Introduction
The development of artificial joints is a major
accomplishment of orthopedic surgery, and joint replacement remains
one of the most common and successful surgical procedures.
Unfortunately, wear debris is formed at prosthetic joint
articulations, modular interfaces, and non-articulating interfaces
(1,2), which is the main reason for
prosthetic failure (3–5). Researchers further believe that the
biological response to wear particles at the bone-implant interface
is considered the main cause of aseptic loosening and osteolysis
(6,7).
During this pathological process, macrophages are
activated to release pro-inflammatory mediators, including tumor
necrosis factor-α (TNF-α), and interleukin (IL)-6 and IL-1β in
vitro (8–10), which have also been shown to be
present in periprosthetic soft tissue in vivo (11). The differentiation and activation
of macrophages is further enhanced by circulating pro-inflammatory
factors. These inflammatory factors induce local chronic
inflammatory response with the activation and recruitment of
osteoclasts to the bone-implant interface, leading to
periprosthetic osteolysis and aseptic loosening.
Previously studies have suggested that TNF-α
augments the osteoblast expression of receptor activation of
nuclear factor (NF)-κB ligand (RANKL) and macrophage
colony-stimulating factor (M-CSF) (12,13). M-CSF and RANKL have been
identified as the 2 principal cytokines of osteoclast
differentiation and activation (14–16). RANKL binds to its membrane-bound
signaling receptor, RANK, and stimulates osteoclast differentiation
and maturation (17,18). Osteoprotegerin (OPG) is a soluble
decoy receptor for RANKL and inhibits its effects by binding to
RANKL (19).
TNF-α blocks the Wnt signaling pathway; the Wnt
signaling pathway positively regulates the osteoblast expression of
OPG and the inhibition of this pathway diminishes the expression of
OPG (20–22). It can be hypothesized that TNF-α
inhibits the expression of OPG. RANKL and OPG stimulate and
inhibit, respectively, osteoclast differentiation. In this respect,
the RANKL/OPG ratio is the principal axis that regulates the
osteoclastogenic event, and this ratio is significantly higher in
severe osteolysis (23). Thus,
reducing the RANKL/OPG ratio would inhibit the formation of
osteoclasts (24). The
OPG/RANKL/RANK signaling pathway is the most important pathway
affecting osteoclast differentiation and functional regulation.
OPG, M-CSF and RANKL are expressed by stromal cells/osteoblasts.
Moreover, in vitro experiments have shown that TNF-α
promotes the expression of RANK and M-CSF on the cell membrane of
osteoclast precursor cells; as a result, the RANKL and M-CSF signal
is amplified (25,26).
The binding of RANKL to RANK prompts the induction
of several intracellular pathways by this receptor, leading to the
activation of key transcription factors, most notably NF-κB. It has
been reported that the NF-κB family of transcription factors is
crucial to pathologic responses and is essential for osteoclast
differentiation (27,28). A number of studies have shown that
the role of TNF-α can be observed from the NF-κB, c-Fos and nuclear
factor of activated T-cells, cytoplasmic, calcineurin-dependent 1
(NFATc1) activity change in osteoclast precursor cells, inducing
the differentiation of osteoclast precursor cells independent of
RANKL/RANK signaling (29–32).
These studies reported that RANKL and TNF-α activate NF-κB, c-Fos
and NFATc1 in a similar manner (29,31); NF-κB, c-Fos and NFATc1 are
essential for the promotion of osteoclast differentiation. In
addition, it has been reported that TNF-α stimulates osteoclast
differentiation through a mechanism independent of the osteoclast
differentiation factor (ODF)/RANKL-RANK system (33). TNF-α only enhances the osteoblast
expression of RANKL to promote osteoclast differentiation; however,
RANKL also independently promotes the differentiation of
osteoclasts.
These cellular mediators from macrophages may act in
an autocrine and paracrine fashion to induce an imbalance between
bone formation and resorption either by enhancing the osteoclastic
lineage or by acting on stromal or osteoblastic cells, leading to
the loss of bone stock (34). It
has previously been reported that TNF-α and IL-1β suppress
osteoblast differentiation (35,36). It has also been reported that
c-Src and IL-6 inhibit osteoblast differentiation (18) and that TNF-α secreted from
macrophages activated by titanium particles suppresses early and
late differentiation markers of osteoprogenitor cells (20). TNF-α increases the expression of
IL-6 through the activation of NF-κB in osteoblast-like cells
(37), and earlier reports have
shown that TNF-α mobilizes and activates c-Src (38,39). It can be hypothesized that TNF-α
directly controls osteoblast function in addition to the induction
of osteoclast differentiation, leading to increased bone resorption
(40).
The formation of the periprosthetic bone bed is
dependent on the balance of bone resorption and bone formation.
TNF-α, IL-6 and IL-1β promote bone loss and inhibit bone formation,
and the reduced bone formation also contributes to periprosthetic
osteolysis.
Based on these views, it can be concluded that
reducing the expression of TNF-α may provide a promising
therapeutic strategy for the treatment of periprosthetic
osteolysis. Therefore, in this study, we used adenovirus carrying
small interfering RNA (siRNA) targeting TNF-α (Ad-TNF-α-siRNA) to
downregulate TNF-α expression. We examined whether the constructed
recombinant adenovirus (Ad-TNF-α-siRNA) can effectively suppress
the TNF-α release from activated mocrophages in response to
titanium particles. We also observed the inhibitory effects of
Ad-TNF-α-siRNA on titanium particle-induced osteoclast formation
and bone resorption in the presence of RANKL, and investigated the
effect of the conditioned medium of macrophages challenged with
titanium particles (Ti CM) and Ad-TNF-α-siRNA (Ti-Ad CM) on
osteoblasts (MC3T3-E1).
Materials and methods
Preparation of titanium particles
Commercially pure titanium particles were obtained
from Zimmer Company (Warsaw, IN, USA). The majority of the titanium
particles (90%) were <10 mm in diameter. Titanuim particles were
prepared as previously described (41). The particles were sterilized by
baking at 180°C for 6 h, followed by treatment with 70% ethanol for
48 h to remove endotoxin. The particle endotoxin level was >0.1
EU/ml, as determined using a commercial detection kit (E-Toxate;
Sigma, St. Louis, MO, USA). Titanium particles were sonicated and
vortexed prior to treatment.
The concentration of particles that was used for
incubation was 0.1 mg/ml, which is similar to the concentration of
wear particles that were retrieved from the periprosthetic tissues
(42,43).
Construction of adenovirus expressing
TNF-α-siRNA
siRNA targeting the TNF-α coding region (sense,
5′-GGUUGCCUC UGUCUCAGAATT-3′ and antisense, 5′-UUCUGAGACAGA
GGCAACCTG-3′) were inserted into the KpnI and SpeI
sites in the pSilencer 2.1-hU6 vector to generate vectors
expressing TNF-α-siRNA, amplified with PCR and cloned into the
pGEMT vectors. TNF-α-siRNA were then cut from the pGEMT vectors
with KpnI and SpeI, and cloned into the same
restriction sites of pAd5 E1-MCS-CMVeGFP. The plasmid was amplified
by transformation into DH5α cells and positive clones were selected
and confirmed by the DNA Miniprep kit and KpnI digestion.
The resultant plasmid, pAd5E1-hU6-TNFα-siRNA-CMVeGFP, was
linearized with PacI digestion and subsequently
co-transformed into HEK293 cells with E3 deleted Ad backbone.
Infection of RAW264.7 with
Ad-TNF-a-siRNA
RAW264.7 cells were plated in 6-well plates at a
density of 4×105 cells/well. After 24 h, the infection
of RAW264.7 cells was carried out. The medium was removed and the
cells were incubated with titanium particles and Ad-TNF-α-siRNA
[multiplicity of infection (MOI) of 50]. After 48 h, total mRNA and
protein were extracted to detect the expression of TNF-α by
real-time RT-PCR and western blot analysis.
Cell culture
RAW264.7 murine macrophage/monocyte cells (BH-AC71;
ATCC) were maintained at 37°C and 5% CO2 in Dulbecco’s
modified Eagle’s medium (DMEM; Sigma) containing 10% FBS (HyClone,
Logan, UT, USA), 100 U/ml penicillin, and 100 U/ml streptomycin at
37°C and 5% CO2. MC3T3-E1 murine osteoblastic cells
(ATCC) were cultured in α-minimum essential medium (α-MEM; Sigma)
supplemented with containing 10% FBS, 100 U/ml penicillin and 100
U/ml streptomycin at 37°C and 5% CO2.
