Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer worldwide and is particularly prevalent in
China (1,2). Currently, treatments for HCC include
surgical resection, chemotherapy and liver transplantation
(3). However, the outcomes remain
dismal. The 5-year survival rate for patients with HCC has been
reported to be 30–50% (4,5). Thus, novel, alternative therapeutic
options for HCC are urgently required. Immunotherapy for cancer has
received much attention in recent years. A number of tumor
antigens, such as human telomerase-reverse transcriptase (hTERT) or
alpha-fetoprotein (AFP) have been identified as immunotherapeutic
targets, and a number of immunotherapeutic trials have been
performed to evaluate the potential therapeutic value of HCC
immunotherapy (6).
Cancer-testis (CT) antigens have been considered
attractive targets for cancer immunotherapy due to their restricted
expression patterns in a variety of tumors and normal tissues
(7). To date, >150 genes or
gene families encoding CT antigens have been identified (8). However, only a limited number of CT
antigens have been shown to elicit both humoral and cellular
responses. NY-ESO-1 (cancer/testis antigen 1B), also known as
CTAG1, is one of the most immunogenic CT antigens (9). It was originally found in esophageal
cancer by serological recombinant cDNA expression cloning (SEREX)
(10) and is expressed in several
tumors, including HCC (11–14). NY-ESO-1 expression is associated
with a poor tumor outcome and is recognized as a potential
biomarker for the prediction of tumor recurrence and treatment
outcomes in patients with gastrointestinal stromal tumors and
cutaneous melanomas (15).
Due to its expression patterns and strong
immunogenicity, NY-ESO-1 has emerged as one of the most attractive
targets for cancer vaccines (7,9).
The NY-ESO-1 vaccine, based on peptide or protein, has been tested
in the treatment of patients with tumors expressing NY-ESO-1,
including lung cancer, ovarian cancer, esophageal cancer and
melanoma (16–20). The majority of the patients showed
enhanced immune responses and disease stabilization was achieved in
some patients (20). Furthermore,
these studies illustrated the safety of various preparations of
NY-ESO-1 vaccines. The use of in vitro-generated, autologous
dendritic cells (DCs) as a cellular adjuvant for vaccine delivery
has been widely tested in cancer patients (21). The potential efficacy of induced
immunity against HCC has been supported by a report that the
immunization of 2 HCC patients with autologous HCC lysate-loaded
DCs resulted in the prolonged survival of >3 years in 1 patient
(22). However, whether DCs
pulsed with NY-ESO-1 protein can induce antigen-specific immune
responses against HCC remains unclear. In addition, the correlation
between NY-ESO-1 expression and clinical parameters has not been
extensively investigated.
In a previous study, we purified the recombinant
NY-ESO-1 protein (rESO) (23). In
this study we aimed to evaluate T cell response against HCC cell
lines following incubation with DCs loaded with rESO. Furthermore,
we assessed the mRNA and protein abundance of NY-ESO-1 in HCC
samples and determined the correlation between NY-ESO-1 expression
and clinical parameters.
Materials and methods
Patients and samples
A total of 190 paraffin-embedded HCC specimens and
their adjacent non-cancerous tissues were collected at the Center
for Liver Disease, the First Affiliated Hospital, Fujian Medical
University, Fuzhou, China, between 2007 and 2009. Frozen tissues
were available in 54 cases. They were frozen immediately in liquid
nitrogen after removal from surgical resection and stored at −80°C
until use. Informed consent was obtained from all patients. The
clinicopathological parameters of these cases are summarized in
Table I. Tissue microarrays
(TMAs) of the HCC and adjacent non-cancerous liver samples were
prepared according to standard procedures (Beecher Instruments
Inc., Silver Spring, MD, USA). The study was approved by the
Ethical Review Board of Fujian Medical University.
 | Table IClinicopathological characteristics of
HCC patients. |
Table I
Clinicopathological characteristics of
HCC patients.
| Characteristic | Paraffin-embeded HCC
specimens | Fresh-frozen HCC
specimens |
|---|
| No. of patients | 190 | 54 |
| Average age
(years) | 49 | 50 |
| Age range
(years) | 21–75 | 32–70 |
| Male/female | 162/28 | 44/10 |
| HBsAg-positive | 155 | 44 |
| AFP (≥20 ng/ml) | 132 | 39 |
| With cirrhosis | 172 | 48 |
| With portal vein
tumor thrombosis | 65 | 18 |
| Edmondson’s
classification (grades I–II/III–IV) | 103/87 | 29/25 |
HCC cell lines
The HCC cell lines, H4M and H2P, were kindly
provided by Dr JianMing Wen at the Department of Pathology, the
First Affiliated Hospital, Sun Yat-sen University, Guangzhou,
China. HCC cells were cultured in RPMI-1640 medium with 10% fetal
bovine serum (FBS), L-glutamine, penicillin (100 IU/ml) and
streptomycin (100 μg/ml) at 37°C.
