Introduction
Cell deviation from the canonical cell cycle
constitutes a critical event in the development of cancer, and the
dysregulation of cell proliferation may result in colonic and
rectal tumorigenesis (1,2). It is known that cancer is most
likely to be caused by the deregulation of transcription factors
that affect cell fate and proliferation (3,4).
Therefore, it is worthwhile to explore the transcription factors
involved in the proliferation of colorectal adenocarcinoma cells
and the underling mechanisms, so as to gain important insight into
effective therapeutic strategies.
Sex determining region Y-box 2 (SOX2), which belongs
to group B of the SOX family, is a high mobility group box
transcription factor involved in the maintenance of pluripotency
and the self-renewal of embryonic and neuronal stem cells (5,6).
SOX2, as well as other SOX family factors, plays a critical role in
cell fate determination, differentiation and proliferation
(7–9). Recent studies have reported that the
anomalous expression of SOX2 is associated with cell proliferation
in several types of human cancer (5,10–13). For instance, SOX2 has been shown
to promote cell proliferation in lung and esophageal squamous cell
carcinomas (5,10,11). However, it has also been shown to
inhibit cell proliferation in gastric cancers (13). To date, the effect of SOX2 on the
proliferation of colorectal adenocarcinoma cells and the underlying
mechanisms remain unclear.
The mammalian target of rapamycin (mTOR) is an
evolutionarily conserved Ser/Thr kinase that belongs to the family
of the phosphatidylinositol 3-kinase-related kinases (PIKKs)
(14). It is upregulated in many
types of cancer, including colorectal adenocarcinoma, contributing
to the dysregulation of cell proliferation, growth, differentiation
and survival (15). mTOR forms
two complexes, termed mTOR complex 1 (mTORC1) and complex 2
(mTORC2). mTORC1 elicits its pleiotropic function mainly through
controlling protein synthesis through the phosphorylation of the
eukaryotic initiation factor 4E-binding protein 1 (4E-BP1), p70
ribosomal S6 kinase 1 (S6K1) and ribosomal protein S6 (S6)
(16,17). Its activity is sensitive to
rapamycin. mTORC2, which phosphorylates (Ser473) and activates Akt,
is known to be rapamycin-insensitive (14,18). A number of recent studies have
suggested that many factors affect the proliferation of tumor cells
through the regulation of mTOR activity (19–22). However, little information is
available on the effect of SOX2 on the mTOR pathway.
In this study, in order to elucidate the role of
SOX2 on the proliferation of human colorectal adenocarcinoma cells,
we performed cell proliferation assay using three cell lines,
HT-29, SW480 and LoVo, after which we selected the HT-29 cells for
further study. Firstly, we investigated whether the mTOR pathway is
involved in the SOX2-induced inhibition of HT-29 cell
proliferation. We also assessed the effects of SOX2 on the cell
cycle progression of HT-29 cells. Moreover, we examined the
expression of SOX2 and downstream targets of the mTOR pathway in
colorectal adenocarcinoma tissues.
Materials and methods
Cell lines and tissue samples
The colorectal adenocarcinoma cell lines, HT-29,
SW480 and LoVo, were provided by the Institute of Basic Medical
Sciences, Qilu Hospital of Shandong University, Jinan, China. The
cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum (FBS) and cultured in a
37°C humidified atmosphere containing 95% air and 5%
CO2. A total of 101 patients with colorectal
adenocarcinoma were selected from the Department of General
Surgery, Qilu Hospital of Shandong University, between October 2010
and April 2012. None of the patients had received pre-operative
adjuvant therapy after being diagnosed with colorectal
adenocarcinoma. The colorectal adenocarcinoma tissues were
subpackaged and frozen at −80°C immediately after surgical removal
and maintained at −80°C for future use. Informed consent was
obtained from each patient, and tissues were collected using
protocols approved by the Ethics Committee of Shandong University.
Histological grading was performed according to the WHO criteria
and staging was based on the TNM classification system, in
affiliation with the International Union against Cancer.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was extracted from the tumor tissues and
cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total
RNA (1 μg) was used for reverse transcription using a
SuperScript kit (Toyobo, Osaka, Japan). All real-time PCR analyses
were performed in triplicate using LightCycler®
FastStart DNA Master SYBR-Green I (Roche Diagnostics, Mannheim,
Germany) on a Roche LightCycler 2.0. β-actin as used the internal
control. The primer sequences were as follows: SOX2,
5′-CATGCACCGCTACGACGTGAG-3′ (forward) and
5′-TGGGAGGAAGAGGTAACCACAGG-3′ (reverse); cyclin D1,
5′-CCGTCCATGCGGAAGATC-3′ (forward) and 5′-ATGGCCAGCGGGAAGAC-3′
(reverse); β-actin, 5′-TGA CGTGGACATCCGCAAAG-3′ (forward) and
5′-CTGGAAGGTGGACAGCGAGG-3′ (reverse). We analyzed the results using
LightCycler software version 4.0 (Roche Diagnostics).
Plasmid DNA and transfection
PCMV-HA-SOX2, the eukaryotic expression plasmid
encoding SOX2, was kindly provided by Dr Dongshi Guan from the
Department of General Surgery, Provincial Hospital Affiliated to
Shandong University. The colorectal adenocarcinoma cells were
transfected with PCMV-HA-SOX2 and PCMV-HA using Lipofectamine 2000
(Invitrogen) at the optimal multiplicity of infection (MOI)
according to the manufacturer’s instructions. The messenger RNA
(mRNA) and protein expression of SOX2 was characterized using
qRT-PCR and western blot analysis.
