Introduction
E3 ubiquitin ligase von Hippel-Lindau (VHL) has been
established as a crucial gatekeeper, inhibiting tumorigenesis
(1); VHL is inactivated in a
number of tumors through diverse mechanisms. VHL deficiency has
been recently identified as a driver of cancer metastatic
colonization (2). The
tumor-suppressive function of VHL lies in its role as a mediator of
the ubiquitin/proteasome-dependent degradation of the α-subunit of
hypoxia inducible factor (HIF) in the presence of oxygen (3,4).
The pro-metastatic effect of VHL inactivation is also ascribed to
the loss of the control of HIF and its transcriptional targets
implicated in metastasis (2).
HIF-1 is the main member of the HIF transcription
factor family, implicated in crucial aspects of cancer biology,
including cancer cell invasion, by transcriptionally activating a
number of protein-coding genes (5). Recently, non-coding microRNAs (miRs)
emerged as a new class of HIF-1-regulated targets (6). Among the HIF-1-responsive miRs,
miR-210 is the master hypoxamir ubiquitously stimulated by HIF-1 in
various cell types (7,8). Of note, miR-210 has been classified
into VHL-regulated and HIF-dependent miRs in renal cancer (9). By targeting a large spectrum of
genes, miR-210 participates in a wide range of cellular functions,
including the cell cycle, cell proliferation and apoptosis
(10–12). miR-210 has been linked to cancer
invasion and metastasis by suppressing vacuole membrane protein 1
(VMP1) expression (13).
Initially defined as an autophagy-related membrane protein highly
expressed in pancreatitis (14),
VMP1 has been established to be a negative regulator of the
cancer-relevant cell cycle, cell-cell adhesion, cell invasion and
metastasis (15).
In epithelial ovarian oncogenesis, the VHL
gene has been shown to be inactivated either by aberrant promoter
methylation (16) or by the loss
of heterozygosity of loci on the short arm of chromosome 3 (3p)
(17). HIF-1 consists of a
constitutively expressed HIF-1 β-subunit and an oxygen- and
growth-factor-regulated HIF-1 α-subunit and is overexpressed in
ovarian cancer biopsies and correlates with patient prognosis
(18). As regards miR-210, its
aberrant overexpression has been found in a variety of cancers
(19,20), apart from epithelial ovarian
cancer, in which miR-210 is exceptionally deleted (21). VMP1 has been reported to be an
inhibitor of the metastasis and proliferation of hepatocellular
carcinoma and a protective factor against invasive ductal carcinoma
(22,23). However, to date, no relation
between VMP1 and ovarian cancer has been reported. Based on these
published results, an aberrant signaling transduction initiating
from VHL inactivation to HIF-1α, miR-210 and VMP1 possibly exists
and is involved in cancer progression. To date, the mechanisms
through which VHL inactivation mediates ovarian cancer metastasis
have not been well defined. It is necessary to explore whether
there is a signaling pathway composed of VHL, HIF-1α, miR-210 and
VMP1 exerting pro-metastatic effects in ovarian cancer. Therefore,
in this study, we silenced VHL expression using siRNA in ovarian
cancer cells, examined the signaling among HIF-1α, miR-210 and
VMP1, and observed the resultant changes in cellular biology.
Materials and methods
Cell culture
The human ovarian cancer cell line, 3AO, was
obtained from the Shandong Academy of Medical Sciences (Jinan,
China). SKOV3 cells were obtained from the Shanghai Cell Bank of
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
in RPMI-1640 medium (Gibco-BRL, Grand Island, NY, USA) supplemented
with 10% newborn bovine serum in a humidified atmosphere with 5%
CO2 at 37°C. For HIF-1α inhibition, the cells were
pre-treated with 50 mM of
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1) (Sigma
Chemical Co., St. Louis, MO, USA) for 24 h before total RNA or
protein extraction.
siRNA transfection
The 3AO and SKOV3 cells were transfected with siRNA
specific to VHL (obtained from Shanghai GenePharma Co., Ltd.,
Shanghai, China) using X-tremeGENE siRNA transfection reagent
(Roche Molecular Biochemicals, Indianapolis, IN, USA) in accordance
with the manufacturer’s instructions. The siRNA sequences used to
silence the human VHL gene were as follows: siVHL-A,
5′-GUCUCAUUCUCAGAGUAAATT-3′; and siVHL-B,
5′-AACUGAAUUAUUUGUGCCAUCTT-3′. A scrambled siRNA
(5′-UUCUCCGAACGUGUCACGUTT-3′) was used in parallel experiments as a
negative control. The cells were plated onto 6-well plates and were
grown to 40–50% confluence at the time of transfection. For each
sample, 1 μg of siRNA and 5 μl of transfection reagent were
incubated in 100 μl of serum- and antibiotics-free medium for 5
min, followed by mixing the solutions together and incubating at
room temperature for a further 20 min; the resultant solution was
layered over the cells at 37°C for the indicated periods of
times.
Total RNA isolation and quantitative
reverse transcription PCR (qRT-PCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The RevertAid first-strand cDNA
synthesis kit (Thermo Fisher Scientific Inc., Rockford, IL, USA)
was used to synthesize the first-strand cDNA. Quantitative PCR was
performed on a Bio-Rad CFX-96 real-time PCR system using a
SYBR-Green Master Mix (Takara Biotechnology Co., Ltd., Dalian,
China). Thermal cycling conditions were as follows: 95°C for 30
sec, ensuing 40 cycles of 95°C for 5 sec and 60°C for 31 sec
Melting curve analysis was performed at the end of the cycles.
Relative expression levels, normalized to those of β-actin, were
calculated automatically based on the formula RQ=2−ΔΔCt.
All samples were assayed in triplicate, and 3 independent
experiments were conducted. The sequences of the primers were as
follows: VHL forward, 5′-GCAGGCGTCGAAGAG TACG-3′ and reverse,
5′-CGGACTGCGATTGCAGAAGA-3′; HIF-1α forward,
5′-ATCCATGTGACCATGAGGAAATG-3′ and reverse,
5′-TCGGCTAGTTAGGGTACACTTC-3′; and β-actin forward,
5′-TCCCTGGAGAAGAGCTACGA-3′ and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′.
