Introduction
Helicobacter pylori (H. pylori)
infection-associated gastritis is one of the most common infectious
diseases worldwide. Epidemiological, pathophysiological and
histological evidence has demonstrated that H. pylori
infection-associated gastritis is a major cause of gastric cancer
(1–3). Although the mechanisms by which
H. pylori induces gastric lesions/malignancies have been
extensively investigated, the role of the H. pylori
infection-associated chronic inflammation microenvironment in this
process is poorly understood.
Mesenchymal stem cells (MSCs) are multipotent stem
cells that have been found in several different tissues (4–6).
MSCs are able to migrate across tissues and differentiate into a
variety of cells, depending on the surrounding microenvironment
(7,8). In addition to normal tissues, MSCs
have been found in injured tissue sites, suggesting a potential for
the application of MSCs in regenerative medicine. MSC tropism for
inflammation and cancer sites has also been reported, which links
MSCs to the development of inflammation-associated cancer (9–14).
In addition, the H. pylori-induced epithelial response can
direct the homing of MSCs into the gastric mucosa (15,16). As a component of the chronic
gastritis microenvironment, MSCs play critical roles in gastric
carcinogenesis and progression; however, little is known about the
mechanisms by which MSCs participate in this process.
Cancer-associated fibroblasts (CAFs), a cell
population that exists in human carcinomas, play a tumor-promoting
role (11,12). CAFs that express fibroblast
activation protein (FAP) and α-smooth muscle actin (α-SMA) create a
niche for cancer cells and promote cancer metastasis (17,18). As the main cellular component of
the tumor stroma, CAFs can also induce the epithelial-mesenchymal
transition (EMT) of malignant cells and promote angiogenesis
(19). MSCs have been identified
as a major source for CAFs (18).
The transition of MSCs into CAFs contributes to tumor progression,
angiogenesis and metastasis (18). We have previously reported that
co-culture with conditioned medium (CM) and microvesicles (MVs)
from gastric cancer cells induces the differentiation of MSCs into
CAFs (20).
To further demonstrate the role of MSCs in H.
pylori-induced gastritis and gastric cancer, in this study, we
designed a human umbilical cord MSC (hucMSC)/H. pylori
co-culture model and evaluated the biological effects of the
infected hucMSCs on normal gastric epithelial cells. Our results
demonstrated that H. pylori infection induced the expression
of CAF markers and cytokines in the hucMSCs. The infected hucMSCs
promoted GES-1 normal gastric epithelial cells to acquire a
mesenchymal phenotype through the process of EMT. The infected
hucMSCs inhibited proliferation and promoted the invasion of GES-1
cells through a paracrine mechanism. Our findings may enhance the
understanding of the role of MSCs in H. pylori
infection-associated gastric cancer.
Materials and methods
HucMSCs isolation and cell culture
HucMSCs were obtained as previously described
(4). Fresh umbilical cords were
collected from informed, consenting mothers and processed within 6
h. The cords were rinsed twice in phosphate-buffered saline (PBS)
containing penicillin and streptomycin and the cord vessels were
removed. The washed cords were cut into sections of 1–3
mm2 in size and were allowed to float in DMEM containing
10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 1%
penicillin and streptomycin. The sections of the cords were
subsequently incubated at 37°C in humidified air with 5%
CO2 and the medium was changed every 3 days after the
initial plating. When well developed colonies of fibroblast-like
cells reached 80% confluence, the cultures were trypsinized and
passaged into new flasks for further expansion. The characteristics
of the isolated hucMSCs, including morphological appearance,
surface antigens, differentiation potential and gene expression
were investigated as previously described (4,21).
It was confirmed that hucMSCs were obtained and the MSCs at passage
3 were used for the experiments. All experimental protocols were
approved by the Ethics Committee of Jiangsu University, Zhenjiang,
China.
Cell culture, H. pylori strain and growth
conditions
Human immortalized GES-1 cells were purchased from
Cowin Biotech Co., Ltd. (Beijing, China) and maintained in
RPMI-1640 medium (Invitrogen) supplemented with 10% FBS at 37°C in
a 5% CO2 humidified atmosphere. The H. pylori
strain 11673 was kindly provided by Dr Shi-He Shao (Jiangsu
University). The H. pylori strain was grown in trypticase
soy agar supplemented with 5% sheep blood and incubated at 37°C
under a microaerophilic atmosphere. For the co-culture experiments,
the H. pylori strain was grown for 48 h, resuspended in DMEM
supplemented with 10% FBS and adjusted to OD600 nm = 1
[corresponding to 1×108 colony-forming units (CFU)/ml]
prior to infection.
Co-culture of hucMSCs with H. pylori
HucMSCs cells were trypsinized, resuspended in DMEM
supplemented with 10% FBS and seeded into culture flask. Twelve
hours after seeding, grown colonies of H. pylori (48 h) were
collected and the bacterial cells were added to the monolayer at a
multiplicity of infection (MOI) of 100 bacteria/cell. Cultures were
maintained at 37°C under a 5% CO2 humidified atmosphere
for 24 h. The culture supernatants were harvested, centrifuged for
5 min at 3,000 rpm, filtered through 0.45-μm filter units, and
stored at −80°C until use. The treated cells were harvested at the
indicated time and subjected to the following experiments. For the
controls, uninfected hucMSCs (hucMSCs) were processed in a similar
manner in the absence of bacteria. Three duplicate wells were
prepared for each experimental condition.
Exposure of GES-1 cells to CM from H.
pylori-infected hucMSCs
The GES-1 cells were harvested as described above
and cultured in RPMI-1640 medium supplemented with 10% FBS and
antibiotics. The GES-1 cells were exposed to freshly harvested CM
from the uninfected hucMSCs or the H. pylori-infected
hucMSCs for 48 h. The control GES-1 cells were processed in a
similar manner in RPMI-1640 medium supplemented with 10% FBS. All
reactions were repeated 3 times independently to ensure the
reproducibility.
RNA extraction and quantitative reverse
transcription PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen) and cDNA was synthesized using a reverse transcription
kit according to the manufacturer’s instructions (Toyobo, Osaka,
Japan). The primers used in this study were produced by Invitrogen
(Shanghai, China). qRT-PCR analysis was performed to detect the
changes in the expression of FAP, α-SMA, E-cadherin, N-cadherin and
vimentin genes (Rotor-Gene 6000 Real-Time PCR Machine; Corbett Life
Science, Sydney, Australia). An endogenous ‘housekeeping’ gene
(β-actin) was quantified to normalize the results. The primers used
in this study were as follows: FAP forward, 5′-ATA
GCAGTGGCTCCAGTCTC-3′ and reverse, 5′-GATAA GCCGTGGTTCTGGTC-3′;
α-SMA forward, 5′-ATAGCAG TGGCTCCAGTCTC-3′ and reverse,
5′-GATAAGCCGTGG TTCTGGTC-3′; E-cadherin forward, 5′-CGCATTGCCACA
TACACTCT-3′ and reverse, 5′-TTGGCTGAGGATGGTGT AAG-3′; N-cadherin
forward, 5′-AGTCAACTGCAACCGT GTCT-3′ and reverse,
5′-AGCGTTCCTGTTCCACTCAT-3′; vimentin forward,
5′-GAGCTGCAGGAGCTGAATG-3′ and reverse, 5′-AGGTCAAGACGTGCCAGAG-3′;
and β-actin forward, 5′-CACGAAACTACCTTCAACTCC-3′ and reverse,
5′-CATACTCCTGCTTGCTGATC-3′. All experiments were performed in
triplicate.
