Introduction
Patients with cirrhosis usually have associated
portal hypertension. Esophageal variceal bleeding (EVB) is one of
the most common and dangerous complications of cirrhosis associated
with portal hypertension (1). The
majority of patients have acute onset of this disease with a large
amount of bleeding which is difficult to stop leading to critical
conditions and high mortality (2). Almost half of cirrhotic patients are
diagnosed with esophageal varices, and those with liver function
classified as Child-Pugh Class B or C are prone to a higher
incidence of esophageal varices (3). Approximately 7% of patients develop
esophageal varices each year (4,5).
The rate of initial hemorrhage is ~12% in the first year (6), the recurrence rate of hemorrhage in
1 year is ~60% (7) and mortality
within 6 weeks of bleeding remains high at up to 20% (8).
Percutaneous transhepatic variceal embolization
(PTVE) has been used since 1974 for the effective treatment of EVB
(9). However, its clinical
application is limited due to a high re-bleeding rate (10–12). Transcatheter splenic arterial
embolization can decrease portal vein blood flow and pressure, and
improve hypersplenic symptoms. However, a disadvantage of this
technique is that 73% of patients have severe complications if the
embolic volume is >70% of the vein (13). We have used the improved phased
joint intervention [PTVE + phased partial splenic embolization
(PSE)] to treat portal hypertension complicated by EVB and
hypersplenism since 2006, which avoids the weaknesses of other
therapeutic methods, thus achieving satisfactory clinical efficacy
(14). This study assessed the
clinical application of phased joint intervention by reviewing
patient data. However, the internal mechanism of this therapeutic
strategy is not yet clear.
Cirrhosis is associated with varying degrees of
splenomegaly and chronic liver disease, with the degree of
splenomegaly being positively correlated with the degree of liver
fibrosis. Immune regulation is one of the most important functions
of the spleen, therefore partial hemisection of the spleen is
conducive to the regeneration of liver cells. The initiation and
promotion of inflammation is an important factor in the progression
of liver fibrosis (15). Studies
have shown that the immune system plays an important role in the
formation of cirrhosis. Hepatic stellate cell (HSC) activation is a
key process in liver fibrosis, and Kupffer cells (KC) (16), blood-derived monocytes/macrophages
(17), natural killer (NK) cells
(18), NK T cells (NKT) (18) and T cells (19,20) synthesize and secrete cytokines,
chemokines and other chemical media that affect the
HSC-cytokine-chemokine immune microenvironment. The interplay of
various factors in this environment forms a complex network system,
affecting the process of liver fibrosis. The proinflammatory
cytokine, interleukin-17 (IL-17), and the anti-inflammatory
cytokine, IL-35, have become an important research topic in the
evaluation of liver fibrosis.
This study investigated the protein and gene
expression levels of IL-35 and IL-17 before and after
interventional embolization, and determined the possible immune
mechanisms of interventional embolization which affect the
progression of liver cirrhosis.
Materials and methods
Patient characteristics
This study retrospectively analyzed 53 cases of
liver cirrhosis complicated by portal hypertension, esophageal
variceal bleeding and hypersplenism in our hospital from October
2006 to December 2011. Portal hypertension was mainly due to
hepatic, autoimmune and alcoholic cirrhosis, and phased joint
intervention (PTVE + phased PSE) was selected as the preferred
treatment method.
In total, 53 patients, 37 males and 16 females with
an average age of 47.92±8.00 years (range, 44–71) were included.
Liver function in these patients was classified as Child-Pugh class
A in 15 cases, class B in 22 cases, and class C in 16 cases. All 53
patients had a history of ≥1 bleeding episodes and all 53 patients
had received emergency interventional treatment including 8 cases
with ascites (Table I).
 | Table IClinical features. |
Table I
Clinical features.
| Clinical
features | Values |
|---|
| No. of cases | 53 |
| Gender |
| Male | 37 |
| Female | 16 |
| Age (years) | 47.92±8.00 |
| Liver function
Child-Pugh |
| A | 15 |
| B | 22 |
| C | 16 |
| Cirrhosis
etiology |
| Hepatic
cirrhosis | 35 |
| Autoimmune
cirrhosis | 10 |
| Alcoholic
cirrhosis | 8 |
| History of
esophageal variceal bleeding | 53 |
| Hypersplenism | 53 |
Following approval by the Shanghai Changning
District Central Hospital Ethics Committee, all patients agreed to
this intervention, and the patients or their authorized family
members signed written informed consent.
Inclusion and exclusion criteria
Preoperative examinations included routine blood
examination, liver and kidney function, hepatitis virus markers,
coagulation, abdominal ultrasound, endoscopy and computed
tomography (CT). Inclusion criteria were as follows: cirrhotic
portal hypertension diagnosed by clinical and imaging examinations;
a history of >1 gastrointestinal bleeding episodes; esophageal
varices diagnosed by endoscopy in the absence of other possible
diseases resulting in upper gastrointestinal hemorrhage; and
obvious hypersplenism. Exclusion criteria were: large ascites;
combination of hepatocellular carcinoma, hepatorenal syndrome or
spontaneous peritonitis; stage III-IV hepatic encephalopathy;
portal vein thrombosis; severe cavernous transformation of portal
vein; superselective failure caused by gastric coronary vein
variation; cardiopulmonary dysfunction; total bilirubin ≥85 μmol/l;
and prolonged prothrombin time (PT) ≥6 sec.
Materials
FTC-3000 real-time polymerase chain reaction (PCR)
kit was purchased from Funglyn Biotech Inc., Scarborough, ON,
Canada. SYBR-Green real-time PCR Master Mix (code #QPK-201) was
obtained from Toyobo Bio-Technology, Co., Ltd., China. IL-35
(EBI3), IL-17 and FOXP3 primers were designed in our laboratory and
synthesized by Shanghai Generay (Shanghai, China) (Table II).
 | Table IIPrimer probe sequences. |
Table II
Primer probe sequences.
| mRNA | Sequence
(5′–3′) | Amplicon size
(bp) |
|---|
| IL-35 (EBI3) | F:
TCATTGCCACGTACAGGCTC
R: GGGTCGGGCTTGATGATGTG | 208 |
| IL-17 | F:
AGATTACTACAACCGATCCACCT
R: GGGGACAGAGTTCATGTGGTA | 151 |
| FOXP3 | F:
GTGGCCCGGATGTGAGAAG
R: GGAGCCCTTGTCGGATGATG | 238 |
| β-actin | F:
TGGAGAAAATCTGGCACCA
R: CAGGCGTACAGGGATAGCAC | 189 |
The following antibodies were used: Rabbit
anti-IL-17 polyclonal antibody (Abcam, cat. #ab79056); mouse
anti-IL-35 monoclonal antibody (R&D Systems, cat. #MAB1570);
rabbit anti-FoxP3 (D25D4) monoclonal antibody (CST, cat. #5298);
mouse anti-β-actin monoclonal antibody (Sigma, cat. #A5316); goat
anti-rabbit IgG (whole molecule)-peroxidase antibody (Sigma, cat.
#A0545); goat anti-mouse IgG (Fc specific)-peroxidase antibody
(Sigma, cat. #A0168); BCA kit: Pierce BCA Protein assay kit (Thermo
Scientific, cat. #23227); ECL reagent: Amersham ECL plus Western
Blot Detection system (GE Healthcare; San Diego, CA, USA) (cat.
#RPN2132); enzyme-linked immunosorbent assay (ELISA) kit (Uscn Life
Science Wuhan, China), the GE3100 flat C-DSA system was purchased
from GE Healthcare; and Color Doppler ultrasound diagnostic
equipment GE730 was purchased from GE Healthcare.
Phased joint embolization
Phased joint intervention (PTVE and PSE) was
utilized for which the PSE embolization range was controlled
between 30 and 40%. PSE was conducted again 3 months later with the
embolization range controlled between 30 and 40%.