Collection of conditioned medium
RAW264.7 cells were plated at a density of
1.0×105 cells/24-well plates in complete DMEM. After 24
h of attachment, the cells were washed with PBS and stabilized with
serum-free DMEM for 1 h. The cells were then cultured with titanium
particles (0.1 mg/ml) or titanium particles and Ad-TNF-α-siRNA.
After 24 h of incubation with control (Cont CM),
titanium-conditioned medium(Ti CM), or titanium with Ad-TNF-α-siRNA
conditioned medium (Ti-Ad CM), the cells were collected,
centrifuged to remove the cell debris if any, and stocked at −20°C
until use. The conditioned medium was prepared as previously
described (20).
RNA isolation and real-time RT-PCR
Total RNA was extracted using TRIzol (Invitrogen)
according to the manufacturer’s instructions. The 260/280
absorbance ratio was measured for verification of the RNA purity
(NanoDrop). The first-strand cDNA was synthesized with 2 μg of
total RNA using the RevertAid First Strand cDNA Synthesis kit
(Fermentas), and one tenth of the cDNA was used for each PCR
mixture containing Express SYBR-Green PCR Supermix (Fermentas). The
reaction was subjected to a 40-cycle amplification at 95°C for 30
sec, at 95°C for 5 sec and at 60°C for 30 sec. Relative mRNA
expression of selected genes was normalized to GAPDH and quantified
using the ΔΔCT method. The sequences of the PCR primers are listed
in Table I.
 | Table IPrimers for real-time RT-PCR. |
Table I
Primers for real-time RT-PCR.
| Target | Forward primer
(5′→3′) | Reverse primer
(3′→5′) |
|---|
| TNF-α |
TCTTCTCATTCCTGCTTGTG |
ACTTGGTGGTTTGCTACG |
| IL-6 |
TCCATCCAGTTGCCTTCTTG |
TTTCTCATTTCCACGATTTCCC |
| IL-1β |
ATCTCGCAGCAGCACATC |
CAGCAGGTTATCATCATCATCC |
| TRAP |
GCAGCCAAGGAGGACTAC |
CCCACTCAGCACATAGCC |
| NFATc1 |
TCTTCCGAGTTCACATCC |
ACAGCACCATCTTCTTCC |
| GADPH |
TCAACGGCACAGTCAAGG |
ACTCCACGACATACTCAGC |
| M-CSF |
TATTGCGACACCGAATCC |
CTTGCTGATCCTCCTTCC |
| RANKL |
CAGGAGGATGAAACAAGCC |
GCAGCATTGATGGTGAGG |
| OPG |
GTGTGAGTGTGAGGAAGG |
TTTATACAGGGTGCTTTCG |
Protein isolation and western blot
analysis
Cells were lysed in RIPA buffer (20 mM Tris-HCl, pH
7.5, 200 mM NaCl, 1% Triton X-100 and 1 mM dithiothreitol)
containing protease inhibitor cocktail (Roche, Indianapolis, IN).
The protein concentration was measured with a protein assay kit
(BCA) following the manufacturer’s instructions. Total protein was
subjected to SDS-polyacrylamide gel electrophoresis and transferred
onto PVDF membranes. The blot was probed with primary antibodies;
anti-TNF-α, NFATc1 (both from Cell Signaling Technology, Inc.,
Danvers, MA, USA) and anti-bone morphogenetic protein (BMP)-2
(Abcam, Cambridge, MA, USA). Anti-β-actin (CWBiotech, Beijing,
China) was used as a loading control. Subsequently, the blots were
washed in TBST (10 mM Tris-HCl, 50 mM NaCl and 0.25% Tween-20) and
incubated with secondary antibody. The presence of target proteins
was detected using enhanced Chemiluminescence reagents (Millipore
Corp., Billerica, MA, USA).
Cell viability:
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay
The effect of recombinant adenovirus and titanium
particles on RAW264.7 cell viability was examined using MTT assay.
RAW264.7 cells (2×104 cells/well) were cultured in RANKL
and titanium particles containing DMEM with Ad-TNF-α-siRNA for 24,
48 and 72 h, and then incubated with 0.5 mg/ml of MTT at 37°C for 4
h. Following the removal of the supernatant, the insoluble formazan
crystal was dissolved in 200 ml of dimethyl sulfoxide (DMSO) and
absorbance was measured using a 490 nm wavelength Synergy
microplate reader (BioTek Instruments).
Tartrate-resistant acid phosphatase
(TRAP)
RAW264.7 cells were plated at a density of
1×104 cells/96-well. After 24 h of attachment, the cells
were cultured in the presence of soluble RANKL (50 ng/ml) and
titanium particles (100 μg/ml) with or without Ad-TNF-α-siRNA (MOI
of 50) After 5 days, RAW264.7 cells with different treatments were
fixed and stained for TRAP expression according to the
manufacturer’s instructions (Sigma). The nuclei were counterstained
with acid hematoxylin solution. The TRAP-positive cells with ≥3
nuclei were counted as osteoclasts (44).
Resorption pit assay for osteoclasts
RAW264.7 cells were plated at a density of
2×104 cells/24-well plates (Corning Inc., Corning, NY,
USA). After 24 h of attachment, the cells were cultured in the
presence of soluble RANKL (50 ng/ml) and titanium particles (100
μg/ml) with or without Ad-TNF-α-siRNA (MOI of 50). After 10 days,
to analyze the surface for pit formation, the medium was aspirated
from the wells, and 100 μl of 10% bleach solution were added. Cells
were incubated with the bleach solution for 5 min at room
temperature. The wells were washed twice with distilled water and
allowed to dry at room temperature for 3 to 5 h. Individual pits or
multiple pit clusters were observed using a microscope at ×10
magnification (Leica). We selected 4 images by the sum of
absorption area in each well for statistical analysis (n=3). Bone
absorption area was measured using image-analysis software
(IPP6.0).
ELISA
RAW264.7 cells were incubated with titanium
particles (100 ng/ml) for 24 h. Ti CM was collected to determine
the concentration of TNF-α. Mouse TNF-α and the MBP-2 ELISA kit
(Ameko) were used for a quantitative measurement according to the
manufacturer’s recommendations.
Statistical analysis
Data are expressed as the means ± SE of ≥3
determinations. Differences between groups were analyzed using
analysis of variance (ANOVA). A P-value <0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS version 17.0 software.
Results
Effects of recombinant adenovirus
(Ad-TNF-α-siRNA) and titanium particles on cell viability
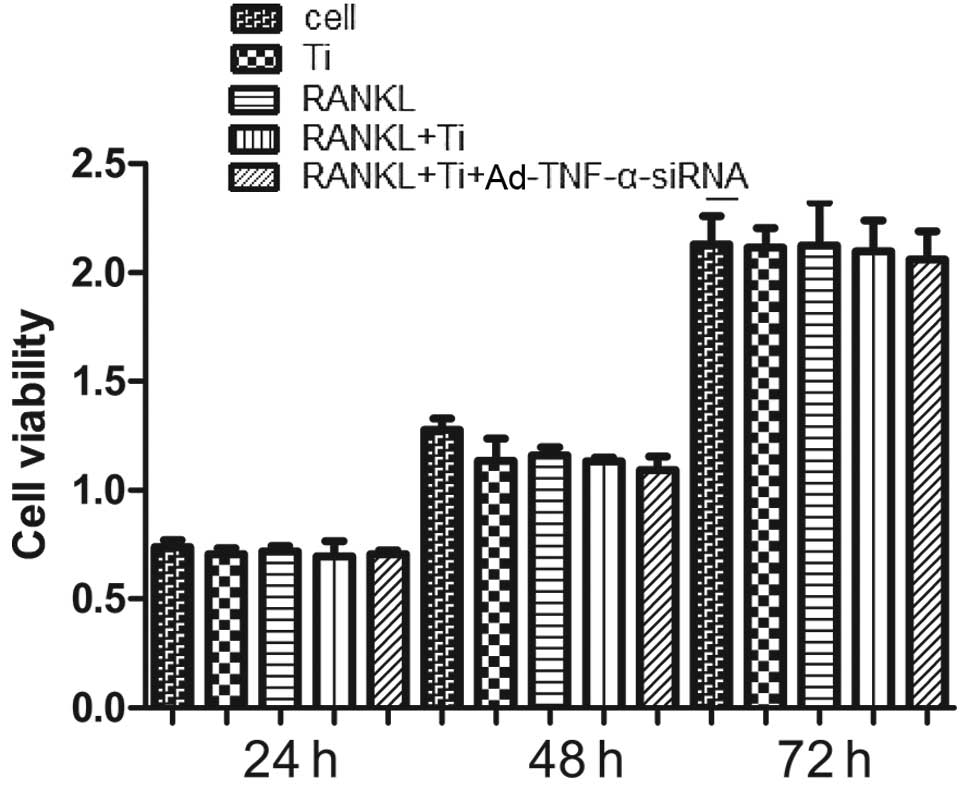
Compared to the controls, neither titanium particles
nor a combination of titanium particles and Ad-TNF-α-siRNA (MOI of
50) affected the viability of RAW264.7 cells (Fig. 1). These results suggest that both
Ad-TNF-α-siRNA (MOI of 50) and titanium particles are not toxic to
RAW264.7 cells.
