Introduction
Microglia, the resident innate immune cells of the
brain, have been suggested to play a role in host defense and
tissue repair in the central nervous system (CNS) and
CNS-associated diseases, such as Parkinson’s, Alzheimer’s and
Huntington’s diseases (1). BV-2
cells are a common microglial cell line that has been widely used
to study inflammatory and necrotic reactions during the course of
neurological diseases (2–4).
Traditional Chinese Medicine (TCM), which has a
history of more than three thousand years, is based on treatments
using compounds extracted from the natural environment (plants).
There are many herbal prescriptions for the treatment of
CNS-associated diseases. Gua Lou Gui Zhi decoction (GLGZD),
consists of extracts of Trichosanthis Radix, Ramulus Cinnamomi,
Paeonia lactiflora, Glycyrrhiza, Zingiber
officinale Roscoe and Fructus Jujubae (5–7).
This treatment has been formulated from the time of the Eastern Han
Dynasty (25–220 AD), and has typically been used in the treatment
of muscular spasticity following stroke, epilepsy, or spinal cord
injury (5–7). However, the precise mechanisms
responsible for its neuroprotective and anti-spasticity effects
remain poorly understood.
Neuronal apoptosis, a form of programmed cell death
that may serve in the regulation of nervous system development, is
an important mechanism of neuronal death in many models of acute
and chronic neurological disorders (8,9).
Glutamate, a major excitatory amino acid neurotransmitter in the
CNS, mediates several physiological processes by engaging the
ionotropic glutamate receptor and the metabotropic glutamate
receptor (10). However,
dysfunction of these glutamate transporters may be a major
contributing factor to the increase in extracellular glutamate
concentration and resulting excitotoxicity. Furthermore, the excess
stimulation of glutamate receptors can induce neuroinflammation and
eventual neurodegeneration (11).
Therefore, glutamate toxicity has been implicated in several acute
and chronic neurological disorders, such as cerebral ischemia,
stroke, epilepsy, Alzheimer’s disease and Parkinson’s disease
(1,10–13).
Thus, in this study, we used glutamate to induce
damage to BV-2 cells, and investigated the protective effects of
GLGZD on this cell model, as well as the underlying mechanisms
involved.
Materials and methods
GLGZD water extract
The prescription of GLGZD was first recorded in ‘Jin
Gui Yao Lue’, a medical book written by Zhongjing Zhang of the
Eastern Han Dynasty during the first century (25–220 AD). The
formula consists of six crude drugs, including Trichosanthis Radix,
Ramulus Cinnamomi, Paeonia lactiflora, Glycyrrhiza,
Zingiber officinale Roscoe and Fructus Jujubae at a
ratio of 3:3:3:2:3:3. Dried crude drugs were purchased from
Tongrentang Chinese Medicine Pharm (Fuzhou, China), a famous and
time-honored pharmaceutical brand in the TCM industry in China.
They were identified and confirmed by the College of Pharmacology,
Fujian University of Traditional Chinese Medicine, Fuzhou, China.
The formula was prepared by boiling the herbs in water. After the
first decoction (2 h), the suspension was filtered and water was
added for the second decoction (1 h). The filtered and mixed
suspension from the two decoctions was concentrated under vacuum by
using a rotary evaporator to a final concentration of 1.16 g/ml.
The samples were then stored at −20°C before use.
Cell culture and treatments
The cells were cultured in DMEM/high glucose medium
supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin
and 100 μg/ml streptomycin solution at 37°C under an atmosphere of
5% CO2 (Thermo Fisher Scientific, Waltham, MA, USA). One
day prior to treatment, the culture medium was changed to DMEM/high
glucose medium with 0.5% FBS in order to reduce the serum effect.
Twenty-four hours after seeding, the medium was renewed with one of
the three types of fresh culture medium (medium without glutamate,
with 30 mM glutamate, and 30 mM glutamate plus various
concentrations of GLGZD), and incubated for 24 h. In a single
experiment each treatment was performed in triplicate.
Cell viability
Cells were seeded into 96-well plates at a
concentration of 5–6×104 cells/ml. Cell viability was
evaluated with a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The MTT assay was performed as follows: 20 μl of 5 mg/ml MTT
dissolved in phosphate-buffered saline (PBS) was added to each
individual well followed by incubation at 37°C for 4 h. The
solution was then removed, and the produced formazan was
solubilized in 100 μl dimethyl sulfoxide (DMSO). Absorbance was
measured at 570 nm using an automated microplate reader (Bio-Rad
Laboratories, Hercules, CA, USA). Cell viability was expressed as a
percentage of the control culture value.
Analysis of cell morphology
Cells were seeded into 6-well microplates. Following
treatment for 24 h, the cells were fixed for 10 min with 4%
paraformaldehyde in PBS, washed with PBS, then visualized and
photographed under a phase contrast microscope (Leica, Wetzlar,
Germany).
Analysis of apoptosis
The apoptosis of BV-2 cells was determined by flow
cytometry on a FACSCalibur flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA) with an Annexin V-FITC Apoptosis Detection
kit (KeyGen Biotech, Nanjing, China). Staining was performed
according to the manufacturer’s instructions. The percentage of
cells found in early apoptosis was calculated by counting the
number of Annexin V-positive and propidium iodide (PI)-negative
cells. The percentage of cells found in late apoptosis was
calculated by counting the number of Annexin V-positive and
PI-positive cells.
Analysis of mitochondrial membrane
potential (MMP)
The JC-1 assay kit (KeyGen Biotech) was employed to
measure the MMP of BV-2 cells according to the manufacturer’s
instructions. Briefly, the cells were seeded into 6-well plates and
exposed to glutamate or GLGZD for 24 h. Thereafter, the cells were
harvested and resuspended in a mixture of 500 μl culture medium and
500 μl JC-1 staining fluid, and incubated in the dark at 37°C for
20 min. Following two washes with JC-1 staining buffer and
incubation in DMEM, the cells were analyzed by flow cytometry.
Mitochondria containing red JC-1 aggregates in healthy cells were
detectable in the FL-2 channel, and those containing green JC-1
monomers in apoptotic cells were detectable in the FL-1 channel.
The values of MMP staining from each sample were expressed as the
ratio of red fluorescence intensity over green fluorescence
intensity.
We also evaluated the MMP of BV-2 cells in
situ. The cells were seeded in a glass-bottom cell sterile
culture Petri dish specific for confocal microscopy (diameter of 15
mm, Nest Biotechnology Co., Ltd., Shanghai, China) for 24 h.
Following 24 h of treatment with glutamate or GLGZD, the cells were
incubated with a mixture of 500 μl JC-1 staining fluid and 500 μl
cell culture medium in the dark at 37°C for 30 min. Subsequently,
the cells were washed twice with staining buffer preserved in 4°C.
Lastly, 1 ml of cell culture medium was added to each specimen and
the cells were analyzed using a LSM 710 laser scanning confocal
microscope (Carl Zeiss, Oberkochen, Germany).