Cell proliferation assay
HT-29, SW480 and LoVo cells were plated at
2×103 cells/well in 96-well plates. Following overnight
incubation, the cells were transfected with PCMV-HA-SOX2 or PCMV-HA
using Lipofectamine 2000 at the optimal MOI. The culture medium was
changed to DMEM with 5% FBS at 4-6 h after transfection. Cell
proliferation was evaluated on days 1, 3 and 5 after transfection
using the Cell Counting kit-8 (CCK-8) (Dojindo Laboratories,
Kumamoto, Japan) at 450 and 630 nm dual wavelengths. The
experiments were performed in duplicate and repeated three times.
All operations were performed according to the manufacturer’s
instructions.
Western blot analysis
The HT-29 cells were plated at 7×104
cells/well in 24-well plates. Cell lysates were harvested at 48 and
72 h after transfection with PCMV-HA-SOX2 or PCMV-HA. Tumor tissues
were resuspended in ice-cold buffer for lysis. The lysates were
then resolved in sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto nitrocellulose
membranes. Proteins were detected with specific antibodies and
revealed using the enhanced chemiluminescence (ECL) kit (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The primary antibodies
specific for SOX2, cyclin D1, phospho-mTOR (S2448), phospho-4E-BP1
(T37/46), phospho-S6K1 (T389), phospho-S6 (S235/236) and
phospho-Akt (S473) were purchased from Cell Signaling Technology,
Inc. (Beverly, MA, USA). The primary antibodies specific for mTOR,
4E-BP1, S6K1, S6 and Akt were purchased from Santa Cruz
Biotechnology, Inc. β-actin antibody was purchased from Bioworld
Technology, Inc. (St. Louis Park, MN, USA).
Cell cycle analysis
HT-29 cells were plated at 7×104
cells/well in 24-well plates. At 48 and 72 h after transfection
with PCMV-HA-SOX2 or PCMV-HA, the cells were harvested and fixed
with 70% ethanol overnight at 4°C. The following day, the cells
were resuspended in phosphate-buffered saline (PBS) containing 50
μg/ml propidium iodide and 10 μg/ml RNase A, and
incubated for 30 min at room temperature in the dark. We measured
the cell cycle progression using flow cytometry (Becton-Dickinson,
San Jose, CA, USA). The percentages of cells in the G0/G1, S and
G2/M phases were analyzed using FCS Express softwarea and ModFit
LT.
Immunohistochemical analysis
Resected fresh tissues from 67 patients with
colorectal adenocarcinoma were formalin-fixed and
paraffin-embedded, and cut into 4-μm-thick sections using a
microtome. Following deparaffinization, antigen retrieval was
performed in 10 mM sodium citrate buffer and endogenous peroxidase
was eliminated using 3% H2O2. All sections
were then blocked with goat serum and incubated with specific
antibodies overnight at 4°C. The primary antibodies used were
anti-SOX2, phospho-S6 (S235/236), phospho-Akt (S473) and cyclin D1
(Cell Signaling Technology, Inc.). The following day, horseradish
peroxidase conjugated IgG and 3,3-diaminobenzidine solution (Vector
Laboratories, Burlingame, CA, USA) were used to visualize the
antibody binding. All sections were counterstained with
hematoxylin. After dehydration and mounting with neutral gummi, the
results were observed under a microscope.
The immunoreactivity in the carcinoma tissues was
graded semiquantitatively according to the intensity and the
percentage of positive cells. The intensity of the samples was
scored as 0, no staining; 1, weak staining; 2, moderate staining;
or 3, strong staining. The percentage of positive cells was scored
as 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; or 4, >75%. The
sum of the score was then graded as -, 0–1; +, 2–3; ++, 4–5; or
+++, 6–7. We defined ‘-’ as negative, and ‘+, ++ and +++’ as
positive.
Statistical analysis
The SPSS software package (version 13.0; SPSS Inc.,
Chicago, IL, USA) was used for all statistical analyses.
