Introduction
Inflammatory bowel disease (IBD) is multifactorial
and involves immunological, environmental and genetic factors.
Although there are no animal models that effectively mimic human
IBD, experimental models allow us to analyze the mechanisms of
chronic intestinal inflammation. Animal models of IBD involve
genetic manipulation, i.e., either the insertion (transgenic) or
selective deletion (knockout) of a gene. In addition, immune
responses evoked in animal models are often used as models of IBD
for the development and evaluation of drug candidates. For example,
2,4,6-trinitrobenzene sulfonic acid (TNBS)-ethanol enema-induced
colitis is associated with chemically induced damage and T cell
immune reactivity. The administration of the sensitizing agent,
TNBS, in a 45–50% ethanol enema induces colitis in rodents. In the
TNBS model, ethanol breaks the mucosal barrier and is a crucial
component; colitis is not induced by TNBS alone (1,2).
In mice, TNBS-induced colitis shows the common delayed
hypersensitivity response, mediated by T cells that are sensitized
by ‘hapten-modified self-antigens’. These self-antigens can be
produced by the covalent binding of the hapten trinitrophenyl (TNP)
to self-peptides. Tissues from mice with TNBS-induced colitis show
an infiltration of CD4+ T helper (Th) cells, mainly Th1
and Th17 (3), and IgG- or
IgA-producing B cells (4) in the
mucosa and submucosa.
Colitis can be also induced in hamsters, rats and
mice by the addition of 30–35 kDa dextran sulfate sodium (DSS) to
drinking water at 3–10% (5). It
results in bloody diarrhea, weight loss, shortening of the colon,
mucosal ulceration and neutrophilic infiltration. This is a fairly
simple and reproducible method used to induce colitis in mice, and
DSS-induced colitis is popular for the screening of potential
therapeutic agents, with a high number of agents having shown
beneficial effects in this model, indicating that it is a sensitive
screening model for IBD. However, limitations of this model include
the fact that DSS-induced colitis does not require T or B cell
responses (6), and that luminal
bacteria may play a role in the development of this type of colitis
(7).
Inflammasomes sense damage signals during infection
(e.g., a norovirus infection) and activate caspase-1, leading to
the activation of interleukin (IL)-1β and IL-18 by cleaving their
pro-forms. Increased levels of pro-inflammatory cytokines,
including IL-1β, IL-6, IL-18 and tumor necrosis factor-α (TNF-α),
have been reported in IBD and correlate with the severity of
inflammation (8,9). Indeed, enhanced levels of IL-1β have
been found in the colonic mucosa and in peritoneal macrophages in
the mouse model of colitis and may hence represent an initial
trigger of intestinal inflammation (10). In the DSS-induced colitis model,
it has been reported that DSS triggers inflammation via the
activation of the NLRP3 inflammasome, leading to caspase-1
activation (11), and also via
the NLRP6 inflammasome (12,13). In addition, studies using
caspase-1 knockout (KO) mice have suggested that caspase-1
contributes to DSS-induced colitis (14).
Myeloid-derived suppressor cells (MDSCs) are a
heterogeneous group of pathologically activated myeloid
CD11b+Gr-1+ cells, consisting of granulocytic
and monocytic subsets. MDSCs are recruited from the blood to the
site of chronic inflammation, cancer, or transplantation, and
modulate innate and adaptive immune responses (15). In the mouse model of colitis, the
transplantation of splenic CD11b+Gr-1+ cells
into mice with DSS-induced colitis improved disease parameters
(16).
In this study, we compared mouse models of TNBS- and
DSS-induced, as well as acute and chronic DSS-induced colitis in
terms of histological parameters and profiles of MDSCs and
CD4+ T cells. We also investigated the role of caspase-1
in TNBS-induced colitis using caspase-1 KO mice.
Materials and methods
Mice
Female, 8-week-old C57BL/6 mice were acquired from
Daehan Biolink Co. Ltd. (Samseong-myeon, Korea) and caspase-1 KO
mice were generously provided by Dr F.S. Sutterwala from the
University of Iowa (Ames, IA, USA). Mice were housed in an animal
care facility, and were provided with food and water ad
libitum; they were exposed to a 12:12 h light-dark cycle at
room temperature. All procedures were approved by The Animal Care
and Use Committee of Ewha Womans University School of Medicine,
Seoul, Korea (ESM 10-0153).
Mouse model of TNBS-induced colitis
Colitis was induced by the rectal administration of
2 mg/100 ml of TNBS (Sigma-Aldrich, St. Louis, MO, USA) in 45%
ethanol (Merck, Darmstadt, Germany) using a vinyl catheter
positioned 3.5 cm proximal to the anus. During the procedure, the
mice were anaesthetized using Rumpun/Zoletil. Following the
instillation of the catheter, the animals were kept vertical for 30
sec. The control mice underwent identical procedures, but were
instilled with 45% ethanol dissolved in phosphate-buffered saline
(PBS). The mice were monitored daily for survival, body weight,
rectal bleeding and stool consistency. All animals were sacrificed
on day 5 of the experiment by cervical dislocation.
Mouse models of DSS-induced colitis
For acute DSS-induced colitis, the mice were
administered 4% DSS (molecular weight, 36,000–50,000; MP
Biomedicals, Solon, OH, USA) in their drinking water for 7 days.
The control animals were administered distilled water. For the
induction of chronic colitis, mice were administered 2% DSS in
their drinking water for 5 days, followed by 5 days of the
administration of distilled water. This cycle was repeated 3 times.
The mice were monitored daily for survival, body weight, rectal
bleeding and stool consistency.
Histological analysis
The large and small intestine and spleen tissues
were fixed in 4% paraformaldehyde and embedded in paraffin. Fixed
tissues were cut into 5-mm-thick sections, placed on glass slides
and deparaffinized. The sections were stained with hematoxylin and
eosin (H&E) and observed under a light microscope.
