Introduction
Endometrial cancer is one of the most common and
lethal gynecological malignancies in Poland. However, the causes of
endometrial carcinogenesis remain to be clarified. Possible causes
include the imbalance of endogenous estrogen and progesterone
levels, obesity, polycystic ovarian syndrome and estrogen
replacement therapy (1,2). Since the 1980s, endometrial cancer
has been classified into two main subsets of sporadic endometrial
cancer that differ in molecular genesis and prognosis (3). Type I endometrioid cancers are
estrogen-dependent cancers that develop from hyperplasia and are
usually low grade with a favorable prognosis. Endometrioid cancers
account for ~70–80% of cases, affecting mainly younger, pre- and
post-menopausal females. In this type of tumor, specific molecular
aberrations such as PTEN gene inactivation by mutation
and/or promoter methylation, mutation of protooncogene
H-RAS, mutation of CTNNB, and microsatellite
instability (MSI) are common (4).
Type II sporadic endometrial cancer is non-endometrioid endometrial
carcinoma (NEEC), which progresses from an atrophic endometrium,
occurs in older females and has a more aggressive course of
disease. This type of cancer is estrogen-independent and
characterized by frequent molecular alterations in oncoprotein
HER2/neu, TP53 mutation and inactivation of CDH1
(4,5). Experiments conducted in this study
focused on endometrioid adenocarcinoma, which is the type I
endometrioid cancer.
Clinicopathologic variables such as age, FIGO stage,
histological grade, myometrial invasion, metastasis to lymph node
and histological type are crucial prognostic factors (6). However, new molecular markers should
be identified to improve the prediction of therapy outcome and
prognosis. Currently, the potential markers available remain
controversial and intensely discussed (6).
WWOX is localized in the common fragile site,
FRA16D (locus 16q23.3–24.1). It has been confirmed to be altered in
various types of cancer including breast, lung, gastric, ovarian,
Wilms' tumor and glioblastoma multiforme (7–13).
Genetic and epigenetic alterations of this gene include loss of
heterozygosity (LOH) and promoter methylation. The gene product is
an oxidoreductase comprising two WW protein interaction domains.
One of the roles of WWOX protein is participation in steroid
hormone metabolism (14,15). Results of previous studies showed
that WWOX is also associated with apoptosis, proliferation,
adhesion and cell signaling pathways (16–19). Additionally, WWOX has been shown
to bind to PPxY motif-containing proteins, and inactivate their
transcription transactivation function by sequestering them in the
cytoplasm (20,21). It is known that WWOX modulates the
Ap2α/γ, p73, ErbB4, Met, Jun, Wnt signaling pathways. Moreover,
Gourley et al identified its role in the decrease of
integrin activity and adhesion of tumor cells to fibronectin
(22).
The expression level of WWOX is known to be
decreased in breast cancer cells and to correlate with poor
prognosis (11). In a series of
in vitro experiments, a WWOX-transfected MDA-MB-231
breast cancer cell line showed increased migratory ability.
However, the results of a test of growth in Matrigel showed that
the transduced cells had more ‘normal’ phenotype and formed mammary
ducts. Control cells in Matrigel grew into spherical structures,
typical of neoplastic cells (22). The improved differentiation
evident in cells with an elevated WWOX level suggests its
involvement in these type of processes. The tumor suppressor
function of WWOX was confirmed in a soft agar growth test,
where WWOX-transfected cells exhibited inhibition of
anchorage-independent growth (23). MDA-MB-231 cells overexpressing
WWOX were also significantly less tumorigenic in vivo
(24). Experiments performed by
Gourley et al on ovarian cancer cells confirmed that WWOX
protein is an inhibitor of anchorage-independent growth also in
this case. Moreover, WWOX silencing was found to result in
enhanced adhesion to fibronectin (22).
No data is currently available on the role of the
WWOX gene in endometrial cancer. The present preliminary
qPCR-based study was conducted on 79 endometrial adenocarcinoma in
relation to 28 tumor-free endometrial tissue samples. The aim of
this study was to investigate the correlation of the expression
levels of WWOX and nine other tumor-related genes:
MKI67, BAX, BCL2, EGFR, CCNE1,
CCND1, CDH1, TP73 and NCOR1.
Additionally, the implications of loss of heterozygosity with
regard to the regulation of WWOX expression in endometrial
cancer were also analyzed.
Materials and methods
In total, 79 samples of endometrial carcinoma
(endometrioid adenocarcinoma) were collected at the Department of
Gynecological Oncology, Medical University of Lodz, Poland. The
tumors were classified according to the FIGO (International
Federation of Gynaecology and Obstetrics) classification system.
