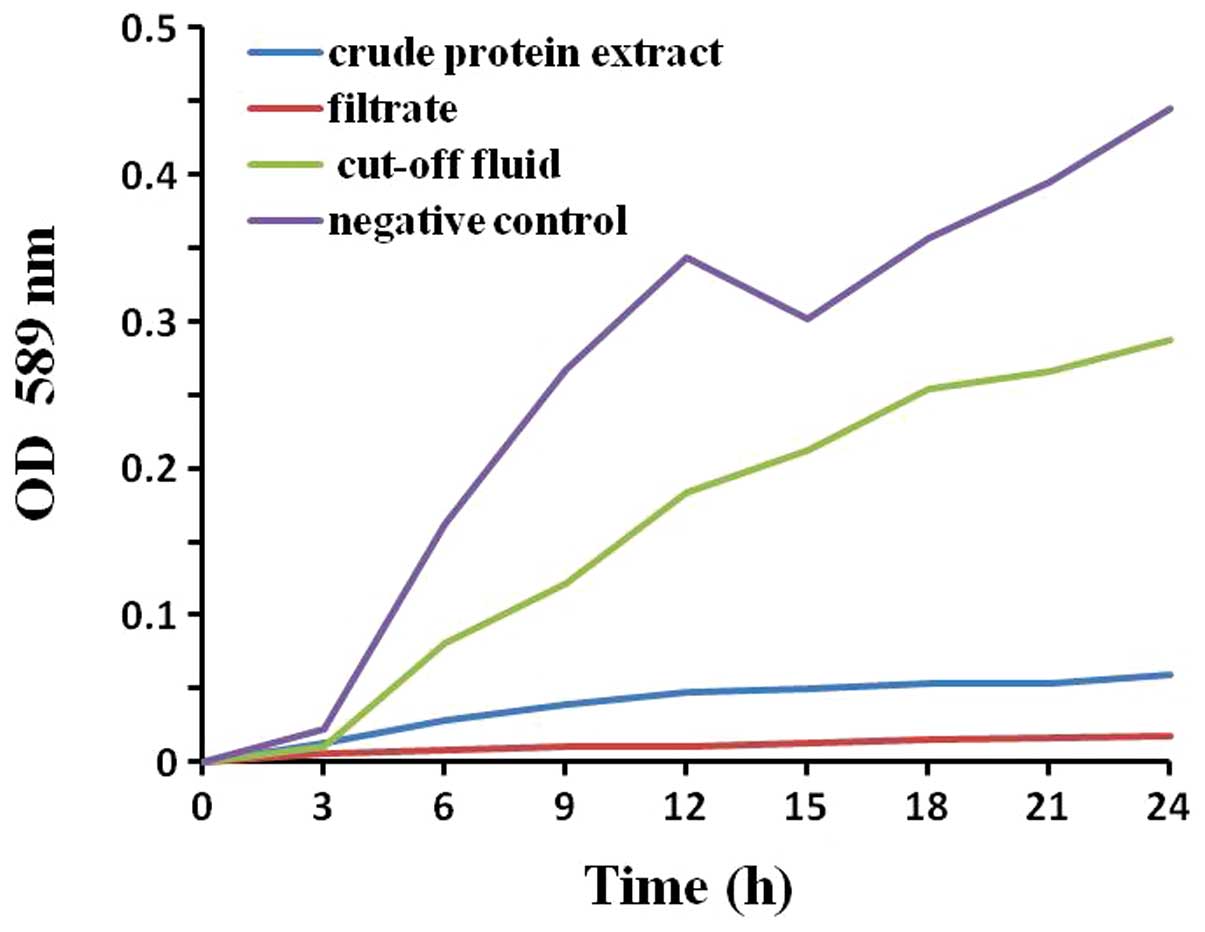
Antimicrobial activity in vitro
A comparison of the sensitivities of the S.
aureus strains to protein extracts from maggots was
investigated using TB assay. All the test crude protein extract,
cut-off fluid and filtrate demonstrated significant antibacterial
activity against S. aureus, compared with the controls. The
crude protein extract and filtrate were more effective than the
cut-off fluid, and the filtrate exhibited the strongest inhibitory
activity (Fig. 1). Therefore, the
filtrate was identified as MAMP and used in subsequent
experiments.
The MIC values for MAMP against S. aureus are
shown in Table I. MAMP showed an
MIC value of 25 μg/ml in the standard strains. The MIC values of
MAMP were 100 and 200 μg/ml against penicillin-resistance S.
aureus and MRSA, respectively.
