Introduction
Diabetic nephropathy (DN) is one of the most common
microvascular chronic complications associated with diabetes, and
is the most common cause of end-stage renal disease (1,2).
DN develops over several years, and involves characteristic
pathological changes, such as the excessive accumulation of
extracellular matrix (ECM), glomerulosclerosis, tubule dilatation
and atrophy, as well as interstitial fibrosis (3). Previous studies have focused on the
accumulation of the ECM (4). A
growing body of evidence suggests that glomerular podocytes are key
players in DN (5). Podocytes are
terminally differentiated cells unable to regenerate and the loss
of podocytes results in permanent alterations in the glomerular
filtration barrier (6,7).
The endoplasmic reticulum (ER) plays a critical role
in controlling the fate of cells. The ER is a highly dynamic
organelle responsible for multiple cellular functions. However, the
ER is highly sensitive to alterations in its homeostasis. A number
of conditions can disturb ER functions, and these conditions induce
a state known as ER stress (ERS) (8,9).
The hyperglycemia-induced increase in reactive
oxygen species (ROS) has been shown to be involved in podocyte
apoptosis and depletion in vitro and in vivo,
suggesting that podocyte apoptosis/depletion represents a novel
early mechanism leading to DN (10,11). There are direct correlations
between ERS and oxidative stress (12). Accumulating evidence indicates
that ERS-related apoptosis may be involved in β-cell loss in type 1
and 2 diabetes mellitus (13,14).
A number of signaling pathways have evolved to cope
with ERS. The first response involved is the unfolded protein
response (UPR), in which ER chaperone proteins are upregulated,
which may alleviate ERS (15).
Glucose-regulated protein 78 (GRP78) is the main modulator of UPR.
It can bind to ER sensors, such as protein kinase R (PKR)-like ER
kinase, inositol requiring 1 (IRE1) and activating transcription
factor 6 (ATF6), inhibiting their activation (16). GRP78 plays a critical role in the
recognition of unfolded proteins (16).
If these adaptive responses cannot alleviate ERS,
apoptosis is triggered by various pathways that are not yet fully
understood. However, two pathways of ER-associated death (ERAD)
have been defined, characterized by the activation of
CCAAT/enhancer-binding protein (C/EBP) homologous protein
(CHOP/GADD153) and of caspase-12 (8,17).
However, the molecular mechanisms underlying the
development of DN remain to be clarified. We hypothesized that ERS
is involved in high glucose (HG)-induced podocyte apoptosis. The
aim of this study was to examine the expression of GRP78 and the
ERAD pathways (CHOP/GADD153- and caspase-12-dependent pathways) in
podocytes exposed to a HG environment. The results revealed that
podocytes treated with HG underwent ERS, which presumably is an
adaptive, protective UPR reaction for cell survival; however, this
protective effect was short-lived, since the continued exposure to
HG eventually overpowered this protective effect, leading to
apoptosis. These results indicate that novel (previously unkown)
mechanisms involved in DN may be targeted by novel therapeutic
interventions.
Materials and methods
Podocyte culture
Conditionally immortalized mouse podocytes purchased
from the Cell Culture Center (Peking Union Medical College,
Beijing, China) were cultured as previously described (6). In this cell line, a
temperature-sensitive SV40 large T-cell antigen (tsA58 Tag) is
controlled by a γ-interferon inducible H-2Kb promoter. To induce
proliferation, the cells were grown on type I collagen-coated
plastic culture bottles (BD Biosciences, Bedford, MA, USA), at 33°C
in RPMI-1640 culture medium (Gibco-BRL, Gaithersburg, MD, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 100 U/ml
penicillin and 100 mg/ml streptomycin (both from Invitrogen,
Carlsbad, CA, USA), to which recombinant mouse γ-interferon 10 U/ml
(PeproTech, Rocky Hill, NJ, USA) was added (growth-permissive
conditions). To induce quiescence and phenotype differentiation,
the podocytes were grown at 37°C and deprived of γ-interferon
(growth-restrictive conditions) in RPMI-1640 supplemented with 10%
FBS, and 1–2 drops of penicillin and streptomycin. The culture
medium was replaced every three days. When the cells grew slowly,
the cell volume increased significantly, and pedicels (foot
processes) extended from the podocytes, visualized under a phase
contrast microscope (Olympus, Tokyo, Japan), indicating that the
podocytes had differentiated. When the podocytes reached 75–85%
confluence under growth-restrictive conditions, they were washed
once with serum-free RPMI-1640 medium, and then growth-arrest was
induced in serum-free RPMI-1640 medium for 24 h to synchronize the
cell growth. The podocytes were then ready for the following
experiments.
Cell stimulation
Differentiated mouse podocytes were stimulated with
normal glucose [NG; 1 g/l D-glucose (Sigma, St. Louis, MO, USA)] or
HG (4.5g/l D-glucose). A third group of podocytes was exposed to 1
g/l D-glucose plus 24.4 mM mannitol (Sigma) as an osmotic control
(M). Cells in each group were collected at 12, 24, 48 and 72 h for
analyses.
