Introduction
Silibinin is a natural polyphenolic flavanoid
extracted from the fruit and seeds of milk thistle (Silybum
marianum). It has been reported that silibinin possesses
antioxidant, anti-apoptotic, anti-inflammatory and anti-fibrotic
properties (1–3). However, the effects of silibinin on
type I collagen expression in human skin fibroblasts (HSFs) and the
related signaling pathways remain unclear.
Type I collagen is a major extracellular matrix
component in the dermis. It is synthesized and secreted in a
soluble form by skin fibroblasts and deposited extracellularly. It
is believed that transforming growth factor-β1 (TGF-β1)/Smad
pathways play key roles in type I collagen synthesis (4–6).TGF-β1 is known to exert a promoting
effect on the extracellular matrix in a variety of cells by
stimulating the synthesis of matrix proteins. An important
physiological feature of TGF-β1 includes the de novo
synthesis of extracellular matrix proteins and the inhibition of
the expression of matrix metalloproteinases (MMPs).
Receptor-activated Smads (R-Smads; Smad2 and Smad3) are the key
intracellular components in the TGF-β1 signaling pathway involved
in the production of type I collagen. Thus, the dysregulation of
the TGF-β1/Smad pathway is crucial for the pathogenesis of
hypertrophic and keloid scar formation (7,8).
In this study, the anti-fibrogenic effects of
silibinin were investigated by inhibiting the expression of type I
collagen through the modulation of TGF-β1/Smad signaling pathways
in HSFs.
Materials and methods
Materials
Antibodies against type I collagen, MMP-1, MMP-2,
tissue inhibitor of metalloproteinase-1 (TIMP-1), Smad2 and Smad3
were obtained from Santa Cruz Biotechnology, Inc., (Santa Cruz, CA,
USA). TGF-β1 was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Smad2, Smad3, phosphorylated Smad2 (p-Smad2) and phosphorylated
Smad3 (p-Smad3) were obtained from Cell Signaling Technologies
(Beverly, MA, USA).
Cell culture
HSFs were maintained at 37°C in a humidified
atmosphere of 95% air and 5% CO2 in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% heat-inactivated FBS, 2
mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. For
the experiments, the cells (5×104 cells/ml) were seeded
in culture dishes, and maintained in a tissue culture incubator.
The cells were treated with TGF-β1 (5 ng/ml) or silibinin (0–200
μM) for 48 h.
Cell counting kit-8 (CCK-8) assay
The cells were cultured in 96-well plates containing
silibinin (0–200 μM) with 3 replicate wells for each concentration.
Following incubation for 48 h, cell viability was measured by CCK-8
assay (Dojindo, Kumamoto, Japan), according to the manufacturer’s
instructions.
Western blot analysis
Whole cell extracts were prepared in lysis buffer
[10 mM Tris (pH 7.4), 5 mM EDTA, 130 mM NaCl, 1% Triton X-100,
phenylmethylsulphonyl fluoride (PMSF, 10 μg/ml), aprotinin (10
μg/ml), leupeptin (10 μg/ml), 5 mM phenanthroline and 28 mM
benzamidine-HCl]. The protein concentration of the extracts was
estimated with Bradford reagent (Bio-Rad, Hercules, CA, USA) using
bovine serum albumin as the standard. Equal amounts of protein (40
μg/lane) were resolved by 6.5–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto a
nitrocellulose membrane. The membrane was then washed with
Tris-buffered saline (10 mM Tris, 150 mM NaCl) containing 0.05%
Tween-20 (TBST) and blocked in TBST containing 5% non-fat dried
milk. The membrane was further incubated with respective specific
antibodies. The membrane was continuously incubated with
appropriate secondary antibodies coupled to horseradish peroxidase,
and blots were developed in the ECL Western blotting detection
reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Chloramphenicol acetyltransferase (CAT)
assay
The construct containing the 3.5-kb COL1A2 promoter
fused to the CAT gene (pMS 3.5/CAT) was derived from a 3.5-kb
EcoRI/SphI genomic subclone that spans the region
from position -3500 to +58 of the COL1A2 promoter. After conversion
of the EcoRI site to BamHI, the eukaryotic insert was
excised with BamHI and HindIII, and was subcloned
into a similarly digested polylinker of the expression vector
p8-CAT, a derivative of the pEMBL plasmid. Thereafter, deletion
mutants were generated in the parental pUC18 subclones and
subsequently transferred to the expression vector. The
transfections were carried out using the calcium phosphate/DNA
co-precipitation method followed by a 1-min (15%) glycerol shock.
Four hours after transfection, the cells were treated with
silibinin. After a total of 48 h of incubation, the cells were
harvested. CAT activity was determined by incubating cell extracts
with [14C]chloramphenicol, followed by separation of its
acetylated and non-acetylated forms by thin-layer chromatography.
CAT activity in the cell extracts was calculated as radioactivity
in the acetylated forms as a percentage of the total radioactivity
in each sample.
Immunofluorescence analysis
To detect the intracellular localization of Smad2
and Smad3, skin fibroblasts were grown on slides in 24-well plates
and then treated under the indicated conditions. After fixing with
4% paraformaldehyde for 30 min, the cells were permeabilized and
blocked with 0.1% saponin and 0.5% bovine serum albumin in
phosphate-buffered saline (PBS) for 30 min at 4°C. Subsequently,
the samples were incubated with each primary antibody (1:50)
overnight at 4°C, then the cells were incubated with fluorescein
isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:100) for 2
h at room temperature. The slides were then mounted with 80%
phosphoglycerol, viewed and photographed under a fluorescence
microscope (Olympus, Tokyo, Japan). In a single experiment, at
least 100 stained cells were analyzed per sample.