 | Table IMIC values of MAMP against standard
stains and clinically isolated antibiotic-resistant strains of
S. aureus. |
Table I
MIC values of MAMP against standard
stains and clinically isolated antibiotic-resistant strains of
S. aureus.
| MIC (μg/ml) |
|---|
|
|
|---|
| Strains | MAMP | Vancomycin | Penicillin |
|---|
| Standard
strains |
| ATCC 25923 | 25 | 3.13 | 6.25 |
| ATCC 29213 | 25 | 3.13 | 12.5 |
| AB 94004 | 25 | 3.13 | 6.25 |
|
Penicillin-resistant strains |
| 12SAU130 | 100 | 3.13 | 10,000 |
| 12SAU133 | 100 | 6.25 | 10,000 |
|
Methicillin-resistant strains |
| 12SAU124 | 200 | 6.25 | 10,000 |
| 12SAU145 | 200 | 6.25 | 20,000 |
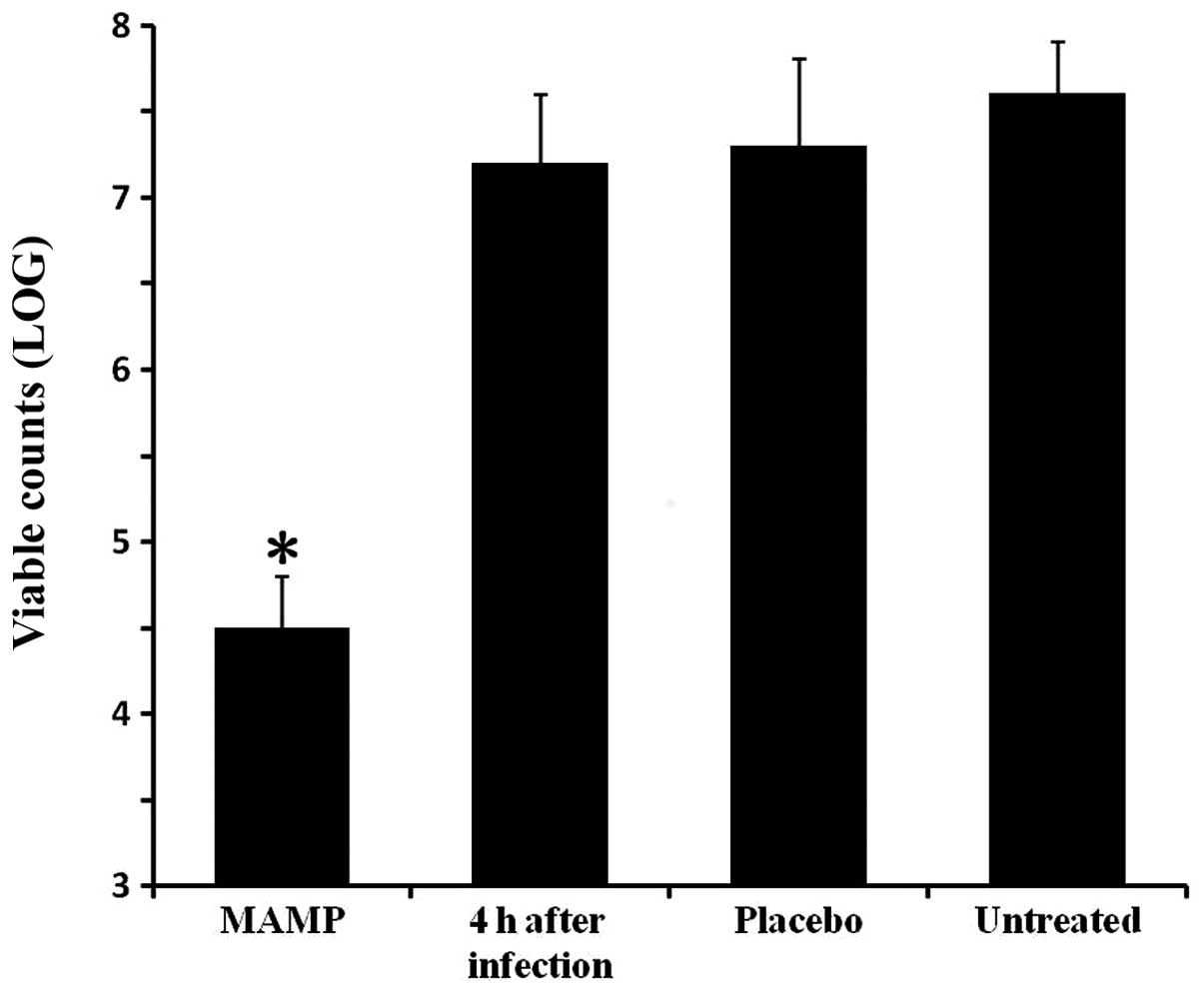
Antimicrobial activity in vivo
The antibacterial activity of MAMP in vivo
was determined by an S. aureus mouse skin infection model.
One group of mice was sacrificed 4 h after formation of the
infected wound, and the viable counts of bacteria on the skin were
measured as a control. The other groups were treated and sacrificed
4 days after infection. The bacterial colony counts in the mouse
wounds in the MAMP group were lower, when compared with these
values in the placebo-treated mice and the untreated mice
(P<0.05; Fig. 2).