TUNEL assay
Apoptotic cells were identified by the TUNEL
technique (Promega, Madison, WI, USA), according to the
manufacturer’s instructions. Differentiated mouse podocytes were
plated on cover slides in six-well plates. Following stimulation
with the indicated treatments, the cells were washed with 0.01 M
phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde
at room temperature for 30 min. The cells were then treated with
proteinase K at room temperature and incubated in terminal
deoxynucleotidyl transferase (TdT) buffer for 1 h at 37°C.
Endogenous peroxidase activity was inhibited using 0.3%
H2O2. The cells were incubated with
horseradish peroxidase (HRP)-conjugated streptavidin at room
temperature for 5 min. DAB working solution was added and the cells
were counterstained with hematoxylin. Negative control cells were
incubated with the labeling solution (without TdT) instead of the
TdT reaction solution. Apoptotic cell nuclei appeared as dark
brown/black under an E600 light microscope (Nikon, Tokyo, Japan).
For the quantification of TUNEL-positive (apoptotic) cells, a
minimum of 200 cells were counted at six random fields (×10) per
group, and the percentage of the positively-labeled cells was
calculated.
Analysis of apoptosis by flow
cytometry
The differentiated mouse podocytes were synchronized
and stimulated with the indicated treatments for 12, 24, 48 and 72
h. The cells were collected and washed twice with 4°C normal
saline. The supernatant was removed following centrifugation at
1,000 rpm for 5 min. The cells were then fixed with 70% ethanol
overnight. The cells were centrifuged (1,000 rpm, 5 min), washed
with normal saline, and then 1 ml DNA dye [propidium iodide (PI) 50
μg/ml, RNase 10 μg/ml and 1% Triton X-100; Sigma] was added. After
staining for 30 min at 4°C, the cells were analyzed by flow
cytometry (Epics-XL II; Beckman Coulter, Brea, CA, USA). Expo32ADC
software was used for analysis: a hypo-diploid peak prior to the
peak of the diploid cells was considered as the apoptotic cell
population. The apoptotic rate was calculated according to the
distribution histogram of the hypo-diploid population.
Immunocytochemistry
PV-9000 two-step immunohistochemical reagent
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., China)
was used to assess GRP78, CHOP/GADD153 and caspase-12 expression.
In brief, the podocytes were plated on cover slides in six-well
plates. Following stimulation with the indicated treatments, the
cells were fixed with 4% paraformaldehyde at room temperature for
15 min. Following pre-treatment with 0.3% Triton X-100 for 20 min
at 37°C, the cells were blocked with goat serum for 30 min at 37°C.
The cells were then incubated with rabbit anti-GRP78 polyclonal
antibody (1:100 dilution; NeoMarkers Inc., Fremont, CA, USA),
rabbit anti-CHOP/GADD153 polyclonal antibody (1:100 dilution; Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rabbit
anti-caspase-12 polyclonal antibody (1:1,000 dilution; Abcam,
Cambridge, MA, USA) overnight at 4°C. Following three washes with
PBS, the cells were incubated with a polymer helper and
polyperoxidase-anti-rabbit IgG (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at 37°C for 30 min, and
the cells were then stained with diaminobenzidine. A negative
control was created by replacing the primary antibody with PBS
buffer. The cells were counterstained with hematoxylin. The entire
stained areas were scanned at ×100 magnification using an E600
light microscope.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from each group using TRIzol
reagent (Invitrogen). RNA purity was estimated by calculating the
260/280 nm absorbance and reverse transcribed into cDNA using an
RT-PCR kit (Promega), according to the manufacturers’ instructions.
Relative levels of target gene mRNA expression normalized to 18S
rRNA were determined by the PCR system using the cDNA as a template
and specific primers synthesized by Beijing AuGCT Biotechnology Co.
Ltd., Beijing, China (Table I).
PCR reactions were performed in duplicate at 95°C for 5 min and
subjected to 36 cycles of 94°C for 1 min, 56–59°C for 45 sec and
72°C for 1 min, followed by 72°C for 10 min. PCR products were
separated by 2% agarose gel electrophoresis with ethidium bromide
staining and analyzed using a GDS-8000 Bioimaging system (UVP Inc.,
Upland, CA, USA) and LabWorks 4.5 software (UVP Inc.).
 | Table IPCR primer sequences. |
Table I
PCR primer sequences.