Western blot analysis
The cells were collected and lysed in RIPA buffer
(10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EGTA, 0.1% SDS, 1 mM
NaF, 1 mM Na3VO4, 1 mM PMSF, 1 mg/ml
aprotinin and 1 g/ml leupeptin) on ice. Aliquots containing
identical amounts of protein were fractionated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto methanol pre-activated polyvinylidene difluoride
(PVDF) membranes (Millipore, Billerica, MA, USA). The membranes
were blocked by 5% w/v non-fat dry milk. Following sequential
incubation with the primary and secondary antibody (Santa Cruz
Biotechnology, Inc., USA), the signal was visualized using HRP
substrate (Millipore) and analyzed using MD ImageQuant Software, as
perviously described (20). The
sources and dilution factors of the primary antibodies were as
follows: rabbit polyclonal anti-FAP (1:500; Abcam, USA),
anti-vimentin (1:2,000; Santa Cruz Biotechnology, Inc.),
anti-N-cadherin (1:400; SAB; Signalway Antibody Co., Ltd., MD,
USA), anti-E-cadherin (1:500), mouse monoclonal anti-α-SMA (1:400),
anti-BMI (1:400) (all from Bioworld Technology Inc., USA),
anti-SOX2 (1:500), anti-Nanog (1:500) (both from Santa Cruz
Biotechnology, Inc.), anti-Oct4 (1:400), anti-p53 (1:800), anti-p21
(1:1,000; all SAB; Signalway Antibody Co., Ltd.) and anti-β-actin
(1:2,000; Bioworld Technology Inc.).
Luminex assay/ELISA
The concentrations of granulocyte-macrophage
colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-8,
platelet-derived growth factor (PDGF), tumor necrosis factor
(TNF)-α, IL-10, IL-1β, epidermal growth factor (EGF), IL-15, IL-17,
IL-2, vascular endothelial growth factor (VEGF) and monocyte
chemoattractant protein-1 (MCP-1) in the supernatants of the
control (uninfected) and H. pylori-infected hucMSCs were
measured using Luminex (Millipore)/ELISA (Dakewe Biotech, Ltd.,
Beijing, China) kits in accordance with the manufacturer’s
instructions.
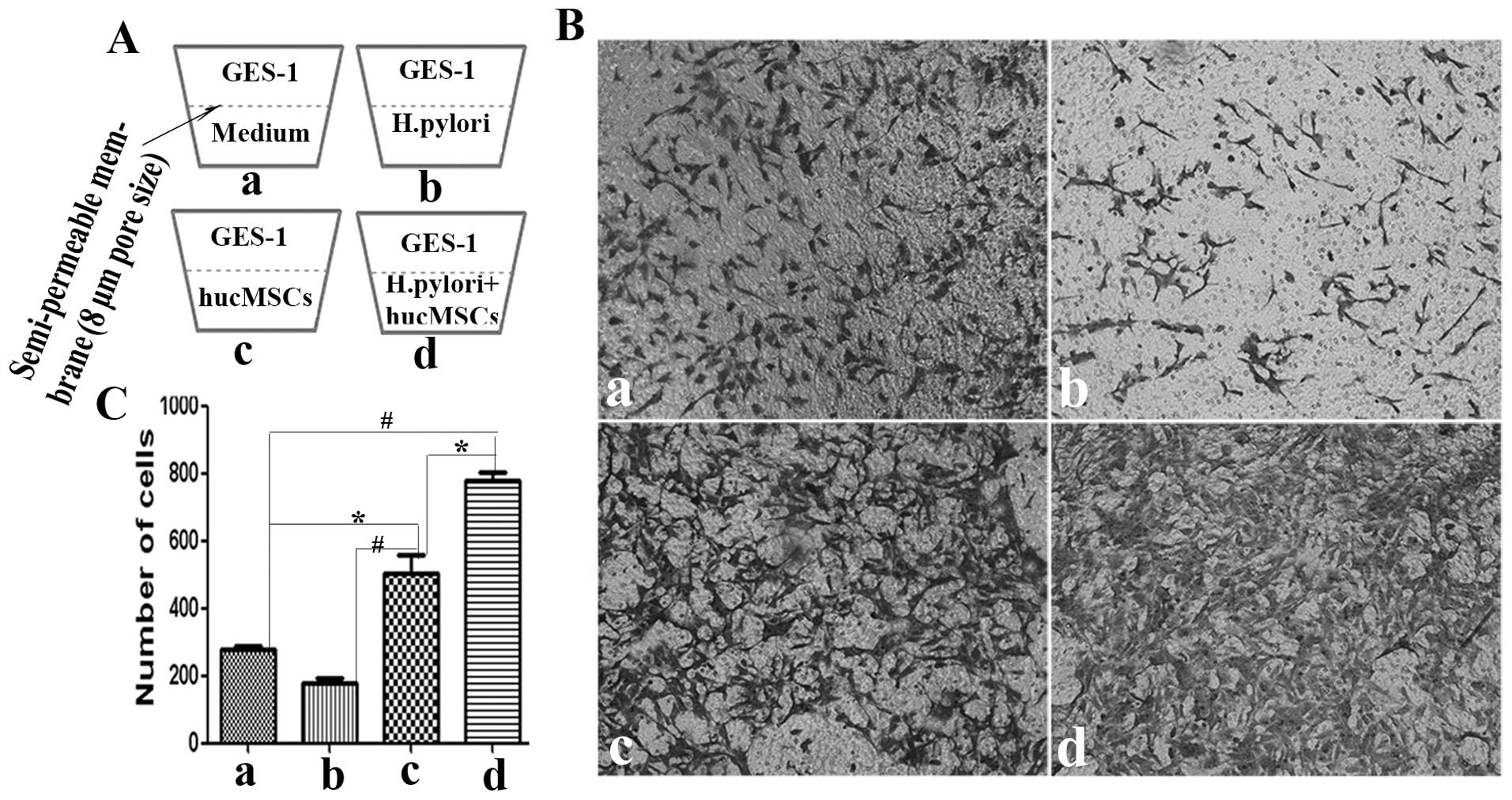
Transwell invasion assay
The invasion assay was carried out as previously
described (20). Briefly, GES-1
cells (5×104 cells/200 μl) suspended in serum-free
medium were loaded into the upper compartment of a Transwell
chamber and 600 μl of 10% FBS-DMEM medium containing hucMSCs
(5×104 cells/well) in the presence or absence of H.
pylori (MOI, 100:1) were added to the bottom well of the
Transwell chamber (Corning, Inc. Life Sciences, MA, USA). Following
culture at 37°C in a humidified atmosphere of 5% CO2 for
8 h, the cells in the upper membrane were wiped with a wet Q-tip.
The cells that had migrated through the membrane (8 μm pore size)
were fixed with 4% paraformaldehyde and stained with crystal
violet. The cells were observed under a microscope and at least 10
fields of cells were assayed for each group. Each assay was
repeated 3 times.