Transfection with miR-210 inhibitor,
small RNA isolationand qRT-PCR
A total of 100 nM of miR-210 inhibitor provided by
RiboBio Co., Ltd. (Guangzhou, China) were transfected into the 3AO
and SKOV3 cells seeded into 6-well plates using the X-tremeGENE
siRNA transfection reagent (Roche Molecular Biochemicals) according
to the manufacturer’s instructions. A negative control was used in
parallel experiments. After 48 h of transfection, small RNA was
isolated with RNAiso as a small RNA reagent (Takara Biotechnology
Co., Ltd.) and reverse-transcribed using stem-loop reverse
transcription primers for miR-210 or U6 with the RevertAid
first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc.)
according to the manufacturer’s instructions. Quantitative PCR was
performed using SYBR-Green Master Mix (Takara Biotechnology Co.,
Ltd.). For the normalization of miR-210, snRNA U6 was used.
Western blot analysis
The cells were lysed with RIPA buffer containing
complete protease inhibitor mixture (Roche Molecular Biochemicals),
incubated for 30 min on ice before they were scraped, transferred
to microfuge tubes and centrifuged at 12,000 rpm, 4°C for 30 min.
The supernatants were collected and protein concentrations were
detected using the Bradford Protein Assay kit (Bio-Rad, Hercules,
CA, USA). Proteins were then boiled for 5 min. Following SDS-PAGE,
the proteins were transferred onto nitrocellulose membranes (Pall
Life Science, Ann Arbor, MI, USA). The membranes were blocked for
60 min at room temperature in 5% non-fan milk prior to incubation
overnight at 4°C with the following antibodies: rabbit anti-VHL
polyclonal antibody (1:1,000; Cell Signaling Technology, Beverly,
MA, USA), mouse anti-HIF-1α polyclonal antibody (1:500; Abcam Inc.,
Cambridge, MA, USA), rabbit anti-matrix metalloproteinase (MMP)2
polyclonal antibody (1:100; Epitomics, Burlingame, CA, USA), rabbit
polyclonal antibody against MMP9 (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), rabbit polyclonal antibody against VMP1
(1:1,000) and mouse anti-β-actin polyclonal antibody (1:1,000)
(both from Cell Signaling Technology). The blots were then reacted
with horseradish peroxidase (HRP)-conjugated goat
anti-mouse/anti-rabbit immunoglobulin (IgG) (Pierce, Rockford, IL,
USA) for 60 min at room temperature. The membranes were washed 5
times with Tris-buffered saline containing 0.05% Tween-20 between
incubations. An enhanced chemiluminescence kit (Santa Cruz
Biotechnology, Inc.) was used to visualize the signal of
immunoreactive bands.
Cell migration assay
Ovarian cancer cells (1×105) in 100 μl of
RPMI-1640 medium without serum were seeded into Millicell modified
Boyden chambers with an 8-μm pore size (Millipore, Bedford, MA,
USA), and were then placed in a 24-well plate containing 500 μl of
medium with 20% fetal bovine serum per well. Following 24 h of
incubation at 37°C under 5% CO2, non-migrating cells
remaining on the upper surface of the filter were removed with a
cotton swab. Migrated cells were fixed with 5% glutaric dialdehyde
and stained with Giemsa. The number of migrated cells was counted
in 5 random fields under an inverted microscope at ×200
magnification.
Statistical analysis
The SPSS18.0 statistical software package was used
for statistical analysis. All assays were independently conducted
at least 3 times and data are presented as the means ± standard
deviation (SD). Differences between groups were analyzed using the
Student’s t-test with a values of P<0.05 and P<0.01
considered to indicate statistically significant and highly
significant differences, respectively.
Results
VHL silencing facilitates ovarian cancer
cell migration
The 3AO and SKOV3 ovarian cancer cell lines, which
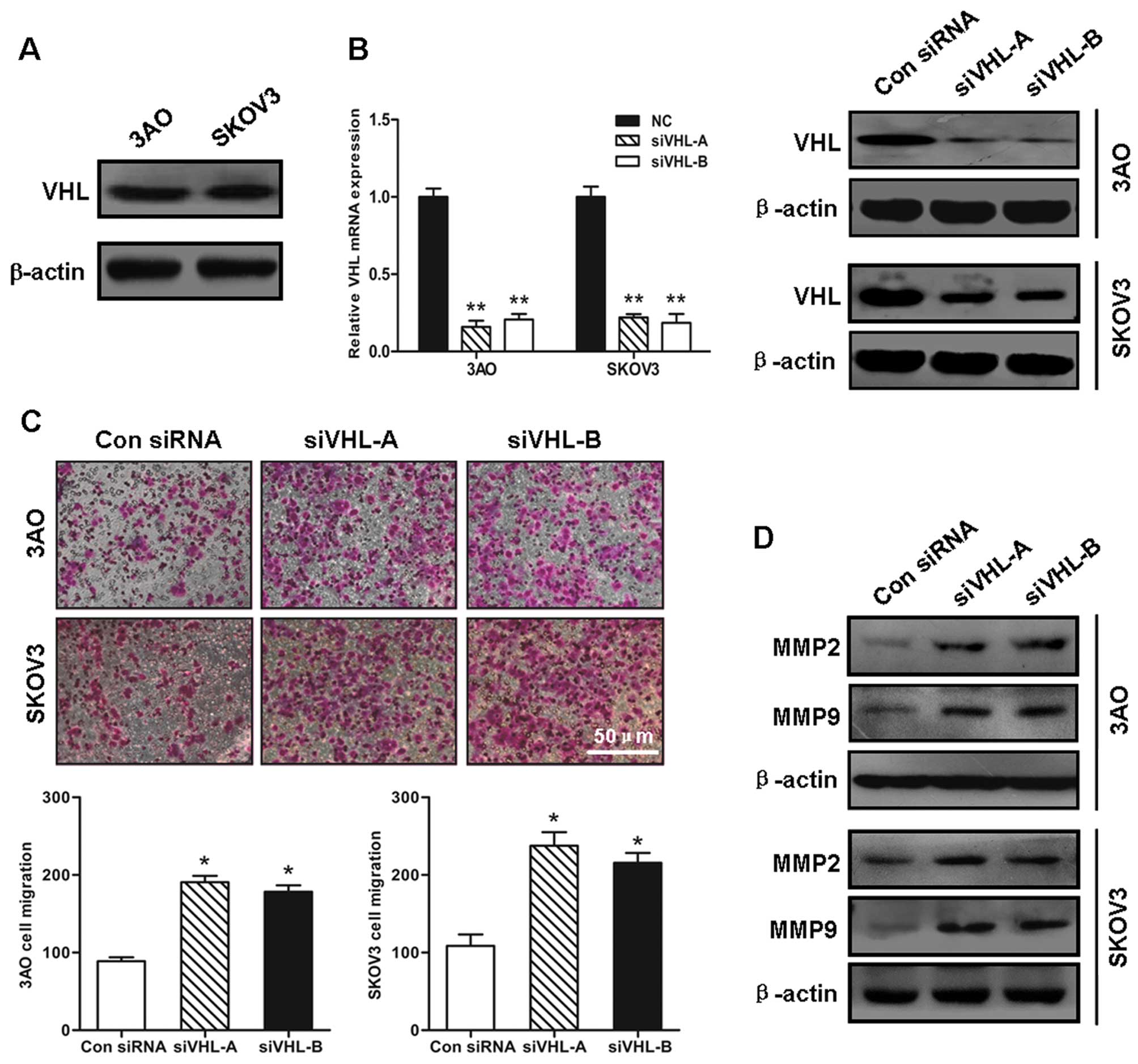
express the VHL protein (Fig.
