Introduction
Cardiovascular diseases, such as atherosclerotic
coronary heart disease, congestive heart failure, hypertensive
heart disease and stroke, are still major health issues worldwide,
particularly in advanced countries (1). According to the American Heart
Association (AHA), ischemic heart disease was the single leading
cause of death in adults in the US, with the estimated direct and
indirect costs of >177.1 billion dollars for the year 2010
(2). However, pharmacological
therapies aimed to reduce symptoms have multiple systemic
side-effects, and do not effectively halt the pathophysiological
progression associated with ischemic heart disease (3,4).
Additionally, intervention or surgical revascularization procedures
in patients with stenotic lesion-induced ischemic heart disease do
not provide long-term relief, and frequently, patients require
repeat revascularization procedures within a few years (5). Therefore, the development of
pharmacological agents effective against this condition is
mandatory.
Over the years, several pharmacological compounds
and agents have been shown to have cardioprotective effects in
animal experiments; however, only a few of these have been
successfully translated to the clinic (6). This may be due, not only to possible
inter-species differences in drug efficacy, but also to multiple
systemic side-effects, as well as the limited therapeutic time
window (7). Studies have found
that many natural products (particularly herbal medicines) provide
an ideal source for the development of safe and effective agents
for the treatment of ischemic heart disease (8,9).
Osthole (7-methoxy-8-isopentenoxycoumarin), a plant coumarin
compound, is an active constituent isolated from a number of
plants, such as Cnidium monnieri and Angelica
pubescens. In traditional Chinese medicine, these plants have
been used as tonics and aphrodisiacs, and have been administered to
humans in clinical practice for several years (10). Modern pharmacological studies have
shown that osthole possesses a variety of pharmacological and
biological activities, such as anti-hepatic (11), anti-inflammatory (12), antitumor (13,14), anti-apoptotic (15), as well as anti-allergic and
estrogen-like effects (16,17). It is considered to have potential
therapeutic applications, and previous studies have shown that
osthole protects against cerebral ischemic reperfusion injury in
vitro and in vivo (18,19). In the present study, we
investigated the potential protective effects of osthole against
myocardial ischemia reperfusion (I/R) injury, as well as its
mechanisms of action, focusing on its anti-inflammatory and
antioxidant activities.
Materials and methods
Animals
Male Sprague-Dawley rats weighing 350–450 g were
housed at 20–22ºC with a 12-h light/dark cycle. Standard animal
chow and water were freely available. All experimental protocols
were approved by the Institutional Animal Care and Use Committee of
the Fourth Military Medical University and were performed in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals (NIH publication no. 80–23, revised 1996). All efforts were
made to minimize the number of animals used, as well as their
suffering.
Myocardial I/R injury
Coronary occlusion and reperfusion were performed as
previously described (20). The
rats were anesthetized and the arterial blood pH and gases were
maintained within normal physiological limits by a rodent
respirator. After the chest was opened by a middle thoracotomy, a
4–0 black silk ligature was placed under the left anterior
descending (LAD) coronary artery, and the ends of the tie were
threaded through a small vinyl tube to form a snare for reversible
LAD coronary artery occlusion. In the vehicle- and osthole-treated
animals, myocardial ischemia was induced by tightening the snare
with a clip for 30 min, and reperfusion was established by
loosening the snare. The ligature was placed under the LAD coronary
artery without occlusion in the control animals.
Drug preparation and experimental
protocol
Osthole (>98% purity) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China) and dissolved in dimethyl sulfoxide
(DMSO) (<0.1%, which had no toxicity). All animals were randomly
assigned to 1 of 5 groups: the control group (control), the vehicle
group (vehicle), and 3 treatment groups, which were treated with
osthole at the concentration of 1, 10 or 50 mg/kg
(intraperitoneally), respectively, upon the initiation of
myocardial ischemia. The control group was only subjected to
surgical procedures (sham-operated), while the other animals were
subjected to myocardial ischemia and reperfusion 30 min later. In
addition, the control group and the vehicle group were
intraperitoneally administered an equal volume of the solution used
to dissolve osthole at the same time.
Hemodynamic assessment
The right common carotid artery was exposed and
cannulated with a 2 F Millar Catheter into the left ventricle
through the ascending aorta to monitor heart function, including
left ventricular systolic pressure (LVSP), left ventricular
end-diastolic pressure (LVEDP), heart rate (HR), mean arterial
pressure (MAP) and first derivative (±dp/dtmax)
of left ventricular pressure in each group. To eliminate the
confounding factor in which the loading conditions of the heart may
influence cardiovascular parameters, additional rats were used to
examine whether osthole on its own has an effect on LVSP, LVEDP, HR
and ±dp/dtmax in normal hearts under
sham-operated conditions.
Analysis of myocardial infarction
Myocardial infarction was determined according to a
previously described method (21). Briefly, 2 ml of 1% Evans blue dye
was injected into the femoral vein to distinguish between the
perfused and non-perfused [area at risk (AAR)] sections of the
heart following 24 h of reperfusion. The AAR was cut into small
sections and incubated with a 1% solution of
2,3,5-triphenyltetrazolium chloride (TTC) for 30 min at 37ºC
followed by fixation in a 4% paraformaldehyde solution to visualize
the infarct area. Infarct size was expressed as the percentage of
the ischemic area (IA) in each heart.