Preparation of DCs
Peripheral blood mononuclear cells (PBMCs) from
healthy volunteers were isolated from blood by Ficoll-Hypaque
density gradient centrifugation (Amersham Biosciences, Uppsala,
Sweden). The cells were then seeded on 6-well plates for 2 h at a
density of 2–3×106 cells/ml. Non-adherent cells were
removed and adherent cells were incubated in RPMI-1640 medium
supplemented with 20% FBS, 1,000 U/ml of granulocyte macrophage
colony-stimulating factor (GM-CSF) and 500 U/ml of interleukin-4
(IL-4; PeproTech Inc., Rocky Hill, NJ, USA). After 3 days of
incubation, the old medium with floating cells was gently removed
and replaced with fresh medium. After 5 days of incubation, 1/3 of
the cells were collected as immature DCs (imDCs). The remaining
cells were treated with rESO or IL-4 at a concentration of 50 μg/ml
for 24 h. The cells were then incubated with 10 ng/ml tumor
necrosis factor-α (TNF-α) for 48 h to induce the formation of
mature DCs (mDCs). ImDCs and mDCs were assayed by flow
cytometry.
Detection of T cell response
T cells (4×105 cells/well) were incubated
with imDCs or mDCs at a ratio of 20:1 at 37°C for 72 h in RPMI-1640
medium with 20% FBS, 100 U/ml interleukin-2 (IL-2) and 20
μg/ml phytohaemagglutinin (PHA). Cell proliferation was
measured by MTT assay as previously described by Li et al
(24). Absorbance was measured at
570 nm using a Multi-Well Plate Reader (Beckman Coulter Inc., Brea,
CA, USA). The proliferation index (PI) of the T cells was
calculated using the following equation: PI = mixed lymphocyte
reaction/lymphocyte reaction. The experiment was conducted 3
times.
Flow cytometry
The level of surface molecules on DCs was determined
by flow cytometry using anti-human antibodies: FITC-CD83, PE-CD86
and APC-HLA-DR (BioLegend, San Diego, CA, USA) as previously
described (25). Negative
controls were fluorochrome-conjugated isotype-matched irrelevant
antibodies (Invitrogen, Carlsbad, CA, USA). Briefly, cells
suspended in PBS were incubated with antibodies at room temperature
for 30 min in the dark. The cells were then analyzed by a BD
FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA).
Cytotoxicity assays
Allogeneic T cells were collected as effector cells,
and H4M/H2P cells were used as the target cells. Effector cells
included the rESO-DC-T group (T cells stimulated with DCs loaded
with rESO), the IL-4-DC-T group (T cells stimulated with DCs
treated with IL-4) and the T cells group (T cells without
stimulation). Effector cells and target cells were incubated at
effector/target ratios of 20:1 or 50:1 for 4 h at 37°C in 96-well
plates. The activity of T cells against the target tumor cells was
measured as previously described in a standard lactate
dehydrogenase (LDH) release assay (26). The cytotoxicity of the T cells was
calculated as a percentage of specific lysis using the following
formula: % specific lysis = (effector/target release − spontaneous
release)/(maximal release − spontaneous release) ×100%. Data are
presented as the means ± standard deviation.
Immunohistochemistry (IHC)
Formalin-fixed slides from TMAs were deparaffinized
by xylene and rehydrated by a series of graded alcohol. Endogenous
horseradish peroxidase activity was blocked by treatment with 3%
(v/v) H2O2. Antigen retrieval was achieved by
heating the samples in a microwave in 10 mM citrate buffer (pH 6.0)
for 20 min. Non-specific binding was blocked by incubation with 1%
(w/v) BSA in phosphate-buffered saline (PBS) for 1 h at room
temperature. The slides were then incubated with 1:200 monoclonal
anti-NY-ESO-1 antibody (clone E978; Zymed Laboratories, Inc., South
San Francisco, CA, USA) overnight at 4°C. The slides were then
incubated with HRP-labeled anti-mouse secondary antibody for 1 h.
Immunoreactivity was visualized by diaminobenzidine (DAB). The
sections were counterstained with hematoxylin. PBS was used for
rinsing between each step. Negative controls were created by
omitting the primary antibody. NY-ESO-1 expression was scored by 2
independent observers. The level of NY-ESO-1 expression was
described semi-quantitatively using a 4-grade scoring system: -, no
staining or focal staining (<5% total); +, 5-<25%; ++,
25–50%; and +++, >50%.
Reverse transcriptase-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the 54 frozen samples
and HCC cell lines using TRIzol reagent (Gibco-BRL, Gaithersburg,
MD, USA) according to the manufacturer’s instructions. The reverse
transcription reaction was performed using the First Strand cDNA
Synthesis kit (MBI Fermentas, Vilnius, Lithuania) according to the
manufacturer’s instructions. Amplification was carried out using
the following primers: ESO-1F (exon 1),
5′-cgcctgcttgagttctacctc-3′; and ESO-1R (exon 3),
5′-agggaaagctgctggagacag-3′. The reaction was conducted under the
following conditions: 5 min at 95°C, followed by 30 sec at 94°C, 1
min at 60°C and 45 sec at 72°C for 35 cycles, with a 10 min
elongation step at 72°C. β-actin was used as the positive control.
The expected PCR product sizes of NY-ESO-1 and β-actin were 219 and
120 bp respectively. Bands were visualized by ethidium bromide
staining after separation on a 1.5% agarose gel. The assay was
carried out at least 2 times.
Statistical analysis
Statistical analyses were carried out using SPSS
version 13.0 software (SPSS, Chicago, IL, USA). Fisher’s exact test
or the χ2 test were used to analyze categorical data.
Variance analysis was used to determine the statistical
significance of the differences between 2 groups. A p-value
<0.05 was considered to indicate a statistically significant
difference.