Correlations of SOX2 protein expression with clinicopathological
factors were examined using the χ2 test. Spearman’s rank
correlation coefficient test was performed to assess the
correlation between SOX2 and cyclin D1. Other data from experiments
were analyzed using the Student’s t-test or analysis of variance
(ANOVA) where appropriate. Data are presented as the means ±
standard deviation (SD) of more than three separate experiments. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
SOX2 inhibits the proliferation of
colorectal adenocarcinoma cells
To evaluate the effect of SOX2 on the proliferation
of colorectal adenocarcinoma cells, the colorectal adenocarcinoma
cells (HT-29, SW480 and LoVo) were transiently transfected with
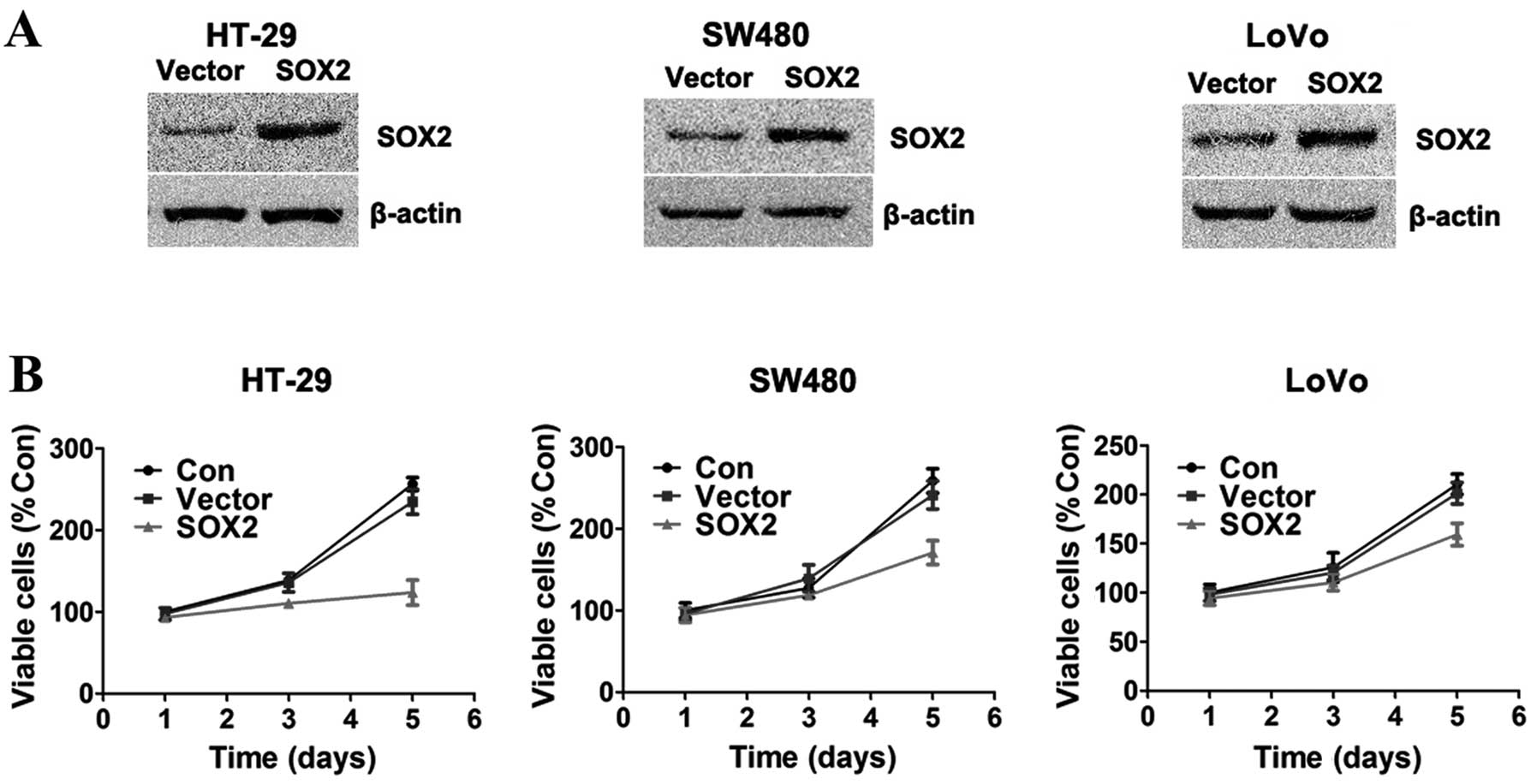
PCMV-HA-SOX2 or PCMV-HA. The expression of SOX2 was confirmed by
western blot analysis (Fig. 1A).
Cell proliferation was evaluated on days 1, 3 and 5 following
transfection with CCK-8. Cell growth curves were made based on the
acquired data (Fig. 1B). As
demonstrated by our results, SOX2-overexpressing cells showed
significant growth inhibition compared to the control cells, in all
three colorectal adenocarcinoma cell lines (all at P<0.05).
Moreover, the inhibitory effect of SOX2 was most obvious in the
HT-29 cells; thus, we selected the HT-29 cells for further
experiments.
SOX2 inhibits the mTOR pathway in HT-29
cells
To investigate whether the mTOR pathway is involved
in the SOX2-induced inhibition of proliferation of HT-29 cells, we
first assessed the phosphorylation status of mTOR (S2448). The
results of western blot analysis indicated that SOX2 decreased the
phosphorylation of mTOR (S2448) (Fig.
2A and B). We then examined whether the activity of mTORC1 and
mTORC2 was inhibited by SOX2. The western blot analysis results
revealed that SOX2 not only inhibited the mTORC1-induced
phosphorylation of S6K1 (T389), 4EBP1 (T37/46) and S6 (S235/236),
but also decreased the mTORC2-induced phosphorylation of Akt at
position Ser473 in the HT-29 cells (Fig. 2C and D). These results revealed
that SOX2 inhibited the mTOR pathway in the HT-29 cells.
