Introduction
In the dermis, fibroblasts that are derived from
mesenchymal cells play a critical role in maintaining homeostasis
(1,2). Dermal fibroblasts synthesize and
secrete extracellular matrix (ECM) components, such as collagen and
elastin (3). The ability to
synthesize ECM components is repressed by chronic and acute solar
ultraviolet (UV) exposure (4,5),
and UV radiation also indirectly causes the destruction of the ECM
through the induction of matrix metalloproteinase (MMP) secretion
by dermal fibroblasts. The impaired ECM forms a non-elastic matrix
in the dermis, which leads to aging (6). Furthermore, UV triggers apoptosis
and growth arrest in fibroblasts (7,8).
Therefore, as dermal fibroblasts play an important role in the
construction and homeostasis of the dermis and UV induces a
decrease in the number of dermal fibroblasts and the destruction of
the ECM, UV radiation is one of the major inducers of disorders of
the dermis.
microRNAs (miRNAs) are 19- to 24-nucleotide (nt)
non-coding RNA sequences that regulate target gene expression
through RNA interference. miRNAs have been shown to modulate
various cellular processes, such as apoptosis, proliferation, cell
cycle progression and differentiation (9). Several lines of evidence suggest
that miRNAs play important roles in the dermis. miRNA-152 and
miRNA-181 act on adhesion proteins and ECM proteins in senescent
dermal fibroblasts (13). The
expression of miRNA-92a leads to a decreased expression of MMP1 and
the accumulation of collagen (14), and miRNA-196a and miRNA-150
directly target collagen type I in scleroderma dermal fibroblasts
(15). Moreover, the expression
of miRNAs is sensitively altered in response to UV radiation
(10–12). In a recent study, we demonstrated
that phytochemicals protect various cell types against UV
radiation, suggesting a role for miRNAs in this protective
mechanism in dermal fibroblasts (16). However, little is known about the
underlying mechanisms.
Troxerutin is a trihydroxyethylated derivative of
the natural bioflavonoid, rutin, which is one of the phytochemical
components extracted from Sophora japonica (17). In addition, as troxerutin has been
shown to have radioprotective, anti-inflammatory and antioxidant
effects, it seems likely that troxerutin may protect cells against
UV-induced oxidative stress and DNA damage (18,19).
In this study, we aimed to determine whether
troxerutin induces a resistance response in dermal fibroblasts
exposed to UVB radiation and, if so, to determine whether miRNAs
are involved in the protective effects of troxerutin against UVB
radiation. We confirm that the treatment of human dermal
fibroblasts (HDFs) with troxerutin induces resistance to
UVB-induced cell damage. In addition, we characterize the miRNA
expression profiles associated with the protective effects of
troxerutin against UVB radiation.
Materials and methods
Cell lines and cell culture
Normal HDFs (nHDFs) were purchased from Lonza
(Basel, Switzerland) and cultured in Dulbecco’s modified Eagle’s
medium (DMEM; Gibco-Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO,
USA) and 1% penicillin/streptomycin (Gibco Invitrogen) in a
humidified atmosphere of 95% air/5% CO2 at 37°C.
WST-1 assay
Cell viability was determined using a water-soluble
tetrazolium salt-1 (WST-1) assay. nHDFs were seeded in a 96-well
plate (SPL Life Sciences, Pocheon, Korea) at a density of
4×103 cells/well. The nHDFs were incubated for 24 h
under growth conditions and then pre-treated with the indicated
concentrations of troxerutin for 4, 8 and 12 h. At each time point,
the cells were exposed to 100 mJ/cm2 UVB radiation using
a G8T5E lamp (Sankyo Denki, Toshima-ku, Japan) and a UV light meter
(Lutron UV-340; Lutron Electronic Enterprise Co., Taipei, Taiwan).
Following exposure to UVB radiation, the nHDFs were incubated for
24 h prior to the addition of WST-1 solution (EZ-Cytox cell
viability assay kit; Itsbio, Seoul, Korea) for 1 h. The optical
density of the colored WST-1 was measured at 450 nm and normalized
using optical density at 620 nm. The results are expressed as the
mean percentage ± standard error (SE) of experiments performed in
triplicate. Statistical significance was determined using a
two-tailed t-test compared with untreated nHDFs or UVB-exposed
nHDFs not treated with troxerutin.
Cell cycle and DNA repair analyses
Cell cycle distribution was determined by measuring
the DNA content. nHDFs were seeded in 60-mm culture dishes (SPL
Life Sciences) at a density of 7×105 cells/dish. The
cells were incubated for 24 h under growth conditions and then
pre-treated with 10 μM troxerutin for 8 h. At various time points,
the cells were exposed to 100 mJ/cm2 UVB and incubated
for a further 24 h. Following incubation, the nHDFs were fixed with
70% ethanol at 4°C for 3 h and incubated with staining solution
containing 50 μg/ml PI (Sigma-Aldrich), 0.5% Triton X-100 (Bioshop,
Burlington, Canada) and 100 μg/ml RNase (Bioshop) at 37°C for 1 h.
DNA content was measured using the FL2-H channel of a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). DNA repair
activity was determined by transfection with a damaged plasmid as
previously described (21).
Before transfection, the pGL3 luciferase reporter plasmids were
damaged with 2,000 J/m2 UVC radiation. The nHDFs were
seeded at 7×105 cells in 60-mm culture dishes and
transiently transfected with either the control pGL3 or the damaged
pGL3 plasmids along with pSV-β-galactosidase plasmid (as a
transfection control). The transfected cells were treated with 10
μM troxerutin for 24 h. After the treatments, DNA repair activity
was determined by using the Luciferase assay system (Promega,
Madison, WI, USA). The results were normalized to β-galactosidase
activity and are presented as the means ± SE of the percentage of
the control. Results shown are the averages of 3 independent
experiments.