Results
DC induction and identification
We obtained 2±0.31×107 mononuclear cells
from 50 ml PBMCs following Ficoll separation. The cells were then
treated as described in Materials and methods. After 5 days of
incubation with GM-CSF and IL-4, 2.2±0.49×106
dendritic-like cells were obtained based on morphology. Flow
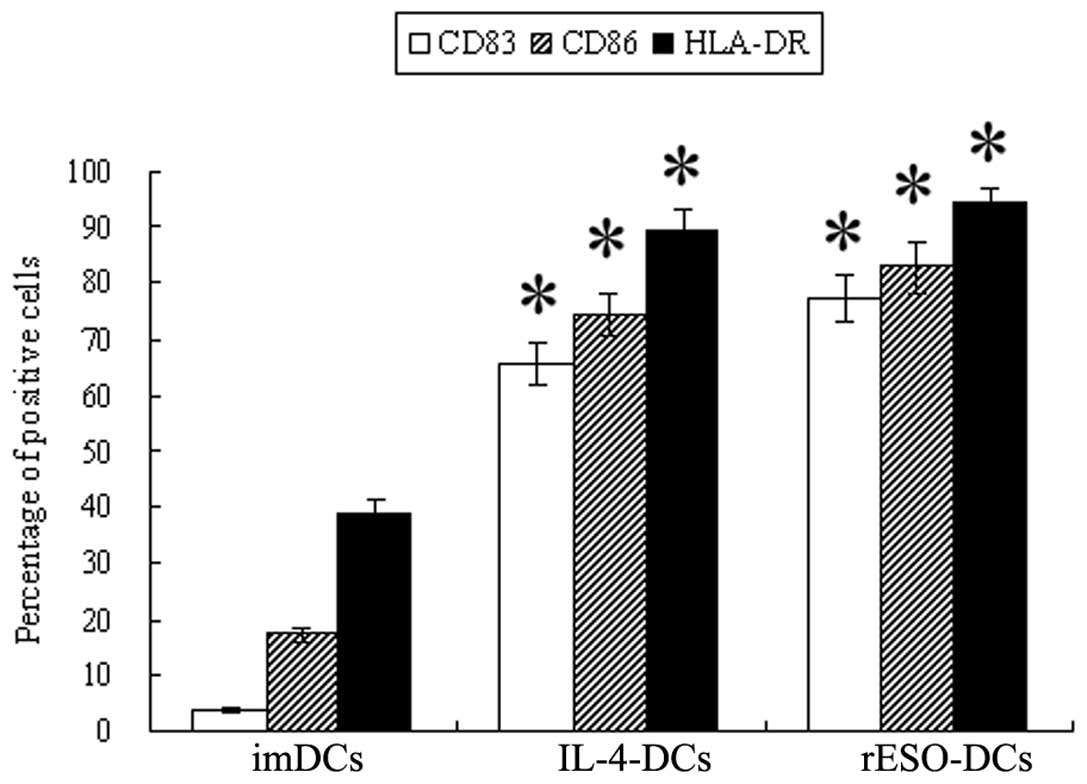
cytometry analysis revealed that 38.90±2.43% of the imDCs were
positive for HLA-DR (Fig. 1). In
addition, 3.67±0.49% and 17.23±1.24% of the imDCs were positive for
CD83 and CD86, respectively. After rESO or IL-4 induction for 24 h
and the addition of TNF-α for 48 h, the rESO-DCs or IL-4-DCs showed
a typical branch-like appearance. These cells exhibited a
significantly higher expression of HLA-DR, CD83 and CD86
(p<0.05) (Fig. 1). Compared
with the imDCs, positivity for HLA-DR, CD83 and CD86 increased by
2- to 21-fold in the rESO-DCs. Similarly, the percentage of HLA-DR,
CD83 and CD86 positivity increased by 2- to 17-fold in the IL-4-DCs
compared with the imDCs.
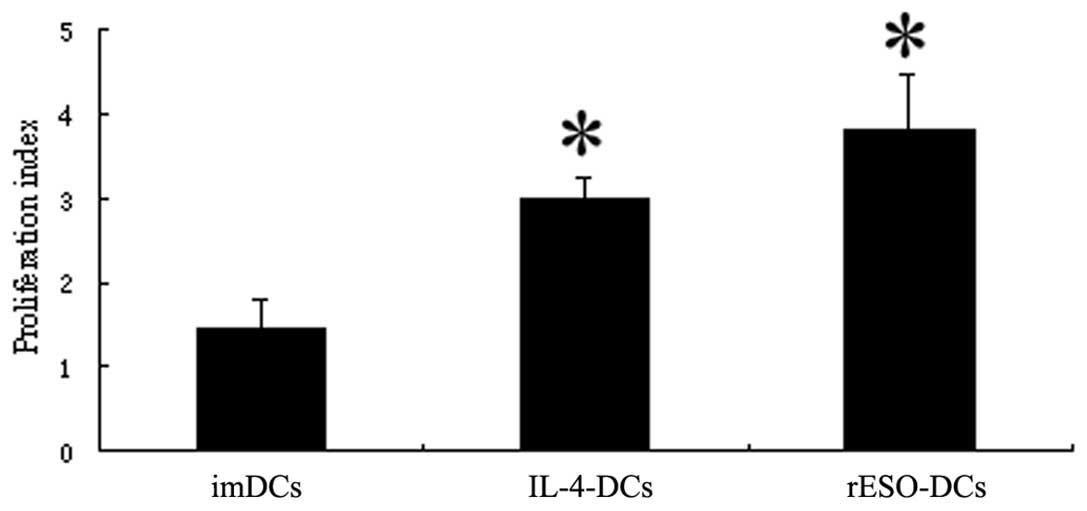
To determine the potential of DCs to stimulate T
cell proliferation, we performed a mixed T lymphocyte reaction by
MTT assay. The PI of the T cells mixed with rESO-DCs was higher
than that of those mixed with IL-4-DCs and the imDCs (3.80±0.66 vs.