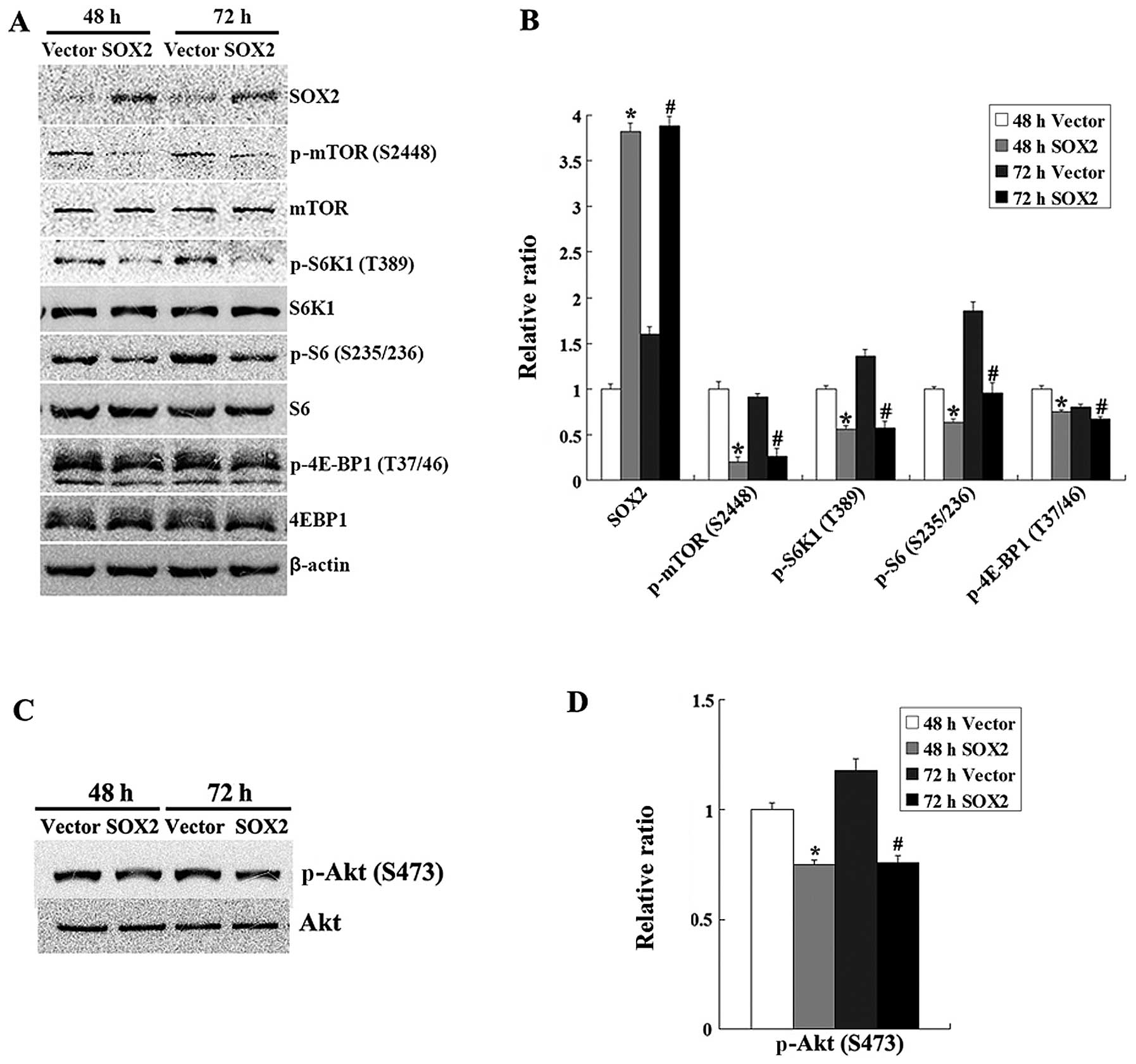 | Figure 2.SOX2 inhibits the mTOR signaling
pathway in HT-29 cells. (A) HT-29 cells were transfected with
PCMV-HA-SOX2 or PCMV-HA. At 48 and 72 h after transfection, the
cells were harvested and western blot analysis was performed to
determine the levels of mTOR, phospho-mTOR (p-mTOR; S2448), S6K1,
phospho-S6K1 (p-S6K1; T389), S6, phospho-S6 (p-S6; S235/236),
4E-BP1, phospho-4E-BP1 (p-4E-BP1; T37/46), Akt and phospho-Akt
(p-Akt; S473). Data are representative of three independent
experiments. (B) Relative ratio of data in (A). The bar graph
represents the mean ± SD of the density (n=3, *P<0.05
vs. control cells at 48 h; #P<0.05 vs. control cells
at 72 h). (C) The level of phospho-Akt (S473) was determined by
western blot analysis. (D) Relative ratio of data in (C). The bar
graph represents the mean ± SD of the density (n=3,
*P<0.05 vs. control cells at 48 h;
#P<0.05 vs. control cells at 72 h). |
SOX2 downregulates cyclin D1 expression
and induces cell cycle arrest at the G0/G1 phase in HT-29
cells
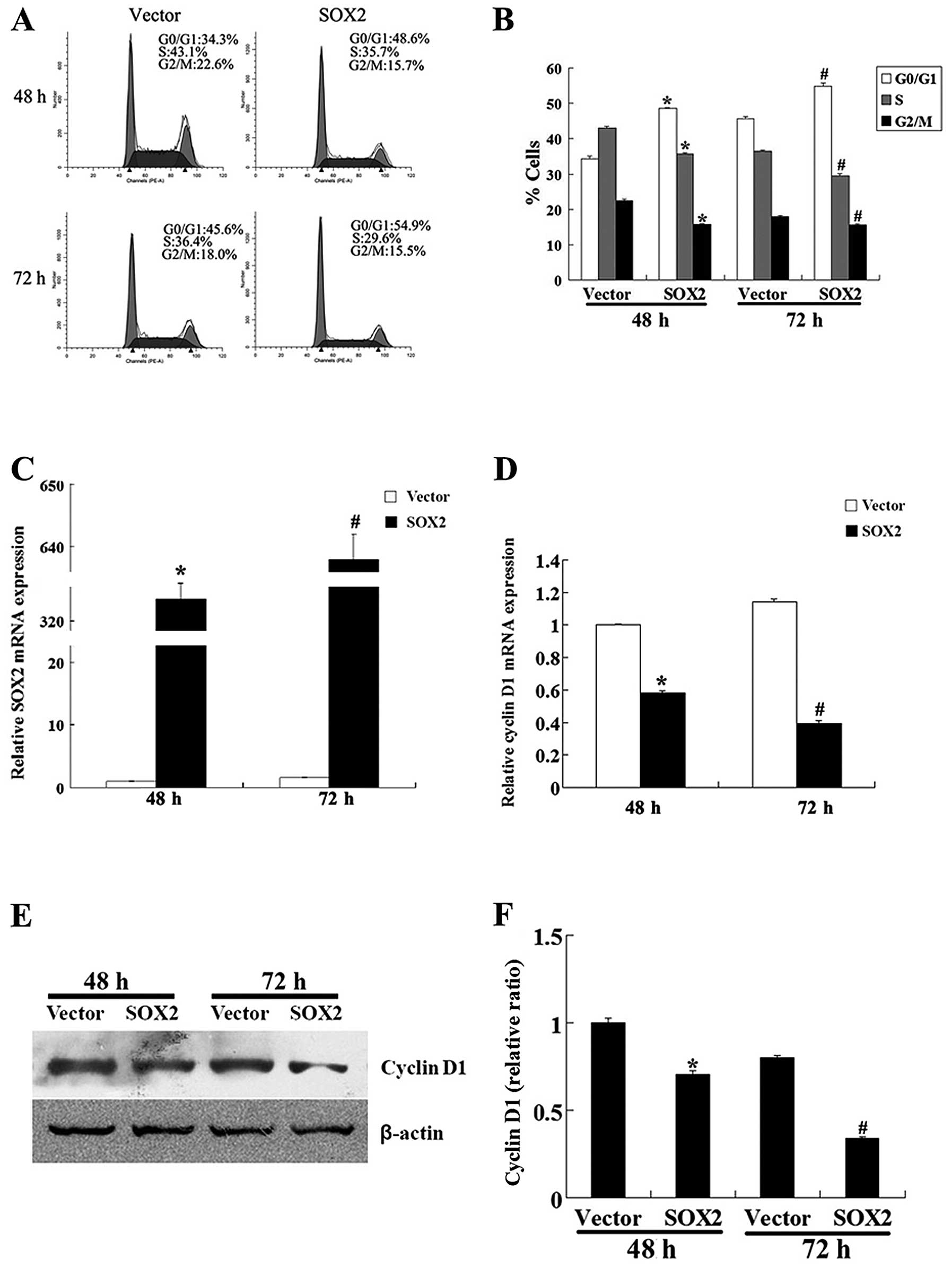
To clarify the mechanism underlying the growth
inhibitory effect of SOX2 on HT-29 cells, we investigated the cell
cycle progression of HT-29 cells using flow cytometry. At 48 h
after transfection, 48.6% of the SOX2-overexpressing cells were in
the G0/G1 phase, while a much lower proportion of the control cells
(34.3%) was in this phase. Similarly, at 72 h after transfection,
54.9% of the SOX2-overexpressing cells and 45.6% of the control
cells were in the G0/G1 phase. The SOX2-overexpressing cells showed
lower proportions of cells in the S and G2/M phase compared to the
control cells (Fig. 3A and
B).
Furthermore, the results from qRT-PCR analysis
revealed that the mRNA expression level of cyclin D1 (a key factor