The mean age of the patients was 61 years (median 60, range 36–83
years). The samples were examined histologically and stored at
−80°C in RNAlater buffer (Ambion, Inc., Austin, TX, USA) until RNA
extraction. Clinical characteristics of the patients are presented
in Table I. Experiments involving
human subjects were conducted according to the Declaration of
Helsinki, and the study was approved by the Ethics Committee at the
Medical University of Lodz. Control samples (n=28) were received
from patients operated on for benign gynecologic disorders.
 | Table IClinical characteristics of
endometrial cancer patients. |
Table I
Clinical characteristics of
endometrial cancer patients.
| Factor | No. of
patients |
|---|
| FIGO stage |
| I | 44 |
| II | 16 |
| III | 10 |
| NS | 9 |
| Lymph node
metastasis |
| Negative | 65 |
| Positive | 8 |
| NS | 6 |
| Histological
grade |
| I | 27 |
| II | 39 |
| III | 12 |
| NS | 1 |
| Myometrial
invasion |
| <1/2 | 41 |
| >1/2 | 33 |
| Without | 4 |
| NS | 1 |
qPCR
RNA was extracted from frozen tissues, stored at
−80°C in RNAlater (Ambion), using TRIzol reagent (Invitrogen,
Carlsbad, CA, USA). cDNA synthesis was performed from 10 μg of
total RNA at a volume of 100 μl using ImProm RT-II™ reverse
transcriptase (Promega, Madison, WI, USA). Reverse transcription
was carried out under the following conditions: incubation at 25°C
for 5 min and 42°C for 60 min, and heating at 70°C for 15 min. cDNA
samples were diluted with sterile deionized water to a total volume
of 150 μl, and 2 μl was added to a PCR reaction. qPCR was performed
using Rotor-Gene™ 3000 (Corbett Research, Australia). PCR products
were detected using SYBR®-Green I and qPCR Core kit for
SYBR-Green I (Eurogentec, Seraing, Belgium). Reactions were
performed in duplicate. The relative expression levels of the
following genes were analyzed: WWOX, TP73,
CCND1, CCNE1, BCL2, BAX, MKI67,
CDH1, EGFR, and NCOR1. The expression levels
of the investigated genes were normalized to three reference genes
(RPS17, H3F3A, RPLP0). Due to the presence of
a relatively low level of WWOX mRNA, a semi-nested RT-PCR
was used for the detection of WWOX expression levels. The
first PCR reaction was performed with primers:
5′-TGCAACATCCTCTTCTCCAACGAGCTGCAC-3′ and
5′-TCCCTGTTGCATGGACTTGGTGAAAGGC-3′ in a 50 μl reaction volume.
Subsequently, 2 μl of 200-fold diluted PCR product (171 bp) was
used as a template for semi-nested PCR. The cycling protocol
consisted of 2 min at 94°C, 30 sec denaturation at 94°C, 30 sec
annealing at 63°C, 1 min extension at 72°C, repeated for 77 cycles,
and additional extension for 7 min at 72°C.
The primer sequences, PCR reaction conditions and
the length of obtained products are available upon request.
Relative gene expression was calculated based on the
Roche company guidebook according to the previously published
algorithm (25). Universal Human
Reference RNA (Stratagene, La Jolla, CA, USA) composed of 10 cell
lines was used as a calibrator.
The primers were designed to be intron-spanning to
avoid amplification of genomic DNA. The detection temperature was
determined above the non-specific/primer-dimer melting
temperature.
Loss of heterozygosity analysis
LOH detection was performed using the
high-resolution melting method in a LightCycler480 (Roche Molecular
Systems, Penzberg, Germany). Genomic DNA was recovered after RNA
isolation using back extraction buffer (BEB, 1 M Tris Base, 4 M
guanidinium thiocyanate, and 50 mM sodium citrate) according to the
manufacturer's instructions. Allelic losses were analyzed by PCR
amplification with two sets of primers for microsatellites D16S518
(intron 1 of WWOX gene) and D16S3096 (intron 8). The primer
sequences were obtained from the Genome database. PCR cycling
programs included one cycle with 95°C for 10 min followed by 35
cycles consisting of 94°C for 30 sec, 56°C (for D16S3096) or 55°C
(for D16S518) for 30 sec, 72°C for 60 sec. The high-resolution
melting conditions involved a temperature increase of 50–95°C, ramp
rate 0.01°C/sec and 40 acquisitions per °C.
Western blot analysis
The tissue fragments were lysed in RIPA protein
extraction buffer supplemented with protease, phosphatase inhibitor
cocktail and PMSF (Sigma-Aldrich, St. Louis, MO, USA). The protein
concentration was measured using the Bradford method (Bio-Rad
Laboratories, Hercules, CA, USA), and 100 μg amounts were run on
10% SDS-PAGE gel electrophoresis and subsequently transferred to a
PVDF membrane (Sigma-Aldrich). The membranes were blocked in 5%
non-fat milk in TBST for 1 h at room temperature and then incubated
for 19 h at 4°C with primary antibodies (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Following incubation, the membranes
were washed three times with TBST and incubated with secondary
antibodies conjugated with alkaline phosphatase (Sigma-Aldrich) for
1 h. Membranes were washed three times in TBST and developed using
Novex® AP Chromogenic Substrate (Invitrogen).
