Introduction
It has been reported that resveratrol
(trans-3,4′,5-trihydroxystilbene), a phytoestrogen which is found
in grape skin and red wine, has numerous beneficial effects,
including the inhibition of tumor growth (1). It affects tumor initiation,
promotion and progression through a wide range of signaling
pathways (2,3). We recently observed that resveratrol
inhibited the growth of prolactinoma and decreased the level of
prolactin through the estrogen receptor (4). Estrogen plays an important role in
prolactinoma (5), as well as in
breast cancer; therefore, prolactinoma is an estrogen-dependent
tumor (6,7). However, some antagonists of
estrogen, such as tamoxifen, cannot exert satisfactory antitumor
effects in prolactinoma as they have been shown to exert in breast
cancer (8). The mechanisms of
anti-estrogen resisitance are still poorly understood in
prolactinoma.
Autophagy is a mechanism by which a cell digests its
own damaged subcellular organelles or unfolded/misfolded/aggregated
proteins, in an attempt to maintain/restore homeostasis (9). Autophagy has been implicated in a
variety of diseases, including neurodegeneration, aging, infection,
myopathy and tumors (10).
However, the role of autophagy in tumors is quite complex and
remains somewhat controversial (11,12). Autophagy appears to play a
suppressive role during tumor development, but contributes to tumor
cell survival during cancer progression (13). Furthermore, tumor cells can use
autophagy to resist various antitumor therapies (14,15). Certain studies have demonstrated
autophagy can potentiate the resensitization of previously
anti-estrogen-resistant breast cancer cells, indicating a close
link between autophagy and antiestrogen resistance (16–18). However, the role of autophagy in
parolacinoma has not yet been clearly elucidated.
The aim of the present study was to determine the
effects of resveratrol and shed further insight into the crosstalk
between autophagy and apoptosis in resveratrol-induced cytotoxicity
in prolactinoma.
Materials and methods
Reagents and chemicals
Reagents and chemical sources were obtained from the
following manufacturers: resveratrol was purchased from Biomol
Research Laboratories, Inc., Plymouth Meeting, PA, USA; Ham’s F-10
medium was from Gibco-BRL, Carlsbad, CA, USA; fetal bovine serum
was obtained from HyClone, Logan UT, USA;
3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium bromide (MTT)
and 3-methyladenine (3-MA) were obtained from Sigma Chemical Co.,
St. Louis, MO, USA; specific inhibitors for caspase-3 (z-DEVE-fmk),
caspase-8 (z-IETD-fmk) and pan-caspase inhibitor (z-VAD-fmk) were
from BioVision, Mountain View, CA, USA; antibodies against Bcl-2,
caspase-3 and caspase-8 were from Sigma Chemical Co.; antibodies
against PARP, microtubule-associated protein 1 light chain 3 (LC3),
beclin-1, phospho-mTOR and mTOR, and donkey anti-goat
immunoglobulin G-fluorescein isothiocyanate were obtained from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; antibodies
against PI3K, Akt, phospho-Akt and p38 were from BD Biosciences,
Franklin Lakes, NJ, USA); and antibodies against ERK1/2,
phospho-ERK1/2, phospho-p38 and phospho-JNK were purchased from
Millipore Corp., Billerica, MA, USA.
Cell culture
GH3 cells are an established estrogen-responsive
cell line from rat pituitary tumor cells, which can secret
prolactin. Therefore, they are a useful model for studying the
effects of resveratrol on prolactinoma. GH3 cells were maintained
in Ham’s F-10 medium containing 12.5% horse serum, 2.5% fetal
bovine serum, 2 mmol/l L-glutamine, 0.25 μg/ml fungizone and 80
μg/ml gentamycin. The cells were plated at various densities
according to purpose and were incubated for 4 days in maintenance
medium. Before treatment, the medium was replaced with experimental
medium, which was a defined serum-free, phenol red-free medium
containing Ham’s F-12 medium with 10 μg/ml insulin, 5 μg/ml
transferrin and 0.5 ng/ml parathyroid hormone for 24 h. As a
negative control, cells were cultured in medium with serum without
treatment; as a positive control, cells were cultured in serum-free
medium without treatment; and the cells in the treatment group were
cultured in serum-free medium with 50 μM resveratrol. All cells
were cultured in a humidified chamber with 5% CO2 at
37°C.
Cell viability assays
At the end of the incubation period, cell viability
was assessed by MTT assay. Briefly, 125 μg/well of MTT were added
to the treatment wells, and 2 h later, 100 ml of developer solution
[50% v/v dimethylformamide (DMF), 20% w/v sodium dodecyl sulfate
(SDS), 0.24% v/v glacial acetic acid and 60 mM sodium acetate] were
added. The optical density at 570 nm was determined. Data are
presented as optical density or as a percentage of the control.
Analysis of apoptosis
GH3 cells were treated with the vehicle (ethanol) or
50 μM resveratrol for 48 h. After treatment, apoptosis was assessed
using the Annexin V-FITC apoptosis detection kit I (BD Biosciences
Pharmingen, San Diego, CA, USA). The cells were washed twice with
PBS, suspended in binding buffer and stained with Annexin V-FITC
and propidium iodide. Cells undergoing apoptosis were detected by
flow cytometry.
Western blot analysis
After treatment, cell lysates were harvested and
then subjected to electrophoresis on 7–12% SDS-PAGE gels.
Fractionated proteins were electrophoretically transferred onto
PVDF membranes. The incubation of the membranes with primary
antibodies (anti-caspase-3, anti-caspase-8, anti-PARP, anti-Bcl-2,
anti-LC3, anti-beclin-1, anti-mTOR, anti-PI3K, anti-Akt and
anti-ERK1/2) was carried out at 4°C. The incubation of the
membranes with horseradish peroxidase (HRP)-conjugated secondary
antibodies was carried out at room temperature for 1 h, and
proteins detected by enhanced chemiluminescence reagents (Pierce
Biotechnology, Rockford, IL, USA), as suggested by the
manufacturer. After normalization to actin, the control sample was
assigned an arbitrary value of 1, and the fold change, in response
to resveratrol treatment, was calculated.