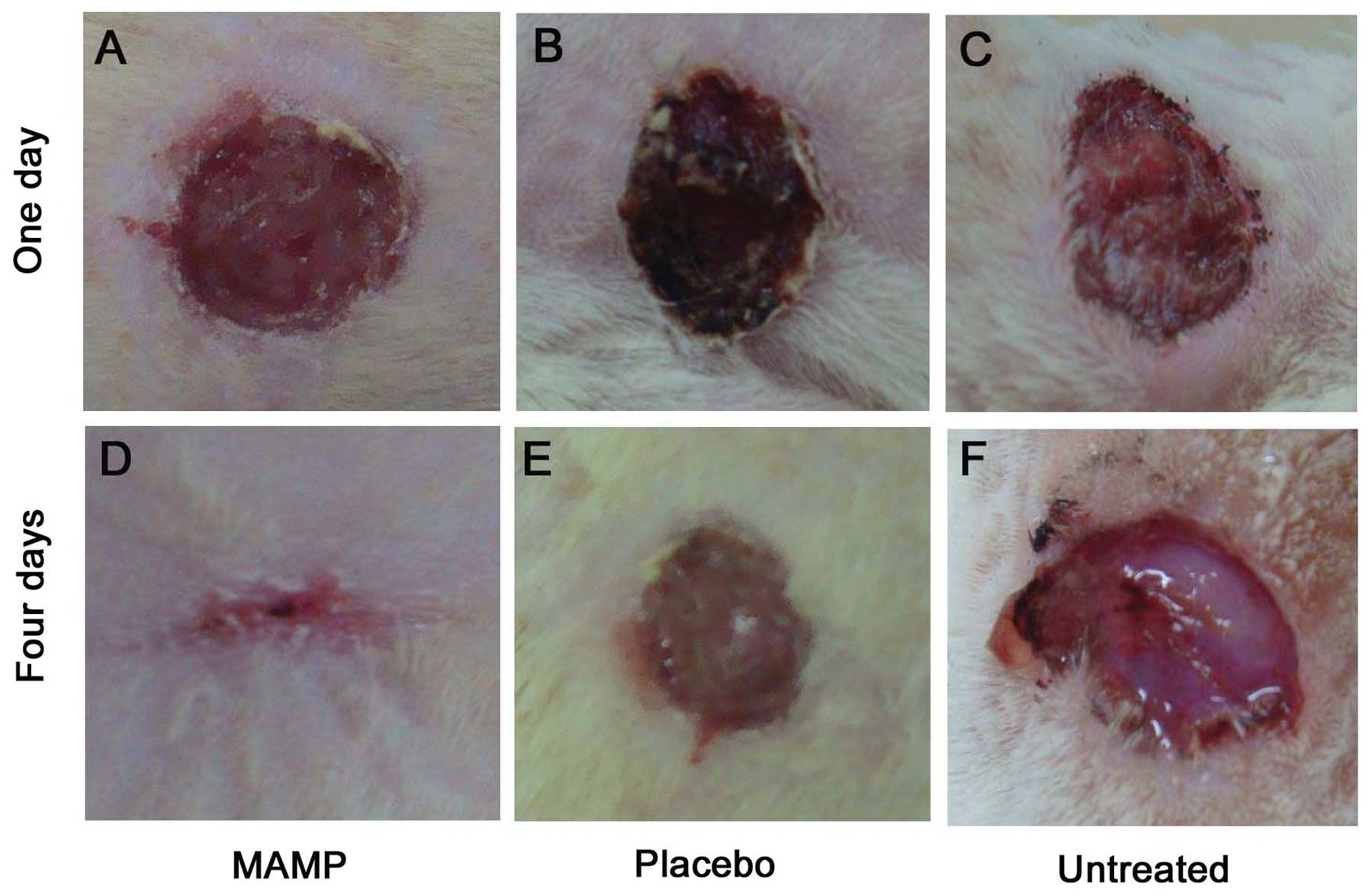
All of the mice were observed daily. On day 1 after
infection, the skin of the mice in the group treated with MAMP
showed slight signs of inflammation and was scabbed over (Fig. 3A), while the wounded skin sections
of the group treated with placebo were characterized by a small
amount of yellow exudates and presented with blistering (Fig. 3B). Additionally, blisters and
light tissue fluid leakage were observed in the wounded skin
sections of the untreated mice (Fig.
3C). Four days after infection, the skin in the MAMP group was
almost closed, accompanied by a well-defined healing ridge
(Fig. 3D). However, the blisters
in the group treated with placebo deteriorated and became larger
(Fig. 3E). Moreover, the skin
sections exhibited a large amount of tissue fluid leakage in the
untreated group (Fig. 3F).
