Introduction
Platelet adhesion and aggregation are the first
steps in thrombus formation at the injured vascular site, and play
a crucial role in hemostasis. Platelets are activated by various
stimuli, resulting in shape change, adhesion, aggregation and
subsequently, thrombus formation. Thrombus formation is associated
with the release of granule contents, such as platelet-derived
growth factor (PDGF)-AB and serotonin, as well as the release of
inflammatory substances, such as soluble CD40 ligand (sCD40L).
These secreted and generated mediators trigger a positive feedback
mechanism that potentiates platelet activation (1,2).
Adenosine diphosphate (ADP), which is released from
damaged cells and secreted from platelet-dense granules,
contributes to the positive feedback mechanism for platelet
activation by acting through P2 receptors on the platelet surface
(1). It is recognized that ADP is
an essential co-factor for the activation of platelets by other
platelet agonists; however, ADP is a weak agonist for platelets
compared to thrombin or collagen (1). ADP induces shape change and platelet
aggregation through P2Y1 and P2Y12 receptors. It has been reported
that P2Y1 or P2Y12 receptors stimulated by ADP induce the
activation of p38 mitogen-activated protein (MAP) kinase and
p44/p42 MAP kinase among the MAP kinase superfamily (1,3–5).
Heat shock proteins (HSPs) are expressed in a
variety of cells in response to various types of biological stress,
such as heat stress and chemical stress (6). HSP27 belongs to the low molecular
weight HSP family (HSPB) with a molecular mass ranging from 10 to
30 kDa (6). It is generally known
that HSP27 activity is regulated by post-translational
modifications, such as phosphorylation (6). HSP27 is promptly phosphorylated in
response to various types of stress, as well as following exposure
to cytokines and mitogens, and changes from an aggregated to a
dissociated form (6). Human HSP27
is phosphorylated at three serine residues (Ser-15, Ser-78 and
Ser-82). It is recognized that the phosphorylation of HSP27 is
catalyzed by members of the MAP kinase superfamily, such as p38 MAP
kinase (6). It has been shown
that ADP induces HSP27 phosphorylation in human platelets (7). We have previously demonstrated that
the ADP-induced phosphorylation of HSP27 via the p44/p42 MAP kinase
and p38 MAP kinase pathway correlates with PDGF-AB secretion and
the release of sCD40L from human platelets (8).
It is well known that thrombopoietin (TPO), which is
recognized as a Mpl ligand or a megakaryocyte growth and
differentiation factor, is a pivotal physiological regulator of
megakaryocytopoiesis and platelet production (9). TPO interacts with its receptor,
c-Mpl, resulting in the activation of a variety of signal
transduction pathways (10–15). It has been reported that TPO alone
fails to induce platelet aggregation, but potentiates platelet
activation stimulated by numerous platelet agonists (12,16–18). However, the exact mechanism behind
the amplification of platelets by agonists and TPO has not yet been
fully elucidated. In the present study, we investigated the effect
of TPO on ADP-induced human platelet activation. We demonstrate
that pre-treatment with TPO amplifies ADP-induced HSP27
phosphorylation via the p38 MAP kinase pathway in human
platelets.
Materials and methods
Materials
ADP was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Recombinant human TPO was purchased from R&D Systems,
Inc. (Minneapolis, MN, USA). p38 MAP kinase antibodies and
phospho-p38 MAP kinase antibodies were obtained from Cell
Signaling, Inc. (Beverly, MA, USA). HSP27 antibodies, phospho-HSP27
(Ser-15) antibodies and phospho-HSP27 (Ser-78) antibodies were from
Stressgen Biotechnologies (Victoria, BC, Canada). Phospho-HSP27
(Ser-82) antibodies were from Biomol Research Laboratories,
(Plymouth Meeting, PA, USA). GAPDH antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The ECL
western blotting detection system was purchased from GE Healthcare
(Buckinghamshire, UK). Other materials and chemicals were obtained
from commercial sources.
Preparation of platelets
Human blood was donated from healthy volunteers and
collected into a 1/10 volume of 3.8% sodium citrate. Platelet-rich
plasma (PRP) was obtained from blood samples by centrifugation at
155 × g for 12 min at room temperature. Platelet-poor plasma (PPP)
was prepared from the residual blood by centrifugation at 2,500 × g
for 15 min. All participants signed an informed consent agreement
after receiving a detailed explanation, and the study was approved
by the Ethics Committee of Gifu University Graduate School of
Medicine, Gifu, Japan.
Measurement of platelet aggregation
induced by ADP
Platelet aggregation using citrated PRP was carried
out in a PA-200 aggregometer (Kowa Co., Ltd., Tokyo, Japan), which
can determine the size of platelet aggregates based upon particle
counting using laser scattering methods (small size, 9–25 mm;
medium size, 25–50 mm; large size, 50–70 mm), at 37°C with a
stirring speed of 800 rpm. The platelets were pre-incubated for 1
min, and then platelet aggregation was monitored for 4 min. The
percentage of transmittance of the isolated platelets was recorded
as 0%, and that of PPP (blank) was recorded as 100%. When
indicated, TPO was administered to PRP 15 min prior to with
stimulation ADP (pre-treatment), simultaneously with ADP
(simultaneous treatment), and 2 min following stimulation with ADP
(post-treatment).
Protein preparation following stimulation
with ADP
Following stimulation with ADP, platelet aggregation
was terminated by the addition of an ice-cold EDTA (10 mM) solution
for 5 min for the western blot analysis samples or for 30 min for
enzyme-linked immunosorbent assay (ELISA) samples. The mixture was
centrifuged at 10,000 × g at 4°C for 2 min. The supernatant was
isolated and stored at −30°C for subsequent ELISA to measure the
levels of PDGF-AB and sCD40L. For western blot analysis, the pellet
was washed twice with PBS and then lysed and immediately boiled in
lysis buffer containing 62.5 mM Tris/Cl, pH 6.8, 2% sodium dodecyl
sulfate (SDS), 50 mM dithiothreitol and 10% glycerol.
Western blot analysis
Western blot analysis was performed as previously
described (8). Briefly,
SDS-polyacrolamide gel electrophoresis (PAGE) was performed
according to the method described in the study by Laemmli (19) in a 10% polyacrylamide gel.