Immunofluorescence of LC3
After being incubated with resveratrol (50 μM) for
24 h, the cells were washed with PBS and then fixed with
paraformaldehyde (4% w:v). After rinsing in PBS, the cells were
blocked with 0.1% Triton X-100 containing 1% bovine serum albumin
in PBS for 1 h. This was followed by incubation in goat polyclonal
antibody against LC3 for 24 h at 4°C in a humidified chamber.
Following 3 washes in PBS, the cells were incubated in donkey
anti-goat immunoglobulin G-fluorescein isothiocyanate for 1 h at
4°C. Finally, the cells were rinsed in PBS, coverslipped and
examined under a confocal microscope (C1si, Nikon, Tokyo,
Japan).
Electron microscopy
The cells were fixed with 2% glutaraldehyde for 2 h,
then post-fixed in 1% osmium tetroxide for 1 h. Dehydration was
carried out in increasing concentrations of ethanol followed by
dehydration in propylene oxide. While being incubated in 70%
ethanol, the pellet was stained en bloc with 1% uranyl acetate.
Finally, the pellet was embedded in Epon resin. Ultrathin sections
were post-stained with uranyl acetate and Reynold’s lead citrate
routinely. Electron micrographs were acquired using an electron
microscope (Hitachi 600, Hitachi, Tokyo, Japan).
Results
Resveratrol suppresses cell viability and
induces apoptosis in GH3 cells
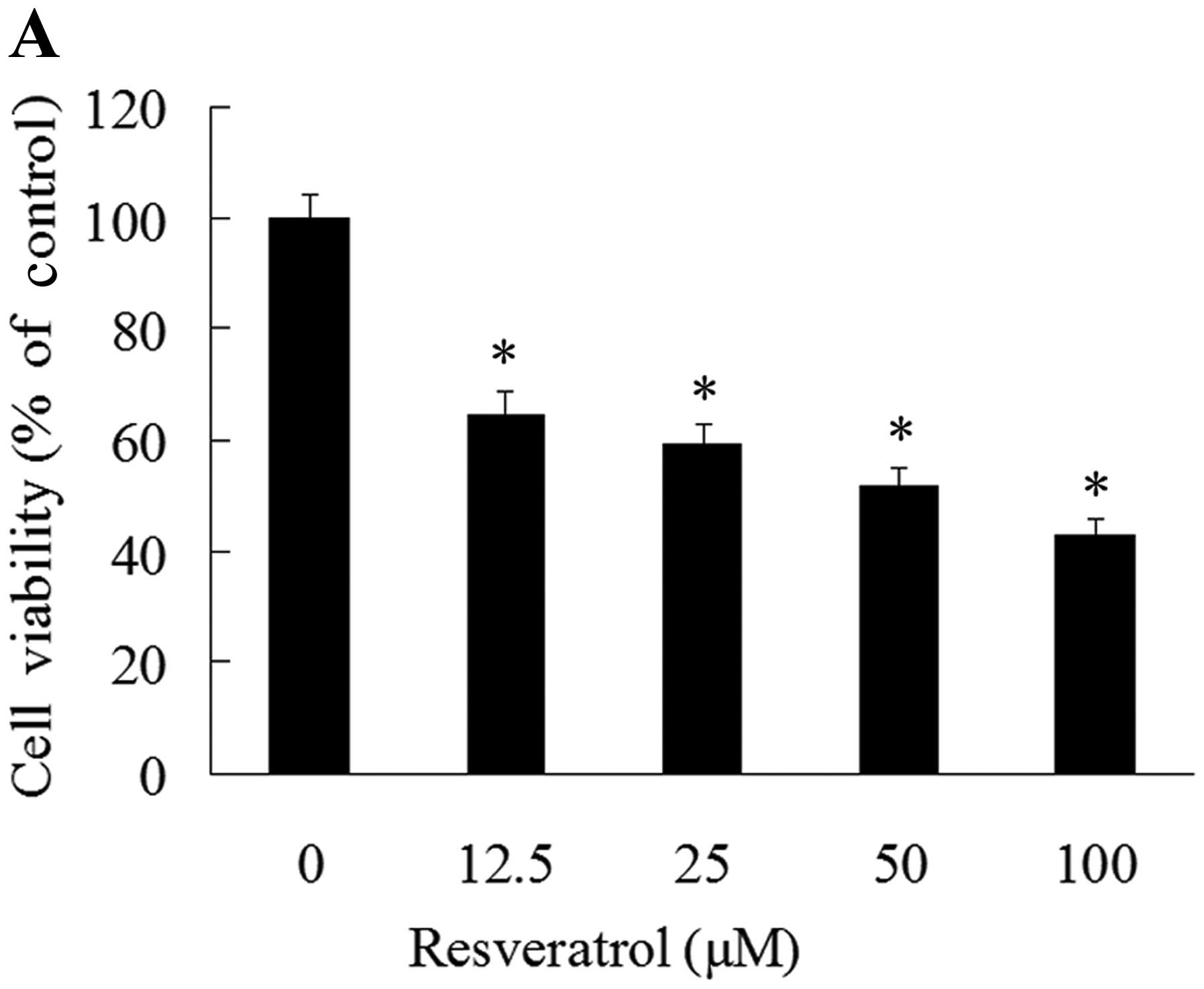
We first investigated the effects of resveratrol on
GH3 cell viability. The GH3 cells were treated with various doses
of resveratrol for 48 h, and cell viability was assessed by MTT
assay. The results revealed that resveratrol inhibited GH3 cell
viability in a dose-dependent manner. A significant inhibition
(62.2%) of cell viability was observed with 12.5 μM, and a maximal
inhibition (42.6%) was observed with 100 μM resveratrol (Fig. 1A).