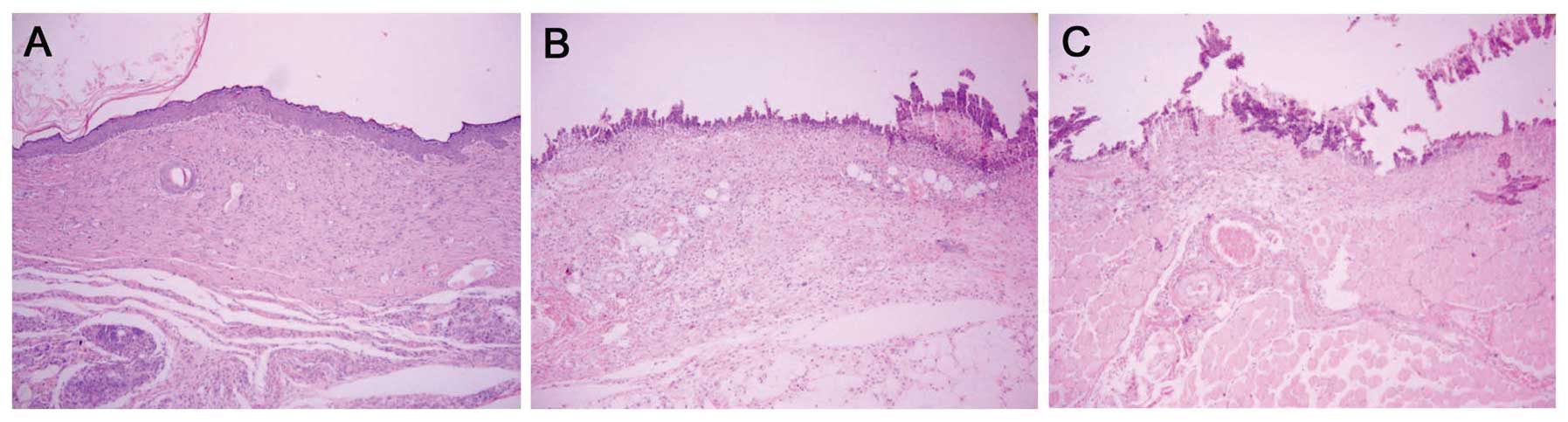
Histological observation demonstrated that on day 4
after infection, the wounds treated with MAMP had no sign of
infection and collagen fiber was mature and well arranged, with
presentation of intact structure. In addition, regenerative
folliculus and sebaceous glands were detected in the fibrous
connective tissues (Fig. 4A). In
contrast, the untreated group and the placebo group lost nearly all
of their epidermis, and the dermis was infected to a certain extent
(Fig. 4B and C).
Mechanisms of action
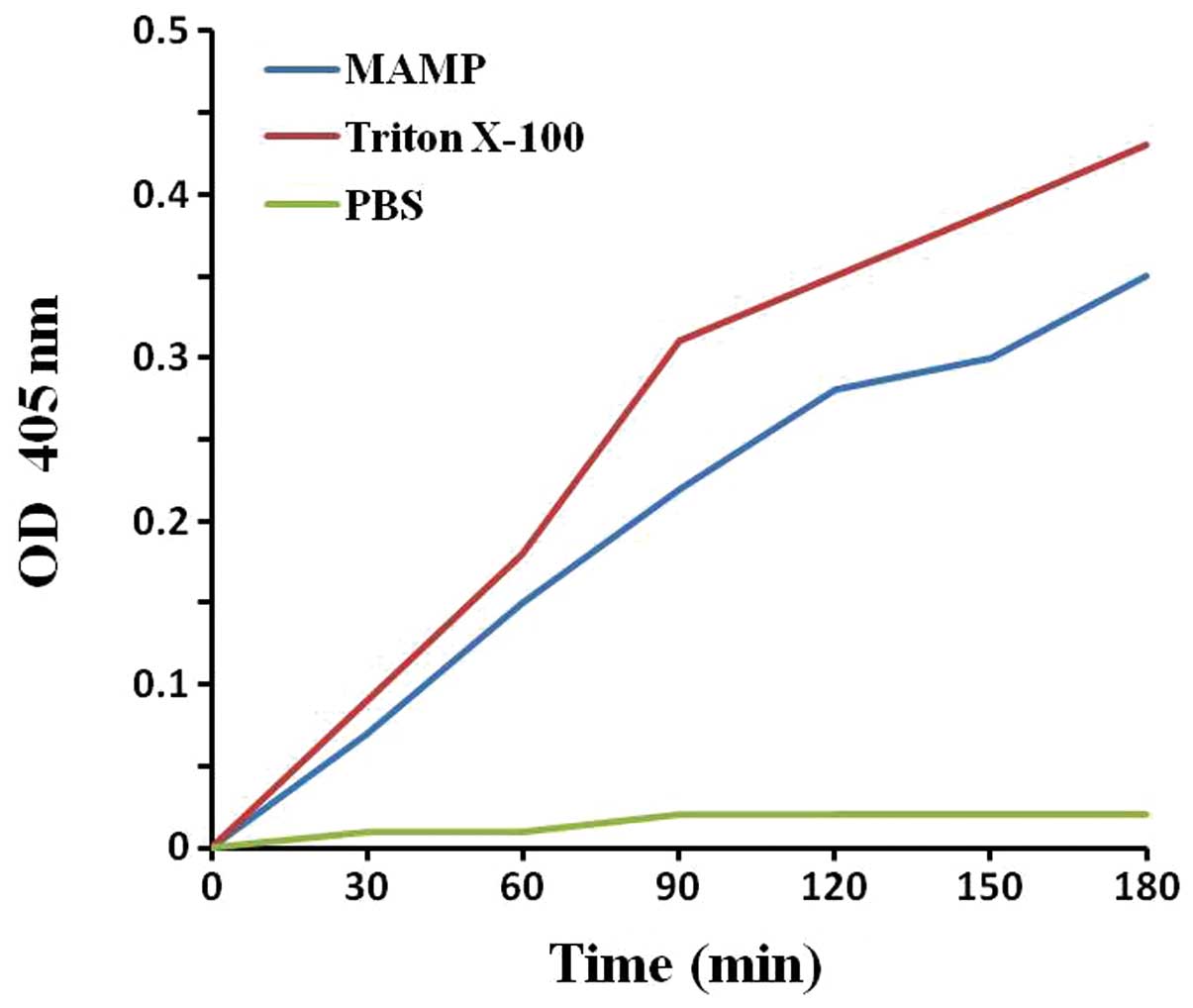
Membrane permeability
The membrane permeability of S. aureus was
determined by the optical density values. As shown in Fig. 5, after 30 min the OD values in the
MAMP group and Triton X-100 group were higher than that of the
negative control group, indicating that MAMP increased the membrane
permeability of S. aureus and exhibited a lytic activity
against S. aureus.