Migration assay
The migration rate was measured as described in a
previous study (20). Briefly,
7×105 nHDFs were seeded in a 60-mm culture dish and
grown to >90% confluence. The cells were pre-treated with 10 μM
troxerutin for 8 h and then exposed to 100 mJ/cm2 UVB.
The cell monolayer was scraped in a straight line with a p200 pipet
tip. At 0 and 48 h after wounding, the distance between the cells
was compared by imaging using a phase contrast microscope.
miRNA microarray
Total RNA was extracted from each sample using
RiboEX (GeneAll, Seoul, Korea) and labeled using Agilent miRNA
labeling kits (Agilent Technologies, Santa Clara, CA, USA)
according to the manufacturer’s instructions. The labeled RNA was
purified by a Micro Bio-Spin P-6 column (Bio-Rad Laboratories,
Hercules, CA, USA) and hybridized with the SurePrint Human v16
miRNA microarray kit (based on miRBase Release 19.0; Agilent
Technologies) as recommended by the manufacturer. The microarray
contained 1,368 probes and detected 1,205 miRNAs. The fluorescence
intensity of each probe was measured using Scanner and Feature
Extraction software (Agilent Technologies). The digitalized
fluorescence intensity was analyzed using GeneSpring GX version
11.5 (Agilent Technologies).
Bioinformatics analysis of miRNAs with
significant changes in expression
Putative targets of miRNAs were identified using
bioinformatics miRNA target prediction software, TargetScan
(www.targetscan.org). The prediction of targets was
performed using a conserved database for all miRNAs that were
either up- or downregulated by >2-fold. The targets of each
significant miRNA were collected in a target pool and Gene Ontology
(GO) of the target pool and were analyzed using DAVID (http://david.abcc.ncifcrf.gov/). After GO
analysis, the significant GO terms were filtered by a >5%
‘percentage of count’, which represented the percentage of each GO
term-related genes.
Statistical analyses
Statistical significance was determined by a
Student’s t-test. Values of p<0.05 were considered to indicate
statistically significant differences.
Results
Cytotoxicity of troxerutin in nHDFs and
protective effects against UVB radiation
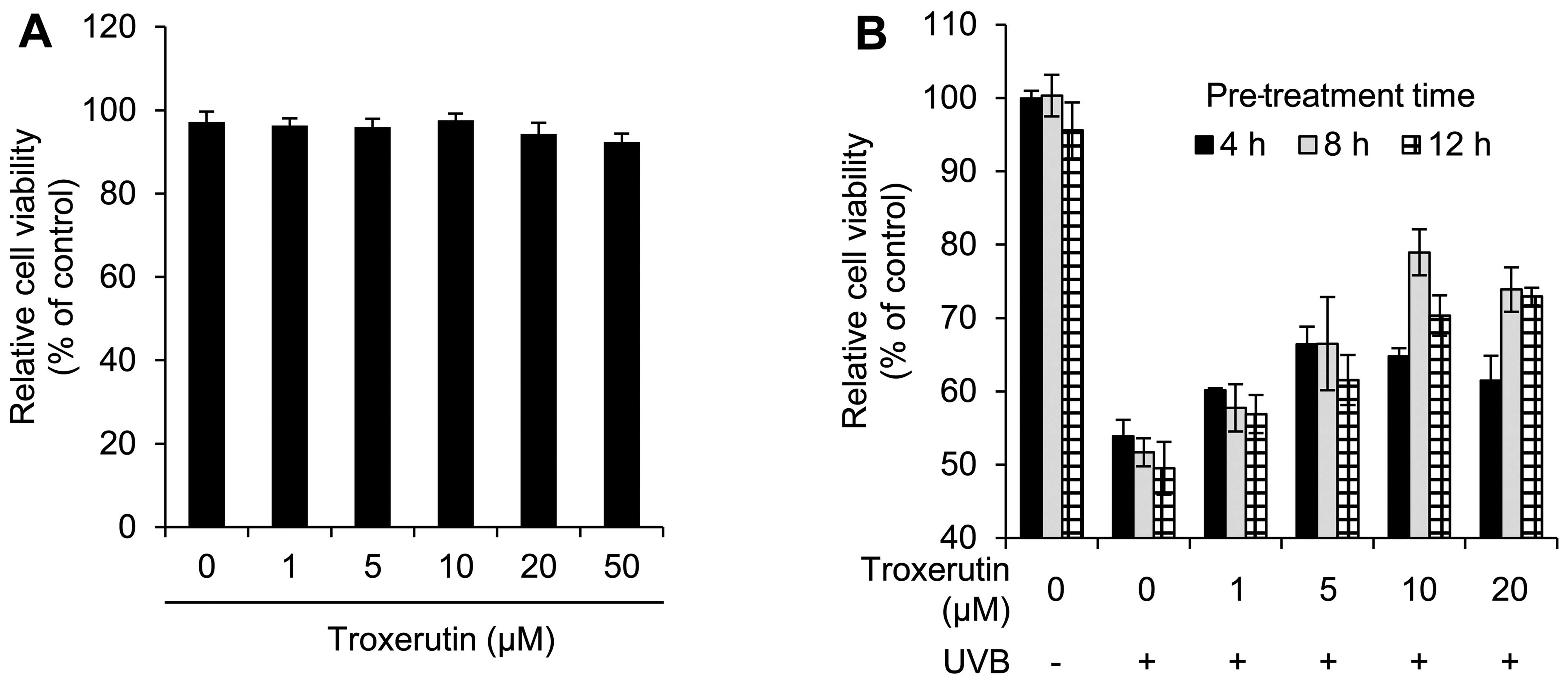
To determine the cytotoxicity of troxerutin we first
performed cell viability assays for nHDFs treated with the
indicated concentrations of troxerutin. Overall, there was no
significant toxicity observed for concentrations up to 50 μM and
treatment with 50 μM troxerutin decreased cell viability to 92.40%
(Fig. 1A). Therefore, we used
troxerutin at concentrations <50 μM in the subsequent
experiments.