In order to determine the apoptosis induced by
resveratrol, the GH3 cells were treated with resveratrol for 48 h.
Apoptosis was detected by flow cytometry. Our results revealed that
in the control (vehicle-treated) group, apoptosis was observed with
a low apoptotic ratio (Fig. 1B);
however, in the resveratrol-treated group, resveratrol increased
apoptosis in a dose-dependent manner, by approximately 2-fold in
the 50 μM resveratrol-treated group compared with the control group
(Fig. 1C-E).
Resveratrol-induced apoptosis is
caspase-dependent with a decrease in Bcl-2 expression in GH3
cells
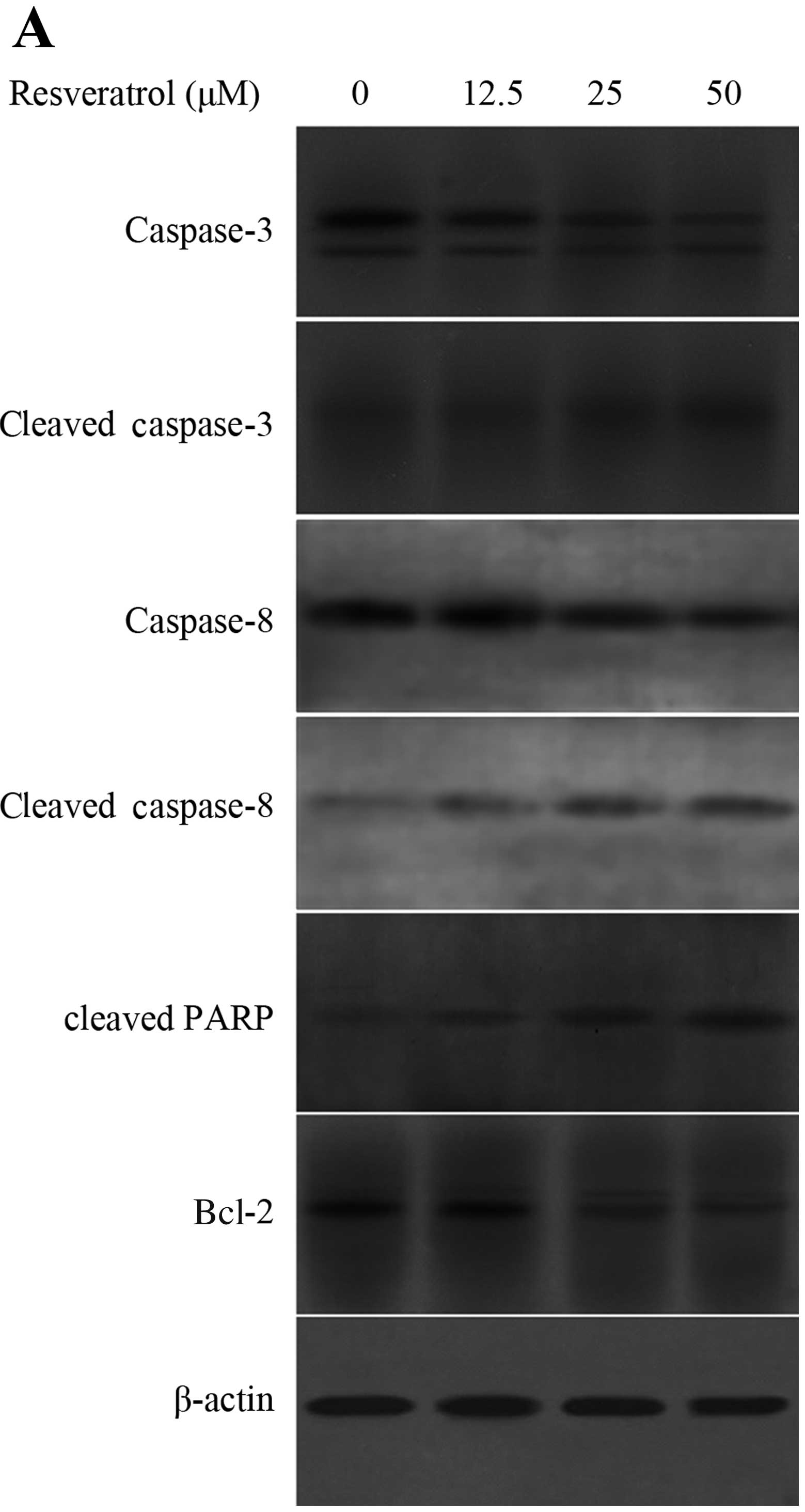
To elucidate the mechanisms behind the apoptosis
induced by resveratrol, we measured the expression levels of Bcl-2,
cleaved PARP, cleaved caspase-3 and caspase-8 following treatment
with 50 μM for 48 h. The results revealed that the levels of
cleaved PARP and cleaved caspase-3 and caspase-8 were increased in
the GH3 cells treated with resveratrol, accompanied by the
decreased expression levels of Bcl-2 (Fig. 2A). To further confirm the
involvement of caspase activation in resveratrol-induced apoptosis,
caspase-specific inhibitors were employed. We found that
pre-treatment with z-DEVE-fmk (a caspase-3-specific inhibitor),
z-IETD-fmk (a caspase-8-specific inhibitor), or z-VAD-fmk (a
pan-caspase inhibitor) effectively attenuated resveratrol-induced
cell apoptosis (Fig. 2B). Taken
together, these results confirm that resveratrol induces apoptosis
through the activation of caspase-3 and caspase-8, and by
decreasing the levels of Bcl-2.