| Gene | Primer sequences | Tm
(°C) | Product size
(bp) |
|---|
| GRP78 | F:
5′-AACCCAGATGAGGCTGTAGCA-3′
R: 5′-ACATCAAGCAGAACCAGGTCAC-3′ | 55 | 91 |
| CHOP/GADDl53 | F:
5′-CCAGCAGAGGTCACAAGCAC-3′
R: 5′-CGCACTGACCACTCTGTTTC-3′ | 42 | 126 |
| Caspase-12 | F:
5′-CACTGCTGATACAGATGAGG-3′
R: 5′-CCACTCTTGCCTACCTTCC-3′ | 56 | 138 |
| 18S rRNA | F:
5′-ACACGGACAGGATTGACAGA-3′
R: 5′-GGACATCTAAGGGCATCACA-3′ | 56 | 238 |
Western blot analysis
The cells were harvested and homogenized on ice-cold
homogenization buffer (10 mM Tris-HCl, pH 7.4, 1.5 mM EDTA, pH 8.0
and 100 mg/l phenylmethylsulfonyl fluoride; Sigma), followed by
centrifugation at 20,000 × g for 20 min at 4°C. Supernatants were
collected for characterizing the relative levels of protein
expression by western blot analysis. In addition, nuclear proteins
in some cells were extracted using nuclear and cytoplasmic protein
extraction kits (KeyGen Biotech, Nanjing, China), according to the
manufacturer’s instructions, followed by the quantification of
protein concentrations using Coomassie brilliant blue.
Total cellular protein (100 μg/lane) or nuclear
protein (60 μg/lane) were separated by 10% SDS-PAGE and transferred
onto polyvinylidene fluoride (PVDF) membranes (Millipore Corp.,
Billerica, MA, USA). After being blocked with 5% non-fat dry milk
in Tris-buffered saline/Tween-20 (TBST) buffer for 2 h, the
membranes were incubated overnight at 4°C with rabbit polyclonal
antibodies against GRP78 (1:1,000 dilution), caspase-12 (1:1,000)
and β-actin (1:1,000; Biosynthesis Biotechnology Co., Ltd.,
Beijing, China), rabbit polyclonal antibodies against CHOP/GADD153
(1:500) and histone H1 (1:500; Boster Biotechnology, Wuhan, China).
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Amersham
Biosciences, Piscataway, NJ, USA) at 37°C for 2 h. After TBST
washing, enhanced chemiluminescence (ECL) reagent (Tiangen Biotech
Co., Ltd., Beijing, China) was added to the membranes. Western blot