We then investigated the protective effects of
troxerutin against UVB radiation. nHDFs were pre-treated with 0–20
μM troxerutin for 4, 8 and 12 h, and then exposed to 100
mJ/cm2 UVB. As shown in Fig. 1B, pre-treatment with troxerutin
reversed the UVB-induced inhibitory effects on cell growth. In
particular, pre-treatment with 10 μM troxerutin for 8 h restored
cell viability to 78.95% compared with 51.68% for the UVB-exposed
nHDFs. We then examined whether troxerutin increases cell viability
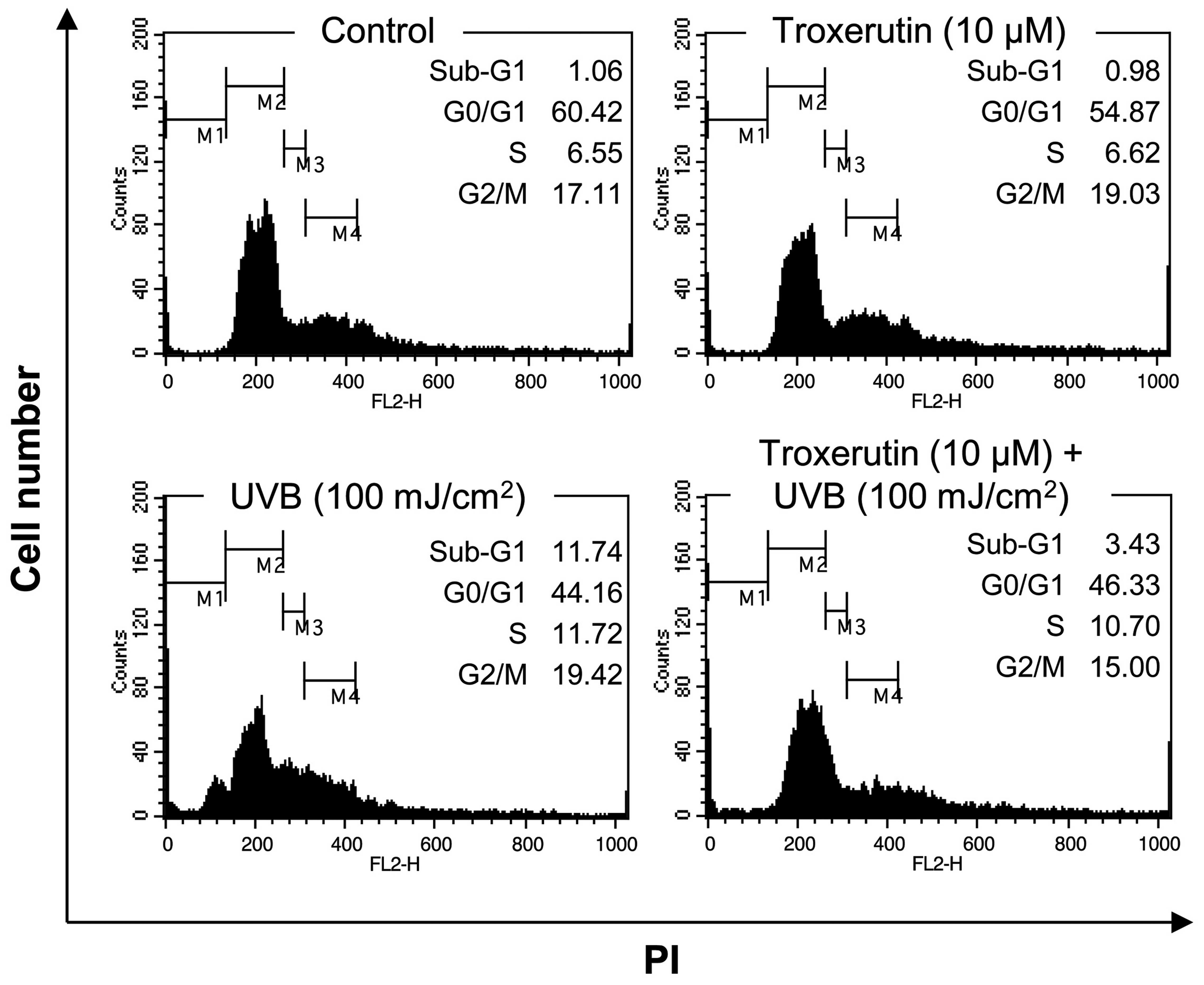
in nHDFs through the regulation of the cell cycle or cell death. To
investigate cell cycle arrest we performed a flow cytometric cell
cycle analysis using PI staining. As shown in Fig. 2, exposure to 100 mJ/cm2
UVB increased the number of cells in the sub-G1 phase, increasing
the population of dead cells from 1.06 to 11.74%. However,
pre-treatment with 10 μM troxerutin for 8 h reversed the
UVB-induced increase in the number of cells in the sub-G1 phase
(percentage of cells in sub-G1 phase decreased to 3.43%).
Therefore, troxerutin blocked the UVB-induced decrease in cell
viability by repressing cell death.