Resveratrol induces autophagy in GH3
cells
To determine whether resveratrol induces autophagy
in GH3 cells, we observed the localization of LC3, a hallmark of
autophagosomes. There are two forms of LC3 proteins: LC3-I and
LC3-II. LC3-I is the cytoplasmic form and is converted into LC3-II,
which is the autophagosome membrane-bound form. Therefore, the
levels of LC3-II correlate with the extent of autophagosome
formation (19). LC3
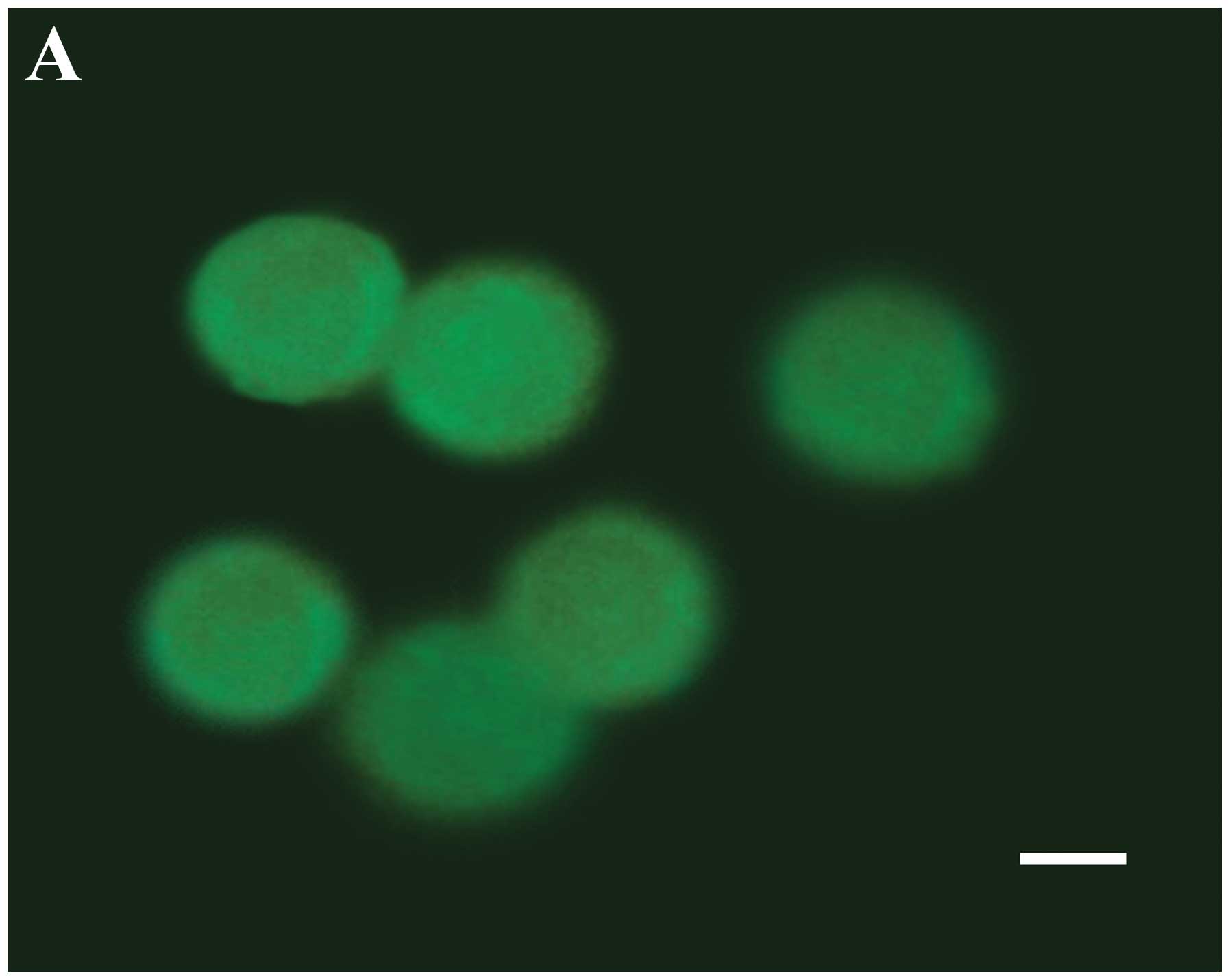
immunoreactivity was examined under a fluorescence microscope. In
the control groups, the cells showed only a diffuse distribution of
LC3-I immunoreactivity in the prensence of serum (Fig. 3A), or with a small amount of
scattered LC3-II puncta in the absence of serum (Fig. 3B). However, punctate distribution
of LC3-II immunoreactivity was detected at 48 h after resveratrol
treatment, representing the increased formation of autophagosomes
(Fig. 3C). In addition,
autophagosomes, which are double membranous vacuoles containing
engulfed cytoplasmic materials, were also observed under an
electron microscope (Fig.
3D).
Beclin-1 is required for the formation of autophagic
vesicles. It interacts with several cofactors to promote the
formation of beclin-1-Vps34-Vps15 core complexes, thereby inducing
autophagy (20). To further
confirm the occurrence of autophagy, we determined the levels of
beclin-1. We observed that the levels of beclin-1 markedly
increased following treatment with resveratrol, indicating
autophagy that was induced by resveratrol treatment (Fig. 3E).
Inhibition of autophagy increases caspase
activation and enhances apoptosis in resveratrol-treated GH3
cells
To investigate the role of autophagy in
resveratrol-induced cell death, 3-MA, an autophagy inhibitor, was
employed to detect its effects on resveratrol-induced cell death.
The results revealed that treatment with 10 mM 3-MA for 1 h prior
to treatment with resveratrol for 48 h led to a decreased level of
the resveratrol-induced LC3-II protein, as well as in increased
levels of cleaved caspase-8 and cleaved caspase-3 (Fig. 4A). Moreover, pre-treatment with
3-MA reduced cell viability (Fig.