Lactate dehydrogenase (LDH)
estimation
The animal hearts were removed from liquid nitrogen,
weighed and a 10% homogenate was prepared in iced phosphate buffer.
The homogenate was then cold centrifuged at 5,000 rpm for 20 min
and the supernatant was used for the estimation of LDH levels using
commercialized assay kits according to the manufacturer's
instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). One unit of LDH is defined as the amount of enzyme required
to reduce 1 μmol of pyruvate to D-lactate/min at pH 7 and 25ºC. LDH
levels are expressed as IU/mg protein.
Creatine kinase-MB (CK-MB) isoenzyme
estimation
CK-MB isoenzyme levels were estimated using a
commercial kit from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA) according to the manufacturer's instructions. Briefly, 50 μl
of each sample were added to tubes containing 1 ml of imidazole
buffer consisting of adenosine monophosphate (AMP, 5.2 mM),
adenosine diphosphate (ADP, 2.1 mM), nicotinamide adenine
dinucleotide phosphate (NADPH, 2.1 mM), glucose-6-phosphate
dehydrogenase (G6PD, 1.6 U/l), phosphocreatine (CP, 31.2 mM) and
N-acetylcysteine (NAC, 21 mM). The cubes consisting of samples and
imidazole buffer were incubated for 2 min at room temperature.
Absorbance was recorded at 340 nm for 180 sec at 60 sec intervals.
One unit of CK-MB isoenzyme is defined as the amount of enzyme that
will transfer 1 μmol of phosphate from CP to ADP/min at pH 7.4 and
30ºC. The absorbance of the reaction mixture was measured at 340 nm
for 3 min every 60 sec. CK-MB levels are expressed as IU/mg
protein.
Biochemical analyses
The rats were sacrificed and the heart tissue
samples were obtained for the measurement of lipid peroxidation
parameters and antioxidant enzyme activities. The homogenate was
centrifuged and the supernatant was used for the assays of
malonyldialdehyde (MDA) and 4-hydroxynonenal (4-HNE) levels by
colorimetric analysis using a spectrophotometer with the associated
detection kits (Cayman Chemical Co., Ann Arbor, MI, USA). The
activities of superoxide dismutase (SOD), glutathione peroxidase
(GPx) and catalase (CAT) were also measured according to the
manufacturer's instructions (Nanjing Jiancheng Bioengineering
Institute). In brief, SOD activity was determined by luciferase
chemiluminescence elicited by the xanthine oxidase system; GPx
activity was assayed by the oxidization of glutathione based on the
development of a yellow color with 5,50-dithiobis-(2-nitrobenzoic
acid) (DTNB); CAT activity was determined by the absorbance of the
remaining H2O2 using a colorimetric
method.
Enzyme-linked immunosorbent assay
(ELISA)
Twenty-four hours following the induction of
myocardial I/R, blood samples were collected and frozen below −70ºC
until analysis. Serum levels of the inflammatory cytokines, tumor
necrosis factor (TNF)-α and interleukin (IL)-6, as well as those of
the myocardial protective cytokine, IL-10, were measured using
ELISA kits according to manufacturer's instructions.
Western blot analysis
The protein concentration was determined using a BCA
protein assay kit. Equivalent amounts of protein (40 μg/lane) were
loaded and separated by 10% SDS-PAGE gels, and transferred onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat milk solution in Tris-buffered saline with
0.1% Triton X-100 (TBST) for 1 h, and then incubated overnight at
4ºC with primary phosphorylated IκB-α antibody (1:600),
phosphorylated nuclear factor (NF)-κB antibody (1:600),
high-mobility group box protein 1 (HMGB1) antibody (1:1,000) or
β-actin antibody (1:800) dilutions in TBST. Subsequently, the
membranes were washed and incubated with a secondary antibody for 1
h at room temperature. ImageJ software was then used to quantify
the optical density of each band.
Statistical analysis
Statistical analysis was performed using SPSS 16.0,
a statistical software package. Statistical evaluation of the data
was performed by one-way analysis of variance (ANOVA). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Osthole reduces myocardial damage
following I/R injury
To investigate the potential protective effects of
osthole against myocardial I/R injury, we first measured the
myocardial infract volume. The IA at risk (ARR/LV) induced by
coronary occlusion did not differ between the vehicle- and
osthole-treated groups (Fig. 1A).
The administration of osthole induced a statistically significant
reduction in IA/AAR values as compared with the vehicle group in a
dose-dependent manner, although 1 mg/kg osthole was not effective
compared with the vehicle group (P>0.05). We then assayed the
level of the myocyte injury marker enzymes, CK-MB and LDH, in the
heart tissues challenged with I/R insults. Osthole significantly
prevented the depletion of myocyte marker enzymes induced by
myocardial I/R injury as evidenced by the significant restoration
of CK-MB isoenzyme and LDH enzyme levels in comparison with the
vehicle group.
Osthole improves functional recovery
following I/R injury
The hemodynamic changes recorded in the anesthetized
animals are presented in Table I.