Results
Effect of silibinin on cell proliferation
rates in HSFs
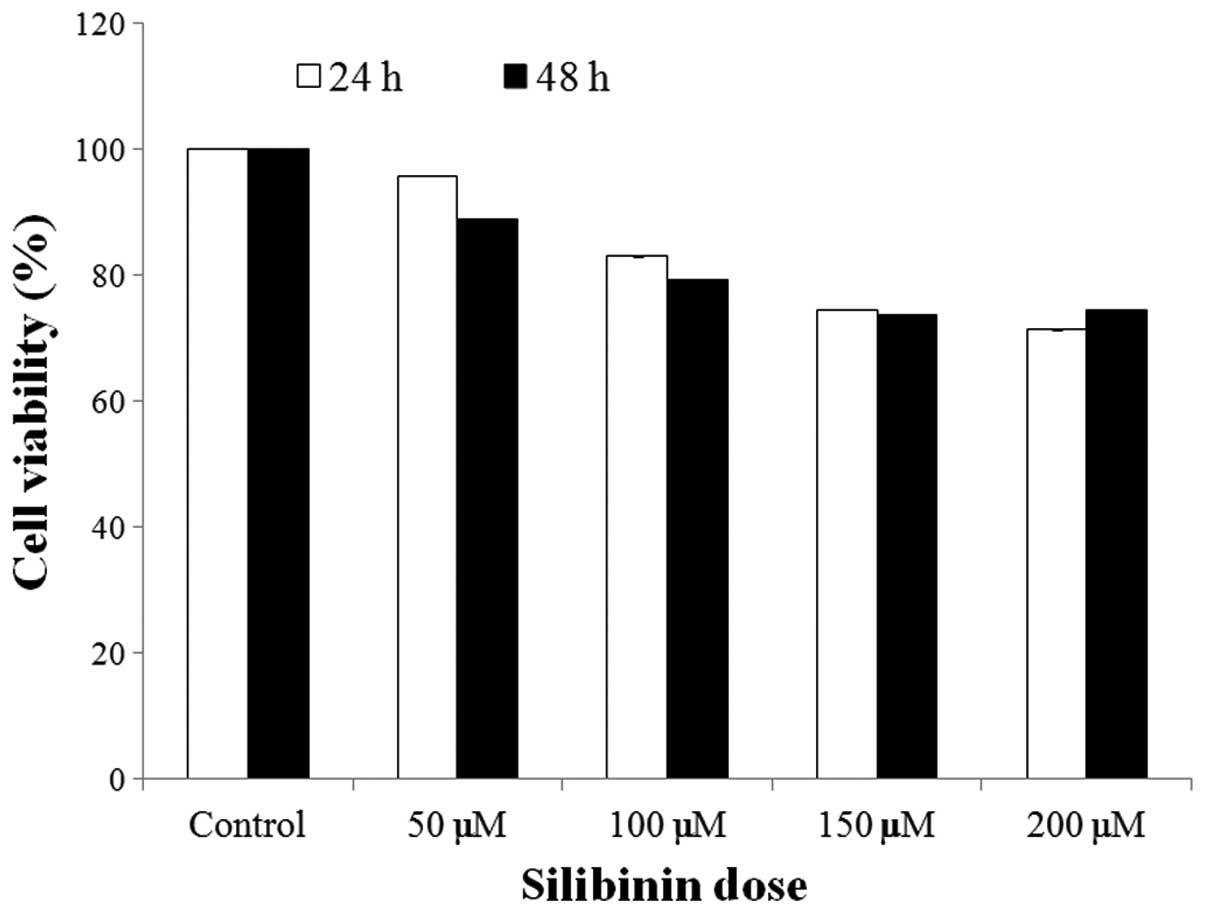
To determine the effect of silibinin on the
proliferation rates of HSFs, cells were exposed to silibinin (0–200
μM) for 2 days. As shown in Fig.
1, the proliferation rates were not markedly decreased
following silibinin treatment. In addition, no cytotoxic effects of
silibinin were observed in the cells (data not shown). These data
indicate that a concentration of silibinin under 200 μM does not
exert any cytotoxic effects on HSFs.