Analysis of mRNA expression by reverse
transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
supplier’s instructions. RNA was quantified by optical density
measurements at 260 and 280 nm. Integrity was confirmed by 1%
agarose gel electrophoresis. We used 2 μg of RNA in a 20 μl
reaction mixture utilizing M-MLV reverse transcriptase (Fermentas,
Waltham, MA, USA) according to the supplier’s instructions. The
resultant reverse transcription products were stored at −20°C until
further use. Mouse polymer, Bcl-2 and Bax primers were synthesized
by Shanghai Sangon Biological Engineering Technology and Services
Co., Ltd. (Shanghai, China) according to the following sequences:
Bax sense, 5′-GAGACACCTGA GCTGACCTTG-3′ and antisense,
5′-GAAGTTGCCATCAG CAAACAT-3′; Bcl-2 sense, 5′-ATGTGTGTGGAGAGCGT
CAAC-3′ and antisense, 5′-CAGCCAGGAGAAATCAAA CAG-3′; β-actin sense,
5′-GAGACACCTGAGCTGACC TTG-3′ and antisense, 5′-GAGACACCTGACCACCCTG
TTGCTGTA-3′. The product size for Bax, Bcl-2 and β-actin sense was
195, 177 and 490 bp, respectively. PCR was carried out with Taq
polymerase (Thermo Fisher Scientific Inc., Rockford, IL, USA)
according to the supplier’s instructions. The PCR conditions were
as follows: 94°C for 4 min, followed by 35 cycles (30 for β-actin)
of 1 min denaturation at 94°C, 1 min annealing at 58°C, 1 min
polymerization at 72°C, and finally 10 min extension at 72°C. The
PCR products were analyzed by 1.5% agarose electrophoresis and
visualized using ethidium bromide (0.25 μg/ml) in 0.5X TBE buffer
(Tris 40 mM, EDTA 1 mM, boric acid 44 mM) at 80 V (constant
voltage). Images of gels were acquired and analyzed by Molecular
Imager software (Bio-Rad Laboratories). The density of the PCR
bands was expressed as a ratio of the band density divided by that
of the housekeeping gene, β-actin.
Western blot analysis of protein
expression
Cells were harvested and lysed in RIPA buffer
containing the protease inhibitor, phenylmethylsulfonyl fluoride
(PMSF) (both from Beyotime, Shanghai, China). Cell lysates were
collected by centrifugation at 12,000 × g for 10 min at 4°C.
Protein concentrations were determined using an enhanced BCA
protein assay kit (Beyotime). Equal amounts of protein from each
sample (40 μg) were resolved by sodium dodecyl sulfate
(SDS)-polyacrylamide gel electrophoresis (12%) and transferred onto
PVDF membranes. The membranes were incubated in blocking buffer
(non-fat milk) and then incubated overnight at 4°C with rabbit
polyclonal antibodies against Bax (CST, 1:1,000, 20 kDa), Bcl-2
(CST, 1:1,000, 26 kDa) or β-actin (1:4,000, 43 kDa) (Beyotime). The
membranes were stringently washed and incubated with HRP-conjugated
secondary antibodies (Proteintech Group, Chicago, IL, USA), for 1 h
at room temperature. After washing, proteins were detected using
enhanced chemiluminescence, and images were acquired using a
Bio-Image Analysis System (Bio-Rad Laboratories).
Detection of cleaved caspase-3 protein by
immunofluorescence
Cells were seeded in a glass-bottom cell sterile
culture Petri dish specific for confocal microscopy (diameter of 15
mm, Nest Biotechnology Co., Ltd.) for 24 h. Following treatment
with glutamate or GLGZD (125, 250, 500 and 1,000 μg/ml) for 24 h,
the cells were fixed with 4% formaldehyde in PBS for 30 min at room
temperature, rinsed three times in PBS, and incubated in blocking
buffer (5% BSA, 10% normal donkey serum, 0.3% Triton X-100 in PBS)
for 60 min at room temperature. The cells were then incubated with
cleaved caspase-3 antibody (Alexa Fluor 488-conjugated) overnight
at 4°C (CST, 1:100). The cells were washed twice with PBS, and
incubated with a rhodamine-conjugated phalloidin (F-actin) antibody
(cytoskeleton, 1:100) at 37°C for 30 min. Lastly, DAPI (Beyotime,
1:1,000) was added to each specimen for nuclei staining and the
cells were analyzed using a LSM 710 laser scanning confocal
microscope under a ×40 water objective (Carl Zeiss).
Statistical analysis
Data are expressed as the means ± standard error of
mean (SEM). Data were first analyzed using Portable IBM SPSS
Statistics software. A paired-sample t-test was then performed to
compare the treated samples, and values of P<0.05 were
considered to indicate statistically significant differences.
Results
Total phenolic compound and sugar content
in the extract
In our previous study (6), a high-performance liquid
chromatography (HPLC) fingerprint was used to control the quality
of the GLGZD extract, which revealed that the method we use to
prepare GLGZD was efficient and that the product used for this
study was pure.
Effect of GLGZD on viability and
apoptosis in glutamate-stimulated BV-2 cells
In order to evaluate the effects of GLGZD on
glutamate-induced cell death, we first determined the optimal
concentration of glutamate which was able to induce BV-2 cell death
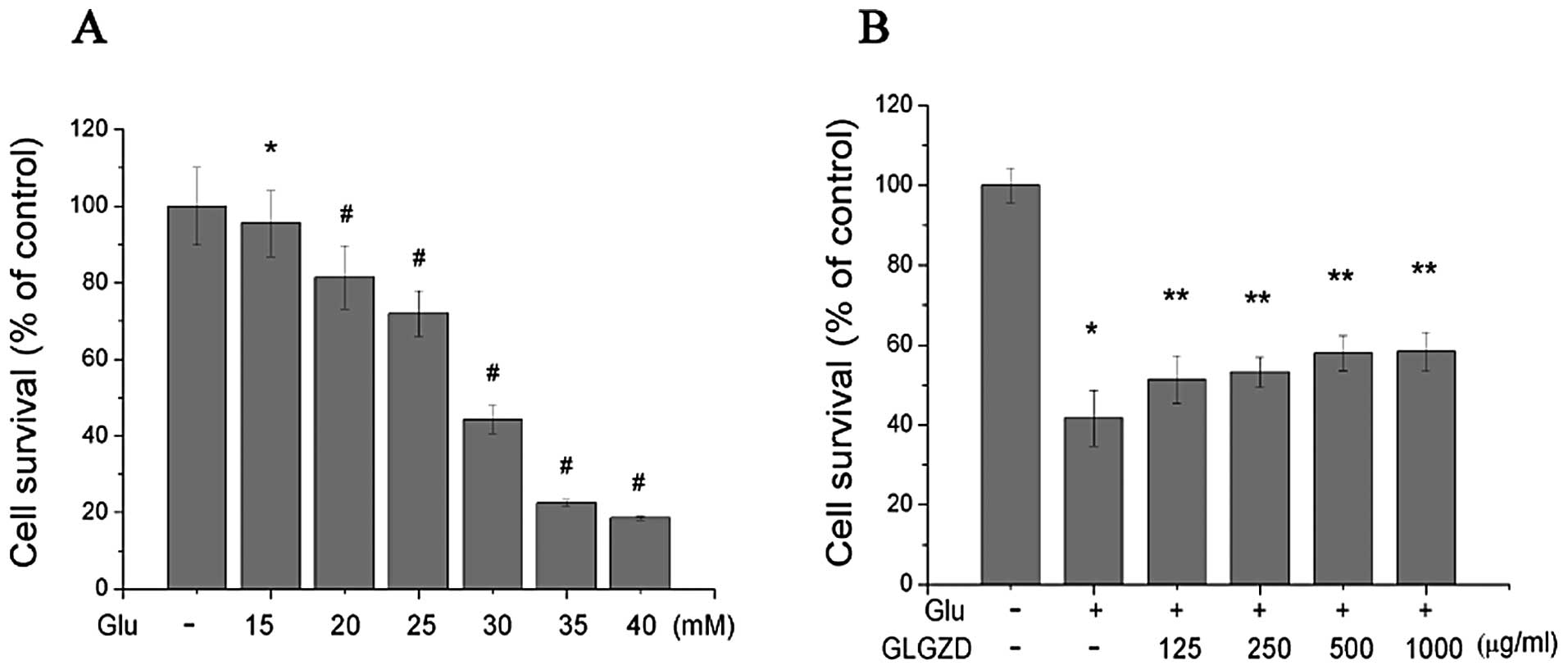
(Fig. 1A). Increasing
concentrations of glutamate were added (15, 20, 25, 30, 35 and 40
mM) and cell survival was measured. The concentration of glutamate
that induced approximately 50% cell death, and the appropriate
concentration for our experiments, was 30.0 mM.