Suppressive effects of Ad-TNF-α-siRNA on
titanium particle-induced inflammatory factor
We investigated the effect of the downregulation of
the mRNA expression of TNF-α by Ad-TNF-α-siRNA from activated
mocrophages in response to titanium particles using comparison
tests with different MOIs (refers to virus particles added/cell).
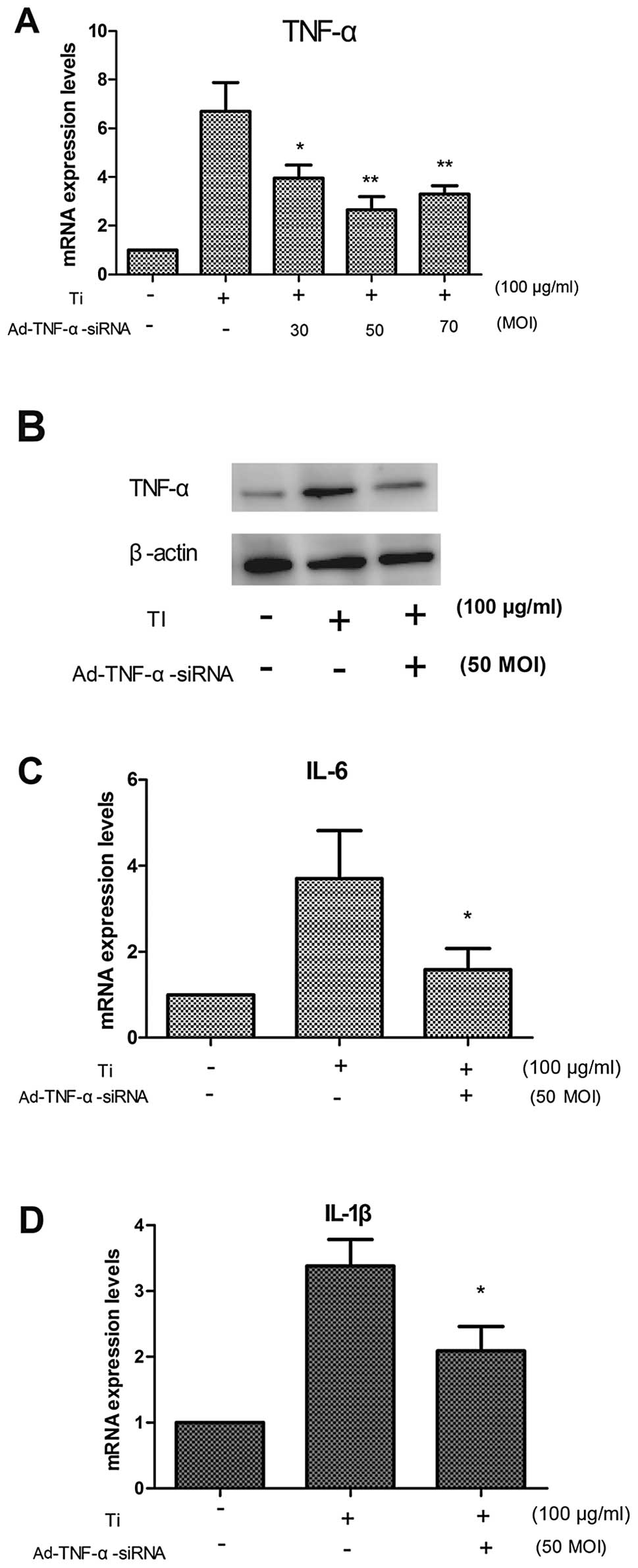
Our results showed that the mRNA expression of TNF-α was
significantly reduced at a MOI of 50 after 48 h (Fig. 2A). The same result was observed
for the TNF-α protein levels by western blot analysis after 48 h
(Fig. 2B).
We also detected the expression of the inflammatory
factors, IL-1β and IL-6. The results showed that the downregulation
of TNF-α mRNA reduced the mRNA expression of inflammatory
cytokines, such as IL-6 (Fig. 2C)
and IL-1β (Fig. 2D). These
results suggest that TNF-α promotes the mRNA expression of IL-6 and
IL-1β.
Ad-TNF-α-siRNA reduces titanium
particle-induced osteoclastogenesis and bone resorption
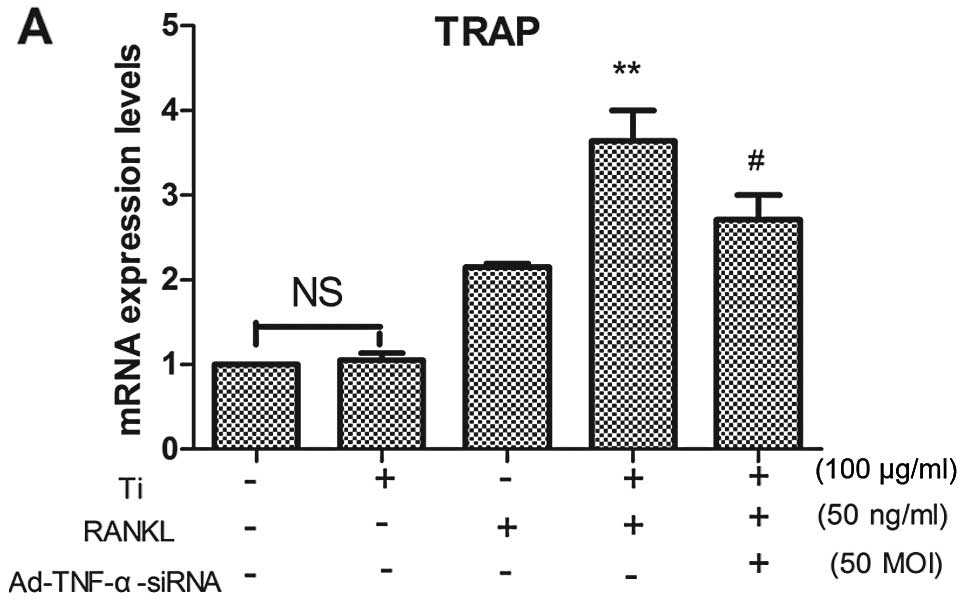
TRAP is a marker that is widely used to identify
osteoclasts. We examined the mRNA expression of TRAP in RAW264.7
cells cultured in the presence of RANKL and/or titanium particles
for 4 days. Our data showed that the mRNA expression of TRAP was
significantly higher in the cells cultured with titanium particles
than in those cultured without titanium particles in the presence
of RANKL (Fig. 3A). The
downregulation TNF-α by Ad-TNF-α-siRNA reduced the mRNA expression
of TRAP (Fig. 3A). Titanium
particles did not stimulate the mRNA expression of TRAP in RAW264.7
cells in the absence of RANKL. The number of TRAP+
multinucleated cells was also significantly higher in the cells
cultured with titanium particles than in those cultured without
titanium particles in the presence of RANKL (Fig. 3B and D). Osteoclastogenesis did
not occur in the cells cultured without RANKL. These results
suggest that TNF-α stimulates osteoclastogenesis only in the
presence of RANKL.