Effect of silibinin on type I collagen
and MMPs in HSFs
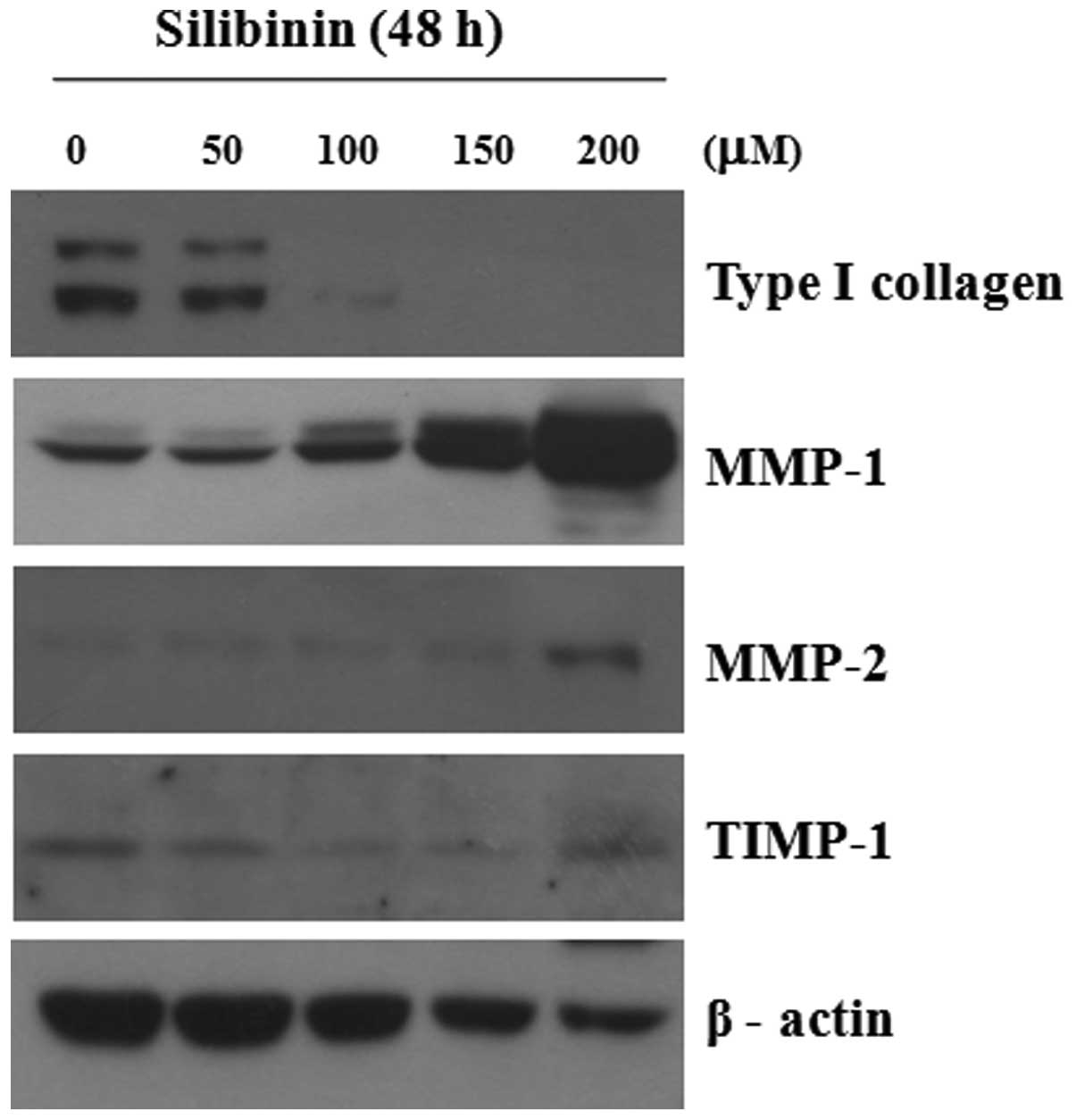
We analyzed the effect of silibinin on the
expression of type I collagen, MMP-1 and MMP-2 in cultured
fibroblasts. As shown in Fig. 2,
silibinin clearly induced the decreased expression of type I
collagen and the increased expression of MMP-1 and MMP-2 proteins
in a dose-dependent manner; however, the expression of TIMP-1 was
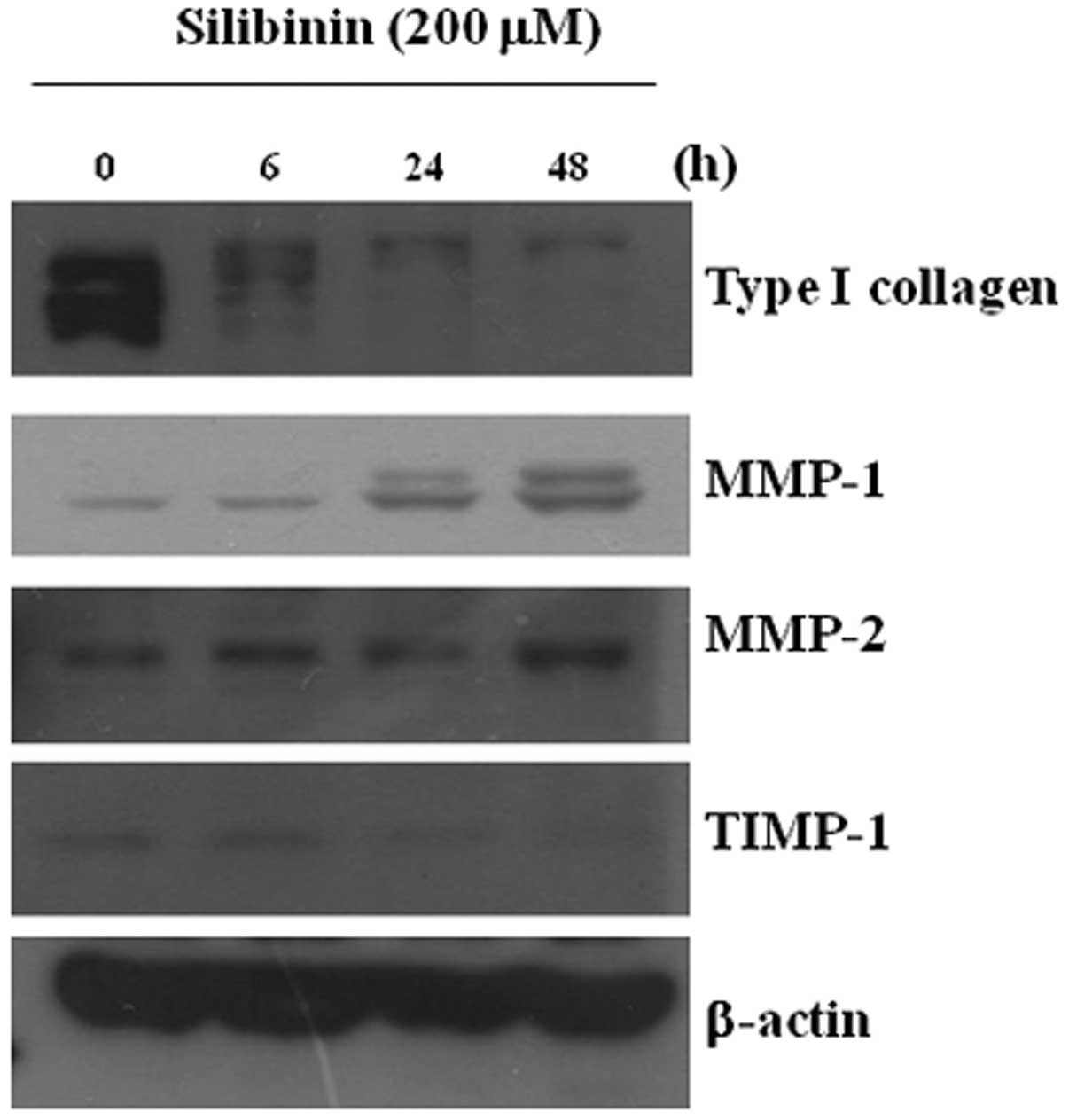
not markedly altered. The decreased expression of MMP-1 was clearly
observed at 6 h and the increased expression of MMP-1 was observed