Glyceraldehyde-3-phosphate dehyrogenase (GAPDH) was used as a
reference protein. The relative protein amount was assessed with
ImageJ software (National Institutes of Health, USA) based on
integrated density of bands.
Statistical analysis
Data analysis was performed by using Statistica
version 8.0 (StatSoft, Tulsa, OK, USA). Gene expression correlation
analysis was performed using the non-parametric Spearman's rank
correlation test. The Mann-Whitney t-test was used to determine
differences between the transcription levels of the WWOX
gene in relation to its hemi/heterozygosity, as well as differences
between quantities of WWOX in different types of tissue. Results
were recognized as being statistically significant at a confidence
level of >95% (P<0.05).
Results
LOH
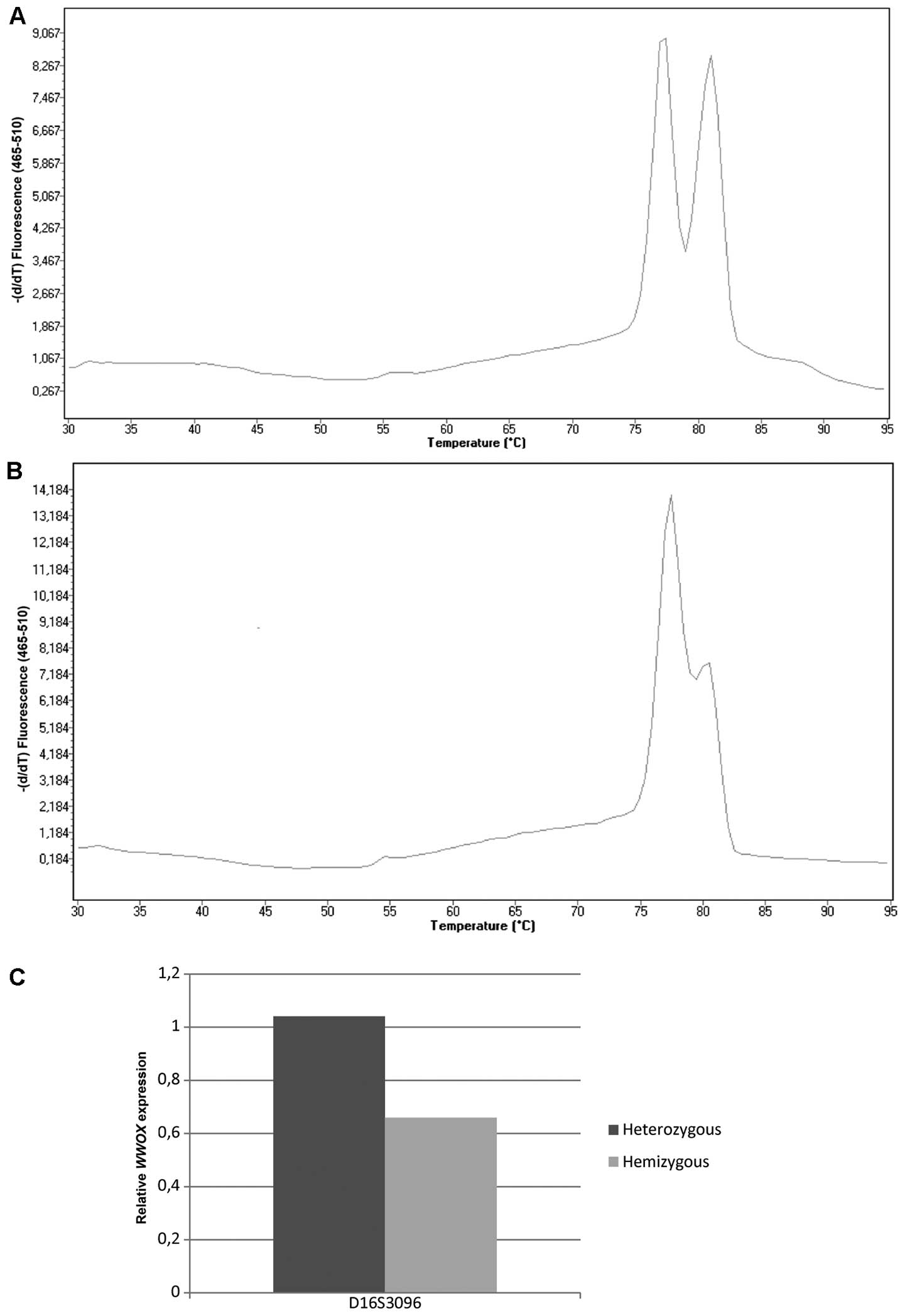
Loss of heterozygosity in the region of the D16S3096
microsatellite marker (localized in intron 8 of WWOX gene)
was found to be a common event in endometrial cancer, with observed
hemizygosity at 38%. Moreover, hemizygous samples exhibited
decreased WWOX gene expression levels compared with
heterozygous samples (medians: 0.66 vs. 1.04, P=0.066). The
percentage of LOH for the microsatellite marker, D16S518, was 40%;
however, hemizygosity did not reveal any tendencies to correlate
with decreased WWOX expression levels (Fig. 1).