Proteins in the gel were transferred onto polyvinylidine fluoride
(PVDF) membranes. The membranes were blocked with 5% fat-free dry
milk in Tris-buffered saline with 0.1% Tween-20 (TBS-T, 20 mM
Tris-HCl; pH 7.6, 137 mM NaCl, 0.1% Tween-20) for 1 h prior to
incubation with the indicated primary antibodies overnight. The
primary antibodies used in the study were against GAPDH, HSP27,
phospho-HSP27 (Ser-15), phospho-HSP27 (Ser-78), phospho-HSP27
(Ser-82), p38 MAP kinase and phospho-p38 MAP kinase antibodies.
Peroxidase-labeled anti-goat IgG or anti-rabbit IgG antibodies were
used as secondary antibodies. The primary and secondary antibodies
were diluted by optimum concentration of 5% fat-free dry milk in
TBS-T. Peroxidase activity on PVDF membranes was visualized on
X-ray films by means of an ECL western blotting detection system as
per the manufacturer’s instructions.
Measurement of PDGF-AB and sCD40L
levels
The PDGF-AB and sCD40L levels in the samples were
determined using PDGF-AB Quantikine and sCD40-Ligand Quantikine
ELIsySA kits (R&D Systems, Inc.), respectively, according to
the manufacturer’s instructions.
Statistical analysis
Unless otherwise stated, representative results from
five independent experiments are shown in the figures. The data are
presented as the means ± SEM. The data were analyzed using the
Student’s t-test, and a p-value <0.05 was considered to indicate
a statistically significant difference.
Results
Effect of TPO on ADP-induced platelet
aggregation
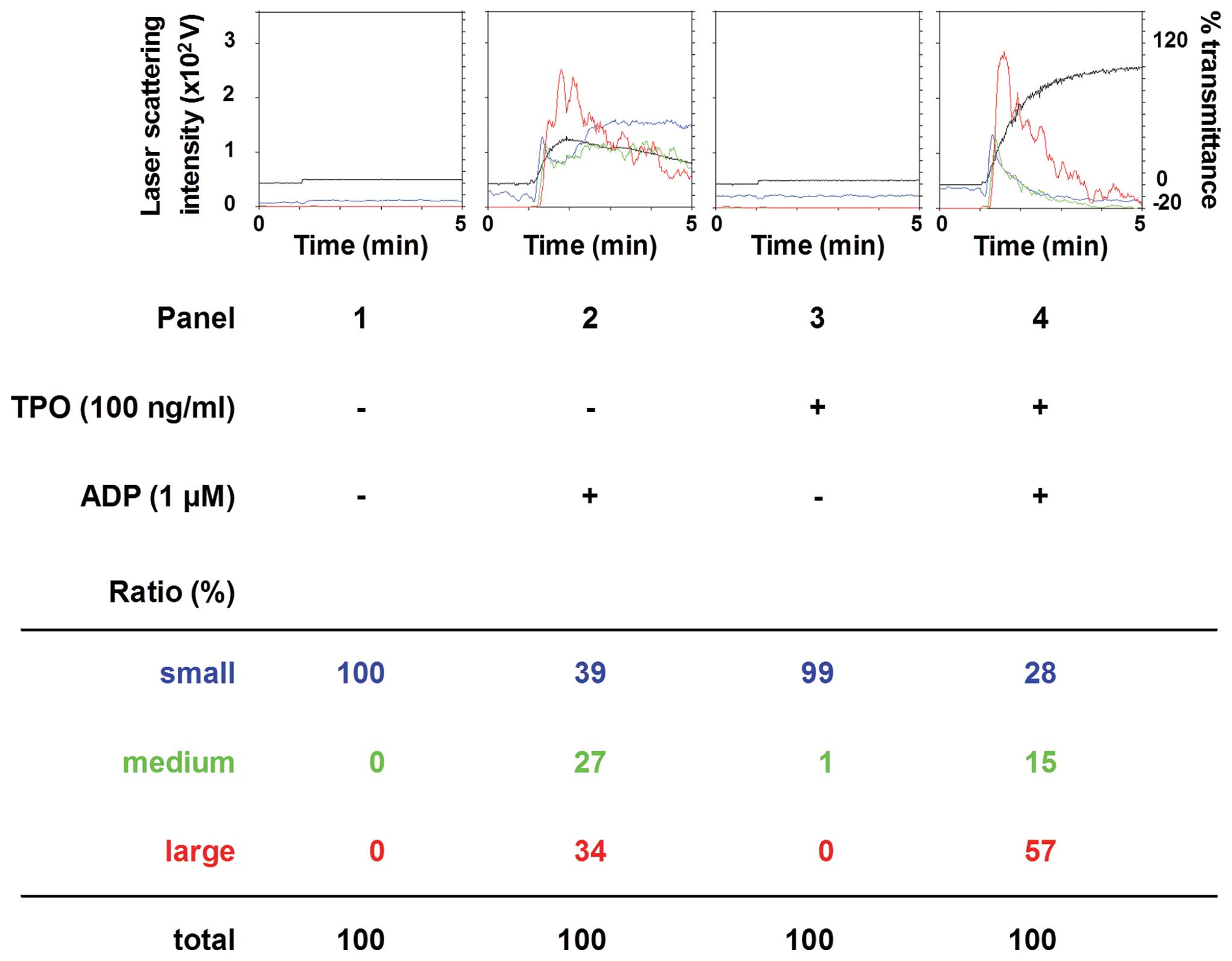
We first examined the effect of pre-treatment with
TPO on ADP-induced platelet aggregation. TPO, which on its own did
not induce platelet aggregation, significantly enhanced the
platelet aggregation induced by 1 mM ADP (Fig. 1). According to the analysis of the
size of the aggregates, the percentage of large-size aggregates
(50–70 mm) was significantly increased from 34 to 57%. On the other
hand, TPO markedly decreased the number of small aggregates.