Cell preparation
Lamina propria (LP) cell
isolation
Segments of the small intestine were incubated for
30 min at 37°C with fluorescence-activated cell sorting (FACS)
buffer [PBS containing 10% fetal bovine serum (FBS), 20 mM Hank’s
balanced salt solution, 100 U/ml penicillin, 100 mg/ml
streptomycin, 1 mM sodium pyruvate, 10 mM
ethylenediaminetetraacetic acid (EDTA) and 10 mg/ml polymyxin B] to
remove epithelial cells, and were washed extensively with PBS. The
segments were then digested with 400 Mandl U/ml collagenase D and
10 mg/ml DNase I (both from Roche Diagnostics GmbH, Mannheim,
Germany) in RPMI-1640/10% FBS solution with continuous stirring at
37°C for 45–90 min. EDTA was added (10 mM), and the cell suspension
was incubated for an additional 5 min at 37°C. After washing, the
cells were subjected to density gradient centrifugation in 40/75%
Percoll (approximate density, 1.058/1.093 g/ml), and LP leukocytes
harvested from the interface were washed with PBS.
Basal membrane (BM) cell
isolation
For BM isolation, mice were sacrificed by cervical
dislocation and their limbs removed. The BM was flushed from the
medullary cavities of femurs and tibias in RPMI-1640 medium using a
25-gauge needle. Cell suspensions were collected though a 70-mm
cell strainer and centrifuged. After the cells were pelleted,
erythrocytes were removed by incubation with RBC lysis solution
(0.15 M NH4Cl, 10 mM NaHCO3, 10 mM
EDTA-disodium in distilled water) and washed with PBS.
Peyer’s patch (PP) and spleen (SP)
cell isolation
Freshly isolated tissues were placed on a cell
strainer (70 μm pore size) in a dish (6 cm in diameter) containing
RPMI-1640 medium and 10% FBS. Tissues were mechanically disrupted
using a plunger and cells were released through a cell strainer.
After collection of the cells in a 15-ml conical tube, the cells
were centrifuged and the erythrocytes removed using RBC lysis
solution.
Flow cytometry
In order to compare the cellular profiles following
the induction of colitis, cells from the BM, SP, PP and LP were
resuspended in FACS buffer. The cells were subsequently incubated
with the following antibodies for 30 min at 4°C (all from
BioLegend, San Diego, CA, USA): fluorescein isothiocyanate
(FITC)-labeled anti-mouse Ly-6G/Ly-6C (Gr-1, RB6-8C5),
phycoerythrin (PE)-labeled anti-mouse CD11b (M1/70), PE-labeled
anti-mouse CD4 (GK1.5), peridinin-chlorophyll-protein complex
(PerCP)-labeled anti-mouse CD4 (GK1.5), FITC-labeled anti-mouse
interferon (IFN)-γ (XMG1.2), PE-labeled anti-mouse IL-9 (RM9A4),
PE-labeled anti-mouse IL-17A (TC11-18H18.1) and Alexa
Fluor®488-labeled anti-mouse FoxP3 (150D). The levels of
labeled proteins were then analyzed on a FACSCalibur instrument (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Values are expressed as the means ± standard error
of the mean (SEM). Survival rates were estimated using Kaplan-Meier
plots. Non-parametric Mann-Whitney tests or two-way analysis of
variance were conducted to determine significance (P<0.05),
using GraphPad Prism software (GraphPad Software Inc., San Diego,
CA, USA).
Results
Induction of colitis with DSS or
TNBS
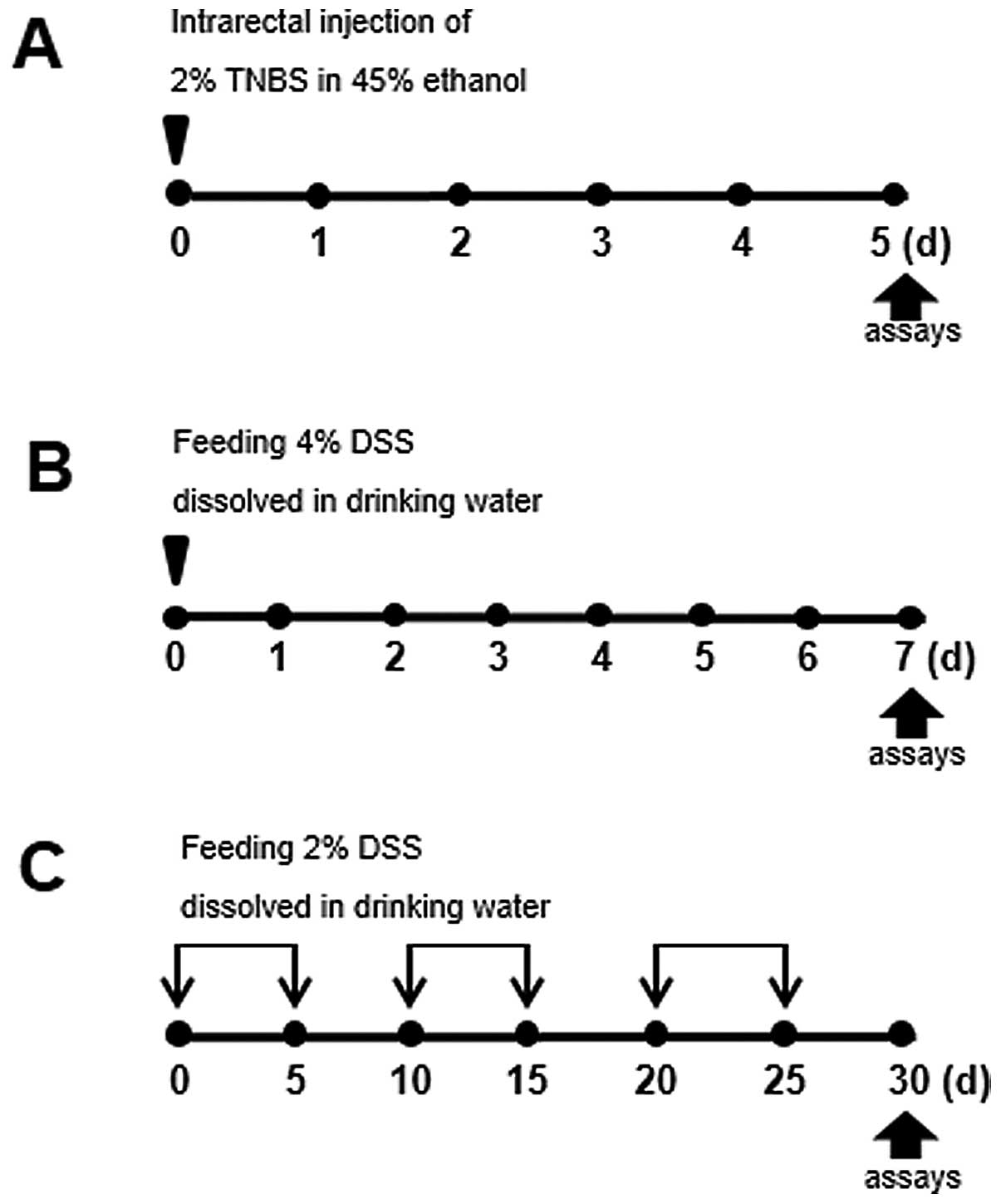
In order to examine the pathogenesis of IBD, we
established mouse models of colitis using TNBS or DSS. To compare
different types of intestinal inflammation caused by different
chemical agents or induced for different periods of time, colitis
was induced as illustrated in Fig.