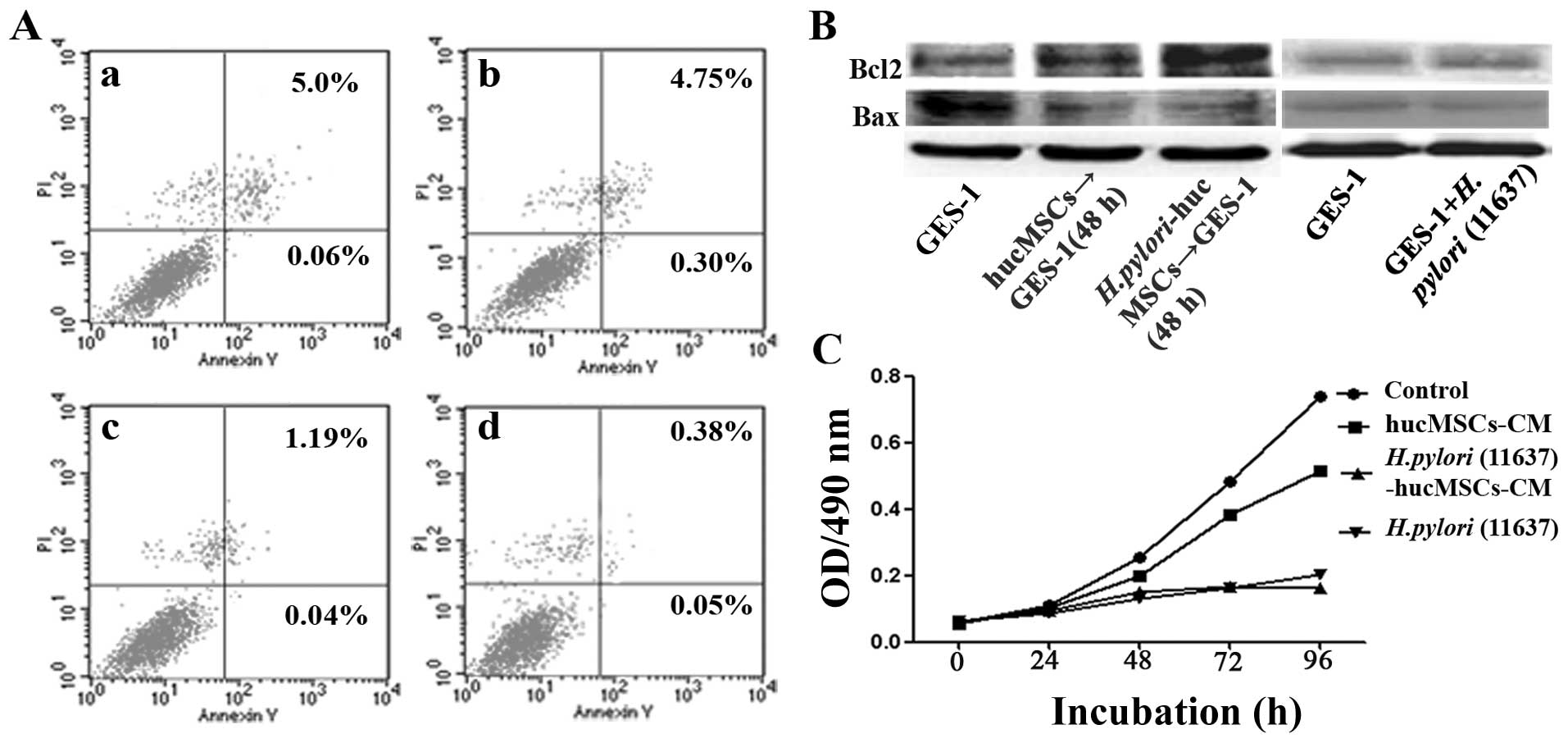
Cell apoptosis assay
Cell apoptosis was evaluated using the FITC-Annexin
V Apoptosis Detection kit I (BioVision Inc., San Francisco, CA,
USA). The GES-1 cells were harvested at 48 h after co-culture with
CM from the infected and uninfected hucMSCs. The fractions of
viable, necrotic and apoptotic cells were detected and quantified
by flow cytometry.
Cell proliferation assay
The GES-1 cells were plated in 96-well plates
(5×103 cells/well) and incubated at 37°C in a humidified
atmosphere with 5% CO2 for 12 h. The cells were then
treated with CM from the infected and uninfected hucMSCs and
incubated for 4 days. At the indicated time points (0, 24, 48, 72
and 96 h), the absorbance of the samples was measured using a
VersaMax Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA,
USA) at wavelength of 450 nm. Each experiment was conducted in
triplicate and repeated twice independently.
Cell colony-forming assay
The GES-1 cells plated in 6-well plates
(1×103 cells/well) were incubated with DMEM (control),
or CM from the uninfected hucMSCs or the H. pylori-infected
hucMSCs for 48 h, and then all groups were incubated at 37°C in a
humidified atmosphere with 5% CO2 for 15 days with
RPMI-1640 medium with 10% FBS (normal medium). The medium was
changed every 3 days. The number of colonies was then evaluated by
crystal violet staining. The results are the mean values of 3
experiments and 3 replicate plates.