Troxerutin enhances cell migration and
DNA repair activity
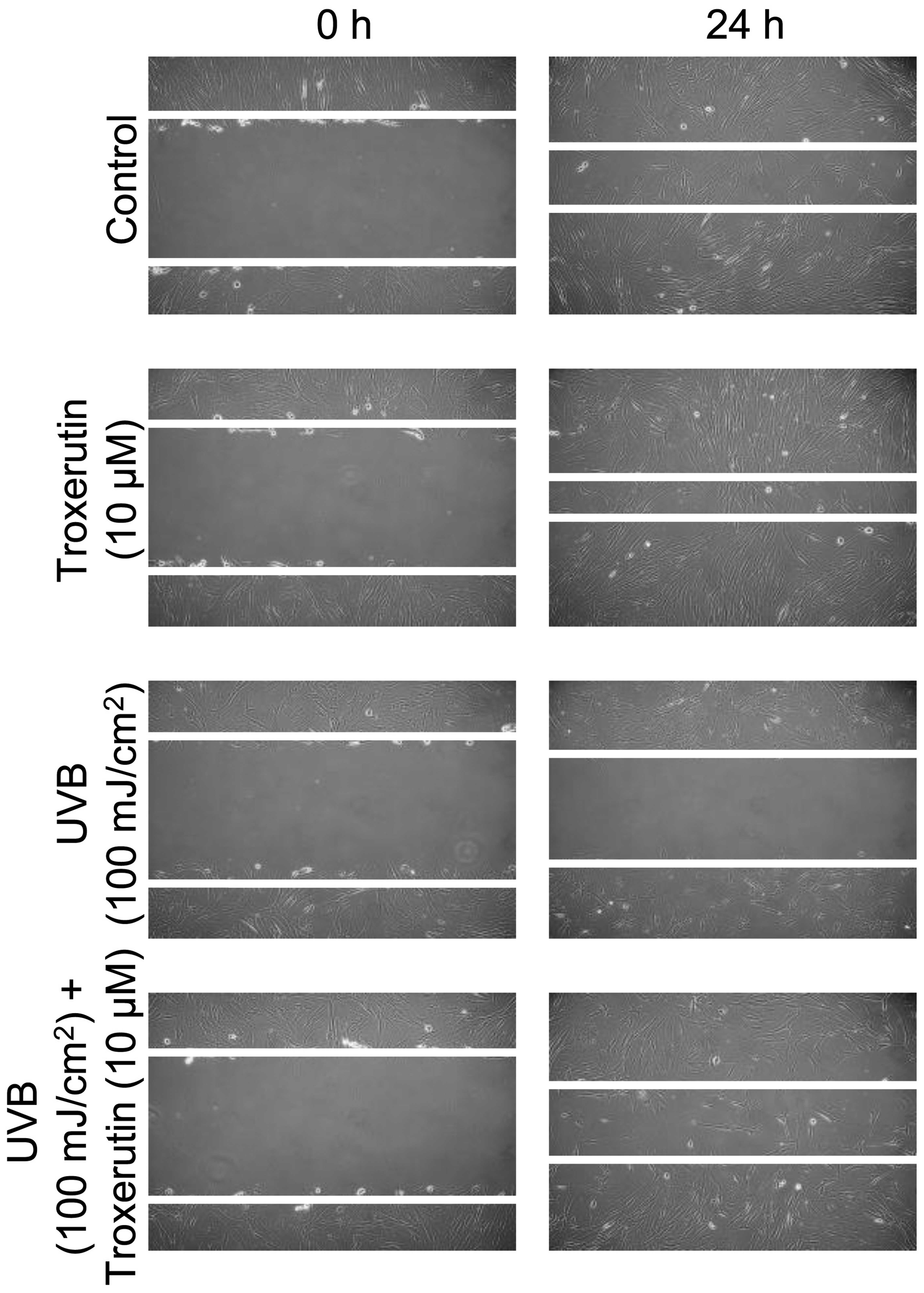
To investigate the effects of troxerutin on the
migration rate we performed a migration assay using a scratch line.
nHDFs were grown to >90% confluence and pre-treated with 10 μM
troxerutin for 8 h. Following pre-treatment, the nHDFs were exposed
to 100 mJ/cm2 UVB and scraped to form a scratch. As
shown in Fig. 3, treatment with
troxerutin enhanced the cell migration rate compared with the
untreated (control) nHDFs and those exposed to UVB radiation and
not treated with troxerutin. In addition, we examined whether
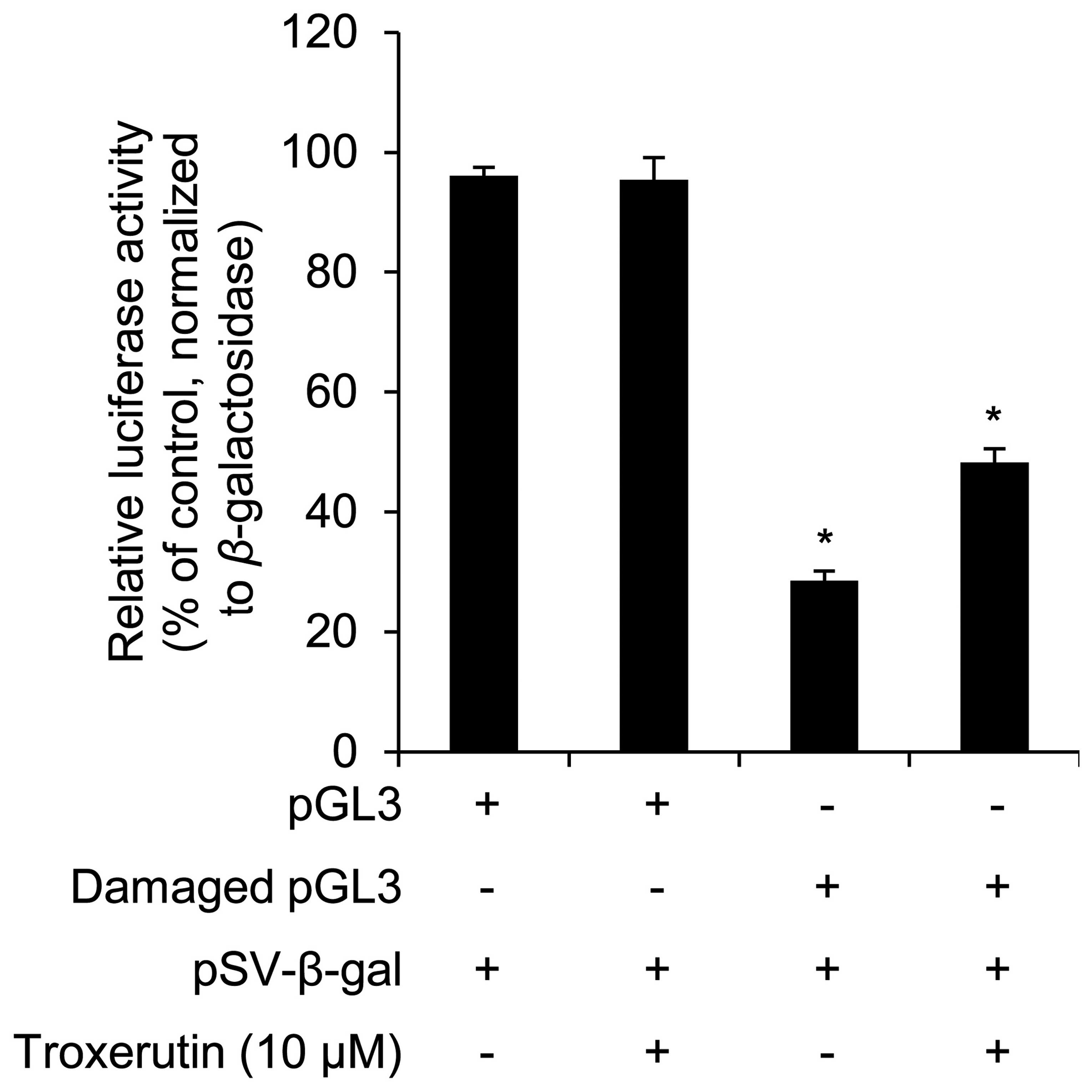
troxerutin increases DNA repair activity. A plasmid containing the
luciferase gene was damaged by UVC and then transfected into the
nHDFs. The damaged vector was broken by endonucleases in the nHDFs,
