Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related mortality worldwide (1,2)
and it has been reported that more than 600,000 individuals succumb
to the disease each year (1). It
is the second most common cause of cancer-related mortality in
China, and 75% of known new cases and deaths in the Asia-Pacific
region (3–5). Currently, the main treatment methods
for liver cancer include surgical resection, radiotherapy and
chemotherapy (4,6). Although surgical resection (which
involves removing the tumor completely) offers the best prognosis
for long-term survival, only 10–15% of patients are suitable for
surgical resection, as the tumor may be too large, or may have
grown into major blood vessels or other vital organs (7–9).
Related data demonstrate that the percentage of HCC cells is
already high at diagnosis with a high expression of the multidrug
resistance gene and conventional chemotherapy of HCC fails to
provide satisfactory remission and may cause serious side-effects
(6,10). Thus, it is necessary to develop a
novel effective drug for the treatment of HCC. Natural products
have attracted much attention in the search for novel anticancer
therapeutic agents as they have relatively few side-effects and
have long been used as alternative therapies for various diseases,
including cancer (11,12). Therefore, determining naturally
occurring agents is a promising approach for anticancer
treatment.
The Janus-activated kinase (JAK) family includes
JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2) (13). The JAK family activate signal
transducer and activator of transcription (STAT) proteins in
response to different cytokines and growth factors (14–17). In normal cells, JAK/STAT signaling
is tightly regulated. However, in cancer cells, it is persistently
activated due to the aberrant activation of JAK family kinases or
other tyrosine kinases (18).
Among the JAK-STAT family members, JAK1-STAT3 plays an important
role in cell proliferation and apoptosis (19). STAT3 can stimulate endothelial
cell migration and differentiation and lead to tumor VEGF
overproduction by direct transcriptional activation (20–22). The JAK1-STAT3 pathway plays a
crucial role in cell survival, angiogenesis, immune evasion and
inflammatory responses through the activation of STAT3, causing
cyclin D1, cyclin-dependent kinase (CDK)4, Bcl-2, Bax and vascular
endothelial growth factor gene transcription (23–26). Thus, the JAK1-STAT3 signaling
pathway as a target for intervention is expected to become a novel
method for the treatment of HCC.
Zanthoxylum nitidum, belonging to the family
Rutaceae, is a medicinal herb widely distributed in Northeastern
Asia. As a well-known traditional Chinese folk medicine, it is used
to promote the flow of Qi, relieve pain, promote blood circulation
and to remove blood stasis (27).
Nitidine is found in the root of Zanthoxylum nitidum and
several studies have demonstrated that nitidine exerts antitumor,
anti-inflammatory, anti-leukemic and even anti-HIV effects
(28,29). Previous studies on nitidine
chloride (NC) have focused on topoisomerase I poison-mediated cell
toxicity (30,31). NC has also been reported to be
involved in the suppression of cell growth, the induction of cell
apoptosis, cell cycle arrest and the inhibition of the activity of
DNA ligases; it exerts its anti-metastatic activity by restraining
the c-Src/focal adhesion kinase (FAK)-associated signaling pathway
in breast cancer cells (28,31) and NC has been shown to inhibit
lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α,
interleukin (IL)-1β and IL-6 production through the suppression of
phosphorylation of mitogen-activated protein kinases (MAPKs) and
the translocation of p65 (29).
In this study, to further elucidate the effects of
NC and the mechanisms behind its antitumor effects, we investigated
the effect of NC on the apoptosis and proliferation of HCC cells
using a mouse xenograft model of HCC and investigated the possible
molecular mechanisms mediating its biological effects.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA, TRIzol
reagent and iBlot Western detection stack/iBlot dry blotting system
were purchased from Invitrogen (Grand Island, NY, USA). Superscript
II reverse transcriptase was provided by Promega (Madison, WI,
USA). Bcl-2, Bax, CDK4, cyclin D1, p21, JAK1, STAT3 antibodies and
horse-radish peroxidase (HRP)-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Proliferating cell nuclear antigen (PCNA) assay and terminal
deoxynucleotidyl transferase-mediated dUTP nick end-labeling
(TUNEL) assay kits were purchased from R&D Systems
(Minneapolis, MN, USA), the Vectastain® Elite ABC kit
was provided by Vector Laboratories, Inc. (Burlingame, CA, USA).
All other chemicals used, unless otherwise stated, were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Drugs
NC was provided by the Sichuan Institute of
Biochemical Technology, Chengdu, China and the purity was
determined to be 98% by high-performance liquid chromatography
(HPLC). A stock solution of NC was prepared in dimethyl sulfoxide
(DMSO; Sigma-Aldrich). Aliquots were stored at −20°C.
Cell culture
HepG2 HCC cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). The cells were grown
in DMEM containing 10% (v/v) FBS and 100 U/ml penicillin and 100
μg/ml streptomycin in a 37°C humidified incubator with 5%
CO2.