Percutaneous transhepatic variceal
embolization
The right portal vein was punctured percutaneously
and transhepatically with a 2lG needle (Cook, Bloomington, IN, USA)
after the liver area was routinely sterilized and positioned. After
successful puncture, a guide wire was inserted into the superior
mesenteric vein along the needle, and then a 5F vascular sheath
(Cook) was inserted along the guide wire. The contrast agent was
injected for splenic and portal vein angiography, respectively,
under digital subtraction angiography (DSA) perspective conditions.
The catheter tip was then superselectively inserted into the
stomach coronary vein to image esophageal varices. According to DSA
development, 5–30 ml of absolute ethyl alcohol were injected for
the distal embolization of the gastric coronary vein. In the case
of slow blood flow, a spring ring of the appropriate specifications
(Cook) was used for main vein thrombosis. When the angiographic
image showed complete occlusion of the gastric coronary vein, short
gastric vein thrombosis was conducted using the same method. In the
case of fast blood flow, a steel ring of 5–10 mm diameter was
applied firstly for embolization to slow the blood flow of
obviously thickened varicose branches. In addition, some patients
required gelatin sponge particles. Absolute ethyl alcohol was then
injected slowly until the varicose vein was no longer visible. DSA
contrast was administered again to determine the effect of
embolization. The catheter tip was inserted into the splenic vein
again to determine the presence of varicose veins. If varicose
veins were observed, the same method was applied for complete
embolization. After treatment, the catheter sheath was slowly
removed from the right portal vein to ensure no bleeding. In the
case of bleeding, a gelatin sponge was used to block the needle
with a pressure dressing to ensure no bleeding for 48 h.
Partial splenic embolization
The catheter was inserted using the Seldinger method
via the femoral artery to reach the splenic hilum, the medium and
lower branch through the proximal splenic artery. DSA was used to
identify the splenic vessels and blood flow, gelatin sponge
particles were injected into the splenic artery and its branches
for embolization, splenic arteriography was conducted, and a
pressure dressing was placed on the puncture point after the
catheter was removed.
Postoperative treatment
Vital signs and abdominal conditions were closely
observed. Patients who received pressure dressings and compression
hemostasis were required to have a 24-h bed rest and measures were
taken to prevent infection and other complications.
Color doppler ultrasound
hemodynamics
B-type ultrasonic color Doppler diagnostic apparatus
with a probe frequency of 3.5 MHz was operated by appointed
personnel from the ultrasound department to determine the diameter
of the portal vein (DPV), diameter of the splenic vein (DSV),
maximum flow velocity (Vmax) and average flow velocity of veins
(V). Blood flow was calculated using the formula, Q =
(D/2)2 × π × 0.57 × Vmax × 60, as described in the study
by Moriyasu et al, when patients were in a quiet inspiratory
breath-hold position (21). These
parameters were measured three times to obtain a mean value, and
each result was double-blinded.
Assessment of efficacy
Patients underwent regular re-examinations of
routine blood measurements, liver function, gastroscopy, color
Doppler ultrasound and CT. The 6-month follow-up period included
examinations of routine blood test, liver function, complications,
and re-bleeding. In the event of gastrointestinal bleeding,
patients were seen by a doctor immediately. Postoperative
hemodynamics of the portal vein system was evaluated using
abdominal ultrasound. Postoperative indicators of liver function
included alanine transaminase (ALT), albumin (ALB), total bilirubin
(TBIL) and the PT/international normalized ratio (INR), while
indicators of hypersplenism improvement included white blood cell
(WBC) and platelet (PLT) counts. The above indicators were
determined prior to the intervention and 1, 4 (one month after
phased PSE) and 6 months after surgery. In addition, temperature
changes, abdominal pain, pleural effusion, peritonitis, splenic
abscess and other adverse reactions were assessed. The serum levels
of IL-35, IL-6 and IL-17 were analyzed by ELISA according to the
manufacturer’s instructions.
Isolation, cryopreservation and
resuscitation of peripheral blood mononuclear cells (PBMC)
When patients visited the doctor at follow up, a 5
ml sample of fresh blood was collected each time [before surgery
and 4 (one month after phased PSE) months after surgery], placed in
an ethylenediaminetetraacetic acid (EDTA) anticoagulant tube, and
sent to the laboratory within 2 h. Peripheral blood samples from 32
healthy subjects were collected and used as controls, and informed
consent forms were signed. The samples were centrifuged at 400 × g
for 5 min to obtain the plasma which was stored at −70°C. The
remaining cell pellets were mixed with 3 ml phosphate-buffered
saline (PBS) solution, added slowly to 3 ml of prepared Ficoll
lymphocyte separation medium along the pipette wall, and
transferred to a horizontal rotor at 800 × g for 15 min.
Mononuclear cells in the intermediate liquid layer were added to a
Falcon tube containing 3 ml PBS solution. The mononuclear cells
were washed at 200 × g for 10 min, 5 ml of PBS solution was added
after the supernatant was removed, washed once at 200 × g for 10
min, counted using a microscope and preserved after the supernatant
was removed. The cells in three tubes were frozen in 500 μl
RPMI-1640 culture medium containing 10% dimethyl sulfoxide (DMSO)
and 10% fetal bovine serum (FBS), placed in the freezer at −80°C
overnight, and stored in liquid nitrogen the following day.
Quantitative PCR
Total RNA was extracted using TRIzol and RNA purity
and concentration were determined using a UV spectrophotometer.
Total RNA (2 μg) was reverse transcribed into cDNA. Real-time
fluorescence was used to quantify PCR with the Toyobo ReverTra Ace
qPCR RT kit system. RT products were amplified for β-actin, EBI3,
FOXP3 and IL-17 genes. The mRNA expression and gradation in each
group were compared with a fully automatic gel imaging analysis
system after the amplified products were subjected to gel
electrophoresis. Results were corrected using β-actin. Table I shows the sequences and fragment
lengths of the PCR primers. PCR was conducted under the following
conditions: 40 cycles in total including initial denaturation at
94°C for 30 sec, denaturation at 94°C for 20 sec, annealing at 61°C
for 30 sec and extension at 72°C for 30 sec. After these cycles,
PCR was extended at 72°C for 1 min and terminated at 4°C.
Western blotting
To extract the protein, 100–400 μl of SDS lysis
solution (containing 50 mM Tris pH 8.1, 1% SDS, and inhibitors such
as sodium pyrophosphate, β-glycerophosphate, sodium orthovanadate,
sodium fluoride, EDTA and leupeptin) was added to the cells in each
tube. The cells were placed on ice in a high-speed electric
homogenizer for 1 min followed by ultrasonic cracking three times
within 20 sec, and centrifugation at 12,000 × g and 4°C for 20 min.
After centrifugation, the supernatant was transferred to a 0.5 ml
centrifuge tube and preserved at −80°C. The BCA protein assay was
used for sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE)
protein electrophoresis, transmembrane immune response, and
development and gel image analysis of EBI3, FOXP3 and IL-17.
Statistical analysis
SPSS 13.0 software was used for statistical analysis
and the main values were expressed as mean ± SD. The enumerative
data were examined using the χ2 test, the differences in
quantitative data between groups were determined by variance
analysis, and pairwise comparison was performed using the Q-test.
Correlations were analyzed by the Pearson’s correlations method,
and all statistical tests were bilateral probability tests
(α=0.05). P<0.05 was considered to indicate statistical
significance.