1. For instance, to induce acute colitis, the intrarectal
administration of TNBS, combined with ethanol to increase
intestinal permeability, was used to elicit intense and sustained
inflammation. In the model of DSS-induced acute colitis, the mice
were administered 4% DSS in their drinking water for 7 days (days
0–6).
Differences between TNBS- and DSS-induced
colitis in C57BL/6 mice
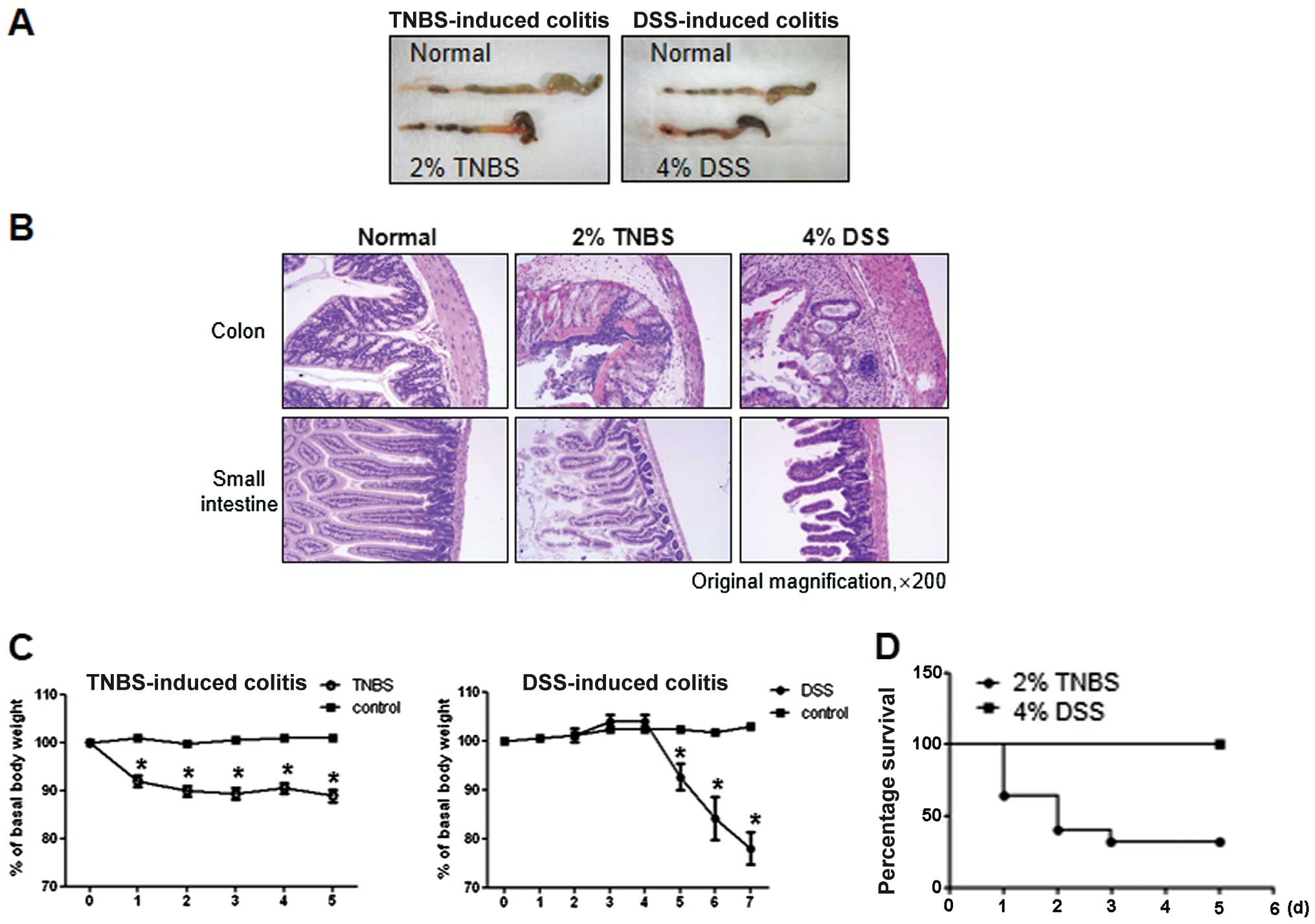
As shown in Fig.
2A, both groups of TNBS- and DSS-treated mice exhibited severe
inflammation, manifested by shortened, thickened and erythematous
colons. In macroscopic histological observations, both groups
showed a massive bowel edema and disruption of epithelial cells by
large ulcerations (Fig. 2B).