Animals
Sixteen nude mice (with an initial body weight of
20–22 g) were provided by the Shanghai Laboratory Animal Center,
Shanghai, China and all animal experiments were carried out
strictly in accordance with the international ethics guidelines and
the National Institutes of Health Guide concerning the Care and Use
of Laboratory Animal and the experiments were approved by the
Ethics Committee of Fujian University of Traditional Chinese
Medicine, Fuzhou, China. All mice were maintained under a
controlled environment [temperature (21±2°C), humidity (50±10%) and
a 12 h light/dark cycle] with free access to food and tap water.
Cells (1.5×106) mixed with Matrigel (1:1) were
inoculated subcutaneously into the right inguinal region of the
mice. The administration began 3 days after inoculation. The mice
were divided into 2 groups: the control group (daily
intraperitoneal injection of saline) and the NC group (daily
intraperitoneal injection of NC 10 mg/kg); each group comprised 8
mice, including 4 males and 4 females. Every 2 days, individual
mice were weighed and each liver tumor was measured using calipers.
After 14 days, the mice were euthanized using 100 mg/kg of
pentobarbital sodium (Nembutal; Boehringer Ingelheim, Artarmon, New
South Wales, Australia), and the tumor tissue was removed and
weighed. Tumor growth was determined by measuring the major (L) and
minor (W) diameter with a caliper. The tumor volume was calculated
according to the following formula: tumor volume = π/6 × L ×
W2.
TUNEL assay for apoptosis
Tumor samples were fixed in 4% buffered
paraformaldehyde for 12 h, and subsequently processed
conventionally for paraffin-embedded tumor sections (4 μm
thick). The tumor sections were then deparaffinized and rehydrated
by treatment with a series of xylenes and graded alcohols. Tissue
sections from paraffin-embedded tumors were examined by TUNEL assay
for the quantitative analyses of apoptosis. TUNEL assay was
performed using an In Situ Cell Death Detection kit (R&D
Systems). Apoptotic cells were counted as DAB-positive cells (brown
stained) at 5 arbitrarily selected microscopic fields at a
magnification of ×400. TUNEL-positive cells were counted as a
percentage of the total cells.
Immunohistochemistry (IHC)
Tumor samples were collected after processing as
described above. The endogenous peroxidase activity of the sections
was quenched by incubating in water containing 0.3%
H2O2 for 30 min. IHC staining was performed
using the Vectastain Elite ABC kit according to the manufacturer’s
instructions. Briefly, after blocking non-specific proteins with
normal serum in PBS (0.1% Tween-20), the sections were treated with
PCNA, Bcl-2, Bax, CDK4, cyclin D1 and p21 antibody (at a dilution
of 1:200). The sections were incubated with a biotinylated
anti-rabbit IgG antibody for 30 min and then treated with the ABC
reagent for 30 min. They were finally treated with DAB for 10 min.
After the sections were washed and air-dried, cover slips were
applied to the sections using permount slide mounting medium.
All indicators were quantified by counting
respective positive cells and total number of cells in 5 high-power
fields (×400) randomly selected in each slide, and the average
proportion of positive cells in each field was counted using the
true color multi-functional cell image analysis management system
(Image-Pro Plus; Media Cybernetics, Bethesda, MD, USA).
Western blot analysis
Six tumors were selected randomly from the control
or NC group, homogenized in non-denaturing lysis buffer using
homogenizer and centrifuged at 15,000 × g for 15 min followed by
the determination of protein concentration in the supernatants.
Equal protein amounts per lysate were separated using NuSep 12%
LongLife Tris Glycine iGels (NuSep Ltd., Lane Cove, New South
Wales, Australia) under a reducing condition using 200 V for 1 h,
on Tris-Glycine gels. They were then transferred onto PVDF
membranes, and blocked for 2 h with 5% non-fat dry milk. The
membranes were incubated with the desired primary antibody to JAK1,
p-JAK1, STAT3, p-STAT3 and β-actin (at a dilution of 1:1,000)
overnight at 4°C and then with appropriate HRP-conjugated secondary
antibody followed by enhanced chemiluminescence detection.
Statistical analysis
All data are presented as the means ± SD.
Statistical calculations were performed using the SPSS package for
Windows (version 17.0). Statistical analysis of the data was
performed using the Student’s t-test and ANOVA. P-values <0.05
were considered to indicate statistically significant
differences.