at 24 h following treatment (Fig.
3). The enzymatic activity of MMP-1 was increased in the
silibinin-treated cultured fibroblasts, as shown by zymography
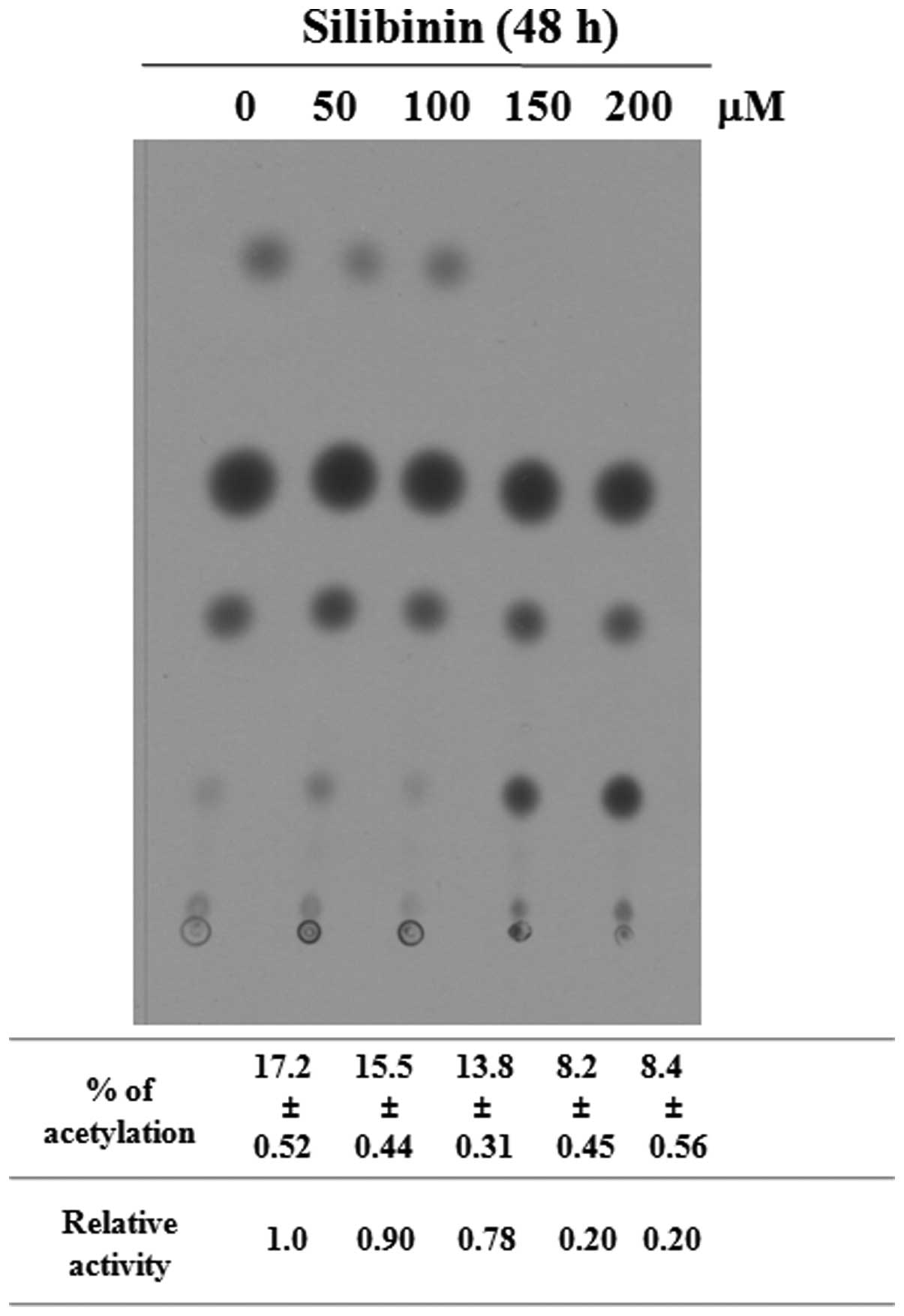
using casein (data not shown). Furthermore, the markedly decreased
promoter activity of type I collagen was confirmed by CAT assay
(Fig. 4). We found that silibinin
induced the decreased expression of type I collagen, as well as the
increased expression of MMP-1 and MMP-2 in the HSFs, demonstrating
the anti-fibrotic effects of silibinin on skin. Thus, we focused on
the role of silibinin in Smad2 and Smad3 in cultured HSFs.