whereas the repaired vector remained and expressed luciferase, as
shown in a previous study (21).
Therefore, an increase in luciferase activity indicates enhanced
DNA repair activity. As shown in Fig.
4, troxerutin increased luciferase activity in the nHDFs
transfected with the damaged vector.
Identification of troxerutin-induced
alterations in miRNA expression in UVB-exposed nHDFs and target
prediction of these miRNAs
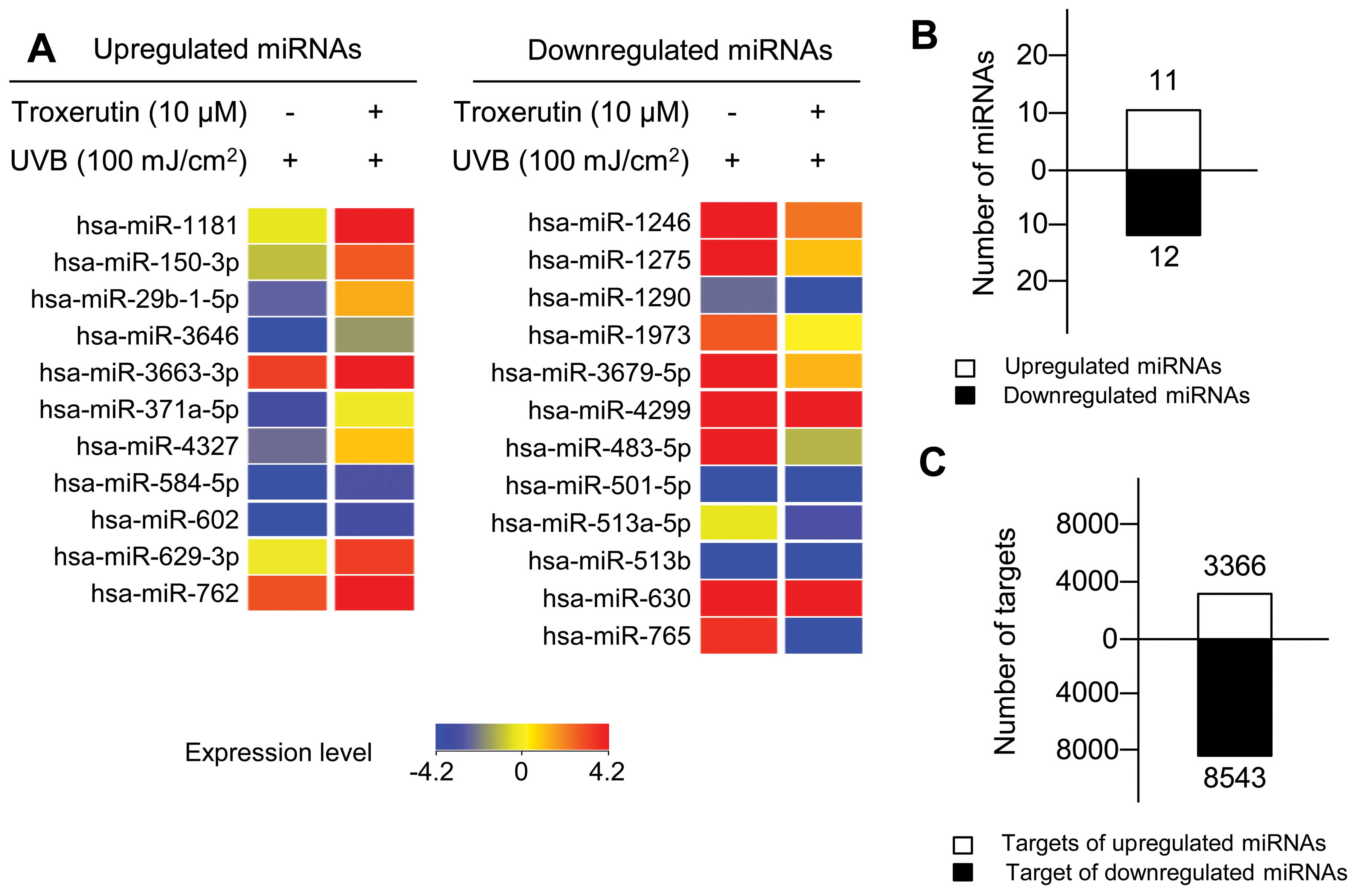
We identified 23 troxerutin-specific miRNAs whose
expression was altered by >2-fold in the troxerutin-treated
nHDFs (Fig. 5A and B), including
11 upregulated miRNAs (Table I)
and 12 downregulated miRNAs (Table
II). To elucidate the biological functions of the
troxerutin-specific miRNAs, we identified putative targets using
TargetScan, a seed sequence-based miRNA target prediction system.