Results
NC inhibits tumor growth in HCC tumor
xenografts in mice
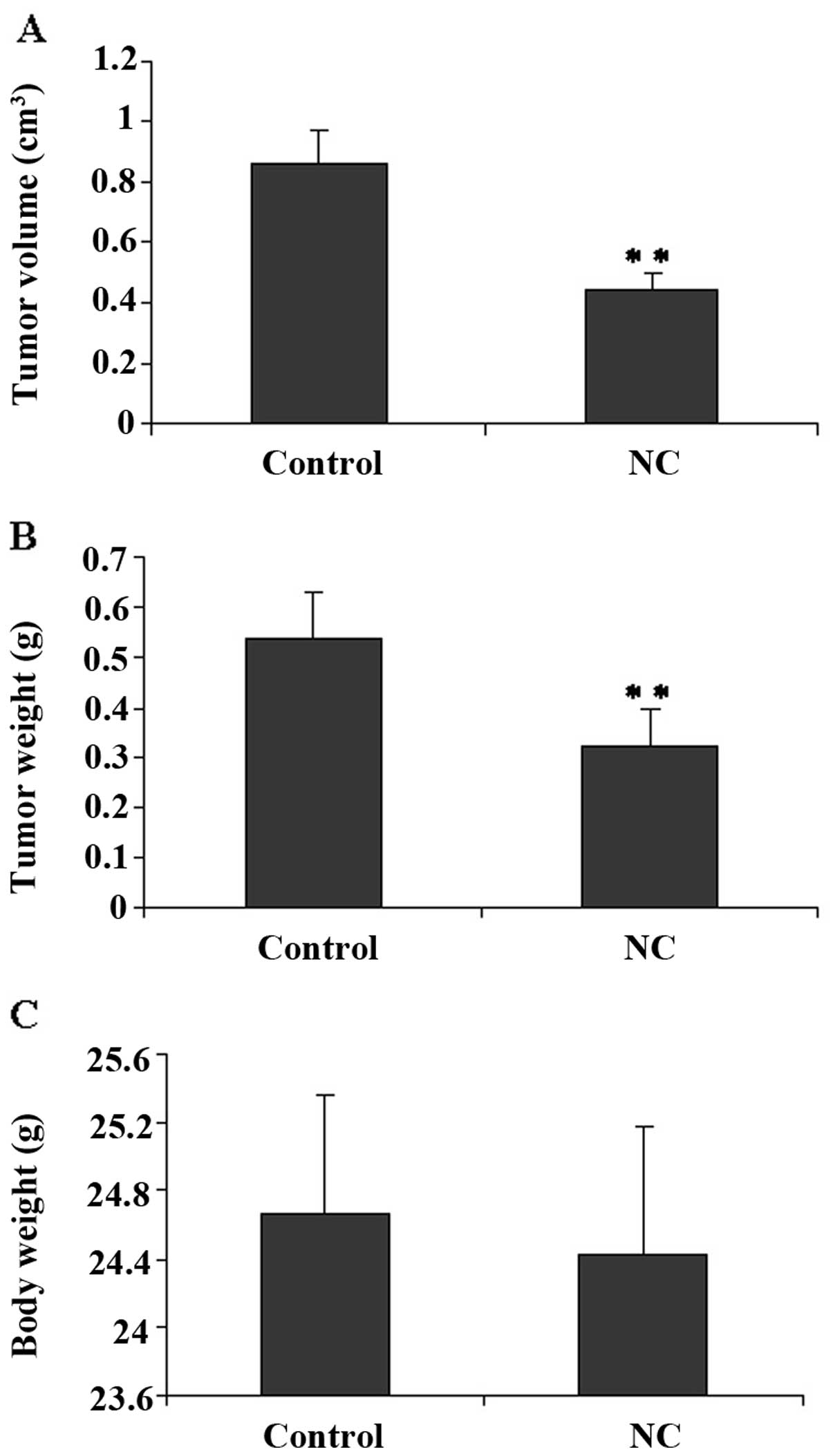
To evaluate the antitumor effect of NC, the tumor
volume and weight were measured in HCC tumor xenografts in mice,
and the side-effect were determined based on the measurement of
body weight gain. In Fig. 1A and
B, tumor volume and weight in the NC group were reduced by 52
and 41%, respectively, as compared to the tumors from the control
mice. Nevertheless, NC treatment exerted no effect on the body
weight gain in the experimental animals (Fig. 1C). These data indicate that NC
inhibits HCC tumor growth in vivo, without evident signs of
toxicity.