2.99±0.26 and 1.44±0.36). A statistically significant difference
was observed when comparing the rESO-DCs with the imDCs (p<0.05)
(Fig. 2). These data illustrated
that DCs pulsed with rESO protein were more effective in
stimulating T lymphocyte proliferation compared with the control
cells.
Cytotoxicity of NY-ESO-1-specific T
lymphocytes
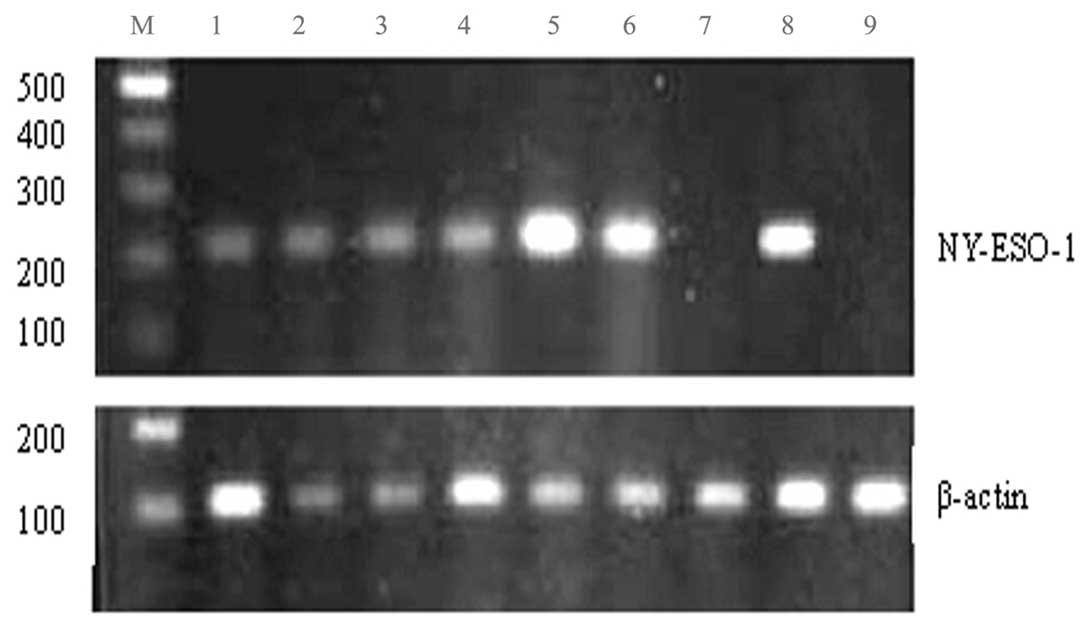
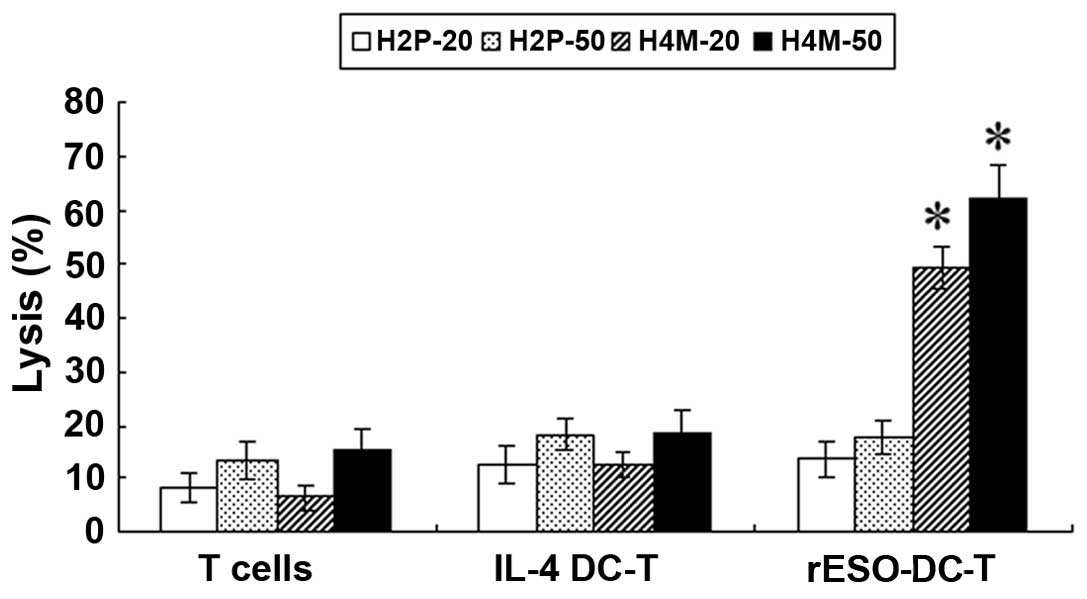
The cytotoxicity of T cells against 2 HCC cell lines
was determined by LDH release assay. Allogeneic T cells were
collected as the effector cells, and H4M and H2P cells were used as
the target cells. H4M cells were NY-ESO-1-positive and H2P cells
were NY-ESO-1-negative, as shown by RT-PCR (Fig. 3). T cells and HCC cells were
incubated at a ratio of 50:1 or 20:1. When the H4M cells were used
as the target cells, the specific NY-ESO-1 T cells lysed the cancer
cells effectively in a dose-dependent manner (62.13±5.89% for ratio
50:1 and 49.23±3.78% for ratio 20:1), significantly higher than
that of the IL-4-DC-T and T cells group (p<0.01) (Fig. 4). By contrast, the specific lysis
among the rESO-DC-T, IL-4-DC-T and T cells did not differ
significantly in the H2P cells, which did not express NY-ESO-1.