Effect of silibinin on Smad2 and Smad3
expression in HSFs
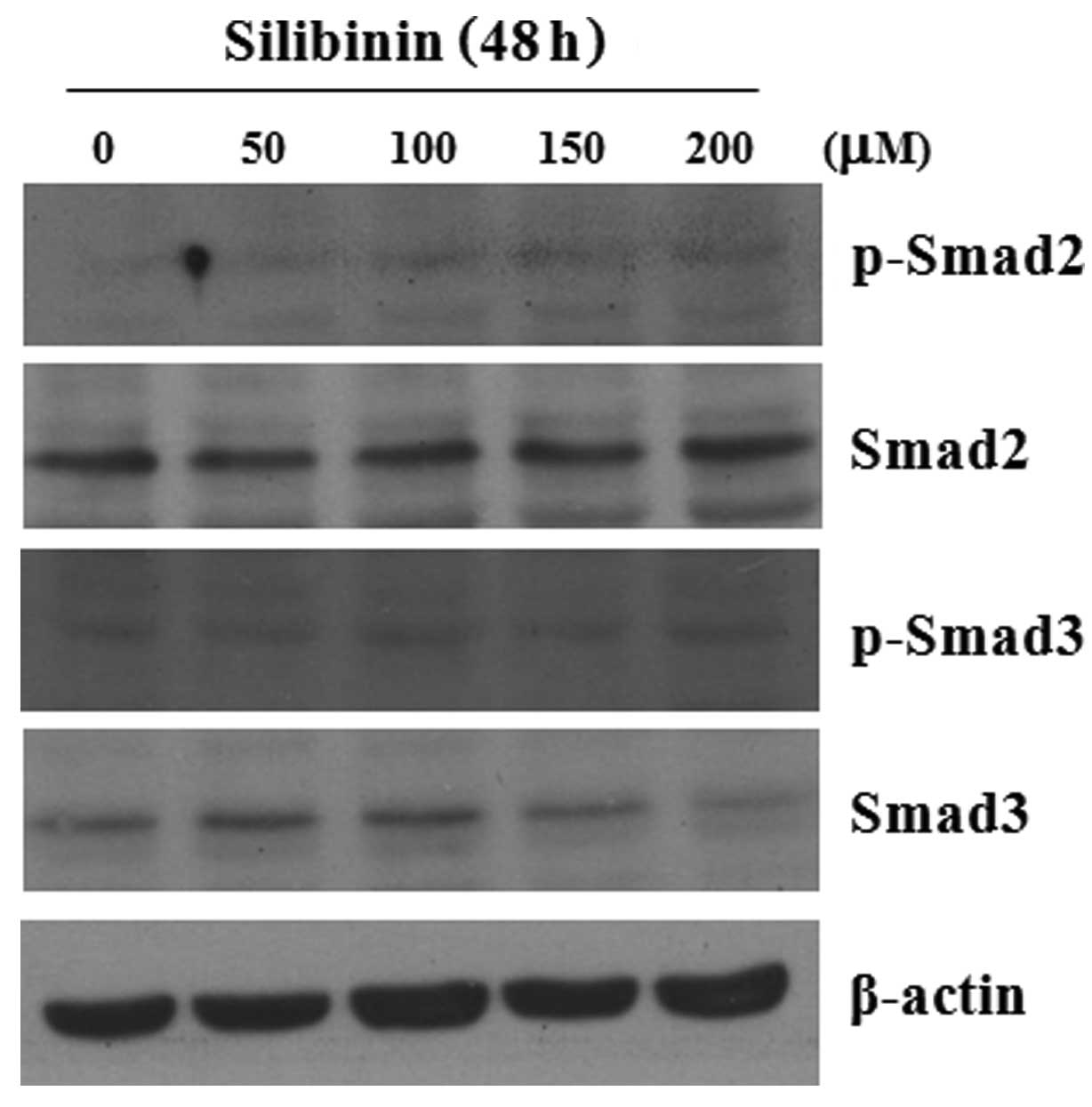
We analyzed the effect of silibinin on the
expression of Smad2 and Smad3 in cultured skin fibroblasts. As
shown in Fig. 5, at a
concentration of >150 μM, silibinin did not alter the basal