NC induces apoptosis and inhibits cell
proliferation in HCC tumor xenografts in mice
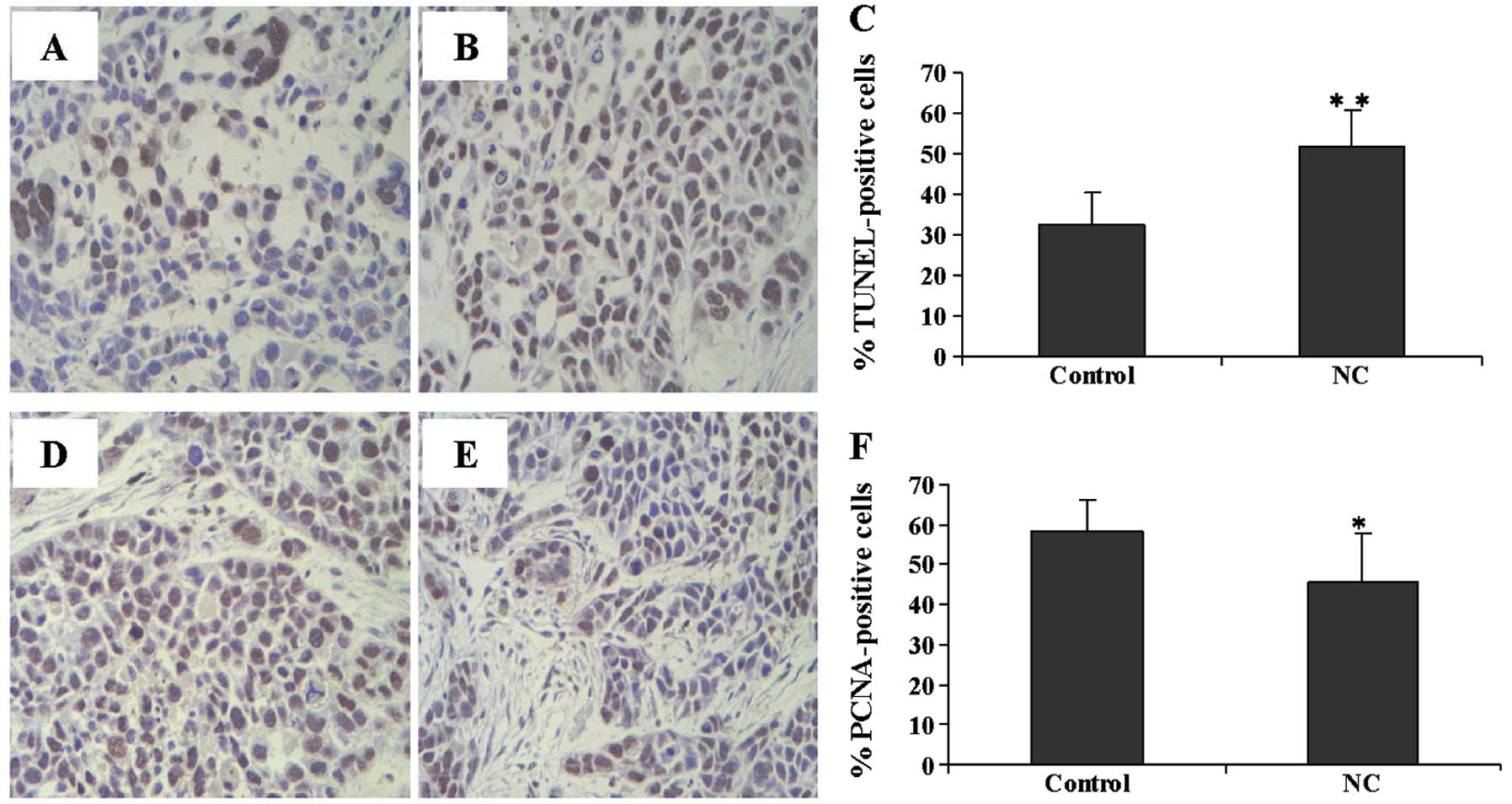
The effect of NC on apoptosis and cell proliferation
in HCC tumor xenografts in mice was examined by TUNEL assay and IHC
staining for PCNA. The number of TUNEL-positive cells was
significantly lower in the tumors from the NC-treated group as
compared to those from the control mice (Fig. 2A–C). The number of PCNA-positive
cells in the control group was markedly higher than that in the
NC-treated mice (Fig. 2D–F).
These results suggest that NC induces apoptosis and inhibits HepG2
cell growth in vivo.
NC inhibits the JAK1-STAT3 signaling
pathway in HCC tumor xenografts in mice
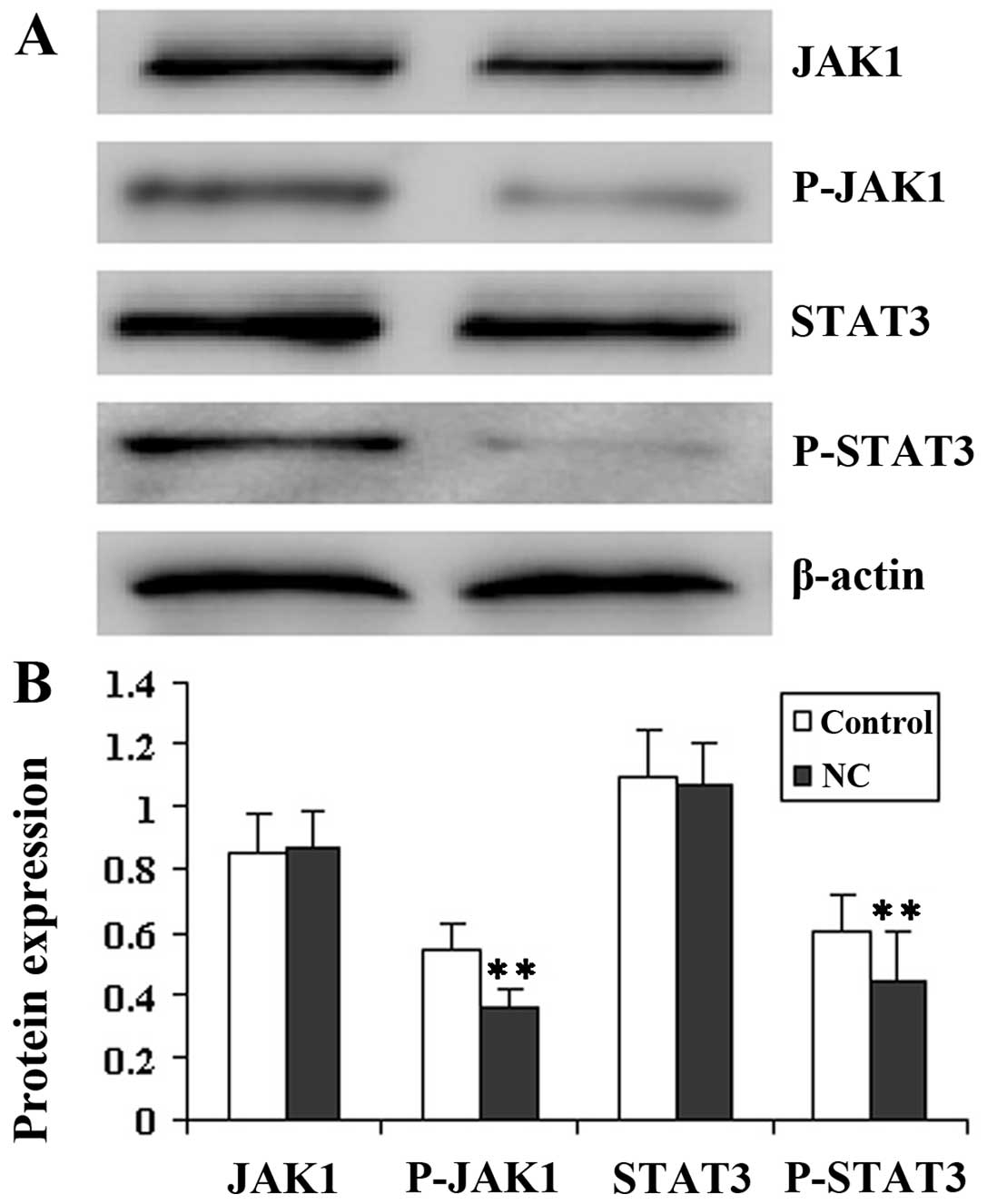
It is well known that STAT3 proteins induce the
phosphorylation of JAK tyrosine kinase. Therefore, in this study,
we examined the effect of NC on JAK1 and STAT3 phosphorylation at
Tyr705 in tumor tissue by western blot analysis.
Fig. 3 illustrates that JAK1 and
STAT3 protein phosphorylation in the NC-treated group was lower
than that in the control mice, while the level of JAK1 and STAT3
remained unaltered following treatment with NC, indicating that NC
evidently inhibited the JAK and STAT3 activation in
vivo.
NC regulates the expression of Bcl-2,
Bax, CDK4, cyclin D1 and p21 in HCC tumor xenografts in mice
To further investigate the mechanism behind the
pro-apoptotic and anti-proliferative effects of NC, we examined the
protein expression of Bcl-2, Bax, CDK4, cyclin D1 and p21. The
results from immunohistochemistry analysis (Fig. 4) showed that NC treatment markedly
reduced the expression of anti-apoptotic Bcl-2, pro-proliferative
cyclin D1 and CDK4 proteinm but increased that of pro-apoptotic Bax
and anti-proliferative p21 in the HCC tumor xenografts in mice