1A), were then used to examine the effects of VHL silencing on
ovarian cancer cell migration. qRT-PCR and western blot analysis
verified that the transient transfection of the synthetic siRNAs
effectively inhibited VHL expression (Fig. 1B). The number of cells transfected
with siVHL that had migrated was 2-fold greater than that of the
cells transfected with the control siRNA (Fig. 1C). In parallel with the increase
in cell motility, the MMP2 and MMP9 protein levels were
substantially elevated when VHL was knocked down, indicating the
augmentation of invasive potential induced by the loss of VHL
(Fig. 1D).
Downregulation of VHL increases HIF-1α
and miR-210 expression
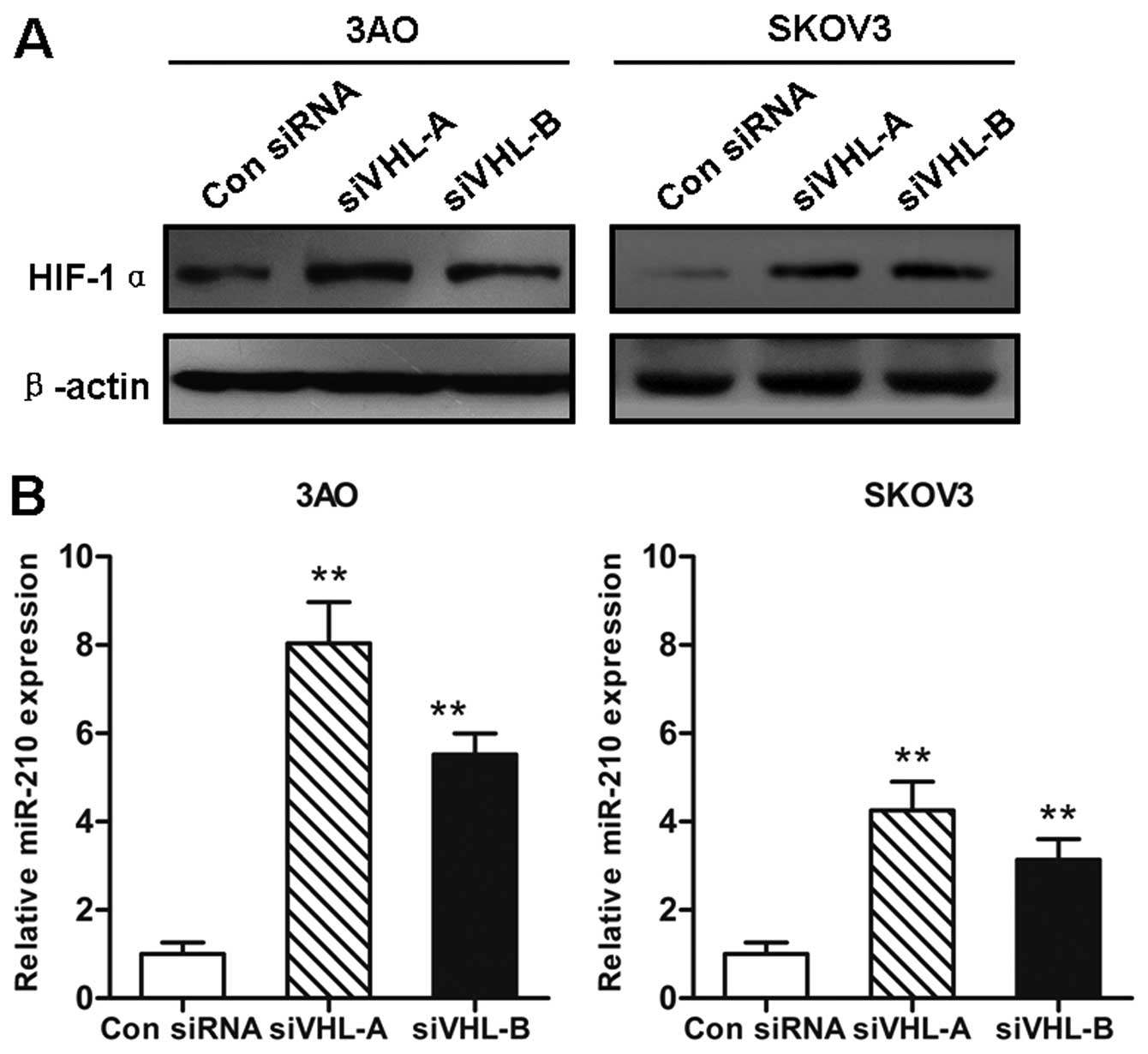
Since VHL is involved in the ubiquitination and
degradation of the HIF-1α protein, we then examined the changes in
HIF-1α protein levels following the silencing of VHL. In line with
previously published data (3),
accumulated HIF-1α protein was detected by western blot analysis in
both the 3AO and SKOV3 cells transfected with siVHL for 72 h; the
effects were more evident in the 3AO than in the SKOV3 cells
(Fig. 2A). Based on the finding
that miR-210 is a direct target of HIF-1α, the relevance between
the loss of VHL and the miR-210 expression level was further
investigated. As expected, a significant increase in the miR-210
expression level was induced by the VHL deficiency. The
augmentation in miR-210 expression in the VHL-deficient 3AO cells
was almost twice that observed in the SKOV3 cells (Fig. 2B).