4B) and increased apoptosis (Fig.
4C). In addition, bafilomycin A1 (BafA1), an inhibitor of
vacuolar ATPase, which prevents the fusion between lysosomes and
autophagosomes at a late stage of autophagy, was also used in the
pre-treatment. BafA1 had no significant effects on GH3 cells;
however, the resvertrol-induced cytotoxicity was greatly
potentiated by BafA1 (Fig. 4B and
C). Therefore, autophagy may play an inhibitory role in the
apoptotic process in GH3 cells following treatment with
resveratrol.
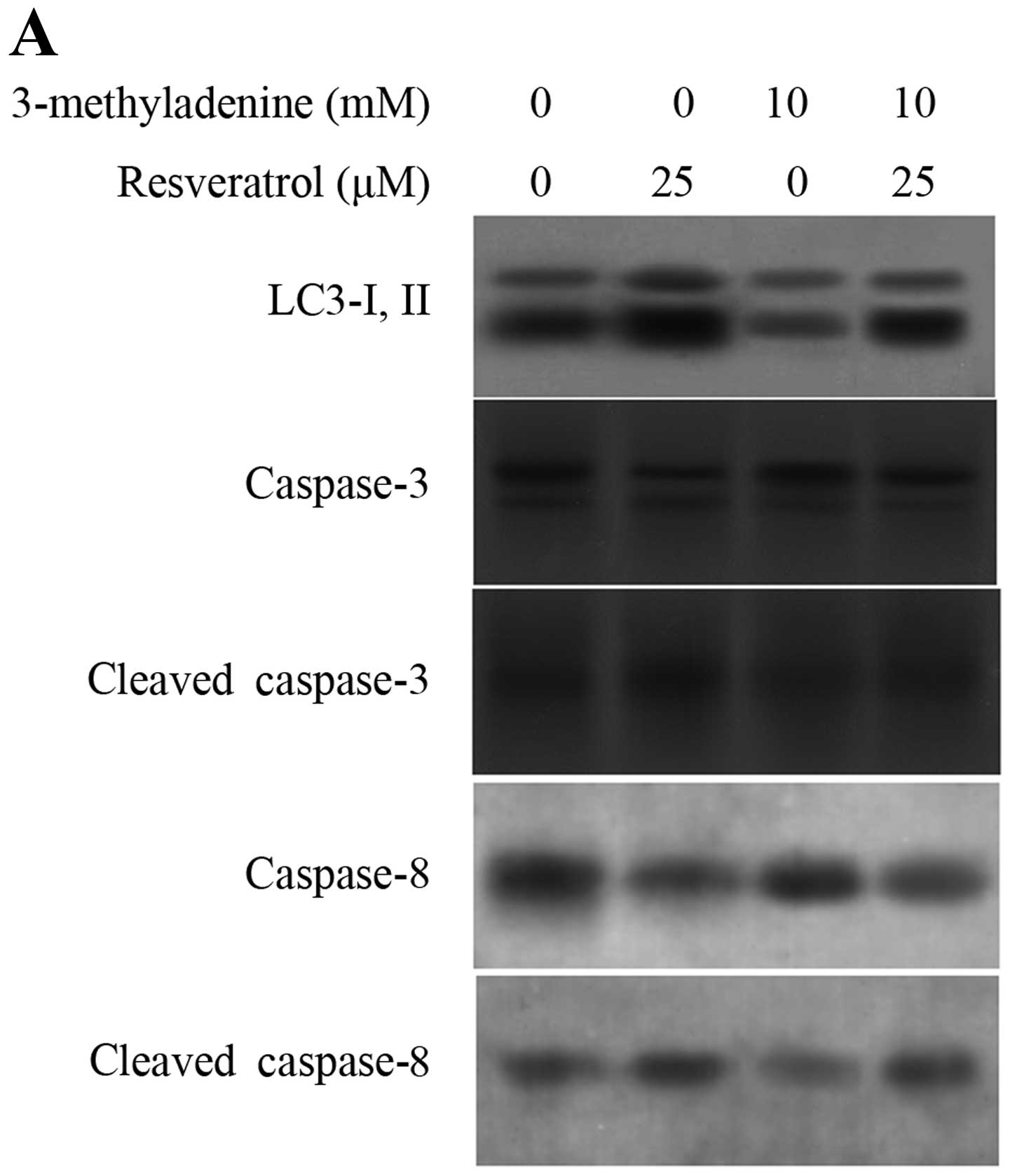 | Figure 4Autophagic inhibitiors enhance
resveratrol-induced apoptosis. Cells were pre-treated with 10 mM
3-MA or 25 nM bafilomycin A1 (BafA1) for 1 h, and then treated with
25 or 50 μM resveratrol for 48 h. (A) After treatment with 10 mM
3-MA plus resveratrol, the expression of LC3-II, cleaved caspase-3
and cleaved caspase-8 was detected by western blot analysis.
β-actin served as an internal control. (B) After treatment, cell
viability was determined by MTT assay. *p<0.05,
**p<0.05, resveratrol vs. 3-MA or BafA1 plus
resveratrol (n=3). (C) After treatment, the cells were harvested
and then subjected to quantitative analysis of cell apoptosis by
Annexin V and PI double-stained flow cytometry.