Statistical analyses
All data are expressed as the means ± SD. SPSS
software was used to analyze the data. The means of different
treatment groups were compared by two-way ANOVA or the Student’s
t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
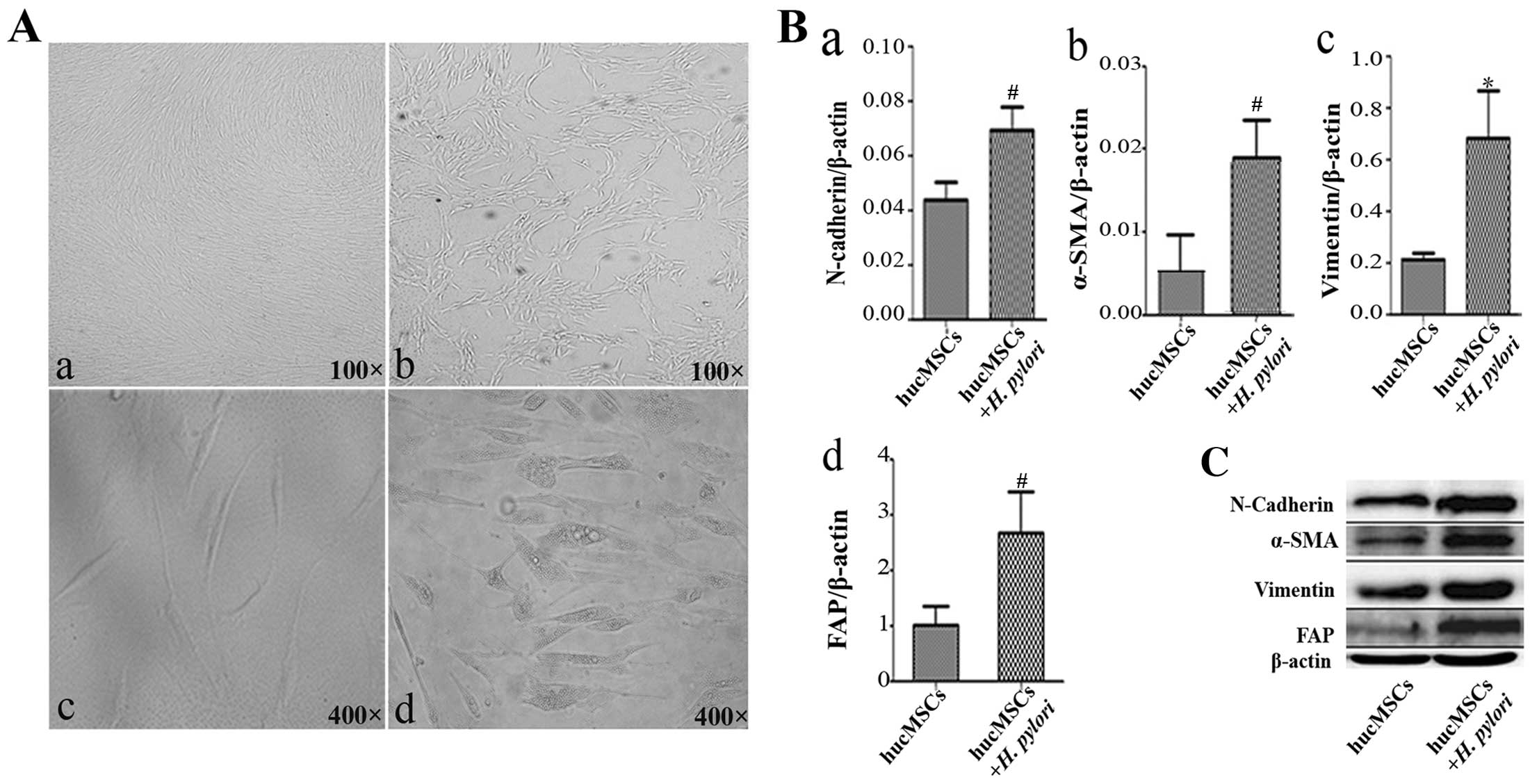
H. pylori infection promotes the
differentiation of hucMSCs into CAFs
To determine the effects of H. pylori
infection on the phenotype of hucMSCs, we infected the cells with
H. pylori at an MOI of 100:1. The hucMSCs acquired a
spindle-shaped morphology at 24 h after H. pylori infection
(Fig. 1A). An increased
expression of fibroblastic proteins (α-SMA, FAP, vimentin and
N-cadherin) has been defined as a marker for CAFs (17,18). Thus, we detected the expression of
CAF markers in the H. pylori-infected hucMSCs by qRT-PCR.
The results revealed that H. pylori infection increased the
expression of FAP, α-SMA, N-cadherin and vimentin genes in the
hucMSCs (Fig. 1B). In agreement
with the gene expression data, FAP, α-SMA, N-cadherin and vimentin
protein levels were also increased in the hucMSCs upon exposure to
H. pylori (Fig. 1C).
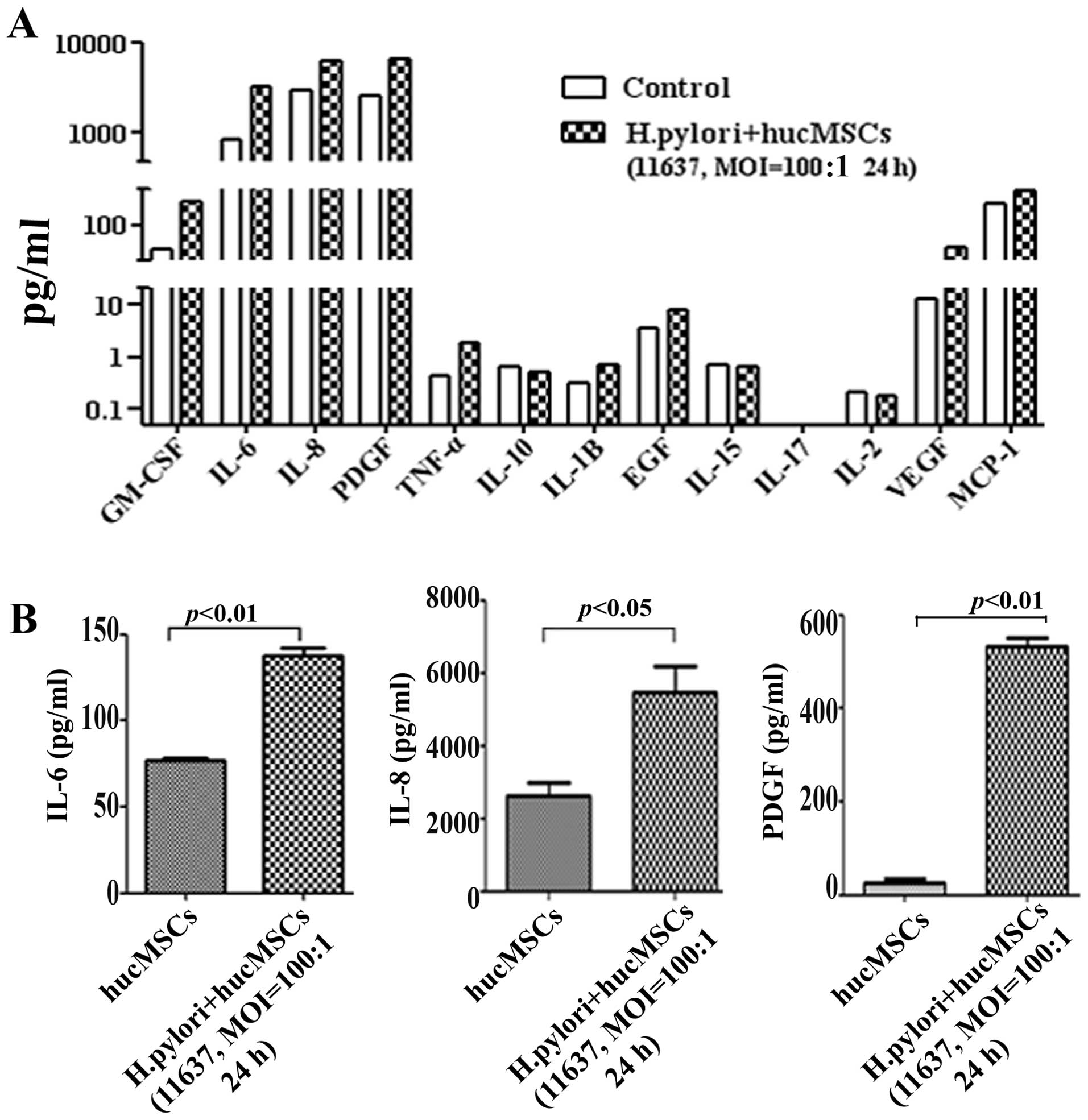
H. pylori infection induces the
production of CAF-associated factors in hucMSCs
The upregulation of specific factors (IL-6, IL-8,
VEGF and EGF) has been suggested as another marker for CAFs
(13,18,22). To that end, we detected
CAF-associated functional factors in H. pylori-infected
hucMSCs by Luminex assay. The results revealed that the expression
of several cytokines and chemokines, such as IL-6, IL-8 and PDGF
was markedly increased in the hucMSCs infected with H.
pylori (Fig. 2A). The
induction of IL-6, IL-8 and PDGF expression in the H.
pylori-infected hucMSCs was further validated by ELISA
(Fig. 2B). These data indicate
that H. pylori infection induces the production of
CAF-associated functional factors in MSCs.