bands were read for integrated optical density (IOD) and quantified
using LabWorks 4.5 software (UVP).
Statistical analysis
Data were processed using SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA). The results are presented as the means ±
standard deviation (SD). Statistical analysis was performed using
one-way ANOVA with the least significant difference (LSD) t-test
for post hoc analysis. Correlation analysis was performed using the
Spearman’s test. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
HG induces apoptosis of differentiated
mouse podocytes
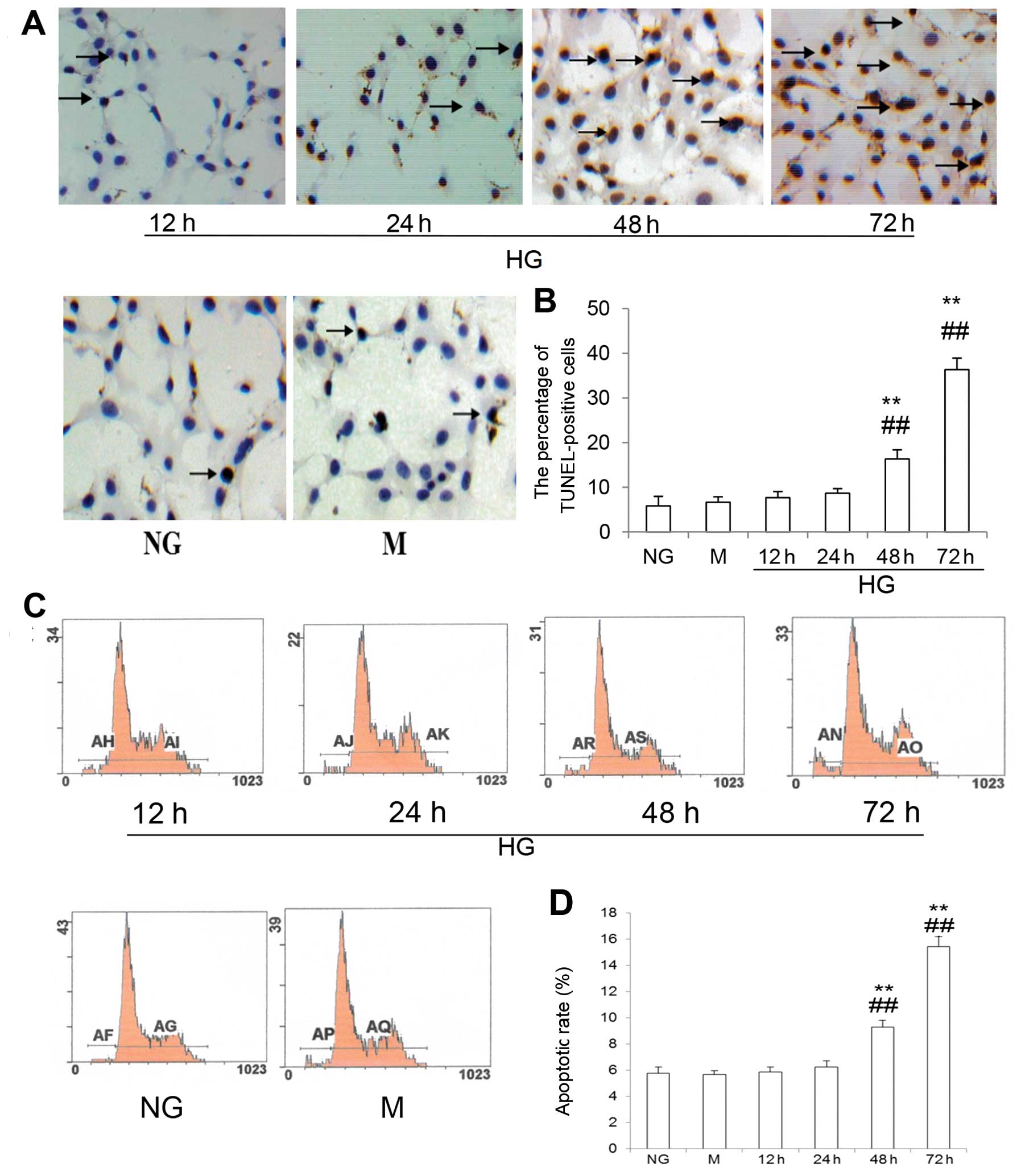
To determine the effects of HG on podocyte
apoptosis, the podocytes were cultured in RPMI-1640 medium
containing 1 g/l D-glucose (NG, control group), 1 g/l D-glucose
plus 24.4 mM mannitol (M group, osmotic control group) and 4.5 g/l
D-glucose (HG, experimental group) for 12, 24, 48 and 72 h.
Apoptotic rates were determined by TUNEL assay and flow cytometry
(PI staining). Fig. 1A and B
shows that compared with the control groups, podocyte apoptotic
rates in the HG group were increased at 48 h, and even more so at
72 h. Indeed, 5.8±2.1% apoptotic cells in the NG group and 6.7±1.2%
apoptotic cells in the M group, compared with 16.3±2.1 and
36.3±2.6% apoptotic cells in the HG group at 48 and 72 h (all
P<0.01), respectively, were detected by TUNEL assay. The results
were consistent with the results from flow cytometric analysis
(Fig. 1C and D). As expected,
mannitol had no effect on podocyte apoptosis as assessed using
these two methods.
HG stimulation upregulates the expression
of the ER chaperone, GRP78, in differentiated mouse podocytes
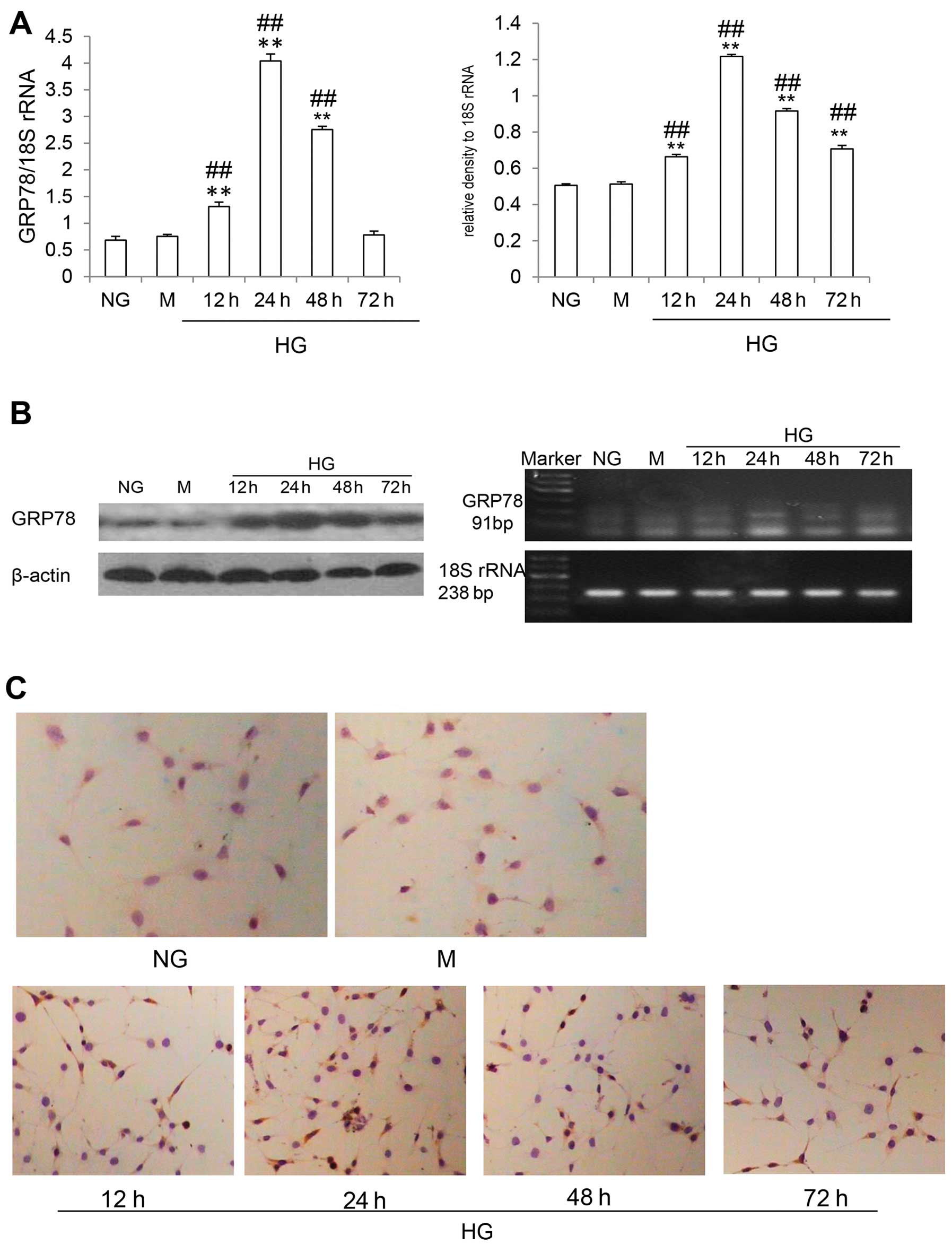
GRP78, an important molecular chaperone localized in
the ER, was used as an indicator of ERS (16). As can be seen in Fig. 2C, immunocytochemistry revealed
that GRP78 was abundantly expressed in the cells in the HG group.