for the transition of the G0/G1-S phase and whose expression is
controlled by mTOR) was significantly decreased in the
SOX2-overexpressing cells (Fig. 3C
and D). The results of western blot analysis also demonstrated
that the protein expression level of cyclin D1 was significantly
decreased in the SOX2-overexpressing cells compared to the control
cells (Fig. 3E and F). All these
results indicate that SOX2 inhibits the proliferation of HT-29
cells by downregulating cyclin D1 and arresting the cells at the
G0/G1 phase.
Significant negative correlation between
the expression of SOX2 and tumor size and phospho-S6 (S235/236),
phospho-Akt (S473) and cyclin D1 levels in colorectal
adenocarcinoma tissues
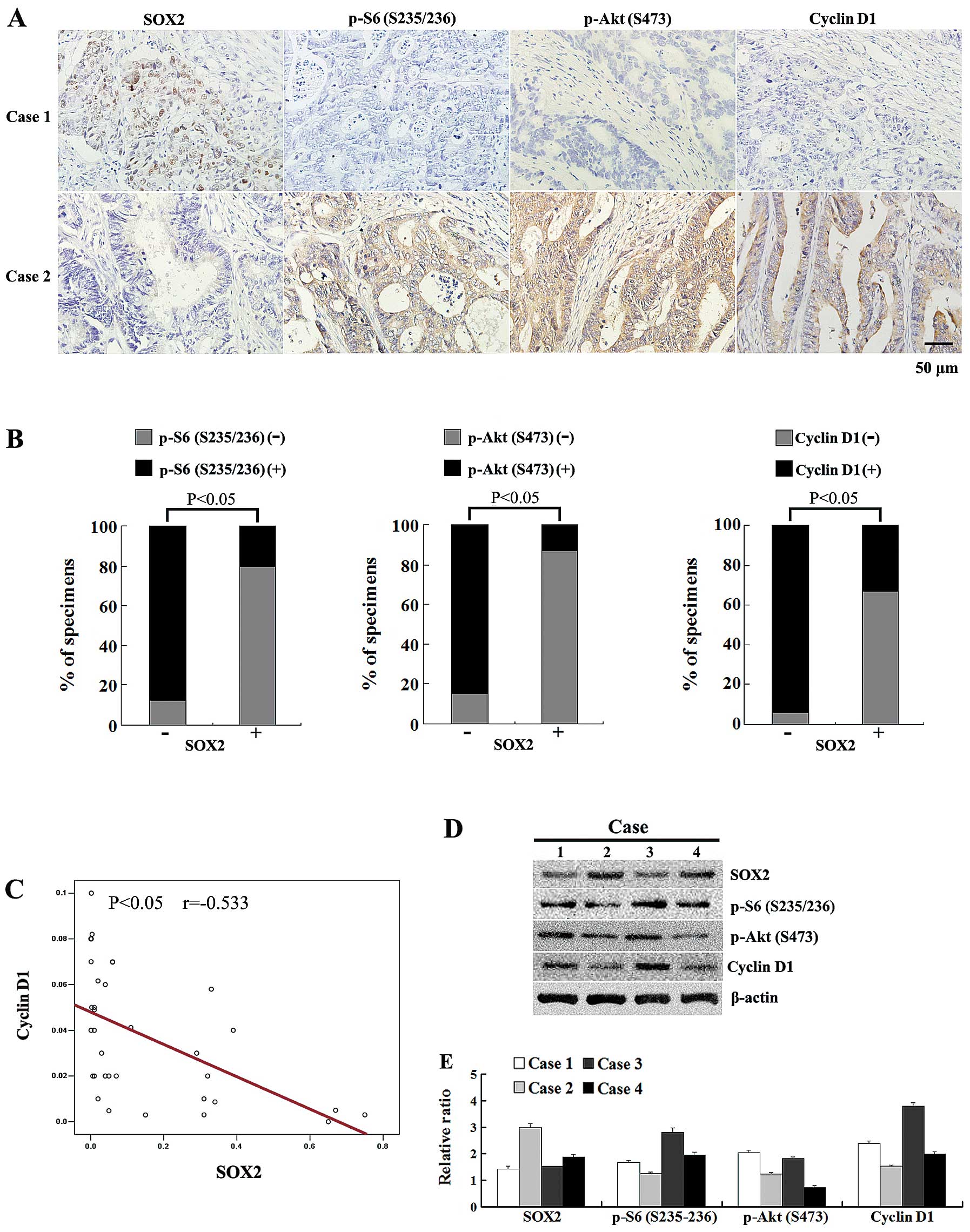
To further determine whether SOX2 affects tumor cell
proliferation through the mTOR pathway, we compared the expression
of SOX2, phospho-S6 (S235/236), phospho-Akt (S473) and cyclin D1 by
the immunohistochemical analysis of 67 colorectal adenocarcinoma
tissues. The results revealed that SOX2 was only detected in 15
sample tissues (22.4%), in which SOX2 was mainly localized in the
nuclei of the cells (Fig. 4A).
The expression of SOX2 significantly correlated with a small tumor
size (P<0.05) (Table I). By
contrast, no correlation was observed between SOX2 expression and
basal clinicopathological characteristics, such as gender, age,
histological grade, tumor stage and lymph node metastasis
(P>0.05) (Table I).
Immunohistochemical staining revealed that phospho-S6 (S235/236),
phospho-Akt (S473) and cyclin D1 expression was detected in 49, 47
and 54 sample tissues, respectively; expression was observed in the
cytoplasm of the tumor cells. SOX2 levels showed a significant
negative correlation with the phospho-S6 (S235/236), phospho-Akt