compared to the control mice.
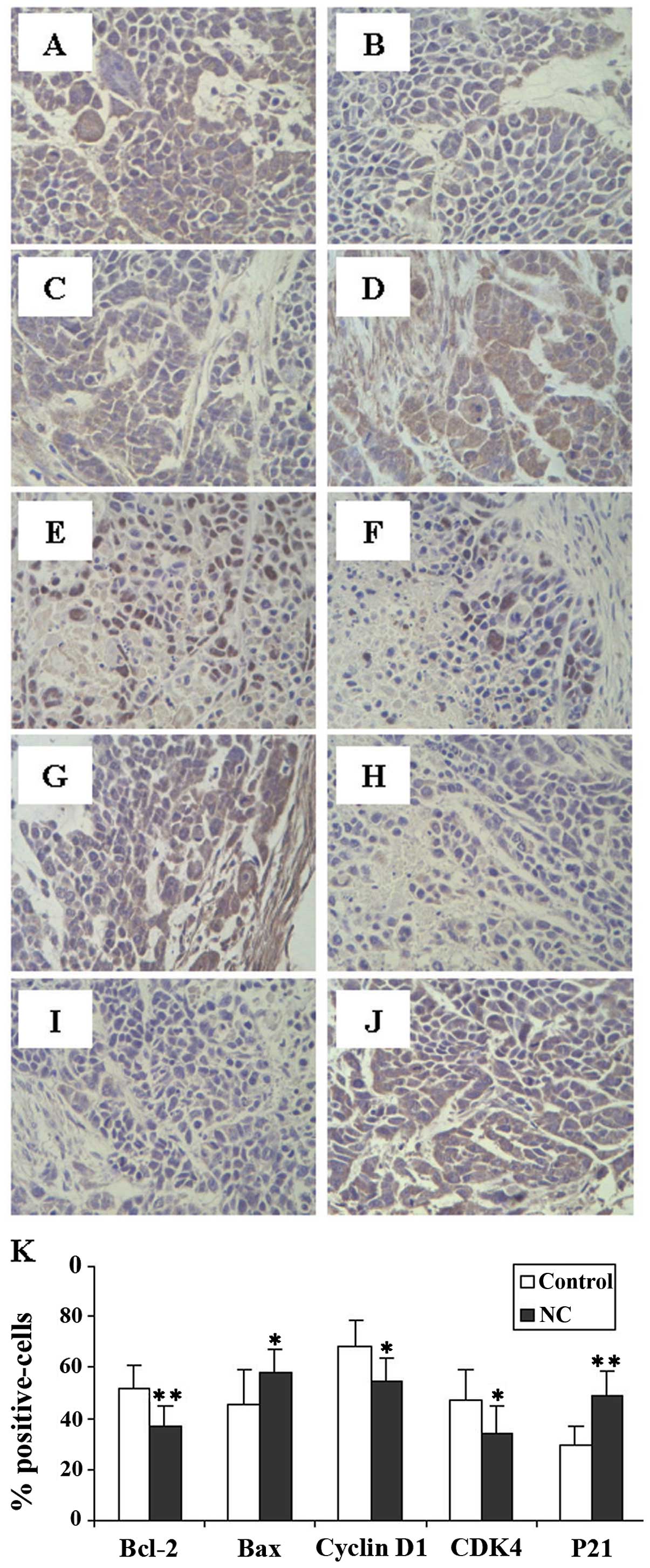 | Figure 4.Effect of nitidine chloride (NC) on
the expression of Bcl-2, Bax, cyclin D1, CDK4 and p21 in
hepatocellular carcinoma (HCC) tumor xenografts in mice. The images
are representative images taken at a magnification of ×400, and the
expression of these tumor tissues was determined by
immunohistochemistry. (A) Bcl-2, (C) Bax, (E) cyclin D1, (G) CDK4
and (I) p21 in the control group; (B) Bcl-2, (D) Bax, (F) cyclin
D1, (H) CDK4 and (J) p21 in the NC-treaded group. (K)
Quantification of immunohistochemical assay is represented as the
percentage of positively-stained cells. Data shown are the means ±
SD (error bars) (n=8). *P<0.05 and
**P<0.01 compared to the control group. |
Discussion
Cancer cells are characterized by an uncontrolled
increase in cell proliferation and/or a reduction in cell
apoptosis, which not only confers a survival advantage to the
cancer cells but also causes resistance to conventional
chemotherapeutic agents (32,33). The currently used anticancer
chemotherapetuic drugs have several side-effects on normal cells,
thus limiting their long-term use. However, natural products with
fewer side-effects, have long been used in Chinese medicine for the
treatment of various types of cancer, including liver cancer, and
these products have shown pro-apoptotic activity and may thus be
promising approach to cancer therapy. Zanthoxylum nitidum, a
well-known traditional Chinese folk medicine, is used to promote
the flow of Qi, relieve pain, promote blood circulation and to
remove blood stasis (27).
However, the precise mechanism behind its potential antitumor
activity has not yet been fully elucidated. Therefore, the
antitumor activity of NC and the underlying molecular mechanisms