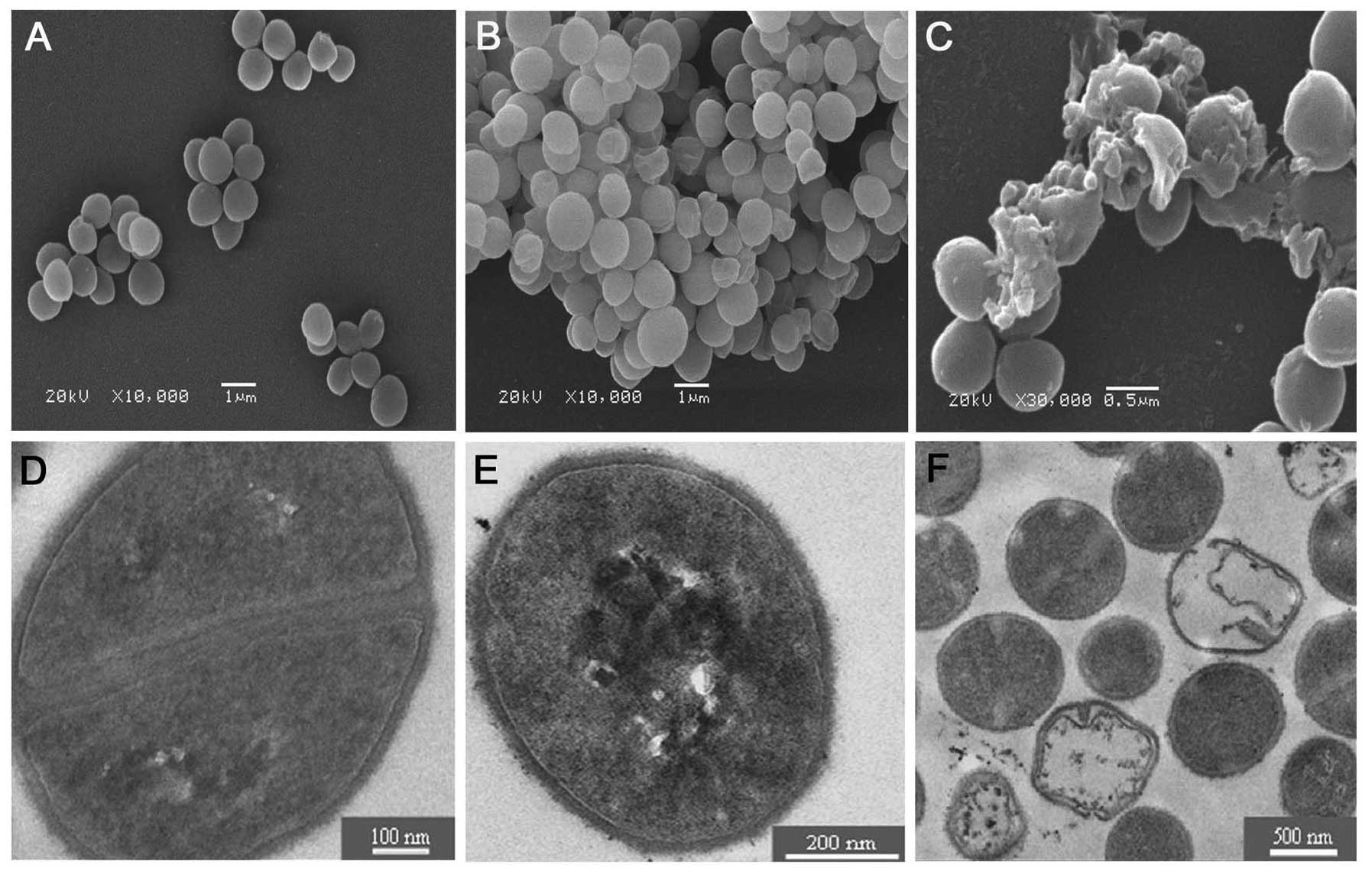
Scanning electron microscopy
SEM indicated that normal S. aureus was
spherical and mellow in a grape cluster-like arrangement. The
surface was smooth with a distinct boundary between the cells
(Fig. 6A). After treated with
MAMP for 6 h, S. aureus exhibited depressions, and cracks or
holes formed on their surface (Fig.