Following myocardial I/R injury, LVEDP was significantly increased,
while LVSP and ±dp/dtmax were decreased in the
vehicle-treated rats as compared with the control rats; these
effects were all partly reversed following treatment with osthole
(10 and 50 mg/kg). Moreover, no significant difference in LVEDP,
LVSP, HR and ±dp/dtmax was observed between the
normal control rats treated with the vehicle or osthole under
sham-operated conditions (Table
II).
 | Table IHemodynamic measurements following
myocardial I/R injury. |
Table I
Hemodynamic measurements following
myocardial I/R injury.
| LVSP (mmHg) | LVEDP (mmHg) |
+dp/dtmax |
−dp/dtmax | HR (bpm) | MAP (mmHg) |
|---|
| Control | 106±8 | 8.8±1.7 | 9038±812 | 8426±730 | 323±23 | 81±6 |
| Vehicle | 62±7a | 12.3±2.3a | 5653±612a | 5153±608a | 353±17 | 74±8 |
| Ost 1 mg/kg | 73±9 | 11.6±1.3 | 6245±533 | 5852±742 | 328±27 | 78±7 |
| Ost 10 mg/kg | 83±8b | 10.4±1.9b | 7547±712b | 6843±603b | 365±22 | 69±8 |
| Ost 50 mg/kg | 89±8b | 9.5±1.1b | 8116±594b | 7335±665b | 346±32 | 77±9 |
 | Table IITime course of hemodynamic changes in
normal control rats treated with osthole (50 mg/kg) or the vehicle
(0.1% DMSO). |
Table II
Time course of hemodynamic changes in
normal control rats treated with osthole (50 mg/kg) or the vehicle
(0.1% DMSO).
| Osthole (50 mg/kg,
n=5) | Vehicle (0.1% DMSO
0.25 ml, n=5) |
|---|
|
|
|
|---|
| Time (h) | LVSP (mmHg) | LVEDP (mmHg) |
+dp/dtmax |
−dp/dtmax | HR (bpm) | LVSP (mmHg) | LVEDP (mmHg) |
+dp/dtmax |
−dp/dtmax | HR (bpm) |
|---|
| 0 | 110±7 | 9.7±2.1 | 10521±747 | 9567±614 | 225±16 | 115±8 | 10.4±2.3 | 10244±818 | 9166±603 | 233±14 |
| 0.5 | 97±6 | 10.8±3.5 | 10123±812 | 9192±523 | 231±22 | 107±7 | 11.2±3.5 | 9875±653 | 9250±750 | 241±18 |
| 1 | 112±9 | 11.8±2.6 | 9834±653 | 8961±496 | 245±19 | 104±9 | 12.1±2.7 | 10144±752 | 8783±565 | 252±21 |
| 2 | 101±8 | 11.5±4.1 | 9699±712 | 8732±568 | 233±17 | 96±8 | 13.3±3.2 | 9793±703 | 8821±784 | 246±19 |
Effects of osthole on lipid peroxidation
following I/R injury
To determine the effects of osthole on lipid
peroxidation, heart tissue samples were obtained 24 h following
myocardial I/R injury. Myocardial I/R injury induced a significant
increase in lipid peroxidation, as reflected by the increased
levels of MDA and 4-HNE (to 378±20 and 252±24% of the control
levels, respectively) (Fig. 2). A
reduction in MDA and 4-HNE formation was observed in the
osthole-treated rats as compared with the vehicle-treated animals,
indicating that osthole prevents lipid peroxidation induced by
myocardial I/R injury.
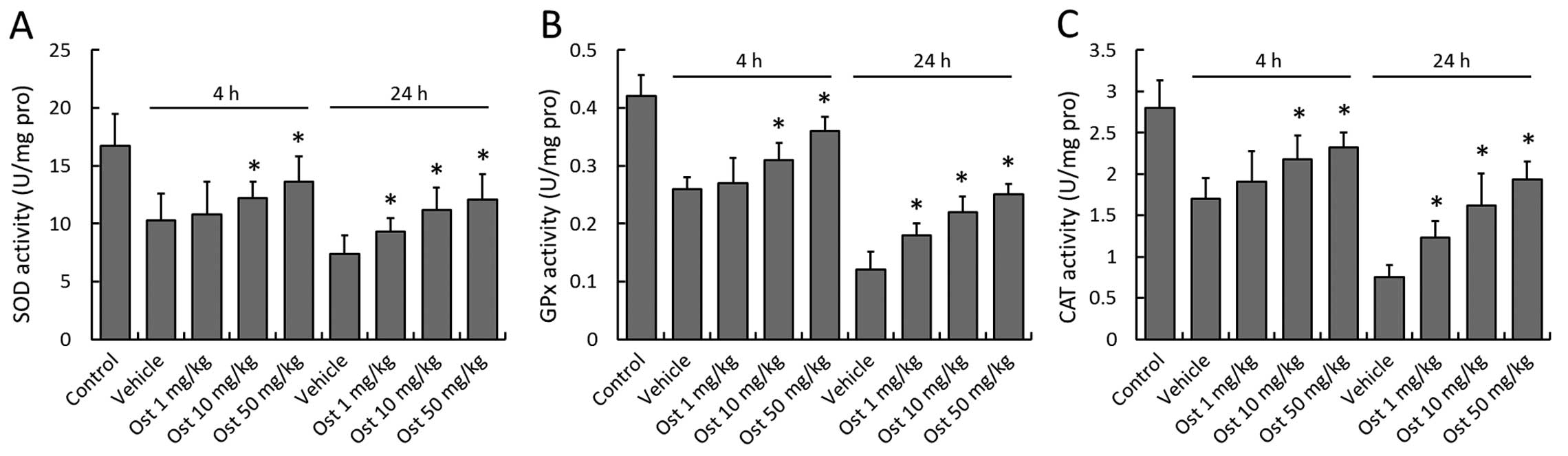
Effects of osthole on antioxidant enzyme
activity following I/R injury
Concomitant to the increased MDA and 4-HNE
formation, a significant decrease in the activities of the
antioxidant enzymes, SOD, GPx and CAT, was observed in the vehicle
group in comparison with the control group at 4 and 24 h following
myocardial I/R injury, demonstrating the damage to the endogenous
antioxidant system induced by myocardial I/R injury (Fig. 3). Treatment with osthole
significantly preserved the enzyme activity of SOD, GPx and CAT at
4 and 24 h following myocardial I/R injury in a dose-dependent
manner, although 1 mg/kg osthole was not effective at 4 h when
compared with the vehicle-treated rats (P>0.05).