*p<0.05, **p<0.05, resveratrol vs. 3-MA
or BafA1 plus resveratrol (n=3). Control, resveratrol treatment
only. |
Induction of autophagy by resveratrol is
dependent on the regulation of the PI3K/Akt/mTOR and ERK1/2
signaling pathways in GH3 cells
Autophagy is regulated by several signaling
pathways, through which other cellular processes, such as apoptosis
or endoplasmic reticulum stress, interact with each other (21–23). To determine whether the
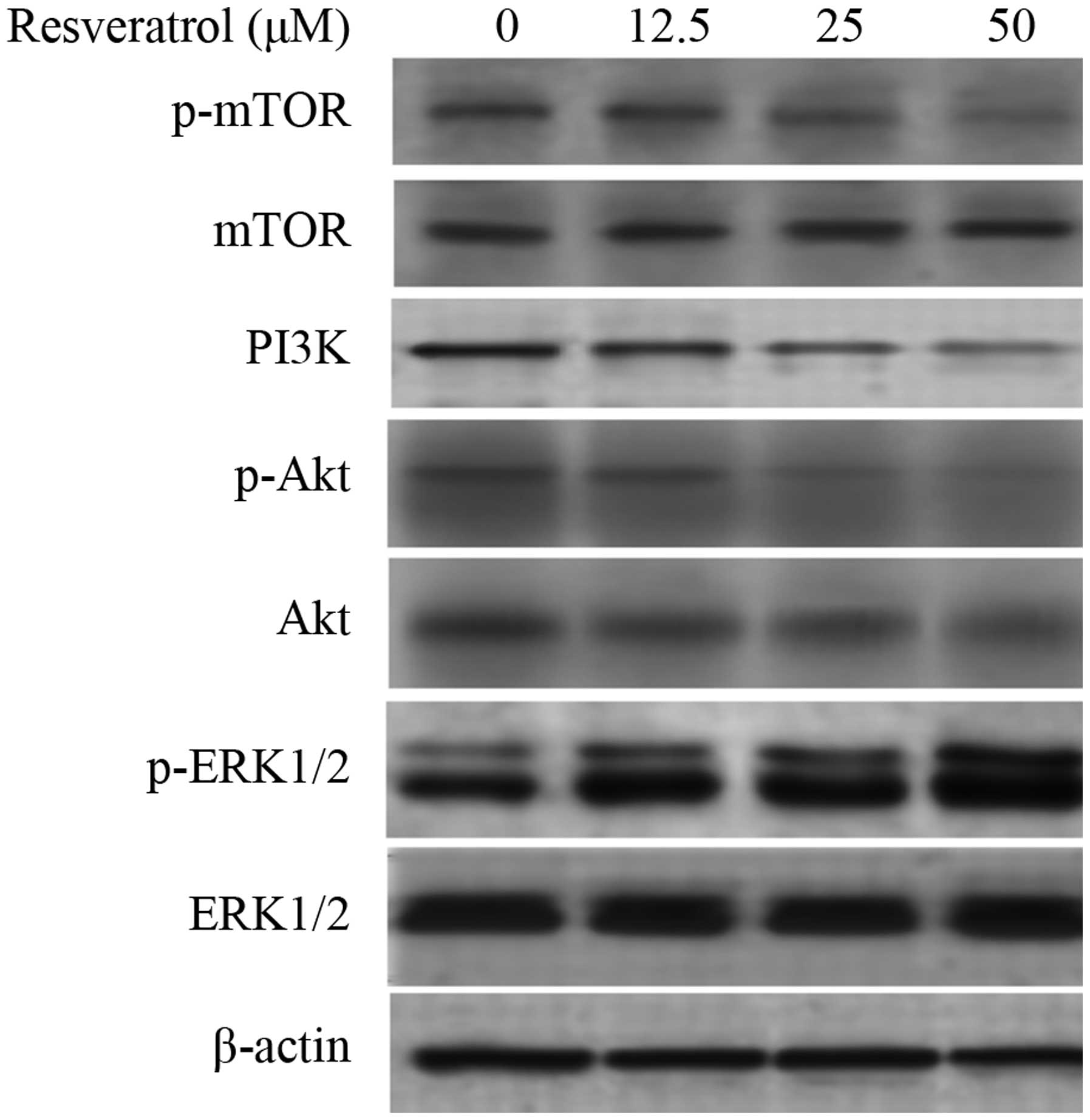
PI3K/Akt/mTOR pathway and the ERK1/2 pathway participate in the
autophagy induced by resveratrol, the expression of PI3K, Akt, mTOR
and ERK1/2 was examined. The results revealed that, after treatment
with resveratrol for 48 h, the expression of PI3K was inhibited and
the phosphorylation of Akt and mTOR was decreased. In addition, the
phosphorylation levels of ERK were upregulated (Fig. 5). These results demonstrate that
resveratrol induces autophagy through the upregulation of ERK1/2
and the downregulation of PI3K/Akt and mTOR phosphorylation.
Discussion
Resveratrol has been identified a potent antitumor
agent. A number of studies have revealed the effects of resveratrol
on cell growth, inflammation, apoptosis, angiogenesis, tumor
invasion and metastasis through multiple intracellular targets
(1–3). The present study demonstrates that
resveratrol inhibits growth and induces apoptosis in prolactinoma.
Previous studies have demonstrated that the resveratrol-induced
growth arrest is followed by apoptotic cell death (24–26). Apoptotic cells exhibit increased
caspase activity, as detected by the cleavage of the
caspase-specific substrate, PARP, that occurs at the onset of
apoptosis. Our results revealed a dose-dependent increase in the
cleaved PARP fragment, indicating caspase activation (Fig. 3A). Moreover, in the presence of
either caspase-3-, caspase-8-specific inhibitor or pan-caspase
inhibitor, the resveratrol-induced cytotoxicity was markedly
attenuated. These data clearly highlight the role of caspase
activation in resveratrol-induced cell death.
The present study demonstrates the induction of
autophagy by resveratrol in GH3 cells. It was found that following
treatment with resveratrol, LC3-I are converted into LC3-II and the
levels of beclin-1 increased. We also observed basal levels of
autophagy in the control groups. This possibly results from the
serum-free medium, which causes starvation as metabolic stress.