6B). After 12 h, dramatic morphologic changes were noted in
S. aureus. The cell membrane of S. aureus ruptured,
and the contents oozed out from the cells (Fig. 6C).
Transmission electron microscopy
TEM demonstrated that the wall and membrane of
normal S. aureus were intact (Fig. 6D). After treatment with MAMP for 6
h, S. aureus showed a slight depression (Fig. 6E). After 12 h, the membrane
integrity was largely damaged, resulting in the release of the cell
content upon cell wall disruption. The residual cell walls showed a
bacterial ‘ghost’ which was displayed as a cavity, indicating the
absence of cytoplasmic contents and chromatin silk shift. Edema
became widespread, with noticeable gaps between the cell membrane
and the cytoplasm (Fig. 6F).
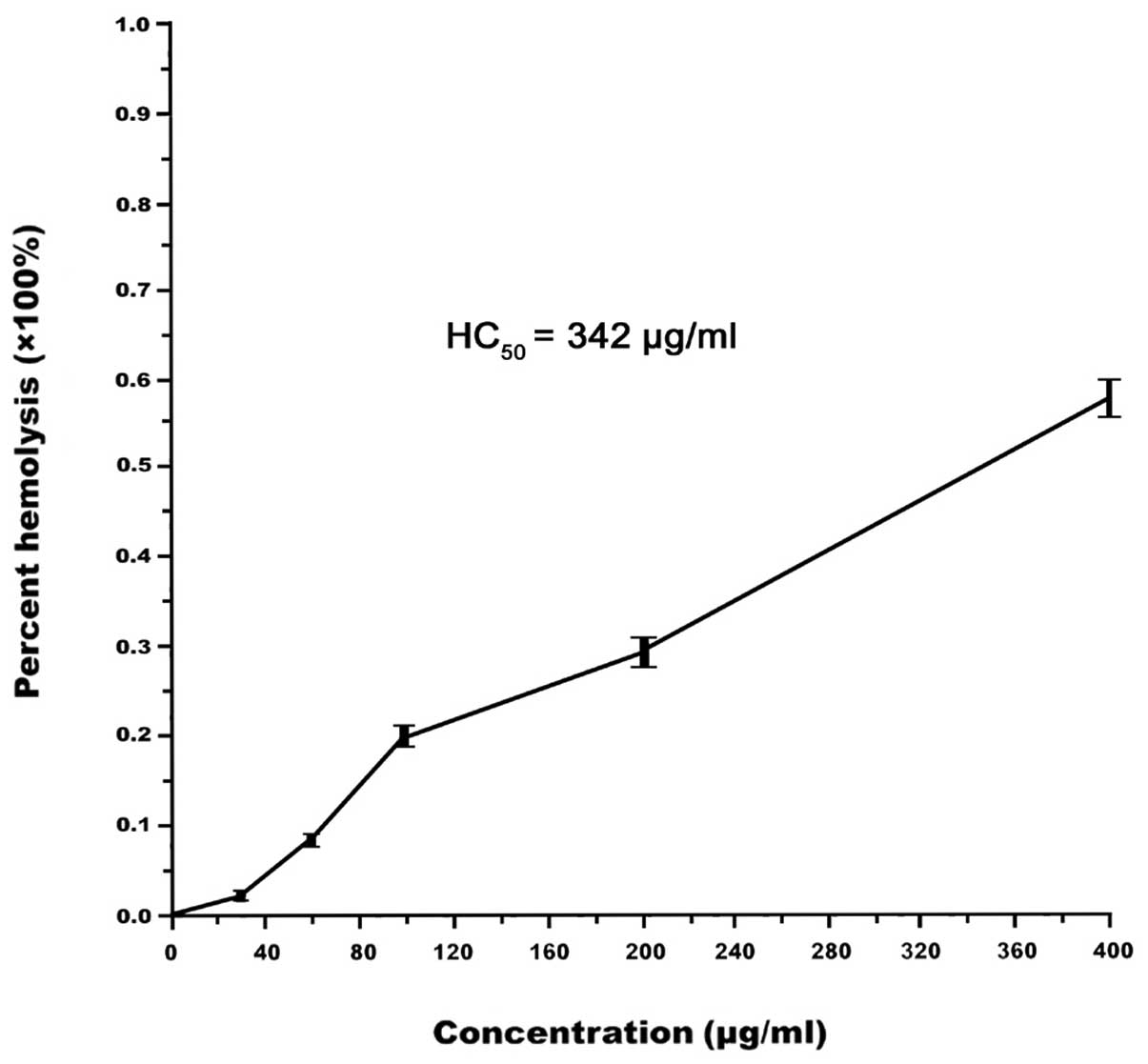
Hemolytic activity
The cytotoxicity of MAMP against mammalian cells was
detected by a hemolysis assay. The results demonstrated that MAMP
had weak hemolytic activity at a high concentration, and the
HC50 value of MAMP was 342 μg/ml. At 200 μg/ml, the
hemolytic activity of MAMP in human erythrocytes was lower than 30%
(Fig. 7). At this concentration,
MAMP effectively inhibited the growth of test bacteria, including
MRSA.