(S473) and cyclin D1 expression in colorectal adenocarcinoma
tissues (P<0.05) (Fig. 4A and
B).
 | Table I.Correlation of SOX2 expression with
clinicopathological characteristics in patients with colorectal
adenocarcinoma. |
Table I.
Correlation of SOX2 expression with
clinicopathological characteristics in patients with colorectal
adenocarcinoma.
| Variables | No. of patients | SOX2
| P-value |
|---|
| Negative (−) | Positive (+, ++,
+++) |
|---|
| | | | |
| Age (years) | | | | 0.281 |
| <50 | 35 | 29 | 6 | |
| ≥50 | 32 | 23 | 9 | |
| Gender | | | | 0.401 |
| Male | 43 | 32 | 11 | |
| Female | 24 | 20 | 4 | |
| Tumor size | | | | 0.008 |
| <5 cm | 25 | 15 | 10 | |
| ≥5 cm | 42 | 37 | 5 | |
| Histological
grade | | | | 0.256 |
|
Well/moderate | 48 | 39 | 9 | |
| Poor | 19 | 13 | 6 | |
| Tumor grade | | | | 0.972 |
| 1/2 | 36 | 28 | 8 | |
| 3/4 | 31 | 24 | 7 | |
| Lymph node
metastasis | | | | 0.533 |
| Negative | 53 | 42 | 11 | |
| Positive | 14 | 10 | 4 | |
We further used qRT-PCR and western blot analysis to
examine the expression of SOX2 and the downstrem targets of mTOR in
colorectal adenocarcinoma tissues. The qRT-PCR results indicated
that in another 34 colorectal adenocarcinoma tissues, the mRNA
expression levels of SOX2 and cyclin D1 showed a significant
negative correlation (r=−0.533, P<0.05) (Fig. 4C). Similarly, the results of
western blot analysis revealed a significant negative correlation
between SOX2 expression levels and phospho-S6 (S235/236),
phospho-Akt (S473) and cyclin D1 expression in several tumor
tissues; this correlation was consistent with that observed by
immunohistochemical analysis (Fig. 4D
and E). Taken together, these results demonstrate that SOX2
levels negatively correlate with mTOR signaling in human colorectal
adenocarcinoma.