require further study before it can be further developed as an
anticancer agent.
Mitochondria-bound Bcl-2 and Bax proteins play a
crucial role in the control of apoptosis. Bcl-2 family proteins are
key regulators of apoptosis, functioning as either promoters (Bax),
or suppressors (Bcl-2). Lower Bax to Bcl-2 ratios due to the
downregulation of Bax expression or the overexpression of Bcl-2 are
commonly observed in cancer cells (32,34–36). In the present study, we
demonstrated that NC inhibited tumor growth and enhanced Bax
expression, but reduced Bcl-2 expression in HCC tumor xenografts in
mice.
Eukaryotic cell proliferation is primarily regulated
by the cell cycle. The orderly progression of the cell cycle is
affected through the periodic activation and inactivation of a
series of CDKs (37). G1/S
checkpoints are located at the end of the G1 phase of the cell
cycle, just before entry into the S phase. The CDK4/cyclin D
complex phosphorylates the retinoblastoma (Rb) protein, promoting
cyclin E expression through its E2F-dependent transcriptional
activation and the disruption of the Rb-histone deacetylase (HDAC)
complex, culminating in CDK2 kinase activation as the S phase
progresses (38,39). Uncontrolled cell division and
malignancy occur due to an unchecked or hyper-activated cyclin
D1/CDK4 complex. Normal p21 function plays a crucial role in the
induction of apoptosis and cell cycle G1/S transition checkpoints
in human and murine cells following DNA damage (40), and the down-regulation of p21
expression is associated with the promotion of tumor formation and
a poor prognosis in many types of cancer (41). In this study, we found that NC
downregulated the expression of cyclin D1/CDK4 and upregulated the
expression of p21 in vivo, suggesting that NC inhibited
cancer cell proliferation by inducing arrest at the G1/S phase.