However, changes in body weight were more prominent in TNBS-induced
colitis compared with DSS-induced colitis. In particular, the
majority of the TNBS-induced mice quickly developed diarrhea and
showed poor coat quality and reduced mobility, followed by
significant weight loss and mortality (data not shown). By
contrast, mice with DSS-induced colitis showed a delay in these
reactions, manifesting on day 5 from the first day of
administration, while survival was 100% (Fig. 2C and D).
Pronounced differences between acute vs.
chronic DSS-induced colitis
Since IBD is characterized by chronic inflammatory
responses and multiple exacerbations during disease progression, a
mouse model of chronic colitis is required. The mice with
TNBS-induced colitis showed a higher rate of mortality, as shown in
Fig. 2D; we therefore used the
model of DSS-induced colitis for studying both the acute and
chronic forms of the disease. While acute colitis was induced by
preparing a 4% DSS solution in drinking water for 5 days, C57BL/6
mice (a highly susceptible strain) were subjected to cyclic
treatment with 2% DSS for 30 days to induce chronic inflammation
(Fig. 1C). Under these
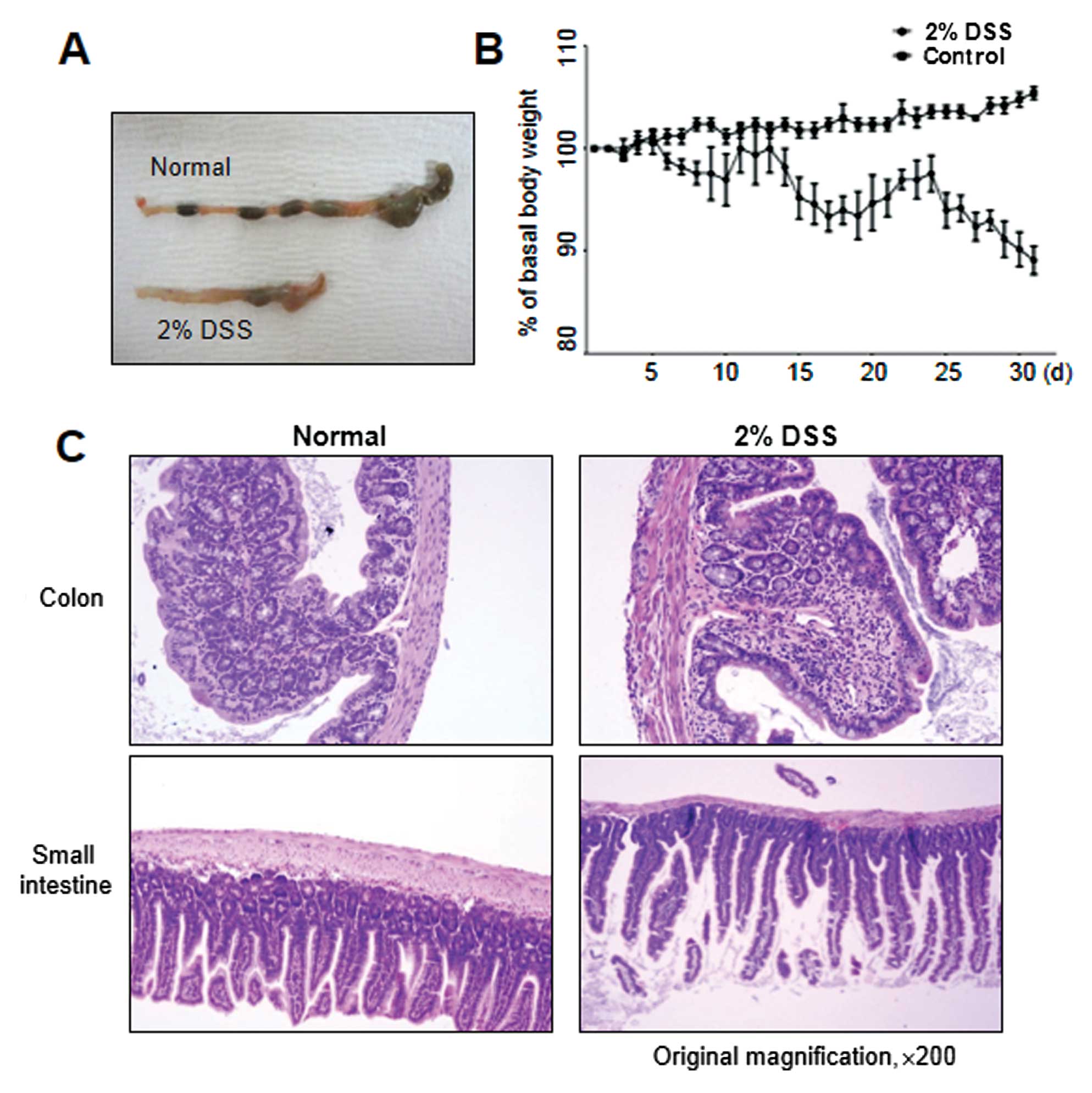
conditions, as shown in Fig. 3A,
chronic colitis developed, accompanied by reduced colon length,
similar to that observed in DSS-induced acute colitis. Moreover,
continuous weight loss was observed, particularly in the 2- to
3-day interval following DSS administration (Fig. 3B). In accordance with these signs
of colitis, mice further exhibited pathological characteristics at
the endpoint of the experiment, i.e., they showed necrosis,
ulcerations, increase in the number of goblet cells, and
infiltration of neutrophils and macrophages into the colonic
mucosal and submucosal layers at 30 days post-DSS treatment
(Fig. 3C). The colonic mucosal
structure of the crypt was broken in both the acute and chronic
disease groups, with the occurrence of colonic inflammation,
resulting in a clear thickening of the mucosal and muscle layers in
the DSS-induced colitis group as compared with the control mouse
group (Figs. 2B and 3C).