Results
Success rate of surgery
The patients successfully received the joint
intervention and all 53 cases underwent emergency hemostasis
resulting in an emergency hemostatic rate of 100%. Bleeding stopped
immediately after surgery following emergency hemostasis and
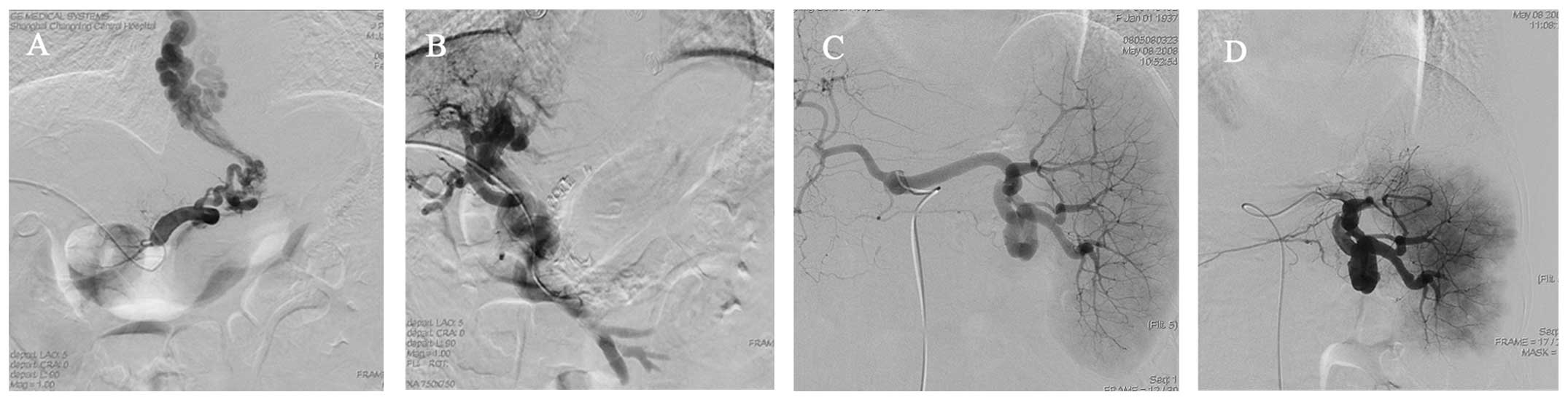
varicose veins disappeared after the intervention (Fig. 1).
Hemodynamic changes
Indicators that were significantly reduced 1 month
after PTVE + PSE included: portal vein diameter (P<0.01), blood
flow (P<0.01), mean blood flow velocity (P<0.01), splenic
vein diameter (P<0.01), blood flow of splenic vein and mean
blood flow velocity of splenic vein (P<0.01). The portal/splenic
vein diameter, portal/splenic blood flow and mean blood flow
velocity 1 month after further PSE were significantly reduced,
compared to these values 1 month after PTVE + PSE (P<0.01). All
indicators increased 6 months later, but were much lower than
preoperative levels (P<0.01) (Fig.
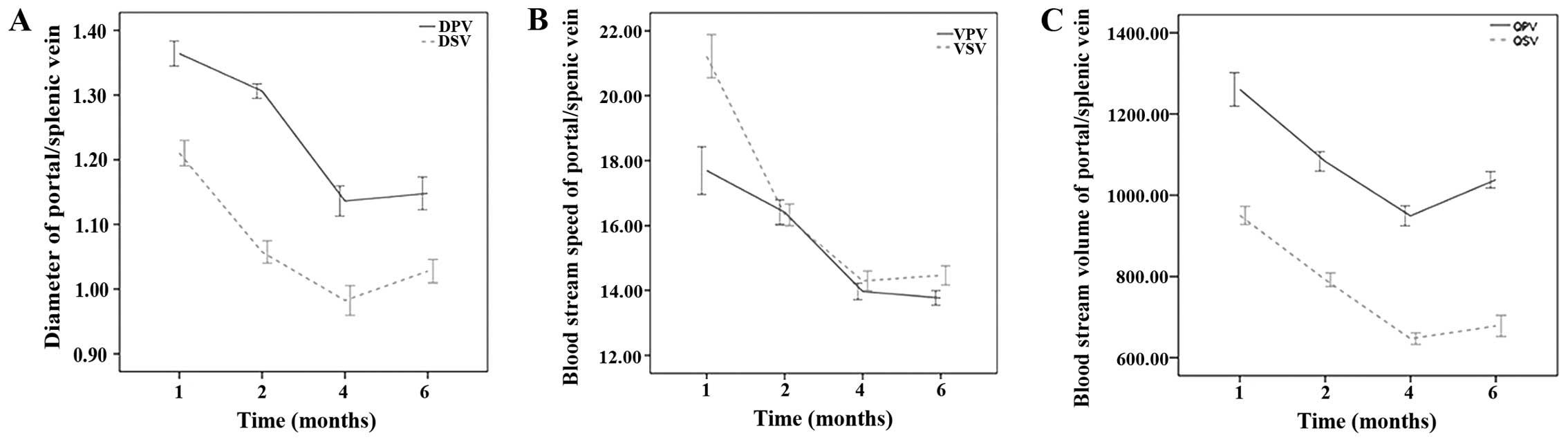
2) and showed statistically significant differences.
Compared with the preoperative levels, the following
indicators were significantly reduced 1 month after PTVE + PSE: DPV
(1.30±0.04 vs. 1.36±0.07, P=0.000) and DSV (1.06±0.06 vs.
1.21±0.07, P=0.000). The portal/splenic vein diameters 1 month
after further PSE were significantly reduced, compared to these
parameters 1 month after PTVE + PSE (DPV 1.13±0.09 vs. 1.36±0.07,
P=0.000; DSV 0.98±0.08 vs. 1.06±0.06, P=0.000). All indicators
increased 6 months later, but were much lower than the preoperative
levels (DPV 1.15±0.09 vs. 1.36±0.07, P=0.000; DSV 1.03±0.07 vs.
1.21±0.07, P=0.000) (Fig.
2A).
Compared with preoperative levels, the following
indicators were significantly reduced 1 month after PTVE + PSE: the
mean blood flow velocity of portal vein (VPV) (16.41±1.39 vs.
17.69±2.67, P=0.000) and mean blood flow velocity of splenic vein
(VSV) (16.33±1.21 vs. 21.22±2.41, P=0.000). The mean blood flow
velocity of portal/splenic veins 1 month after further PSE was
significantly reduced, compared to these levels 1 month after PTVE
+ PSE (VPV 13.96±0.93 vs. 16.41±1.39, P=0.000; VSV 14.29±1.12 vs.
16.33±1.21, P=0.000). All indicators increased 6 months later, but
were much lower than the preoperative levels (VPV 13.77±0.81 vs.
17.69±2.67, P=0.000; VSV 14.46±1.07 vs. 21.22±2.41, P=0.000)
(Fig. 2B).
Indicators that were significantly reduced 1 month
after PTVE + PSE included: quantity of blood flow in the portal
vein (QPV) (1083.46±87.84 vs. 1260.65±150.98, P=0.000) and quantity
of blood flow in the splenic vein (QSV) (792.18±60.46 vs.
950.44±80.56, P=0.000). The QPV and QSV 1 month after further PSE
were significantly reduced, compared to the levels 1 month after
PTVE + PSE (QPV 949.37±89.80 vs. 1083.46±87.84, P=0.000; QSV
646.84±51.88 vs. 792.18±60.46, P=0.000). All indicators increased 6
months later, but were much lower than the preoperative levels (QPV
1038.14±74.24 vs. 1260.65±150.98, P=0.000; QSV 678.16±94.38 vs.
950.44±80.56, P=0.000) (Fig.
2C).
Determination of blood chemistry and
liver function
Patients with obvious hypersplenism had WBC and PLT
counts below the normal reference ranges prior to the intervention
(Fig. 3). A gradual increase in
postoperative WBC and PLT counts was observed, with marked
improvement 1 and 4 months after treatment compared to the
preoperative levels, respectively (P<0.01). WBC and PLT counts
gradually decreased 6 months later, and were significantly
different (P<0.01) compared with preoperative levels. Patients
showed different degrees of liver dysfunction prior to the
intervention, which gradually improved following the intervention.