expression level of Smad2, but induced the decreased expression
level of Smad3. Of note, silibinin clearly inhibited the
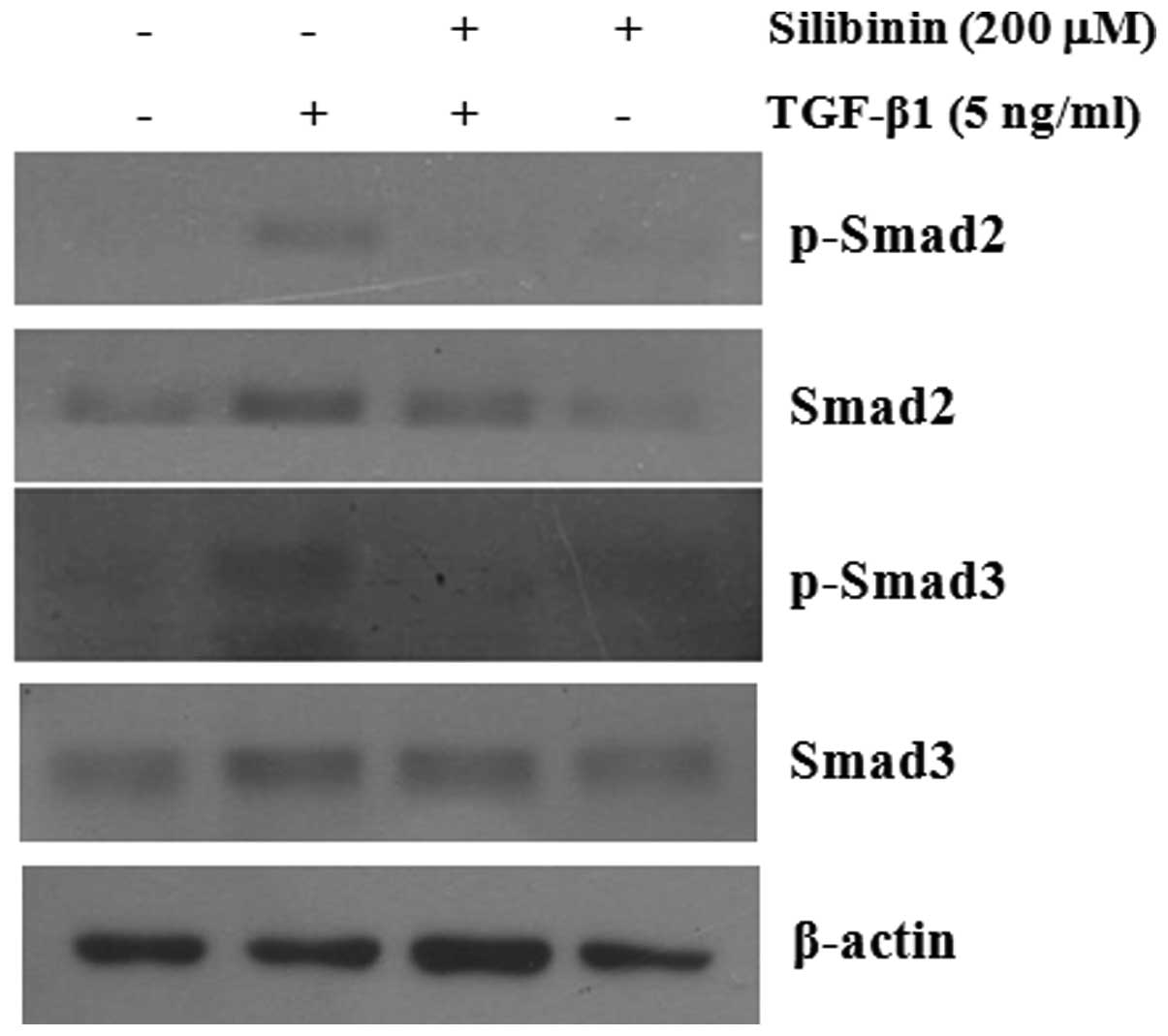
TGF-β1-induced phosphorylation levels of Smad2 and Smad3 (Fig. 6).