Comparison of cellular profiles between
acute and chronic DSS-induced colitis
We then compared acute and chronic DSS-induced
colitis in terms of the mucosal innate immune response, focusing on
cellular profiles. IBD is a chronic intestinal inflammatory disease
characterized by the infiltration and activation of various immune
cells, leading to prolonged inflammation in the intestine. In order
to identify the colonic cellular populations involved in acute and
chronic DSS-induced colitis, LP leukocyte cells isolated from
DSS-treated mice were examined for their MDSC profile through the
determination of CD11b+Gr-1+ levels, as well
as of the levels of Th cells, including Th1 (IFNγ-secreting,
IFNγ+), Th2 (IL-4-secreting, IL-4+), Th9
(IL-9-secreting, IL-9+), Th17 (IL-17-secreting,
IL-17+) and Treg (Foxp3+). Other
lymphoid organs, including the BM, SP and PP, were also included in
the determination of cellular profiles following the administration
of DSS.
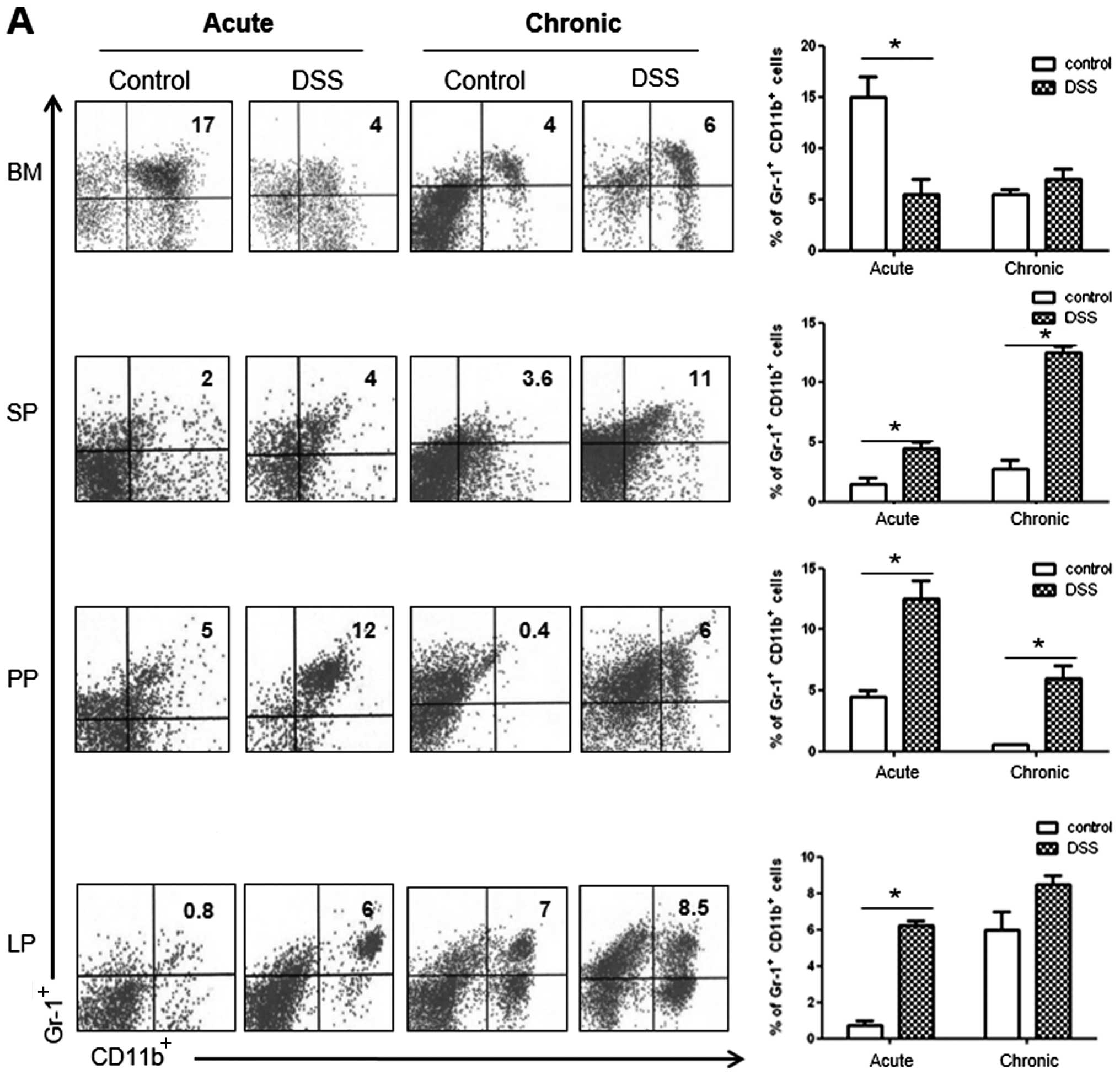
As shown in Fig.
4A, we observed a significant increase in the number of
CD11b+Gr-1+cells in the SP and PP of the
acute and chronic colitis mouse groups, as compared with the
controls. However, the number of CD11b+Gr-1+
cells decreased in the BM and increased in the LP only in the acute
colitis group. As regards CD4+ T cell subsets in the LP,
we observed a significantly higher percentage of IFNγ+
and IL-17+ cells in the mice with DSS-induced colitis in
both the acute and the chronic models of colitis (Fig. 4B). Notably, the number of
IL-9+ cells was markedly increased only in the chronic
colitis group, whereas the number of Foxp3+ cells showed
a significant decrease in both groups of mice with colitis.