By contrast, the cells in the NG and M groups showed only a modest
or weak GRP78 signal. Western blot analysis revealed that GRP78
expression was significantly increased at 12 and 24 h (both
P<0.05), and then decreased at 48 h, approximately returning to
the control levels by 72 h (Fig.
2B). Furthermore, RT-PCR analysis revealed the same trend in
GRP78 mRNA levels. However, GRP78 mRNA levels remained higher at 72
h (P<0.05) (Fig. 2A). These
findings suggested that UPR was induced and that ERS was activated
in the differentiated mouse podocytes exposed to HG.
HG stimulation induces the activation of
the CHOP/GADD153-dependent apoptotic pathway in differentiated
mouse podocytes
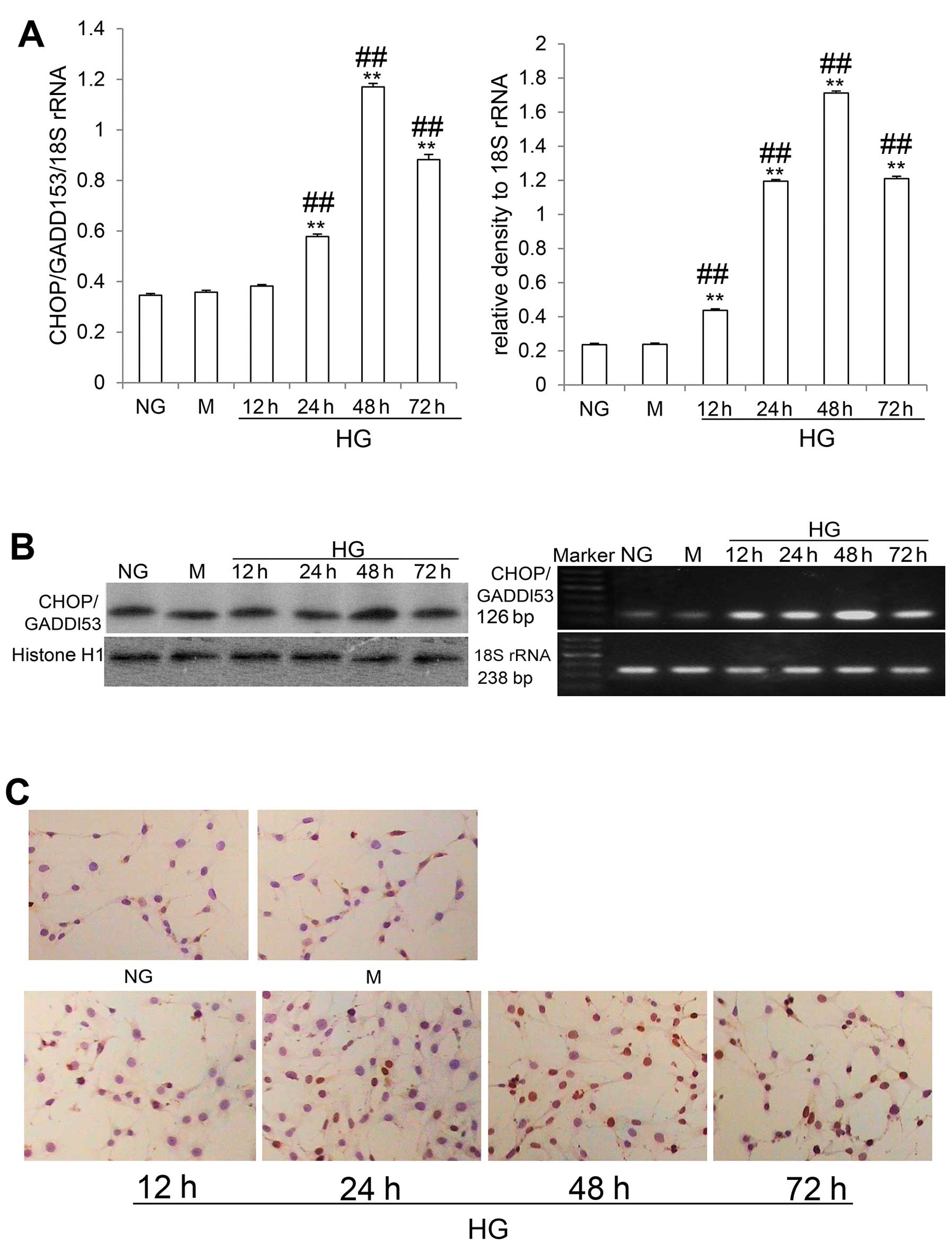
CHOP/GADD153 is a nuclear protein forming stable
heterodimers with C/EBP family members (8,17).