Expression correlation of WWOX and
various tumor-related genes
The expression correlation between WWOX and
nine other tumor-related genes is assessed. MKI67 was
connected with the rate of proliferation, BAX, BCL2
and TP73 are involved in the course of apoptosis,
CCNE1 and CCND1 encode cyclins crucial for cell cycle
progression, EGFR and NCOR1 act as receptors and
transcriptional factors, while CDH1 is a gene of one of
proteins responsible for cell-cell adhesion. A positive correlation
was observed between the expression of WWOX and BCL2
(Rs = 0.3822; P=0.0005), CCND1 (Rs =
0.3821; P=0.0005) as well as with the BCL2/BAX
anti-apoptotic ratio (Rs = 0.4496; P=0.0001).
WWOX expression correlated inversely with BAX
(Rs = −0.2302; P=0.0412), CDH1 (Rs =
−0.4126; P=0.0002) and NCOR1 (Rs = −0.3061;
P=0.0064) expression levels. Details of the expression correlations
between WWOX and the nine investigated genes are presented
in Table II.
 | Table IICorrelation analysis between the
expression levels of WWOX and other tumor-related genes. |
Table II
Correlation analysis between the
expression levels of WWOX and other tumor-related genes.
| Gene | Spearman's rank
correlation | P-value |
|---|
|
BCL2/WWOX | 0.3822 | 0.0005 |
|
BAX/WWOX | −0.2302 | 0.0412 |
|
CCND1/WWOX | 0.3821 | 0.0005 |
|
CDH1/WWOX | −0.4126 | 0.0002 |
|
NCOR1/WWOX | −0.3061 | 0.0064 |
| BCL2_BAX
ratio/WWOX | 0.4496 | 0.0001 |
|
EGFR/WWOX | 0.1004 | 0.3786 |
|
CCNE1/WWOX | −0.0162 | 0.8872 |
|
MKI67/WWOX | −0.1132 | 0.3314 |
No correlation was found between WWOX mRNA
levels and clinicopathological factors such as grade, FIGO stage,
lymph node metastasis or myometrial invasion (Table III). However, the highest
expression of WWOX was observed in normal endometrium tissue
(NT) in comparison to tumor tissue (median expression 2.826, NT vs.
G1, P=0.003; NT vs. G2, P<0.0001; NT vs. G3, P=0.002).
 | Table IIIDependence of the WWOX
expression levels and clinical characteristics of endometrial
cancer patients. |
Table III
Dependence of the WWOX
expression levels and clinical characteristics of endometrial
cancer patients.