HIF-1α/miR-210 mediate VHL
inactivation-induced migration
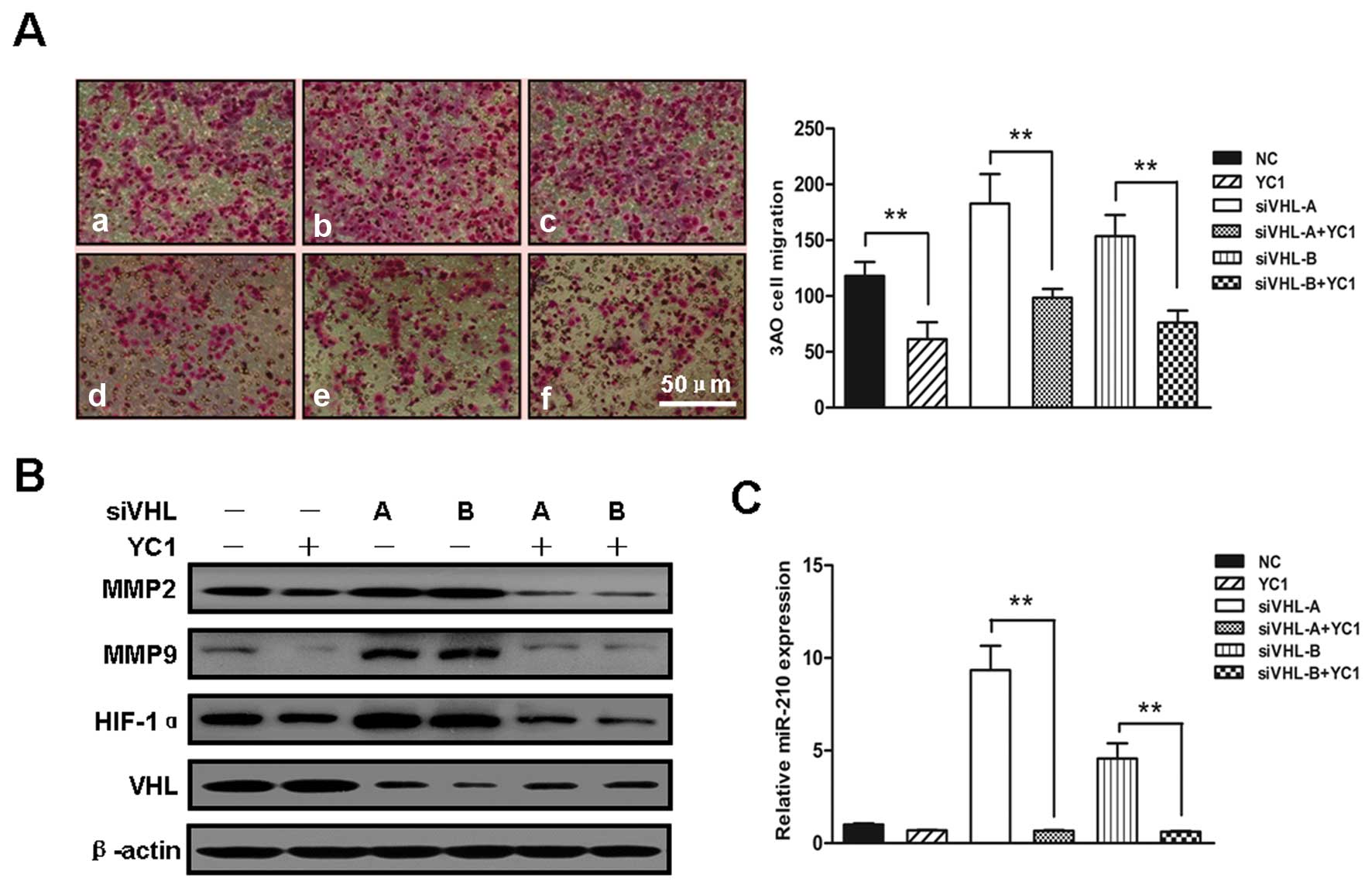
To determine the function of increased HIF-1α
expression in the enhanced cell migration induced by VHL silencing,
the VHL-silenced 3AO cells were treated with the HIF-1α inhibitor,
YC-1. In vitro cell migration assay revealed that compared
with the untreated VHL-silenced 3AO cells, treatment with 50 mM
YC-1 for 24 h decreased the number of migrated cells by almost
2-fold (Fig. 3A). The
expression-enhancing effect of VHL silencing on HIF-1α, MMP2 and
MMP9 was effectively antagonized by YC-1 (Fig. 3B). Additionally, the increase in
miR-210 expression induced by VHL silencing was greatly diminished
by YC-1 (Fig. 3C), verifying that
miR-210 is a downstream target of HIF-1α.