Discussion
Maggot therapy, also known as bio-debridement, is a
highly successful clinical approach to the treatment of infected
wounds. With the rising occurrence of antibiotic-resistant
pathogens, maggot therapy has been widely utilized in many
countries (22,23). However, little is known concerning
the antibacterial mechanisms of maggots, in particular no reports
on the mechanism of healing promotion and the effects on the
bacterial ultrastructure exist. In the present study, we isolated
and purified MAMP using ultrafiltration from the crude protein
extract. Based on the TB assay, the filtrate (<10 kDa) exhibited
the strongest inhibitory activity and therefore was identified as
MAMP. The MIC values also indicated that MAMP effectively inhibited
the growth of both standard S. aureus strains and MRSA. The
inhibition of standard strains and clinically isolated
antibiotic-resistant strains of S. aureus was in agreement
with previously published research, which determined the activity
of maggot secretions against bacterial liquid cultures (18). The results obtained in the present
study confirmed that the proteins from maggots effective against
S. aureus were of low molecule weight (<10 kDa).
Furthermore, the hemolytic activity of MAMP has always been an
obstacle to the application of AMPs in the clinic (24). In our study, the weak hemolytic
activity observed in the experimental group demonstrated that MAMP
had little influence on normal human cells.
Recently, clinical success of maggot therapy in
wound healing has been well documented (25,26). However, little is known about the
role of MAMP in infected tissue repair in vivo. In the
present study, MAMP prepared in gel (0.5% hydroxypropylcellulose)
was applied to treat an S. aureus mouse skin infection
model, and the effect of MAMP upon infected wounds of mice was
investigated. At a dosage of 200 times the MIC in vitro,
topical application of MAMP resulted in the cure of a wound on the
skin of mice infected with S. aureus. Significant lower
bacterial colony counts were found in the MAMP group, compared with
the controls. Based on morphological and histological evaluations,
MAMP may have dual functions in the healing process: antibacterial
activity and improvement in wound healing quality; the latter was
evidenced by well-arranged collagen fibers, mature fusiform
fibrocytes, and regenerative folliculus and sebiferous glands as
observed in the MAMP group. In addition, the MAMP-treated mice did
not show any signs of restlessness or scratching of the wound site,
suggesting that MAMP did not cause irritation or pain to the
animals. These results in vitro and in vivo imply
that MAMP prepared in gel has potential for clinical application to
treat wounds infected with S. aureus in a human setting.
Several antimicrobial peptides have been isolated
and identified from a variety of organisms (27–30). Their mode of action includes
disruption of membranes, interference with metabolism, and the
targeting of cytoplasmic components (31,32). Several biolayer interaction and
disruption models have been proposed for those AMPs that depend on
membrane interference for their antibacterial activity, such as
‘barrel-stave pore’, ‘carpet mechanism’, ‘toroidal pore’ and
‘disordered toroidal pore’ (33).
However, there are no reports concerning the influence of MAMP on
the bacterial membrane. In the present study, following the
treatment of MAMP for 30 min, the membrane permeability of S.
aureus began to increase. Additionally, SEM and TEM
examinations further demonstrated the effects of MAMP on the
membrane structure of S. aureus, including cell membrane
rupture and release of cellular contents.
In conclusion, MAMP with a molecular weight of less
than 10 kDa, isolated and purified by ultrafiltration, had
antimicrobial activities against standard strains and clinically
isolated antibiotic-resistant strains of S. aureus in vitro
and in vivo. The possible mechanism of action included the
interaction with the bacterial cell membrane and disruption of the
cell surface structure. Our data strongly suggest that MAMP can be
developed as a topical therapeutic agent for the treatment of
bacterial infections in wound healing.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81270052), from
Dalian Scientific and Technological Foundation (no. 2010J21DW022)
and from Program for Liaoning Excellent Talents in University.
References
|
1
|
Allen HK, Donato J, Wang HH, et al: Call
of the wild: antibiotic resistance genes in natural environments.
Nat Rev Microbiol. 8:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baer WS: The classic: the treatment of
chronic osteomyelitis with the maggot (larva of the blow fly).