| Factor | n | Median of
WWOX mRNA level (range) | P-value |
|---|
| FIGO stage |
| I | 44 | 1.004
(0.678–1.613) | 0.701 (I/II) |
| II | 16 | 0.716
(0.357–1.370) | 0.527 (II/III) |
| III | 10 | 0.871
(0.554–4.267) | 0.982 (III/I) |
| NS | 9 | | |
| Lymph node
metastasis |
| Negative | 65 | 0.870
(0.613–1.295) | 0.646 |
| Positive | 8 | 0.871
(0.554–1.436) | |
| NS | 6 | | |
| Histological
grade |
| G1 | 27 | 1.125
(0.494–1.824) | 0.386 (I/II) |
| G2 | 39 | 0.732
(0.613–0.974) | 0.756 (II/III) |
| G3 | 12 | 0.722
(0.306–1.613) | 0.523 (III/I) |
| NS | 1 | | |
| Myometrial
invasion |
| <1/2 | 41 | 1.050
(0.641–1.563) | 0.279 |
| >1/2 | 33 | 0.856
(0.410–1.226) | |
| Without | 4 | | |
| NS | 1 | | |
Western blot analysis
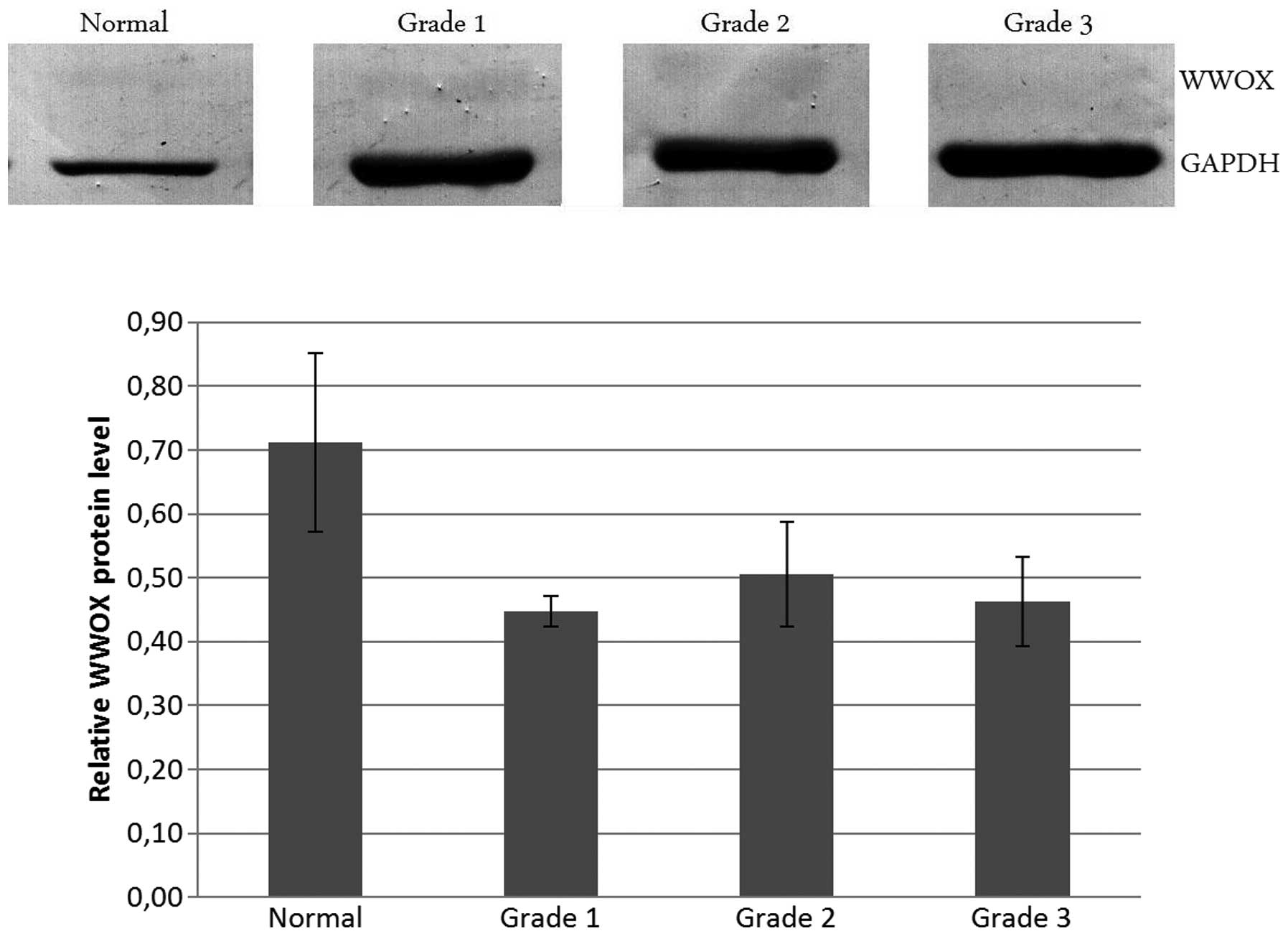
To assess the level of WWOX protein in normal and
cancer tissues, a western blot assay was conducted, which revealed
that the protein amount was greater in normal endometrium tissue
compared with cancer samples, although this tendency did not
achieve statistical significance (P>0.05). There was no
difference between tumor grades. Such results are reflected in the
amounts of mRNA. However, in the qPCR analysis of gene expression
the differences between normal and cancer tissues were
statistically significant. The smaller differences in protein
between normal and tumor tissues may depend on the low level of the
WWOX protein, which hindered western blot sensitivity. The
densitometric analysis and protein bands are presented in Fig. 2.
Discussion
Apoptosis is a natural process for the elimination
of senescent cells from a layer of endometrium during late
secretory and menstrual phases. The mRNA levels of
apoptosis-related genes change during phases of the ovulation
cycle. The expression level of the antiapoptotic Bcl2 gene
increases during the late proliferative phase but decreases during
the late secretory and menstruating phases when the expression of
the Bax proapoptotic gene increases. Anomalies in the
expression of apoptosis-related genes may lead to pathological
changes including endometriosis and cancer (26).