As illustrated in Fig. 3C, it was
detectable at low levels in the cells in the NG and M groups,
whereas in the cells in the HG group, CHOP/GADD153 expression was
increased as detected by immunocytochemistry. Western blot analysis
revealed that CHOP/GADD153 expression was increased at 24 h,
reaching a peak at 48 h, and then decreasing at 72 h, but without
returning to the control levels (all P<0.05) (Fig. 3B). RT-PCR analysis revealed that
CHOP/GADD153 mRNA levels were increased at 12 h, also reaching a
peak at 48 h, and then decreasing at 72 h, but without returning to
the control levels (all P<0.05) (Fig. 3A). These data suggested that the
CHOP/GADD153-dependent apoptotic pathway was activated by HG
stimulation in the differentiated mouse podocytes.
HG stimulation activates the
caspase-12-dependent apoptotic pathway in the differentiated mouse
podocytes
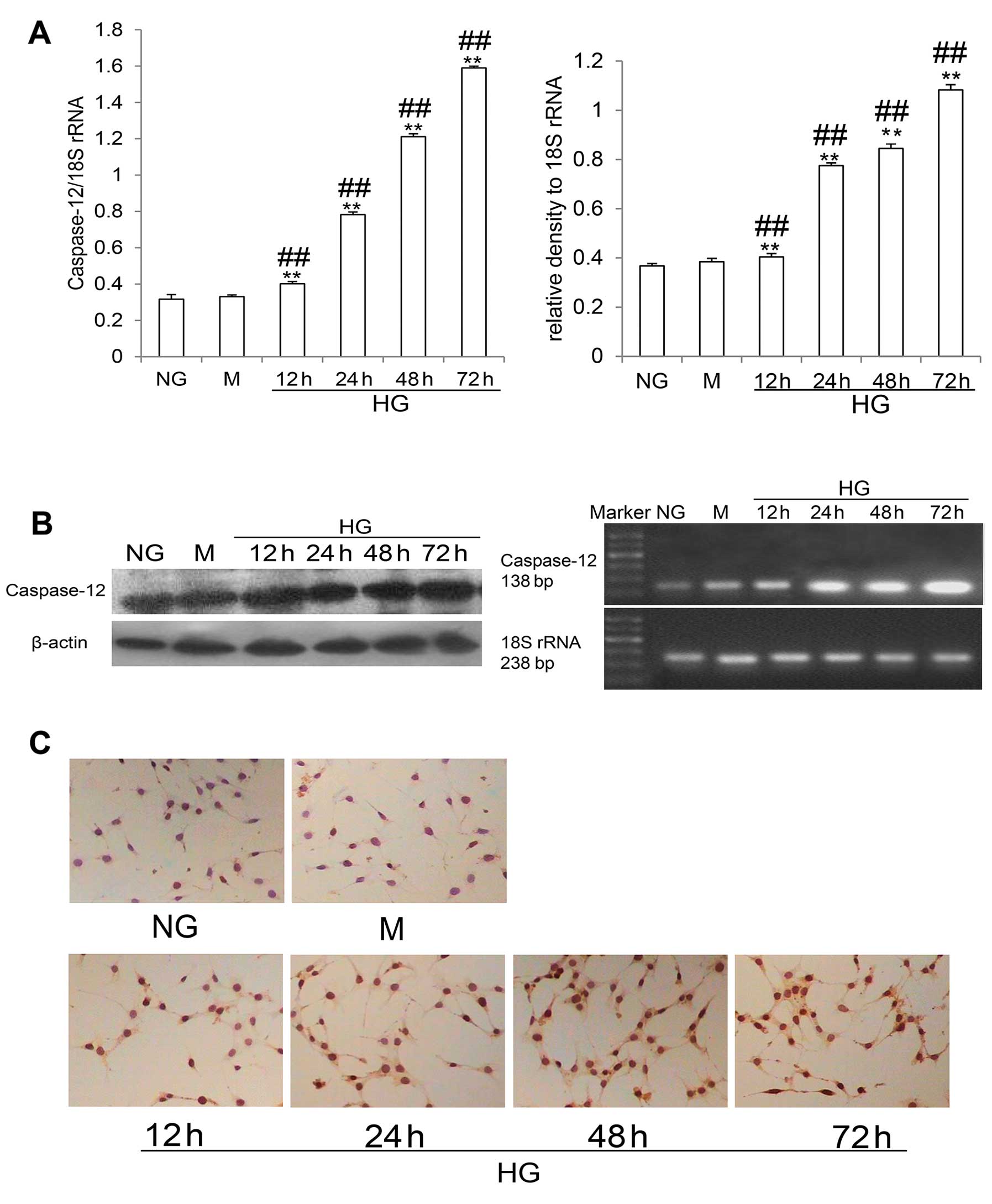
Caspase-12 is exclusively located in the ER. It is
ubiquitously and constitutively expressed, but unlike other
caspases, caspase-12 is specifically activated by ERS (18). As shown in Fig. 4C, immunocytochemistry revealed
that caspase-12 expression was increased in the cells in the HG
group compared with the control cells (NG and M group cells).