Pronounced differences between WT and
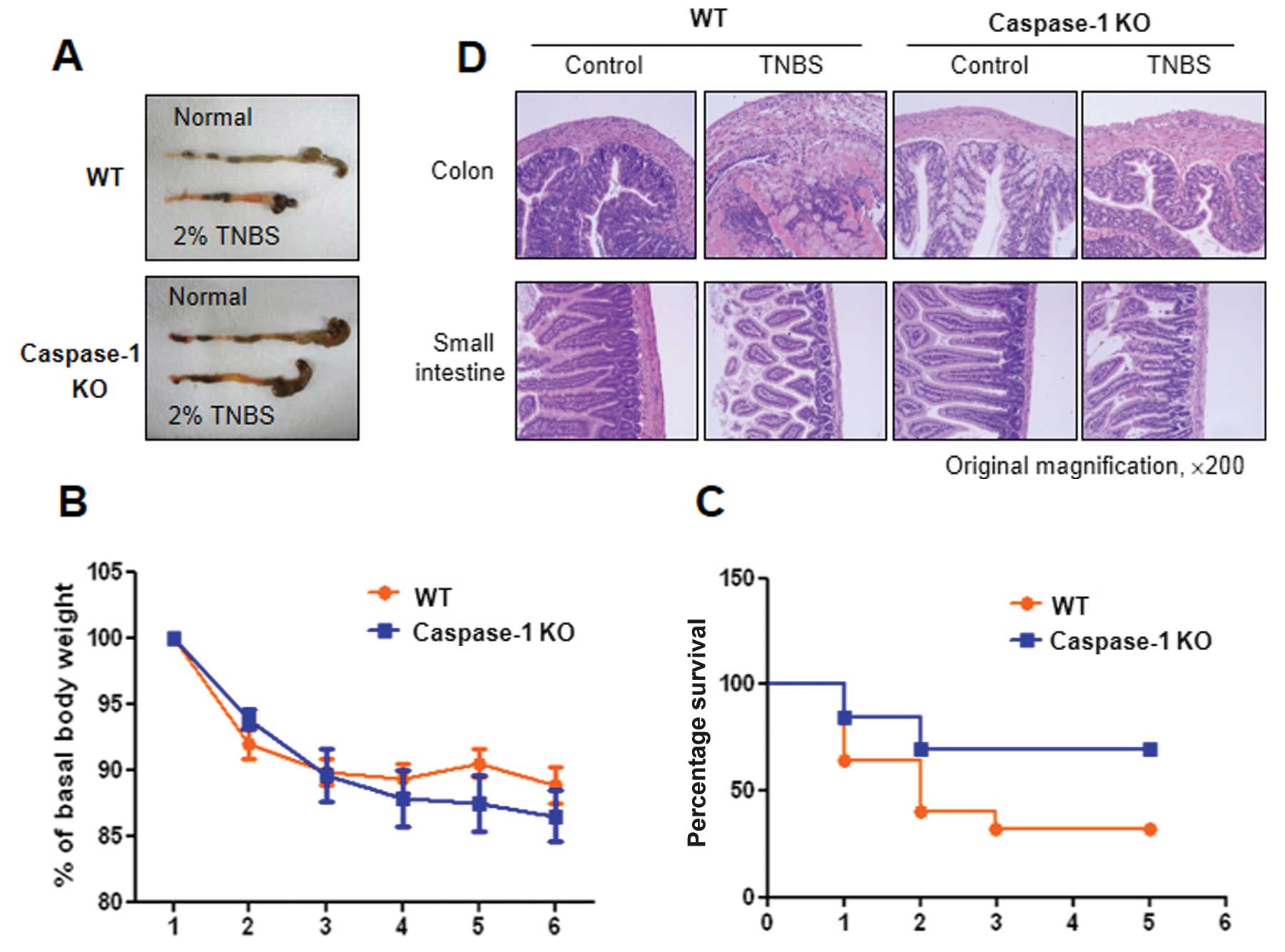
caspase-1 KO mice in TNBS-induced colitis
IL-1β and IL-18 are activated by caspase-1;
therefore, studies using caspase-1 KO mice are necessary to
elucidate the roles of IL-1β and IL-18 in TNBS-induced colitis. In
this study, we established acute colitis in wild-type (WT, C57BL/6)
and caspase-1 KO mice by the administration of TNBS (Fig. 1A) and examined the progress of the
disease. Colon shortening was more prominent in the WT mice than in
the caspase-1 KO mice, and severe colitis with the thickening of
the colonic wall and ulcerations were observed in TNBS-treated WT
mice. By contrast, TNBS-treated caspase-1 KO mice showed smaller
erosions and a mild edema in the colon (Fig. 5A). Furthermore, lower mortality
rates were observed in the caspase-1 KO mice compared with the WT
mice, although a similar decrease in body weight was observed in
both groups (Fig. 5C). The
caspase-1-dependent phenotypes were also confirmed by histological
examination. Fig. 5D shows
representative histological features of the colon and the small
intestine in healthy WT, TNBS-treated WT and TNBS-treated caspase-1
KO mice. TNBS administration induced a marked thickening of the
colonic wall, with transmural infiltration and aggregation of
numerous inflammatory cells; all these features are absent in the
healthy colon. Moreover, an alteration in the crypt structure and
an erosion in the surface of the epithelium were clearly visible in
the colons of the TNBS-treated WT mice. The TNBS-treated caspase-1
KO mice showed moderate leukocyte infiltration, a submucosal edema,
and partial retention of the crypt structure and of the surface of
the epithelium.