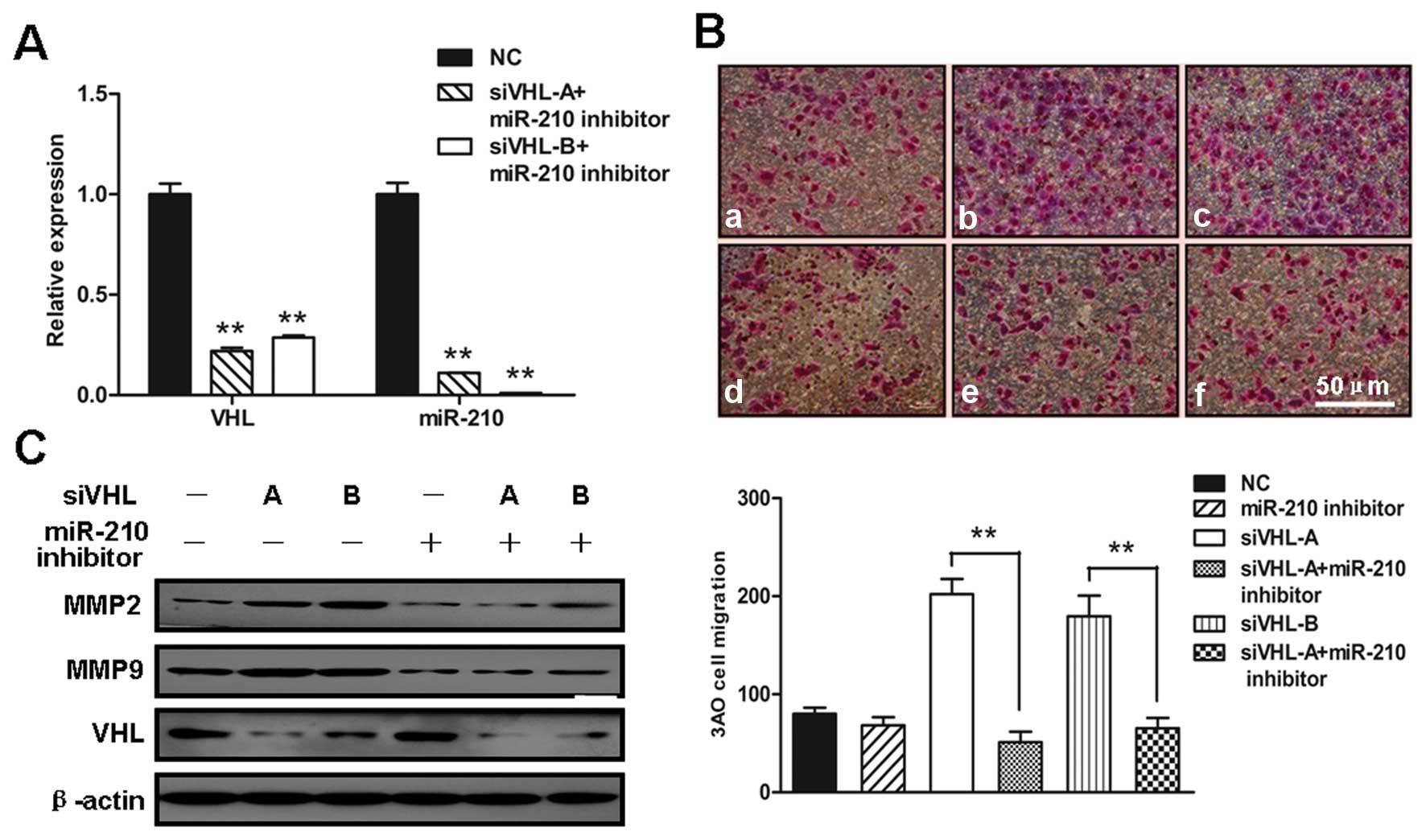
To further explore the role of upregulated miR-210
in the enhanced cell migration induced by VHL silencing, VHL and
miR-210 were simultaneously inhibited by co-transfection with siVHL
and miR-210 inhibitor into 3AO cells (Fig. 4A). The inhibition of miR-210
abolished the contribution of VHL silencing to the enhanced cell
motility (Fig. 4B) and to the
increased expression of MMP2 and MMP9 (Fig. 4C); these changes were similar to
those induced by YC-1. Of note, neither YC-1, nor miR-210 inhibitor
had an effect on VHL protein levels, indicating that HIF-1α and
miR-210 are downstream targets of VHL.
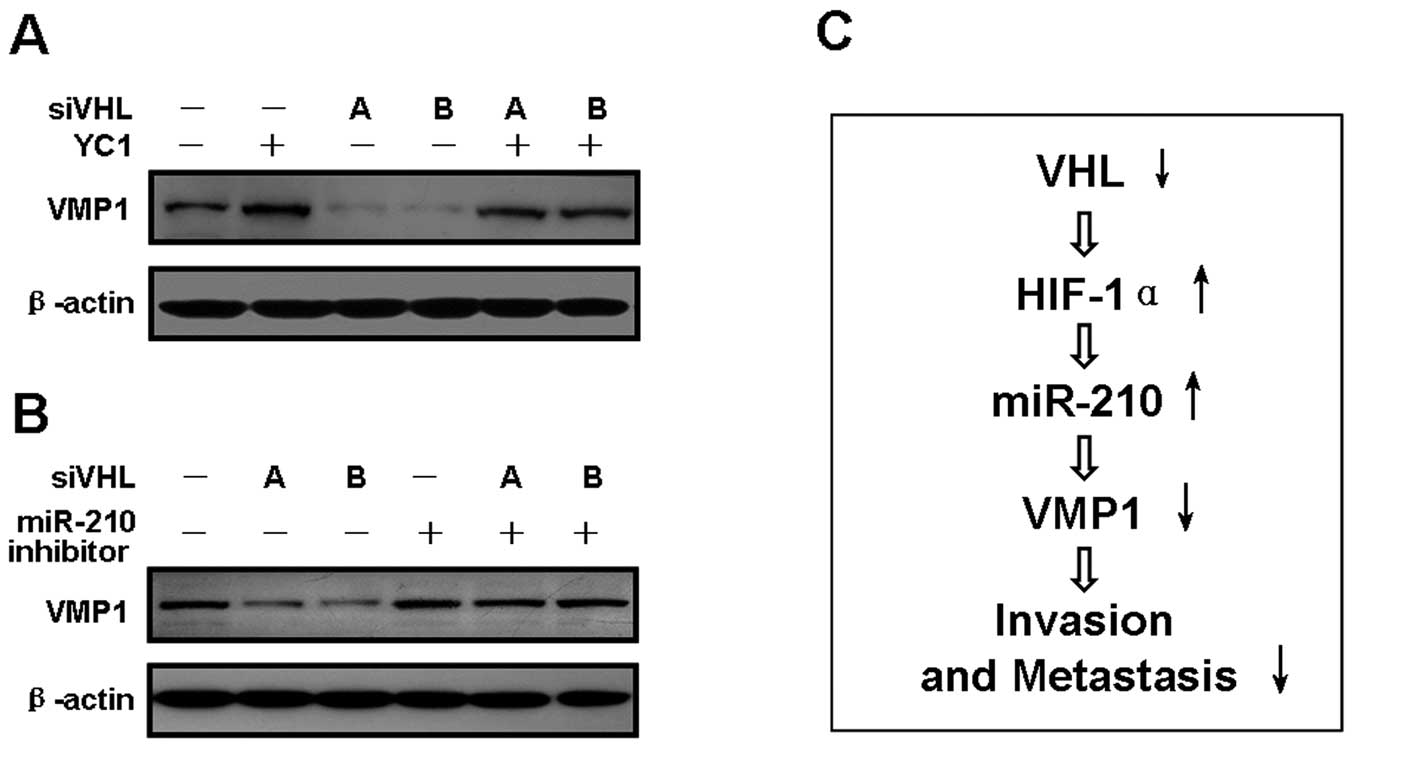
VHL/HIF-1α/miR-210 downregulate VMP1,
promoting ovarian cancer cell migration
miR-210 has been shown to mediate hypoxia-induced
hepatocarcinoma cell migration and invasion by directly targeting
VMP1 (13). Therefore, we
investigated the involvement of VMP1 in the VHL/HIF-1α/miR-210
pathway. When VHL alone was silenced, the VMP1 protein level was
substantially diminished (Fig. 5A and
B). By contrast, VMP1 protein expression was markedly augmented
with HIF-1α alone was inhibited. The suppressive effects of VHL
silencing on VMP1 expression were reversed by the YC-1 and miR-210
inhibitors (Fig. 5A and B),
indicating that VMP1 is a downstream effector of the
VHL/HIF-1α/miR-210 pathway (Fig.