Western blot analysis revealed that HG stimulation increased
caspase-12 protein levels in a time-dependent manner (all
P<0.05) (Fig. 4B). HG
stimulation also increased the mRNA levels of caspase-12 in a
time-dependent manner (all P<0.05) (Fig. 4A). These data indicated that HG
stimulation activated the caspase-12-dependent apoptosis pathway in
the differentiated mouse podocytes.
Correlation between caspase-12 and
CHOP/GADD153 protein expression and apoptosis induced by HG
Spearman’s correlation analysis revealed that
caspase-12 and CHOP/GADD153 positively correlated with the
apoptotic rate (r=0.915, P<0.01 and r=0.639, P<0.01).
Discussion
Podocytes, a type of glomerular epithelial cell, are
unique, highly specialized and terminally differentiated cells
(6). The loss of podocytes leads
to the stripping of areas of the glomerular basement membrane,
which contributes to impaired renal function, as is evident by
proteinuria and the development of glomerulosclerosis (7). It is generally accepted that
apoptosis is a major cause of podocytes loss, occurring early in
the development of DN and closely correlating with its progression
(19,20).
ERS is generally present under physiological and
pathological conditions, and is an important inducer of cell
apoptosis (15,16). A growing number of studies have
demonstrated that ERS plays a key role in the pathogenesis of
several renal diseases, including DN (21). In the present study, we
investigated the hypothesis that ERS is partly responsible for
podocyte apoptosis induced by HG. We observed that some podocyte
apoptosis was detected after 12 and 24 h in the cells exposed to HG
and the control cells, but without significant differences between
these two groups. However, the number of apoptotic cells markedly
increased with the increasing exposure time to HG.
The levels of GRP78, a key UPR modulator (15), were increased in the cells exposed
to HG at 12 and 24 h, and decreased by 72 h. These results
suggested that UPR was induced and that ERS was activated when the
podocytes were exposed to HG. Based on current knowledge, the
function of UPR is to adapt to the changing environment and to
re-establish a normal ER function (15). However, our data indicated that
this protective effect of the UPR lasted only for a short time,
even if the stress was persistent. These results suggest that this
protective mechanism is eventually overpowered and that apoptosis
ensues.
CHOP/GADD153 is a nuclear protein that forms stable
heterodimers with C/EBP family members (22). It is barely detectable under
normal physiological conditions, but it is strongly induced in
response to ERS (23). The
overexpression of CHOP/GADD153 and the microinjection of
CHOP/GADD153 protein have been reported to lead to cell cycle
arrest and/or apoptosis (24–27). The overexpression of GRP78 (also
known as BiP) may attenuate the induction of CHOP/GADD153 in ERS
and may reduce ERS-induced apoptosis. Our results revealed that
just as GRP78 expression began to weaken at 48 h, CHOP/GADD153
expression reached its peak. Furthermore, CHOP/GADD153 expression
correlated with the apoptotic rate in cells exposed to HG.
Caspase-12 is exclusively located in the ER
(18). It is ubiquitously and
constitutively expressed, but unlike other caspases, caspase-12 is
specifically activated by insults inducing ERS and not by other
death stimuli (29). Following
its activation, it can directly process downstream caspases in the
cytosol, mainly caspase-9 and -3 (30). Our results demonstrated that
caspase-12 expression gradually increased with time in response to
HG, and that its levels correlated with the apoptotic rate in cells
exposed to HG.
The results from the present study demonstrated that
hyperglycemia induced ERS in podocytes, and that this stress
gradually exceeded the capacity of different protective mechanisms,
including GRP78 response. CHOP/GADD153 and caspase-12 were
activated according to different patterns, suggesting that these
mechanisms contribute differently to podocyte apoptosis.
Nevertheless, these mechanisms partially contribute to the
pathogenesis of DN.