Postoperative ALB levels increased significantly, and TBIL and the
PT of INR decreased gradually. These indicators improved each month
following treatment (P<0.01). During the postoperative follow-up
period, the level of ALT did not change significantly
(P>0.05).
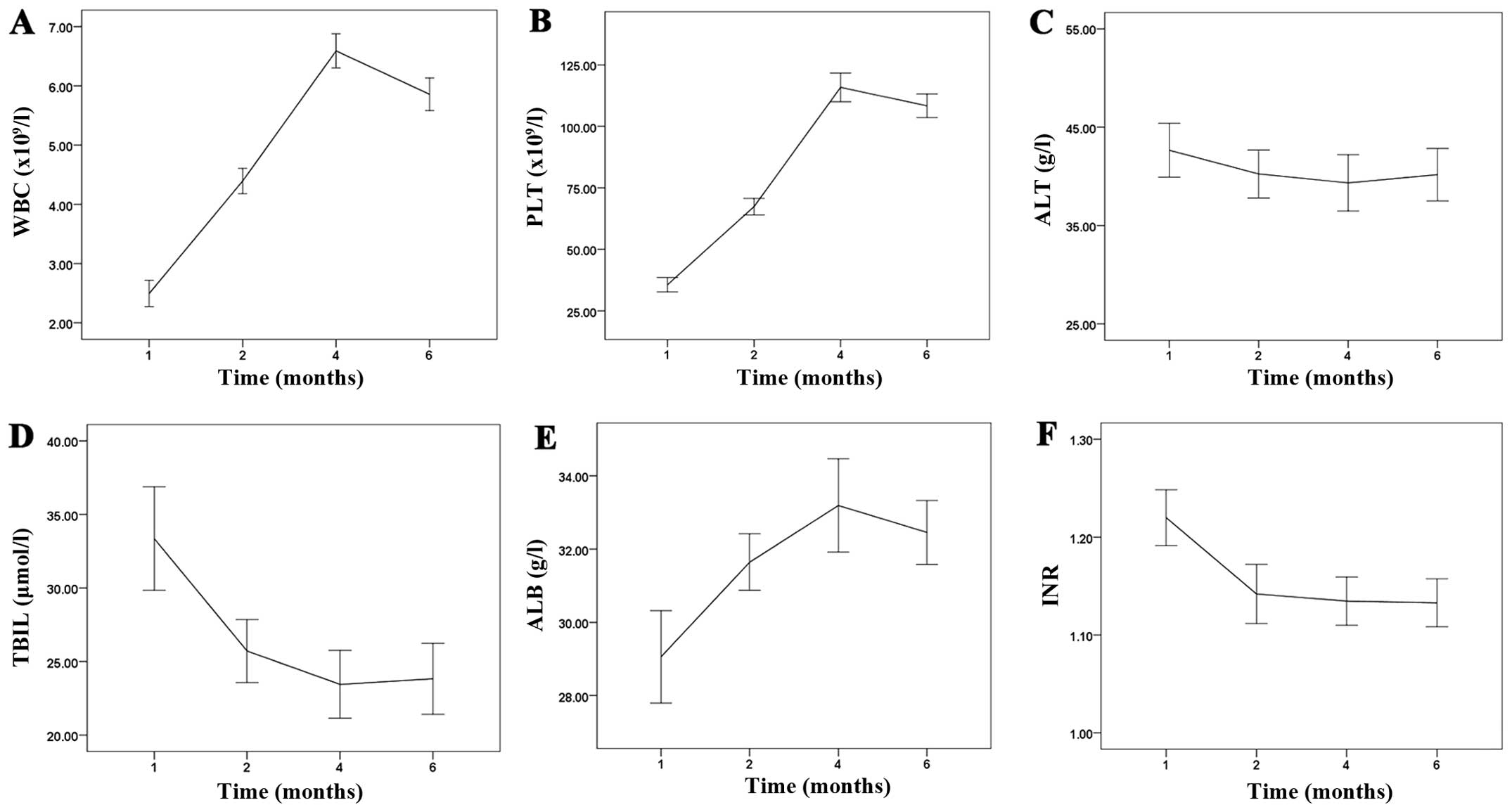 | Figure 3Changes in blood chemistry and liver
function in patients before and after treatment. (A, B and E)
Compared with the preoperative WBC and PLT count, a significant
increase was observed 1 month after PTVE + PSE which increased
further 1 month after additional PSE. The WBC and PLT counts
decreased slightly 6 months later, but were significantly increased
compared with the preoperative level. (C) The level of ALT did not
change significantly. (D and F) Compared with the preoperative TBIL
and INR levels, a significant decrease was observed 1 month after
PTVE + PSE which did not decrease 1 month after further PSE. TBIL
and INR increased slightly 6 months later, but were significantly
decreased compared with the preoperative levels. WBC, white blood
cell; PLT, platelet; TBIL, total bilirubin; ALB, albumin; INR, the
prothrombin time/international normalized ratio; ALT, alanine
transaminase. |
Compared with the preoperative WBC count, a
significant increase was observed 1 month after PTVE + PSE
(4.39±0.78 vs. 2.49±0.82, P=0.000) and increased further 1 month
after additional PSE (6.58±1.04 vs. 4.39±0.78, P=0.000). The WBC
count decreased slightly 6 months later, but was significantly
increased compared with the preoperative level (5.86±1.01 vs.
2.49±0.82, P=0.000) (Fig.
3A).
Compared with the preoperative PLT count, a
significant increase was observed 1 month after PTVE + PSE
(67.37±12.21 vs. 35.60±10.55, P=0.000) and increased further 1
month after additional PSE (115.86±21.09 vs. 67.37±12.21, P=0.000).
The PLT count decreased slightly 6 months later, but was
significantly increased compared with the preoperative level
(108.38±17.46 vs. 35.60±10.55, P=0.000) (Fig. 3B). In the postoperative follow-up
period, the level of ALT did not change significantly (P>0.05)
(Fig. 3C).
Compared with the preoperative TBIL level, a
significant decrease was observed 1 month after PTVE + PSE
(25.71±7.78 vs. 33.35±12.81, P=0.000), but did not decrease 1 month
after further PSE (23.45±8.41 vs. 25.71±7.78, P=0.22). TBIL
increased slightly 6 months later, but was significantly decreased
compared with the preoperative level (23.83±8.76 vs. 33.35±12.81,
P=0.000) (Fig. 3D).
Compared with the preoperative ALB level, a
significant increase was observed 1 month after PTVE + PSE
(31.64±2.81 vs. 29.05±4.59, P=0.001) and increased slightly 1 month
after further PSE (33.18±4.64 vs. 31.64±2.81, P=0.042). ALB
decreased slightly 6 months later, but was significantly increased
compared with the preoperative level (32.45±3.17 vs. 29.05±4.59,
P=0.000) (Fig. 3E).
Compared with the preoperative INR, a significant
decrease was observed 1 month after PTVE + PSE (1.14±0.10 vs.
1.22±0.10, P=0.000), but did not decrease 1 month after further PSE
(1.13±0.08 vs. 1.14±0.10, P=0.70). INR increased slightly 6 months
later, but was significantly decreased compared with the
preoperative level (1.13±0.09 vs. 1.22±0.10, P=0.000) (Fig. 3F).
Re-bleeding rate and survival rate
During the 6-month follow-up period, no patient
experienced re-bleeding or died.
Complications
Following phased joint interventional embolization,
32 patients showed different degrees of fever with body temperature
ranging between 37.5 and 39.4°C (including 21 cases <38.0°C and
11 cases >38.0°C), which was alleviated by symptomatic
treatment. Twenty-nine patients experienced short-term
postoperative pain and recovered following symptomatic treatment.