As one of the more recently recognized oncogenic
signaling pathways, the JAK1-STAT3 signaling pathway has been shown
to be important for tumorigenesis. The persistent activation of
JAK1/STAT3 signaling contributes to the malignancy of tumors by
promoting tumor cell proliferation and survival, angiogenesis and
immune evasion (10, 34, 42, 43). Thus, the JAK1-STAT3 signaling
pathway is a promising molecular target in cancer therapy. Numerous
studies have revealed that STAT3 contributes to oncogenesis through
several mechanisms, including the inhibition of apoptosis, the
enhancement of cell proliferation, the induction of angiogenesis
and the suppression of immune responses (24,44). Our study demonstrates that NC
blocks the phosphorylation of JAK1-STAT3 in HCC tumor xenografts in
mice.
In conclusion, in the present study, we demonstrate
that NC inhibits tumor growth in HCC tumor xenografts in mice. In
addition, our results revealed that NC blocked the activation of
the JAK1-STAT3 pathway. Consequently, the inhibitory effect of NC
on JAK1-STAT3 activation led to the induction of cell apoptosis
which resulted from the decrease in the Bcl-2/Bax ratio, and the
suppression of cell proliferation which originated from G1/S phase
arrest through the decrease in CDK4 and cyclin D1 expression and
the increase in p21 expression.
Abbreviations:
|
HCC
|
hepatocellular carcinoma;
|
|
JAK1
|
Janus-activated kinase 1;
|
|
STAT3
|
signal transducer and activator of
transcription 3;
|
|
NC
|
nitidine chloride;
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling;
|
|
PCNA
|
proliferating cell nuclear antigen
|
Acknowledgements
The study was supported by grants from
the National Natural Science Foundation of China (nos. 81273836 and
81001554).
References
|
1.
|
Ferenci P, Fried M, Labrecque D, et al:
Hepatocellular carcinoma (HCC): a global perspective. J Clin
Gastroenterol. 44:239–245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Cheng AL, Kang YK, Chen Z, et al: Efficacy
and safety of sorafenib in patients in the Asia-Pacific region with
advanced hepatocellular carcinoma: a phase III randomised,
double-blind, placebo-controlled trial. Lancet Oncol. 10:25–34.
2009. View Article : Google Scholar
|
|
3.
|
Bridges JF, Dong L, Gallego G, Blauvelt
BM, Joy SM and Pawlik TM: Prioritizing strategies for comprehensive
liver cancer control in Asia: a conjoint analysis. BMC Health Serv
Res. 12:3762012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wörns MA and Galle PR: Future perspectives
in hepatocellular carcinoma. Dig Liver Dis. 42(Suppl 3): S302–S309.
2010.
|
|
5.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
6.
|
Bruix J, Sherman M, Llovet JM, et al:
Clinical management of hepatocellular carcinoma. Conclusions of the
Barcelona-2000 EASL conference. European Association for the Study
of the Liver. J Hepatol. 35:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Levin B and Amos C: Therapy of
unresectable hepatocellular carcinoma. N Engl J Med. 332:1294–1296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Abou-Alfa GK, Huitzil-Melendez F-D,
O’Reilly EM and Saltz LB: Current management of advanced
hepatocellular carcinoma. Gastrointest Cancer Res. 2:64–70.
2008.PubMed/NCBI
|
|
9.
|
Boos G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Auyeung KK and Ko JK: Coptis
chinensis inhibits hepatocellular carcinoma cell growth through
nonsteroidal anti-inflammatory drug-activated gene activation. Int
J Mol Med. 24:571–577. 2009.
|
|
11.
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yamaoka K, Saharinen P, Pesu M, Holt VE
III, Silvennoinen O and O’Shea JJ: The Janus kinases (Jaks). Genome
Biol. 5:2532004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Aaronson DS and Horvath CM: A road map for
those who don’t know JAK-STAT. Science. 296:1653–1655. 2002.
|
|
15.
|
O’Shea JJ, Gadina M and Schreiber RD:
Cytokine signaling in 2002: new surprises in the Jak/Stat pathway.
Cell. 109(Suppl): S121–S131. 2002.PubMed/NCBI
|
|
16.
|
Darnell JE Jr, Kerr IM and Stark GR:
Jak-STAT pathways and transcriptional activation in response to
IFNs and other extracellular signaling proteins. Science.
264:1415–1421. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kisseleva T, Bhattacharya S, Braunstein J
and Schindler C: Signaling through the JAK/STAT pathway, recent
advances and future challenges. Gene. 285:1–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu L, Nam S, Tian Y, et al:
6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces
apoptosis of human melanoma cells. Cancer Res. 71:3972–3979.
2011.
|
|
19.
|
Bromberg J and Darnell JE Jr: The role of
STATs in transcriptional control and their impact on cellular
function. Oncogene. 19:2468–2473. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Xu Q, Briggs J, Park S, et al: Targeting
Stat3 blocks both HIF-1 and VEGF expression induced by multiple
oncogenic growth signaling pathways. Oncogene. 24:5552–5560. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liao J, Ke M, Xu T and Lin L:
Electroacupuncture inhibits apoptosis in annulus fibrosis cells
through suppression of the mitochondria-dependent pathway in a rat
model of cervical intervertebral disc degradation. Genet Mol Biol.