The glutamate-induced loss of cell viability was
markedly attenuated by treatment with GLGZD (Fig. 1B). Following treatment with 30 mM
glutamate for 24 h, cells survived for an average of 41.72±6.95% of
the control value. Treatment with 125, 250, 500 and 1,000 μg/ml of
GLGZD in the presence of 30.0 mM glutamate markedly increased cell
viability to 51.37±5.99, 53.24±3.68, 58.02±4.34 and 58.39±4.81%,
respectively. These results demonstrated that the glutamate-induced
loss of cell viability was partially attenuated by GLGZD in a
dose-dependent manner.
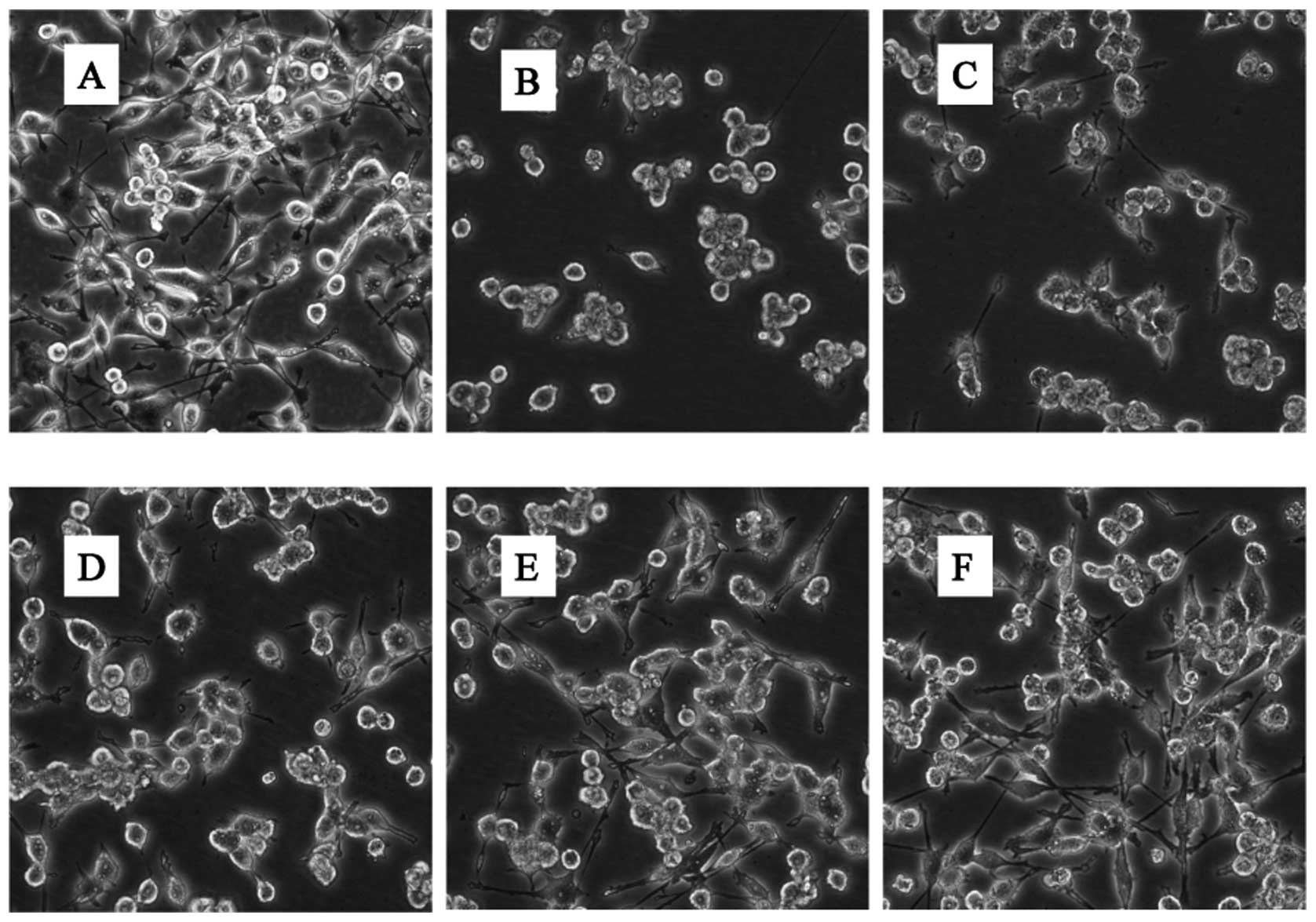
In addition to cell viability, we also sought to
analyze the morphological characteristics of BV-2 cells cultured in
the presence of glutamate with or without GLGZD. BV-2 cells display
a characteristic small spherical morphology with more than half of
the cells displaying process-bearing sites, often bipolar and
tripolar (Fig. 2A). The addition
of 30 mM glutamate induced contraction, rounding and even floating
of the majority of cells (Fig.
2B). This suggests the involvement of microglial cell apoptosis
and necrosis induced by treatment with glutamate. This
morphological change was effectively inhibited by 250, 500 and
1,000 μg/ml of GLGZD (Fig.
2D–F).
A quantitative evaluation of apoptosis was then
carried out by flow cytometry with an Annexin V/PI test. The
apoptotic rate of the cells treated with 30 mM glutamate alone for
24 h markedly increased to 36.46% (Fig. 3). However, treatment with GLGZD
reversed this effect. The proportion of apoptotic cells decreased
from 36.46% to 24.86, 19.32, 17.11 and 16.06%, when the cells were
co-incubated with concentrations of 125, 250, 500 and 1,000 μg/ml
of GLGZD, respectively (Fig.