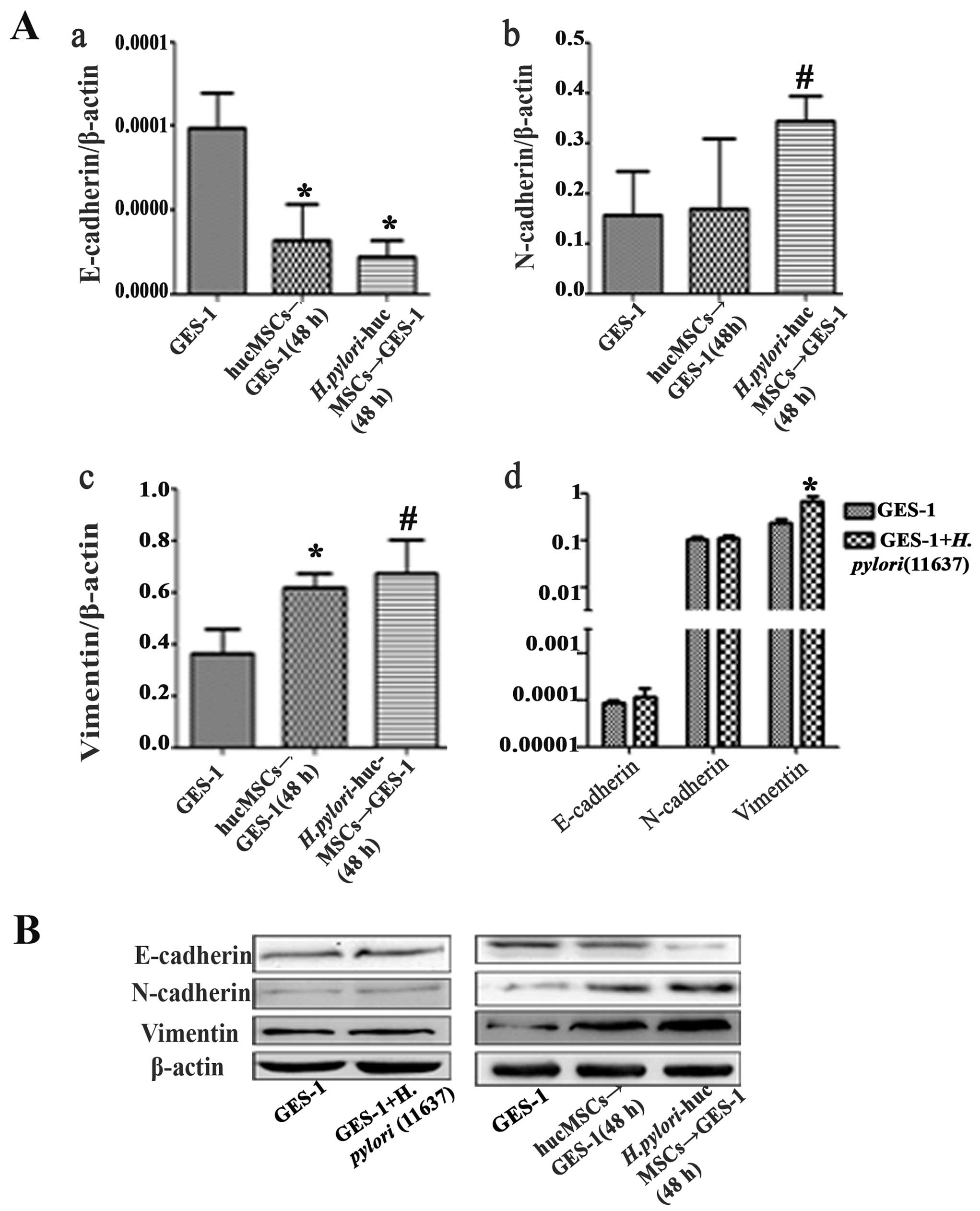
H. pylori-infected hucMSCs induce EMT in
GES-1 cells
To determine the effects of H.
pylori-infected hucMSCs on gastric epithelial cells, we
observed morphological changes in the control GES-1 cells, those
cultured with H. pylori (11637; MOI, 100:1), and those
cultured with uninfected and H. pylori-infected hucMSCs. The
morphological shift from an epithelial to a fibroblastic phenotype
was observed in the GES-1 cells treated with CM from both the
uninfected and H. pylori-infected hucMSCs. However, the
H. pylori infection of the hucMSCs significantly enhanced
their ability to induce the acquirement of a fibroblastic phenotype
in GES-1 cells (Fig. 3). We then
analyzed the expression of EMT markers, including E-cadherin,
N-cadherin and vimentin, in the GES-1 cells that were treated with
CM from the uninfected and H. pylori-infected hucMSCs. The
results revealed that GES-1 cells exhibited a mesenchymal phenotype
characterized by an impaired E-cadherin expression and an increased
expression of vimentin and N-cadherin (Fig. 4).
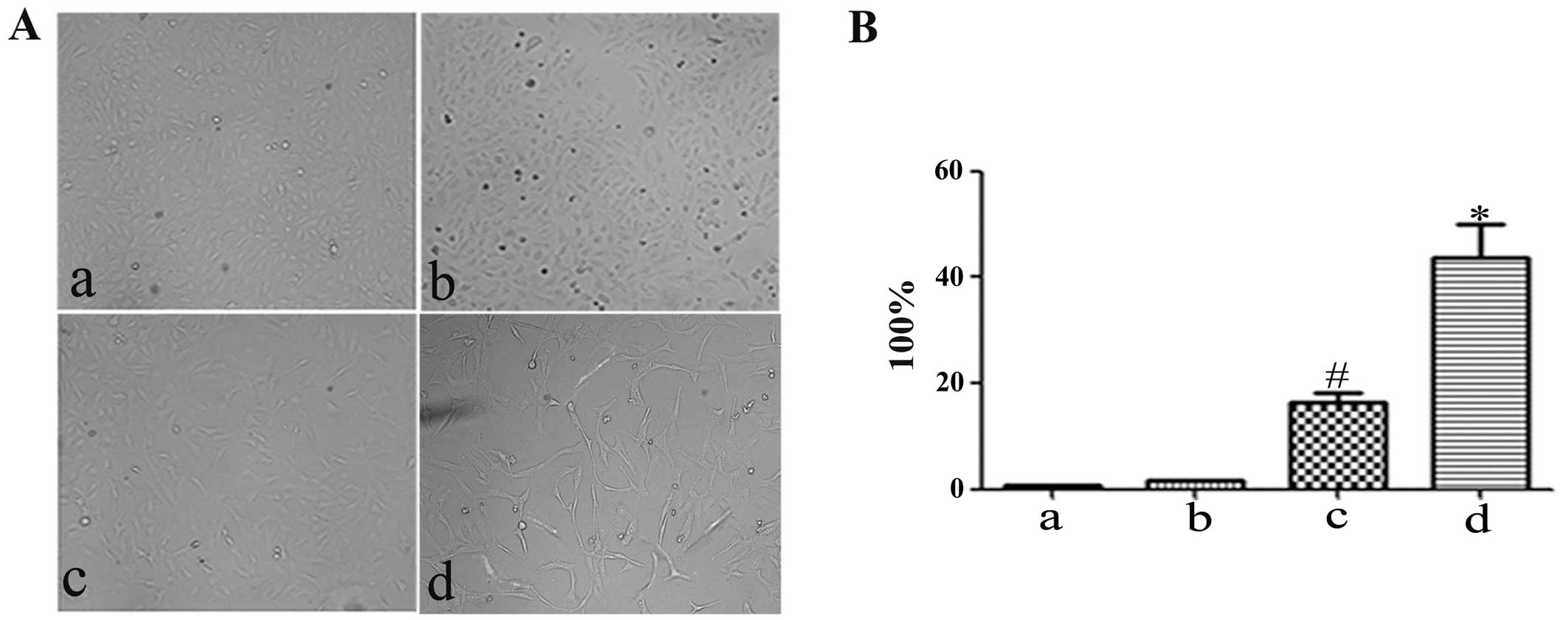
H. pylori-infected hucMSCs enhance the
invasive ability of GES-1 cells
EMT phenotypes are associated with an enhanced
invasive ability. To determine whether H. pylori infection
affects the pro-invasive capacity of the hucMSCs in GES-1 cells, an
in vitro Transwell cell migration assay was performed
(Fig. 5A). CM from the hucMSCs
induced the migration of GES-1 cells. Compared with the control
(control GES-1 cells) and hucMSC group, CM from the H.