Since the miRNAs and miRNA target sites are conserved in various
species (22), we performed
TargetScan in the conserved database. Through TargetScan, we
identified 3,366 targets of troxerutin-specific upregulated miRNAs
and 8,543 targets of troxerutin-specific downregulated miRNAs
(Fig. 5C).
 | Table ITroxerutin-induced upregulated miRNAs
in UVB-exposed nHDFs. |
Table I
Troxerutin-induced upregulated miRNAs
in UVB-exposed nHDFs.
| Accession no. | miRNA | Fold change | Chromosome |
|---|
| MIMAT0005826 | hsa-miR-1181 | 3.45 | chr19 |
| MIMAT0004610 | hsa-miR-150-3p | 2.82 | chr19 |
| MIMAT0004514 |
hsa-miR-29b-1-5p | 2.95 | chr7 |
| MIMAT0018065 | hsa-miR-3646 | 2.29 | chr20 |
| MIMAT0018085 |
hsa-miR-3663-3p | 2.30 | chr10 |
| MIMAT0004687 |
hsa-miR-371a-5p | 2.43 | chr19 |
| MIMAT0016889 | hsa-miR-4327 | 2.54 | chr21 |
| MIMAT0003249 | hsa-miR-584-5p | 2.77 | chr5 |
| MIMAT0003270 | hsa-miR-602 | 5.24 | chr9 |
| MIMAT0003298 | hsa-miR-629-3p | 2.51 | chr15 |
| MIMAT0010313 | hsa-miR-762 | 3.60 | chr16 |
 | Table IITroxerutin-induced downregulated
miRNAs in UVB-exposed nHDFs. |
Table II
Troxerutin-induced downregulated
miRNAs in UVB-exposed nHDFs.
| Accession no. | miRNA | Fold change | Chromosome |
|---|
| MIMAT0005898 | hsa-miR-1246 | −3.31 | chr2 |
| MIMAT0005929 | hsa-miR-1275 | −2.95 | chr6 |
| MIMAT0005880 | hsa-miR-1290 | −2.32 | chr1 |
| MIMAT0009448 | hsa-miR-1973 | −2.02 | chr4 |
| MIMAT0018104 |
hsa-miR-3679-5p | −3.20 | chr2 |
| MIMAT0016851 | hsa-miR-4299 | −2.42 | chr11 |
| MIMAT0004761 | hsa-miR-483-5p | −10.23 | chr11 |
| MIMAT0002872 | hsa-miR-501-5p | −2.38 | chrX |
| MIMAT0002877 |
hsa-miR-513a-5p | −2.13 | chrX |
| MIMAT0005788 | hsa-miR-513b | −2.49 | chrX |
| MIMAT0003299 | hsa-miR-630 | −3.86 | chr15 |
| MIMAT0003945 | hsa-miR-765 | −13.79 | chr1 |
Biological functions of putative targets
of troxerutin-specific miRNAs
To identify the biological functions of the putative
targets of miRNAs that were either up- or downregulated in response
to troxerutin, we performed GO analysis using DAVID and obtained
the GO distribution of the putative targets. As shown in Table III, GO analysis of the putative
targets of troxerutin-specific upregulated miRNAs revealed the
following distribution of biological functions: regulation of
transcription (17.5%, percentage of GO term-related gene in the
putative target pool), positive regulation of macromolecule
metabolic processes (5.5%), the regulation of programmed cell death
(5.2%) and cell cycle (5.0%). GO analysis of putative targets of
troxerutin-specific downregulated miRNAs revealed the following
distribution of biological functions: regulation of transcription
(15.9%), intracellular signaling cascades (7.6%), phosphorus
metabolic processes (5.9%), protein localization (5.5%), positive
regulation of macromolecule metabolic processes (5.4%) and the
regulation of cell proliferation (5.0%).
 | Table IIIGene Ontology (GO) analysis of
potential target genes of troxerutin-regulated miRNAs. |
Table III
Gene Ontology (GO) analysis of
potential target genes of troxerutin-regulated miRNAs.
| GO no. | GO term | Percentage of
count |
|---|
| Targets of
upregulated miRNAs |
| GO:0045449 | Regulation of
transcription | 17.5 |
| GO:0010604 | Positive regulation
of macromolecule metabolic processes | 5.5 |
| GO:0043067 | Regulation of
programmed cell death | 5.2 |
| GO:0007049 | Cell cycle | 5.0 |
| Targets of
downregulated miRNAs |
| GO:0045449 | Regulation of
transcription | 15.9 |
| GO:0007242 | Intracellular
signaling cascade | 7.6 |
| GO:0006793 | Phosphorus
metabolic processes | 5.9 |
| GO:0008104 | Protein
localization | 5.5 |
| GO:0010604 | Positive regulation
of macromolecule metabolic processes | 5.4 |
| GO:0042127 | Regulation of cell
proliferation | 5.0 |
Discussion
In the present study, we investigated the protective
effects of troxerutin against UVB radiation in nHDFs and showed
that the beneficial effects of troxerutin were mediated by miRNAs.