These results demonstrate that T cells stimulated with DCs pulsed
with rESO exert significant antigen-specific lysis on HCC cells
which express NY-ESO-1.
NY-ESO-1 expression in HCC patients
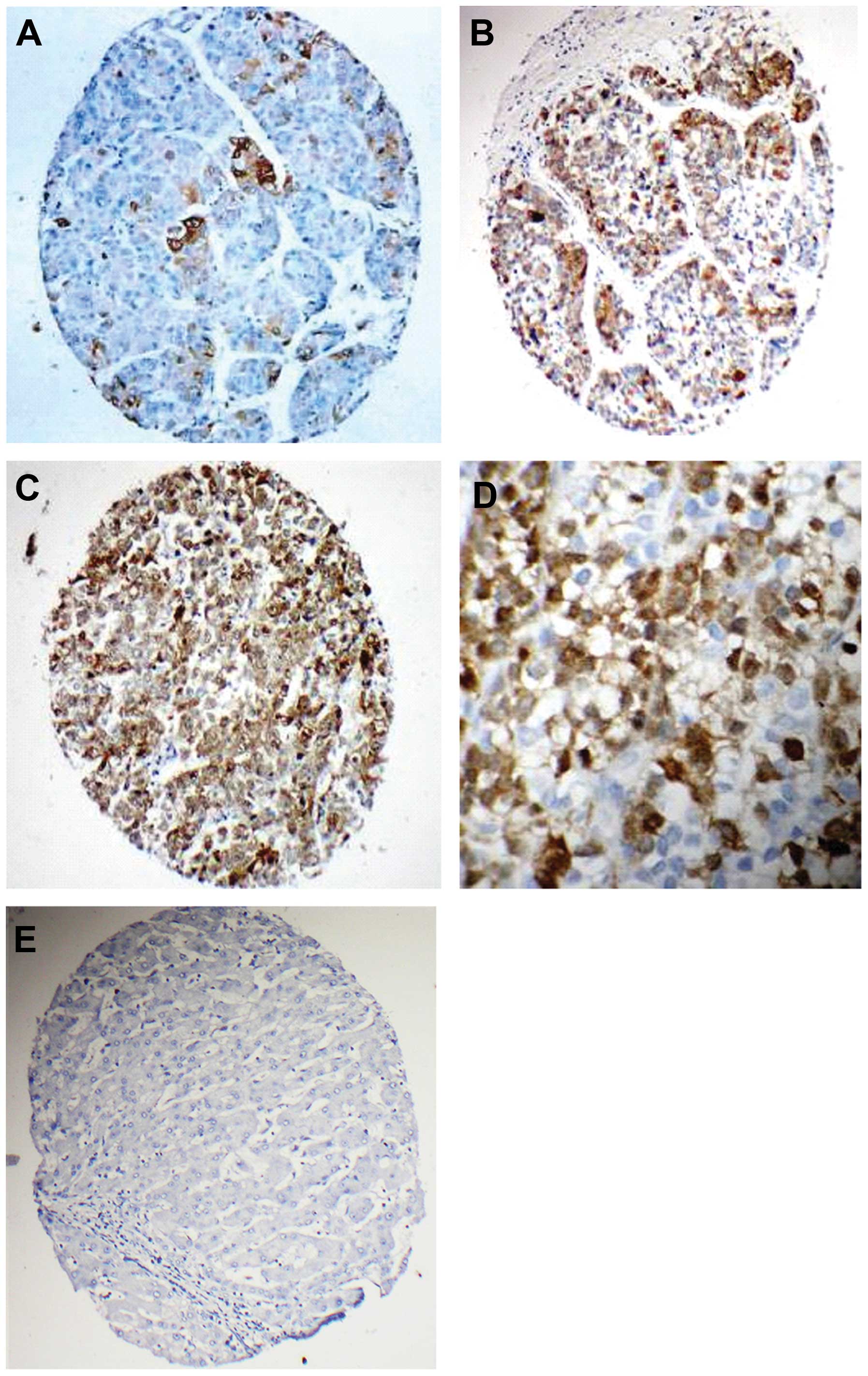
IHC analyses of NY-ESO-1 protein indicated that a
total of 30 out of the 190 HCC specimens expressed NY-ESO-1
(15.8%). Among these, 8 (4.2%) were graded as +++, 12 (6.3%) as ++,
and 10 (5.3%) as + (Fig. 5A–C).
NY-ESO-1 was located predominantly in the cytoplasm, although
nuclear staining was observed in a few cells (Fig. 5D). No staining was observed in the
tissue adjacent to HCC (Fig. 5E).
We also determined the expression profile of NY-ESO-1 mRNA in 54
tumors by RT-PCR. A detectable NY-ESO-1 transcript was observed in
10 tumors (18.5%). Representative results are shown in Fig. 3.