In the present study, positive correlations were
found between WWOX and the antiapoptotic BCL2 gene as
well as between WWOX and the BCL2/BAX ratio. Findings
of a previous study demonstrated the increasing expression of the
proapoptotic BAX gene and decreasing expression of the
antiapoptotic BCL2 gene during progression from endometrial
hyperplasia to cancer (27).
Geisler et al (28)
demonstrated that a higher expression level of the Bcl2 protein
correlates with favorable clinicopathological variables such as
well-differentiated tumor cells, reduced FIGO stage, lack of
invasion into lymph node and superficial myometrial invasion
(28,29). Chao et al observed a
correlation of BAX overexpression in endometrial cancer
specimens in relation to normal endometrium and premalignant
lesions (30). A negative
correlation between WWOX and BAX gene was also
identified in the present study. These results are similar to our
previously reported gene expression analysis conducted on breast
cancer (11) and glioblastoma
multiforme patients (9).
Investigations on apoptosis of an A2780 ovarian cancer cell line
transfected with the WWOX gene demonstrated a decreased
ability for anchorage-independent growth with a simultaneous
increase of apoptosis (31).
An important regulator of the cell cycle is cyclin
D1. Expression of CCND1 can be regulated by several
signaling pathways, such as RAS or PTEN. In type I
endometrioid cancer, expression of the CCND1 gene is
connected with the proliferation process Wnt signalling pathways
(32). Findings of a previous
study identified an increase in expression of cyclin D1 from normal
endometria to hyperplasia and carcinoma (33). Moreno-Bueno et al suggest
two different causes of cyclin D1 overexpression: an amplification
of the gene in NEEC and a microsatellite instability in
endometrioid cancer (34). In the
present study, a positive correlation of WWOX and
CCND1 was observed.
Additionally, results of the present study
demonstrate that WWOX expression level correlates negatively
with the NCOR1 (nuclear receptor corepressor 1) ERα
corepressor gene. The corepressor suppressess transcription
estrogen-responsive genes by modeling chromatin structures by
incorporating histone deacetylases (HDAC) (35). Using a microarray method
Moreno-Bueno et al noted a 2-fold higher expression of
NCOR1 in endometrioid cancer in comparison to NEEC samples
(36). However, such differences
between tumor groups were not observed by Kershah et al,
although they demonstrated the upregulation of nuclear receptor
coregulators including NCOR1 in a malignant endometrium, as
compared to a normal one. The ratio between coactivators SRC-1 and
SRC-2 and corepressor NCOR1 decreased in malignant tissues. No
significant differences were identified between tumor groups
regarding NCOR expression, categorized on the basis of such
clinical parameters as grades or stages (37). Upregulation of NCOR1 was
also observed in breast cancer, however, a low expression of this
gene was associated with worse prognosis and serves as a potential
predicting factor for tamoxifen treatment in estrogen receptor
α-positive breast cancer (38).
In a previous study on ER-positive breast cancer patients, a
positive correlation was noted between the expression level of
WWOX and NCOR1 (data not shown).
The CDH1 gene encoding the cell-cell adhesion
protein E-cadherin is located near WWOX on chromosome 16
(CDH1 locus 16q22.1, WWOX locus 16q23.3–24.1). As
previously shown, CDH1 expression is often reduced or
completely inactivated by promoter methylation. A low E-cadherin
expression is associated with worse prognosis, higher stage and
greater metastatic potential (39). Our previous experiments conducted
on breast and colon cancer lines confirm that an increase of
WWOX expression level results in changes in cell behavior
(23, unpublished data). Cancer
cells with a high WWOX express a higher motility, which has
an effect on improved migration through the basal membrane.
Additionally, they are less malignant due to the suppression of
anchorage-independent growth. This change in cell motility may
explain the observed correlation of WWOX expression with the
reduced expression of the cell adhesion gene CDH1. A
previous in vivo study revealed the role of WWOX protein in
the attachment and adhesion of ovarian cancer cells.
WWOX-transfected PEO1 cells showed a decrease in the
adhesion to fibronectin in comparison to vector-transfected control
cells, which suggests a WWOX influence on processes such as
tumor invasiveness and spread (40). Gourley et al also confirmed
these results on the ovarian cancer cell line, A2780, and showed
that WWOX overexpression reduces adhesion through membranous
integrin α3 protein (22).