We further investigated osteoclast function induced
by titanium particles and RANKL with or without Ad-TNF-α-siRNA. We
examined resorption pits on osteoclast culture plates. The results
showed that the area of the resorption pits was significantly
greater on the osteoclast culture plates cultured with titanium
particles than on those cultured without particles in the presence
of RANKL (Fig. 3C and E). The
downregulation of TNF-α by Ad-TNF-α-siRNA significantly reduced the
area of the resorption pits on the osteoclast culture plates
compared to that on the plates cultured with titanium particles and
RANKL (Fig. 3C and E). These
results suggest that TNF-α may exert a strong synergistic effect
with RANKL, increasing local bone resorption in vivo.
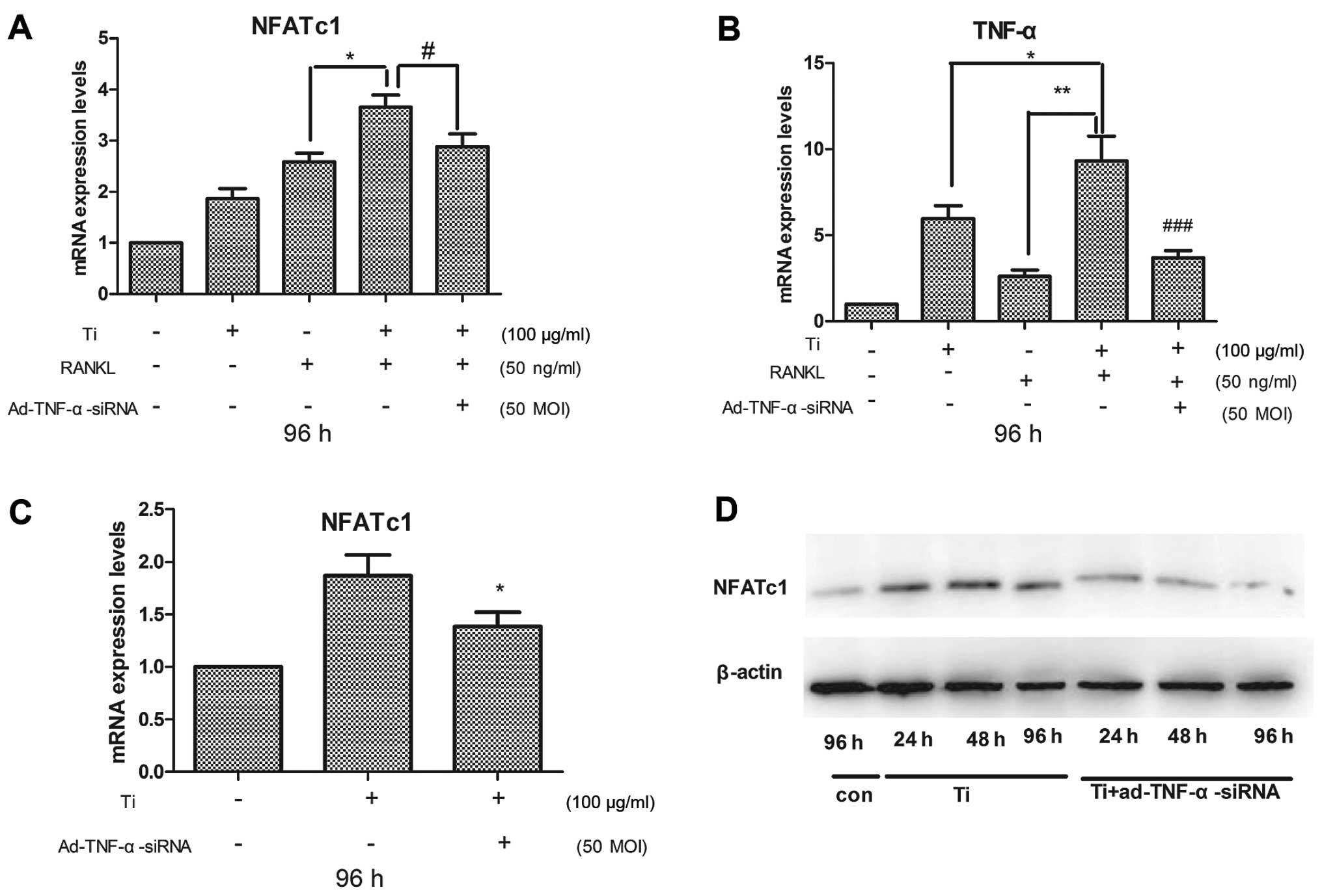
NFATc1 plays a crucial role in osteoclastogenesis
(45,46). Real-time PCR demonstrated that in
the presence of RANKL, the mRNA expression of NFATc1 in the
RAW264.7 cells cultured with titanium particles was greater than in
the cells cultured without titanium particles (Fig. 4A). The downregulation of TNF-α by
Ad-TNF-α-siRNA in the cells cultured with titanium particles and
RANKL reduced the expression of NFATc1 compared to that in cells
cultured without Ad-TNF-α-siRNA (Fig.
4A). These results showed that TNF-α expression was induced in
the cells cultured with RANKL (Fig.
4B). Real-time RT-PCR revealed that the downregulation of TNF-α
by Ad-TNF-α-siRNA reduced NFATc1 expression in the cells cultured
with titanium particles and RANKL (Fig. 4A). These results suggest that
NFATc1 is activated by TNF-α.
We further determined NFATc1 expression in RAW264.7
cells cultured with titanium particles in the absence of RANKL. We
examined NFATc1 expression by real-time PCR and western blot
analysis. The results showed that the titanium particles activated
the expression of NFATc1, and that the downregulation of TNF-α by
Ad-TNF-α-siRNA reduced the expression of NFATc1 in the cells
cultured without RANKL (Fig. 4C).
Western blot analysis revealed that the downregulation of TNF-α
reduced NFATc1 protein expression in the cells in a time-dependent
manner (Fig. 4D). These results
demonstrated that TNF-α activated NFATc1 expression, and suggest
that the synergistic effect of TNF-α with RANKL increases local
osteoclastogenesis and bone resorption; this synergistic effect
activates the expression of NFATc1. NFATc1 is essential to promote
osteoclast differentiation (47).
Effects of conditioned medium on MC3T3-E1
mouse osteoblastic cell line
RANKL has been identified as the principal cytokine
of osteoclast differentiation and activation. RANKL is synthesized
by stromal cells/osteoblasts. We examined the effect of Ti CM on
RANKL and M-CSF expression in MC3T3-E1 cells. The results showed
that Ti CM significantly increased the mRNA expression of RANKL in
MC3T3-E1 cells (Fig. 5A), and Ti
CM had absolutely no effect on the mRNA expression of M-CSF in the
MC3T3-E1 cells. OPG expression was not detected by real-time RT-PCR
under our culture conditions. Ti-Ad CM significantly reduced the
mRNA expression of RANKL (Fig.
5A). In addition, RANKL stimulated RAW264.7 mouse macrophages
cell differentiation into osteoclasts on its own in a
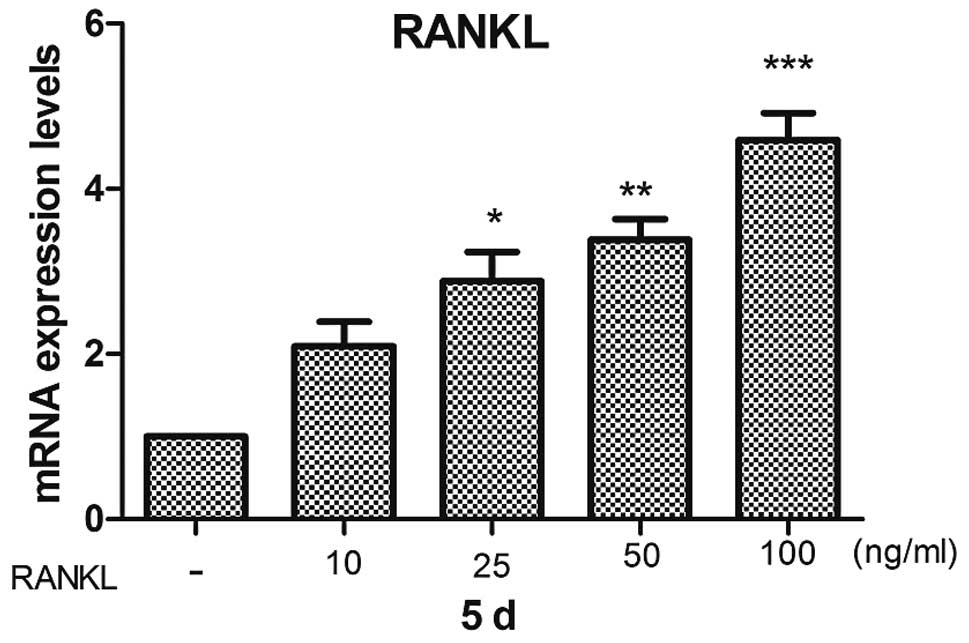
dose-dependent manner in vitro (Fig. 7). Thus, these results suggest that
TNF-α can indirectly promote osteoclast differentiation by
increasing RANKL expression in MC3T3-E1 cells. Ad-TNF-α-siRNA
significantly reduced the expression of RANKL.