5C).
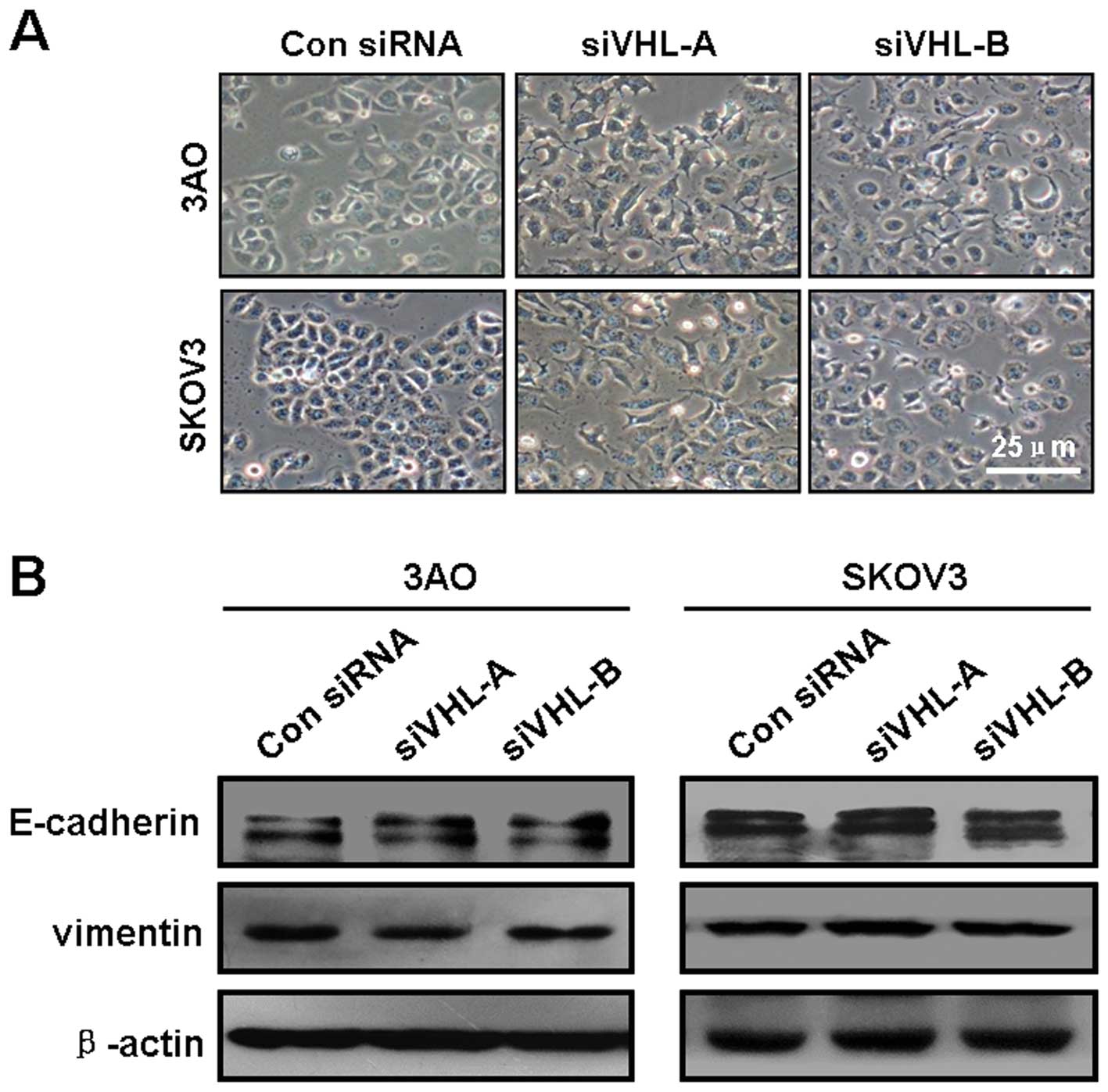
The VHL/HIF-1α/miR-210/VMP1 pathway
promotes cell motility independent of epithelial-mesenchymal
transition (EMT)
Since EMT is a critical mechanism initiating cancer
invasion and metastasis, and HIF-1α is an important mediator of
EMT, we examined the potential implication of the loss of VHL in
inducing EMT in ovarian cancer cells. When VHL was knocked down,
cell morphology did not undergo a typical EMT change from a
cobble-stone shape to a dispersed fibroblastoid shape (Fig. 6A). Consistently, the protein level
of the epithelial marker, E-cadherin, and the mesenchymal marker,
vimentin, remained unaltered when VHL was silenced (Fig. 6B). These observations suggest that
the pro-motility function of the VHL downregulation is irrelevant
to EMT.
Discussion
The VHL protein has long been characterized as an
important gatekeeper, inhibiting tumor initiation (24,25). The crucial aspect of VHL function
is to act in a ubiquitin ligase complex targeting the
prolyl-hydroxylated α-subunit of HIF-1 (26) for ubiquitination and further
proteolysis under normoxic conditions (3). During tumor development and
progression, the decrease or inactivation of VHL stabilizes the
α-subunit of HIF, which then heterodimerizes with the β-subunit and
gives rise to HIF (27). The
increase in HIF levels results in the transcriptional activation of
a number of genes, to facilitate the adaptation of angiogenesis and
cell metabolism to the hypoxic microenvironment (28). VHL has been shown to negatively
regulate tumor metastasis (29).