In previous studies, a decrease in the expression of
the WWOX gene was found to be associated with loss of
heterozygosity (LOH) in gastric (7), pancreatic (18), esophageal (41) and lung cancer (13). In the present study, the
percentage of hemizygosity at two analyzed loci of the WWOX
gene was ~40%. LOH in microsatellite marker D16S3096, exhibited a
tendency towards a correlation with the reduced expression level of
the WWOX gene. This observation suggest that this process is
involved in the regulation of the WWOX mRNA level.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate the potential role of WWOX in
endometrial cancer through the regulation of Wnt (CDH1 and
CCND1), apoptosis (BCL2 and BAX) and
estrogen-related genes (NCOR1). Results of the present study
have also shown that WWOX mRNA and protein levels decrease
in transformed endometrial tissue. The fact that no significant
differences exist between tumor grades suggests that WWOX
silencing is an early event in endometrial cancerogenesis. Similar
to other types of cancer, WWOX expression in endometrial
adenocarcinoma correlates with the expression level of apoptosis
and cell cycle regulators. The results of our preliminary
experiment have shown that additional investigations should be
conducted that may enable the better elucidation of the role of
WWOX in endometrial cancer. Future experiments are to be
conducted on endometrial cell lines, which may shed some light on
the functions performed by the WWOX protein and its relevance to
endometrial cancer promotion and progression.
Acknowledgements
This study was funded by the National Center of
Sciences N N407 168940.
References
|
1
|
Kaaks R, Lukanova A and Kurzer MS:
Obesity, endogenous hormones, and endometrial cancer risk: a
synthetic review. Cancer Epidemiol Biomarkers Prev. 11:1531–1543.
2002.PubMed/NCBI
|
|
2
|
Salazar EL, Paredes A and Calzada L:
Endometrial thickness of postmenopausal breast cancer patients
treated with tamoxifen. Gynecol Endocrinol. 21:312–316. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu FS: Molecular carcinogenesis of
endometrial cancer. Taiwan J Obstet Gynecol. 46:26–32. 2007.
View Article : Google Scholar
|
|
5
|
Merritt MA and Cramer DW: Molecular
pathogenesis of endometrial and ovarian cancer. Cancer Biomark.
9:287–305. 2010.PubMed/NCBI
|
|
6
|
Kim JW, Kim SH, Kim YT and Kim DK:
Clinicopathologic and biological parameters predicting the
prognosis in endometrial cancer. Yonsei Med J. 43:769–778. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Aqeilan RI, Kuroki T, Pekarsky Y, et al:
Loss of WWOX expression in gastric carcinoma. Clin Cancer Res.
10:3053–3058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen T, Sahin A and Aldaz CM: Deletion map
of chromosome 16q in ductal carcinoma in situ of the breast:
refining a putative tumor suppressor gene region. Cancer Res.
56:5605–5609. 1996.PubMed/NCBI
|
|
9
|
Kosla K, Pluciennik E, Kurzyk A, et al:
Molecular analysis of WWOX expression correlation with
proliferation and apoptosis in glioblastoma multiforme. J
Neurooncol. 101:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nunez MI, Rosen DG, Ludes-Meyers JH, et
al: WWOX protein expression varies among ovarian carcinoma
histotypes and correlates with less favorable outcome. BMC Cancer.
5:642005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pluciennik E, Kusinska R, Potemski P,
Kubiak R, Kordek R and Bednarek AK: WWOX - the FRA16D cancer gene:
expression correlation with breast cancer progression and
prognosis. Eur J Surg Oncol. 32:153–157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pluciennik E, Nowakowska M, Wujcicka WI,
et al: Genetic alterations of WWOX in Wilms' tumor are
involved in its carcinogenesis. Oncol Rep. 28:1417–1422. 2012.
|
|
13
|
Yendamuri S, Kuroki T, Trapasso F, et al:
WW domain containing oxidoreductase gene expression is altered in
non-small cell lung cancer. Cancer Res. 63:878–881. 2003.PubMed/NCBI
|
|
14
|
Saluda-Gorgul A, Seta K, Nowakowska M and
Bednarek AK: WWOX oxidoreductase - substrate and enzymatic
characterization. Z Naturforsch C. 66:73–82. 2011. View Article : Google Scholar
|
|
15
|
Aqeilan RI, Hagan JP, de Bruin A, et al:
Targeted ablation of the WW domain-containing oxidoreductase tumor
suppressor leads to impaired steroidogenesis. Endocrinology.
150:1530–1535. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aderca I, Moser CD, Veerasamy M, et al:
The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced
by the tumor suppressor WWOX. J Hepatol. 49:373–383. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X and
Sun ZQ: WWOX induces apoptosis and inhibits proliferation of human
hepatoma cell line SMMC-7721. World J Gastroenterol. 18:3020–3026.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroki T, Yendamuri S, Trapasso F, et al:
The tumor suppressor gene WWOX at FRA16D is involved in pancreatic
carcinogenesis. Clin Cancer Res. 10:2459–2465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qin HR, Iliopoulos D, Semba S, et al: A
role for the WWOX gene in prostate cancer. Cancer Res.