Effects of silibinin on TGF-β1-induced
nuclear translocation of Smad2
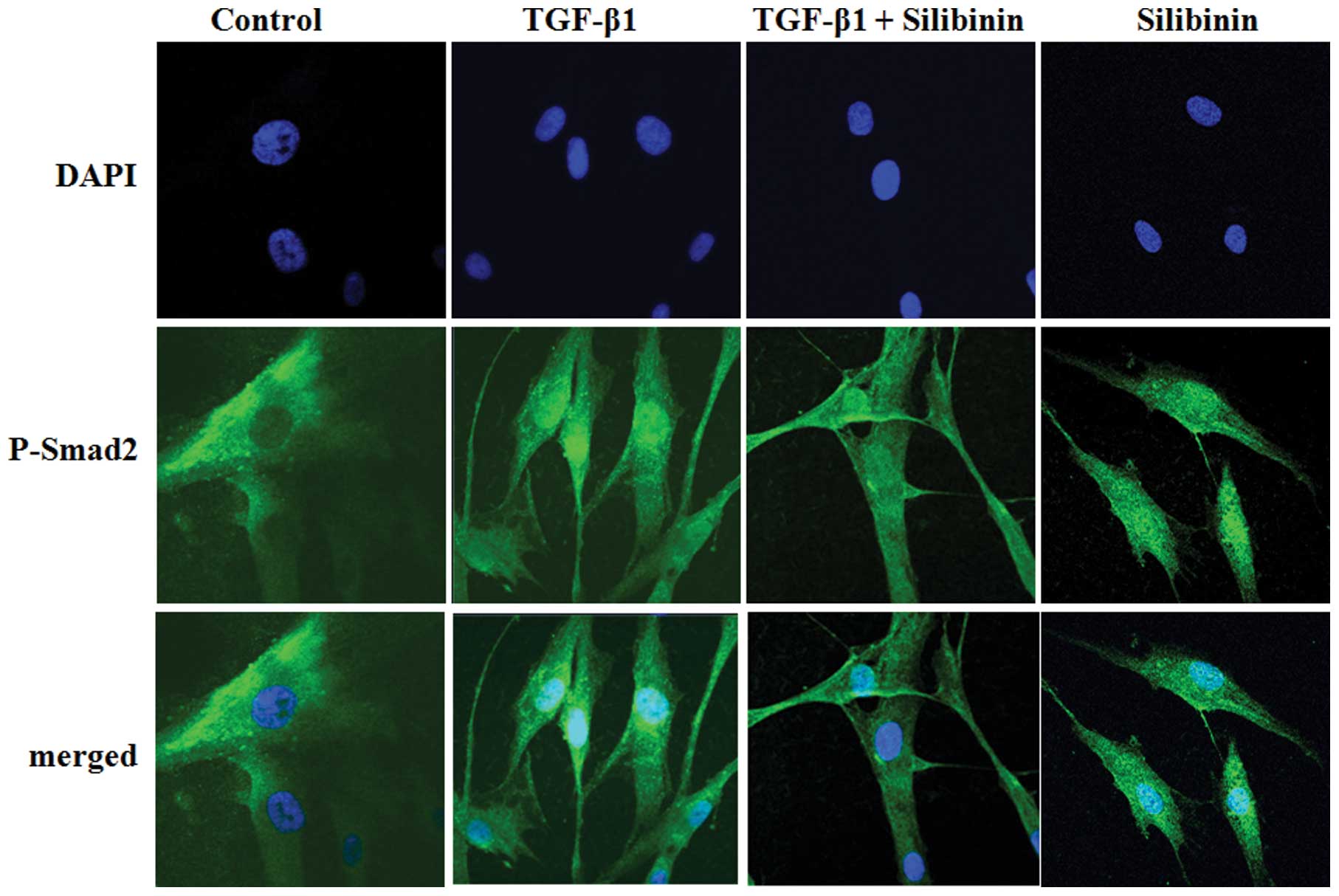
We further investigated whether silibinin inhibits
the nuclear translocation of Smad2 in TGF-β1-treated fibroblasts.
The intracellular localization of p-Smad2 was examined by
immunofluorescence microscopy using anti-p-Smad2 antibody. At least
100 stained cells were analyzed per sample. As shown in Fig. 7, Smad2 was scarcely phosphorylated
in the absence of exogenous TGF-β1. The majority of Smad2 proteins
that were phosphorylated at the C-terminal region following TGF-β1
treatment translocated to the nuclei. Treatment with silibinin (200
μM) interrupted the TGF-β1-mediated nuclear translocation of
p-Smad2 in the cells. The nuclear translocation of p-Smad3 was also
attenuated by silibinin treatment in the fibroblasts (data not
shown).
Discussion
The investigation of the molecular mechanisms
underlying fibrosis or hypertrophic scar formation has attracted
considerable attention in recent years. It has been widely accepted
that the dysregulation of the TGF-β/Smad pathway plays a key role
in hypertrophic or keloid scar formation in human skin tissues. A
number of studies have clearly demonstrated that the increased
expression of TGF-β receptors (types I and II) and the increased
phosphorylation of Smad3 in keloid fibroblasts compared to normal
HSFs, as well as the suppression of the TGF-β1/Smad pathway by
certain chemicals such as curcumin, may exert chemopreventive
effects, thus preventing keloid formation (7–9).
In the current study, we demonstrated that silibinin
induced the decreased expression of type I collagen at both the
protein and mRNA levels in cultured skin fibroblasts. In addition,
the silibinin-induced downregulation of type I collagen expression
was partly mediated by the inhibition of the phosphorylation and
nuclear translocation of Smad2 and Smad3. In accordance with our
findings, silibinin has been shown to attenuate cardiac hypertrophy
and fibrosis by blocking epidermal growth factor receptor
(EGFR)-dependent signaling, as well as the nuclear factor-κB
(NF-κB) and TGF-β1/Smad signaling pathways (10). These findings suggest that
silibinin may be an effective therapeutic candidate against
hypertrophic or keloid scarring through the downregulation of type
I collagen expression.
The phosphorylation and nuclear translocation of
Smad2, Smad3 with Smad4 in response to TGF-β1 stimulation are
critical for the activation of the TGF-β1/Smad signaling pathway
(11–13). TGF-β1 induces a complex formation
between p-Smad2 and p-Smad3 at the C-terminal and linker region
with Smad4. The Smad2/3/4 complex translocates to the nucleus to
regulate the expression of target genes, such as type I collagen.
Namely, TGF-β-stimulated α2(I)-collagen expression occurs via the
cooperation between Smad3 and Smad4, Sp1, CBP/p300 and Egr-1
(14–16). Our results demonstrated that
silibinin attenuated the nuclear translocation of Smad2 and Smad3,
as well as the inhibition of the TGF-β-induced phosphorylation of
Smad2 and Smad3. It was observed that the signaling activity of
Smad was modulated through phosphorylation and cytosol-nucleus
translocation. Our data suggest that silibinin may be an effective
therapeutic candidate against hypertrophic or keloid scarring
through the inhibition of the nuclear translocation and
phosphorylation of Smad2 and Smad3.