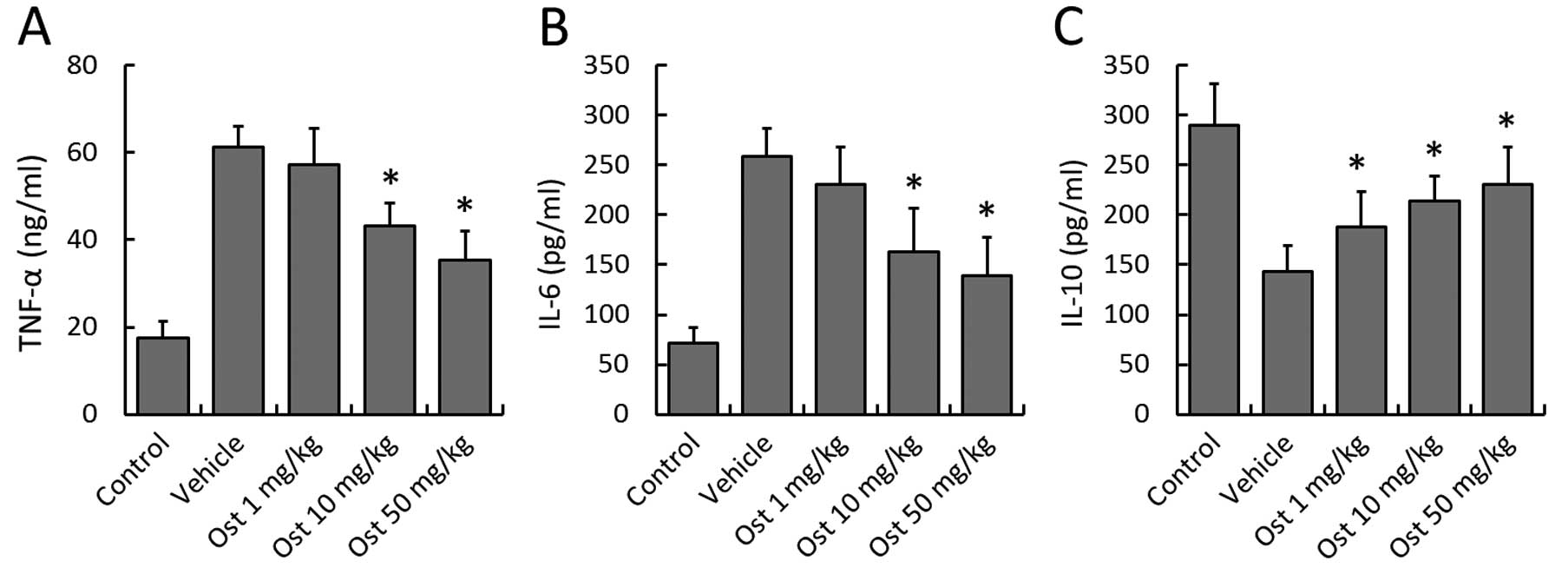
Effects of osthole on the expression of
inflammatory cytokines
To investigate the potential anti-inflammatory
activity of osthole following myocardial I/R injury, the levels of
cytokines associated with inflammation, such as TNF-α, IL-6 and
IL-10 were measured in the blood samples using ELISA. Osthole (10
and 50 mg/kg) markedly decreased the expression of TNF-α as
compared with the vehicle group, and a similar pattern was observed
in the IL-6 concentration (Fig. 4A
and B). By contrast, the concentration of IL-10, an
anti-inflammatory cytokine, was significantly higher in the
osthole-treated groups compared with the vehicle group (Fig. 4C).