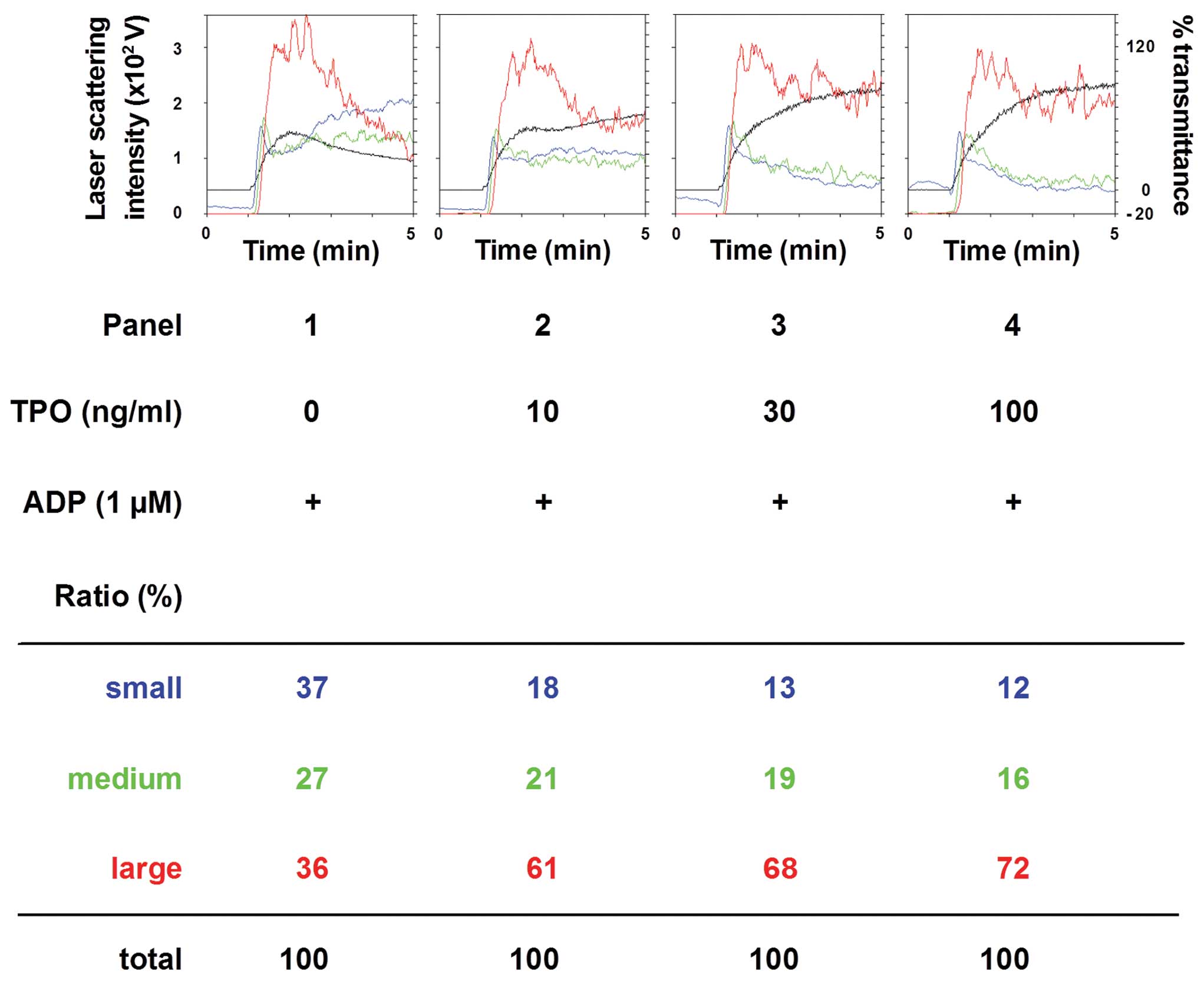
We then examined the dose-dependent effect of TPO on
the ADP (1 mM)-induced platelet aggregation. The amplifying effect
of TPO (between 10 and 100 mM) was dose-dependent (Fig. 2). The number of large aggregates
was dose-dependently increased by TPO, whereas the number of small
aggregates was decreased.
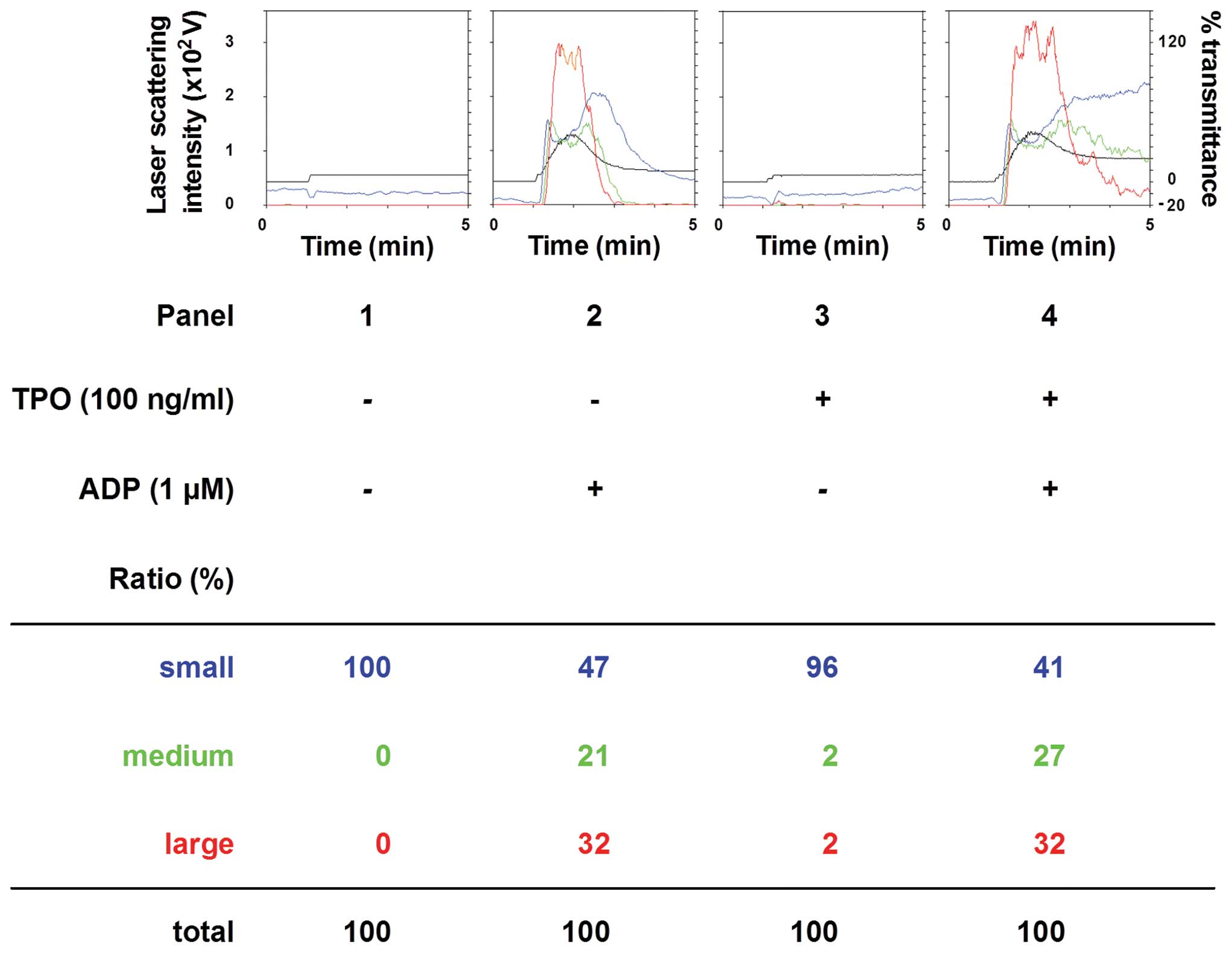
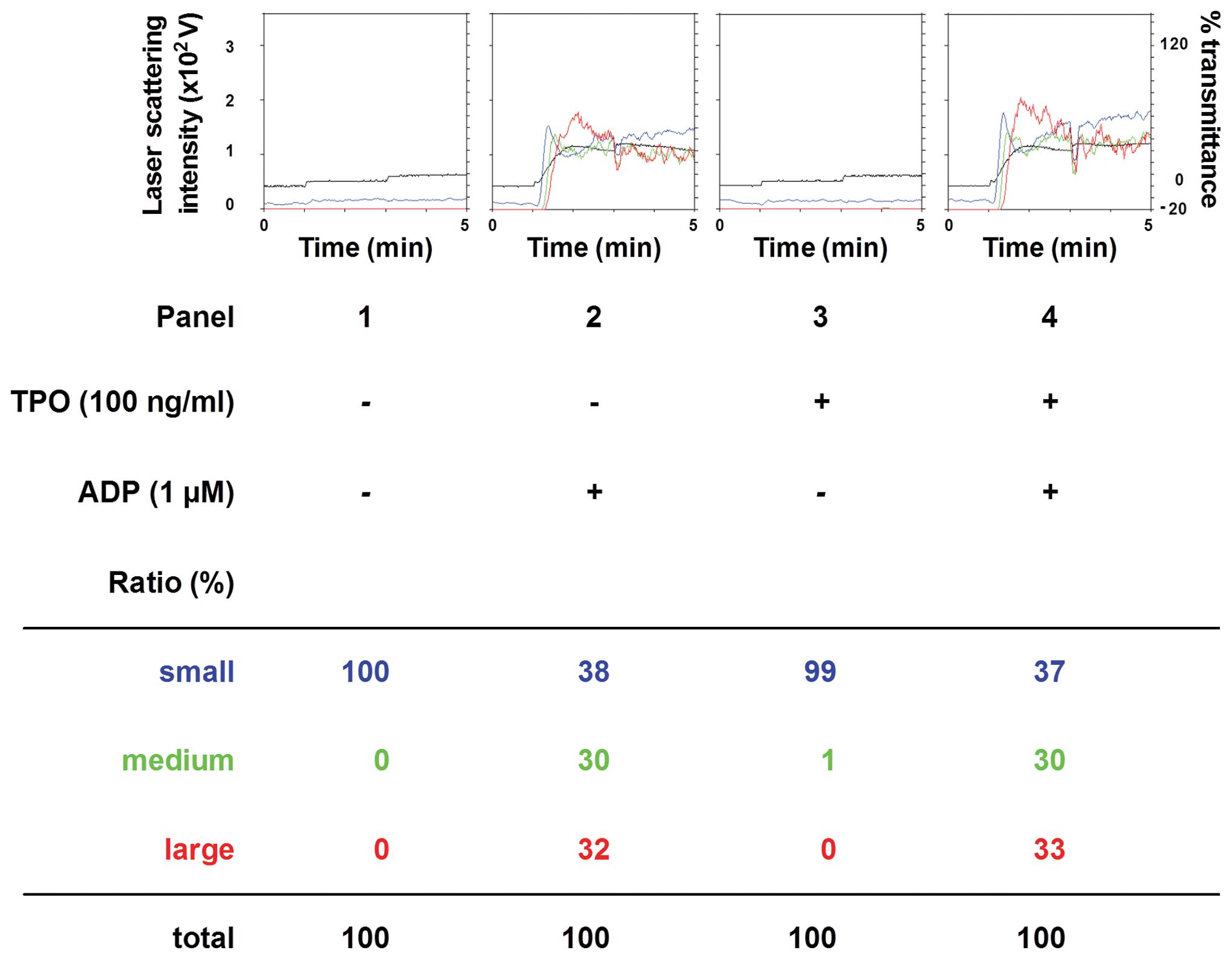
In order to clarify the exact mechanism of action of
TPO in ADP-induced platelet activation, we examined the effect of a
combination of TPO and ADP on platelet aggregation. In contrast to
pre-treatment with TPO, the simultaneous stimulation with TPO and
ADP did not further enhance platelet aggregation compared to
treatment with ADP alone (Fig.
3). In addition, post-treatment with TPO following stimulation
with ADP had no additional effect on the platelet aggregation
induced by ADP alone (Fig.
4).