TGF-β represses the expression of MMP-1 via Smad3
and Smad4 with dominant-negative Smad3 or Smad4 mutants abrogating
this response (17). If the
imbalance of MMP-1 activity occurs in type I collagen synthesis
during the wound healing process, the excessive accumulation of the
extracellular matrix may result in hypertrophic scarring or keloid
formation. In this study, we found that silibinin-treated
fibroblasts showed increased MMP-1 and MMP-2 expression levels, as
well as a decreased type I collagen expression level. These results
suggest that silibinin positively regulates the expression of MMP-1
and MMP-2 through the partial suppression of Smad2/3
activation.
In conclusion, the data from the present study
suggest that silibinin has the potential to prevent fibrotic skin
changes by inducing the downregulation of type I collagen
expression; this effect is is partly mediated by blocking the
Smad2/3-dependent signaling pathway in HSFs.
Acknowledgements
This study was supported by a research promotion
grant from the Keimyung University Dongsan Medical Center in
2012.
References
|
1
|
Ramasamy K and Agarwal R: Multitargeted
therapy of cancer by silymarin. Cancer Lett. 269:352–362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muriel P, Moreno MG, Hernández Mdel C,
Chávez E and Alcantar LK: Resolution of liver fibrosis in chronic
CCl4 administration in the rat after discontinuation of treatment:
effect of silymarin, silibinin, colchicine and trimethylcolchicinic
acid. Basic Clin Pharmacol Toxicol. 96:375–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh RP, Dhanalakshmi S, Agarwal C and
Agarwal R: Silibinin strongly inhibits growth and survival of human
endothelial cells via cell cycle arrest and downregulation of
survivin, Akt and NF-kappaB: implications for angioprevention and
antiangiogenic therapy. Oncogene. 24:1188–1202. 2005. View Article : Google Scholar
|
|
4
|
Rahimi RA and Leof EB: TGF-beta signaling:
a tale of two responses. J Cell Biochem. 102:593–608. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie JL, Qi SH, Pan S, Xu YB, Li TZ, Liu XS
and Liu P: Expression of Smad protein by normal skin fibroblasts
and hypertrophic scar fibroblasts in response to transforming
growth factor beta1. Dermatol Surg. 34:1216–1225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zi Z, Chapnick DA and Liu X: Dynamics of
TGF-β/Smad signaling. FEBS Lett. 586:1921–1928. 2012.
|
|
7
|
Hsu YC, Chen MJ, Yu YM, Ko SY and Chang
CC: Suppression of TGF-β1/SMAD pathway and extracellular matrix
production in primary keloid fibroblasts by curcuminoids: its
potential therapeutic use in the chemoprevention of keloid. Arch
Dermatol Res. 302:717–724. 2010.
|
|
8
|
He S, Liu X, Yang Y, Huang W, Xu S, Yang
S, Zhang X and Roberts MS: Mechanisms of transforming growth factor
beta(1)/Smad signalling mediated by mitogen-activated protein
kinase pathways in keloid fibroblasts. Br J Dermatol. 162:538–546.
2010.
|
|
9
|
Chin GS, Liu W, Peled Z, Lee TY,
Steinbrech DS, Hsu M and Longaker MT: Differential expression of
transforming growth factor-beta receptors I and II and activation
of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 108:423–429.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ai W, Zhang Y, Tang QZ, Yan L, Bian ZY,
Liu C, Huang H, Bai X, Yin L and Li H: Silibinin attenuates cardiac
hypertrophy and fibrosis through blocking EGFR-dependent signaling.
J Cell Biochem. 110:1111–1122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y and Massagué J: Mechanisms of
TGF-beta signaling from cell membrane to the nucleus. Cell.
113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moustakas A, Pardali K, Gaal A and Heldin
CH: Mechanisms of TGF-beta signaling in regulation of cell growth
and differentiation. Immunol Lett. 82:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen HB, Rud JG, Lin K and Xu L: Nuclear
targeting of transforming growth factor-beta-activated Smad
complexes. J Biol Chem. 280:21329–21336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poncelet AC and Schnaper HW: Sp1 and Smad
proteins cooperate to mediate transforming growth factor-beta
1-induced alpha 2(I) collagen expression in human glomerular
mesangial cells. J Biol Chem. 276:6983–6992. 2001. View Article : Google Scholar
|
|
15
|
Ghosh AK, Yuan W, Mori Y and Varga J:
Smad-dependent stimulation of type I collagen gene expression in
human skin fibroblasts by TGF-beta involves functional cooperation
with p300/CBP transcriptional coactivators. Oncogene. 19:3546–3555.
2000. View Article : Google Scholar
|
|
16
|
Chen SJ, Ning H, Ishida W, Sodin-Semrl S,
Takagawa S, Mori Y and Varga J: The early-immediate gene EGR-1 is
induced by transforming growth factor-beta and mediates stimulation
of collagen gene expression. J Biol Chem. 281:21183–21197. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan W and Varga J: Transforming growth
factor-beta repression of matrix metalloproteinase-1 in dermal
fibroblasts involves Smad3. J Biol Chem. 276:38502–38510. 2001.
View Article : Google Scholar : PubMed/NCBI
|