3A–F). A dose-dependent effect was evident, as the highest
concentration of GLGZD (1,000 μg/ml) demonstrated the least number
of apoptotic cells (Fig. 3G).
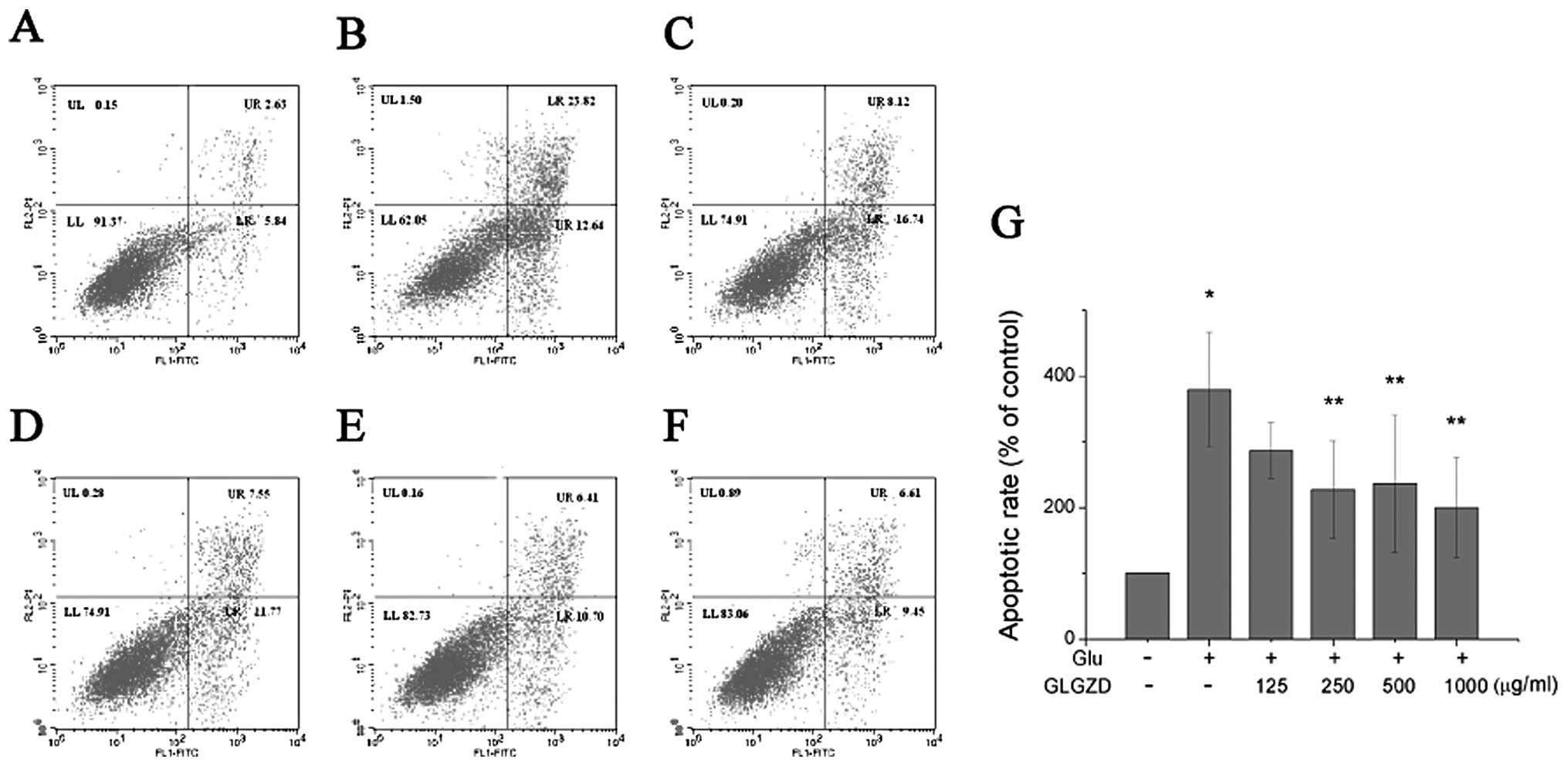 | Figure 3Effect of Gua Lou Gui Zhi decoction
(GLGZD) on cell apoptosis of BV-2 cells. Cells were collected,
stained with Annexin V/propidium iodide (PI) and analyzed by flow
cytometry. (A) Control, (B) glutamate (Glu), (C) Glu + GLGZD (125
μg/ml), (D) Glu + GLGZD (250 μg/ml), (E) Glu + GLGZD (500 μg/ml),
(F) Glu + GLGZD (1,000 μg/ml). Upper right quadrant, Annexin V/PI
double-positive stained cells (late apoptosis); lower right
quadrant, Annexin V-positive/PI-negative stained cells (early
apoptosis). (G) Results from flow cytometric analysis are expressed
as the means ± SEM of three independent experiments. The apoptotic
rate was calculated using Annexin V/PI double-positive stained cell
plus Annexin V-positive/PI-negative stained cell populations.
*P<0.01 as compared with control group.