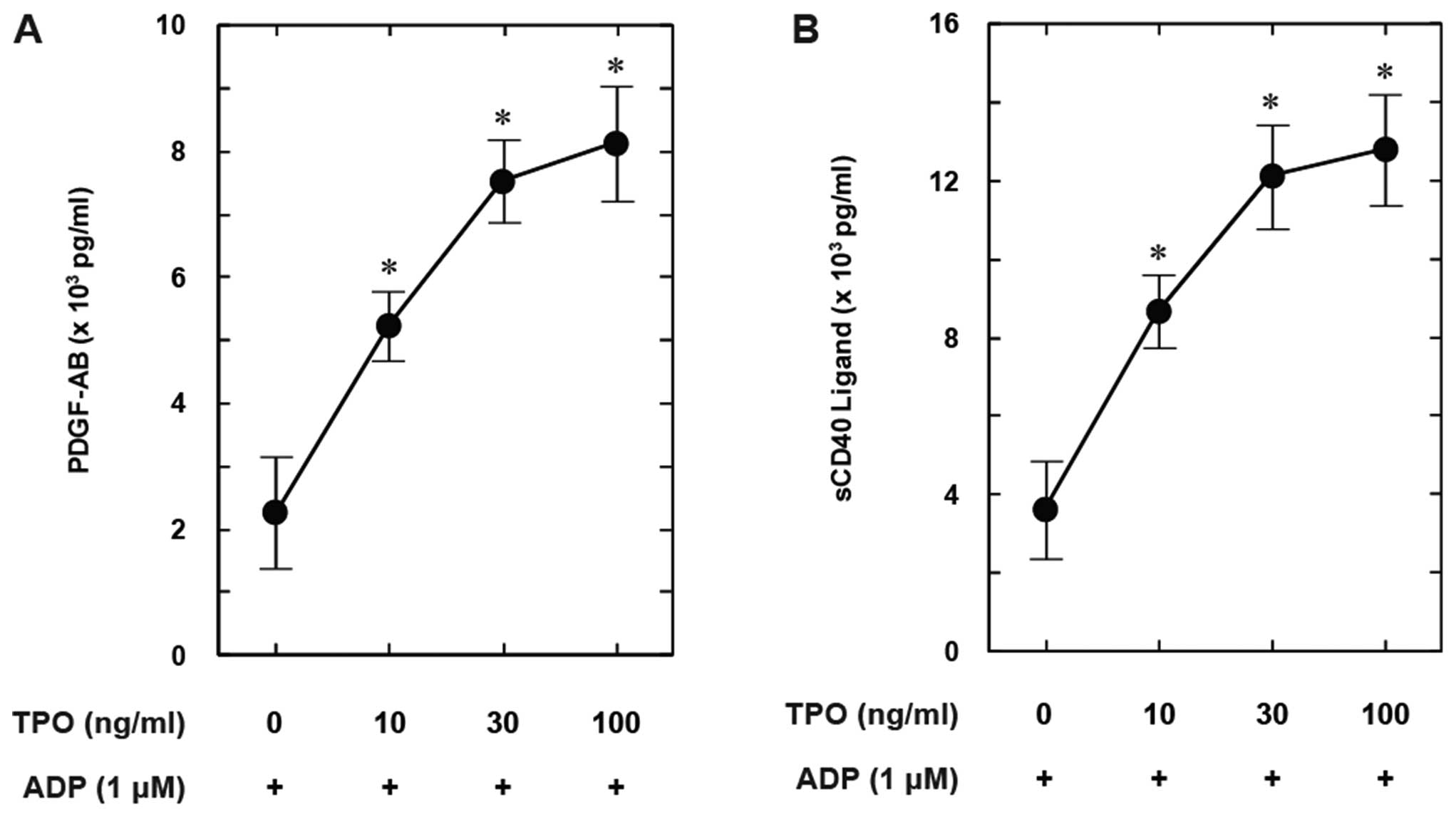
Effects of TPO on ADP-induced PDGF-AB
secretion and release of sCD40L
Pre-treatment with TPO (between 10 and 100 ng/ml)
significantly enhanced the ADP-induced PDGF-AB secretion in a
dose-dependent manner (Fig. 5A).
In addition, the release of sCD40L stimulated by ADP was
dose-dependently amplified following pre-treatment with TPO
(Fig. 5B).
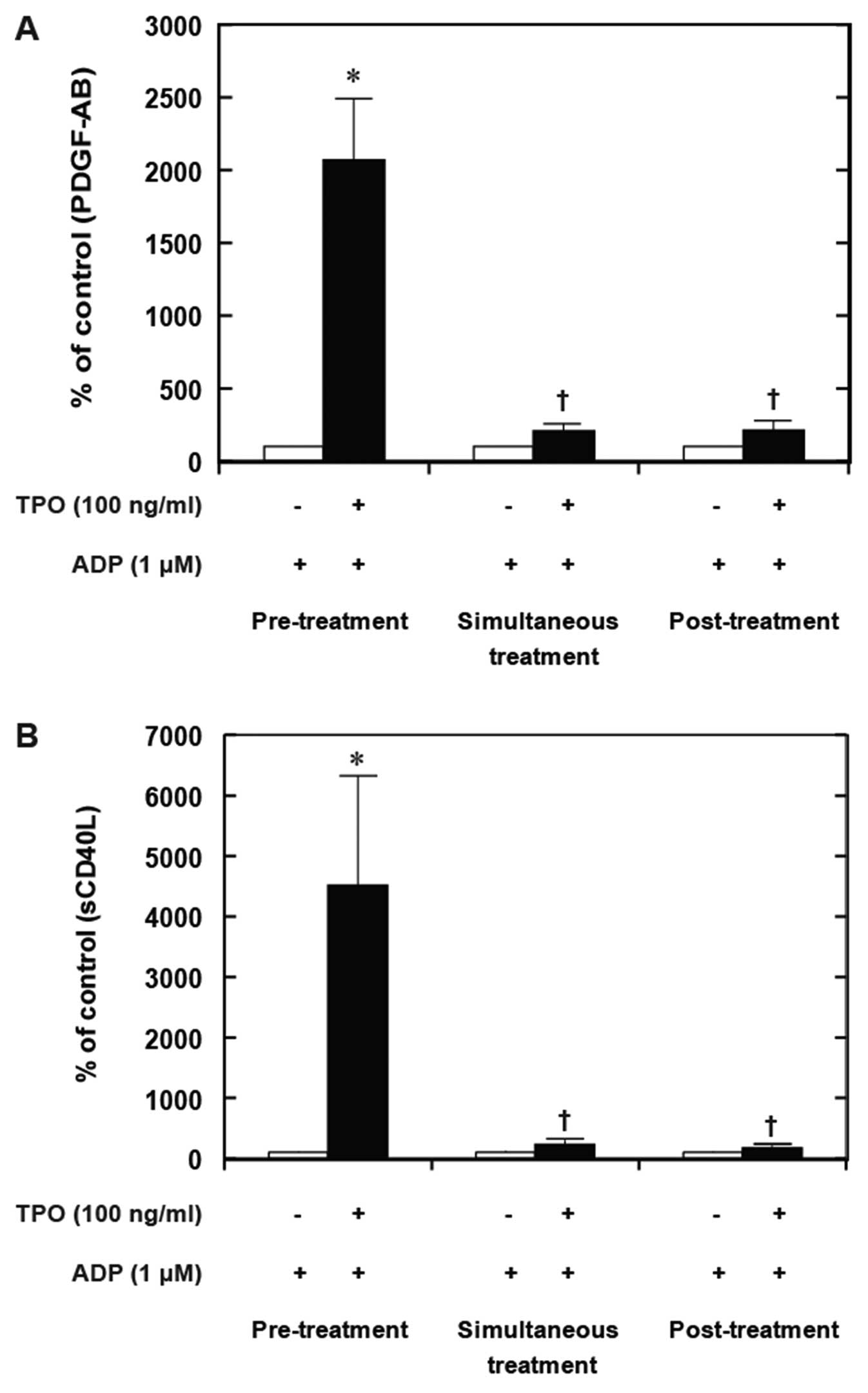
On the other hand, the simultaneous stimulation with
TPO and ADP did not further enhance the PDGF-AB secretion compared
to treatment with ADP alone (Fig.