Moreover, prolatinoma must balance the extensive production of
prolactin with the risk that an excessive load of protein may
result in endoplasmic reticulum stress (27), which has been reported to induce
autophagy in many types of cancer (21,23). This requires further
investigation.
The contribution of autophagy to physiological
homeostasis has been previously demonstrated (28). However, an excessive level of
autophagy has been suggested to promote cell death due to the
overconsumption of critical cellular constituents; this process has
been termed ATG-dependent or type II-programmed cell death.
Therefore, the outcome of the induction of autophagy can be
variable in terms of cell fate and depends not only on the genotype
of the cell, but also on the environment (29). It is now well established that
autophagy correlates with tumorigenesis, although the exact roles
are not yet clear and in some cases, contradictory (11,12). Several anticancer agents have been
reported to induce autophagy, and there is a close correlation
between resistance to chemotherapeutic agents and autophagy
(16–18). Previous studies have suggested
that resveratrol induces autophagy, which plays a pro-survival role
in multiple cancer cell types (23,30–32). In our study, 3-MA, an autophagic
inhibitor, increased the levels of caspase-3 and caspase-8, and
enhanced the apoptosis induced by resveratrol. Another specific
inhibitor of autophagy, BafA1, increased resveratrol-induced
cytotoxicity. These findings suggest that resveratrol-induced
autophagy may represent a pro-survival mechanism in GH3 cells.
Moreover, in our previous study, we demonstrated that estrogen
receptor mediates the effects of resveratrol on GH3 cells (4). Therefore, autophagy may result in
antiestrogen resistance in prolactinoma, and agents that inhibit
autophagy may be potential candidates for combination treatment
with resveratrol.
A number of signaling pathways have been implicated
in the regulation of autophagy. Previous studies have suggested
that tge PI3K/Akt/mTOR pathway and the ERK1/2 pathway regulate
autophagy (33,34), and the important roles of these
pathways have also have been identified in pituitary tumors
(35,36). To identify the underlying
mechanisms of resveratrol-induced autophagy, the PI3K/Akt/mTOR
pathway and ERK1/2 pathway were examined. The results demonstrated
that resveratrol inhibited the activation of the PI3K/Akt/mTOR and
ERK1/2 signaling pathways (Fig.
5). The results of this study provide evidence that
resveratrol-induced autophagy is regulated by the PI3K/Akt/mTOR
pathway and ERK1/2 pathway.
Bcl-2 is an antiapoptotic protein that exhibits
oncogenic potential through its ability to regulate the apoptotic
pathway. Some compounds inhibit the proliferation of prolactinoma
by decreasing the levels of Bcl-2 (37,38). In our study, resveratrol
attenuated the expression of Bcl-2 followed by the inhibition of
proliferation. Beclin-1 was originally identified as a
Bcl-2-interacting protein, and binding of the BH3 domain of
beclin-1 inhibits PI3K activation, subsequently inhibiting
autophagy (20). In cases of
nutrient starvation, or when cells are treated with Bcl-2
inhibitors that reduce Bcl-2 protein levels, Bcl-2 and beclin-1
dissociate and autophagy is stimulated (39). In our study, we observed the
increase in beclin-1 and the decrease in Bcl-2 levels following
treatment with resveratrol. Therefore, through its effects on
beclin-1 and Bcl-2, resveratrol influences their binding, and then
regulates apoptosis and autophagy. The functional outcome of the
interaction between beclin-1 and Bcl-2 is unidirectional, and
results in the inhibition of autophagy without having a reciprocal
effect on apoptosis (40,41). Autophagy can also directly
contribute to the induction of apoptosis. Caspase-8 can be
recruited to autophagosomes, which serve as intracellular platforms
for caspases activation (40,41). In the present study, resveratrol
induced the activation of caspase-8; however, it remains to be
determined whether caspase-8 is involved in the regulation of
apoptosis by resveratrol-induced autophagy. Our present results
indicate a the crosstalk between autophagy and apoptosis, which
possibly mediates the antitumor effects of resveratrol.
In our study, resveratrol inhibited proliferation
and induced apoptosis in prolactinoma, and the PI3K/Akt/mTOR
pathway and ERK1/2 pathway were implicated in resveratrol
induced-autophagy, which plays a pro-survival role. The inhibition
of autophagy enhances resveratrol-induced caspase activation and
apoptosis. Therefore, resveratrol may act as a novel and
potential/promising antitumor agent for prolactinoma.
Acknowledgements
This study was supported partly by grants from the
Creative Talent Special Foundation of Harbin Science and Technology
Bureau, Heilongjiang Province, China (2008RFQXS090), and the
Foundation of Health Department of Heilongjiang Province
(2009-199).
References
|
1
|
Kundu JK and Surh YJ: Cancer
chemopreventive and therapeutic potential of resveratrol:
mechanistic perspectives. Cancer Lett. 269:243–261. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gusman J, Malonne H and Atassi G: A
reappraisal of the potential chemo preventive and chemotherapeutic
properties of resveratrol. Carcinogenesis. 22:1111–1117. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muqbil I, Beck FW, Bao B, Sarkar FH,
Mohammad RM, Hadi SM and Azmi AS: Old wine in a new bottle: the
Warburg effect and anticancer mechanisms of resveratrol. Curr Pharm
Des. 18:1645–1654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang C, Hu ZQ, Chu M, et al: Resveratrol
inhibited GH3 cell growth and decreased prolactin level via
estrogen receptors. Clin Neurol Neurosurg. 114:241–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heaney AP, Horwitz GA, Wang Z, Singson R
and Melmed S: Early involvement of estrogen-induced pituitary tumor
transforming gene and fibroblast growth factor expression in
prolactinoma pathogenesis. Nat Med. 5:1317–1321. 1999. View Article : Google Scholar
|
|
6
|
Li C, Sun Z, Gui S, Liu F and Zhang Y:
Effects of fulvestrant, an estrogen receptor antagonist, on MMQ
cells and its mechanism. Neuro Endocrinol Lett. 30:268–274.