pylori-infected hucMSCs induced a more aggressive phenotype in
the GES-1 cells (Fig. 5B). The
number of migrated cells was then quantified (Fig. 5C).
H. pylori-infected hucMSCs reduce the
apoptosis of GES-1 cells
The GES-1 cells were treated with CM from the H.
pylori-infected hucMSCs for 48 h, and cell apoptosis was
analyzed by Annexin V-FITC/PI staining. The apoptotic rate
significantly decreased following exposure to CM from the H.
pylori-infected hucMSCs (5.06 vs. 0.43%, p<0.05) (Fig. 6A). We also detected the expression
of apoptosis-related proteins in the GES-1 cells treated with CM
from the H. pylori-infected hucMSCs. We found that the
expression of Bax, a pro-apoptotic effector, decreased in the
treated GES-1 cells, whereas the expression of the anti-apoptotic
protein, Bcl-2, increased (Fig.
6B). These results indicated that the H. pylori-infected
hucMSCs inhibited apoptosis in gastric epithelial cells.
H. pylori-infected hucMSCs inhibit the
proliferation of GES-1 cells
The GES-1 cells were treated with CM from the H.
pylori-infected hucMSCs for 4 days, and cell proliferation was
analyzed by MTT assay. The results revealed that CM obtained from
the infected hucMSCs significantly inhibited the proliferation of
GES-1 cells in vitro (Fig.
6C).
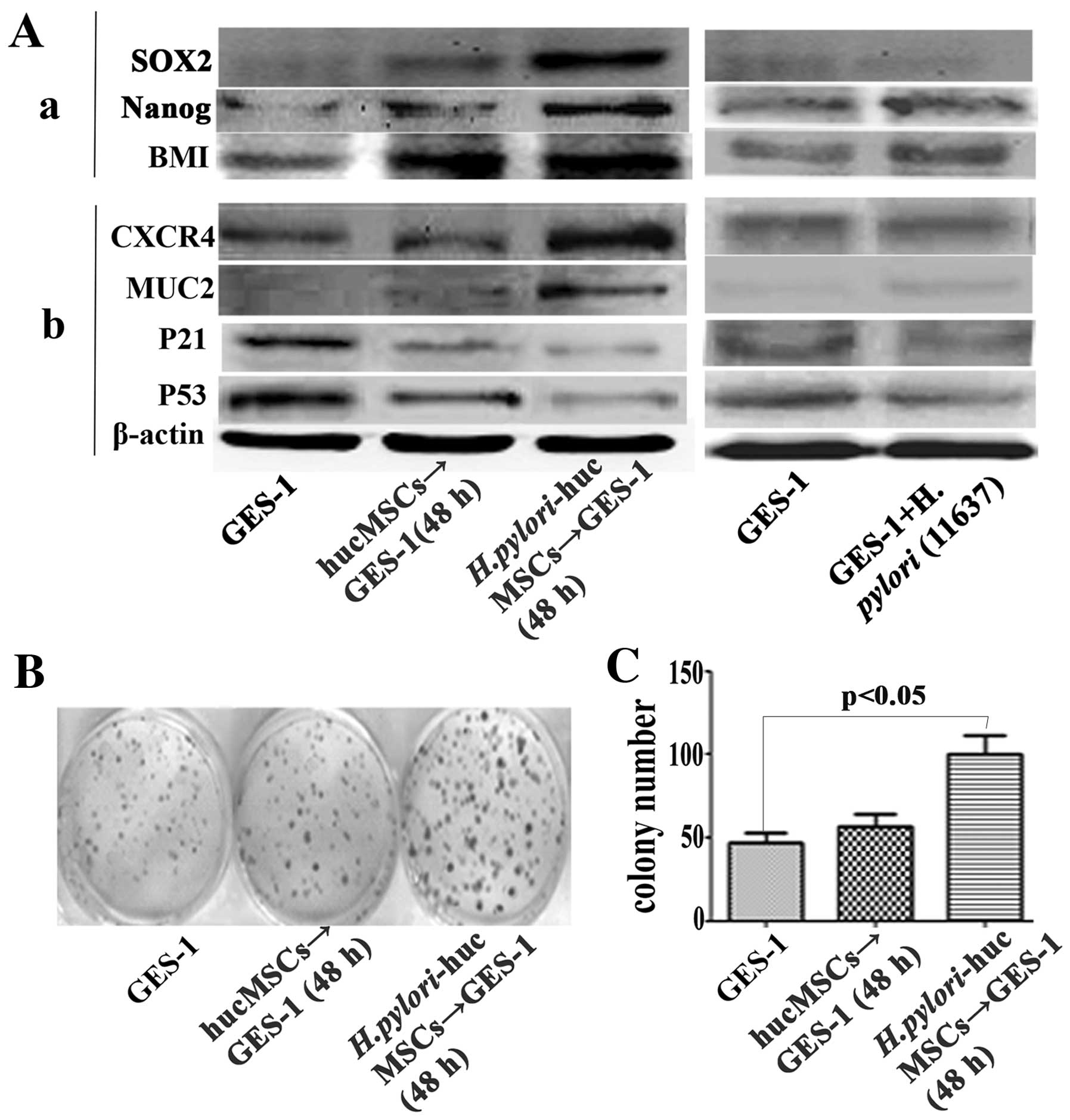
H. pylori-infected hucMSCs induce stem
cell properties in GES-1 cells
Several stem cell-related proteins, including SOX2,
Nanog and BMI were detected in the GES-1 cells treated with CM from
the H. pylori-infected hucMSCs. The H.
pylori-infected hucMSCs significantly upregulated the
expression of SOX2, Nanog and BMI-1 in the GES-1 cells (Fig. 7A-a).