**P<0.05, compared with the group treated only with
glutamate. |
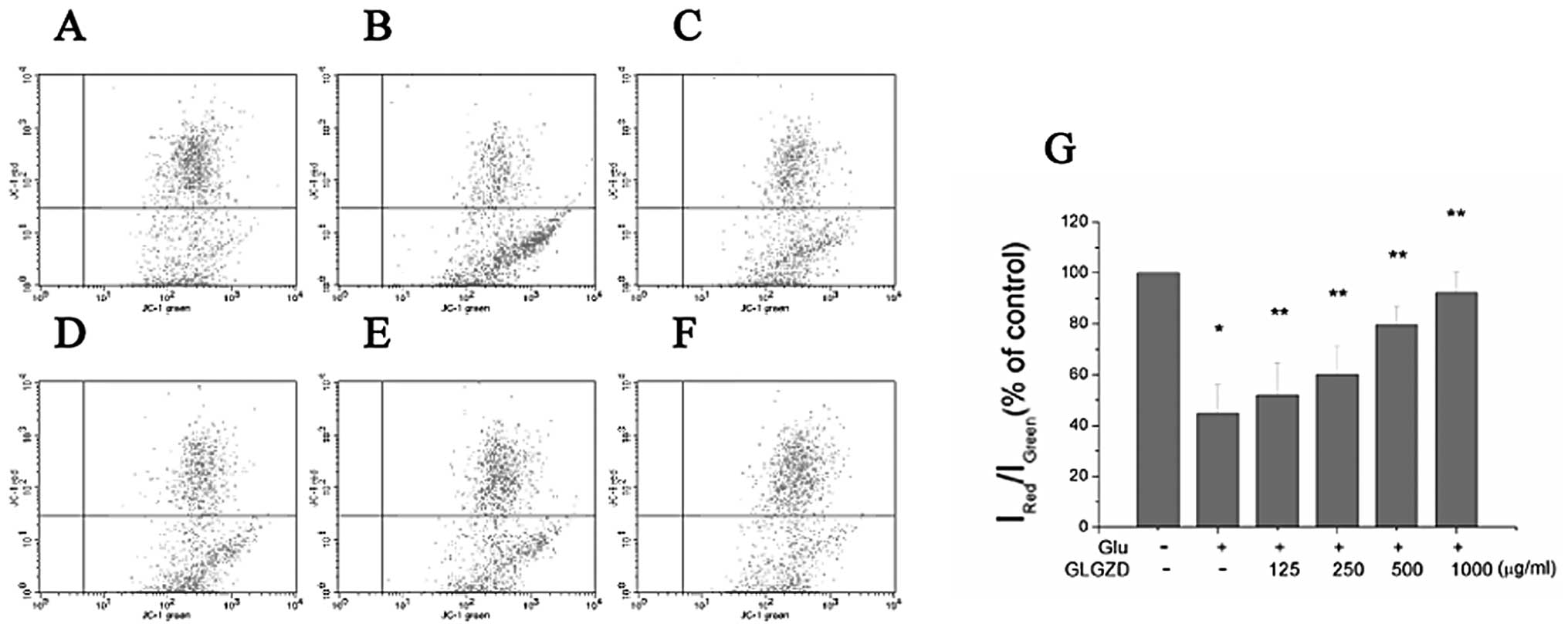
Effects of GLGZD on MMP in
glutamate-stimulated BV-2 cells
Apoptosis is often accompanied by mitochondrial
dysfunction, and the decline in MMP is considered as a symbolic
event of early cellular apoptosis (14,15). In this study, to investigate the
effects of glutamate and GLGZD on mitochondrial function,
indicators of mitochondrial activity were monitored using JC-1
staining. The MMP of BV-2 cells treated with glutamate for 24 h was
markedly reduced (Fig. 4). The
ratio of aggregated JC-1 (FL-2 channel) to monomeric JC-1 (FL-1
channel) was decreased from 79.8 (control group) to 33.7%
(glutamate-treated only group) (P<0.01) after 24 h of treatment.
When the cells were incubated with 125, 250, 500 and 1,000 μg/ml of
GLGZD, the ratio increased from 33.7% to 42.7, 54.5, 78.4 and
79.7%, respectively (Fig. 4A–F).
Treatment with GLGZD attenuated the decline in MMP in a
dose-dependent manner, as indicated by the increase in red (JC-1
aggregates)/green (JC-1 monomers) ratio (Fig. 4G).
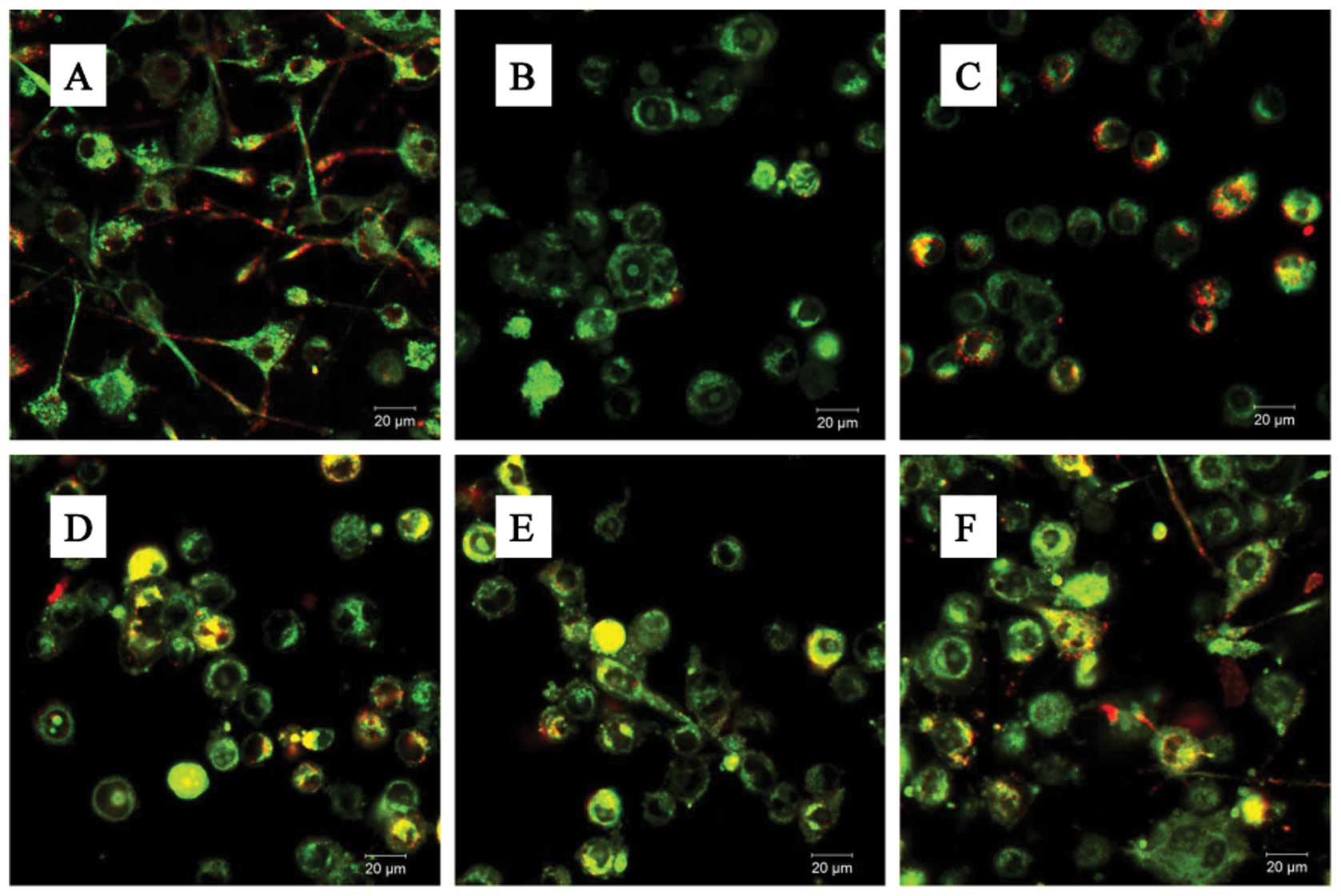
In addition, the BV-2 cells stained with JC-1
exhibited mitochondrial red fluorescence with a little green
fluorescence, suggesting that the cells were in a normal polarized
state (Fig. 5A). The JC-1
aggregates were dispersed to the monomeric form (green
fluorescence) in the glutamate-treated cells (Fig. 5B). However, treatment with GLGZD
attenuated the dissipation of the MMP (Fig. 5C–F), corroborating our results
from flow cytometry (Fig. 4).