6A). Additionally, post-treatment with TPO following
stimulation with ADP failed to amplify the ADP-induced release of
sCD40L (Fig. 6B).
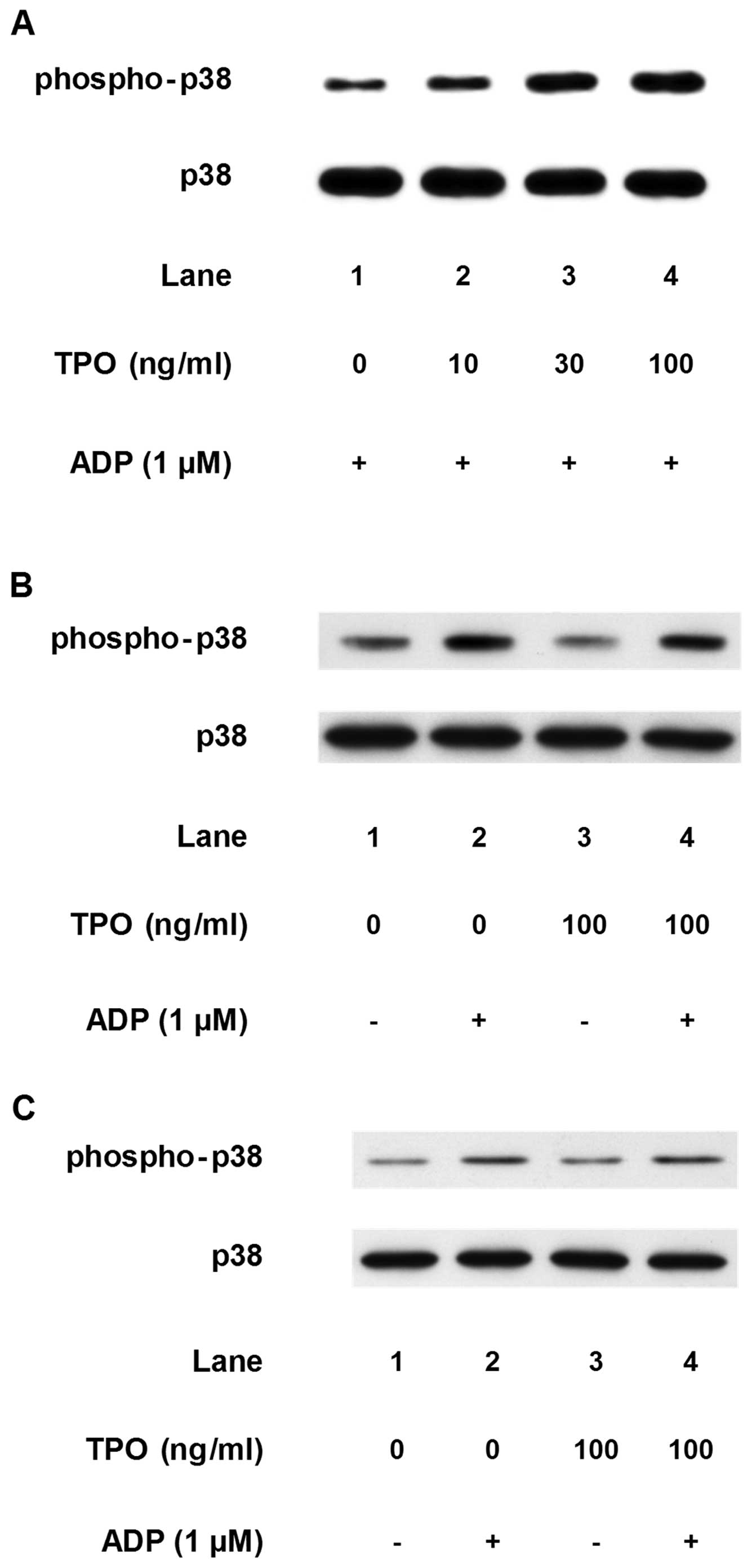
Effects of TPO on the ADP-induced
phosphorylation of p38 MAP kinase and HSP27 in human platelets
We have previously shown that ADP induces HSP27
phosphorylation via the activation of the p38 MAP kinase in human
platelets, resulting in the stimulation of PDGF-AB secretion and
the release of sCD40L (8).
Therefore, we examined the effect of pre-treatment with TPO on the
ADP-induced phosphorylation of p38 MAP kinase and HSP27.
Pre-treatment with TPO (between 10 and 100 mM), which on its own
had little effect on p38 MAP kinase phosphorylation,
dose-dependently enhanced the ADP-induced phosphorylation of p38
MAP kinase in a dose-dependent manner (Fig. 7A). However, as regards the
simultaneous stimulation with TPO and ADP or post-treatment with
TPO, the phosphorylated levels of p38 MAP kinase were similar to
those induced by treatment with ADP alone (Fig. 7B and C).