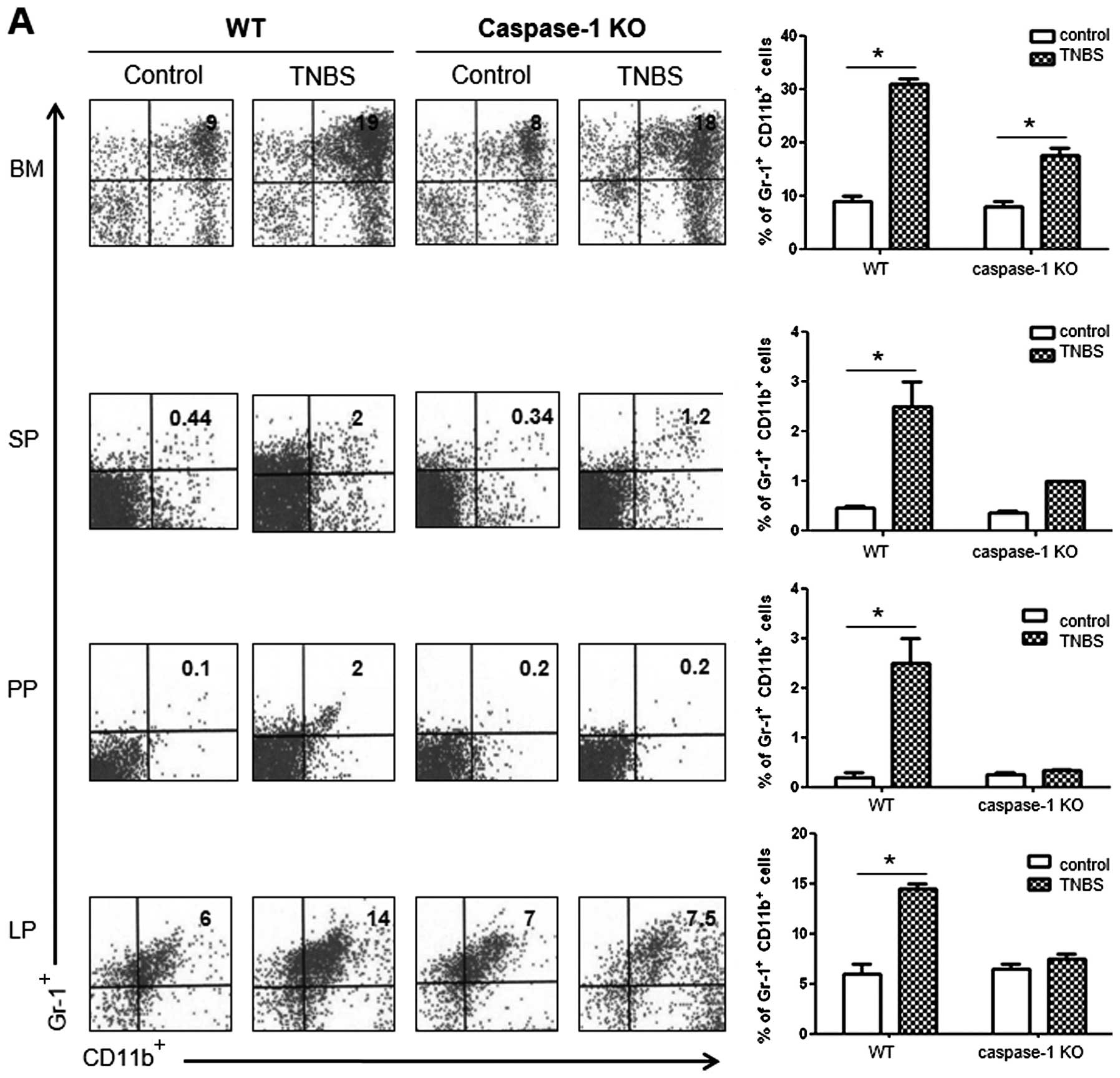
Comparison of cellular profiles between
colitis in WT vs. caspase-1 KO mice
To compare the cellular profiles between WT and
caspase-1 KO mice following the administration of TNBS, we
performed flow cytometry. The number of
CD11b+Gr-1+ MDSCs was significantly higher in
the BM, SP, PP and LP of the TNBS-treated WT mice compared with the
control mice. However, the TNBS-treated caspase-1 KO mice did not
show a significant increase in the numbers of these cells apart
from the BM cells (Fig. 6A). As
regards the CD4+ T cell subsets in the LP, the number of
IL-9+ and IL-17+ cells significantly
increased in TNBS-treated WT mice, whereas the proportions of these
cells were not increased in the TNBS-treated caspase-1 KO mice
compared with the corresponding control group. The number of
Foxp3+ cells showed a significant increase only in the
TNBS-treated caspase-1 KO mice (Fig.
6B).
Discussion
In this study, we compared 3 different experimental
mouse models of IBD (colitis): 4% DSS-induced (acute), 2%
DSS-induced (chronic) and 2% TNBS-induced acute colitis, the latter
studied in both WT and caspase-1 KO mice. We found that mice with
chronic DSS-induced colitis showed periodic fluctuations in weight
loss during the 30-day experimental period (Fig. 3B); this phenotype is more similar
to the progression of IBD in humans compared with the one observed
in the mouse models of acute colitis (Fig. 2C). All 3 WT mouse models of
colitis showed an increase in the number of IFN-γ+ and
IL-17+ T cells and a decrease in the number of
IL-4+ T cells. TNBS-induced colitis was less severe in
the caspase-1 KO mice and was accompanied by an increase in the
number of Foxp3+ CD4 T cells (Fig. 6).
Although the model of TNBS-induced colitis has been
reported to involve T cell responses (3), mucosal damage from ethanol challenge
is a prerequisite for establishing this model. In this study, we
evaluated epithelial layer damage in terms of inflammasome
activation. The final step in inflammasome activation involves
caspase-1 activation, leading to the cleavage of pro-IL-1β to the
active form of IL-1β. We challenged caspase-1 KO mice with TNBS to
induce colitis, and found that acute TNBS-induced colitis was
accompanied by an increase in the number of IFNγ+ and
Foxp3+ cells but not in the number of IL-17+
cells. Considering that IL-1β enhances Th17 (IL-17+)
cell differentiation, the observed decrease in the number of
IFNγ+ cells upon TNBS challenge in the caspase-1 KO mice
was expected (Fig. 6B).
It was previously reported that NLRP3 KO mice are
protected against DSS- and TNBS-induced colitis, possibly due to an
increase in the number of tolerogenic CD103+ dendritic
cells (11). In the present
study, we focused on the immune-suppressive cell population of
MDSCs. MDSCs are poorly characterized in human and mouse models of
IBD; there are only limited studies available on MDSCs in colitis.
It has been previously reported that the transplantation of
CD11b+Gr-1+ splenic cells into mice with
DSS-induced colitis improved disease parameters (16) and suppressed the development of
CD8-induced autoimmune enterocolitis (17). By contrast, there are several
reports on the pro-inflammatory role of myeloid cells in models of
IBD. For example, during chronic colitis, adoptively transferred
Ly6Chigh monocytes are recruited to the colon and
differentiate into inflammatory DCs and macrophages (18), while neutrophils
(CD11b+Ly6G+Ly6Cint) act as
antigen-presenting cells to trigger T cell proliferation (19). These results suggest that MDSCs
can provide activation signals for effector T cells in the LP of
the colon. In our study, since severe inflammatory changes occured
in the DSS- and TNBS-challenged mice and the proportion of
CD11b+Gr-1+ cells was increased in the SP, PP
and LP, MDSCs may contribute to the pro-inflammatory response.