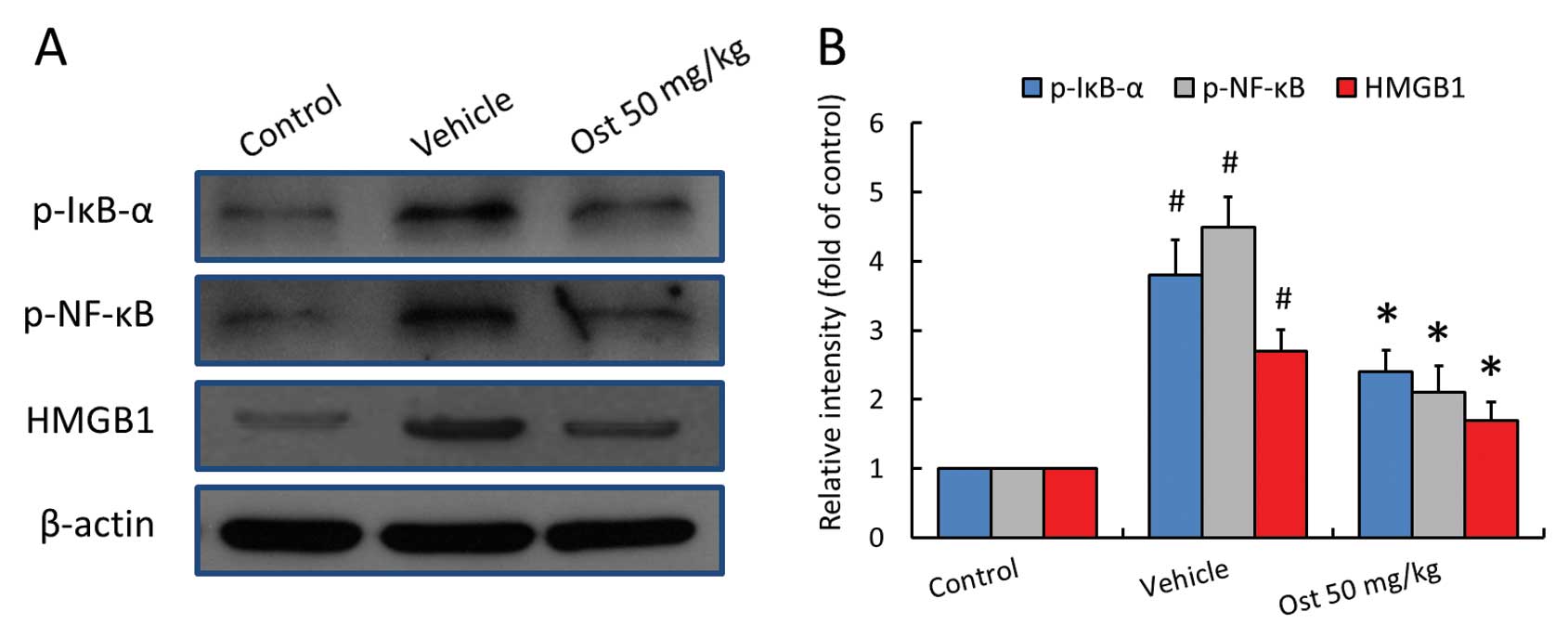
Effects of osthole on the activities of
IκB-α and NF-κB, and HMGB1 expression
To further shed light on the potential molecular
mechanisms behind the cardioprotective effects of osthole, the
expression levels of HMGB1, and the phosphorylation levels of IκB-α
and NF-κB in the ischemic myocardial tissues were measured by
western blot analysis (Fig. 5A).
A significant increase in the levels of HMGB1, and the
phosphorylation of IκB-α at Ser32 and NF-κB at Ser536 was detected
in the myocardial tissues following the induction of I/R (3.8-,
4.5- or 2.7-fold of the control levels, respectively); these
increased levels were markedly reduced following treatment with
osthole.
Discussion
Under myocardial ischemic conditions, the oxygen and
nutrient demands of the heart tissues are decreased, and blood
supply is also severely diminished (22). Therefore, the ensuing relative
insufficiency of oxygen and ATP in turn induce a myriad of changes
in cellular biomolecules and the activation of signaling pathways
that in severe cases, result in cell demise (23,24). With no effects on relieving the
primary injury, re-establishment of the blood flow following
prolonged ischemia aggravates the damage to the myocardium and
eventually leads to structural and functional changes in patients
(25). However, both the ischemic
and reperfusion insults present opportunities for pharmacological
agents to intervene and help salvage the injured heart tissues
(26). In the present study, we
found that osthole significantly reduced the infract volume induced
by myocardial I/R injury, and also prevented the depletion of CK-MB
isoenzyme and LDH enzymes in the ischemic heart tissues when
administrated upon the initiation of I/R injury. In addition,
treatment with osthole significantly preserved left ventricular
function, as reflected by a significant increase in the indices of
contraction (+dp/dtmax), relaxation
(−dp/dtmax), LVSP and a decrease in LVEDP in the
I/R insulted rat heart. All these data clearly demonstrate that
osthole exertes protective effects against myocardial I/R injury in
rats.