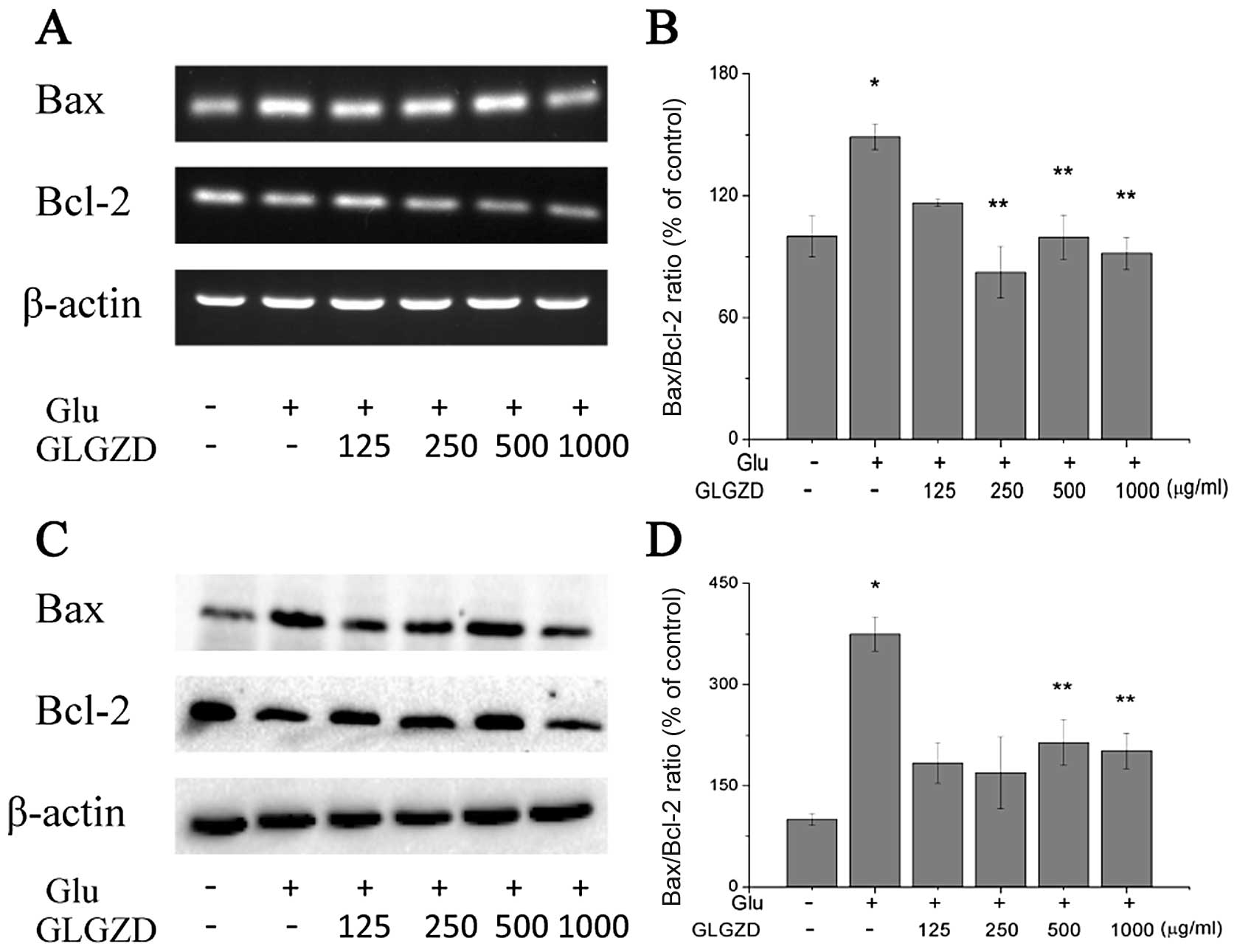
Effects of GLGZD on the mRNA and protein
expression levels of Bax and Bcl-2 in glutamate-stimulated BV-2
cells
To determine whether GLGZD protects the BV-2 cells
from glutamate-induced apoptosis by modulating the Bcl-2 family of
proteins, the mRNA and protein levels of Bax and Bcl-2 were
estimated using RT-PCR and western blot analysis. We detected an
increase in Bax and a decrease in Bcl-2 mRNA levels following
exposure to glutamate (Fig. 6A and
B). Treatment with GLGZD inhibited the upregulation of Bax and
enhanced the upregulation of Bcl-2 slightly at 24 h of glutamate
exposure. Thus, GLGZD attenuated the increase in the Bax to Bcl-2
ratio induced by glutamate, a sign of apoptosis inhibition. In
addition to the GLGZD modulation of Bax and Bcl-2 mRNA levels, we
observed similar results with the protein levels (Fig. 6C and D).
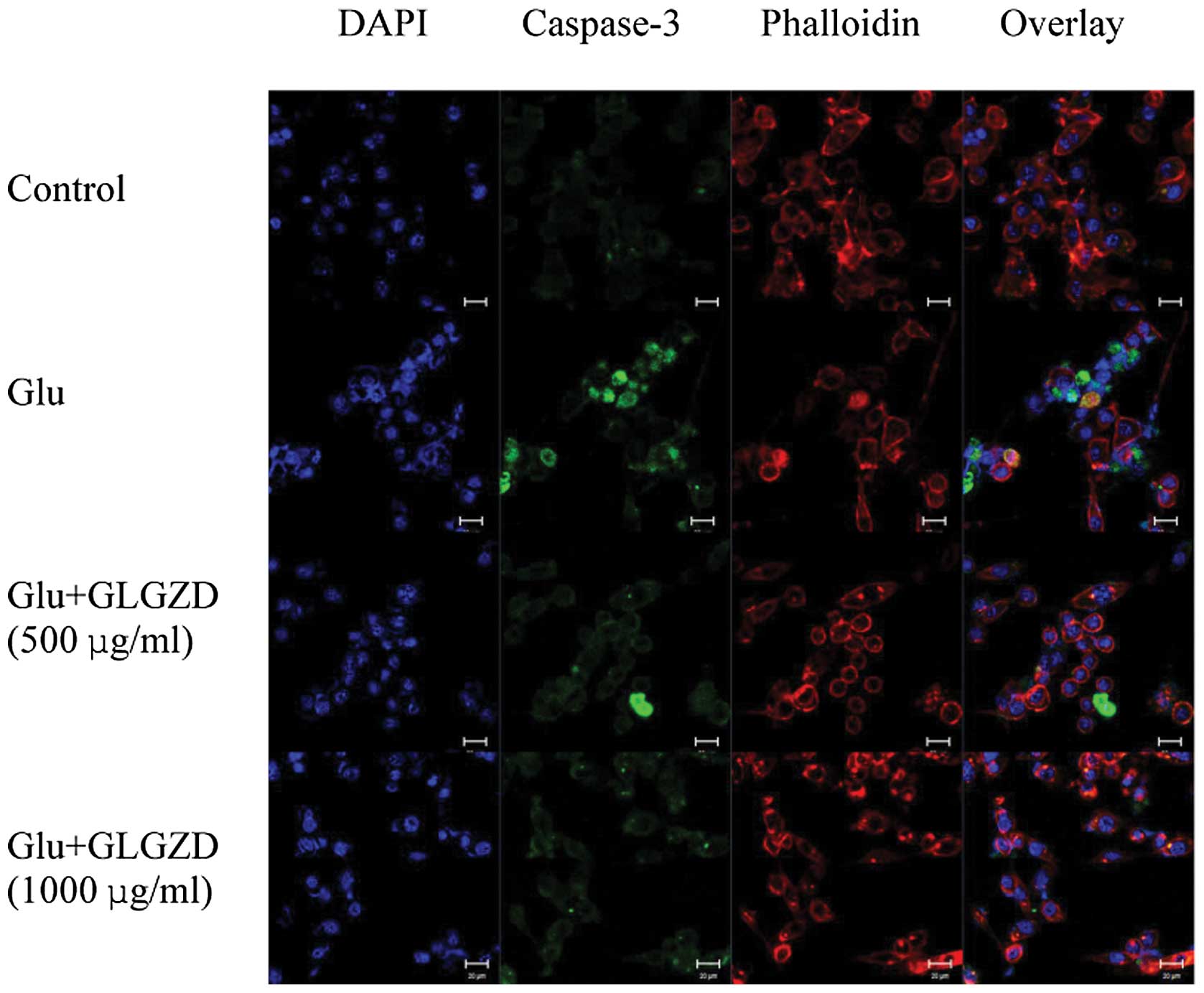
Effects of GLGZD on the expression of
cleaved caspase-3 protein in glutamate-stimulated BV-2 cells
We utilized an antibody specific for the activated
form of caspase-3, a caspase that plays an important role in a
number of neuronal apoptotic pathways, as an ‘executioner’ of cell
death (16). Treatment with
glutamate led to the formation of apoptotic nuclei, as assessed by
an abundance of green puncta labeled with the antibody to activated
caspase-3 (Fig. 7). Cleaved
caspase-3 was localized in the nuclei, as it overlapped with
apoptotic cell nuclei. Treatment with GLGZD induced the
re-localization of caspase-3, out of the nucleus, representing a
decrease in caspase-3 expression levels (Fig. 7)
Discussion
Increasing evidence indicates that a number of
herbal medicinal plants, including some formulations used in TCM,
have beneficial effects on neurodegenerative diseases, such as
Artemisia annua L. (17),
baicalein (2,14), cassia twig (18), 6-Shogaol (a ginger product)
(19), Gastrodia elata
Blume. (20), Chrysanthemum
indicum Linné (21),
pinocembrin (11), Buyang Huanwu
decoction (22), Guizhi-Fuling
capsules (23), Xiao-Xu-Ming
decoction (24),
Danggui-Shaoyao-San (25) and
Yi-Gan San (13). Similar to
other Chinese medicinal compounds, GLGZD is thought to possess
various traditional and ethnopharmacological benefits for
neurodegenerative diseases, and it has long been clinically
employed in the treatment of stroke (5). Although the underlying mechanisms
remain largely unknown, a rat model of focal cerebral
ischemia-reperfusion (I/R) injury demonstrated that GLGZD exerts
neuroprotective and anti-spasticity effects in a model of cerebral
ischemia through the modulation of glutamate levels and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptor expression (6). In
addition, GLGZD has been shown to induce an anti-inflammatory
response through the suppression of the LPS-stimulated TLR4/NF-κB
pathway in BV-2 murine microglial cells (7). However, molecular studies on
cellular models of toxin-based Parkinson’s disease have suggested
that oxidative stress-mediated mitochondrial dysfunction, apoptosis
and microglial-mediated neuroinflammation play a major etiological
role in neurotoxicity (26).
Therefore, in the present study, we focused on the ability of GLGZD
to suppress neurodegeneration and neuroinflammation in a cellular
model of glutamate-induced apoptosis and to explore the intrinsic
mechanisms involved.
We examined the neuroprotective effects of GLGZD on
the glutamate-induced apoptosis of BV-2 cells. Glutamate
significantly decreased cell viability and increased cell