No serious complications such as splenic rupture, peritonitis,
pancreatitis, hepatic coma and ectopic embolism were noted.
Quantitative determination of EBI3, FOXP3
and IL-17 expression levels by PCR
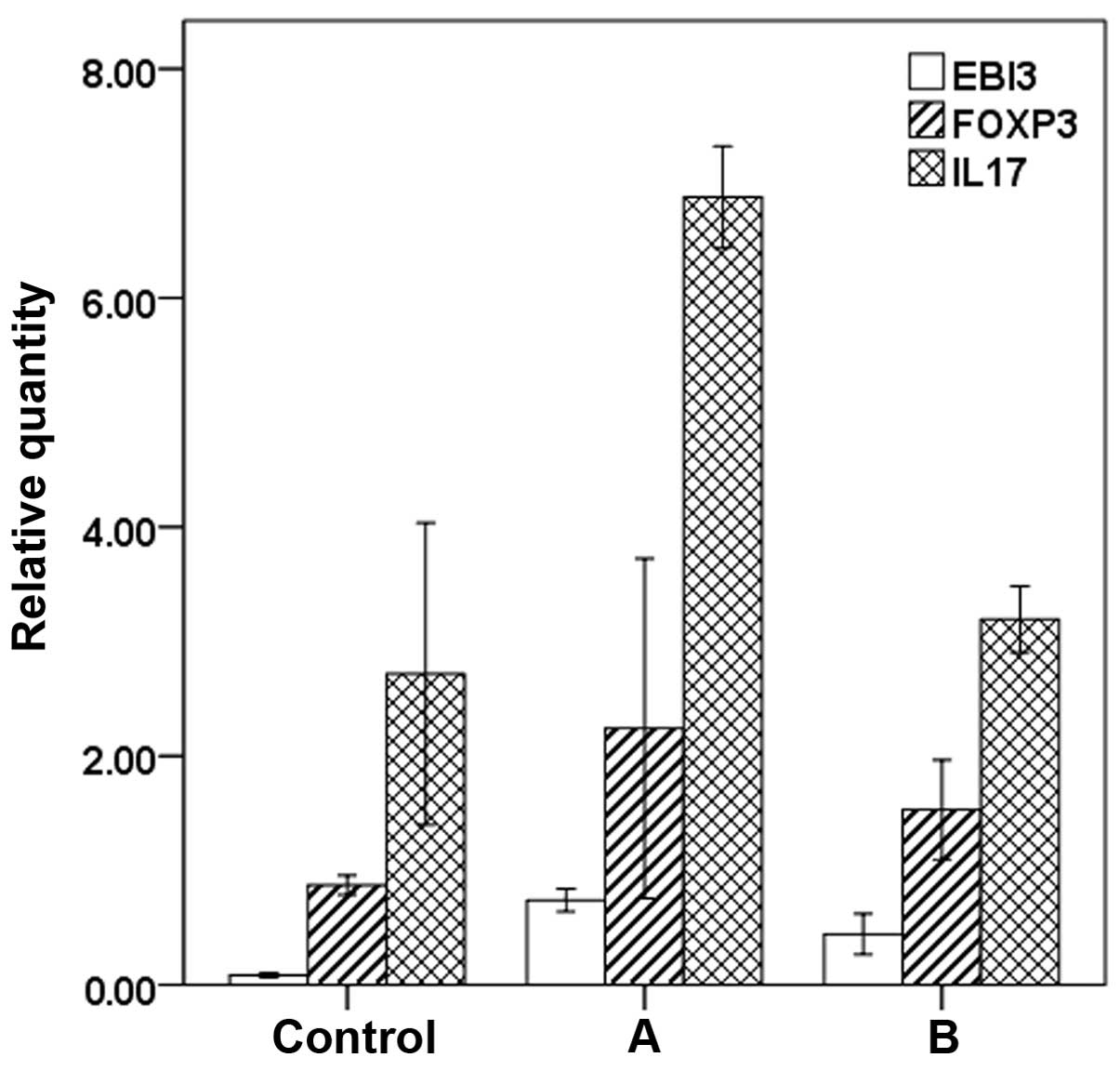
The results of RT-qPCR showed that preoperative
expression levels of EBI3 (P<0.01), FOXP3 (P<0.05) and IL-17
(P<0.01) mRNA in patients were higher than those in the normal
control group. Postoperative expression levels of EBI3 mRNA were
higher than the normal control group (P<0.01). There was no
significant difference (P>0.05) in the expression levels of
FOXP3 and IL-17 between the postoperative group and normal control
group. The expression levels of EBI3, FOXP3 and IL-17 mRNA in the
postoperative group were significantly lower than those noted
preoperatively (P<0.01) (Fig.
4).
The results of real-time RT-PCR showed that the
expression levels of EBI3, FOXP3 and IL-17 mRNA were higher in
patients undergoing phased joint interventional embolization than
in the normal control group (EBI3: pre-operation: 0.74±0.09 vs.
0.09±0.02, P=0.000; FOXP3: pre-operation: 2.24±0.74 vs. 0.87±0.07,
P=0.034 and IL-17: pre-operation: 6.88±0.38 vs. 2.71±1.14,
P=0.000). The expression levels of EBI3 mRNA were higher in
patients who underwent phased joint interventional embolization,
than in the normal control group (0.44±0.15 vs. 0.09±0.02,
P=0.005). There were no significant differences (P>0.05) in the
expression levels of FOXP3 and IL-17 between the postoperative
group and normal control group (FOXP3: post-operation: 1.53±0.37
vs. 0.87±0.07, P=0.338 and IL-17: post-operation: 3.19±0.25 vs.
2.71±1.14, P=0.443). The expression levels of EBI3, FOXP3 and IL-17
mRNA in the postoperative group were significantly lower than
preoperative levels (EBI3: 0.44±0.15 vs. 0.74±0.09, P=0.013; FOXP3:
1.53±0.37 vs. 2.24±0.74, P=0.037; IL-17: 3.19±0.25 vs. 6.88±0.38,
P=0.001).
Determination of EBI3, FOXP3 and IL-17
expression levels using western blotting
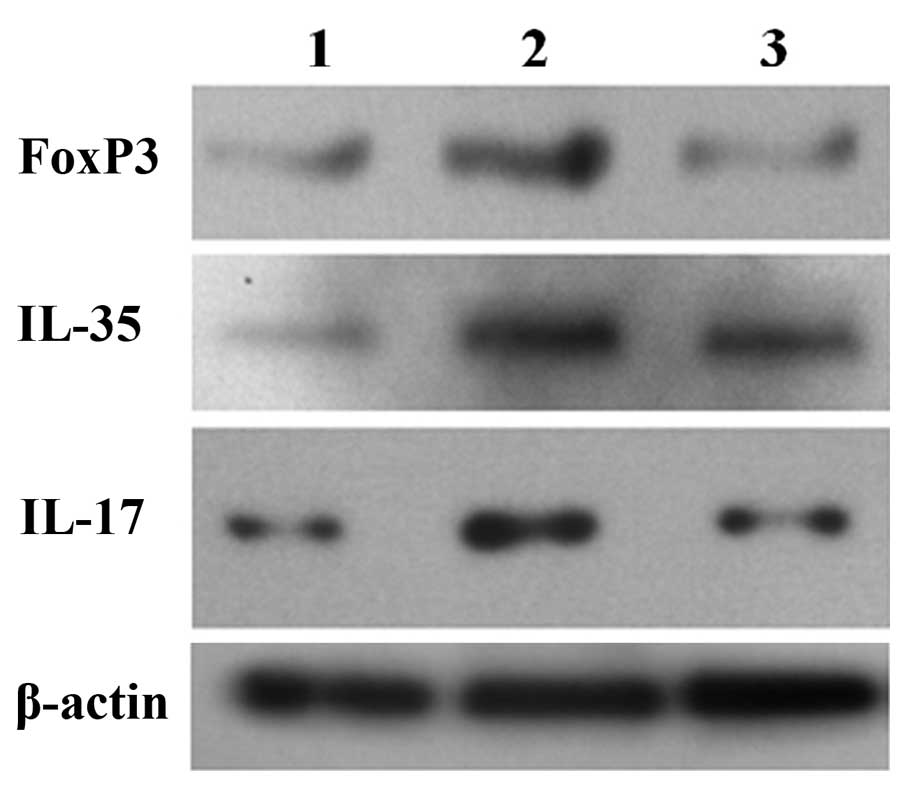
The results of western blotting showed that the
expression levels of EBI3, FOXP3 and IL-17 were higher in patients
who underwent surgery than in the normal control group before and
after phased joint interventional embolization. The expression
levels of EBI3, FOXP3 and IL-17 in the postoperative group were
significantly lower than the preoperative levels. The changes in
protein expression were consistent with mRNA expression (Fig. 5).