However, the increase in the number of MDSCs that we observed may
also be due to the modulation of immune responses. The most notable
difference between the caspase-1 KO and WT mice was that no
significant increase was observed in the number of
CD11b+Gr-1+ cells in the PP and LP after TNBS
challenge in the caspase-1 KO mice (Fig. 6A). It has been reported that IL-1β
expression correlates with the early recruitment of MDSCs to
inflammatory tissue (20), and
this result could explain the decrease in the number of MDSCs in
the caspase-1 KO mice, where IL-1β expression is expected to be
low. MDSCs are defined as CD11b+Gr-1+ cells
and are subdivided into granulocytic
(CD11b+Ly6G+Ly6Cint) and monocytic
(CD11b+Ly6G−Ly6C+high). As we used
anti-mouse Ly-6G/Ly-6C (Gr-1, RB6-8C5) antibody binding to both
Ly6G and Ly6C, we could not differentiate granulocytic from
monocytic MDSCs in our study and thus could not compare these
populations of cells.
In conclusion, among CD4+ T cell subsets
in the LP, the number of IFNγ+ and IL-17+,
but not that of IL-4+ cells, increased in the 3
different mouse models of IBD. The discrepancies in
histopathological changes between the WT and caspase-1 KO mice
further suggest that IBD pathogenesis may be associated with the
caspase-1-mediated inflammasome pathway in TNBS-induced colitis,
and is accompanied by an increase in the number of Treg
(Foxp3+) cells.
Acknowledgements
This study was partly supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(2013R1A1A3A04006995 to S.-Y.W.) and by the Korean Government
(MRC-2010-0029353 to J.L.K).
References
|
1
|
Morris GP, Beck PL, Herridge MS, Depew WT,
Szewczuk MR and Wallace JL: Hapten-induced model of chronic
inflammation and ulceration in the rat colon. Gastroenterology.
96:795–803. 1989.PubMed/NCBI
|
|
2
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neurath MF, Fuss I, Kelsall BL, Stuber E
and Strober W: Antibodies to interleukin 12 abrogate established
experimental colitis in mice. J Exp Med. 182:1281–1290. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elson CO, Beagley KW, Sharmanov AT, et al:
Hapten-induced model of murine inflammatory bowel disease: mucosa
immune responses and protection by tolerance. J Immunol.
157:2174–2185. 1996.PubMed/NCBI
|
|
5
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI
|
|
6
|
Dieleman LA, Ridwan BU, Tennyson GS,
Beagley KW, Bucy RP and Elson CO: Dextran sulfate sodium-induced
colitis occurs in severe combined immunodeficient mice.
Gastroenterology. 107:1643–1652. 1994.PubMed/NCBI
|
|
7
|
Yamada M, Ohkusa T and Okayasu I:
Occurrence of dysplasia and adenocarcinoma after experimental
chronic ulcerative colitis in hamsters induced by dextran sulphate
sodium. Gut. 33:1521–1527. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiguro Y: Mucosal proinflammatory
cytokine production correlates with endoscopic activity of
ulcerative colitis. J Gastroenterol. 34:66–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monteleone G, Trapasso F, Parrello T, et
al: Bioactive IL-18 expression is up-regulated in Crohn’s disease.
J Immunol. 163:143–147. 1999.PubMed/NCBI
|
|
10
|
Kwon KH, Murakami A, Hayashi R and
Ohigashi H: Interleukin-1beta targets interleukin-6 in progressing
dextran sulfate sodium-induced experimental colitis. Biochem
Biophys Res Commun. 337:647–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer C, Duewell P, Mayer C, et al:
Colitis induced in mice with dextran sulfate sodium (DSS) is
mediated by the NLRP3 inflammasome. Gut. 59:1192–1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elinav E, Strowig T, Kau AL, et al: NLRP6
inflammasome regulates colonic microbial ecology and risk for
colitis. Cell. 145:745–757. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nam JH: Regulation of obesity and
non-alcoholic fatty liver disease by modulation of the gut
micobiota through inflammasome; its mechanism and potential for
clinical use. J Bacteriol Virol. 42:359–362. 2012. View Article : Google Scholar
|
|
14
|
Siegmund B, Lehr HA, Fantuzzi G and
Dinarello CA: IL-1beta-converting enzyme (caspase-1) in intestinal
inflammation. Proc Natl Acad Sci USA. 98:13249–13254. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gabrilovich DI and Nagaraj S:
Myeloid-derived suppressor cells as regulators of the immune
system. Nat Rev Immunol. 9:162–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang R, Ito S, Nishio N, Cheng Z, Suzuki
H and Isobe KI: Dextran sulphate sodium increases splenic
Gr1(+)CD11b(+) cells which accelerate recovery from colitis
following intravenous transplantation. Clin Exp Immunol.
164:417–427. 2011.PubMed/NCBI
|
|
17
|
Haile LA, von Wasielewski R,
Gamrekelashvili J, et al: Myeloid-derived suppressor cells in
inflammatory bowel disease: a new immunoregulatory pathway.
Gastroenterology. 135:871–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rivollier A, He J, Kole A, Valatas V and
Kelsall BL: Inflammation switches the differentiation program of
Ly6Chi monocytes from antiinflammatory macrophages to inflammatory
dendritic cells in the colon. J Exp Med. 209:139–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ostanin DV, Kurmaeva E, Furr K, et al:
Acquisition of antigen-presenting functions by neutrophils isolated
from mice with chronic colitis. J Immunol. 188:1491–1502. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu S, Bhagat G, Cui G, et al:
Overexpression of interleukin-1beta induces gastric inflammation
and cancer and mobilizes myeloid-derived suppressor cells in mice.
Cancer Cell. 14:408–419. 2008. View Article : Google Scholar : PubMed/NCBI
|