We further examined IL-6 expression in MC3T3-E1
cells cultured with Ti CM or Ti-Ad CM. Our results showed that IL-6
expression was increased in the MC3T3-E1 cells cultured with Ti CM,
and was reduced in the cells cultured with Ti-Ad CM (Fig. 5B). These results suggest that
TNF-α promotes IL-6 expression in MC3T3-E1 cells.
Discussion
The present study describes a novel approach for
inflammatory reduction in activated macrophages in response to
titanium particles by siRNA-mediated TNF-α silencing in the
RAW264.7 mouse macrophage cell line. Recombinant adenovirus
(Ad-TNF-α-siRNA) significantly inhibited the TNF-α release from
activated macrophages in response to particle debris. We detected
TNF-α expression in macrophages activated by titanium particles by
real-time PCR and western blot analysis (Fig. 2A and B). The downregulation of
TNF-α by Ad-TNF-α-siRNA also reduced the gene expression of
inflammatory cytokines, such as IL-1β (Fig. 2D) and IL-6 (Fig. 2C); these inflammatory factors play
a critical role in osteolysis (48). Our data suggest that titanium
particles stimulate RAW264.7 mouse macrophage cells to secrete
pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6; these
cytokines may not directly induce RAW264.7 mouse macrophage cells
to differentiate into osteoclasts in the absence of RANKL, but
these cytokines can activate NFATc1 mRNA expression in the absence
of RANKL (Fig. 4A). A previous
study indicated that inactivated NFATc1 can suppress titanium
particle-induced osteoclast formation (10). NFATc1 is essential to promote
osteoclast differentiation (47,49). The downregulation of TNF-α by
Ad-TNF-α-siRNA reduced the expression of NFATc1 mRNA. Thus, we
hypothesized that NFATC1 was activated by TNF-α. This result is in
agreement with that of a previous study (29). TNF-α cannot promote the formation
of osteoclasts in the absence of RANKL, but TNF-α promotes the
formation of osteoclasts in the presence of RANKL, in accordance
with previous studies (50,51). RANKL stimulates osteoclast
precursor differentiation into osteoclasts by activating c-Fos,
NF-κB and NFATc1 (29,47,51). TNF-α activates NF-κB, c-Fos and
NFATc1 in a similar manner (29).
Despite activating similar signaling, TNF-α does not effectively
induce osteoclast differentiation in the absence of RANKL (Fig. 3D). Thus, it can be hypothesized
that NFATc1, TNF-α, IL-1β and IL-6 cannot activate the terminal
differentiation of osteoclasts. TNF-α has already been proven to
stimulate osteoclast differentiation in the presence of M-CSF.
Therefore, we hypothesized that TNF-α cannot directly induce
osteoclast differentiation, but indirectly induces osteoclast
differentiation in the presence of RANKL and/or M-CSF. Thus, RANKL
and M-CSF can activate the terminal differentiation of
osteoclasts.
Titanium particles in conjunction with RANKL
stimulate osteoclast formation and bone absorption; this effect is
more evident in the presence of both titanium particles and RANKL,
than with RANKL alone. The downregulation of TNF-α by
Ad-TNF-α-siRNA in activated macrophages in response to titanium
particles reduced the formation of osteoclasts and bone absorption
in the presence of RANKL and titanium particles compared with group
cultured with titanium particles and RANKL without Ad-TNF-α-siRNA
(Fig. 3). Our results show that
TNF-α cannot directly effectively promote osteoclast formation in
the absence of RANKL, but TNF-α and RANKL have a synergistic
effect, promoting osteoclast formation and bone absorption. At the
same time, our results showed that the expression of NFATc1 in the
presence of RANKL and titanium particles was significantly higher
than in the presence of RANKL alone, and that the downregulation of
TNF-α by recombinant adenovirus significantly reduced the
expression of NFATc1. However, the above results confirmed that
TNF-α slightly activated the expression of NFATc1; thus, we
detected the expression of TNF-α in the titanium, RANKL and RANKL
and titanium group. Our results confirmed that RANKL also
stimulated the expression of TNF-α. The increased expression of
TNF-α increased the expression of NFATc1. Furthermore, it was
confirmed that TNF-α promotes the expression of RANK on the cell
membrane of osteoclast precursor cells; as a result, the RANKL
signal is amplified, further activating NFATc1 expression. Thus, we
hypothesized that the mechanism behind the synergistic promoting
effect of TNF-α and RANKL on osteoclast formation and bone
absorption may well be that TNF-α activates NFATc1 to facilitate
osteoclast formation and bone absorption in the presence of RANKL.
Recombinant adenovirus (Ad-TNF-α-siRNA) effectively reduces
osteoclasts formation and bone resorption.
RANKL and M-CSF are essential to promote the
osteoclast differentiation factor, which is expressed in
osteoblasts during each stage, and TNF-α augments the osteoblast
expression of RANKL and M-CSF (12,52,53). RANKL has been demonstrated to not
only deliver a final osteoclast differentiation signal, but to also
activate osteoclasts and promote their survival (54–57).
Our results indicated that RANKL stimulated RAW264.7
mouse macrophages cell differentiation into osteoclasts on its own
in a dose-dependent manner in vitro (Fig. 7). RANKL expression was increased
in the conditioned medium collected from macrophages activated by
Ti CM in vitro (Fig. 5A).
Furthermore, the mRNA expression of RANKL was downregulated in the
conditioned medium collected from macrophages activated by titanium
particles and pre-treated with Ad-TNF-α-siRNA (Ti-Ad CM) compared
to that in conditioned medium of the cells from the Ti CM
pre-treated group (Fig. 5A).
Therefore, we speculated that TNF-α in the macrophages activated by
titanium particles augments the osteoblast expression of RANKL.
Taken together, these data suggest that TNF-α indirectly promotes
osteoclast differentiation by increasing RANKL expression in
MC3T3-E1 cells.
Bone remodeling is a physiological process that
involves the resorption and synthesis of bone by osteoclasts and
osteoblasts, respectively (59).
During the pathological process, TNF-α, IL-1β and IL-6 are
overexpressed in macrophages stimulated by titanium particles
(58). These inflammatory factors
have been shown to suppress osteoblast differentiation (35,36,60). It has been proven that Ti CM
reduces osteogenic parameters, such as alkaline phosphatase (ALP)
activity and the expression of Runt-related transcription factor 2
(Runx2), and suppresses late differentiation markers of
osteoprogenitor cells (20).
TNF-α, IL-1β and IL-6 are overexpressed in macrophages activated by
titanium particles, but only TNF-α mimicks the effects of Ti CM on
the ALP activity of osteoprogenitor cells, compared to IL-1β and
IL-6. IL-1β and IL-6 have no significant effect on ALP activity
(20). Thus, it can be
hypothesized that TNF-α suppresses osteoblast differentiation. This
idea is consistent with previous studies (36,61). Thus, Ad-TNF-α-siRNA can inhibit
the suppressive effect of TNF-α on osteoblast differentiation.