Effect of H. pylori-infected hucMSCs on
the expression of oncoproteins and tumor suppressor proteins in
GES-1 cells
To determine whether H. pylori-infected
hucMSCs can induce the transformation of GES-1 cells, we analyzed
the expression of oncoproteins and tumor suppressor proteins in the
GES-1 cells. The GES-1 cells were treated with CM derived from the
H. pylori-infected hucMSCs for 48 h. The results revealed
that the H. pylori-infected hucMSCs increased the expression
of mucin 2 (MUC2) and chemokine (C-X-C motif) receptor 4 (CXCR4)
oncoproteins, and decreased the expression of the tumor suppressor
proteins, p53 and p21, in the GES-1 cells (Fig. 7A-b).
H. pylori-infected hucMSCs stimulate the
colony-forming ability of GES-1 cells
We further performed a cell colony-forming assay to
assess the oncogenic potential of GES-1 cells that were treated
with CM from the H. pylori-infected hucMSCs. Compared with
the control GES-1 cells and those treated with CM from the
uninfected hucMSCs, the cells treated with CM from the H.
pylori-infected hucMSCs showed a significantly enhanced
colony-forming ability (Fig. 7B and
C).
Discussion
CAFs that express FAP and α-SMA are key determinants
in the malignant progression of cancer growth, vascularization and
metastasis (11,17,18). After being passaged successively
10 times in vitro without ongoing interaction with carcinoma
cells, CAFs still retain their ability to promote tumor growth when
co-injected with carcinoma cells into immunodeficient mice
(11,12). MSCs have been shown to be involved
in H. pylori infection-associated gastric carcinogenesis
(15). However, the mechanisms
responsible for the promoting roles of MSCs in cancer initiation
and progression are not yet well understood. In this study, we
demonstrated that H. pylori infection induced typical CAF
differentiation with the increased expression of CAF markers and
cytokines in hucMSCs. Recent studies have demonstrated well-defined
roles for CAF-associated cytokines, such as IL-6, IL-8, PDGF, VEGF,
EGF and GM-CSF in tumorigenesis (18,22,23). The results presented in this study
suggest that H. pylori infection may induce the
differentiation of MSCs into CAFs and enhance the secretion of
multiple cytokines.
EMT is essential for the generation of new tissues
during embryogenesis and plays pivotal roles in inflammation and
wound healing (24,25). EMT is defined as a biological
process, in the course of which epithelial cell-cell adhesion is
decreased by the downregulation of adhesion molecules, such as
E-cadherin, and cell morphology becomes fibroblast-like with the
upregulation of vimentin and N-cadherin (24). In addition to a loss in epithelial
characteristics, EMT frequently coincides with the acquisition of
motility and invasiveness, as well as an increase in the resistance
to apoptosis and a markedly altered production of extracellular
matrix components. Cancer is often viewed as the corrupted form of
normal development (26–29). EMT has been implicated as a
fundamental step of carcinogenesis (30) and represents one of the steps
required for tumor progression through invasion and metastatic
spread (31). We hypothesized
that H. pylori-infected MSCs may promote gastric
lesions/malignancies under the conditions of chronic gastritis
through the induction of EMT in gastric epithelial cells. To prove
this hypothesis, we analyzed the phenotype of GES-1 cells
co-cultured with CM from H. pylori-infected hucMSCs. We
found that GES-1 cells exposed to CM from H. pylori-infected
hucMSCs not only displayed a morphological shift from an epithelial
to a fibroblastic phenotype, but also presented decreased
E-cadherin expression, increased vimentin and N-cadherin
expression, and an enhanced invasive ability in vitro. These
results are in agreement with data from previous studies,
demonstrating that the loss of E-cadherin expression augments
cellular dissemination and metastasis (32). These results support the
hypothesis that H. pylori infection-induced MSC transition
into CAFs results in gastric lesions/malignancies by promoting the
occurrence of EMT.
Tumor development involves multiple steps and
factors, including the activation of oncogenes, the inactivation of
tumor suppressor genes, and the aberrant expression of
apoptosis-related genes (33). In
this study, we demonstrated that the infected hucMSCs enhanced the
expression of the oncoproteins, MUC2 and CXCR4, whereas they
inhibited the expression of the tumor suppressor proteins, p53 and
p21, in GES-1 cells. We also demonstrated that the proliferation
rate of the GES-1 cells was reduced by CM from H.
pylori-infected hucMSCs. These results are consistent with
those of other studies, suggesting that the proliferation rate of
cancer cells decreases when the cells move and migrate (34,35). Evidence suggests that cancer cells
acquire stem cell-like properties through EMT (36). Mani et al (37) demonstrated an upregulation of stem
cell markers through the induction of EMT in mammary epithelial
cells and breast cancer cells. Additional studies have demonstrated
that the induction of EMT not only promotes tumor cell invasion and
metastasis, but also contributes to the acquisition of stem cell
properties (38). In this study,
we detected the expression of stem cell markers and evaluated the
capacity of gastric epithelial cells to form colonies. We
demonstrated that GES-1 cells expressed higher levels of the
stemness markers, Nanog, BMI and SOX2, following exposure to CM
from H. pylori-infected hucMSCs. Nanog, SOX2 and BMI have
been shown to be critical factors for cancer initiation and
progression (39–41). The upregulation of these
stemness-related factors indicated the acquirement of stem cell
properties in the GES-1 cells following exposure to CM from H.
pylori-infected hucMSCs. In addition, the H.
pylori-infected MSCs markedly enhanced the colony-forming
ability of gastric epithelial cells, suggesting that incubation
with CM from H. pylori-infected MSCs endows gastric
epithelial cells with both oncogenic potential and self-renewal
ability. However, the specific factors that mediate this process
and the signaling pathways involved remain to be identified.
Taken together, we demonstrate that H. pylori
infection induces the differentiation of MSCs into CAF-like cells
and that incubation with CM from H. pylori-infected MSCs
destroys cell junctions, promotes cell invasion and enhances the
colony-forming ability of gastric epithelial cells though the
induction of EMT. Our findings suggest that H. pylori
infection causes gastric lesions/malignancies by converting MSCs
into CAFs, creating a unique microenvironment for the malignant
transformation of the normal gastric epithelium. This study may aid
in the understanding of the role and mechanisms of action of MSCs
in the initiation and progression of H. pylori-associated
gastric cancer.
Acknowledgements
This study was supported by the Major Research Plan
of the National Natural Science Foundation of China (grant no.
91129718), the National Natural Science Foundation of China (grant
nos. 81071421, 81302119, 81000181 and 81201660), the Jiangsu
Province Project of Scientific and Technological Innovation and
Achievements Transformation (grant no. BL2012055), the Jiangsu
Province Outstanding Medical Academic Leader and Sci-tech
Innovation Team Program (grant no. LJ201117), the Doctoral Program
Foundation of State Education Ministry (grant no. 20113227110011),
the Jiangsu Province Natural Science Foundation (grant no.