apoptosis. An MTT assay and an Annexin V/PI test revealed that
GLGZD markedly inhibited the glutamate-induced apoptosis of BV-2
cells. The Annexin V/PI test results revealed that glutamate
increased early and late apoptosis; however, treatment with GLGZD
suppressed the percentage of early apoptotic cells.
Genes of the Bcl-2 family play a key role in the
mitochondrial pathway of apoptosis, reflecting the balance between
the pro- and anti-apoptotic members of the Bcl-2 family, of which
Bax and Bcl-2 are the two main members (27). In this study, we found that
glutamate had a profound effect on the gene expression and protein
levels of Bax and Bcl-2. Our results indicated that GLGZD provided
neuroprotection partly by inhibiting Bax overexpression and
increasing anti-apoptotic Bcl-2 expression. Treatment with GLGZD
reduced the expression of pro-apoptotic Bax and increased the
expression of anti-apoptotic Bcl-2 significantly in a
dose-dependent manner, thereby attenuating the elevated
glutamate-induced Bax/Bcl-2 ratio in the BV-2 cells. This finding
indicate that the protective effects of GLGZD against neurological
damage are likely attributed to its anti-apoptotic properties.
Caspases are cysteine proteases that are essential
for apoptosis in a variety of in vitro and in vivo
models. Caspase-3 is the major executioner protease, responsible
for initiating the mitochondrial-regulated apoptotic program
(16,27,28). We found that the levels of
caspase-3, which is normally expressed at low levels, increased
significantly when the cells were treated with glutamate for 24 h.
However, following treatment with GLGZD, the increased expression
levels of caspase-3 were slightly lower, and these changes occurred
in a dose-dependent manner.
The activation of microglial cells plays a crucial
role in the initiation and progression of brain inflammation, and
BV-2 cells have been used to study the expression of various
pro-inflammatory and anti-inflammatory cytokines (8). Although in a previous study, we
revealed that GLGZD exhibited an anti-inflammatory response on
LPS-induced BV-2 cell damage (7),
it remains unknown whether the pro-apoptotic effects of glutamate
are due to the excitotoxic properties of pro-inflammatory cytokines
or to the direct activation of microglial phagocytosis (4). Therefore, the similarity and
differences between the possible anti-apoptotic and
anti-inflammatory mechanisms of GLGZD on microglial cells require
further investigation.
In conclusion, GLGZD exerts protective effects
against glutamate-induced cellular injury. To our knowledge, this
is the first report revealing the role of GLGZD in protecting BV-2
cells against glutamate-induced neurotoxicity. Further studies on
mature primary neurons, as well as on animal models of Parkinson’s
disease and comparisons with known anti-parkinsonian agents are,
however, required to establish both efficacy and safety. Based on
the protective effects of GLGZD on glutamate-induced cellular
injury, GLGZD may be used as a potential therapeutic candidate for
the treatment of neurodegenerative disorders.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (no. 81273835), the Guidance Project of
the Fujian Provincial Department of Science and Technology (no.
2012D012), the Key Project of Fujian Provincial Department of
Science and Technology (no. 2012Y0041), the Project of Fujian
Education Department (no. JK2012024), and the Project of Fujian
Education Department (no. JA2012179).