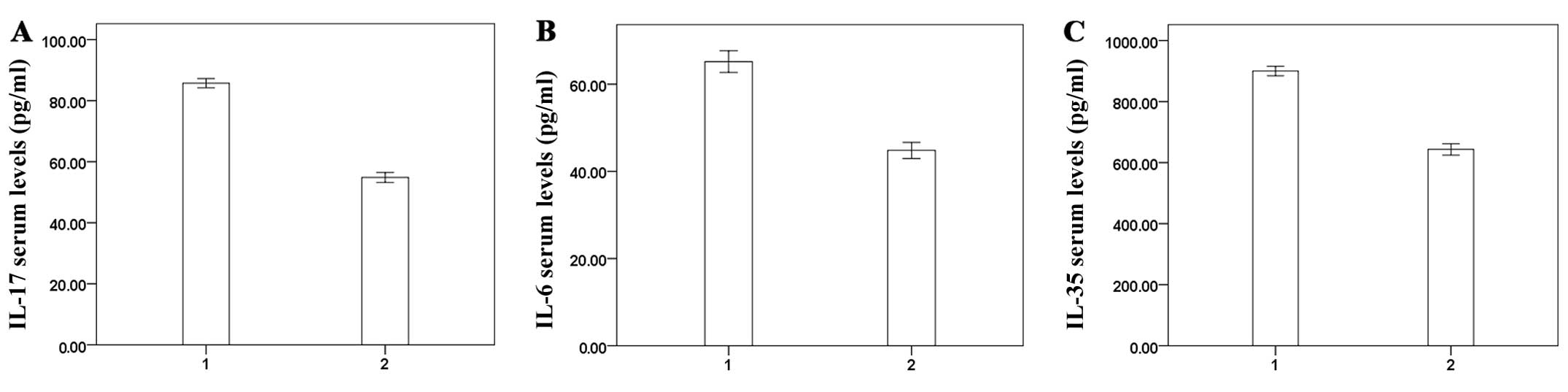
Determination of IL-17, IL-6 and IL-35
concentrations in peripheral blood by ELISA
The results of ELISA showed that the IL-17, IL-6 and
IL-35 concentrations in peripheral blood after phased joint
intervention were significantly lower than those of the
preoperative concentrations (P<0.01) (Fig. 6).
Correlation analysis
Correlation analysis of serum ALT, TBIL, ALB and INR
with concentrations of IL-17, IL-6 and IL-35 in peripheral blood
showed that serum IL-17, IL-6 and IL-35 levels were positively
correlated with TBIL and INR and negatively correlated with ALB,
the differences were statistically significant, ALT showed no
correlations (Table III).
 | Table IIICorrelation between liver function
and serum concentrations of IL-35, IL-6 and IL-17. |
Table III
Correlation between liver function
and serum concentrations of IL-35, IL-6 and IL-17.
| IL-17 | IL-6 | IL-35 |
|---|
|
|
|
|
|---|
| Factors | r | P-value | r | P-value | r | P-value |
|---|
| TBIL | 0.454 | 0.000 | 0.426 | 0.000 | 0.412 | 0.000 |
| ALB | −0.369 | 0.000 | −0.264 | 0.006 | −0.372 | 0.000 |
| INR | 0.331 | 0.001 | 0.293 | 0.002 | 0.320 | 0.001 |
| ALT | 0.174 | 0.740 | 0.130 | 0.184 | 0.120 | 0.221 |
Discussion
PTVE mainly embolizes the gastric coronary vein and
short gastric vein to block the blood flow of varicose veins and
effectively stops bleeding. It is a proven method for treating
portal hypertension and esophageal varices bleeding (9,22).
However, due to the promotion of portal vein pressure and bleeding
rate using this method, its clinical applications are limited. PSE
decreases the blood flow in the spleen and the return flow in the
splenic vein, releases high posthepatic pressure, reduces portal
blood flow and portal pressure, lowers the recurrence rate of
esophageal and gastric variceal bleeding and improves symptoms of
hypersplenism.
Findings of previous studies (23) have suggested that the hemogram
does not return to the normal level if the splenic embolization
area is <40%, while serious adverse reactions may occur if the
embolization area of the splenic artery is >80%. In previous
studies (24,25) it has been shown that in patients
with a moderately or severely enlarged spleen, at least two
embolization treatments with a single range no >50% are
effective in reducing postoperative reactions and complications.
Pålsson et al (26)
demonstrated that the embolism area correlated with increasing PLT
levels and Tajiri et al (27) observed that the significant
increase in postoperative RBC and PLT counts can be maintained 7.5
and 8 years after PSE if the degree of embolism reached ~70%. Based
on national and international research results, we conducted this
phased joint intervention using a small area of PSE each time
(30–40%) and a total embolism area of 60–80%, which not only
ensured greater efficacy, but also minimized the incidence of
complications.
This study showed that the phased joint intervention
immediately controlled bleeding in 53 patients with an emergency
hemostasis rate of 100%, and resolved or significantly improved
varices. Portal hemodynamics were improved following the phased
joint intervention with portal vein diameter, splenic vein
diameter, blood flow parameters, and mean blood flow velocity
significantly reduced compared with the preoperative levels. These
indicators increased 6 months later, but were much lower than the
preoperative levels. The WBC and PLT counts increased significantly
following this intervention, and were improved 1, 4 and 6 months
after treatment compared with preoperative counts, respectively.
Liver function after treatment was improved, with a significant
increase in ALB, and decrease in TBIL and INR. These parameters
remained improved 1, 4 and 6 months after treatment. The ideal
range of splenic artery embolization is 60–80% without serious
adverse reactions. Most patients only experience low/medium fever
with short duration.
This study confirmed the satisfactory efficacy of
joint interventional embolization. However, the immunological
mechanisms related to the improvement in liver function remain
unknown. The spleen is involved in the incidence and development of
cirrhosis (28), liver cirrhosis
is associated with varying degrees of splenomegaly, and the degree
of splenomegaly is positively correlated with liver fibrosis in
patients with chronic liver disease (29). Immune function is the most
important role of the spleen, (30) in which a variety of immune cells
and immune factors restrict and interact with each other. In portal
hypertension and spleen enlargement, hypoxia leads to changes in
the morphology and function of immune cells, and lymphocyte density
is significantly reduced, although spleen immune function is
maintained, and the total number of lymphocytes is increased when
its volume increases (31,32).
Partial hemisection of the spleen is conducive to liver cell
regeneration (33).
We suggest that phased joint embolization
intervention improves hemodynamics in the liver, retains partial
spleen function, and regulates the immune status thereby affecting
the biological behavior of HSCs. This study has examined the
effects of improved immune status following phased joint
embolization on cytokine expression.