It is now well established that oxidative stress
resulting from reactive oxygen species (ROS) and lipid
peroxidation, which are generated in cardiac myocytes subjected to
I/R injury, plays a causative role in the development of ischemic
heart disease and may contribute to the induction of cell death
(27). Antioxidant enzymes are
essential in keeping the physiological functions and play a
fundamental role in coping with oxidative stress from endogenous or
exogenous sources (28). Several
antioxidant enzymes, such as SOD, GPx and CAT function
synergistically to provide a line of defense against oxidative
damage under a variety of stressful conditions, including I/R
injury (29). Therefore,
treatment with pharmacological agents with the ability to enhance
the activities of antioxidant enzymes, or the enforced
overexpression of antioxidant enzymes by using gene transfer
approaches, represents a potential therapeutic option to attenuate
tissue damage induced by I/R injury. For example, the combination
of SOD and CAT gene delivery has achieved additive effects in
preventing I/R-induced liver injury in mice, and herpes simplex
virus (HSV)-mediated GPx overexpression in the striatum has been
shown to reduce neuronal injury caused by cerebral I/R injury
(30,31). Furthermore, several natural
products have been shown to exert protective effects against I/R
injury by increasing the activities of antioxidant enzymes
(19,32,33). In the present study, osthole
administration significantly increased the activities of SOD, GPx
and CAT in the myocardial I/R-challenged rats in a dose-dependent
manner, and suppressed the formation of the lipid peroxidation
products, MDA and 4-HNE. These data demonstrate that treatment with
osthole enhances the capacity of antioxidant enzymes and that this
mechanism of action is attributed to its protective effects on
oxidative stress-mediated injury in myocardial I/R-challenged
animals.
Inflammatory processes play a crucial role in
myocardial I/R injury, and myocardial cell damage is thought to be
aggravated by the secondary intense inflammatory response initiated
by the infiltration of leukocytes and the production of
pro-inflammatory cytokines (34,35). Numerous experimental studies have
demonstrated that inflammatory mediators exacerbate myocardial
damage not only during acute ischemic injury, but also in the
ensuing reperfusion phase (36,37). Treatments targeting cellular
recruitment and the modulation of inflammatory cytokine secretion
and activity have proven to be a potent therapeutic strategy for
the reduction of myocardial I/R injury (34,38). Inflammation-related cytokines are
usually classified into pro- and anti-inflammatory mediators based
on their ability to promote or suppress the activation of the
immune system (39). In the
present study, we demonstrated that ischemia and the ensuing
reperfusion injury induced an increase in the plasma levels of
pro-inflammatory mediators and a decrease in the expression of
anti-inflammatory cytokines in the infarct area; these results are
in accordance with those from previous studies (40–42). Treatment with osthole
significantly decreased the levels of TNF-α and IL-6, while it
increased IL-10 levels in the serum in a dose-dependent manner, as
compared to the vehicle-treated animals. Therefore, we hypothesized
that the protective effects of osthole against myocardial I/R
injury may be attributed, at least in part, to the suppression of
the inflammatory response via the inhibition of pro-inflammatory
mediators and the promotion of anti-inflammatory cytokines.
HMGB1, a nuclear non-histone DNA-binding protein, is
highly conserved and can be found in the nuclei and cytoplasm of
almost all cell types (43). It
is a necessary and sufficient mediator of inflammation, and
targeting HMGB1 with antibodies and specific antagonists has been
shown to exert protective effects in several established
inflammation-related disease models, including I/R-induced tissue
injury (44). As shown in a
previous study, HMGB1 expression increased following myocardial I/R
injury as early as 30 min following ischemia, peaked at 12 h, and
remained significantly increased up to 7 days later (45). It has been demonstrated that HMGB1
is not only released in response to pro-inflammatory stimuli, but
also induces the production of inflammatory mediators through
multiple downstream signaling pathways, such as the receptor for
advanced glycation endproducts (RAGE)-dependent sustained NF-κB
activation (46,47). Treatment with anti-HMGB1
antibodies has been shown to prevent hepatic injury in response to
ischemic insult-enhanced activation of the NF-κB pathway in
vivo (48). Several agents
have been demonstrated to have protective effects against
myocardial I/R injury through the HMGB1-NF-κB pathway-dependent
anti-inflammatory activities (49,50). The results from the present study
revealed that myocardial I/R injury induced significant increases
in HMGB1 expression and the phosphorylation of IκB-α and NF-κB;
these increased levels were markedly reduced following treatment
with osthole, with the analogous decreased expression levels of
pro-inflammatory cytokines. Therefore, we hypothesized that the
protective effects of osthole may be attributed, at least part, to
the suppression of inflammatory cascades through an HMGB1-dependent
NF-κB signaling pathway.
In conclusion, in this study, we determined that
osthole reduces myocardial injury and improves functional recovery
following myocardial I/R injury (Fig.