66:6477–6481. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matteucci E, Bendinelli P and Desiderio
MA: Nuclear localization of active HGF receptor Met in aggressive
MDA-MB231 breast carcinoma cells. Carcinogenesis. 30:937–945. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aqeilan RI, Donati V, Palamarchuk A, et
al: WW domain-containing proteins, WWOX and YAP, compete for
interaction with ErbB-4 and modulate its transcriptional function.
Cancer Res. 65:6764–6772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gourley C, Paige AJ, Taylor KJ, et al:
WWOX gene expression abolishes ovarian cancer tumorigenicity in
vivo and decreases attachment to fibronectin via integrin alpha3.
Cancer Res. 69:4835–4842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewandowska U, Zelazowski M, Seta K,
Byczewska M, Pluciennik E and Bednarek AK: WWOX, the tumour
suppressor gene affected in multiple cancers. J Physiol Pharmacol.
60(Suppl 1): 47–56. 2009.PubMed/NCBI
|
|
24
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
et al: WWOX, the FRA16D gene, behaves as a suppressor of tumor
growth. Cancer Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
25
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harada T, Kaponis A, Iwabe T, et al:
Apoptosis in human endometrium and endometriosis. Hum Reprod
Update. 10:29–38. 2004. View Article : Google Scholar
|
|
27
|
Kokawa K, Shikone T, Otani T, et al:
Apoptosis and the expression of Bax and Bcl-2 in hyperplasia and
adenocarcinoma of the uterine endometrium. Hum Reprod.
16:2211–2218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geisler JP, Geisler HE, Wiemann MC, Zhou
Z, Miller GA and Crabtree W: Lack of bcl-2 persistence: an
independent prognostic indicator of poor prognosis in endometrial
carcinoma. Gynecol Oncol. 71:305–307. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saegusa M and Okayasu I: Bcl-2 is closely
correlated with favorable prognostic factors and inversely
associated with p53 protein accumulation in endometrial carcinomas:
immunohistochemical and polymerase chain reaction/loss of
heterozygosity findings. J Cancer Res Clin Oncol. 123:429–434.
1997. View Article : Google Scholar
|
|
30
|
Chao H, Sun J and Lu S: Bax gene
expression in endometrial carcinoma. Zhonghua Zhong Liu Za Zhi.
23:214–216. 2001.(In Chinese).
|
|
31
|
Xiong Z, Hu S and Wang Z: Cloning of WWOX
gene and its growth-inhibiting effects on ovarian cancer cells. J
Huazhong Univ Sci Technolog Med Sci. 30:365–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
KEGG Database. http://www.genome.jp/kegg-bin/show_pathway?ko05213+K045032013.
Accessed 03.10.2013
|
|
33
|
Ruhul Quddus M, Latkovich P, Castellani
WJ, et al: Expression of cyclin D1 in normal, metaplastic,
hyperplastic endometrium and endometrioid carcinoma suggests a role
in endometrial carcinogenesis. Arch Pathol Lab Med. 126:459–463.
2002.
|
|
34
|
Moreno-Bueno G, Rodriguez-Perales S,
Sanchez-Estevez C, et al: Cyclin D1 gene (CCND1) mutations in
endometrial cancer. Oncogene. 22:6115–6118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Leo C, Schroen DJ and Chen JD:
Characterization of receptor interaction and transcriptional
repression by the corepressor SMRT. Mol Endocrinol. 11:2025–2037.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moreno-Bueno G, Sanchez-Estevez C, Cassia
R, et al: Differential gene expression profile in endometrioid and
nonendometrioid endometrial carcinoma: STK15 is frequently
overexpressed and amplified in nonendometrioid carcinomas. Cancer
Res. 63:5697–5702. 2003.
|
|
37
|
Kershah SM, Desouki MM, Koterba KL and
Rowan BG: Expression of estrogen receptor coregulators in normal
and malignant human endometrium. Gynecol Oncol. 92:304–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Girault I, Lerebours F, Amarir S, et al:
Expression analysis of estrogen receptor alpha coregulators in
breast carcinoma: evidence that NCOR1 expression is predictive of
the response to tamoxifen. Clin Cancer Res. 9:1259–1266.
2003.PubMed/NCBI
|
|
39
|
Yi TZ, Guo J, Zhou L, et al: Prognostic
value of E-cadherin expression and CDH1 promoter methylation in
patients with endometrial carcinoma. Cancer Invest. 29:86–92. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang JQ, Li L, Song HL, Paige A and Gabra
H: Effect of WWOX gene on the attachment and adhesion of ovarian
cancer cells. Zhonghua Fu Chan Ke Za Zhi. 44:529–532. 2009.(In
Chinese).
|
|
41
|
Kuroki T, Trapasso F, Shiraishi T, et al:
Genetic alterations of the tumor suppressor gene WWOX in esophageal
squamous cell carcinoma. Cancer Res. 62:2258–2260. 2002.PubMed/NCBI
|