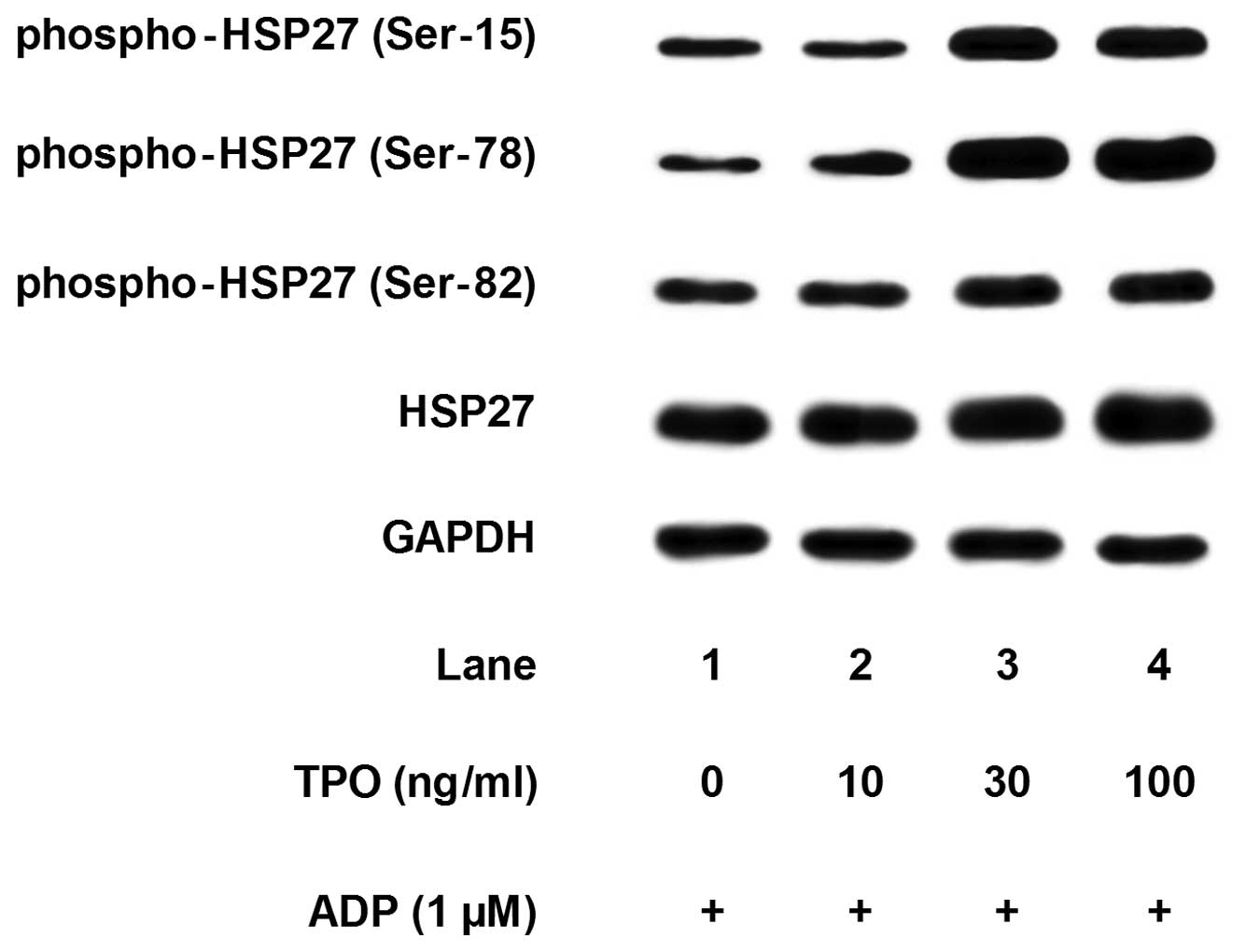
Pre-treatment with TPO, which alone had little
effect on the HSP27 phosphorylation (data not shown), markedly
enhanced the ADP-induced phosphorylation of HSP27 at three serine
residues (Ser-15, Ser-78 and Ser-82) (Fig. 8). The amplifying effects of TPO
(between 10 and 100 mM) were dose-dependent. On the contrary,
simultaneous treatment with TPO or post-treatment with TPO failed
to enhance the ADP-induced phosphorylation of HSP27 (data not
shown).
Discussion
In the present study, we demonstrate that only
pre-treatment with TPO, but not the simultaneous stimulation with
TPO and ADP, or post-treatment with TPO significantly enhances
ADP-induced platelet aggregation, PDGF-AB secretion from granules
and the release of sCD40L from human platelets. It has been
reported that TPO enhances the agonist-induced platelet aggregation
and the activation of various signaling pathways (10–14,17,20). We found that TPO significantly
amplified ADP-induced platelet activation even at a low dose of ADP
(1 mM). Therefore, our findings suggest the importance of
pre-treatment with TPO in the enhancement of ADP-induced human
platelet activation. Based on these results, it is possible that
TPO plays a preconditioning role, and acts synergistically with ADP
in human platelet activation.
We have previously demonstrated that ADP stimulates
the phosphorylation of HSP27 via the activation of p38 MAP kinase
in human platelets and that the ADP-induced phosphorylation of
HSP27 via the p38 MAP kinase pathway correlates with PDGF-AB
secretion and the release of sCD40L from human platelets (8). Thus, in this study, we examined the
effect of TPO administration on the phosphorylation of p38 MAP
kinase and HSP27. It has been reported that TPO amplifies the
ADP-stimulated activation of p38 MAP kinase in platelets (14). We found that pre-treatment with
TPO markedly enhanced the ADP-induced phosphorylation levels of
HSP27 at three serine residues (Ser-15, Ser-78 and Ser-82) in
addition to p38 MAP kinase in human platelets. However, the
simultaneous stimulation with TPO and ADP or post-treatment with
TPO failed to affect the ADP-induced phosphorylation of p38 MAP
kinase and HSP27. It appears that the PDGF-AB secretion and release
of sCD40L enhanced by TPO correlate with the enhanced
phosphorylation of p38 MAP kinase and HSP27. Based on these
findings, it is possible that amplification of the ADP-induced
platelet activation by pre-treatment with TPO is at least in part,
due to the upregulation of HSP27 phosphorylation via the p38 MAP
kinase pathway.
It is well recognized that activated platelets
result in degranulation, such as PDGF-AB secretion and the release
of a variety of agents, such as sCD40L. It is well known that
PDGF-AB is a potent growth factor which induces the proliferation
of vascular smooth muscle cells and plays a crucial role in the
development of atherosclerosis. On the other hand, the release of
sCD40L from platelets activates CD40 in vascular endothelial cells
and smooth muscle cells, and induces a variety of pro-inflammatory
and pro-atherogenic responses (21). Elevated sCD40L levels have been
observed in patients with acute coronary syndrome (22). sCD40L has been reported to
stimulate the release of inflammatory substances from dense
granules in human platelets (23). In the present study, we
demonstrated that TPO enhanced the PDGF-AB secretion and the
release of sCD40L from platelets activated by ADP. Thus, it is
possible that TPO acts as a pro-inflammatory mediator, resulting in
inflammation. It has been shown that serum TPO levels in patients
with serious diseases, such as sepsis, trauma and thrombocytopenia
are higher than normal (24–26). It has also been shown that in
patients with sepsis, serum TPO levels are closely associated with
the severity of the disease (25,26). Taking these findings into account,
it is possible that inflammatory agents, whose release is increased
from activated platelets by TPO in patients with these disorders,
may aggravate the pathological conditions. Thus, the blockade of
TPO-amplifying effects on platelet function seems to be crucial in
preventing the acceleration of pathological states. Further studies
are required to clarify the precise mechanism of action of TPO in
platelet activation.