2009.PubMed/NCBI
|
|
7
|
Torres-Arzayus MI, Zhao J, Bronson R and
Brown M: Estrogen-dependent and estrogen-independent mechanisms
contribute to AIB1-mediated tumor formation. Cancer Res.
70:4102–4111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SY, Ahn BT, Baik SH and Lee BL:
Tamoxifen inhibits GH3 cell growth in culture via enhancement of
apoptosis. Neurosurgery. 43:116–123. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizushima N and Komatsu M: Autophagy:
renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang J and Klionsky DJ: Autophagy and
human disease. Cell Cycle. 6:1837–1849. 2007. View Article : Google Scholar
|
|
11
|
Eskelinen EL: The dual role of autophagy
in cancer. Curr Opin Pharmacol. 11:294–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosenfeldt MT and Ryan KM: The multiple
roles of autophagy in cancer. Carcinogenesis. 32:955–956. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar
|
|
14
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grandér D and Panaretakis T: Autophagy:
cancer therapy’s friend or foe? Future Med Chem. 2:285–297.
2010.
|
|
16
|
Cook KL, Shajahan AN and Clarke R:
Autophagy and endocrine resistance in breast cancer. Expert Rev
Anticancer Ther. 11:1283–1294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan L, Motchoulski N, Danzer B,
Davidovich I, Shariat-Madar Z and Levenson VV:
Prolylcarboxypeptidase regulates proliferation, autophagy, and
resistance to 4-hydroxytamoxifen-induced cytotoxicity in estrogen
receptor-positive breast cancer cells. J Biol Chem. 286:2864–2876.
2011. View Article : Google Scholar
|
|
18
|
Schoenlein PV, Periyasamy-Thandavan S,
Samaddar JS, Jackson WH and Barrett JT: Autophagy facilitates the
progression of ERalpha-positive breast cancer cells to antiestrogen
resistance. Autophagy. 5:400–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clarke R, Cook KL, Hu R, et al:
Endoplasmic reticulum stress, the unfolded protein response,
autophagy, and the integrated regulation of breast cancer cell
fate. Cancer Res. 72:1321–1331. 2012.PubMed/NCBI
|
|
22
|
Yin JJ, Li YB, Wang Y, Liu GD, Wang J, Zhu
XO and Pan SH: The role of autophagy in endoplasmic reticulum
stress-induced pancreatic β cell death. Autophagy. 8:158–164.
2012.
|
|
23
|
Moretti L, Cha YI, Niermann KJ and Lu B:
Switch between apoptosis and autophagy: radiation-induced
endoplasmic reticulum stress? Cell Cycle. 6:793–798. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin HY, Tang HY, Davis FB and Davis PJ:
Resveratrol and apoptosis. Ann NY Acad Sci. 1215:79–88. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delmas D, Solary E and Latruffe N:
Resveratrol, a phytochemical inducer of multiple cell death
pathways: apoptosis, autophagy and mitotic catastrophe. Curr Med
Chem. 18:1100–1121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan E, Jiang S, Zhang L and Bai Y:
Molecular mechanism of apoptosis induction by resveratrol, a
natural cancer chemopreventive agent. Int J Vitam Nutr Res. 78:3–8.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zarzynska J and Motyl T: Apoptosis and
autophagy in involuting bovine mammary gland. J Physiol Pharmacol.
59:275–288. 2008.
|
|
28
|
Uchiyama Y, Shibata M, Koike M, Yoshimura
K and Sasaki M: Autophagy-physiology and pathophysiology. Histochem
Cell Biol. 129:407–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roy S and Debnath J: Autophagy and
tumorigenesis. Semin Immunopathol. 32:383–396. 2010. View Article : Google Scholar
|
|
30
|
Tang Q, Li G, Wei X, et al:
Resveratrol-induced apoptosis is enhanced by inhibition of
autophagy in esophageal squamous cell carcinoma. Cancer Lett.
336:325–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH,
Lin YF and Shih CM: Resveratrol enhances the therapeutic effect of
temozolomide against malignant glioma in vitro and in vivo by
inhibiting autophagy. Free Radic Biol Med. 52:377–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamamoto M, Suzuki SO and Himeno M:
Resveratrol-induced autophagy in human U373 glioma cells. Oncol
Lett. 1:489–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coward J, Ambrosini G, Musi E, et al:
Safingol (L-threo-sphinganine) induces autophagy in solid tumor
cells through inhibition of PKC and the PI3-kinase pathway.
Autophagy. 5:184–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai C, Zhang B, Liu X, et al: Inhibition
of PI3K/AKT/mTOR pathway enhances temozolomide-induced cytotoxicity
in pituitary adenoma cell lines in vitro and xenografted pituitary
adenoma in female nude mice. Endocrinology. 154:1247–1259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cakir M and Grossman AB: Targeting MAPK
(Ras/ERK) and PI3K/Akt pathways in pituitary tumorigenesis. Expert
Opin Ther Targets. 13:1121–1134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang QH, Xu JN, Xu RK and Pang SF:
Antiproliferative effects of melatonin on the growth of rat
pituitary prolactin-secreting tumor cells in vitro. J Pineal Res.
42:172–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leng L and Zhang Y: Effects of an estrogen
receptor antagonist on proliferation, prolactin secretion and
growth factor expression in the MMQ pituitary prolactinoma cell
line. J Clin Neurosci. 18:1694–1698. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pattingre S, Tassa A, Qu X, et al: Bcl-2
antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell.
122:927–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rubinstein AD and Kimchi A: Life in the
balance - a mechanistic view of the crosstalk between autophagy and
apoptosis. J Cell Sci. 125:5259–5268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|