In conclusion, hyperglycemia induced apoptosis
partly through ERS in differentiated mouse podocytes, and this may
contribute to the pathogenesis of DN. HG-stimulated podocytes
undergo ERS, which presumably is an adaptive, protective UPR
reaction for cell survival; however, this protective effect was
short-lived, since continued exposure to HG eventually overpowered
this effect and led to apoptosis. These results indicate that novel
(previously unknown) mechanisms involved in DN may be targeted by
novel therapeutic interventions.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81000301 and 81100517)
and the Technology Bureau of Handan (no. 1223108149).
References
|
1
|
Pavkov ME, Knowler WC, Bennett PH, Looker
HC, Krakoff J and Nelson RG: Increasing incidence of proteinuria
and declining incidence of end-stage renal disease in diabetic Pima
Indians. Kidney Int. 70:1840–1846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cowie CC, Port FK, Wolfe RA, Savage PJ,
Moll PP and Hawthorne VM: Disparities in incidence of diabetic
end-stage renal disease according to race and type of diabetes. N
Engl J Med. 321:1074–1079. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adler S: Diabetic nephropathy: linking
histology, cell biology, and genetics. Kidney Int. 66:2095–2106.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mason RM and Wahab NA: Extracellular
matrix metabolism in diabetic nephropathy. J Am Soc Nephrol.
14:1358–1373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shankland SJ: The podocyte’s response to
injury: role in proteinuria and glomerulosclerosis. Kidney Int.
69:2131–2147. 2006.
|
|
6
|
Mundel P and Shankland SJ: Podocyte
biology and response to injury. J Am Soc Nephrol. 13:3005–3015.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kriz W, Gretz N and Lemley KV: Progression
of glomerular diseases: is the podocyte the culprit? Kidney Int.
54:687–697. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marciniak SJ, Yun CY, Oyadomari S, et al:
CHOP induces death by promoting protein synthesis and oxidation in
the stressed endoplasmic reticulum. Genes Dev. 18:3066–3077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siu B, Saha J, Smoyer WE, Sullivan KA and
Brosius FC III: Reduction in podocyte density as a pathologic
feature in early diabetic nephropathy in rodents: prevention by
lipoic acid treatment. BMC Nephrol. 7:62006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
King GL and Loeken MR:
Hyperglycemia-induced oxidative stress in diabetic complications.
Histochem Cell Biol. 122:333–338. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Feng D, Li Y, Iida K, McGrath B
and Cavener DR: PERK EIF2AK3 control of pancreatic beta cell
differentiation and proliferation is required for postnatal glucose
homeostasis. Cell Metab. 4:491–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laybutt DR, Preston AM, Akerfeldt MC, et
al: Endoplasmic reticulum stress contributes to beta cell apoptosis
in type 2 diabetes. Diabetologia. 50:752–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao RV, Peel A, Logvinova A, et al:
Coupling endoplasmic reticulum stress to the cell death program:
role of the ER chaperone GRP78. FEBS Lett. 514:122–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zinszner H, Kuroda M, Wang X, et al: CHOP
is implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Butt A and Riaz S: Study of protein
profiling of human urine in diabetic hypertensive nephropathy
versus normal healthy controls. Diabetes Technol Ther. 12:379–386.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Felix K and Wang J: Immune
regulation through mitochondrion-dependent dendritic cell death
induced by T regulatory cells. J Immunol. 187:5684–5692. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Sun Y, Li Z, et al: Apoptosis
induced by endoplasmic reticulum stress involved in diabetic kidney
disease. Biochem Biophys Res Commun. 370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ron D and Habener JF: CHOP, a novel
developmentally regulated nuclear protein that dimerizes with
transcription factors C/EBP and LAP and functions as a
dominant-negative inhibitor of gene transcription. Genes Dev.
6:439–453. 1992. View Article : Google Scholar
|
|
23
|
Wang XZ, Lawson B, Brewer JW, et al:
Signals from the stressed endoplasmic reticulum induce
C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol.
16:4273–4280. 1996.PubMed/NCBI
|
|
24
|
Matsumoto M, Minami M, Takeda K, Sakao Y
and Akira S: Ectopic expression of CHOP (GADD153) induces apoptosis
in M1 myeloblastic leukemia cells. FEBS Lett. 395:143–147. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maytin EV, Ubeda M, Lin JC and Habener JF:
Stress-inducible transcription factor CHOP/gadd153 induces
apoptosis in mammalian cells via p38 kinase-dependent and
-independent mechanisms. Exp Cell Res. 267:193–204. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oyadomari S, Takeda K, Takiguchi M, et al:
Nitric oxide-induced apoptosis in pancreatic beta cells is mediated
by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci
USA. 98:10845–10850. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gotoh T, Oyadomari S, Mori K and Mori M:
Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated
by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J
Biol Chem. 277:12343–12350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu H and Baliga R: Endoplasmic reticulum
stress-associated caspase 12 mediates cisplatin-induced LLC-PK1
cell apoptosis. J Am Soc Nephrol. 16:1985–1992. 2005. View Article : Google Scholar : PubMed/NCBI
|