Correlation between NY-ESO-1 expression
and clinical parameters
Table II
summarizes the correlation between NY-ESO-1 expression and the
clinicopathological characteristics of the tumor samples. In the
HCC patients with portal vein tumor thrombosis, the frequency of
NY-ESO-1 positivity was 24.6% (16/65). By contrast, the frequency
of NY-ESO-1 positivity in the HCC patients without portal vein
tumor thrombosis was 11.2% (14/125), which was significantly lower
compared with the HCC patients with portal vein tumor thrombosis
(p=0.013). Statistical analysis also revealed that the frequency of
the detectable NY-ESO-1 transcript was higher in HCC patients with
portal vein tumor thrombosis as compared with the HCC patients
without portal vein tumor thrombosis (33.3 vs. 19.4%, p=0.21).
Statistical analysis did not reveal a correlation between NY-ESO-1
expression and age, gender, tumor size, histological grade or
hepatitis B surface antigen (HBsAg)/AFP status in the HCC
samples.
 | Table IINY-ESO-1 expression in the HCC
patients. |
Table II
NY-ESO-1 expression in the HCC
patients.
| IHC | RT-PCR |
|---|
|
|
|
|---|
| Group | N | Positive | (%) | N | Positive | (%) |
|---|
| Gender |
| Male | 162 | 25 | 15.4 | 44 | 10 | 22.7 |
| Female | 28 | 5 | 17.9 | 10 | 3 | 30.0 |
| Tumor size |
| <5 cm | 52 | 7 | 13.5 | 13 | 3 | 23.1 |
| >5 cm | 138 | 23 | 16.7 | 41 | 10 | 24.4 |
| Portal vein tumor
thrombosis |
| Positive | 65 | 16 | 24.6a | 18 | 6 | 33.3 |
| Negative | 125 | 14 | 11.2 | 36 | 7 | 19.4 |
| Edmondson’s
classification |
| Grades I–II | 103 | 14 | 13.6 | 29 | 6 | 20.7 |
| Grades III–IV | 87 | 16 | 18.4 | 25 | 7 | 28 |
| HBsAg |
| Positive | 155 | 24 | 15.5 | 44 | 10 | 22.7 |
| Negative | 35 | 6 | 17.1 | 10 | 3 | 30.0 |
| AFP |
| <20 ng/ml | 58 | 9 | 15.5 | 15 | 3 | 20.0 |
| ≥20 ng/ml | 132 | 21 | 15.9 | 39 | 10 | 25.6 |
| Total | 190 | 30 | 15.8 | 54 | 13 | 24.1 |
Correlation between NY-ESO-1 mRNA and
protein levels in HCC patients
A total of 54 HCC samples were examined for both the
transcript and protein levels of NY-ESO-1. A total of 10 cases were
positive for NY-ESO-1 protein, as shown by by IHC and 13 tumors
expressed NY-ESO-1 mRNA, as shown by RT-PCR. As presented in
Table III, 4 cases were
positive for NY-ESO-1 mRNA expression, as shown by RT-PCR, but were
shown to be negative by IHC. Out of the 10 tumors with positive
immunostaining, 1 tumor was shown to be negative by RT-PCR. This
case displayed positive IHC staining for NY-ESO-1.
 | Table IIIExpression of NY-ESO-1 in 54 HCC
samples detected by IHC and RT-PCR. |
Table III
Expression of NY-ESO-1 in 54 HCC
samples detected by IHC and RT-PCR.
| IHC |
|---|
|
|
|---|
| RT-PCR | + (n=5) | ++ (n=2) | +++ (n=3) | Negative
(n=44) | Total |
|---|
| Positive | 4 | 2 | 3 | 4 | 13 |
| Negative | 1 | 0 | 0 | 40 | 41 |
| Total | 5 | 2 | 3 | 44 | 54 |
Discussion
The survival of HCC patients is poor despite
advancements in HCC therapy (3).
In recent years, immunotherapy has become a promising strategy for
tumor therapy. A variety of immunotherapy regimens have emerged for
HCC patients, including HCC lysates (27), tumor cell-DC fusion (28) and cytokines (29). However, the effects of these
methods have been limited. The major barrier to antigen-specific
immunotherapy in HCC is a lack of well-defined immunogenic tumor
antigens. It has been shown that NY-ESO-1 is one of the most
immunogenic CT antigens (7).
Immune responses against NY-ESO-1 have been induced in a number of
tumors, such as melanoma (30),
ovarian cancer (31) and lung
cancer (12). The NY-ESO-1
vaccine has been investigated in clinical trials of melanoma and
ovarian cancer (32). Recently, a
NY-ESO-1 vaccine has also been examined in esophageal and prostate
cancer patients (33). Patients
bearing NY-ESO-1-expressing tumors displayed an effective induction
of NY-ESO-1 antibody and CD4/CD8 T cell responses. Although only a
few studies have reported the immune responses induced by NY-ESO-1
in HCC patients (34),
NY-ESO-1-specific immune responses in HCC are not yet well
understood. In our study, our results demonstrated that
NY-ESO-1-specific T cell responses were induced, which
significantly increased the lysis of NY-ESO-1-expressing HCC cells
in vitro. These data provide evidence supporting the use of
NY-ESO-1-based immunotherapy and suggest that NY-ESO-1 may be a
useful target for the immunotherapy of HCC patients.