The anti-metastatic mechanism of VHL is realized through
controlling the degradation of the α-subunit of HIF and influencing
the transcription of its target genes associated with metastasis.
Nevertheless, the VHL/HIF-1α axis has been extensively examined in
certain types of cancer, particularly renal cell carcinoma
(30). Evidence to verify the
role of VHL/HIF-1α in ovarian cancer is limited, even though a
negative correlation has been found between the VHL expression
level and the nuclear expression of HIF-1α in ovarian clear cell
carcinomas tissues (17).
Furthermore, the detailed mechanisms through which VHL functions as
a suppressor of tumor metastasis remain to be clarified. The direct
cellular and molecular evidence provided in this study indicates
that in ovarian cancer cells, the depletion of VHL enhances cell
motility and the invasion potential by stabilizing HIF-1α
protein.
HIF modulates cellular functions by acting as a
transcription factor. Recent studies have revealed that its
transcriptional targets are not merely confined to protein-coding
genes, as several non-coding miRs have been found as a new class of
HIF-responsive molecules. Among the HIF-regulated miRs, including
miR-21 (31) and miR-373
(32), miR-210 is unique as it is
consistently upregulated by HIF in diverse cell types. An increase
in the miR-210 level has been reported in a number of tumor types
and correlates with poor prognosis (33). Exceptionally, the diminished
expression of miR-210 has been found in more than half of ovarian
cancer patients (21). However,
miR-210 cannot be simply assumed as a candidate tumor-suppressor
gene in ovarian cancer. In SKOV3 ovarian cancer cells, miR-210 has
been shown to be constantly induced by hypoxia (21,34), and is considered a crucial factor
for tumor development and progression (35,36), indicating that miR-210 may play a
certain role under a specific temporal-spatial condition without
necessarily maintaining a high level of expression. The
observations made in this study indicate that miR-210 is essential
for the deficient VHL-induced aggressiveness of ovarian cancer
cells, indicating that miR-210 potentially possesses pro-malignant
activity in ovarian cancer.
Consistent with previously published data (37), the miR-210 expression level was
found to be dependent on HIF-1α protein expression, which was
stabilized by the loss of VHL. miR-210 targets a complex set of
genes involved in a number of processes, including cell metabolism,
cell cycle, proliferation and apoptosis (8). Moreover, miR-210 has been reported
to promote the migration and invasion capability of hepatocellular
carcinoma cells by directly repressing the autophagy-associated
protein, VMP1 (13). VMP1
inhibition had been associated with the enhanced invasion and
metastatic potential of cancer cells (15). In agreement with these findings,
we found that VMP1 was a downstream effector of VHL in restraining
ovarian carcinoma malignant progression, since the loss of VHL
suppressed the VMP1 protein level in a HIF-1α/miR-210-dependent
manner.
EMT has been regarded as a significant mechanism
implicated in cancer metastasis, featured by an increase in cell
motility and invasion capability, cell morphological changes from a
cobble-stone shape to a fibroblastoid shape, a decrease in the
levels of the epithelial marker, E-cadherin, and an increase in the
levels of the mesenchymal marker, vimentin. VHL is capable of
elevating the expression of E-cadherin in clear-cell renal cell
carcinoma (34,35), and the silencing of VHL promotes
EMT in lung cancer cells (36).
However, no obvious EMT phenomena were observed after the silencing
of VHL in this study. We assumed that VHL may restrain ovarian
cancer cell metastasis independent of EMT.
In conclusion, we found that VHL suppreses the
invasion and migration capability of ovarian cancer cells by
promoting the suppressive effects of HIF-1α/miR-210 on VMP1.
Although the role of the aberrant signaling pathway composed of
VHL/HIF-1α/miR-210/VMP1 in ovarian cancer metastasis and invasion
has not been extensively defined, the results reported in this
study provided new insight into the mechanisms behind ovarian
cancer progression.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30973429).
References
|
1
|
de Paulsen N, Brychzy A, Fournier MC, et
al: Role of transforming growth factor-alpha in von Hippel-Lindau
(VHL)(−/−) clear cell renal carcinoma cell proliferation: a
possible mechanism coupling VHL tumor suppressor inactivation and
tumorigenesis. Proc Natl Acad Sci USA. 98:1387–1392. 2001.
|
|
2
|
Vanharanta S, Shu W, Brenet F, et al:
Epigenetic expansion of VHL-HIF signal output drives multiorgan
metastasis in renal cancer. Nat Med. 19:50–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kapitsinou PP and Haase VH: The VHL tumor
suppressor and HIF: insights from genetic studies in mice. Cell
Death Differ. 15:650–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gong K, Zhang N, Zhang K and Na Y: The
relationship of erythropoietin overexpression with von
Hippel-Lindau tumour suppressor gene mutations between
hypoxia-inducible factor-1α and -2α in sporadic clear cell renal
carcinoma. Int J Mol Med. 26:907–912. 2010.PubMed/NCBI
|
|
5
|
Minet E, Michel G, Remacle J and Michiels
C: Role of HIF-1 as a transcription factor involved in embryonic
development, cancer progression and apoptosis (Review). Int J Mol
Med. 5:253–259. 2000.PubMed/NCBI
|
|
6
|
Shen G, Li X, Jia YF, Piazza GA and Xi Y:
Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin.
34:336–341. 2013. View Article : Google Scholar
|
|
7
|
Chan YC, Banerjee J, Choi SY and Sen CK:
miR-210: the master hypoxamir. Microcirculation. 19:215–223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan SY and Loscalzo J: MicroRNA-210: a
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neal CS, Michael MZ, Rawlings LH, Van der
Hoek MB and Gleadle JM: The VHL-dependent regulation of microRNAs
in renal cancer. BMC Med. 8:642010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang W, Sun T, Cao J, Liu F, Tian Y and
Zhu W: Downregulation of miR-210 expression inhibits proliferation,
induces apoptosis and enhances radiosensitivity in hypoxic human
hepatoma cells in vitro. Exp Cell Res. 318:944–954. 2012.