A recent study illustrated that IL-6 impairs
osteoblast maturation in immature osteoblasts in vitro and
in vivo. By contrast, in mature osteoblasts, IL-6 is no
longer able to impair osteoblast maturation (60). It has been reported that TNF-α
increases the expression of IL-6 in osteoblast-like cells (37). This would aggravate the inhibitory
effect on osteoblast differentiation. Nevertheless, the
downregulation of TNF-α by Ad-TNF-α-siRNA reduced the expression of
IL-1β and IL-6 in macrophages activated by titanium particles
(Fig. 2C and D). The expression
of IL-6 in the MC3T3-E1 cells cultured in conditioned medium
challenged with titanium particles and Ad-TNF-α-siRNA (Ti-Ad CM)
was reduced compared with that in the cells in the Ti CM group
(Fig. 5B). Thus, Ad-TNF-α-siRNA
can inhibit the suppressive effect of IL-6 on osteoblast
differentiation through the reduced exogenous and endogenous IL-6
expression during the pathological process of titanium particle
induced-osteolysis.
Moreover, the Wnt and BMP pathways are regulated by
several processes; TNF-α is able to hamper osteoblast
differentiation process by annulling Wnt and BMP signaling
(20). Wnt and BMP pathways
tightly regulate osteoblast differentiation (62). Furthermore, during the
differentiation process of osteoblasts, OPG expression is
significantly downregulated in osteocytes by abolishing Wnt
signaling (63), suggesting that
the reduction in osteocytic OPG and the concomitant increase in the
osteocytic RANKL/OPG ratio contribute to the increased number of
osteoclasts and resorption. In fact, Wnt serves as an autocrine
stimulator of OPG expression in osteoblasts, and Wnt signaling
promotes bone formation by upregulating OPG and downregulating
RANKL (64). This shows that
TNF-α not only inhibits bone formation by annulling Wnt and BMP-2,
but also promotes bone absorption through the upregulation the
RANKL/OPG ratio.
TNF-α and IL-6 suppressed osteoblast
differentiation, which likely establishes a functional loop to
maintain osteoblasts in a less mature status. A previous study
suggested that the RANKL/OPG ratio is increased in immature
osteoblasts and is reduced in mature osteoblasts (65). TNF-α and IL-6 suppress osteoblast
differentiation, leading to an increase in the number of immature
osteoblasts and the concomitant increase in the immature osteoblast
RANKL/OPG ratio contributes to the increased number of osteoclasts
and resorption, and reduced bone formation.
Osteoclasts and osteoblasts contribute individually
to bone remodeling, whereas their interactions control their
cellular activities, as well as the intensity of bone remodeling.
These interactions can be established either through a cell-cell
contact, in which molecules of the integrin family may be involved,
or by the release of many polypeptide factors and/or their soluble
receptor chains. During the pathological process of titanium
particle-induced osteolysis, TNF-α not only promotes osteoclast
formation and bone resorption in the presence of RANKL, but also
suppresses osteoblast differentiation and increases the RANKL/OPG
ratio, leading to bone mass loss, and the reduced bone formation
also contributes to periprosthetic bone tissue; as a result
periprosthetic osteolysis and subsequent aseptic loosening are the
most common causes of TJA failure.
Thus far, there is no effective treatment for
aseptic joint loosening apart from reoperation; the reoperation of
patients causes serious physical and psychological trauma and
increases the economic burden; reoperation also increases the risk
of subsequent multiple peri-operative complications (particularly
in elderly patients). Therefore, the discovery of non-surgical
methods to combat aseptic joint loosening is a hot research topic;
however, no effective treatment has yet been discovered. Although
the mechanism behind aseptic joint loosening is not clear, it
involves the generation of wear debris particles which are
phagocytosed by macrophages and other inflammatory cells, resulting
in cellular activation and the release of pro-inflammatory
mediators and cytokines, which cause periprosthetic osteolysis and
subsequent aseptic loosening, the most common causes of TJA failure
(8,66–69). Our results confirmed that TNF-α
plays an important role in the particle-induced osteoclastogenic
and osteolytic events. This view is also supported by previous
studies using animal models, in vitro cell cultures and
clinic trials (70–73). Although therapeutic agents against
pro-inflammatory mediators, such as TNF have shown promise in
animal models, no approved treatments are yet available to
osteolysis patients. There are concerns as to the side-effects of
pharmaceutical drugs during the systemic administration of certain
drugs. As a result, there is no approved pharmacological treatment
for periprosthetic osteolysis thus far. Therefore, gene therapy,
instead of drug therapy, targeting inflammatory factors may provide
a therapeutic approach with which to avoid the side-effects of
anti-inflammatory drugs during long-term systemic
administration.
Gene therapy has been applied to the field of
orthopedics. Clinical trials have already been initiated for
arthritis and the aseptic loosening of prosthetic joints, and the
development of bone-healing applications is at an advanced,
pre-clinical stage (74).
Orthopedic gene therapy originated during the early 1990s to
deliver genes to joints (75,76). The aim is to engineer intra- and
peri-articular tissus to synthesize antiarthritic gene products,
thereby providing a sustained, local therapy for individual
arthritic joints. This approach is attractive since joints are
discrete, accessible cavities that can be readily injected.
RNAi interference (RNAi) is a standard method for
the knockdown of any target gene of interest in vitro,
exploring a naturally occurring catalytic mechanism. The
downregulation of pathologically relevant genes which are
aberrantly expressed in a given disease will offer novel
therapeutic approaches. The mechanism behind RNAi involves siRNAs,
which are cleaved into the fragments known as siRNA (21–23
nucleotides in length) by the enzyme Dicer (77). siRNA therapeutics have developed
rapidly, with ongoing or planned clinical trials, and thus we used
this siRNA targeting TNF-α to inhibit titanium particle-induced
bone resorption. siRNA delivery in vivo is of critical
importance for its implementation.
Adenoviral gene therapy, due to its carrier safety,
and the fact that it does not need not to be integrated into the
host cell genome, has relative stability and high transfection
efficiency (78–80), is currently the most widely used
viral vectors. Moreover, RNAi has been applied to the clinical
practice for the treatment of cancer, and as shown in our study,
recombinant adenovirus (Ad-TNF α-siRNA) does not exert toxic
effects on RAW264.7 murine macrophage cells (Fig. 1). It is thus clear that its safety
is reliable and the method is feasible. Ad-TNF-α-siRNA effectively
suppressd osteoclast differentiation following exposrure to
titanium particles and reduced the inhibitory effect of TNF-α
secretion from macrophages activated by titanium particles on
osteoblast differentiation, which may be useful for the prevention
and/or treatment of osteolysis and aseptic loosening, thus
preventing TJA. Our in vitro results revealed that TNF-α
promoted osteoclast differentiation by stimulating the expression
of RANKL in osteoblasts, and that TNF-α synergizes with RANKL to
promote osteoclast differentiation. Furthermore, Ad-TNF-α-siRNA
effectively suppressed osteoclast differentiation following
exposure to titanium particles and may well reduce the inhibitory
effect of TNF-α secretion from macrophages activated by titanium
particles, thus preventing osteoblast differentiation. Taken
together, our data suggest that the downregulation of the
expression of TNF-α by Ad-TNF-α-siRNA provides a promising
therapeutic strategy for the treatment of periprosthetic
osteolysis.
Our results also highlight the need for more
effective treatment methods. Further evidence of the effect of
Ad-TNF-α-siRNA on particle-induced osteolysis is required in in
vivo experiments on animal models. This would involve the
creation of an animal model of aseptic loosening, and would then
involve the local administration of an intra-articular injection of
recombinant adenovirus in order to detect the effect of recombinant
adenovirus on titanium particle-induced pro-inflammatory cytokine
expression and periprosthetic osteolysis. Our data may aid in the
continued advances in research for the prevention and/or treatment
of particle-induced osteolysis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NSFC, grant no. 81060146).
References
|
1
|
Harris WH: The problem is osteolysis. Clin
Orthop Relat Res. 311:46–53. 1995.PubMed/NCBI
|
|
2
|
Goldring SR, Clark CR and Wright TM: The
problem in total joint arthroplasty: aseptic loosening. J Bone
Joint Surg Am. 75:799–801. 1993.PubMed/NCBI
|
|
3
|
Kadoya Y, Kobayashi A and Ohashi H: Wear
and osteolysis in total joint replacements. Acta Orthop Scand
Suppl. 278:1–16. 1998.PubMed/NCBI
|
|
4
|
Friedman RJ, Black J, Galante JO, Jacobs
JJ and Skinner HB: Current concepts in orthopaedic biomaterials and
implant fixation. Instr Course Lect. 43:233–255. 1994.PubMed/NCBI
|
|
5
|
Harris WH: Wear and periprosthetic
osteolysis: the problem. Clin Orthop Relat Res. 393:66–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacobs JJ, Roebuck KA, Archibeck M, Hallab
NJ and Glant TT: Osteolysis: basic science. Clin Orthop Relat Res.