For cancer clinical trials targeted against defined
antigens, a detailed knowledge of the antigen expression is
crucial. Our data indicated that the positive rate of NY-ESO-1
protein was 15.8% and the NY-ESO-1 mRNA expression was 24.1% in the
HCC samples, which was higher than that found in the study by Luo
et al (35), and is
comparable to the results of Wang et al (13). We also illustrated a high
concordance between RT-PCR and IHC for NY-ESO-1 expression in the
HCC samples. The HCC patients displayed moderate or high levels of
NY-ESO-1 and as shown by IHC, they were positive for the NY-ESO-1
transcript. However, we detected positivity for the NY-ESO-1
transcript in 4 HCC samples without a detectable NY-ESO-1 protein
expression. A likely explanation for this is that RT-PCR has a
higher sensitivity to detect NY-ESO-1 than IHC. In this study, we
also observed heterogeneity for NY-ESO-1 expression by IHC in a few
cases, and this information could not be achieved by RT-PCR. It may
therefore be desirable to use both IHC and RT-PCR to obtain
information regarding the expression level and antigen
distribution.
NY-ESO-1 has been speculated to be a prognostic
marker in gastrointestinal stromal tumors, melanoma and HCC
(15,36,37). NY-ESO-1 expression is associated
with a worse tumor outcome. The present study also revealed that
NY-ESO-1 expression in HCC patients with portal vein tumor
thrombosis had a significantly higher intensity and positivity
compared with that in HCC patients without portal vein tumor
thrombosis, as shown by IHC and RT-PCR. Thus, it can be
hypothesized that the NY-ESO-1 gene may play an important role in
tumor invasion and progression. Although the mechanisms involved
are unclear, a NY-ESO-1 vaccine may play an important role in the
advanced stages of disease, as also found in esophageal carcinoma
by Bujas et al (38).
In conclusion, we identified specific T cell
responses stimulated with DCs pulsed with NY-ESO-1 in HCC cell
lines. We also demonstrate that NY-ESO-1 is heterogeneously
expressed in HCC patients, specifically in advanced HCC patients
with portal vein tumor thrombosis. This suggests that a DC-based
NY-ESO-1 vaccine may prove to be effective for immunotherapy in
advanced HCC. Moreover, a combination of demethylation or
preparation of multiple CT antigens may increase the efficacy of
HCC immunotherapy.
Acknowledgements
This study was supported by the Natural Science
Foundation of Fujian Province (C0610023, 2012J01362). We are
grateful to Dr JianMing Wen (Department of Pathology, the First
Affiliated Hospital, Sun Yat-sen University) for providing the HCC
cell lines in this study. We also thank Kay Ka-Wai Li. (Department
of Anatomical and Cellular Pathology, the Chinese University of
Hong Kong) for revising this article.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen MS, Peng ZW, Xu L, Zhang YJ, Liang HH
and Li JQ: Role of radiofrequency ablation in the treatment of
hepatocellular carcinoma: experience of a cancer center in China.
Oncology. 81(Suppl 1): S100–S104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rossi L, Zoratto F, Papa A, et al: Current
approach in the treatment of hepatocellular carcinoma. World J
Gastrointest Oncol. 2:348–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimozawa N and Hanazaki K: Longterm
prognosis after hepatic resection for small hepatocellular
carcinoma. J Am Coll Surg. 198:356–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang CH, Chau GY, Lui WY, Tsay SH, King
KL and Wu CW: Long-term results of hepatic resection for
hepatocellular carcinoma originating from the noncirrhotic liver.
Arch Surg. 139:320–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greten TF, Manns MP and Korangy F:
Immunotherapy of HCC. (Review). Rev Recent Clin Trials. 3:31–39.
2008. View Article : Google Scholar
|
|
7
|
Caballero OL and Chen YT: Cancer/testis
(CT) antigens: potential targets for immunotherapy. Cancer Sci.
100:2014–2021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hofmann O, Caballero OL, Stevenson BJ, et
al: Genome-wide analysis of cancer/testis gene expression. Proc
Natl Acad Sci USA. 105:20422–20427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gnjatic S, Nishikawa H, Jungbluth AA, et
al: NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer
Res. 95:1–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen YT, Scanlan MJ, Sahin U, et al: A
testicular antigen aberrantly expressed in human cancers detected
by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oba-Shinjo SM, Caballero OL, Jungbluth AA,
et al: Cancer-testis (CT) antigen expression in medulloblastoma.
Cancer Immun. 8:72008.
|
|
12
|
Kim SH, Lee S, Lee CH, et al: Expression
of cancer-testis antigens MAGE-A3/6 and NY-ESO-1 in non-small-cell
lung carcinomas and their relationship with immune cell
infiltration. Lung. 187:401–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang XY, Chen HS, Luo S, Zhang HH, Fei R
and Cai J: Comparisons for detecting NY-ESO-1 mRNA expression
levels in hepatocellular carcinoma tissues. Oncol Rep. 21:713–719.
2009.PubMed/NCBI
|
|
14
|
Grigoriadis A, Caballero OL, Hoek KS, et
al: CT-X antigen expression in human breast cancer. Proc Natl Acad
Sci USA. 106:13493–13498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Svobodová S, Browning J, MacGregor D, et
al: Cancer-testis antigen expression in primary cutaneous melanoma
has independent prognostic value comparable to that of Breslow
thickness, ulceration and mitotic rate. Eur J Cancer. 47:460–469.
2011.
|
|
16
|
Jäger E, Karbach J, Gnjatic S, et al:
Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral
and cellular NY-ESO-1-specific immune responses in cancer patients.