These findings provide new insight into the protective mechanisms
against UVB-induced damage in dermal fibroblasts. UVB radiation is
linked to the majority of skin disorders, including cancer,
photoaging, sunburn and hyperpigmentation (23). In the dermis, UVB exposure induces
disorders, such as inflammation and photoaging through cellular
senescence and apoptosis (7,24–28). Thus, protection from UVB radiation
is important to maintain the physiological functions of the
dermis.
We first demonstrated that troxerutin markedly
protected nHDFs from UVB-induced cell growth arrest and death, as
indicated by the restoration of cell viability and an increase in
the sub-G1 cell population following exposure to UVB radiation
(Figs. 1B and 2). In addition, we demonstrated that
troxerutin enhanced cell migration and increased DNA repair
activity in the nHDFs (Fig. 4).
Under the same conditions, our data indicated that troxerutin
altered the expression of 23 miRNAs by at least 2-fold in
UVB-exposed nHDFs.
The targets of several of these miRNAs have been
reported in previous studies. The overexpression of miR-602
(5.24-fold upregulation by troxerutin) has been shown to induce
proliferation and to target RASSF1A and p73, a member of the p53
family that represses proliferation and induces cell cycle arrest
(29). miR-483-5p (downregulated
10.23-fold by troxerutin) has been shown to suppress proliferation
through interference with ERK1 translation (30). miR-630 (3.86-fold downregulation
by troxerutin) has been shown to target BCL2, BCL2L2, YAP1 and
IGF-1R (31,32). BCL2 and BCL2L2, members of the
pro-apoptotic BCL-2 family, repress intrinsic apoptosis by blocking
the release of cytochrome c from the mitochondria (33). YAP1 is a co-transcription factor
that increases the expression of growth-stimulated protein
(34). Lastly, IGF-1R has been
implicated in growth factor-mediated cell growth in various cells
(31). In addition, in
cisplatin-induced apoptosis, the expression of miR-630 has been
shown to be increased by p63, a member of the p53 tumor suppressor
family (32). Thus, it is
reasonable to suggest that troxerutin mediates its UVB protective
effects through the regulation of the expression of these
miRNAs.
We then demonstrated biological function by GO
analysis of the putative targets of troxerutin-specific miRNAs
using DAVID (Table III). The
putative targets of the troxerutin-specific upregulated miRNAs were
involved in ‘regulation of transcription’, ‘positive regulation of
macromolecule metabolic processes’, ‘regulation of programmed cell
death’ and ‘cell cycle’. Targets of troxerutin-specific
downregulated miRNAs were involved in ‘regulation of
transcription’, ‘intracellular signaling cascade’, ‘phosphorus
metabolic processes’, ‘protein localization’, ‘positive regulation
of macromolecule metabolic processes’ and ‘regulation of cell
proliferation’. In particular, GO analysis revealed that the
protective effects of troxerutin against UVB-induced cell death
were mediated by alterations in the expression of miRNAs that
targeted genes within specific functional categories, including
programmed cell death, cell cycle and cell proliferation.
Overall, our study provides information on a number
of putative miRNA-target associations. Further studies are required
to mechanistically explore the involvement of these miRNAs in the
protective effects of troxerutin against UVB-induced cell damage in
nHDFs. In addition, the discovery of previously unidentified
functional associations may lead to the development of novel
therapeutic approaches in UVB-induced skin disorders.
Acknowledgements
We would like to thank all the other members of
Coreana Cosmetics Co., Ltd. for their support. This study was
supported by the KU Research Professor Program of Konkuk University
and a grant from the Ministry of Science, ICT and Future Planning
(grant no. 20110028646) of the Republic of Korea.
References
|
1
|
Brown RA, Prajapati R, McGrouther DA,
Yannas IV and Eastwood M: Tensional homeostasis in dermal
fibroblasts: mechanical responses to mechanical loading in
three-dimensional substrates. J Cell Physiol. 175:323–332. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sha W, Thompson K, South J, Baron M and
Leask A: Loss of PPARγ expression by fibroblasts enhances dermal
wound closure. Fibrogenesis Tissue Repair. 5:52012.
|
|
3
|
Johansson S, Hedman K, Kjellén L,
Christner J, Vaheri A and Höök M: Structure and interactions of
proteoglycans in the extracellular matrix produced by cultured
human fibroblasts. Biochem J. 232:161–168. 1985.PubMed/NCBI
|
|
4
|
Quan T, He T, Kang S, Voorhees JJ and
Fisher GJ: Solar ultraviolet irradiation reduces collagen in
photoaged human skin by blocking transforming growth factor-beta
type II receptor/Smad signaling. Am J Pathol. 165:741–751. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uitto J: The role of elastin and collagen
in cutaneous aging: intrinsic aging versus photoexposure. J Drugs
Dermatol. 7(Suppl 2): S12–S16. 2008.PubMed/NCBI
|
|
6
|
Yaar M and Gilchrest BA: Photoageing:
mechanism, prevention and therapy. Br J Dermatol. 157:874–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naik E, Michalak EM, Villunger A, Adams JM
and Strasser A: Ultraviolet radiation triggers apoptosis of
fibroblasts and skin keratinocytes mainly via the BH3-only protein
Noxa. J Cell Biol. 176:415–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vincent F, Deplanque G, Ceraline J, Duclos
B and Bergerat JP: p53-independent regulation of cyclin B1 in
normal human fibroblasts during UV-induced G2-arrest. Biol Cell.