35:686–692. 2012. View Article : Google Scholar
|
|
22.
|
Yarani R, Mansouri K, Mohammadi-Motlagh
HR, Mahnam A and Emami Aleagha MS: In vitro inhibition of
angiogenesis by hydroalcoholic extract of oak (Quercus
infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9
secretion. Pharm Biol. 51:361–368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chen J, Wang J, Lin L, et al: Inhibition
of STAT3 signaling pathway by nitidine chloride suppressed the
angiogenesis and growth of human gastric cancer. Mol Cancer Ther.
11:277–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Buettner R, Mora LB and Jove R: Activated
STAT signaling in human tumors provides novel molecular targets for
therapeutic intervention. Clin Cancer Res. 8:945–954.
2002.PubMed/NCBI
|
|
25.
|
Calvisi DF, Pascale RM and Feo F:
Dissection of signal transduction pathways as a tool for the
development of targeted therapies of hepatocellular carcinoma. Rev
Recent Clin Trials. 2:217–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Imada K and Leonard WJ: The Jak-STAT
pathway. Mol Immunol. 37:1–11. 2000. View Article : Google Scholar
|
|
27.
|
Bencao Z: Chinese Herbal Medicine. Chinese
Edition. Shanghai Science and Technology Publishing House;
Shanghai: 1996
|
|
28.
|
Pan X, Han H, Wang L, et al: Nitidine
chloride inhibits breast cancer cells migration and invasion by
suppressing c-Src/FAK associated signaling pathway. Cancer Lett.
313:181–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Wang Z, Jiang W, Zhang Z, Qian M and Du B:
Nitidine chloride inhibits LPS-induced inflammatory cytokines
production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J
Ethnopharmacol. 144:145–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Liu LM and Liu HG: Anti-hepatoma activity
of nitidine chloride and its effect on topoisomerase. Chin
Pharmacol Bull. 4:497–500. 2010.(In Chinese).
|
|
31.
|
Cushman M, Mohan P and Smith EC: Synthesis
and biological activity of structural analogs of the anticancer
benzophenanthridine alkaloid nitidine chloride. J Med Chem.
27:544–547. 1984. View Article : Google Scholar
|
|
32.
|
Adams J and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Cai Q, Lin J, Wei L, et al: Hedyotis
diffusa Willd inhibits colorectal cancer growth in vivo via
inhibition of STAT3 signaling pathway. Int J Mol Sci. 13:6117–6128.
2012. View Article : Google Scholar
|
|
34.
|
Hall C, Troutman SM, Price DK, Figg WD and
Kang MH: Bcl-2 family of proteins as therapeutic targets in
genitourinary neoplasms. Clin Genitourin Cancer. 11:10–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Paul-Samojedny M, Kokocińska D, Samojedny
A, et al: Expression of cell survival/death genes: Bcl-2 and Bax at
the rate of colon cancer prognosis. Biochim Biophys Acta.
1741:25–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chae HD, Kim J and Shin DY: NF-Y binds to
both G1- and G2-specific cyclin promoters; a possible role in
linking CDK2/Cyclin A to CDK1/Cyclin B. BMB Rep. 44:553–557. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Tin AS, Sundar SN, Tran KQ, Park AH,
Poindexter KM and Firestone GL: Antiproliferative effects of
artemisinin on human breast cancer cells requires the downregulated
expression of the E2F1 transcription factor and loss of E2F1-target
cell cycle genes. Anticancer Drugs. 23:370–379. 2012. View Article : Google Scholar
|
|
40.
|
Zafonte BT, Hulit J, Amanatullah DF, et
al: Cell-cycle dysregulation in breast cancer: breast cancer
therapies targeting the cell cycle. Front Biosci. 5:D938–D961.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Domagala W, Welcker M, Chosia M, et al:
p21/WAF1/Cip1 expression in invasive ductal breast carcinoma:
relationship to p53, proliferation rate, and survival at 5 years.
Virchows Arch. 439:132–140. 2001.PubMed/NCBI
|
|
42.
|
Whang-Peng J, Cheng AL, Hsu C and Chen CM:
Clinical development and future direction for the treatment of
hepatocellular carcinoma. J Exp Clin Med. 2:93–103. 2010.
View Article : Google Scholar
|
|
43.
|
Fracchiolla N, Capaccio P, Carboni N, et
al: Immunohistochemical and molecular analysis of bax, bcl-2 and
p53 genes in laryngeal squamous cell carcinomas. Anticancer Res.
19:1043–1051. 1999.PubMed/NCBI
|
|
44.
|
Diaz N, Minton S, Cox C, et al: Activation
of stat3 in primary tumors from high-risk breast cancer patients is
associated with elevated levels of activated SRC and survivin
expression. Clin Cancer Res. 12:20–28. 2006. View Article : Google Scholar : PubMed/NCBI
|