Proc Natl Acad Sci USA. 103:14453–14458. 2006.PubMed/NCBI
|
|
17
|
Bender A, Karbach J, Neumann A, et al: LUD
00-009: phase 1 study of intensive course immunization with
NY-ESO-1 peptides in HLA-A2 positive patients with
NY-ESO-1-expressing cancer. Cancer Immun. 7:162007.PubMed/NCBI
|
|
18
|
Odunsi K, Qian F, Matsuzaki J, et al:
Vaccination with an NY-ESO-1 peptide of HLA class I/II
specificities induces integrated humoral and T cell responses in
ovarian cancer. Proc Natl Acad Sci USA. 104:12837–12842. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicholaou T, Ebert L, Davis ID, et al:
Directions in the immune targeting of cancer: lessons learned from
the cancer-testis Ag NY-ESO-1. Immunol Cell Biol. 84:303–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kakimi K, Isobe M, Uenaka A, et al: A
phase I study of vaccination with NY-ESO-1f peptide mixed with
Picibanil OK-432 and Montanide ISA-51 in patients with cancers
expressing the NY-ESO-1 antigen. Int J Cancer. 129:2836–2846. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Engell-Noerregaard L, Hansen TH, Andersen
MH, Thor Straten P and Svane IM: Review of clinical studies on
dendritic cell-based vaccination of patients with malignant
melanoma: assessment of correlation between clinical response and
vaccine parameters. Cancer Immunol Immunother. 58:1–14. 2009.
View Article : Google Scholar
|
|
22
|
Ladhams A, Schmidt C, Sing G, et al:
Treatment of non-resectable hepatocellular carcinoma with
autologous tumor-pulsed dendritic cells. J Gastroenterol Hepatol.
17:889–896. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Xiao G, Zhang M, Xie D, Guo A and
Wen J: The prokaryotic expression, purification and preliminary
application of human NY-ESO-1 gene. Zhongguo Kang Ai Xie Hui.
32:626–629. 2005.(In Chinese).
|
|
24
|
Li DY, Gu C, Min J, Chu ZH and Ou QJ:
Maturation induction of human peripheral blood mononuclear
cell-derived dendritic cells. Exp Ther Med. 4:131–134.
2012.PubMed/NCBI
|
|
25
|
Della Bella S, Nicola S, Riva A, Biasin M,
Clerici M and Villa ML: Functional repertoire of dendritic cells
generated in granulocyte macrophage-colony stimulating factor and
interferon-alpha. J Leukoc Biol. 75:106–116. 2004.
|
|
26
|
Wang XH, Qin Y, Hu MH and Xie Y: Dendritic
cells pulsed with gp96-peptide complexes derived from human
hepatocellular carcinoma (HCC) induce specific cytotoxic T
lymphocytes. Cancer Immunol Immunother. 54:971–980. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan K, Zhao JJ, Wang H, et al: Comparative
analysis of cytotoxic T lymphocyte response induced by dendritic
cells loaded with hepatocellular carcinoma-derived RNA or cell
lysate. Int J Biol Sci. 6:639–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao DY, Yang JY, Yue SQ, et al:
Comparative analysis of DC fused with allogeneic hepatocellular
carcinoma cell line HepG2 and autologous tumor cells as potential
cancer vaccines against hepatocellular carcinoma. Cell Immunol.
259:13–20. 2009. View Article : Google Scholar
|
|
29
|
Rinaldi M, Iurescia S, Fioretti D,
Ponzetto A and Carloni G: Strategies for successful vaccination
against hepatocellular carcinoma. Int J Immunopathol Pharmacol.
22:269–277. 2009.PubMed/NCBI
|
|
30
|
Gedye C, Quirk J, Browning J, et al:
Cancer/testis antigens can be immunological targets in clonogenic
CD133+ melanoma cells. Cancer Immunol Immunother.
58:1635–1646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Milne K, Barnes RO, Girardin A, et al:
Tumor-infiltrating T cells correlate with NY-ESO-1-specific
autoantibodies in ovarian cancer. PLoS One. 3:e34092008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Odunsi K, Matsuzaki J, Karbach J, et al:
Efficacy of vaccination with recombinant vaccinia and fowlpox
vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma
patients. Proc Natl Acad Sci USA. 109:5797–5802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawada J, Wada H, Isobe M, et al:
Heteroclitic serological response in esophageal and prostate cancer
patients after NY-ESO-1 protein vaccination. Int J Cancer.
130:584–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Korangy F, Ormandy LA, Bleck JS, et al:
Spontaneous tumor-specific humoral and cellular immune responses to
NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res.
10:4332–4341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo G, Huang S, Xie X, et al: Expression
of cancer-testis genes in human hepatocellular carcinomas. Cancer
Immun. 2:112002.PubMed/NCBI
|
|
36
|
Perez D, Hauswirth F, Jäger D, et al:
Protein expression of cancer testis antigens predicts tumor
recurrence and treatment response to imatinib in gastrointestinal
stromal tumors. Int J Cancer. 128:2947–2952. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu H, Gu N, Liu ZB, et al: NY-ESO-1
expression in hepatocellular carcinoma: A potential new marker for
early recurrence after surgery. Oncol Lett. 3:39–44.
2012.PubMed/NCBI
|
|
38
|
Bujas T, Marusic Z, Peric Balja M, Mijic
A, Kruslin B and Tomas D: MAGE-A3/4 and NY-ESO-1 antigens
expression in metastatic esophageal squamous cell carcinoma. Eur J
Histochem. 55:e72011. View Article : Google Scholar : PubMed/NCBI
|