1931. Clin Orthop Relat Res. 469:920–944. 2011. View Article : Google Scholar
|
|
3
|
Sherman RA: Maggot versus conservative
debridement therapy for the treatment of pressure ulcers. Wound
Repair Regen. 10:208–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mumcuoglu KY: Clinical applications for
maggots in wound care. Am J Clin Dermatol. 2:219–227. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtenay M, Church JC and Ryan TJ: Larva
therapy in wound management. J R Soc Med. 93:72–74. 2000.PubMed/NCBI
|
|
6
|
Davies CE, Turton G, Woolfrey G, et al:
Exploring debridement options for chronic venous leg ulcers. Br J
Nurs. 14:393–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang S, Zhao G, et al: Treatment
of infected wounds with maggot therapy after replantation. J
Reconstr Microsurg. 22:277–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SY, Wang JN, Lv DC, et al: Clinical
research on the bio-debridement effect of maggot therapy for
treatment of chronically infected lesions. Orthop Surg. 2:201–206.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kerridge A, Lappin-Scott H and Stevens JR:
Antibacterial properties of larval secretions of the blowfly,
Lucilia sericata. Med Vet Entomol. 19:333–337. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huberman L, Gollop N, Mumcuoglu KY, et al:
Antibacterial substances of low molecular weight isolated from the
blowfly, Lucilia sericata. Med Vet Entomol. 21:127–131.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bexfield A, Nigam Y, Thomas S, et al:
Detection and partial characterisation of two antibacterial factors
from the excretions/secretions of the medicinal maggot Lucilia
sericata and their activity against methicillin-resistant
Staphylococcus aureus (MRSA). Microbes Infect. 6:1297–1304.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hancock RE and Lehrer R: Cationic
peptides: a new source of antibiotics. Trends Biotechnol. 16:82–88.
1998.PubMed/NCBI
|
|
13
|
Gauri SS, Mandal SM, Pati BR, et al:
Purification and structural characterization of a novel
antibacterial peptide from Bellamya bengalensis: activity
against ampicillin and chloramphenicol resistant Staphylococcus
epidermidis. Peptides. 32:691–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menzies BE and Kenoyer A:
Staphylococcus aureus infection of epidermal keratinocytes
promotes expression of innate antimicrobial peptides. Infect Immun.
73:5241–5244. 2005. View Article : Google Scholar
|
|
15
|
Knobloch JK, Horstkotte MA, Rohde H, et
al: Evaluation of different detection methods of biofilm formation
in Staphylococcus aureus. Med Microbiol Immunol.
191:101–106. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowy FD: Staphylococcus aureus
infections. N Engl J Med. 339:520–532. 1998. View Article : Google Scholar
|
|
17
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomas S, Andrews AM, Hay NP and Bourgoise
S: The anti-microbial activity of maggot secretions: results of a
preliminary study. J Tissue Viability. 9:127–132. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou Z, Lu J, Fang C, et al: Underlying
mechanism of in vivo and in vitro activity of C-terminal-amidated
thanatin against clinical isolates of extended-spectrum
beta-lactamase-producing Escherichia coli. J Infect Dis.
203:273–282. 2011. View Article : Google Scholar
|
|
20
|
Cao L, Dai C, Li Z, et al: Antibacterial
activity and mechanism of a scorpion venom peptide derivative in
vitro and in vivo. PLoS One. 7:e401352012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marri L, Dallai R and Marchini D: The
novel antibacterial peptide ceratotoxin A alters permeability of
the inner and outer membrane of Escherichia coli K-12. Curr
Microbiol. 33:40–43. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dumville JC, Worthy G, Bland JM, et al:
Larval therapy for leg ulcers (VenUS II): randomised controlled
trial. BMJ. 338:b7732009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Whitaker IS, Twine C, Whitaker MJ, et al:
Larval therapy from antiquity to the present day: mechanisms of
action, clinical applications and future potential. Postgrad Med J.
83:409–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Levy O: Antimicrobial proteins and
peptides: anti-infective molecules of mammalian leukocytes. J
Leukoc Biol. 76:909–925. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davydov L: Maggot therapy in wound
management in modern era and a review of published literature. J
Pharm Pract. 24:89–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Opletalová K, Blaizot X, Mourgeon B, et
al: Maggot therapy for wound debridement: a randomized multicenter
trial. Arch Dermatol. 148:432–438. 2012.PubMed/NCBI
|
|
27
|
Steiner H, Hultmark D, Engström A, et al:
Sequence and specificity of two antibacterial proteins involved in
insect immunity. Nature. 292:246–248. 1981. View Article : Google Scholar
|
|
28
|
Cole AM, Weis P and Diamond G: Isolation
and characterization of pleurocidin, an antimicrobial peptide in
the skin secretions of winter flounder. J Biol Chem.
272:12008–12013. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park CB, Lee JH, Park IY, et al: A novel
antimicrobial peptide from the loach, Misgurnus
anguillicaudatus. FEBS Lett. 411:173–178. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nelson A, Hultenby K, Hell E, et al:
Staphylococcus epidermidis isolated from newborn infants
express pilus-like structures and are inhibited by the
cathelicidin-derived antimicrobial peptide LL37. Pediatr Res.
66:174–178. 2009. View Article : Google Scholar
|
|
31
|
Brogden KA: Antimicrobial peptides: pore
formers or metabolic inhibitors in bacteria. Nat Rev Microbiol.
3:238–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Weiss TM, Lehrer RI, et al:
Crystallization of antimicrobial pores in membranes: magainin and
protegrin. Biophys J. 79:2002–2009. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melo MN, Ferre R and Castanho MA:
Antimicrobial peptides: linking partition, activity and high
membrane-bound concentrations. Nat Rev Microbiol. 7:245–250. 2009.
View Article : Google Scholar : PubMed/NCBI
|