91:665–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cai Y, Yu X, Hu S and Yu J: A brief review
on the mechanisms of miRNA regulation. Genomics Proteomics
Bioinformatics. 7:147–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou BR, Xu Y, Permatasari F, et al:
Characterization of the miRNA profile in UVB-irradiated normal
human keratinocytes. Exp Dermatol. 21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou BR, Xu Y and Luo D: Effect of UVB
irradiation on microRNA expression in mouse epidermis. Oncol Lett.
3:560–564. 2012.PubMed/NCBI
|
|
12
|
Li W, Zhou BR, Hua LJ, Guo Z and Luo D:
Differential miRNA profile on photoaged primary human fibroblasts
irradiated with ultraviolet A. Tumour Biol. Jul 7–2013.(Epub ahead
of print).
|
|
13
|
Mancini M, Saintigny G, Mahé C,
Annicchiarico-Petruzzelli M, Melino G and Candi E: MicroRNA-152 and
-181a participate in human dermal fibroblasts senescence acting on
cell adhesion and remodeling of the extra-cellular matrix. Aging.
4:843–853. 2012.PubMed/NCBI
|
|
14
|
Sing T, Jinnin M, Yamane K, et al:
microRNA-92a expression in the sera and dermal fibroblasts
increases in patients with scleroderma. Rheumatology. 51:1550–1556.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honda N, Jinnin M, Kajihara I, et al:
TGF-β-mediated downregulation of microRNA-196a contributes to the
constitutive upregulated type I collagen expression in scleroderma
dermal fibroblasts. J Immunol. 188:3323–3331. 2012.
|
|
16
|
An IS, An S, Park S, Lee SN and Bae S:
Involvement of microRNAs in epigallocatechin gallate-mediated UVB
protection in human dermal fibroblasts. Oncol Rep. 29:253–259.
2013.PubMed/NCBI
|
|
17
|
Lu J, Wu DM, Zheng YL, Hu B, Cheng W,
Zhang ZF and Li MQ: Troxerutin counteracts domoic acid-induced
memory deficits in mice by inhibiting CCAAT/enhancer binding
protein β-mediated inflammatory response and oxidative stress. J
Immunol. 190:3466–3479. 2013.PubMed/NCBI
|
|
18
|
Ping X, Junqing J, Junfeng J and Enjin J:
Radioprotective effects of troxerutin against gamma irradiation in
mice liver. Int J Radiat Biol. 88:607–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu J, Wu DM, Zheng ZH, Zheng YL, Hu B and
Zhang ZF: Troxerutin protects against high cholesterol-induced
cognitive deficits in mice. Brain. 134:783–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tran H, Brunet A, Grenier JM, et al: DNA
repair pathway stimulated by the forkhead transcription factor
FOXO3a through the Gadd45 protein. Science. 296:530–534. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saetrom P, Snøve O, Nedland M, et al:
Conserved microRNA characteristics in mammals. Oligonucleotides.
16:115–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Armstrong BK and Kricker A: The
epidemiology of UV induced skin cancer. J Photochem Photobiol B.
63:8–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Son ED, Shim JH, Choi H, et al: Cathepsin
G inhibitor prevents ultraviolet B-induced photoaging in hairless
mice via inhibition of fibronectin fragmentation. Dermatology.
224:352–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Ye Y, Liu W, et al: Dual effects
of silibinin treatment on autophagy-regulated dermal apoptosis
retardation and epidermal apoptosis up-regulation in UVB-induced
skin inflammation. J Asian Nat Prod Res. 14:688–699. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong MJ, Ko EB, Park SK and Chang MS:
Inhibitory effect of Astragalus membranaceus root on matrix
metalloproteinase-1 collagenase expression and procollagen
destruction in ultraviolet B-irradiated human dermal fibroblasts by
suppressing nuclear factor kappa-B activity. J Pharm Pharmacol.
65:142–148. 2013. View Article : Google Scholar
|
|
27
|
Averbeck M, Gebhardt CA, Voigt S, et al:
Differential regulation of hyaluronan metabolism in the epidermal
and dermal compartments of human skin by UVB irradiation. J Invest
Dermatol. 127:687–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meeran SM, Akhtar S and Katiyar SK:
Inhibition of UVB-induced skin tumor development by drinking green
tea polyphenols is mediated through DNA repair and subsequent
inhibition of inflammation. J Invest Dermatol. 129:1258–1270. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Ma Z, Wang D, Zhao W, Chen L and
Wang G: MicroRNA-602 regulating tumor suppressive gene RASSF1A is
overexpressed in hepatitis B virus-infected liver and
hepatocellular carcinoma. Cancer Biol Ther. 9:803–808. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Shi M, Hou S, et al: MiR-483-5p
suppresses the proliferation of glioma cells via directly targeting
ERK1. FEBS Lett. 586:1312–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Farhana L, Dawson MI, Murshed F, Das JK,
Rishi AK and Fontana JA: Upregulation of miR-150* and miR-630
induces apoptosis in pancreatic cancer cells by targeting IGF-1R.
PLoS One. 8:e610152013.
|
|
32
|
Huang Y, Chuang A, Hao H, et al:
Phospho-ΔNp63α is a key regulator of the cisplatin-induced
microRNAome in cancer cells. Cell Death Differ. 18:1220–1230.
2011.
|
|
33
|
Sasi N, Hwang M, Jaboin J, Csiki I and Lu
B: Regulated cell death pathways: new twists in modulation of BCL2
family function. Mol Cancer Ther. 8:1421–1429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao B, Kim J, Ye X, Lai ZC and Guan KL:
Both TEAD-binding and WW domains are required for the growth
stimulation and oncogenic transformation activity of yes-associated
protein. Cancer Res. 69:1089–1098. 2009. View Article : Google Scholar : PubMed/NCBI
|