Cell immunity mainly relies on the secretion of
various cytokines. Cytokines are important chemical mediators
synthesized and secreted by immune cells, which act on
corresponding receptors and regulate immune cell differentiation
and proliferation, simultaneously, and coordinate the body’s immune
and inflammatory response and fibrosis progression. The role of
various cytokines is not isolated, and they are synthesized and
secreted through mutual adjustment. Cytokines can regulate receptor
expression, affect the biological effects of the cytokine network
to achieve a synergistic, antagonistic or functional expansion
effect. IL-17 is mainly produced by activated Th17 cells, and as a
characterized cytokine, serum IL-17 reflects the number and
function of Th17 cells, to a certain extent (34–36). Sun et al (37) showed that increased Th17 cells in
cirrhosis patients promoted HSC activity which led to disease
progression. Additionally, Lemmers et al (38) that HSCs express IL-17R, while Wang
et al (39) and Ye et
al (40) found that IL-17
expression was elevated with an increase in liver fibrosis stage.
This may be connected with transforming growth factor-β1 (TGF-β1)
which is crucial in the development of liver fibrosis (41). The collaboration between TGF-β1
and IL-6 may initiate CD4+ T-cell rapid maturation of Th17 cells
(42–44), which then produce IL-17. IL-6
promotes chronic liver inflammation, leading to cirrhosis.
Results of the present study have demonstrated that
expression of preoperative IL-17 gene and protein was significantly
increased, and the expression levels of serum IL-17 and IL-6 were
significantly increased, which suggests that they are important in
the development of liver fibrosis. The expression levels of IL-17
mRNA and protein were significantly decreased after the
intervention, and the concentrations of IL-17 and IL-6 in
peripheral blood were significantly reduced in the postoperative
group, suggesting that phased joint embolization may regulate the
immune status, and thus reduce IL-17, TGF-β1 and IL-6 expression,
interrupting their positive feedback loop interaction, and delaying
the process of liver fibrosis.
IL-35 is a heterodimer consisting of the EB
virus-induced gene 3 protein-coding (EBI3) and IL-12 p35 subunits
(45) and belongs to the IL-12
family. IL-35 is secreted mainly by Treg cells, its primary
physiological role is to induce the formation of Th1 cells and
facilitate the proliferation of Treg cells (46). Previous studies have shown that
IL-35 can inhibit the differentiation of Th17 cells and production,
spleen cells from EBI3-deficient mice can produce more IL-17, and
purified CD4+ T cells from BALB/c mice cultured in medium coated
with CD3 and CD28 antibody, with IL-35 added at the initial stage,
resulted in significant inhibition of IL-17 generation compared
with medium alone (45). The
study found that TGF-β enhanced the pro-inflammatory response by
accelerating Th17 differentiation, and IL-35 upregulated
interferon-γ (IFN-γ), which inhibited TGF-β receptor downstream
effector Smad-3 phosphorylation in order to block TGF-β binding to
its receptor, and prevent the differentiation of Th17 cells
(36).Orphan receptor γt (RORγt)
is an important transcription factor of Th17 cell differentiation,
and EBI3 deletion increased the expression of IL-17, IL-22 and
RORγt (47). The studies of
Fontenot (48), Hori (49) and Khattri (50) demonstrated that the specific
expression of FOXP3 in Treg cells is required for Treg cell
development and function.
We found that mRNA and protein expression levels of
IL-35, FOXP3 and IL-17 were higher than those in the normal control
group before and after phased joint embolization, and serum levels
of IL-17, IL-6 and IL-35 were significantly increased, which
indicated that pro-inflammatory cytokines during cirrhosis can lead
to pathological liver damage, while increased anti-inflammatory
cytokines play a role in protecting liver cells. The mRNA
expression of IL-17 in the postoperative group was significantly
lower than that preoperatively, and both IL-35 and FOXP3 decreased
correspondingly. Serum concentrations of expressed proteins were
consistent with mRNA expression. Serum IL-35, IL-6 and IL-17 levels
were positively correlated with TBIL and INR and negatively
correlated with ALB, with the differences being statistically
significant. No correlations were observed for ALT. ALT is an
important indicator of hepatic inflammatory activation, however,
ALT is susceptible to reducing enzyme drugs and other factors, and
in some patients with active liver inflammation, ALT appears
normal, which may be a reason for there being no correlations
between serum cytokines and ALT observed in this study.
This preliminary study shows that phased joint
embolization can treat esophageal varices, improve hypersplenism
symptoms and liver function, effectively reduce complications,
improve liver hemodynamics, retain partial spleen function which
may regulate immune status, and have an impact on the expression of
cytokines, which affects the biological behavior of HSCs.
This may have a significant impact on the activation
and proliferation of HSCs and on expression of the extracellular
matrix. The present study has shown that phased joint embolization
reduced the protein and mRNA expression levels of IL-35, FOXP3 and
IL-17, and inhibited the generation of IL-17, IL6, IL-35 and other
cytokines, thus inhibiting the activation and proliferation of HSC,
improving liver function, and delaying cirrhosis progression. As an
insufficient number of cases were included in this study with a
short follow-up and assessment of long-term outcome after surgery,
further studies based on more cases and longer follow-up are
needed.
Acknowledgements
Funding sources were received from the Shanghai
Municipal Health Bureau (grant no. 2009232) and the Shanghai
Municipal Health Bureau Key Disciplines (grant no. ZK2012A05).
Abbreviations:
|
PTVE
|
percutaneous transhepatic variceal
embolization
|
|
PSE
|
partial splenic embolization
|
|
PBMC
|
peripheral blood mononuclear cells
|
References
|
1
|
Comar KM and Sanyal AJ: Portal
hypertensive bleeding. Gastroenterol Clin North Am. 32:1079–1105.
2003. View Article : Google Scholar
|
|
2
|
Augustin S, González A and Genescà J:
Acute esophageal variceal bleeding: Current strategies and new
perspectives. World J Hepatol. 2:261–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kovalak M, Lake J, Mattek N, Eisen G,
Lieberman D and Zaman A: Endoscopic screening for varices in
cirrhotic patients: data from a national endoscopic database.
Gastrointest Endosc. 65:82–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Groszmann RJ, Garcia-Tsao G, Bosch J, et
al: Beta-blockers to prevent gastroesophageal varices in patients
with cirrhosis. The N Engl J Med. 353:2254–2261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merli M, Nicolini G, Angeloni S, et al:
Incidence and natural history of small esophageal varices in
cirrhotic patients. J Hepatol. 38:266–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D’Amico G, Pagliaro L and Bosch J:
Pharmacological treatment of portal hypertension: an evidence-based
approach. Semin Liver Dis. 19:475–505. 1999.PubMed/NCBI
|
|
7
|
Bosch J and Garcia-Pagan JC: Prevention of
variceal rebleeding. Lancet. 361:952–954. 2003. View Article : Google Scholar
|
|
8
|
de Franchis R: Evolving consensus in
portal hypertension. Report of the Baveno IV consensus workshop on
methodology of diagnosis and therapy in portal hypertension. J
Hepatol. 43:167–176. 2005.
|
|
9
|
Lunderquist A and Vang J: Sclerosing
injection of esophageal varices through transhepatic selective
catheterization of the gastric coronary vein. A preliminary report.
Acta Radiol Diagn (Stockh). 15:546–550. 1974.
|
|
10
|
Benner KG, Keeffe EB, Keller FS and Rösch
J: Clinical outcome after percutaneous transhepatic obliteration of
esophageal varices. Gastroenterology. 85:146–153. 1983.PubMed/NCBI
|
|
11
|
Chikamori F, Kuniyoshi N, Shibuya S and
Takase Y: Correlation between endoscopic and angiographic findings
in patients with esophageal and isolated gastric varices. Dig Surg.
18:176–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
L’Hermine C, Chastanet P, Delemazure O,
Bonniere PL, Durieu JP and Paris JC: Percutaneous transhepatic
embolization of gastroesophageal varices: results in 400 patients.