View Article : Google Scholar
|
|
11
|
Fasanaro P, Greco S, Lorenzi M, et al: An
integrated approach for experimental target identification of
hypoxia-induced miR-210. J Biol Chem. 284:35134–35143. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuchiya S, Fujiwara T, Sato F, et al:
MicroRNA-210 regulates cancer cell proliferation through targeting
fibroblast growth factor receptor-like 1 (FGFRL1). J Biol Chem.
286:420–428. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ying Q, Liang L, Guo W, et al:
Hypoxia-inducible microRNA-210 augments the metastatic potential of
tumor cells by targeting vacuole membrane protein 1 in
hepatocellular carcinoma. Hepatology. 54:2064–2075. 2011.
View Article : Google Scholar
|
|
14
|
Ropolo A, Grasso D, Pardo R, et al: The
pancreatitis-induced vacuole membrane protein 1 triggers autophagy
in mammalian cells. J Biol Chem. 282:37124–37133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sauermann M, Sahin O, Sültmann H, et al:
Reduced expression of vacuole membrane protein 1 affects the
invasion capacity of tumor cells. Oncogene. 27:1320–1326. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozdemir F, Altinisik J, Karateke A,
Coksuer H and Buyru N: Methylation of tumor suppressor genes in
ovarian cancer. Exp Ther Med. 4:1092–1096. 2012.PubMed/NCBI
|
|
17
|
Osada R, Horiuchi A, Kikuchi N, et al:
Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible
factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian
neoplasms and allelic loss of von Hippel-Lindau gene: nuclear
expression of hypoxia-inducible factor 1alpha is an independent
prognostic factor in ovarian carcinoma. Hum Pathol. 38:1310–1320.
2007.
|
|
18
|
Semenza GL: HIF-1 and tumor progression:
pathophysiology and therapeutics. Trends Mol Med. 8(Suppl 4):
S62–S67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rothe F, Ignatiadis M, Chaboteaux C, et
al: Global microRNA expression profiling identifies MiR-210
associated with tumor proliferation, invasion and poor clinical
outcome in breast cancer. PLoS One. 6:e209802011. View Article : Google Scholar
|
|
20
|
Redova M, Poprach A, Besse A, et al:
MiR-210 expression in tumor tissue and in vitro effects of its
silencing in renal cell carcinoma. Tumour Biol. 34:481–491. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giannakakis A, Sandaltzopoulos R, Greshock
J, et al: miR-210 links hypoxia with cell cycle regulation and is
deleted in human epithelial ovarian cancer. Cancer Biol Ther.
7:255–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo L, Yang LY, Fan C, Chen GD and Wu F:
Novel roles of Vmp1: inhibition metastasis and proliferation of
hepatocellular carcinoma. Cancer Sci. 103:2110–2119. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Niu Y, Liu N, et al: Expression of
vacuole membrane protein 1 and its prognostic value in invasive
ductal carcinoma of the breast. Zhonghua Bing Li Xue Za Zhi.
42:86–89. 2013.(In Chinese).
|
|
24
|
Moch H: Von-Hippel-Lindau (VHL) protein
function by initiation and progression of renal cancer. Pathologe.
29(Suppl 2): 149–152. 2008.(In German).
|
|
25
|
Ivanov SV, Ivanova AV, Salnikow K,
Timofeeva O, Subramaniam M and Lerman MI: Two novel VHL targets,
TGFBI (BIGH3) and its transactivator KLF10, are up-regulated in
renal clear cell carcinoma and other tumors. Biochem Biophys Res
Commun. 370:536–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu F, White SB, Zhao Q and Lee FS:
HIF-1alpha binding to VHL is regulated by stimulus-sensitive
proline hydroxylation. Proc Natl Acad Sci USA. 98:9630–9635. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaelin WG Jr: The von Hippel-Lindau
protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res
Commun. 338:627–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
29
|
Zia MK, Rmali KA, Watkins G, Mansel RE and
Jiang WG: The expression of the von Hippel-Lindau gene product and
its impact on invasiveness of human breast cancer cells. Int J Mol
Med. 20:605–611. 2007.PubMed/NCBI
|
|
30
|
Choueiri TK, Fay AP, Gagnon R, et al: The
role of aberrant VHL/HIF pathway elements in predicting clinical
outcome to pazopanib therapy in patients with metastatic clear-cell
renal cell carcinoma. Clin Cancer Res. 19:5218–5226. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mace TA, Collins AL, Wojcik SE, Croce CM,
Lesinski GB and Bloomston M: Hypoxia induces the overexpression of
microRNA-21 in pancreatic cancer cells. J Surg Res. 2:855–860.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crosby ME, Kulshreshtha R, Ivan M and
Glazer PM: MicroRNA regulation of DNA repair gene expression in
hypoxic stress. Cancer Res. 69:1221–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Camps C, Buffa FM, Colella S, et al:
hsa-miR-210 is induced by hypoxia and is an independent prognostic
factor in breast cancer. Clin Cancer Res. 14:1340–1348. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Feng Y, Zhang S and Huang X: A robust
TALENs system for highly efficient mammalian genome editing. Sci
Rep. 4:36322014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Han Y, Zhang H, et al: Synthetic
miRNA-mowers targeting miR-183–96–182 cluster or miR-210 inhibit
growth and migration and induce apoptosis in bladder cancer cells.
PLoS One. 7:e522802012.PubMed/NCBI
|
|
36
|
Cui H, Grosso S, Schelter F, Mari B and
Kruger A: On the pro-metastatic stress response to cancer
therapies: evidence for a positive co-operation between TIMP-1,
HIF-1α, and miR-210. Front Pharmacol. 3:1342012.PubMed/NCBI
|
|
37
|
Huang X, Ding L, Bennewith KL, et al:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans AJ, Russell RC, Roche O, et al: VHL
promotes E2 box-dependent E-cadherin transcription by HIF-mediated
regulation of SIP1 and snail. Mol Cell Biol. 27:157–169. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esteban MA, Tran MG, Harten SK, et al:
Regulation of E-cadherin expression by VHL and hypoxia-inducible
factor. Cancer Res. 66:3567–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou Q, Chen T, Ibe JC, Raj JU and Zhou G:
Knockdown of von Hippel-Lindau protein decreases lung cancer cell
proliferation and colonization. FEBS Lett. 586:1510–1515. 2012.
View Article : Google Scholar : PubMed/NCBI
|