Discussion
SOX2, a high mobility group box transcription
factor, plays a critical role in tumor cell proliferation. In
gastric cancer, SOX2 has been shown to suppress cell proliferation
by inducing cell-cycle arrestment (13). In lung squamous cell carcinoma,
SOX2 has been shown to promote cell proliferation by affecting key
regulators of the cell cycle (10). The contradictory effect of SOX2 in
gastric cancer and lung squamous cell carcinoma suggests that SOX2
plays a differential role in adenocarcinomas and squamous cell
carcinomas. In the current study, we demonstrate that SOX2
effectively inhibits the proliferation of colorectal adenocarcinoma
cells. Our results are consistent with those of a previous study,
demonstrating the effects of SOX2 in gastric cancer (13). However, in contrast to our
results, another previous study reported that SOX2 promoted the
growth of SW620 colorectal cancer cells (23). The reason for these different
results may be the different cell lines and experimental methods
used, such as reaction temperature and time; thus, we performd
further experiments to confirm our results.
To further determine the effects of SOX2 on HT-29
cell proliferation, we performed western blot analysis. The results
showed that the mTOR pathway was inhibited by SOX2 in the HT-29
cells. Generally, the mTOR pathway plays a central role in the
regulation of cell growth and proliferation. Hay and Sonenberg
(14) suggested that mTOR
contributes to cancer development through its effect on cell cycle
progression. mTOR mediates the cell cycle progression partly
through increasing the translation of cyclin D1 mRNA and other
regulators of G0/G1-S phase progression (14,24). In certain cell types, the
inhibition of the mTOR pathway induces G0/G1 phase cell cycle
arrest, which correlates with the downregulation of cyclin D1
levels (25–28). In our study, flow cytometric
analysis revealed that the cell cycle of SOX2-overexpressing cells
was arrested at the G0/G1 phase. Cyclin D1, an important regulator
of G0/G1-S phase celly cycle progression in many cell types, is
often overexpressed in cancer cells and plays a critical role in
the development and progression of many types of cancer, including
colorectal adenocarcinoma (29).
We performed qRT-PCR and western blot analysis, and found that the
mRNA and protein expression level of cyclin D1 was significantly
reduced in the SOX2-overexpressing cells, which indicated that SOX2
downregulated cyclin D1 expression in the HT-29 cells. Cyclin D1 is
also known as a key factor whose expression is controlled by mTOR.
The data presented above suggest that SOX2 inhibits the
proliferation of colorectal adenocarcinoma cells, possibly by
downregulating cyclin D1 expression via the mTOR pathway. On the
basis of the aforementioned data, SOX2 may be a potential target
for the inhibition of the proliferation of colorectal
adenocarcinoma cells.
However, a causal link of SOX2 with mTOR signaling
in cancers has not yet been reported. To examine whether SOX2
levels correlate with mTOR signaling in human colorectal
adenocarcinoma, we carried out a series of experiments to explore
the association of SOX2 and the mTOR pathway in colorectal
adenocarcinoma tissues. Immunostaining analysis revealed that SOX2
was largely absent in the colorectal adenocarcinoma tissues, which
is consistent with the notion that SOX2 is highly expressed in
squamous cell carcinomas, but sparsely expressed in adenocarcinomas
(30). Based on the
clinicopathological analysis of SOX2, we reported a significant
negative correlation between the expression of SOX2 and tumor size.
The study by Gontan et al (31) also reported that
SOX2-overexpressing lungs are reduced in size, which is possibly
due to the slight reduction in cell proliferation. Of note,
immunostaining and western blot analyses revealed that SOX2
negatively correlated with phospho-S6 (S235/236), phospho-Akt
(S473) and cyclin D1 in the colorectal adenocarcinoma tissues,
which is in accordance with our results obtained in vitro.
The qRT-PCR results revealed a significant negative correlation
between SOX2 and cyclin D1 at the mRNA level in the colorectal
adenocarcinoma tissues. Taken together, these data further suggest
that SOX2 inhibits the proliferation of colorectal adenocarcinoma
cells through the mTOR pathway.
In conclusion, to our knowledge, our study
demonstrates for the first time that SOX2 exerts potential
anti-proliferative effects on colorectal adenocarcinoma cells
through the mTOR pathway. We also indicate that the inhibition of
the mTOR pathway by the overexpression of SOX2 may contribute to
the downregulation of cyclin D1 in colorectal adenocarcinoma cells.
These findings confirm the important role of SOX2 in human
colorectal adenocarcinoma, and may aid in the understanding of the
molecular mechanisms behind the anti-proliferative effects of SOX2
in colorectal adenocarcinoma. Therefore, SOX2 can be considered a
potential novel therapeutic agent for the treatment of colorectal
adenocarcinoma.
Acknowledgements
The authors would like to thank Shuhai
Li (Department of Thoracic Surgery, Qilu Hospital, Shandong
University, Jinan, China) for his technical assistance. This study
was supported by grants from the National Natural Science
Foundation of China (no. 30672010); the Shandong Province Natural
Science Foundation of China (nos. ZR2010H004 and ZR2009CQ038) and
the National Key Clinical Medical Specialties Foundation.