AJR Am J Roentgenol. 152:755–760. 1989.PubMed/NCBI
|
|
13
|
Koconis KG, Singh H and Soares G: Partial
splenic embolization in the treatment of patients with portal
hypertension: a review of the english language literature. J Vasc
Interv Radiol. 18:463–481. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Y, Wang Y, Shi M, et al: Clinical
study of combined interventional treatment of portal hypertension.
Chin J Ethnomed Ethnopharm. 11:63–65. 2010.(In Chinese).
|
|
15
|
Henderson NC and Iredale JP: Liver
fibrosis: cellular mechanisms of progression and resolution. Clin
Sci (Lond). 112:265–280. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Leclercq I, Brymora JM, et al:
Kupffer cells mediate leptin-induced liver fibrosis.
Gastroenterology. 137:713–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pesce JT, Ramalingam TR, Mentink-Kane MM,
et al: Arginase-1-expressing macrophages suppress Th2
cytokine-driven inflammation and fibrosis. PLoS Pathog.
5:e10003712009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao B, Radaeva S and Park O: Liver natural
killer and natural killer T cells: immunobiology and emerging roles
in liver diseases. J Leukoc Biol. 86:513–528. 2009.PubMed/NCBI
|
|
19
|
Delhem N, Carpentier A, Morales O, et al:
Regulatory T-cells and hepatocellular carcinoma: implication of the
regulatory T lymphocytes in the control of the immune response.
Bull Cancer. 95:1219–1225. 2008.(In French).
|
|
20
|
Delhem N, Cottrez F, Carpentier A, et al:
Role of the regulatory T lymphocytes in hepatitis C fibrosis
progression. Bull Cancer. 95:1029–1038. 2008.(In French).
|
|
21
|
Moriyasu F, Nishida O, Ban N, et al:
Measurement of portal vascular resistance in patients with portal
hypertension. Gastroenterology. 90:710–717. 1986.PubMed/NCBI
|
|
22
|
Scott J, Dick R, Long RG and Sherlock S:
Percutaneous transhepatic obliteration of gastro-oesophageal
varices. Lancet. 2:53–55. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sangro B, Bilbao I, Herrero I, et al:
Partial splenic embolization for the treatment of hypersplenism in
cirrhosis. Hepatology. 18:309–314. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alwmark A, Bengmark S, Gullstrand P,
Joelsson B, Lunderquist A and Owman T: Evaluation of splenic
embolization in patients with portal hypertension and
hypersplenism. Ann Surg. 196:518–524. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moro E, Pais M, Benvegnu M, Ferrari M and
Bittolo Bon G: Decrease of insulin resistance after splenectomy in
a diabetic patient with liver cirrhosis and portal hypertension.
Physiopathologic evaluation. Minerva Gastroenterol Dietol.
40:213–218. 1994.(In Italian).
|
|
26
|
Pålsson B, Hallén M, Forsberg AM and
Alwmark A: Partial splenic embolization: long-term outcome.
Langenbecks Arch Surg. 387:421–426. 2003.PubMed/NCBI
|
|
27
|
Tajiri T, Onda M, Yoshida H, Mamada Y,
Taniai N and Kumazaki T: Long-term hematological and biochemical
effects of partial splenic embolization in hepatic cirrhosis.
Hepatogastroenterology. 49:1445–1448. 2002.PubMed/NCBI
|
|
28
|
Yamaguchi S, Kawanaka H, Yoshida D,
Maehara Y and Hashizume M: Splenic hemodynamics and decreased
endothelial nitric oxide synthase in the spleen of rats with liver
cirrhosis. Life Sci. 80:2036–2044. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoefs JC, Wang FW, Lilien DL, Walker B and
Kanel G: A novel, simple method of functional spleen volume
calculation by liver-spleen scan. J Nucl Med. 40:1745–1755.
1999.PubMed/NCBI
|
|
30
|
Chadburn A: The spleen: anatomy and
anatomical function. Semin Hematol. 37(Suppl 1): S13–S21. 2000.
View Article : Google Scholar
|
|
31
|
Okamoto A, Fujio K, van Rooijen N, et al:
Splenic phagocytes promote responses to nucleosomes in (NZB × NZW)
F1 mice. J Immunol. 181:5264–5271. 2008.PubMed/NCBI
|
|
32
|
Sekiguchi T, Nagamine T, Takagi H and Mori
M: Autoimmune thrombocytopenia in response to splenectomy in
cirrhotic patients with accompanying hepatitis C. World J
Gastroenterol. 12:1205–1210. 2006.PubMed/NCBI
|
|
33
|
Li ZF, Zhang S, Lv GB, et al: Changes in
count and function of splenic lymphocytes from patients with portal
hypertension. World J Gastroenterol. 14:2377–2382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harrington LE, Hatton RD, Mangan PR, et
al: Interleukin 17-producing CD4+ effector T cells
develop via a lineage distinct from the T helper type 1 and 2
lineages. Nat Immunol. 6:1123–1132. 2005.PubMed/NCBI
|
|
35
|
Infante-Duarte C, Horton HF, Byrne MC and
Kamradt T: Microbial lipopeptides induce the production of IL-17 in
Th cells. J Immunol. 165:6107–6115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park H, Li Z, Yang XO, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS
and Jia JH: Increased Th17 cells contribute to disease progression
in patients with HBV-associated liver cirrhosis. J Viral Hepat.
19:396–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lemmers A, Moreno C, Gustot T, et al: The
interleukin-17 pathway is involved in human alcoholic liver
disease. Hepatology. 49:646–657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang L, Chen S and Xu K: IL-17 expression
is correlated with hepatitis B-related liver diseases and fibrosis.
Int J Mol Med. 27:385–392. 2011.PubMed/NCBI
|
|
40
|
Ye Y, Xie X, Yu J, et al: Involvement of
Th17 and Th1 effector responses in patients with Hepatitis B. J
Clin Immunol. 30:546–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li H, Zheng HW, Chen H, et al: Hepatitis B
virus particles preferably induce Kupffer cells to produce TGF-β1
over pro-inflammatory cytokines. Dig Liver Dis. 44:328–333.
2012.PubMed/NCBI
|
|
42
|
Bettelli E, Carrier Y, Gao W, et al:
Reciprocal developmental pathways for the generation of pathogenic
effector TH17 and regulatory T cells. Nature. 441:235–238. 2006.
View Article : Google Scholar
|
|
43
|
Mangan PR, Harrington LE, O’Quinn DB, et
al: Transforming growth factor-beta induces development of the
T(H)17 lineage. Nature. 441:231–234. 2006. View Article : Google Scholar
|
|
44
|
Veldhoen M, Hocking RJ, Atkins CJ,
Locksley RM and Stockinger B: TGFbeta in the context of an
inflammatory cytokine milieu supports de novo differentiation of
IL-17-producing T cells. Immunity. 24:179–189. 2006. View Article : Google Scholar
|
|
45
|
Niedbala W, Wei XQ, Cai B, et al: IL-35 is
a novel cytokine with therapeutic effects against collagen-induced
arthritis through the expansion of regulatory T cells and
suppression of Th17 cells. Eur J Immunol. 37:3021–3029. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Collison LW, Chaturvedi V, Henderson AL,
et al: IL-35-mediated induction of a potent regulatory T cell
population. Nat Immunol. 11:1093–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang J, Yang M, Htut TM, et al:
Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22
and RORgamma t. Eur J Immunol. 38:1204–1214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fontenot JD, Gavin MA and Rudensky AY:
Foxp3 programs the development and function of
CD4+CD25+ regulatory T cells. Nat Immunol.
4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khattri R, Cox T, Yasayko SA and Ramsdell
F: An essential role for Scurfin in CD4+CD25+
T regulatory cells. Nat Immunol. 4:337–342. 2003. View Article : Google Scholar : PubMed/NCBI
|