6). Osthole significantly increased the activities of SOD, GPx
and CAT in a dose-dependent manner, and suppressed the formation of
the lipid peroxidation products, MDA and 4-HNE, in the injured
heart tissues. These effects of osthole correlated with the
decrease in the expression of pro-inflammatory factors, the
increase in the expression of anti-inflammatory cytokines in the
serum, as well as with the inhibition of the expression of HMGB1
and phosphorylated IκB-α and NF-κB proteins. These protective
effects of osthole against myocardial I/R injury appeared to be
mainly mediated by its anti-inflammatory and antioxidant
activities. Taken together, these data suggest that osthole may
prove to be an important therapeutic agent for limiting the
severity and functional deficits associated with myocardial I/R
injury.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (no. 81200633) and the
Guangzhou Science and Technology Plan Project (no.
2011J4100021).
References
|
1
|
Thom T, Haase N, Rosamond W, et al: Heart
disease and stroke statistics - 2006 update: a report from the
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee. Circulation. 113:e85–e151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lloyd-Jones D, Adams RJ, Brown TM, et al:
Heart disease and stroke statistics - 2010 update: a report from
the American Heart Association. Circulation. 121:e46–e215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renda G and de Caterina R: Impact of
antiplatelet therapy in heart disease. Adv Cardiol. 47:5–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gnecchi M, Danieli P and Cervio E:
Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol.
57:48–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Songco AV and Brener SJ: Initial strategy
of revascularization versus optimal medical therapy for improving
outcomes in ischemic heart disease: a review of the literature.
Curr Cardiol Rep. 14:397–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji X, Tan BK, Zhu YC, Linz W and Zhu YZ:
Comparison of cardioprotective effects using ramipril and DanShen
for the treatment of acute myocardial infarction in rats. Life Sci.
73:1413–1426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bolli R, Becker L, Gross G, Mentzer R Jr,
Balshaw D and Lathrop DA: Myocardial protection at a crossroads:
the need for translation into clinical therapy. Circ Res.
95:125–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J, Tan BK, Huang SH, Whiteman M and
Zhu YZ: Effects of natural products on ischemic heart diseases and
cardiovascular system. Acta Pharmacol Sin. 23:1142–1151.
2002.PubMed/NCBI
|
|
9
|
He H, Xu J, Xu Y, et al: Cardioprotective
effects of saponins from Panax japonicus on acute myocardial
ischemia against oxidative stress-triggered damage and cardiac cell
death in rats. J Ethnopharmacol. 140:73–82. 2012.
|
|
10
|
Ko FN, Wu TS, Liou MJ, Huang TF and Teng
CM: Vasorelaxation of rat thoracic aorta caused by osthole isolated
from Angelica pubescens. Eur J Pharmacol. 219:29–34. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang RL, Chen CC, Huang YL, et al:
Osthole increases glycosylation of hepatitis B surface antigen and
suppresses the secretion of hepatitis B virus in vitro. Hepatology.
24:508–515. 1996.PubMed/NCBI
|
|
12
|
Liu JH, Zschocke S, Reininger E and Bauer
R: Inhibitory effects of Angelica pubescens f. biserrata on
5-lipoxygenase and cyclooxygenase. Planta Med. 64:525–529.
1998.
|
|
13
|
Xu XM, Zhang Y, Qu D, Feng XW, Chen Y and
Zhao L: Osthole suppresses migration and invasion of A549 human
lung cancer cells through inhibition of matrix metalloproteinase-2
and matrix metallopeptidase-9 in vitro. Mol Med Rep.
6:1018–1022. 2012.PubMed/NCBI
|
|
14
|
Lin VC, Chou CH, Lin YC, et al: Osthole
suppresses fatty acid synthase expression in HER2-overexpressing
breast cancer cells through modulating Akt/mTOR pathway. J Agric
Food Chem. 58:4786–4793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okamoto T, Kawasaki T and Hino O: Osthole
prevents anti-Fas antibody-induced hepatitis in mice by affecting
the caspase-3-mediated apoptotic pathway. Biochem Pharmacol.
65:677–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XX, Hara I and Matsumiya T: Effects of
osthole on postmenopausal osteoporosis using ovariectomized rats;
comparison to the effects of estradiol. Biol Pharm Bull.
25:738–742. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsuda H, Tomohiro N, Ido Y and Kubo M:
Anti-allergic effects of Cnidii Monnieri Fructus (dried fruits of
Cnidium monnieri) and its major component, osthol. Biol
Pharm Bull. 25:809–812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chao X, Zhou J, Chen T, et al:
Neuroprotective effect of osthole against acute ischemic stroke on
middle cerebral ischemia occlusion in rats. Brain Res.
1363:206–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen T, Liu W, Chao X, et al:
Neuroprotective effect of osthole against oxygen and glucose
deprivation in rat cortical neurons: involvement of
mitogen-activated protein kinase pathway. Neuroscience.
183:203–211. 2011. View Article : Google Scholar
|
|
20
|
Lee YS, Kang YJ, Kim HJ, et al: Higenamine
reduces apoptotic cell death by induction of heme oxygenase-1 in
rat myocardial ischemia-reperfusion injury. Apoptosis.
11:1091–1100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwa JS, Jin YC, Lee YS, et al:
2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat
myocardial ischemia and reperfusion injury in vivo due to HO-1
induction. J Ethnopharmacol. 139:605–615. 2012.
|
|
22
|
Raedschelders K, Ansley DM and Chen DD:
The cellular and molecular origin of reactive oxygen species
generation during myocardial ischemia and reperfusion. Pharmacol
Ther. 133:230–255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanada S, Komuro I and Kitakaze M:
Pathophysiology of myocardial reperfusion injury: preconditioning,
postconditioning, and translational aspects of protective measures.