In conclusion, the results from the present study
strongly suggest that pre-treatment with TPO amplifies ADP-induced
HSP27 phosphorylation via the p38 MAP kinase pathway in human
platelets.
Acknowledgements
We are very grateful to Yumiko
Kurokawa for her skillful technical assistance. This study was
supported in part by Grants-in-Aid for Scientific Research (nos.
20590565 and 20591825) from the Ministry of Education, Science,
Sports and Culture of Japan and Research Grants for Longevity
Sciences (22A-4) and Research on Proteomics from the Ministry of
Health, Labour and Welfare of Japan.
References
|
1
|
Kahner BN, Shankar H, Murugappan S, Prasad
GL and Kunapuli SP: Nucleotide receptor signaling in platelets. J
Thromb Haemost. 4:2317–2326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Delaney MK, O’Brien KA and Du X:
Signaling during platelet adhesion and activation. Arterioscler
Thromb Vasc Biol. 30:2341–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dangelmaier C, Jin J, Daniel JL, Smith JB
and Kunapuli SP: The P2Y1 receptor mediates ADP-induced p38
kinase-activating factor generation in human platelets. Eur J
Biochem. 267:2283–2289. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Falker K, Lange D and Presek P: ADP
secretion and subsequent P2Y12 receptor signalling play a crucial
role in thrombin-induced ERK2 activation in human platelets. Thromb
Haemost. 92:114–123. 2004.PubMed/NCBI
|
|
5
|
Stegner D and Nieswandt B: Platelet
receptor signaling in thrombus formation. J Mol Med (Berl).
89:109–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Y, O’Neill S, Saklatvala J, Tassi L
and Mendelsohn ME: Phosphorylated HSP27 associates with the
activation-dependent cytoskeleton in human platelets. Blood.
84:3715–3723. 1994.PubMed/NCBI
|
|
8
|
Kato H, Takai S, Matsushima-Nishiwaki R,
Adachi S, Minamitani C, Otsuka T, Tokuda H, Akamatsu S, Doi T,
Ogura S and Kozawa O: HSP27 phosphorylation is correlated with
ADP-induced platelet granule secretion. Arch Biochem Biophys.
475:80–86. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ellis MH, Avraham H and Groopman JE: The
regulation of megakaryocytopoiesis. Blood Rev. 9:1–6. 1995.
View Article : Google Scholar
|
|
10
|
Ezumi Y, Takayama H and Okuma M:
Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of
Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in
platelets in vitro. FEBS Lett. 374:48–52. 1995. View Article : Google Scholar
|
|
11
|
Kojima H, Hamazaki Y, Nagata Y, Todokoro
K, Nagasawa T and Abe T: Modulation of platelet activation in vitro
by thrombopoietin. Thromb Haemost. 74:1541–1545. 1995.PubMed/NCBI
|
|
12
|
Rodriguez-Linares B and Watson SP:
Thrombopoietin potentiates activation of human platelets in
association with JAK2 and TYK2 phosphorylation. Biochem J.
316:93–98. 1996.PubMed/NCBI
|
|
13
|
Kubota Y, Arai T, Tanaka T, Yamaoka G,
Kiuchi H, Kajikawa T, Kawanishi K, Ohnishi H, Yamachi M, Takahara J
and Iriono S: Thrombopoietin modulates platelet activation in vitro
through protein-tyrosine phosphorylation. Stem Cells. 14:439–444.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ezumi Y, Nishida E, Uchiyama T and
Takayama H: Thrombopoietin potentiates agonist-stimulated
activation of p38 mitogen-activated protein kinase in human
platelets. Biochem Biophys Res Commun. 261:58–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kojima H, Shinagawa A, Shimizu S, Kanada
H, Hibi M, Hirano T and Nagasawa T: Role of phosphatidylinositol-3
kinase and its association with Gab1 in thrombopoietin-mediated
up-regulation of platelet function. Exp Hematol. 29:616–622. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Herceg-Harjacek L, Groopman JE and
Grabarek J: Regulation of platelet activation in vitro by the c-Mpl
ligand, thrombopoietin. Blood. 86:4054–4062. 1995.PubMed/NCBI
|
|
17
|
Oda A, Miyakawa Y, Druker BJ, Ozaki K,
Yabusaki K, Shirasawa Y, Handa M, Kato T, Miyazaki H, Shimosaka A
and Ikeda Y: Thrombopoietin primes human platelet aggregation
induced by shear stress and by multiple agonists. Blood.
87:4664–4670. 1996.PubMed/NCBI
|
|
18
|
Eilers M, Schulze H, Welte K and Ballmaier
M: Thrombopoietin acts synergistically on Ca(2+) mobilization in
platelets caused by ADP or thrombin receptor agonist peptide.
Biochem Biophys Res Commun. 263:230–238. 1999.
|
|
19
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wun T, Paglieroni T, Hammond WP,
Kaushansky K and Foster DC: Thrombopoietin is synergistic with
other hematopoietic growth factors and physiologic platelet
agonists for platelet activation in vitro. Am J Hematol.
54:225–232. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Henn V, Slupsky JR, Grafe M,
Anagnostopoulos I, Forster R, Muller-Berghaus G and Kroczek RA: D40
ligand on activated platelets triggers an inflammatory reaction of
endothelial cells. Nature. 391:591–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heeschen C, Dimmeler S, Hamm CW, van den
Brand MJ, Boersma E, Zeiher AM and Simooms ML: Soluble CD40 ligand
in acute coronary syndromes. N Engl J Med. 348:1104–1111. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inwald DP, McDowall A, Peters MJ, Callard
RE and Klein NJ: CD40 is constitutively expressed on platelets and
provides a novel mechanism for platelet activation. Circ Res.
92:1041–1048. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaushansky K: Thrombopoietin. N Engl J
Med. 339:746–754. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zakynthinos SG, Papanikolaou S,
Theodoridis T, Zakynthinos EG, Christopoulou-Kokkinou V, Katsaris G
and Mavrommatis AC: Sepsis severity is the major determinant of
circulating thrombopoietin levels in septic patients. Crit Care
Med. 32:1004–1010. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lupia E, Bosco O, Mariano F, Dondi AE,
Goffi A, Spatola T, Cuccurullo A, Tizzani P, Brondino G, Stella M
and Montrucchio G: Elevated thrombopoietin in plasma of burned
patients without and with sepsis enhances platelet activation. J
Thromb Haemost. 7:1000–1008. 2009. View Article : Google Scholar : PubMed/NCBI
|