BK20130540) and the Jiangsu Province Department of Education
Science Research Foundation (grant no. 13KJB320001).
References
|
1
|
Machado AM, Figueiredo C, Touati E, et al:
Helicobacter pylori infection induces genetic instability of
nuclear and mitochondrial DNA in gastric cells. Clin Cancer Res.
15:2995–3002. 2009. View Article : Google Scholar
|
|
2
|
Milne AN, Carneito F, O’Morain C and
Offerhaus GJ: Nature meets nurture: molecular genetics of gastric
cancer. Hum Genet. 126:615–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chan AO, Chu KM, Huang C, et al:
Association between Helicobacter pylori infection and
interleukin 1beta polymorphism predispose to CpG island methylation
in gastric cancer. Gut. 56:595–597. 2007.
|
|
4
|
Qiao C, Xu W, Zhu W, et al: Human
mesenchymal stem cells isolated from the umbilical cord. Cell Biol
Int. 32:8–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao H, Xu W, Qian H, et al: Mesenchymal
stem cell-like cells derived from human gastric cancer tissues.
Cancer Lett. 274:61–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schäffler A and Büchler C: Concise review:
adipose tissue-derived stromal cells - basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007.PubMed/NCBI
|
|
7
|
Charbord P: Bone marrow mesenchymal stem
cells: historical overview and concepts. Hum Gene Ther.
21:1045–1056. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phinney DG and Prockop DJ: Concise review:
mesenchymal stem/multipotent stromal cells: the state of
transdifferentiation and modes of tissues repair - current views.
Stem Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma
D and Shimizu H: Mesenchymal stem cells are recruited into wounded
skin and contribute to wound repair by transdifferentiation into
multiple skin cell type. J Immunol. 180:2581–2587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spaeth E, Kloop A, Dembinski J, Andreeff M
and Marini F: Inflammation and tumor microenvironments: defining
the migratory itinerary of mesenchymal stem cells. Gene Ther.
15:730–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orimo A and Weinberg RA: Stromal
fibroblasts in cancer: a novel tumor-promoting cell type. Cell
Cycle. 5:1597–1601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Orimo A, Gupta PB, Sqroi DC, et al:
Stromal fibroblasts present in invasive human breast carcinomas
promote tumor growth and angiogenesis through elevated SDF-1/CXCL12
secretion. Cell. 121:335–348. 2005. View Article : Google Scholar
|
|
13
|
Glaire MA, EI-Omar EM, Wang TC and
Worthley DL: The mesenchyme in malignancy: a partner in the
initiation, progression and dissemination of cancer. Pharmacol
Ther. 136:131–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: mesenchymal stem cells: their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar
|
|
15
|
Houghton J, Stoicov C, Nomura S, et al:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrand J, Lehours P, Schmid-Alliana A,
Mégraud F and Varon C: Helicobacter pylori infection of
gastrointestinal epithelial cells in vitro induces mesenchymal stem
cell migration through an NF-κB-dependent pathway. PLoS One.
6:e290072011. View Article : Google Scholar
|
|
17
|
Dudás J, Fullár A, Bitsche M, et al:
Tumor-produced, active interleukin-1β regulates gene expression in
carcinoma-associated fibroblasts. Exp Cell Res. 317:2222–2229.
2011.PubMed/NCBI
|
|
18
|
Spaeth EL, Dembinski JL, Sasser AK, et al:
Mesenchymal stem cell transition to tumor-associated fibroblasts
contributes to fibrovascular network expansion and tumor
progression. PLoS One. 4:e49922009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giannoni E, Bianchini F, Masieri L, Serni
S, Torre E, Calorini L and Chiarugi P: Reciprocal activation of
prostate cancer cells and cancer-associated fibroblasts stimulates
epithelial- mesenchymal transition and cancer stemness. Cancer Res.
70:6945–6956. 2010. View Article : Google Scholar
|
|
20
|
Gu J, Qian H, Shen L, et al: Gastric
cancer exosomes trigger differentiation of umbilical cord derived
mesenchymal stem cells to carcinoma-associated fibroblasts through
TGF-β/Smad pathway. PLoS One. 7:e524652012.PubMed/NCBI
|
|
21
|
Qian H, Yang H, Xu W, et al: Bone marrow
mesenchymal stem cells ameliorate rat acute renal failure by
differentiation into renal tubular epithelial-like cells. Int J Mol
Med. 22:325–332. 2008.PubMed/NCBI
|
|
22
|
Räsänen K and Vaheri A: Activation of
fibroblasts in cancer stroma. Exp Cell Res. 316:2713–2722.
2010.
|
|
23
|
Augsten M, Hägglöf C, Olsson E, et al:
CXCL14 is an autocrine growth factor for fibroblasts and acts as a
multi-modal stimulator of prostate tumor growth. Proc Natl Acad Sci
USA. 106:3414–3419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiery JP: Epithelial-mesenchymal
transitions in development and pathologies. Curr Opin Celll Biol.
15:740–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allan GJ, Beattie J and Flint DJ:
Epithelial injury induces an innate repair mechanism linked to
cellular senescence and fibrosis involving IGF-binding protein-5. J
Endocrinol. 199:155–164. 2008. View Article : Google Scholar
|
|
26
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massagué J: TGFbeta in cancer. Cell.
134:215–230. 2008.
|
|
28
|
Padua D and Massagué J: Roles of TGFbeta
in metastasis. Cell Res. 19:89–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Buijs JT, Henriquez NV, van Overveld PG,
van der Horst G, ten Dijke P and van der Pluijm G: TGF-beta and
BMP7 interactions in tumour progression and bone metastasis. Clin
Exp Metastasis. 24:609–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsukamoto H, Shibata K, Kajiyama H,
Terauchi M, Nawa A and Kikkawa F: Irradiation-induced
epithelial-mesenchymal transition (EMT) related to invasive
potential in endometrial carcinoma cells. Gynecol Oncol.
107:500–504. 2007. View Article : Google Scholar
|
|
33
|
SI PH and HU QG: Progress of RNA interfere
in gene therapy for oral tumor. Int J Stomatol. 134:281–283.
2007.
|
|
34
|
Berdiel-Acer M, Bohem ME, López-Doriga A,
et al: Hepatic carcinoma-associated fibroblasts promote an
adaptative response in colorectal cancer cells that inhibit
proliferation and apoptosis: nonresistant cells die by nonapoptotic
cell death. Neoplasia. 13:931–946. 2011.
|
|
35
|
Vega S, Morales AV, Ocaña OH, Valdés F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mani SA, Guo W, Liao MJ, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thiery JP, Aclogue H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeter CR, Badeaux M, Choy G, et al:
Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu Y, Futtner C, Rock JR, Xu X, Whitworth
W, Hogan BL and Onaitis MW: Evidence that SOX2 overexpression is
oncogenic in the lung. PLoS One. 5:el110222010. View Article : Google Scholar
|
|
41
|
Qiao B, Chen Z, Hu F, Tao Q and Lam AK:
BMI-1 activation is crucial in hTERT-induced epithelial-mesenchymal
transition of oral epithelial cells. Exp Mol Pathol. 95:57–61.
2013. View Article : Google Scholar : PubMed/NCBI
|