References
|
1
|
Eun SY, Hong YH, Kim EH, Jeon H, Suh YH,
Lee JE, Jo C, Jo SA and Kim J: Glutamate receptor-mediated
regulation of c-fos expression in cultured microglia. Biochem
Biophys Res Commun. 325:320–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang KY, Oh YT, Yoon H, Lee J, Kim H,
Choe W and Kang I: Baicalein suppresses hypoxia-induced HIF-1alpha
protein accumulation and activation through inhibition of reactive
oxygen species and PI 3-kinase/Akt pathway in BV2 murine microglial
cells. Neurosci Lett. 444:264–269. 2008. View Article : Google Scholar
|
|
3
|
Kim BW, Koppula S, Kim JW, Lim HW, Hwang
JW, Kim IS, Park PJ and Choi DK: Modulation of LPS-stimulated
neuroinflammation in BV-2 microglia by Gastrodia elata:
4-hydroxybenzyl alcohol is the bioactive candidate. J
Ethnopharmacol. 139:549–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tremblay MÈ, Stevens B, Sierra A, Wake H,
Bessis A and Nimmerjahn A: The role of microglia in the healthy
brain. J Neurosci. 31:16064–16069. 2011.
|
|
5
|
Chen YL, Chen LD and Tao J: Clinical
research on treating limbs spasm from cerebral apoplexy with the
Gualou Guizhi decoction. Clin J Chin Med. 5:7–9. 2013.(In
Chinese).
|
|
6
|
Huang J, Tao J, Xue X, Yang S, Han P, Lin
Z, Xu W, Lin J, Peng J and Chen L: Gua Lou Gui Zhi decoction exerts
neuroprotective effects on post-stroke spasticity via the
modulation of glutamate levels and AMPA receptor expression. Int J
Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
7
|
Hu H, Li Z, Zhu X, Lin R, Lin J, Peng J,
Tao J and Chen L: Gua Lou Gui Zhi decoction suppresses LPS-induced
activation of the TLR4/NF-kB pathway in BV-2 murine microglial
cells. Int J Mol Med. 31:1327–1332. 2013.PubMed/NCBI
|
|
8
|
Lin HC, Yang CM, Liu CL and Hu ML:
Synergistic effects of homocysteine, S-adenosylhomocysteine and
adenosine on apoptosis in BV-2 murine microglial cells. BioFactors.
34:81–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin CH, Kuo SC, Huang LJ and Gean PW:
Neuroprotective effect of N-acetylcysteine on neuronal apoptosis
induced by a synthetic gingerdione compound: involvement of ERK and
p38 phosphorylation. J Neurosci Res. 84:1485–1494. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Platt SR: The role of glutamate in central
nervous system health and disease-a review. Vet J. 173:278–286.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao M, Zhang WC, Liu QS, Hu JJ, Liu GT and
Du GH: Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y
neuronal cells via decrease of bax/bcl-2 ratio. Eur J Pharmacol.
591:73–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Y, Li J, Liu P, Chen X, Guo DH, Li QS
and Rahman K: Protection of SH-SY5Y neuronal cells from
glutamate-induced apoptosis by 3,6′-disinapoyl sucrose, a bioactive
compound isolated from Radix Polygala. J Biomed Biotechnol. 1–5.
2012.PubMed/NCBI
|
|
13
|
Kawakami Z, Kanno H, Ikarashi Y and Kase
Y: Yokukansan, a kampo medicine, protects against glutamate
cytotoxicity due to oxidative stress in PC12 cells. J
Ethnopharmacol. 134:74–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang SH, Ye JL and Dong GX:
Neuroprotective effect of baicalein on hydrogen peroxide- mediated
oxidative stress and mitochondrial dysfunction in PC12 cells. J Mol
Neurosci. 40:311–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Ma H, Xie B, Han C, Wang C, Qing
H and Deng Y: Alpha-synuclein overexpression induced mitochondrial
damage by the generation of endogenous neurotoxins in PC12 cells.
Neurosci Lett. 547:65–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharifi AM, Eslami H, Larijani B and
Davoodi J: Involvement of caspase-8, −9, and −3 in high
glucose-induced apoptosis in PC12 cells. Neurosci Lett. 459:47–51.
2009.
|
|
17
|
Lee IS, Ryu DK, Lim J, Cho S, Kang BY and
Choi HJ: Artesunate activates Nrf2 pathway-driven anti-inflammatory
potential through ERK signaling in microglial BV2 cells. Neurosci
Lett. 509:17–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui F, Lin N, Guo JY, Zhang CB, Du XL,
Zhao BS, Liu HB, Yang N, Li LF, Guo SY, Huo HR and Jiang TL:
Cinnamaldehyde up-regulates the mRNA expression level of TRPV1
receptor potential ion channel protein and its function in primary
rat DRG neurons in vitro. J Asian Nat Prod Res. 12:76–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ha SK, Moon E, Ju MS, Kim DH, Ryu JH, Oh
MS and Kim SY: 6-Shogaol, a ginger product, modulates
neuroinflammation: a new approach to neuroprotection.
Neuropharmacology. 63:211–223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
An H, Kim IS, Koppula S, Kim BW, Park PJ,
Lim BO, Choi WS, Lee KH and Choi DK: Protective effects of
Gastrodia elata Blume on MPP+-induced
cytotoxicity in human dopaminergic SH-SY5Y cells. J Ethnopharmacol.
130:290–298. 2010.
|
|
21
|
Kim IS, Ko HM, Koppula S, Kim BW and Choi
DK: Protective effect of Chrysanthemum indicum Linne against
1-methyl-4-phenylpridinium ion and lipopolysaccharide-induced
cytotoxicity in cellular model of Parkinson’s disease. Food Chem
Toxicol. 49:963–973. 2011.
|
|
22
|
Zhao LD, Wang JH, Jin GR, Zhao Y and Zhang
HJ: Neuroprotective effect of Buyang Huanwu decoction against focal
cerebral ischemia/reperfusion injury in rats - time window and
mechanism. J Ethnopharmacol. 140:339–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li TJ, Qiu Y, Mao JQ, Yang PY, Rui YC and
Chen WS: Protective effects of Guizhi-Fuling-Capsules on rat
brain ischemia/reperfusion injury. J Pharmacol Sci. 105:34–40.
2007.
|
|
24
|
Zhu XH, Li SJ, Hu HH, Sun LR, Das M and
Gao TM: Neuroprotective effects of Xiao-Xu-Ming decoction against
ischemic neuronal injury in vivo and in vitro. J Ethnopharmacol.
127:38–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian YF, Wang H, Yao WB and Gao XD:
Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San,
inhibits apoptosis in hydrogen peroxide-induced PC12 cells by
preventing cytochrome c release and inactivating of caspase
cascade. Cell Biol Int. 32:304–311. 2008.
|
|
26
|
Block ML, Zecca L and Hong JS:
Microglia-mediated neurotoxicity: uncovering the molecular
mechanisms. Nat Rev Neurosci. 8:57–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lang-Rollin I, Maniati M, Jabado O,
Vekrellis K, Papantonis S, Rideout HJ and Stefanis L: Apoptosis and
the conformational change of Bax induced by proteasomal inhibition
of PC12 cells are inhibited by bcl-xL and bcl-2. Apoptosis.
10:809–820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|