393:71–77. 2001. View Article : Google Scholar
|
|
7
|
Ingham E and Fisher J: The role of
macrophages in osteolysis of total joint replacement. Biomaterials.
26:1271–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hallab NJ and Jacobs JJ: Biologic effects
of implant debris. Bull NYU Hosp Jt Dis. 67:182–188.
2009.PubMed/NCBI
|
|
9
|
Kaufman AM, Alabre CI, Rubash HE and
Shanbhag AS: Human macrophage response to UHMWPE, TiAlV, CoCr, and
alumina particles: analysis of multiple cytokines using protein
arrays. J Biomed Mater Res A. 84:464–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu F, Zhu Z, Mao Y, Liu M, Tang T and Qiu
S: Inhibition of titanium particle-induced osteoclastogenesis
through inactivation of NFATc1 by VIVIT peptide. Biomaterials.
30:1756–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shanbhag AS, Jacobs JJ, Black J, Galante
JO and Glant TT: Cellular mediators secreted by Interfacial
membranes obtained at revision total hip arthroplasty. J
Arthroplasty. 10:498–506. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimble RB, Srivastava S, Ross FP,
Matayoshi A and Pacifici R: Estrogen deficiency increases the
ability of stromal cells to support murine osteoclastogenesis via
an interleukin-1and tumor necrosis factor-mediated stimulation of
macrophage colony-stimulating factor production. J Biol Chem.
271:28890–28897. 1996. View Article : Google Scholar
|
|
13
|
Mori T, Miyamoto T, Yoshida H, Asakawa M,
Kawasumi M, Kobayashi T, et al: IL-1β and TNFα-initiated IL-6-STAT3
pathway is critical in mediating inflammatory cytokines and RANKL
expression in inflammatory arthritis. Int Immunol. 23:701–712.
2011.
|
|
14
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, et al: Osteoprotegerin (OPG)
ligand is a cytokine that regulates osteoclast differentiation and
activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quinn JM, Elliott J, Gillespie MT and
Martin TJ: A combination of osteoclast differentiation factor and
macrophage-colony stimulating factor is sufficient for both human
and mouse osteoclast formation in vitro. Endocrinology.
139:4424–4427. 1998. View Article : Google Scholar
|
|
16
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, et al: Osteoclast differentiation
factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar
|
|
17
|
Takayanagi H, Ogasawara K, Hida S, Chiba
T, Murata S, Sato K, et al: T-cell-mediated regulation of
osteoclastogenesis by signalling cross-talk between RANKL and
IFN-gamma. Nature. 408:600–605. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goater JJ, O’Keefe RJ, Rosier RN, et al:
Efficacy of ex vivo OPG gene therapy in preventing wear debris
induced osteolysis. J Orthop Res. 20:169–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SS, Sharma AR, Choi BS, Jung JS, Chang
JD, Park S, et al: The effect of TNFα secreted from macrophages
activated by titanium particles on osteogenic activity regulated by
WNT/BMP signaling in osteoprogenitor cells. Biomaterials.
33:4251–4263. 2012.
|
|
21
|
Glass DA II, Bialek P, Ahn JD, Starbuck M,
Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, et al:
Canonical Wnt signaling in differentiated osteoblasts controls
osteoclast differentiation. Dev Cell. 8:751–764. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holmen SL, Zylstra CR, Mukherjee A, Sigler
RE, Faugere MC, Bouxsein ML, et al: Essential role of beta-catenin
in postnatal bone acquisition. J Biol Chem. 280:21162–21168. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grimaud E, Soubigou L, Couillaud S,
Coipeau P, Moreau A, Passuti N, et al: Receptor activator of
nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is
increased in severe osteolysis. Am J Pathol. 163:2021–2031. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gori F, Hofbauer L, Dunstan CR, Spelsberg
TC, Khosla S and Riggs BL: The expression of osteoprotegerin and
RANK ligand and the support of osteoclast formation by
stromal-osteoblast lineage cells is developmentally regulated.
Endocrinology. 141:4768–4776. 2000.PubMed/NCBI
|
|
25
|
Yao Z, Li P, Zhang Q, et al: Tumor
necrosis factor-increases circulating osteoclast precursor numbers
by promoting their proliferation and differentiation in the bone
marrow through up-regulation of c-Fms expression. J Biol Chem.
281:11846–11855. 2006. View Article : Google Scholar
|
|
26
|
Zhang YH, Heulsmann A, Tondravi MM, et al:
Tumor necrosis factor-alpha (TNF) stimulates RANKL-induced
osteoclastogenesis via coupling of TNF type 1 receptor and RANK
signaling pathways. J Biol Chem. 276:563–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abu-Amer Y: Advances in osteoclast
differentiation and function. Curr Drug Targets Immune Endocr
Metabol Disord. 5:347–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teitelbaum S: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamashita T, Yao Z, Li F, et al: NF-kappaB
p50 and p52 regulate receptor activator of NF-kappaB ligand (RANKL)
and tumor necrosis factor-induced osteoclast precursor
differentiation by activating c-Fos and NFATc1. J Biol Chem.
282:18245–18253. 2007. View Article : Google Scholar
|
|
30
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao Z, Xing L and Boyce BF: NF-κB p100
limits TNF-induced bone resorption in mice by a TRAF3-dependent
mechanism. J Clin Invest. 119:3024–3034. 2009.
|
|
32
|
Fullerb K and Murphy C: TNF alpha potently
activates osteoclasts, through a direct action independent of and
strongly synergistic with RANKL. Endocrinology. 143:1108–1118.
2002.PubMed/NCBI
|
|
33
|
Kobayashi K, Takahashi N, Jimi E, Udagawa
N, Takami M, Kotake S, et al: Tumor necrosis factor alpha
stimulates osteoclast differentiation by a mechanism independent of
the ODF/RANKL-RANK interaction. J Exp Med. 191:275–286. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gallo J, Raska M, Mrazek F and Petrek M:
Bone remodeling, particle disease and individual susceptibility to
periprosthetic osteolysis. Physiol Res. 57:339–349. 2008.PubMed/NCBI
|
|
35
|
Hikiji H, Shin WS, Koizumi T, Takato T,
Susami T, Koizumi Y, et al: Peroxynitrite production by TNF-alpha
and IL-1beta: implication for suppression of osteoblastic
differentiation. Am J Physiol Endocrinol Metab. 278:E1031–E1037.
2000.PubMed/NCBI
|
|
36
|
Gilbert L, He X, Farmer P, Boden S,
Kozlowski M, Rubin J, et al: Inhibition of osteoblast
differentiation by tumor necrosis factor-alpha. Endocrinology.
141:3956–3964. 2000.PubMed/NCBI
|
|
37
|
Kurokouchi K, Kambe F, Yasukawa K, Izumi
R, Ishiguro N, Iwata H and Seo H: TNF-alpha increases expression of
IL-6 and ICAM-1 genes through activation of NF-kappaB in
osteoblast-like ROS17/2.8 cells. J Bone Miner Res. 13:1290–1299.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abu-Amer Y, Ross FP, Edwards J and
Teitelbaum SL: Lipopolysaccharide-stimulated osteoclastogenesis is
mediated by tumor necrosis factor via its P55 receptor. J Clin
Invest. 100:1557–1565. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abu-Amer Y, Ross FP, McHugh KP, Livolsi A,
Peyron JF and Teitelbaum SL: Tumor necrosis factor-α activation of
nuclear transcription factor-κB in marrow macrophages is mediated
by c-Src tyrosine phosphorylation of IκBα. J Biol Chem.
273:29417–29423. 1998.
|
|
40
|
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A,
Torisu T and Athanasou NA: Proinflammatory cytokine
(TNF-alpha/IL-1alpha) induction of human osteoclast formation. J
Pathol. 198:220–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rakshit DS, Ly K, Sengupta TK, Nestor BJ,
Sculco TP, Ivashkiv LB and Purdue PE: Wear debris inhibition of
anti-osteoclastogenic signaling by interleukin-6 and
interferon-gamma. Mechanistic insights and implications for
periprosthetic osteolysis. J Bone Joint Surg Am. 88:788–799. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
von Knoch M, Jewison DE, Sibonga JD,
Sprecher C, Morrey BF, Loer F, et al: The effectiveness of
polyethylene versus titanium particles in inducing osteolysis in
vivo. J Orthop Res. 22:237–243. 2004.PubMed/NCBI
|
|
43
|
Lee SS, Woo CH, Chang JD and Kim JH: Roles
of Rac and cytosolic phospholipase A2 in the intracellular
signalling in response to titanium particles. Cell Signal.
15:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zheng H, Yu X, Collin-Osdoby P and Osdoby
P: RANKL stimulates inducible nitricoxide synthase expression and
nitric oxide production in developing osteoclasts. An autocrine
negative feedback mechanism triggered by RANKL induced
interferon-beta via NF-kappaB that restrains osteoclastogenesis and
bone resorption. J Biol Chem. 281:15809–15820. 2006.
|
|
45
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takayanagi H: The role of NFAT in
osteoclast formation. Ann NY Acad Sci. 1116:227–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, et al: Induction and activation of the
transcription factor NFATc1 (NFAT2) integrate RANKL signaling in
terminal differentiation of osteoclasts. Dev Cell. 3:889–901. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Taki N, Tatro JM, Lowe R, Goldberg VM and
Greenfield EM: Comparison of the roles of IL-1, IL-6, and TNF-α in
cell culture and murine models of aseptic loosening. Bone.
40:1276–1283. 2007.
|
|
49
|
Yamanaka Y, Abu-Amer W, Foglia D, Otero J,
Clohisy JC and Abu-Amer Y: NFAT2 is an essential mediator of
orthopedic particle-induced osteoclastogenesis. J Orthop Res.
26:1577–1584. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lam J, Takeshita S, Barker JE, Kanagawa O,
Ross FP and Teitelbaum SL: TNF-alpha induces osteoclastogenesis by
direct stimulation of macrophages exposed to permissive levels of
RANK ligand. J Clin Invest. 106:1481–1488. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsuo K, Galson DL, Zhao C, Peng L,
Laplace C, Wang KZ, et al: Nuclear factor of activated T-cells
(NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J
Biol Chem. 279:26475–26480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hofbauer LC, Lacey DL, Dunstan CR,
Spelsberg TC, Riggs BL and Khosla S: Interleukin-1beta and tumor
necrosis factoralpha, but not interleukin-6, stimulate
osteoprotegerin ligand gene expression in human osteoblastic cells.
Bone. 25:255–259. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crotti TN, Smith MD, Findlay DM, Zreiqat
H, Ahern MJ, Weedon H, et al: Factors regulating osteoclast
formation in human tissues adjacent to peri-implant bone loss:
expression of receptor activator NFkappaB, RANK ligand and
osteoprotegerin. Biomaterials. 25:565–573. 2004. View Article : Google Scholar
|
|
54
|
Arai F, Miyamoto T, Ohneda O, Inada T,
Sudo T, Brasel K, Miyata T, et al: Commitment and differentiation
of osteoclast precursor cells by the sequential expression of c-Fms
and receptor activator of nuclear factor kappaB (RANK) receptors. J
Exp Med. 190:1741–1754. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bucay N, Sarosi I, Dunstan CR, Morony S,
Tarpley J, Capparelli C, et al: Osteoprotegerin-deficient mice
develop early onset osteoporosis and arterial calcification. Genes
Dev. 12:1260–1268. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Burgess TL, Qian Y, Kaufman S, Ring BD,
Van G, Capparelli C, et al: The ligand for osteoprotegerin (OPGL)
directly activates mature osteoclasts. J Cell Biol. 145:527–538.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nakagawa N, Kinosaki M, Yamaguch K, Shima
N, Yasuda H, Yano K, et al: RANK is the essential signaling
receptor for osteoclast differentiation factor in
osteoclastogenesis. Biochem Biophys Res Commun. 253:395–400. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Purdue PE, Koulouvaris P, Nestor BJ and
Sculco TP: The central role of wear debris in periprosthetic
osteolysis. HSS J. 2:102–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tanaka Y, Nakayamada S and Okada Y:
Osteoblasts and osteoclasts in bone remodeling and inflammation.
Curr Drug Targets Inflamm Allergy. 4:325–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Peruzzi B, Cappariello A, Del Fattore A,
Rucci N, De Benedetti F and Teti A: c-Src and IL-6 inhibit
osteoblast differentiation and integrate IGFBP5 signalling. Nat
Commun. 3:6302012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Abbas S, Zhang YH, Clohisy JC and Abu-Amer
Y: Tumor necrosis factor-alpha inhibits pre-osteoblast
differentiation through its type-1 receptor. Cytokine. 22:33–41.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, et al: Osteocyte Wnt/beta-catenin signaling is
required for normal bone homeostasis. Mol Cell Biol. 30:3071–3085.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kamiya N, Ye L, Kobayashi T, et al: BMP
signaling negatively regulates bone mass through sclerostin by
inhibiting the canonical Wnt pathway. Development. 135:3801–3811.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thomas GP, Baker SU, Eisman JA and
Gardiner EM: Changing RANKL/OPG mRNA expression in differentiating
murine primary osteoblasts. J Endocrinol. 170:451–460. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sabokbar A and Rushton N: Role of
inflammatory mediators and adhesion molecules in the pathogenesis
of aseptic loosening in total hip arthroplasties. J Arthroplasty.
10:810–816. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kim KJ, Rubash HE, Wilson SC, D’Antonio JA
and McClain EJ: A histologic and biochemical comparison of the
interface tissues in cementless and cemented hip prostheses. Clin
Orthop Relat Res. 287:142–152. 1993.PubMed/NCBI
|
|
68
|
Al-Saffar N, Khwaja HA, Kadoya Y and
Revell PA: Assessment of the role of GM-CSF in the cellular
transformation and the development of erosive lesions around
orthopaedic implants. Am J Clin Pathol. 105:628–639.
1996.PubMed/NCBI
|
|
69
|
Wang W, Ferguson DJ, Quinn JM, Simpson AH
and Athanasou NA: Biomaterial particle phagocytosis by
bone-resorbing osteoclasts. J Bone Joint Surg Br. 79:849–856. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gallo J, Kamínek P, Tichá V, Riháková P
and Ditmar R: Particle disease. A comprehensive theory of
periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 146:21–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Archibeck MJ, Jacobs JJ, Roebuck KA and
Glant TT: The basic science of periprosthetic osteolysis. Instr
Course Lect. 50:185–195. 2001.PubMed/NCBI
|
|
72
|
Schwarz E, Lu P, Goater J, Benz E, Kollias
G, Rosier R, et al: Tumor necrosis factor-alpha/nuclear
transcription factor-kappaB signaling in periprosthetic osteolysis.
J Orthop Res. 18:472–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Xu JW, Konttinen YT, Lassus J, Natah S,
Ceponis A, Solovieva S, et al: Tumor necrosis factor-alpha
(TNF-alpha) in loosening of total hip replacement (THR). Clin Exp
Rheumatol. 14:643–648. 1996.PubMed/NCBI
|
|
74
|
Evans CH, Ghivizzani SC and Robbins PD:
Orthopedic gene therapy in 2008. Mol Ther. 17:231–244. 2009.
View Article : Google Scholar
|
|
75
|
Bandara G, Robbins PD, Georgescu HI,
Mueller GM, Glorioso JC and Evans CH: Gene transfer to
synoviocytes: prospects for gene treatment of arthritis. DNA Cell
Biol. 11:227–231. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bandara G, Mueller GM, Galea-Lauri J,
Tindal MH, Georgescu HI, Suchanek MK, et al: Intraarticular
expression of biologically active interleukin 1-receptor-antagonist
protein by ex vivo gene transfer. Proc Natl Acad Sci USA.
90:10764–10768. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Bao JJ, Zhang WW and Kuo MT: Adenoviral
delivery of recombinant DNA into transgenic mice bearing
hepatocellular carcinomas. Hum Gene Ther. 7:355–365. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ali M, Lemoine NR and Ring CJ: The use of
DNA viruses as vectors for gene therapy. Gene Ther. 1:367–384.
1994.PubMed/NCBI
|
|
80
|
Smith AE: Viral vectors in gene therapy.
Annu Rev Microbiol. 49:807–838. 1995. View Article : Google Scholar : PubMed/NCBI
|