References
|
1.
|
Sherr CJ: The Pezcoller lecture: cancer
cell cycles revisited. Cancer Res. 60:3689–3695. 2000.PubMed/NCBI
|
|
2.
|
Sancho E, Batlle E and Clevers H:
Signaling pathways in intestinal development and cancer. Annu Rev
Cell Dev Biol. 20:695–723. 2004. View Article : Google Scholar
|
|
3.
|
Ruiz i Altaba A, Sanchez P and Dahmane N:
Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat
Rev Cancer. 2:361–372. 2002.PubMed/NCBI
|
|
4.
|
Singh G, Singh SK, Konig A, et al:
Sequential activation of NFAT and c-Myc transcription factors
mediates the TGF-beta switch from a suppressor to a promoter of
cancer cell proliferation. J Biol Chem. 285:27241–27250. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ben-Porath I, Thomson MW, Carey VJ, et al:
An embryonic stem cell-like gene expression signature in poorly
differentiated aggressive human tumors. Nat Genet. 40:499–507.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Schmitz M, Temme A, Senner V, et al:
Identification of SOX2 as a novel glioma-associated antigen and
potential target for T cell-based immunotherapy. Br J Cancer.
96:1293–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wegner M: From head to toes: the multiple
facets of Sox proteins. Nucleic Acids Res. 27:1409–1420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kamachi Y, Uchikawa M and Kondoh H:
Pairing SOX off: with partners in the regulation of embryonic
development. Trends Genet. 16:182–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wilson M and Koopman P: Matching SOX:
partner proteins and co-factors of the SOX family of
transcriptional regulators. Curr Opin Genet Dev. 12:441–446. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hussenet T, Dali S, Exinger J, et al: SOX2
is an oncogene activated by recurrent 3q26.3 amplifications in
human lung squamous cell carcinomas. PLoS One. 5:e89602010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Bass AJ, Watanabe H, Mermel CH, et al:
SOX2 is an amplified lineage-survival oncogene in lung and
esophageal squamous cell carcinomas. Nat Genet. 41:1238–1242. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chen Y, Shi L, Zhang L, et al: The
molecular mechanism governing the oncogenic potential of SOX2 in
breast cancer. J Biol Chem. 283:17969–17978. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Otsubo T, Akiyama Y, Yanagihara K and
Yuasa Y: SOX2 is frequently downregulated in gastric cancers and
inhibits cell growth through cell-cycle arrest and apoptosis. Br J
Cancer. 98:824–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar
|
|
15.
|
Rosenwald IB, Chen JJ, Wang S, Savas L,
London IM and Pullman J: Upregulation of protein synthesis
initiation factor eIF-4E is an early event during colon
carcinogenesis. Oncogene. 18:2507–2517. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Burnett PE, Barrow RK, Cohen NA, Snyder SH
and Sabatini DM: RAFT1 phosphorylation of the translational
regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA.
95:1432–1437. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Brunn GJ, Hudson CC, Sekulic A, et al:
Phosphorylation of the translational repressor PHAS-I by the
mammalian target of rapamycin. Science. 277:99–101. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Easton JB and Houghton PJ: mTOR and cancer
therapy. Oncogene. 25:6436–6446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Sengupta S, Peterson TR and Sabatini DM:
Regulation of the mTOR complex 1 pathway by nutrients, growth
factors, and stress. Mol Cell. 40:310–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bai X and Jiang Y: Key factors in mTOR
regulation. Cell Mol Life Sci. 67:239–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liu J, Li M, Song B, et al: Metformin
inhibits renal cell carcinoma in vitro and in vivo xenograft. Urol
Oncol. 31:264–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wen ZH, Su YC, Lai PL, et al: Critical
role of arachidonic acid-activated mTOR signaling in breast
carcinogenesis and angiogenesis. Oncogene. 32:160–170. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Fang X, Yu W, Li L, et al: ChIP-seq and
functional analysis of the SOX2 gene in colorectal cancers. OMICS.
14:369–384. 2010. View Article : Google Scholar
|
|
24.
|
Gera JF, Mellinghoff IK, Shi Y, et al: AKT
activity determines sensitivity to mammalian target of rapamycin
(mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J
Biol Chem. 279:2737–2746. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hashemolhosseini S, Nagamine Y, Morley SJ,
Desrivieres S, Mercep L and Ferrari S: Rapamycin inhibition of the
G1 to S transition is mediated by effects on cyclin D1 mRNA and
protein stability. J Biol Chem. 273:14424–14429. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Grewe M, Gansauge F, Schmid RM, Adler G
and Seufferlein T: Regulation of cell growth and cyclin D1
expression by the constitutively active FRAP-p70s6K pathway in
human pancreatic cancer cells. Cancer Res. 59:3581–3587.
1999.PubMed/NCBI
|
|
27.
|
Law M, Forrester E, Chytil A, et al:
Rapamycin disrupts cyclin/cyclin-dependent kinase/p21/proliferating
cell nuclear antigen complexes and cyclin D1 reverses rapamycin
action by stabilizing these complexes. Cancer Res. 66:1070–1080.
2006. View Article : Google Scholar
|
|
28.
|
Inoki K, Ouyang H, Zhu T, et al: TSC2
integrates Wnt and energy signals via a coordinated phosphorylation
by AMPK and GSK3 to regulate cell growth. Cell. 126:955–968. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Alao JP: The regulation of cyclin D1
degradation: roles in cancer development and the potential for
therapeutic invention. Mol Cancer. 6:242007. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Long KB and Hornick JL: SOX2 is highly
expressed in squamous cell carcinomas of the gastrointestinal
tract. Hum Pathol. 40:1768–1773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gontan C, de Munck A, Vermeij M, Grosveld
F, Tibboel D and Rottier R: Sox2 is important for two crucial
processes in lung development: branching morphogenesis and
epithelial cell differentiation. Dev Biol. 317:296–309. 2008.
View Article : Google Scholar : PubMed/NCBI
|