Am J Physiol Heart Circ Physiol. 301:H1723–H1741. 2011. View Article : Google Scholar
|
|
24
|
Garcia-Dorado D, Agullo L, Sartorio CL and
Ruiz-Meana M: Myocardial protection against reperfusion injury: the
cGMP pathway. Thromb Haemost. 101:635–642. 2009.PubMed/NCBI
|
|
25
|
Bell RM and Yellon DM: There is more to
life than revascularization: therapeutic targeting of myocardial
ischemia/reperfusion injury. Cardiovasc Ther. 29:e67–e79. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akhlaghi M and Bandy B: Mechanisms of
flavonoid protection against myocardial ischemia-reperfusion
injury. J Mol Cell Cardiol. 46:309–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rakotovao A, Berthonneche C, Guiraud A, et
al: Ethanol, wine, and experimental cardioprotection in
ischemia/reperfusion: role of the prooxidant/antioxidant balance.
Antioxid Redox Signal. 6:431–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu J, Hecker JG and Chiamvimonvat N:
Antioxidant enzyme gene transfer for ischemic diseases. Adv Drug
Deliv Rev. 61:351–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Limon-Pacheco J and Gonsebatt ME: The role
of antioxidants and antioxidant-related enzymes in protective
responses to environmentally induced oxidative stress. Mutat Res.
674:137–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He SQ, Zhang YH, Venugopal SK, et al:
Delivery of antioxidative enzyme genes protects against
ischemia/reperfusion-induced liver injury in mice. Liver Transpl.
12:1869–1879. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoehn B, Yenari MA, Sapolsky RM and
Steinberg GK: Glutathione peroxidase overexpression inhibits
cytochrome C release and proapoptotic mediators to protect neurons
from experimental stroke. Stroke. 34:2489–2494. 2003. View Article : Google Scholar
|
|
32
|
Zhu JW, Chen T, Guan J, Liu WB and Liu J:
Neuroprotective effects of allicin on spinal cord
ischemia-reperfusion injury via improvement of mitochondrial
function in rabbits. Neurochem Int. 61:640–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye R, Yang Q, Kong X, et al: Ginsenoside
Rd attenuates early oxidative damage and sequential inflammatory
response after transient focal ischemia in rats. Neurochem Int.
58:391–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frangogiannis NG, Smith CW and Entman ML:
The inflammatory response in myocardial infarction. Cardiovasc Res.
53:31–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jordan JE, Zhao ZQ and Vinten-Johansen J:
The role of neutrophils in myocardial ischemia-reperfusion injury.
Cardiovasc Res. 43:860–878. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steffens S, Montecucco F and Mach F: The
inflammatory response as a target to reduce myocardial ischaemia
and reperfusion injury. Thromb Haemost. 102:240–247.
2009.PubMed/NCBI
|
|
38
|
Frangogiannis NG: Chemokines in ischemia
and reperfusion. Thromb Haemost. 97:738–747. 2007.PubMed/NCBI
|
|
39
|
Chen T, Liu W, Chao X, et al: Salvianolic
acid B attenuates brain damage and inflammation after traumatic
brain injury in mice. Brain Res Bull. 84:163–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandrasekar B and Freeman GL: Induction
of nuclear factor kappaB and activation protein 1 in postischemic
myocardium. FEBS Lett. 401:30–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chandrasekar B, Colston JT and Freeman GL:
Induction of proinflammatory cytokine and antioxidant enzyme gene
expression following brief myocardial ischaemia. Clin Exp Immunol.
108:346–351. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li C, Gao Y, Xing Y, Zhu H, Shen J and
Tian J: Fucoidan, a sulfated polysaccharide from brown algae,
against myocardial ischemia-reperfusion injury in rats via
regulating the inflammation response. Food Chem Toxicol.
49:2090–2095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang H and Tracey KJ: Targeting HMGB1 in
inflammation. Biochim Biophys Acta. 1799:149–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Andrassy M, Volz HC, Igwe JC, et al:
High-mobility group box-1 in ischemia-reperfusion injury of the
heart. Circulation. 117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schmidt AM, Yan SD, Yan SF and Stern DM:
The multiligand receptor RAGE as a progression factor amplifying
immune and inflammatory responses. J Clin Invest. 108:949–955.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Volz HC, Kaya Z, Katus HA and Andrassy M:
The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb
Hemost. 36:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsung A, Sahai R, Tanaka H, et al: The
nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang WL, Zhang SP, Zhu HB and Hou J:
Cardioprotection of Asperosaponin X on experimental myocardial
ischemia injury. Int J Cardiol. 155:430–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang ZC, Jiang WL, Xu Y, Zhu HB and Hou J:
Cardioprotection with 8-O-acetyl shanzhiside methylester on
experimental myocardial ischemia injury. Eur J Pharm Sci.
47:124–130. 2012. View Article : Google Scholar : PubMed/NCBI
|